Abstract
In this chapter, we will introduce the concept of regenerative approaches in orthodontic and orthopedic treatments. We will specifically focus on the current tissue engineering approaches in bone/cartilage remodeling, which could be stem cell based, biomaterial based, or a combination. We will then discuss the potential applications of bone/cartilage engineering technologies to address clinical problems, including jaw discrepancies, bone loss due to periodontal diseases, and temporomandibular disorders. In each disease model settings, the current evidence (preclinical/clinical) in applying the regenerative approaches will be discussed and the foreseeable opportunities and challenges will be addressed. Finally, a summary of the main findings and future directions will be provided.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Bone Remodeling and Regeneration Through Tissue Engineering
Bone is a dynamic tissue that undergoes remodeling all the time. Bone tissue has an intrinsic ability to repair small defects and some fractures. However, if bone defects exceed the critical size (which depends on the location and anatomy, usually >2 cm in humans) [1], the body usually cannot repair them unaided.
In defect situations, the bone needs to be reconstructed, to providing it with the necessary mechanical integrity and o aid rehabilitation. Current clinical treatments of large bone defects using bone grafts, including autografts and allografts, have considerable limitations [2, 3]. Autografts require a second operation to harvest bone from other sites in the body. Associated complications may include donor site morbidity, intraoperative morbidity, and prolonged hospitalization. Limitations also include an inadequate quantity of donor bone and difficulties in shaping the graft to the correct shape to restore complex 3-dimensional defects. The use of allografts is associated with the potential transmission of infection and with host immune responses.
Recently, engineered bone scaffolds have been receiving increasing attention as alternatives to conventional bone grafts [4]. For bone tissue engineering, three factors are crucial for the long-term success: osteogenic cells to produce the bone matrix, biomaterials, or scaffolds with suitable mechanical properties, to provide the desired microenvironment, and biomolecular cues such as growth factors to attract osteogenic cells and/or modify their functions. In the following part, those key factors for bone tissue engineering are discussed in detail.
2 Cell Sources for Bone Regeneration
Mesenchymal Stem Cells (MSCs)
MSCs have long been recognized for their potential use because they can differentiate and form bone during the natural bone development process. MSCs have been defined through the expression of various markers (i.e., negative for CD34, CD45, CD14, CD11a, CD19, and HLA-DR, and positive for STRO-1, CD29, CD73, CD90, CD105, CD106, CD166, CD146, and CD44) [5, 6]. The high proliferative potential of MSCs, combined with their ability to withstand freezing conditions, allows for their expansion in vitro, to obtain clinically relevant cell numbers.
Dental Stem Cells (DSCs)
In addition to MSCs, various DSCs such as dental pulp stem cells (DPSCs), stem cells from the human exfoliated deciduous tooth (SHED), dental follicle stem cells (DFSCs) and periodontal ligament stem cells (PDLSCs) have been used for bone tissue engineering due to their ease of harvesting and high proliferation rate [7, 8].
DPSCs express typical MSC biomarkers, such as CD90, CD29, CD73, CD44, and CD105 [9, 10], and they differentiate into odontoblast-like cells [11]. In vivo transplantation of such DPSCs into nude rats generates living fibrous lamellar bone tissues containing osteocytes [12].
Induced Pluripotent Stem Cells (iPSCs)
The generation of iPSCs was first reported by the Yamanaka group and others in 2006. This approach involves directly introducing specific reprogramming genes (Oct4, Sox2, cMyc, and Klf4) into somatic cells to give them pluripotent abilities [13]. iPSCs have been induced into osteoblast-like cells [14, 15] for bone regeneration.
Other Cells
Other stem cells such as adipose-derived stem cells [16], and peripheral blood-derived stem cells [17] are also used for bone and cartilage repair.
3 Biomaterials for Bone Regeneration
Bioceramics
Bone contains an inorganic component of carbonated apatite minerals. Bioceramics are a class of inorganic biomaterials which have similar composition to the mineral parts of natural bone. Calcium phosphates such as β-tricalcium phosphate and hydroxyapatite are the most common types of bioactive ceramics used for bone tissue engineering. They are widely used in clinical practice as bone cements or as coatings on implants [18].
Bioactive glass is another important type of bioceramic. It is composed of silicates, calcium, and phosphate [19]. Compared to hydroxyapatite, bioactive glass is reported to have a faster bone regeneration rate in vivo as a bone graft [20]. Recently, the capacity of releasing bioactive ions such as Ca2+, Si4+, Mg2+, Cu2+, Sr2+, Li+, and Ag+ from bioceramics has been receiving interest [21].
Polymers
Various naturally derived and synthetic polymers are used for bone tissue engineering. Natural polymers, such as collagen, gelatine, and alginate exhibit several desirable characteristics such as good biocompatibility, degradability, and cell attachment. Collagen is the main protein component of natural bone. These biopolymers contain aminoacid sequences (specifically, the adhesion ligand arginine–glycine–aspartic acid (RGD)) to which cells readily attach. For natural polymers, concerns exist over their immunogenicity and their relatively weak mechanical properties.
Synthetic polymers such as polycaprolactone, polylactic acid (PLA ) , polyglycolic acid (PGA ) , copolymers of PLA and PGA (PLGA) offer a versatile alternative. PCL is a popular polymer for use in bone tissue engineering systems. It has high mechanical strength and is included in the list of US Food and Drug Administration (FDA) approved products. Synthetic polymers usually lack features that promote cell adhesion, and they undergo very slow hydrolytic degradation in vivo. A combination with natural polymers such as a surface coating can address this particular concern for synthetic polymers.
Composites and Hybrids
Composites are an increasingly important class of biomaterials used for bone tissue engineering, owing to their ability to combine the strength of both bioceramics and polymers. Inorganic-organic composites aiming to “mimic” the composite nature of native bone combine the toughness of a polymer phase with the compressive strength of an inorganic phase, to generate bioactive materials with improved mechanical properties and degradation profiles. These composites and hybrid usually include a biodegradable polymer phase, in which bioceramic particles are incorporated as fillers. Tissue-engineered porous PEO layered polymer-magnesium system, for example, is emerging and are showing promising [22].
3.1 Growth Factors for Bone Regeneration
Bone is a dynamic tissue that constantly undergoes remodeling, with a coupled process of bone formation by osteoblasts and resorption by osteoclasts. When bone defects occur, a bone healing process is triggered. Osteoblasts differentiate and are activated to form new bone. During this process, growth factors particularly bone morphogenetic proteins (BMP) [23] are expressed to promote the differentiation of osteoblasts and to enhance the bone-forming activity [24]. Preclinical and clinical studies have shown that BMP-2 has a strong osteoinductive ability, and can be utilized in therapeutic interventions for bone defects, non-union fractures, spinal fusion, osteoporosis, and root canal surgery [23, 25]. Recombinant human BMP-2 (rhBMP-2) has been approved by the US FDA for clinical use [26].
In addition to BMPs, fibroblast growth factors (FGFs) and vascular endothelial growth factor (VEGF ) are also important in bone tissue engineering. VEGF is an angiogenic protein that regulates endothelial cell proliferation. FGFs are group of proteins that induce angiogenesis through endothelial and osteoblast cell proliferation. The design of scaffolds with the localized release of growth factors has attracted significant attention, due to the potential for dose reduction, a controlled release pattern, and lower side effects compared to systemic delivery. Various advanced fabrication techniques such as 3D printing, electro-spinning, and electro-spraying are used for growth factor and drug delivery to enhance bone growth when scaffolds are used [1].
4 Regenerative Approaches for Jaw Discrepancies
A jaw size discrepancy is commonly seen in orthodontic patients and can cause a mild to a severe malocclusion. The latter usually requires a multi-disciplinary treatment plan including orthodontic treatment and orthognathic surgery. Conventionally, orthodontic treatment is used to compensate for the size difference between jaws in moderate cases, and to de-compensate the inclined teeth to facilitate orthognathic surgery in severe cases.
As shown in Fig. 1, in the past, when there is no skeletal discrepancy, no treatment or simple orthodontic treatment is needed for an ideal occlusion. When there exists q moderate level of jaw discrepancy, orthodontic treatment can camouflage the skeletal difference via the repositioning of teeth. When severe skeletal problems are identified in patients, usually a combination of orthodontic and orthognathic treatment is planned. At this point, there is not much tissue regeneration involved to correct the jaw discrepancy.
As is now well accepted, orthodontic tooth movement relies on alveolar bone remodeling, which is initiated by force. This bone remodeling process involves both osteoblastic and osteoclastic activities. With the understanding of this process and the development of bone tissue engineering, the treatment efficacy of orthodontic approaches for dealing with jaw discrepancies has been greatly improved.
Unlike moderate jaw discrepancy, when a severe discrepancy exists, this indicates that a large volume of bone is needed. Besides grafting, bone tissue engineering techniques have been used widely in this field. In this section, first, tissue engineering techniques that further developed the capacity of conventional orthodontic treatment will be introduced. This part addresses moderate jaw discrepancies. Secondly, bone tissue engineering technique advancements for treating severe skeletal jaw discrepancies will be discussed. Currently, with the aid of rapidly developing tissue engineering techniques, less invasive procedures should be possible for treating jaw discrepancies in the future (Fig. 1).
4.1 Orthodontic Treatment Tissue Engineering Approaches for Jaw Discrepancies
Modern orthodontics has adopted so many tissue engineering techniques that the capacity of orthodontists to correct jaw discrepancy has been expanded considerably. The border between camouflage orthodontic treatment and surgical treatment has been pushed outwardly (Fig. 1, lower panel). Implant anchorage, maxillary expansion, micro-osteoperforation, and corticotomy are de facto common surgical techniques that expand the capacity of traditional orthodontic appliances to enable faster tooth movement. These also allow clinicians to move teeth across a longer distance. While most clinicians may not instantly relate these techniques to tissue engineering, they all rely heavily on the bone remodeling capabilities of alveolar bone [27] and/or jaw bone. This section focuses on tissue engineering techniques adopted by orthodontists, and experimental approaches for orthodontic treatment.
4.1.1 Osteoclastic Activity Accelerates Orthodontic Tooth Movement Rate
Surgically facilitated orthodontic treatment is performed widely in modern orthodontic practice. Liou and coworkers showed that orthognathic surgery could create a window period of up to 3 months for active osteoclast activity and alveolar bone metabolism, which resulted in accelerated tooth movement [28]. A regional acceleration phenomenon relates to an active osteosis response around a corticotomy site. Cortical bone is regarded as the main resistance during tooth movement, and the cortical layer is removed in front of the tooth along the track of its movement. After corticotomy, the accelerating effect persists for the first 3 months. This effect is not caused by the removal of resistance, but rather by the activation of resorption and formation processes in the alveolar bone itself. To initiate a burst of acceleration, a corticotomy requires a full-thickness gingival and mucosa flap to provide direct access to the surgical site. As such, a conventional corticotomy is relatively complex, with postoperative complications such as pain and discomfort. A series of minimally invasive modifications are now available, including corticision, piezocision, micro-osteoperforation, and discision [29]. A tissue flap is avoided in all these modified versions, and this reduces soft tissue reaction.
In general, corticotomy is suitable for many types of tooth movement. Its application is limited to patients who take anti-inflammatory medicines (e.g., corticosteroids, non-steroidal anti-inflammatory agents) and bisphosphonates, as these interfere with bone remodeling [30, 31]. There is a delicate balance between the choice of a corticotomy to maintain a satisfactory acceleration of tooth movement, and the risk of harm to the individual. More research is needed in this field, as inconsistent results have been found in animals versus patients. A recent animal study indicated that even remote corticotomy can effectively accelerate tooth movement [32]. A review of clinical studies has pointed out that strong clinical evidence is lacking, mostly due to poorly designed studies [33].
Micro-osteoperforation has been used in both animal and clinical studies, with little or no complications. As micro-osteoperforation avoids raising a flap, irritation and tissue swelling are minimized. Most patients tolerate this procedure very well [34, 35]. In one study, three micro-osteoperforations were performed on the buccal side of the target canine. There was no significant acceleration of tooth movement observed, which may reflect the low frequency of the procedure. A higher frequency may be needed to achieve an obvious acceleration [36]. A 2020 systematic review and meta-analysis concluded that micro-osteoperforation was not effective in enhancing orthodontic tooth movement [37].
4.1.2 Osseointegration Enables Definite Anchorage in Orthodontic Treatment
A mini-screw implant used in orthodontic treatment provides stable anchorage, reduces the need for patient compliance, and avoids unfavorable tooth movement. Osseointegration, as first introduced by Branemark in the 1960s, serves as the biological basis for the min-screw implants used as orthodontic anchorage. The application of mini-screw implants has changed the management of many cases from extraction to non-extraction, and from surgical cases to extraction cases. OTM facilitated by these implants is comparable to what occurs with conventional anchorage [38, 39].
4.1.3 Intramembranous Osteogenesis Corrects Jaw Discrepancy
Maxillary expansion is to address a discrepancy between the upper and lower jaws in the transverse plane. Usually in such cases, the width of the maxilla is below normal, while the width of the mandible is within the normal range. Correction of mandibular width is sometimes carried out at the same time as the expansion of maxilla. The optimal age to undergo expansion is in the teenage years, from 13 to 15 years old. The mechanism behind the maxillary expansion is the same as distraction osteogenesis (DO ) (as described in the next section). Patients with skeletal jaw discrepancy also can benefit from the surgically facilitated maxillary expansion, e.g., for mature adult patients.
4.1.4 Factors Regulating Orthodontic Tooth Movement
It is well accepted that a continuous light force can provide an optimal rate of tooth movement with minimum tissue damage, e.g., root resorption. Current research on tooth movement indicates that it involves three separate but interacting osteosis activities: resorption, formation, and remodeling. Bone resorption is regarded as rate-limiting aspect for tooth movement rate [40], and animal studies have proved a direct relationship with bone resorption via osteoclast activation [41, 42]. The sympathetic nervous system regulates bone remodeling (including osteoblast mediated bone formation and osteoclast-mediated bone resorption) through β-2 adrenergic receptors (Adrb2) [43].
A number of chemicals and drugs have been studied for possible use in accelerating the rate of orthodontic tooth movement [44, 45], e.g., hydrogen sulfide [46, 47], triptolide [48], and asperosaponin [49]. On the other hand, resveratrol has been found to reduce the rate of orthodontic tooth movement [50].
Low level laser therapy has been proposed to accelerate orthodontic tooth movement, with most studies using near-infrared gallium–aluminum–arsenic (GaAlAs) diode lasers for photobiomodulation [51,52,53]. Low level laser therapy increases IL-1β secretion and faster tooth movement is observed [53]. A 2014 systematic review and meta-analysis concluded that low level laser therapy accelerated tooth movement, with a moderate level of evidence. Further research is needed to optimize this technique so that it becomes a part of the normal routine [54]. In 2020, a triple-blind, split-mouth, randomized controlled trial found no accelerating effect on tooth movement [55]. This may be due to sub-optimal laser parameters being used.
4.2 Orthodontic/Orthopedic Treatment Tissue Engineering Approaches for Jaw Discrepancies
Depending on the type of discrepancy, jaw correction sometime requires orthopedic intervention with additional orthodontic treatment. For instance, in an adult patient with a palatal cleft, dentition compensation (including changes to the alveolar bone) always exists. In order to achieve the mastication function, orthodontic treatment is needed to correct the malocclusion (a de-compensation), before treatment of the jaw discrepancy.
Most cases of jaw discrepancy are well compensated with teeth, alveolar bone and soft tissues, which results in a normal facial appearance, progressing to moderate asymmetry and/or malocclusion. Only a small proportion of patients with a moderate to severe jaw discrepancy present with an obviously asymmetric facial appearance and severe malocclusion [56]. This latter situation is seen commonly in cases of cleft palate, or of benign tumors that progressively disrupt bone (e.g., aggressive giant cell lesions, or ameloblastoma). On the other hand, trauma in the maxillofacial area, especially cases of TMJ trauma in young patients, often result in a severe jaw discrepancy.
Conventionally, there are two types of treatment approaches. Distraction osteogenesis (DO ) was proposed in 1989 [57], and was first applied in maxillofacial cases in 1998 [58]. DO has now become a standard procedure for elongating the jawbone and the expanding maxilla. DO uses the patient’s own bone formation capacity to increase the bone volume. Its application in the maxillofacial area is limited by the complex and irregular shapes of the facial bones in individual cases. Each person has a unique facial contour. Another issue is damage to accompanying nerves, since this can impact significantly on the quality of the newly formed bone [59].
4.2.1 DO in the Maxilla
Distraction osteogenesis as a treatment for skeletal deformities relies on achieving an increase in bone volume. It avoids or reduces the need for bone grafting, and the surgery is less invasive. Because this technique can expand bone in any direction, it has often been used for correcting jaw discrepancies in cases of congenital or acquired deformities, where the bone volume deficit is large.
In DO, an osteotomy separates the bone, the two parts are then fixed a distraction device, after which a gradual distraction period allows intramembranous bone formation to occur. In this process, mesenchymal stem cells from the bone marrow differentiate into osteoblasts to form neo-callus. Bone formation follows the “tension-stress principle,” as proposed by Ilizarov [60]. Neovascularization is critically required for successful bone formation in this process and for this reason, systemic factors that impair neovascularization also affect bone regeneration at the DO site [61].
Midface deficiency is common in cleft patients due to unavoidable scar tissue formation during surgical closure of clefts at an early age. When the growth of the maxillofacial complex ends, a severe crossbite and maxillary hypoplasia are often the end result, with a low bone volume. A simple maxillary advancement (Le Fort I) is not enough. DO can provide reliable advancement of the maxilla with substantial bone deposition. Results are stable 12 months after treatment. Using a rigid external distraction device, it is possible to perform a stable advancement of the maxilla in both the horizontal and vertical planes.
4.2.2 DO in the Mandible
In bone tissue, the areas with the highest metabolic activity receive the richest sympathetic innervation [62]. Bone cells express neuronal signal receptors, which mediate neuro-osteogenic interactions [63]. The sympathetic nervous system regulates the bone remodeling process [43, 64, 65], stimulating osteoclastic activity via β-2 adrenergic receptors (Adrb2). In Adrb2 knockout animals, tooth movement is significantly reduced.
Nerve integrity has an impact on bone remodeling. In sagittal DO of the mandible, there is a risk that DO surgery procedures may injure or transect the inferior dental nerve. If this occurs, there would be a reduction in new bone formation following DO [59]. In the mandible, the approach of using DO is limited to cases where correction in the transverse dimension is required, such as in hypoplasia of the mandible [66].
4.3 Bone Tissue Engineering for the Maxillofacial Region
Besides auto-transplantation, heterogeneric osteogenesis has been explored extensively for bone tissue engineering. Bone tissue grafting [67, 68] and the implantation of synthetic bone substitutes have been the two main approaches used for the treatment of jaw discrepancies. A recently review suggested that using allogenic bone chips could be a safe technique, [69] however this approach has limited source material and is not likely to achieve the maximum extent of bone regeneration capacity. This next section will focus on synthetic bone substitutes used in bone tissue engineering for the maxillofacial region [70, 71].
Bone substitutes include both organic and inorganic-metal substitutes. This has been a greatly expanding topic within tissue engineering over recent decades.
4.3.1 Vascularization in Bone Regeneration
Scaffold materials have been applied to repair bone defects in the maxillofacial area. When the bone defect size exceeds a certain limit, or the bone volume needed to correct a jaw discrepancy is large, achieving sufficient tissue in -growth into the scaffold becomes a major challenge. The importance of achieving blood vessel in-growth into scaffolds used in bone regeneration is acknowledged widely. To promote angiogenesis in bone regeneration, many approaches have been reported, including the addition of growth factors (e.g., VEGF and bFGF) [72, 73], incorporating blood vessels [74], pre-vascularizing the scaffold material with a cell sheet [75], and optimizing the scaffold microstructure [76]. In yet another approach, an arteriovenous loop was microsurgically created and introduced inside a scaffold used to repair a critical size bone defect in the mandible. There was a significant blood vessel formation inside that scaffold, and more bone formation throughout the scaffold [74].
4.3.2 Research Models
To study the bone regeneration capacity of bone substitutes used in the maxillofacial region, many in vivo models have been proposed [77, 78]. Bone in the maxillofacial area follows intramembranous formation, like the bones of the cranium. The calvarial bone defect model has become the most common critical size defect model used to evaluate the bone formation capacity of new regeneration techniques and materials used in the maxillofacial region. The calvarial model is typically employed with small animals, e.g., mice [73], rats [79], and rabbits [80, 81]. A mandible bone defect model is often used with large animals, e.g., dogs [82, 83], pigs [84], sheep [85] and non-human primates [86, 87]. Recently, a mandible defect model in small animals has also been reported [88]. In the maxilla, the research model is usually alveolar bone regeneration of clefts [89, 90]. Recently, a rat mandible model has been introduced to study clefts [91].
5 Regenerative Aspects in Orthodontic Treatments for Periodontal Diseases
Orthodontic tooth movement is not limited to biological events within the periodontal ligament. It involves two interacting biological activities: one involves remodeling of alveolar bone, and the other involves remodeling of periodontal tissues. Orthodontic intervention, essentially applied as a light force, initiates mechano-transduction, and triggers a series of osteosis and angiogenesis processes that are yet to be understood fully.
The concept of guided orthodontic regeneration has been proposed by Paolone and coworkers [92, 93]. This recognizes the regenerative potency of orthodontics in periodontal tissues, including both soft and hard tissues. From a tissue engineering standpoint, using orthodontic treatment (with light force) appears to be a feasible approach to tissue regeneration with less complications than surgical methods (Fig. 2) [94, 95]. In this section, the regeneration of periodontal tissue achieved via orthodontic treatment will be discussed.
Orthodontic force-initiated tooth movement and tissue responses. Orthodontic force is applied to the labial side of the tooth crown. The tooth rotates around the center of resistance: the crown moves to the lingual side, while the root moves to the labial side (a). At the cervical level of the root, periodontal tissue on the labial side is expanded, while periodontal tissue on the lingual side is compressed (b). Expansion stress activates osteoblast precursors, and bone formation is observed; compression stress promotes osteoclast formation via fusion of mononuclear cells, and bone resorption occurs. Usually these mononuclear cells migrate in from nearby blood vessels (c)
Tissue responses to applied forces vary depending on the force type that is used. Take alveolar bone, for example. Bone formation can be observed under an expansion force, while bone resorption occurs when a compression force is applied. The periodontium plays a conductor role by transferring the applied mechanical forces into biological signals, to initiate tissue responses. As shown in vivo, periodontal tissue can be reshaped as needed by applying different forces. An orthodontic force can regenerate gingival tissue, alveolar bone and periodontium. It can also aid periodontal treatment to preserve a tooth.
An insufficient alveolar bone volume is a common problem in edentulous ridge areas where teeth have been lost. To prepare the periodontal tissue to support an implant, orthodontic approaches can be used to increase the alveolar crest height prior to implant insertion.
Besides implants, as an alternative treatment for a missing tooth, tooth auto-transplantation has been undertaken, and this has proved effective in the long term. An active periodontium has been regarded as the most important factor for the success of tooth auto-transplantation.
Orthodontic force can create an activated periodontium. In the following part, orthodontic application in periodontal tissue regeneration is discussed for gingival, alveolar bone and periodontal regeneration.
5.1 Orthodontics Improves Periodontal Esthetics: Gingival Recession/Black Triangle
The etiology of the gingival recession is multifactorial. It is generally regarded that growing patient can gain spontaneous re-growth of gingival while it is almost impossible to regain recessed gingiva in adults. Few studies have reported the regain of recessed labial gingiva and gingival papilla. The mechanism behind the success of gingiva re-growth lies in the dynamically remodeling alveolar bone. Orthodontic force or surgical procedures (for instance, corticotomy introduced in other parts) could activate a series of osteosis process including resorption, formation, and remodeling of the alveolar bone. Research has gradually better understood the relation between orthodontics and regeneration of periodontal tissue reaction. Gingival attachment is determined by supracrestal tissue attachment [96], which is related to the shape of alveolar bone, mostly the height. In practice, treating recessed gingival tissue can be (partially) achieved with orthodontic intervention.
Gingival attachment is determined by the alveolar bone. When the front tooth is labially positioned, it is not surprising to observe the gingival recession. Hence obvious regain of recessed gingiva is seen in repositioning the teeth in the center of the alveolar ridge [97,98,99,100,101,102]. Depending on the original position of the recessed tooth and the occlusion, common orthodontic approaches include torque (incline the crown while maintain the position of the root), retracting, and intrusion. Orthodontic correction can greatly improve the recession grading, which is correlated to the treatment prognosis of periodontal plastic surgery [103,104,105,106,107]. Hence in the treatment of the gingival recession, the position of the tooth inside the alveolar ridge should be prioritized.
As the cause of gingival recession is multifactorial, there has not been a systematic treatment protocol or guideline. There exists a controversial debate in the relation of orthodontic treatment and gingival recession. Conclusive research on gingival recession is lacking in the literature. Yet some evidence showed it is possible to regrow gingival when dental plaque is well controlled. A multi-disciplinary team management is required for the succeed in the treatment of gingival recession. For instance, under a carefully monitored plaque control, orthodontic treatment could significantly minimize the recessed area followed by gingival reconstructive surgery. This combined strategy shall provide a good prognosis of treatment of gingival recession.
5.2 Orthodontics in Prosthesis
The esthetics of labial gingival contour (height, width, and symmetry) plays an essential role in dentofacial esthetics in the front view. When performing implantation at the anterior maxillary region, gingiva usually recesses to a noticeable level in the first 3–6 months post-surgery [108,109,110,111]. To prepare alveolar bone and gingival for esthetic implant placement [112], orthodontic extrusion, a coronal tooth movement, can be performed to activates the osteosis process [93]. As a result, the alveolar process will be remodeled at the coronal direction (increasing crest height) as well as a reduction in periodontal pocket depth could be observed [113]. Orthodontic appliance can be used to develop implant site, which is not restricted by the residual attachment level. The efficacy of gingival and alveolar bone regeneration was reported about 70% and 60% [114]. Due to this plastic nature of periodontal tissue with force, orthodontic techniques have been adopted to grow the periodontal structure prior to an implant placement [115,116,117,118,119,120,121].
In some cases, to gain biological distance restoration, the clinical crown (the part of a tooth that is exposed to oral cavity) needs to be elongated. Usually orthodontic extrusion is preferred. Conventional orthodontic extrusion creates bone apposition at the alveolar crest broadening the width of the attached gingival. This tissue regeneration is appreciated in implant placement where there is lacking enough bone to support the implant. On the contrary, in periodontal treatment of crown lengthening, this tissue regeneration becomes undesired. Then gingival fiber resection (fiberotomy) and root surface scaling are needed to avoid the coronal migration of periodontal tissue [122]. The reason is because that alveolar bone grows coronally as the tooth being extruded. And periodontal soft tissue attachment is determined by biological width. The term has been adopted as a clinical term that describes the variable dimension of the supracrestal attached periodontal tissue in apicocoronal (vertical) direction. It has been recently replaced by supracrestal tissue attachment. Supracrestal tissue attachment is a concise, descriptive definition of the histological structure: junctional epithelium and supracrestal connective tissue attachment [96]. The relation between alveolar bone crest to the periodontal attachment remained acknowledged. This concept of attachment centers on the important role of alveolar height in periodontal treatment.
5.3 Orthodontics Helps Reconstruct Periodontium
Missing tooth brings functional and esthetic issues. Prosthetic implant is commonly used to resolve the problem. Yet it lacks periodontal ligament which provides individuals with a physiologic proprioception and sensory reflection. In growing patients, implants fail to provide physiologic eruption which results with an unleveled gingival margin. Tooth auto-transplantation can be a good alternative as the tooth comes with viable periodontal ligament which enables a high successful rate [123]. Ankylosis and root resorption are common complications. Orthodontic treatment (force) activates physiological tissue response. Upon distortion of collagen fibers within periodontal ligament, mechanical strain transduces to cells inside ligament and neighboring tissues. Macrophages response early and appear in periodontal ligament when orthodontic force is applied. They then response to mechanotransductively released cytokines. Proinflammatory and angiogenic cytokines are produced by macrophages under compression strain, which alter the microenvironment of periodontal ligament [124]. These cytokines are influential to the survival of periodontal ligament. Bone lining cells in periodontal ligament play an important role in tooth movement and osteoclastogenesis in response to mechanical force [125]. Short term application of orthodontic force on donor’s tooth can activate the periodontal ligament with upregulated expression of inflammation, osteoclastogenesis. After transplantation, donor tooth activated by orthodontic force showed higher tissue regenerative potency than normal tooth. Genes relating to periodontal ligament regeneration, cell proliferation, osteoblastogenesis, and osteoclastogenesis are highly expressed in orthodontically activated donor tooth in the first-week post-transplantation. Osteoclastogenic gene expression in orthodontically activated donor tooth is reduced to significant lower level than that in normal donor tooth in the fourth-week post-transplantation. This consisted of histologic observation where root resorption was less and inflammatory activity subsided in 4 weeks [126].
The vitality of periodontal ligament is a key factor in the prognosis of transplanted teeth [127]. Hypofunctional, un-occluded, teeth have narrow periodontal ligament. Donor teeth with functional periodontal ligament survived much better than unerupted or partially erupted teeth [128]. They survive longer, have less complications, e.g., root resorption. This updates previous belief that unerupted or partially erupted teeth are preferable in tooth transplantation as they have wide periapical foramen and higher chance to maintain the vitality of pulp. The application of orthodontic force also increases the survival chance of transplanted tooth [127]. Early orthodontic force engagement (within 4 weeks post-transplantation) could reduce the incidence of ankylosis [128].
5.4 Orthodontic Application in Periodontal Treatment
Orthodontic force creates stress of periodontal tissue and receives a complicate set of signaling responses including osteoclastogenic and angiogenic activation. The former results in alveolar bone change and the latter may impact on the alteration in the gingival tissue and periodontal fiber. Orthodontic tooth movement can create periodontal tissue, which benefits perio-restorative patients. As discussed in the previous section “Orthodontics in prosthesis,” increased amount of soft and hard tissue is generated during extrusion. In perio-restorative patients, these excessive tissues could be used in periodontal regenerative surgery [94].
Aggressive periodontitis featured by disruption in periodontal tissue, extrusion of front teeth, and loss of teeth leaves esthetics and mastication disabilities [129]. In chronic periodontitis, with loss of periodontal tissue, crown/root proportion becomes large, which creates a greater unfavorable force to remained periodontium under normal mastication compared a healthy tooth. Orthodontic tooth intrusion is performed to correct the crown/root proportion [130]. In both aggressive and chronic periodontitis, there is tooth loss and space remained, which compromises mastication efficiency. To restore these spaces, orthodontic treatment is often applied to re-arrange remained teeth and space for a balanced and periodontal tissue-friendly restoration.
Zasciurinskiene and colleagues concluded that no evidence indicate either beneficial or deteriorating role of orthodontic treatment to periodontally compromised dentition [131]. A recent clinical study demonstrated that under strict plaque control periodontal assessment of aggressive periodontitis was similar to that of the orthodontic patient who has healthy periodontal tissue [129]. There have been a few case reports of orthodontic treatment on periodontitis [132].
6 Regenerative Approaches for Temporomandibular Joint Disorders (TMD)
During basic daily functions (i.e., speaking, swallowing, and eating), the temporomandibular joint (TMJ) plays an extremely important role in coordinating the jaw movements. The TMJ is described as a bilateral synovial joint formed by fibrocartilaginous articular surfaces of the mandibular condyle and glenoid fossa, muscles, ligaments, and the articular disc [133]. It is estimated that 10–40% of the population are affected by temporomandibular joint disorders (TMD), with a predominance among young adults under 45 years of age [134].
The main signs and symptoms of TMDs include limited mouth opening, mandibular deviation during the opening, displacement, clicking, locking, and muscle pain during mandibular movements. TMJ pathologies that require clinical treatment are internal derangement, degenerative joint disease, and ankylosis [133, 134].
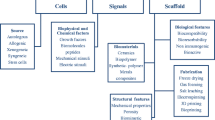
Three categories are described for the clinical treatment of TMJ pathologies: non-invasive, minimally invasive, and invasive. When in an advanced stage of degenerative disease, the total alloplastic reconstruction of the TMJ is considered the treatment of choice. To possibly eliminate the need for total TMJ replacement, tissue engineering may provide a functional and permanent biological replacement of the TMJ structures [135,136,137,138,139]. Figure 3 shows a general overview of main TMJ pathologies, current therapies, and the tissue engineering approach of the different TMJ structures.
6.1 Tissue Engineering of the TMJ
Many tissues of the body, after an injury, exhibit an ability to repair themselves, however, some tissues have little or no ability to self-repair. Among these, the TMJ tissues can be included. Given an advanced pathological process of the TMJ (i.e., osteoarthritis), coupled with limited repair capacity of the TMJ tissues (i.e., fibrocartilage, cartilage, bone), the current treatment options for clinicians and surgeons, in order to maintain normal function and eliminate the patient’s pain, are limited and considered semi-permanent [133, 134, 137,138,139].
To overcome the current obstacles, tissue engineering may provide permanent, biomimetic replacement tissue for the TMJ [136,137,138]. Thus, scientists have used the tissue engineering paradigm [139]. The first step is to characterize the native and healthy tissues, providing parameters for the appropriate design using the concepts of tissue engineering. Considering the tissues to be regenerated and its characteristics and properties, the parameters obtained will guide the optimal selection of cells, of scaffold materials (extracellular matrix), of proper growth factors and, in specific tissues, the necessity of biomechanical stimulation (mainly for the articular disc), making it possible to obtain an implantable biomimetic tissue [135,136,137,138,139]. Table 1 describes the main cell sources, scaffold materials, and growth factors used to tissue engineer the TMJ disc, the condylar cartilage, and the mandibular condyle.
Over the past decade, advances in the field of biomaterials science, tissue engineering, and stem cell therapies have led to the development of less invasive and alternative treatments for diseased joint tissues [133, 136, 137, 139]. Cell-based therapies involving expansion and transplantation of stem cells combined with different scaffold materials and growth factors have been shown regenerative capabilities to repair diseased TMJ tissues [140, 141]. Also, cell-free scaffolds have been used for TMJ cartilage regeneration, for fibrocartilage (disc) regeneration, and for osteochondral regeneration with regenerative capabilities in animal models [142,143,144].
Two methods have been extensively described in the literature for bone tissue engineering and for TMJ tissue engineering: (a) in situ tissue engineering, which consists of using cell-free scaffolds to attract local cells (cell homing) that will guide the regeneration process; (b) and scaffolds seeded with competent cells to guide the regeneration process [145].
Preclinical studies using small and large animals using different cell sources, combined with scaffolds made of a wide range of materials and enriched with a variety of growth factors have been described to regenerate the TMJ disc, the condylar cartilage and the mandibular condyle with promising outcomes [136, 146]. Some clinical trials [147] have demonstrated the efficacy of autologous or allogeneic MSCs in cartilage repair [148] (Table 2).
Besides the promising results from preclinical data, there are still challenges to overcome to bring tissue-engineered TMJ structures to the clinic. Among them, the total restoration and incorporation of fibrocartilage in the articular surfaces, the possible displacement of the implanted material, and the lack of long-term results of the regenerative approaches of the TMJ structures.
6.2 Future Treatment for the TMD Treatment
In view of the challenges that the unique TMJ environment represents (i.e., mechanically demanding and biochemically active), the field of tissue engineering has made significant progress over the past decade with promising results to replace diseased, displaced, or degenerated tissues. Currently, research has focused on biological substitutions of the mandibular disc, adjacent structures of the TMJ, and engineering of craniofacial tissues (i.e., bone, soft tissue, nerve, muscle). In addition, tissue engineering strategies to provide treatment options for the glenoid fossa and the articular eminence should be considered.
The scientific and technological advances available provide a solid basis for scientists and surgeons to overcome the challenges that still exist in the field of TMJ tissue engineering, such as the proper selection of cell sources, scaffold materials, and growth factors. A detailed understanding of the native tissues of the TMJ and their respective complex pathologies are essential for scientists who wish to develop and increase the success of permanent biological TMJ replacements.
7 Conclusions and Future Perspectives
Current Status
The regenerative approaches and tissue engineering combine stem cells with scaffolding biomaterials as well as growth factors. This has potential applications in surgical correction of jaw discrepancies, bone loss due to periodontal disease, congenital bone defect such as cleft lips and palates, TMJ disorders related to bone/cartilage defect, and alveolar bone lesions. Recent advances in stem cells indicate effective treatment and improved clinical outcomes in bone tissue regeneration in orthodontic/orthopedic treatment. Studies also showed that stem cells based regenerative approaches can reduce morbidity and speed up the recovery process comparing to the conventional surgical approaches. Taken together, we can conclude that regenerative approaches through tissue engineering are promising for orthodontic/orthopedic treatments.
Future Perspectives
The contemporary evidence indicates the feasibility of regenerative approaches in orthodontic/orthopedic treatments, yet the majority of the evidence is from preclinical studies with animal models. It is worthwhile to notice that there is still a long distance from bench to bed. The future study in this field need to focus on the followings:
-
Advances technologies: Nanoscale biomaterials will be applied as control delivery systems for tissue engineering. The nanomaterials can also regulate the immuno-response as such to enhance the regeneration efficiency. Among all the stem cells, dental pulp stem cells (DPSCs) are an emerging source that has drawn more and more intention. The advantages of DPSCs include but not limited to: low immunogenicity, high differentiative capacity, and easy to access through bio-banking of the deciduous teeth or young adult teeth.
-
Clinical translation: More clinical trials are required to assess the safety and efficacy of the stem cell-based regenerative approaches for orthodontic and orthopedic patients. Ideally such clinical trials should be conducted in double-blinded, randomized, and controlled manner in order to produce the high quality of clinical evidence.
References
Koons GL, Diba M, Mikos AG. Materials design for bone-tissue engineering. Nat Rev Mater. 2020;5:584. https://doi.org/10.1038/s41578-020-0204-2.
Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40(5):363–408. https://doi.org/10.1615/critrevbiomedeng.v40.i5.10.
Stevens MM. Biomaterials for bone tissue engineering. Mater Today. 2008;11(5):18–25. https://doi.org/10.1016/S1369-7021(08)70086-5.
Wei GB, Ma PX. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. 2004;25(19):4749–57. https://doi.org/10.1016/j.biomaterials.2003.12.005.
Gomez-Barrena E, Rosset P, Muller I, Giordano R, Bunu C, Layrolle P, et al. Bone regeneration: stem cell therapies and clinical studies in orthopaedics and traumatology. J Cell Mol Med. 2011;15(6):1266–86. https://doi.org/10.1111/j.1582-4934.2011.01265.x.
Arvidson K, Abdallah BM, Applegate LA, Baldini N, Cenni E, Gomez-Barrena E, et al. Bone regeneration and stem cells. J Cell Mol Med. 2011;15(4):718–46. https://doi.org/10.1111/j.1582-4934.2010.01224.x.
Nakamura S, Yamada Y, Katagiri W, Sugito T, Ito K, Ueda M. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J Endod. 2009;35(11):1536–42. https://doi.org/10.1016/j.joen.2009.07.024.
Chen X, Zhang T, Shi J, Xu P, Gu Z, Sandham A, et al. Notch1 signaling regulates the proliferation and self-renewal of human dental follicle cells by modulating the G1/S phase transition and telomerase activity. PLoS One. 2013;8(7):e69967.
Volpe G, Bernstock JD, Peruzzotti-Jametti L, Pluchino S. Modulation of host immune responses following non-hematopoietic stem cell transplantation: translational implications in progressive multiple sclerosis. J Neuroimmunol. 2019;331:11–27.
Luo L, Albashari AA, Wang X, Jin L, Zhang Y, Zheng L, et al. Effects of transplanted heparin-poloxamer hydrogel combining dental pulp stem cells and bFGF on spinal cord injury repair. Stem Cells Int. 2018;2018:1.
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97(25):13625–30. https://doi.org/10.1073/pnas.240309797.
Laino G, d’Aquino R, Graziano A, Lanza V, Carinci F, Naro F, et al. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Miner Res. 2005;20(8):1394–402. https://doi.org/10.1359/Jbmr.050325.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. https://doi.org/10.1016/j.cell.2006.07.024.
Cai J, Zhang Y, Liu P, Chen S, Wu X, Sun Y, et al. Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regener. 2013;2(1):6. https://doi.org/10.1186/2045-9769-2-6.
Abdullah AN, Miyauchi S, Onishi A, Tanimoto K, Kato K. Differentiation of mouse-induced pluripotent stem cells into dental epithelial-like cells in the absence of added serum. In Vitro Cell Dev-An. 2019;55(2):130–7. https://doi.org/10.1007/s11626-019-00320-z.
Storti G, Scioli MG, Kim BS, Orlandi A, Cervelli V. Adipose-derived stem cells in bone tissue engineering: useful tools with new applications. Stem Cells Int. 2019;2019:1. https://doi.org/10.1155/2019/3673857.
Chen YR, Yan X, Yuan FZ, Ye J, Xu BB, Zhou ZX, et al. The use of peripheral blood-derived stem cells for cartilage repair and regeneration in vivo: a review. Front Pharmacol. 2020;11:404. https://doi.org/10.3389/fphar.2020.00404.
Jeong J, Kim JH, Shim JH, Hwang NS, Heo CY. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater Res. 2019;23:4. https://doi.org/10.1186/s40824-018-0149-3.
Xie H, Gu Z, Li C, Franco C, Wang J, Li L, Meredith N, Ye Q, Wan C. A novel bioceramic scaffold integrating silk fibroin in calcium polyphosphate for bone tissue-engineering. Ceram Int. 2016;42(2):2386–92.
Fujishiro Y, Hench LL, Oonishi H. Quantitative rates of in vivo bone generation for Bioglass(R) and hydroxyapatite particles as bone graft substitute. J Mater Sci-Mater M. 1997;8(11):649–52. https://doi.org/10.1023/A:1018527621356.
Zhou YL, Wu CT, Chang J. Bioceramics to regulate stem cells and their microenvironment for tissue regeneration. Mater Today. 2019;24:41–56. https://doi.org/10.1016/j.mattod.2018.07.016.
Alabbasi A, Mehjabeen A, Kannan MB, Ye Q, Blawert C. Biodegradable polymer for sealing porous PEO layer on pure magnesium: an in vitro degradation study. Appl Surf Sci. 2014;301:463–7.
Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22(4):233–41. https://doi.org/10.1080/08977190412331279890.
Deal C. Future therapeutic targets in osteoporosis. Curr Opin Rheumatol. 2009;21(4):380–5. https://doi.org/10.1097/Bor.0b013e32832cbc2a.
Park SB, Park SH, Kim NH, Chung CK. BMP-2 induced early bone formation in spine fusion using rat ovariectomy osteoporosis model. Spine J. 2013;13(10):1273–80. https://doi.org/10.1016/j.spinee.2013.06.010.
Zhang SF, Kucharski C, Doschak MR, Sebald W, Uludag H. Polyethylenimine-PEG coated albumin nanoparticles for BMP-2 delivery. Biomaterials. 2010;31(5):952–63. https://doi.org/10.1016/j.biomaterials.2009.10.011.
Kapoor P, Kharbanda OP, Monga N, Miglani R, Kapila S. Effect of orthodontic forces on cytokine and receptor levels in gingival crevicular fluid: a systematic review. Prog Orthod. 2014;15(1):65.
Liou EJ, Chen PH, Wang YC, Yu CC, Huang CS, Chen YR. Surgery-first accelerated orthognathic surgery: postoperative rapid orthodontic tooth movement. J Oral Maxillofac Surg. 2011;69(3):781–5.
Alfawal AM, Hajeer MY, Ajaj MA, Hamadah O, Brad B. Evaluation of piezocision and laser-assisted flapless corticotomy in the acceleration of canine retraction: a randomized controlled trial. Head Face Med. 2018;14(1):4.
Al-Naoum F, Hajeer MY, Al-Jundi A. Does alveolar corticotomy accelerate orthodontic tooth movement when retracting upper canines? A split-mouth design randomized controlled trial. J Oral Maxillofac Surg. 2014;72(10):1880–9.
Kundi I, Alam MK, Shaheed S. Micro-osteo perforation effects as an intervention on canine retraction. Saudi Dent J. 2020;32(1):15–20.
Dutra EH, Ahmida A, Lima A, Schneider S, Nanda R, Yadav S. The effects of alveolar decortications on orthodontic tooth movement and bone remodelling in rats. Eur J Orthodont. 2018;40(4):423–9.
Patterson BM, Dalci O, Darendeliler MA, Papadopoulou AK. Corticotomies and orthodontic tooth movement: a systematic review. J Oral Maxillofac Surg. 2016;74(3):453–73.
Tsai CY, Yang TK, Hsieh HY, Yang LY. Comparison of the effects of micro-osteoperforation and corticision on the rate of orthodontic tooth movement in rats. Angle Orthod. 2016;86(4):558–64.
Alikhani M, Raptis M, Zoldan B, Sangsuwon C, Lee YB, Alyami B, Corpodian C, Barrera LM, Alansari S, Khoo E, Teixeira C. Effect of micro-osteoperforations on the rate of tooth movement. Am J Orthod Dentofac Orthop. 2013;144(5):639–48.
Alkebsi A, Al-Maaitah E, Al-Shorman H, Alhaija EA. Three-dimensional assessment of the effect of micro-osteoperforations on the rate of tooth movement during canine retraction in adults with class II malocclusion: a randomized controlled clinical trial. Am J Orthod Dentofac Orthop. 2018;153(6):771–85.
Sivarajan S, Ringgingon LP, Fayed MM, Wey MC. The effect of micro-osteoperforations on the rate of orthodontic tooth movement: a systematic review and meta-analysis. Am J Orthod Dentofac Orthop. 2020;157(3):290–304.
Ali D, Mohammed H, Koo SH, Kang KH, Kim SC. Three-dimensional evaluation of tooth movement in class II malocclusions treated without extraction by orthodontic mini-implant anchorage. Korean J Orthod. 2016;46(5):280–9.
Antoszewska-Smith J, Sarul M, Łyczek J, Konopka T, Kawala B. Effectiveness of orthodontic miniscrew implants in anchorage reinforcement during en-masse retraction: a systematic review and meta-analysis. Am J Orthod Dentofac Orthop. 2017;151(3):440–55.
Roberts WE, Huja SS. Bone physiology, metabolism, and biomechanics in orthodontic practice. Orthodontics: current principles and techniques. 6th ed. Oxford: Elsevier Health Sciences; 2016. p. 99–152.
Chen N, Sui BD, Hu CH, Cao J, Zheng CX, Hou R, Yang ZK, Zhao P, Chen Q, Yang QJ, Jin Y. microRNA-21 contributes to orthodontic tooth movement. J Dent Res. 2016;95(12):1425–33.
Wu L, Su Y, Lin F, Zhu S, Wang J, Hou Y, Du J, Liu Y, Guo L. MicroRNA-21 promotes orthodontic tooth movement by modulating the RANKL/OPG balance in T cells. Oral Dis. 2020;26(2):370–80.
Cao H, Kou X, Yang R, Liu D, Wang X, Song Y, Feng L, He D, Gan Y, Zhou Y. Force-induced Adrb2 in periodontal ligament cells promotes tooth movement. J Dent Res. 2014;93(11):1163–9.
Kouskoura T, Katsaros C, von Gunten S. The potential use of pharmacological agents to modulate orthodontic tooth movement (OTM). Front Physiol. 2017;8:67.
Makrygiannakis MA, Kaklamanos EG, Athanasiou AE. Does common prescription medication affect the rate of orthodontic tooth movement? A systematic review. Eur J Orthod. 2018;40(6):649–59.
Qin J, Hua Y. Effects of hydrogen sulfide on the expression of alkaline phosphatase, osteocalcin and collagen type I in human periodontal ligament cells induced by tension force stimulation. Mol Med Rep. 2016;14(4):3871–7.
Pu H, Hua Y. Hydrogen sulfide regulates bone remodeling and promotes orthodontic tooth movement. Mol Med Rep. 2017;16(6):9415–22.
Yang F, Wang XX, Ma D, Cui Q, Zheng DH, Liu XC, Zhang J. Effects of Triptolide on tooth movement and root resorption in rats. Drug Des Devel Ther. 2019;13:3963.
Ma D, Wang X, Ren X, Bu J, Zheng D, Zhang J. Asperosaponin VI injection enhances orthodontic tooth movement in rats. Med Sci Mon Int Med J Exp Clin Res. 2020;26:e922372–1.
Liu XC, Wang XX, Zhang LN, Yang F, Nie FJ, Zhang J. Inhibitory effects of resveratrol on orthodontic tooth movement and associated root resorption in rats. Arch Oral Biol. 2020;111:104642.
Youssef M, Ashkar S, Hamade E, Gutknecht N, Lampert F, Mir M. The effect of low-level laser therapy during orthodontic movement: a preliminary study. Lasers Med Sci. 2008;23(1):27–33.
Qamruddin I, Alam MK, Mahroof V, Fida M, Khamis MF, Husein A. Effects of low-level laser irradiation on the rate of orthodontic tooth movement and associated pain with self-ligating brackets. Am J Orthod Dentofac Orthop. 2017;152(5):622–30.
Varella AM, Revankar AV, Patil AK. Low-level laser therapy increases interleukin-1β in gingival crevicular fluid and enhances the rate of orthodontic tooth movement. Am J Orthod Dentofac Orthop. 2018;154(4):535–44.
Gkantidis N, Mistakidis I, Kouskoura T, Pandis N. Effectiveness of non-conventional methods for accelerated orthodontic tooth movement: a systematic review and meta-analysis. J Dent. 2014;42(10):1300–19.
Mistry D, Dalci O, Papageorgiou SN, Darendeliler MA, Papadopoulou AK. The effects of a clinically feasible application of low-level laser therapy on the rate of orthodontic tooth movement: a triple-blind, split-mouth, randomized controlled trial. Am J Orthod Dentofac Orthop. 2020;157(4):444–53.
Karamesinis K, Basdra EK. The biological basis of treating jaw discrepancies: an interplay of mechanical forces and skeletal configuration. BBA-Mol Basis Dis. 2018;1864(5):1675–83.
Ilizarov GA. The tension stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res. 1989;238:249–81.
Rachmiel A, Laufer D, Jackson IT, Lewinson D. Midface membranous bone lengthening: a one-year histological and morphological follow-up of distraction osteogenesis. Calcif Tissue Int. 1998;62:370–6.
Cao J, Zhang S, Gupta A, Du Z, Lei D, Wang L, Wang X. Sensory nerves affect bone regeneration in rabbit mandibular distraction osteogenesis. Int J Med Sci. 2019;16(6):831.
Ilizarov GA. Clinical application of the tension–stress effect for limb lengthening. Clin Orthop Relat Res. 1990;250:8–26.
Weiss S, Zimmermann G, Baumgart R, Kasten P, Bidlingmaier M, Henle P. Systemic regulation of angiogenesis and matrix degradation in bone regeneration—distraction osteogenesis compared to rigid fracture healing. Bone. 2005;37(6):781–90.
Chartier SR, Mitchell SA, Majuta LA, Mantyh PW. The changing sensory and sympathetic innervation of the young, adult and aging mouse femur. Neuroscience. 2018;387:178–90.
Tomlinson RE, Li Z, Zhang Q, Goh BC, Li Z, Thorek DL, Rajbhandari L, Brushart TM, Minichiello L, Zhou F, Venkatesan A. NGF-TrkA signaling by sensory nerves coordinates the vascularization and ossification of developing endochondral bone. Cell Rep. 2016;16(10):2723–35.
Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305–17.
Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032):514–20.
Kishore SN, Yuvraj I, Ankur T. Mandibular distraction osteogenesis. J Craniofac Surg. 2019;30(8):e743–6.
Yoda N, Zheng K, Chen J, Liao Z, Koyama S, Peck C, Swain M, Sasaki K, Li Q. Biomechanical analysis of bone remodeling following mandibular reconstruction using fibula free flap. Med Eng Phys. 2018;56:1–8.
Li X, Jiang C, Gao H, Wang C, Wang C, Ji P. Biomechanical analysis of various reconstructive methods for the mandibular body and ramus defect using a free vascularized fibula flap. Biomed Res Int. 2020;13:2020.
Stopa Z, Siewert-Gutowska M, Abed K, Szubińska-Lelonkiewicz D, Kamiński A, Fiedor P. Evaluation of the safety and clinical efficacy of allogeneic bone grafts in the reconstruction of the maxilla and mandible. Transplant Proc. 2018;50(7):2199–201.
Henkel J, Woodruff MA, Epari DR, Steck R, Glatt V, Dickinson IC, Choong PF, Schuetz MA, Hutmacher DW. Bone regeneration based on tissue engineering conceptions—a 21st century perspective. Bone Res. 2013;1(1):216–48.
Jazayeri HE, Tahriri M, Razavi M, Khoshroo K, Fahimipour F, Dashtimoghadam E, Almeida L, Tayebi L. A current overview of materials and strategies for potential use in maxillofacial tissue regeneration. Mater Sci Eng C. 2017;70:913–29.
Rumney RM, Lanham SA, Kanczler JM, Kao AP, Thiagarajan L, Dixon JE, Tozzi G, Oreffo RO. In vivo delivery of VEGF RNA and protein to increase osteogenesis and intraosseous angiogenesis. Sci Rep. 2019;9(1):17745.
Qu D, Li J, Li Y, Gao Y, Zuo Y, Hsu Y, Hu J. Angiogenesis and osteogenesis enhanced by bFGF ex vivo gene therapy for bone tissue engineering in reconstruction of calvarial defects. J Biomed Mater Res A. 2011;96(3):543–51.
Eweida AM, Nabawi AS, Abouarab M, Kayed M, Elhammady H, Etaby A, Khalil MR, Shawky MS, Kneser U, Horch RE, Nagy N. Enhancing mandibular bone regeneration and perfusion via axial vascularization of scaffolds. Clin Oral Investig. 2014;18(6):1671–8.
Dong QN, Kanno T, Bai Y, Sha J, Hideshima K. Bone regeneration potential of uncalcined and unsintered hydroxyapatite/poly L-lactide bioactive/osteoconductive sheet used for maxillofacial reconstructive surgery: an in vivo study. Materials. 2019;12(18):2931.
Cao Y, Xiao L, Cao Y, Nanda A, Xu C, Ye Q. 3D printed β-TCP scaffold with sphingosine 1-phosphate coating promotes osteogenesis and inhibits inflammation. Biochem Biophys Res Commun. 2019;512(4):889–95.
Mardas N, Dereka X, Donos N, Dard M. Experimental model for bone regeneration in oral and cranio-maxillo-facial surgery. J Investig Surg. 2014;27(1):32–49.
Shanbhag S, Pandis N, Mustafa K, Nyengaard JR, Stavropoulos A. Alveolar bone tissue engineering in critical-size defects of experimental animal models: a systematic review and meta-analysis. J Tissue Eng Regen Med. 2017;11(10):2935–49.
Liu G, Guo Y, Zhang L, Wang X, Liu R, Huang P, Xiao Y, Chen Z, Chen Z. A standardized rat burr hole defect model to study maxillofacial bone regeneration. Acta Biomater. 2019;86:450–64.
Xie H, Wang J, He Y, Gu Z, Xu J, Li L, Ye Q. Biocompatibility and safety evaluation of a silk fibroin-doped calcium polyphosphate scaffold copolymer in vitro and in vivo. RSC Adv. 2017;7(73):46036–44.
Deng N, Sun J, Li Y, Chen L, Chen C, Wu Y, Wang Z, Li L. Experimental study of rhBMP-2 chitosan nano-sustained release carrier-loaded PLGA/nHA scaffolds to construct mandibular tissue-engineered bone. Arch Oral Biol. 2019;102:16–25.
Wang S, Zhao J, Zhang W, Ye D, Zhang X, Zou D, Zhang X, Sun X, Sun S, Zhang W, Yang C. Comprehensive evaluation of cryopreserved bone-derived osteoblasts for the repair of segmental mandibular defects in canines. Clin Implant Dent Relat Res. 2015;17(4):798–810.
Yang C, Liu X, Zhao K, Zhu Y, Hu B, Zhou Y, Wang M, Wu Y, Zhang C, Xu J, Ning Y. miRNA-21 promotes osteogenesis via the PTEN/PI3K/Akt/HIF-1α pathway and enhances bone regeneration in critical size defects. Stem Cell Res Ther. 2019;10(1):65.
Konopnicki S, Sharaf B, Resnick C, Patenaude A, Pogal-Sussman T, Hwang KG, Abukawa H, Troulis MJ. Tissue-engineered bone with 3-dimensionally printed β-tricalcium phosphate and polycaprolactone scaffolds and early implantation: an in vivo pilot study in a porcine mandible model. J Oral Maxillofac Surg. 2015;73(5):1016–e1.
Schliephake H, Knebel JW, Aufderheide M, Tauscher M. Use of cultivated osteoprogenitor cells to increase bone formation in segmental mandibular defects: an experimental pilot study in sheep. J Oral Maxillofac Surg. 2001;30(6):531–7.
Chanchareonsook N, Tideman H, Feinberg SE, Jongpaiboonkit L, Lee S, Flanagan C, Krishnaswamy G, Jansen J. Segmental mandibular bone reconstruction with a carbonate-substituted hydroxyapatite-coated modular endoprosthetic poly (ɛ-caprolactone) scaffold in Macaca fascicularis. J Biomed Mater Res B Appl Biomater. 2014;102(5):962–76.
Xie Y, Su Y, Min S, Tang J, Goh BT, Saigo L, Ansari S, Moshaverinia A, Zhang C, Wang J, Liu Y. Collagen sponge functionalized with chimeric anti-BMP-2 monoclonal antibody mediates repair of critical-size mandibular continuity defects in a nonhuman primate model. Biomed Res Int. 2017;16:2017.
Trejo-Iriarte CG, Serrano-Bello J, Gutiérrez-Escalona R, Mercado-Marques C, García-Honduvilla N, Buján-Varela J, Medina LA. Evaluation of bone regeneration in a critical size cortical bone defect in rat mandible using microCT and histological analysis. Arch Oral Biol. 2019;101:165–71.
Zhang D, Chu F, Yang Y, Xia L, Zeng D, Uludağ H, Zhang X, Qian Y, Jiang X. Orthodontic tooth movement in alveolar cleft repaired with a tissue engineering bone: an experimental study in dogs. Tissue Eng Part A. 2011;17(9–10):1313–25.
Ahn G, Lee JS, Yun WS, Shim JH, Lee UL. Cleft alveolus reconstruction using a three-dimensional printed bioresorbable scaffold with human bone marrow cells. J Craniofac Surg. 2018;29(7):1880–3.
Sasayama S, Hara T, Tanaka T, Honda Y, Baba S. Osteogenesis of multipotent progenitor cells using the epigallocatechin gallate-modified gelatin sponge scaffold in the rat congenital cleft-jaw model. Int J Mol Sci. 2018;19(12):3803.
Kaitsas R, Paolone MG, Paolone G. Guided orthodontic regeneration: a tool to enhance conventional regenerative techniques in implant surgery. Int Orthod. 2015;13(4):539–54.
Paolone MG, Kaitsas R. Orthodontic-periodontal interactions: orthodontic extrusion in interdisciplinary regenerative treatments. Int Orthod. 2018;16(2):217–45.
Meikle MC. The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur J Orthod. 2006;28(3):221–40.
Liu Z, Yin X, Ye Q, He W, Ge M, Zhou X, Hu J, Zou S. Periodontal regeneration with stem cells-seeded collagen-hydroxyapatite scaffold. J Biomater Appl. 2016;31(1):121–31.
Jepsen S, Caton JG, Albandar JM, Bissada NF, Bouchard P, Cortellini P, Demirel K, de Sanctis M, Ercoli C, Fan J, Geurs NC. Periodontal manifestations of systemic diseases and developmental and acquired conditions: consensus report of workgroup 3 of the 2017 world workshop on the classification of periodontal and Peri-implant diseases and conditions. J Clin Periodontol. 2018;45:S219–29.
Engelking G, Zachrisson BU. Effects of incisor repositioning on monkey periodontium after expansion through the cortical plate. Am J Orthod. 1982;82(1):23–32.
Pazera P, Fudalej P, Katsaros C. Severe complication of a bonded mandibular lingual retainer. Am J Orthod Dentofac Orthop. 2012;142(3):406–9.
Machado AW, MacGinnis M, Damis L, Moon W. Spontaneous improvement of gingival recession after correction of tooth positioning. Am J Orthod Dentofac Orthop. 2014;145(6):828–35.
Farret MM, Farret MM, da Luz VG, Assaf JH, de Lima EM. Orthodontic treatment of a mandibular incisor fenestration resulting from a broken retainer. Am J Orthod Dentofac Orthop. 2015;148(2):332–7.
Laursen MG, Rylev M, Melsen B. Treatment of complications after unintentional tooth displacement by active bonded retainers. J Clin Orthod. 2016;50(5):290–7.
Laursen MG, Rylev M, Melsen B. The role of orthodontics in the repair of gingival recessions. Am J Orthod Dentofac Orthop. 2020;157(1):29–34.
Holbrook T, Ochsenbein C. Complete coverage of the denuded root surface with a one-stage gingival graft. Int J Periodontics Restorative Dent. 1983;3(3):8.
Miller PD Jr. A classification of marginal tissue recession. Int J Periodont Rest Dent. 1985;5:9.
Prato GP, Tinti C, Vincenzi G, Magnani C, Cortellini P, Clauser C. Guided tissue regeneration versus mucogingival surgery in the treatment of human buccal gingival recession. J Periodontol. 1992;63(11):919–28.
Trombelli L, Schincaglia GP, Scapoli C, Calura G. Healing response of human buccal gingival recessions treated with expanded polytetrafluoroethylene membranes. A retrospective report. J Periodontol. 1995;66(1):14–22.
Cortellini P, Bissada NF. Mucogingival conditions in the natural dentition: narrative review, case definitions, and diagnostic considerations. J Periodontol. 2018;89:S204–13.
Saadoun AP, LeGall M, Touati B. Selection and ideal tridimensional implant position for soft tissue aesthetics. Pract Periodontics Aesthet Dent. 1999;11(9):1063.
Small PN, Tarnow DP. Gingival recession around implants: a 1-year longitudinal prospective study. Int J Oral Maxillofac Implants. 2000;15(4):527–32.
Kan JY, Kois JC. Predictable peri-implant gingival aesthetics: surgical and prosthodontic rationales. Pract Proced Aesthet Dent. 2001;13(9):691–8.
Block MS, Mercante DE, Lirette D, Mohamed W, Ryser M, Castellon P. Prospective evaluation of immediate and delayed provisional single tooth restorations. J Oral Maxillofac Surg. 2009;67(11):89–107.
Conserva E, Fadda M, Ferrari V, Consolo U. Predictability of a new orthodontic extrusion technique for implant site development: a retrospective consecutive case-series study. Sci World J. 2020.
Brown IS. The effect of orthodontic therapy on certain types of periodontal defects I—clinical findings. J Periodontol. 1973;44(12):742–56.
Amato F, Mirabella D, Macca U, Tarnow DP. Implant site development by orthodontic forced extraction: a preliminary study. Int J Oral Maxillofac Implants. 2012;27(2).
Heithersay GS. Combined endodontic-orthodontic treatment of transverse root fractures in the region of the alveolar crest. Oral Surg Oral Med Oral Pathol. 1973;36(3):404–15.
Ingber JS. Forced eruption: Part I. A method of treating isolated one and two wall infrabony osseous defects-rationale and case report. J Periodontol. 1974;45(4):199–206.
Ingber JS. Forced eruption: part II. A method of treating nonrestorable teeth – periodontal and restorative considerations. J Periodontol. 1976;47(4):203–16.
Potashnick SR, Rosenberg ES. Forced eruption: principles in periodontics and restorative dentistry. J Prosthet Dent. 1982;48(2):141–8.
Chambrone L, Chambrone LA. Forced orthodontic eruption of fractured teeth before implant placement: case report. J Can Dent Assoc. 2005;71(4):257–61.
Brindis MA, Block MS. Orthodontic tooth extrusion to enhance soft tissue implant esthetics. J Oral Maxillofac Surg. 2009;67(11):49–59.
Sun H, Wang Y, Sun C, Ye Q, Dai W, Wang X, Xu Q, Pan S, Hu R. Root morphology and development of labial inversely impacted maxillary central incisors in the mixed dentition: a retrospective cone-beam computed tomography study. Am J Orthod Dentofac Orthop. 2014;146(6):709–16.
da Silva VC, de Molon RS, Martins RP, Ribeiro FS, Pontes AE, Zandim-Barcelos DL, Leite FR, Neto CB, Marcantonio RA, Cirelli JA. Effects of orthodontic tooth extrusion produced by different techniques, on the periodontal tissues: a histological study in dogs. Arch Oral Biol. 2020;20:104768.
Schwartz O, Frederiksen K, Klausen B. Allotransplantation of human teeth. A restrospective study of 73 transplantations over a period of 28 years. Int J Oral Maxillofac Surg. 1987;16(3):285–301.
Schröder A, Käppler P, Nazet U, Jantsch J, Proff P, Cieplik F, Deschner J, Kirschneck C. Effects of compressive and tensile strain on macrophages during simulated orthodontic tooth movement. Mediat Inflamm. 2020;28:2020.
Yang CY, Jeon HH, Alshabab A, Lee YJ, Chung CH, Graves DT. RANKL deletion in periodontal ligament and bone lining cells blocks orthodontic tooth movement. Int J Oral Sci. 2018;10(1):1–9.
Park WY, Kim MS, Kim MS, Oh MH, Lee SY, Kim SH, Cho JH. Effects of pre-applied orthodontic force on the regeneration of periodontal tissues in tooth replantation. Korean J Orthod. 2019;49(5):299–309.
Suzaki Y, Matsumoto Y, Kanno Z, Soma K. Preapplication of orthodontic forces to the donor teeth affects periodontal healing of transplanted teeth. Angle Orthod. 2008;78(3):495–501.
Yang S, Jung BY, Pang NS. Outcomes of autotransplanted teeth and prognostic factors: a 10-year retrospective study. Clin Oral Investig. 2019;23(1):87–98.
Carvalho CV, Saraiva L, Bauer FP, Kimura RY, Souto ML, Bernardo CC, Pannuti CM, Romito GA, Pustiglioni FE. Orthodontic treatment in patients with aggressive periodontitis. Am J Orthod Dentofac Orthop. 2018;153(4):550–7.
Re S, Cardaropoli D, Abundo R, Corrente G. Reduction of gingival recession following orthodontic intrusion in periodontally compromised patients. Orthod Craniofac Res. 2004;7(1):35–9.
Zasciurinskiene E, Lindsten R, Slotte C, Bjerklin K. Orthodontic treatment in periodontitis-susceptible subjects: a systematic literature review. J Clin Exp Dent. 2016;2(2):162–73.
Cosendey VL, Frossard W, Feu D. Treatment of chronic adult periodontitis in a patient with negative overjet and multiple tooth loss. J Clin Orthod. 2016;50(4):239–49.
Murphy MK, MacBarb RF, Wong ME, Athanasiou KA. Temporomandibular joint disorders: a review of etiology, clinical management, and tissue engineering strategies. Int J Oral Max Impl. 2013;28:e393.
List T, Jensen RH. Temporomandibular disorders: old ideas and new concepts. Cephalalgia. 2017;37:692–704.
Willard VP, Zhang L, Athanasiou KA. Tissue engineering of the temporomandibular joint. In: Ducheyne P, Healy K, Hutmacher DW, et al., editors. Comprehensive biomaterials, vol. 5. London: Elsevier; 2011. p. 221–35.
Aryaei A, Vapniarsky N, Hu JC, Athanasiou KA. Recent tissue engineering advances for the treatment of temporomandibular joint disorders. Curr Osteoporos Rep. 2016;14:269–79.
Salash JR, Hossameldin RH, Almarza AJ, Chou JC, McCain JP, Mercuri LG, Wolford LM, Detamore MS. Potential indications for tissue engineering in temporomandibular joint surgery. J Oral Maxillofac Surg. 2016;74:705–11.
Acri TM, Shin K, Seol D, Laird NZ, Song I, Geary SM, Chakka JL, Martin JA, Salem AK. Tissue engineering for the temporomandibular joint. Rev Adv Healthc Mater. 2019;8:e1801236.
Donahue RP, Hu JC, Athanasiou KA. Remaining hurdles for tissue-engineering the temporomandibular joint disc. Rev Trends Mol Med. 2019;25:241–56.
Brady MA, Sivananthan S, Mudera V, Liu Q, Wiltfang J, Warnke PH. The primordium of a biological joint replacement: coupling of two stem cell pathways in biphasic ultrarapid compressed gel niches. J Cranio Maxill Surg. 2011;39:380–6.
Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol. 2013;9:584.
Chin AR, Gao J, Wang Y, Taboas JM, Almarza AJ. Regenerative potential of various soft polymeric scaffolds in the temporomandibular joint condyle. J Oral Maxillofac Surg. 2018;76:2019–26.
Helgeland E, Shanbhag S, Pedersen TO, Mustafa K, Rosen A. Scaffold-based temporomandibular joint tissue regeneration in experimental animal models: a systematic review. Tissue Eng Part B Rev. 2018;24:300–16.
Cheng B, Tu T, Shi X, Liu Y, Zhao Y, Zhao Y, Li Y, Chen H, Chen Y, Zhang M. A novel construct with biomechanical flexibility for articular cartilage regeneration. Stem Cell Res Ther. 2019;10:298.
Kinoshita Y, Maeda H. Recent developments of functional scaffolds for craniomaxillofacial bone tissue engineering applications. Sci World J. 2013;2013:863157.
Xia T, Yu F, Zhang K, Wu Z, Shi D, Teng H, Shen J, Yang X, Jiang Q. The effectiveness of allogeneic mesenchymal stem cells therapy for knee osteoarthritis in pigs. Ann Transl Med. 2018;6:404.
Iijima H, Isho T, Kuroki H, Takahashi M, Aoyama T. Effectiveness of mesenchymal stem cells for treating patients with knee osteoarthritis: a meta-analysis toward the establishment of effective regenerative rehabilitation. NPJ Regen Med. 2018;3:15.
Lee YH, Park HK, Auh QS, Nah H, Lee JS, Moon HJ, Heo DN, Kim IS, Kwon IK. Emerging potential of exosomes in regenerative medicine for temporomandibular joint osteoarthritis. Int J Mol Sci. 2020;21:E1541.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
He, Y., Guastaldi, F., Xu, C., Ye, Q. (2021). Regenerative Approaches in Orthodontic and Orthopedic Treatment. In: Hosseinpour, S., Walsh, L.J., Moharamzadeh, K. (eds) Regenerative Approaches in Dentistry. Springer, Cham. https://doi.org/10.1007/978-3-030-59809-9_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-59809-9_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-59808-2
Online ISBN: 978-3-030-59809-9
eBook Packages: MedicineMedicine (R0)