Abstract
Gender plays an important role in the pathophysiology, clinical presentation, and outcomes of various medical illnesses. Among those, special interest is directed towards ischemic heart disease, being the most common cause of mortality in the United States and the developed world. The prevalence and pathogenesis of traditional cardiovascular risk factors differ between women and men, including hypertension, diabetes, dyslipidemia, as well as smoking and psychosocial risk factors such as depression, emotional stress, and low socioeconomic status. However, the difference in the pathophysiology of atherosclerosis represents the hallmark of gender-related discrepancies in ischemic heart disease. While men are more likely to have obstructive coronary artery disease (CAD), women have a higher prevalence of endothelial dysfunction, more tendency for vasospasm, lower coronary flow reserve, and higher incidence of plaque erosion than rupture compared with men. These differences are, at least in part, attributed to variations in sex hormones. Not only that gender affects the pathophysiology of CAD, but also impacts the difference in its clinical presentation. Women are more likely to present with atypical symptoms, and hence are more likely to present late or to be misdiagnosed. This has been shown to adversely impact the outcomes of CAD in women compared with men. Further, the utilization of various modalities for the diagnosis of CAD in women may be challenging due to multiple factors including limited exercise capacity, lower sensitivity of exercise-induced changes in electrocardiogram, higher prevalence of single-vessel disease, attenuation artifacts from breast tissue, and the higher likelihood of endothelial dysfunction rather than obstructive CAD. Clinicians should pay special attention to such differences aiming to improve outcomes in women with ischemic heart disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Among both women and men, ischemic heart disease remains the most common cause of mortality in the United States and the developed world [1]. Furthermore, it represents a heavy burden on the healthcare system in the United States with more than 1.4 million hospitalizations per year [1]. There is emerging evidence that ischemic heart disease presentation and outcome differ between women and men. In this chapter, we highlight the gender-related differences in the pathogenesis and diagnosis of ischemic heart disease.
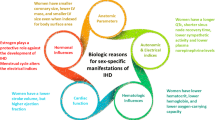
Gender-Related Differences in the Pathogenesis of Ischemic Heart Disease
-
(A)
Risk factors for coronary artery disease
Hypertension
Among the traditional risk factors for coronary artery disease (CAD), there are important differences in the prevalence and pathogenesis of hypertension between men and women. While the overall prevalence of hypertension appears to be similar among women and men, the prevalence is higher among women over the age of 65 [2], especially black women [3], and could be attributed to hormonal changes [4]. Estrogen plays a beneficial role in systemic blood pressure regulation through vascular vasodilation (either via a direct effect on the endothelial cells or indirectly through promoting nitric oxide release), as well as modulation of the renin-angiotensin system (via increasing the synthesis of angiotensinogen and suppressing the activity of pro-hypertensive angiotensin type 1 receptor and reducing the activity of angiotensin type 2 receptor) [5]. The drop in estrogen levels after menopause may be the basis of higher prevalence of hypertension in elderly women. Higher prevalence of hypertension in women > 65 years may relate to higher rates of CAD in elderly women.
Diabetes, obesity, and insulin resistance
Diabetes is among the most important risk factors for CAD. Diabetic women have comparable outcomes as age-matched men, indicating that diabetes may counteract the advantage of female sex hormones in the pathogenesis of CAD [6]. The peripheral adipose tissue distribution as well as the higher levels of adiponectin in women are associated with improved insulin sensitivity and provides some protection from insulin resistance, the hallmark of metabolic syndrome, compared to men [7,8,9,10]. Obesity is an important risk factor for diabetes and CAD. While the burden of obesity affects women and men equally, women above the age of 60 years have higher prevalence of metabolic syndrome [11,12,13], and among women, black women have the highest prevalence [14]. On the other hand, it is important to recognize that polycystic ovarian syndrome, which is present in about 10% of women, and includes a constellation of obesity, diabetes, and metabolic syndrome-like picture may relate to the higher prevalence of CAD. Treatment of this disease may reverse these risk factors and reduce the risk of cardiovascular disease [15].
Dyslipidemia
While pre-menopausal women (age 20–55 years) have lower cholesterol levels compared to men, the levels increase significantly after the age of 55 years in women. That is usually linked, at least in part, to the increased cardiovascular risk in elderly women [16]. On the cellular level, not only that women have higher levels of high-density lipoprotein (HDL) cholesterol and lower levels of low-density lipoprotein (LDL) cholesterol, very low-density lipoprotein (VLDL) cholesterol, total plasma triglyceride and VLDL triglyceride compared to age-matched men [5], their circulating VLDL particles are smaller in size, while HDL are larger in size, which appears to be inversely associated with cardiovascular disease [17, 18]. Furthermore, hepatic lipase activity in women is much lower compared with men, leading to relatively larger size of HDL and LDL particles. Small HDL and LDL were found to be linked to increased cardiovascular disease risk [19, 20]. Further, low HDL and high triglyceride levels are more predictive of the risk of cardiovascular disease in women compared with men [21,22,23].
Smoking
Smoking is the single most important modifiable risk factor for CAD and contributed to 480,000 premature deaths in the U.S. from 2005–2009 [2], one-third of which were a result of secondhand smoke. The prevalence of smoking remains higher in men than women, however, the recent decline in tobacco use is less pronounced in women than in men [16]. Tobacco is known to be a major cause of endothelial dysfunction. It promotes inflammatory environment, stimulates platelet aggregation, increases oxidative stress, and induces vasospasm [24, 25]. Interestingly, the impact of smoking as a risk factor for cardiovascular disease was found to be at least 25% greater in women than age-matched men [26]. Smoking cessation is associated with a decline in the risk of cardiovascular disease to the levels of non-smokers in 5–10 years [27, 28].
Psychosocial risk factors
Psychosocial stress plays a role in the pathophysiology of CAD [29]. Among the important psychosocial factors, depression is more prevalent in women and was found to be present in up to 40% of young women presenting with acute myocardial infarction [30]. Not only depression increases the risk of cardiac events in women by at least 50% [31,32,33], it is also related to poor adherence to medical therapy after cardiac events and worse overall outcomes. In addition, emotional stress, either acute or chronic, is a known risk factor for acute coronary syndromes in women. Multiple studies have shown a relationship between anxiety and marital dissatisfaction with the risk of cardiac events and progression of CAD [34,35,36,37]. Importantly, low level of education, poor socioeconomic status, smoking, obesity, metabolic syndrome, and lack of exercise, all of which are highly associated with CAD [38], may be more pronounced in women than in men. The gender differences among different risk factors are summarized in Table 1.1.
-
(B)
Plaque burden and morphology
While atherosclerosis is an inflammatory process that involves multiple factors such as dyslipidemia, endothelial dysfunction and oxidative stress [39,40,41,42,43], the resultant atherosclerotic plaques differ in morphology and burden between women and men [13, 44]. Men have been shown to have more obstructive CAD than women in multiple studies [45,46,47]. Atheromatous plaques in men have more volume, are more commonly eccentric [13, 44], more likely to have calcification [48], with more structural and functional abnormalities in epicardial coronary arteries than women [11], while women have less dense fibrous tissue in their plaques [49, 50], and are more likely to have angiographically normal coronaries on angiogram. Women have higher prevalence of endothelial dysfunction, more tendency for vasospasm [2, 4, 51, 52], and lower coronary flow reserve compared with men [11, 44]. Furthermore, compared with men, women exhibit higher incidence of plaque erosion than rupture [53], and increased hyaluronan deposition, as well as elevated levels of serum myeloperoxidase [54, 55].
The differences in plaque burden and morphology among women and men are summarized in Table 1.2.
-
(C)
Vascular biology and microvascular disease
Studies relating to vascular biology have led to better understanding of the pathogenesis of atherosclerosis. As mentioned above, while the burden of epicardial CAD may be less in women compared with men, coronary microvasculature plays a major role in the development of atherosclerosis and CAD is women. Women have higher incidence of impaired NO-dependent vasodilation of the coronary microvasculature, resulting in more microvascular spasm and vasospastic angina [56]. Microvascular dysfunction is considered an important part of the pathophysiology of chest pain in the absence of significant coronary obstruction, also known as microvascular angina [57, 58]. The authors of the WISE (Women’s Ischemia Syndrome Evaluation) study [59], showed a clear role for estrogen in mediating vasodilation of coronary microvasculature through myocyte hyperpolarization, increase in prostacyclin production, and inhibition of myocyte-mediated vasoconstriction induced by calcium and endothelin-1 [59]. Of note, other studies showed that part of estrogen-induced coronary vasodilation is independent of these classical pathways and is mediated through changes in ATP-sensitive potassium or calcium channels [60]. As levels of estrogen decrease, women become more predisposed to microvascular dysfunction. Further etiologies of microvascular dysfunction in women without obstructive CAD include abnormal response of phosphocreatine/ATP to exercise indicating a shift to anaerobic metabolism consistent with myocardial ischemia [2, 52], as well as impairment of coronary flow velocity reserve [61].
Estrogen plays multiple other roles at the cellular level. For example, it accelerates the catabolism of reactive oxygen species through up-regulation of multiple enzymes such as superoxide dismutase and catalase. Thus, it reduces oxidative stress and increases free NO levels [9, 62]. Besides the classic NO/cGMP mediated pathway for vasodilation, estrogen also causes direct smooth muscle relaxation via endothelium derived hyperpolarizing factor (EDHF) [9, 63]. Furthermore, estrogen plays an important role in modulating the shear stress-mediated regulation in arteriolar diameter through G-protein-coupled estrogen receptors (GPER) in the vascular endothelium [18, 64, 65].
The role of estrogen and androgen in vascular biology and pathogenesis of atherosclerosis is shown in Fig. 1.1.
-
(D)
Coronary artery dissection
Although spontaneous coronary dissection (SCAD) was considered to be a rare cause of acute coronary syndrome, evidence is emerging that SCAD is the underlying etiology in 10–25% of patients with myocardial infarction, predominantly in women younger than 50 years of age [66,67,68] without atherosclerotic CAD. SCAD most commonly affects the left anterior descending artery [68], and is caused either by an intimal tear or bleeding of vasa vasorum leading to intramural hematoma and false lumen between the media and adventitia [69, 70]. While the underlying trigger remains unclear, hormonal changes with subsequent weakening of the arterial walls are among the main theories behind SCAD [71]. Multiple other factors include inflammatory disorders, hemodynamic stress during pregnancy or peripartum period, coronary vasospasm, and connective tissue disorders [72, 73]. In a recent National Inpatient Sample analysis of 7,347 women with SCAD in the period from 2009–2014, in-hospital mortality with acute myocardial infarction in the setting of SCAD was 6.8%, and outcomes appeared to favor conservative management especially in patients with non-ST-segment elevation myocardial infarction (NSTEMI) [74].
-
(E)
Acute myocardial infarction
Acute myocardial infarction is a leading cause of death in the United States, with more than 610,000 deaths annually [75]. Development of atherosclerotic plaque is usually the initial step in the pathophysiology of AMI. Disruption of the endothelium overlying these plaques triggers a cascade of pro-inflammatory events that in turn lead to activation of multiple pro-thrombotic factors. Ann et al. described gender-related differences in plaque morphology in patients with STEMI [76]. Yahagi et al. [77] observed that AMI in men was more often associated with thin cap vulnerable plaques than in women. Hence, men are more likely to present with a ruptured plaque than women. Ruptured plaques are characterized by abundant foamy macrophages which are potent stimulator of the coagulation cascade, resulting in more frequently in an occlusive thrombus [78]. Although plaque rupture remains the main etiology of AMI in women, it is more prevalent in men than women [79]. In contrast, plaque erosions are seen more commonly in women than men and are associated with a less dramatic coronary obstruction and micro-embolization [80].
Another gender-related difference is the severity of CAD at the time of AMI. Early studies showed less plaque burden and obstructive CAD in women by angiogram in the setting of acute coronary syndrome, and normal appearing coronaries are not uncommon [47]. Intravascular ultrasound (IVUS) examination of plaques showed less eccentric location, more fibrous tissue, and less prevalence of thin-cap fibroatheromas [80, 81]. Women are more likely to have microvascular dysfunction and impaired coronary flow reserve [82, 83], which may explain acute coronary syndromes in women without significant CAD. SCAD is another major entity resulting in AMI that predominantly occurs in women as mentioned before.
These differences in plaque burden and morphology as well as the pathophysiology of AMI are, at least in part, attributed to variations in sex hormones. On the cellular level, estrogen promotes re-endothelialization, reduces leukocyte adhesion molecules, and inhibits smooth muscle proliferation as well as matrix deposition in response to any vascular injury, hence acts as a major protective agent against atherosclerosis [84,85,86]. Estrogen also enhances vasodilation of coronary arteries through the production of NO and vasodilator prostaglandins PGE2 and PGI2 through estrogen receptors ERa and ERb [84]. These protective effects of estrogen are believed to be the main reason for the reduced burden of atherosclerosis and the lower incidence of AMI in premenopausal women.
Gender-Related Differences in the Diagnosis of Ischemic Heart Disease
The Symptomatic Conundrum
The symptomatic conundrum is a common term in the current cardiology literature. It describes the fact that women present with different CAD symptoms compared with men. Reasons for higher CAD mortality in women include older age at initial presentation and relatively smaller use of diagnostic tools [87]. Symptoms in women are often “atypical” and may include dyspnea, heartburn, bloating, or generalized fatigue. The symptomatic conundrum often leads to underappreciation of presenting symptoms and feeds the unawareness of association of these symptoms with CAD [88]. In ACS, despite the fact that most women present with typical symptoms including chest and/or arm pain, the likelihood of presenting with atypical symptoms is still high [89, 90].
The symptomatic conundrum not only leads to underappreciation of the symptoms by medical practitioners, but also leads to significant delay of care as the symptoms are also underappreciated by the patient. In a study by Rosenfeld et al. in 2005, 52 US women who presented with AMI were investigated; a large proportion of them attributed their symptoms to alternative causes or minimized their importance [91]. This could be partly explained by the common underestimation of CAD risk in women. In The Berlin Female Risk Evaluation (BEFRI) study, only half of urban women correctly estimated their CAD risk [92].
The symptomatic conundrum can have serious consequences. In stable CAD patients, it might lead to delay of care and lack of appropriate therapy that could help with the patients’ symptoms and improve their quality of life. On the other hand, it might lead to late presentation in AMI, especially STEMI, delaying effective reperfusion therapy and leading to more serious long-term sequelae [93].
It is also important to understand that despite the underestimation of CAD risk, women actually have about 20% higher prevalence of angina compared with men. Women also report persistent or worsening symptoms at a rate that is double that of men [94,95,96,97]. There is a much larger pool of women with nonobstructive and obstructive CAD who report a heavy burden of symptoms compared with men [98]. Younger women tend to present with absence of chest pain and suffer worse outcomes in comparison with their male counterparts. But, this is attenuated by age, with the difference between genders in the absence of chest pain narrowing or disappearing as age advances [99].
The impact of symptomatic conundrum has led to gender-based differences in the work-up of CAD. Women presenting with CAD symptoms might not be thoroughly investigated as men. Data from Euro Heart Survey in the management and clinical outcomes of stable angina (3779 patients, 42% women) showed that women were less likely to be referred for an exercise test or coronary angiography [100]. Another cross-sectional survey of 1162 patients with angina showed that physicians were more likely to note the risk factors in men and refer them for further work-up [88]. However, an Italian study investigating the use of cardiac procedures in relation to age and sex found that there was an age bias but no gender bias in referral to cardiac catheterization [101]. It is not unreasonable to assume that the underappreciation of CAD because of atypical symptoms in women would result in incorrectly reassuring patients without appropriate diagnostic tests.
Diagnostic Modalities
-
(A)
Exercise EKG test
The choice of diagnostic testing and risk stratification in women suspected to have CAD can be challenging. Patients with low pretest probabilities of CAD, both men and women, are less likely to benefit from the addition of stress imaging test [102]. Therefore, for most patients at low-risk, exercise treadmill testing (ETT) without imaging is the initial test of choice for diagnosis and risk stratification. In the low-risk cohort who were able to exercise, the WOMEN trial [What is the Optimal Method of Ischemia Elucidation in Women] showed that the ETT alone was equivalent to ETT plus myocardial perfusion imaging (MPI) [103]. This is supported by guidelines and consensus statements of the ACC (American College of Cardiology) and American Society of Nuclear Cardiology (ASNC) [87, 94, 104, 105].
Exercise stress testing is physiologic and provides a constellation of clinical, electrocardiographic (EKG) and hemodynamic data; therefore, it is generally preferred over pharmacological testing [106]. However, exercise-induced ST depression may be less sensitive in women compared with men [107]. Exercise-induced ST depression in the absence of CAD could be secondary to changes in estrogen levels during the menstrual cycle [108, 109] or due to menopausal hormone therapy which renders EKG changes less accurate in women compared to men [110]. A recent study by Fitzgerald et al. found that the prevalence of false-positive exercise ST depression might be equal in men and women but with less predictable causes in women such as left ventricular hypertrophy and hypertension [111] Although endothelial dysfunction is thought to be a hallmark of atherosclerosis, a study found no relationship between endothelial dysfunction and exercise-induced ST depression in women [112].
Most women referred for stress testing often have limited exercise capacity as they are typically older with significant comorbidities. This is one of the major difficulties as they are often unable to reach a sufficient workload, resulting in submaximal tests of limited sensitivity. In women who are unable to exercise adequately, pharmacological stress testing is reasonable (e.g., adenosine MPI or dobutamine stress echocardiogram).
-
(B)
Stress imaging
In symptomatic women who are at intermediate to high risk for CAD and have baseline EKG ST-T abnormalities, stress imaging with echocardiography, radionuclide single-photon emission computed tomography (SPECT), and positron emission tomography (PET) are recommended as the initial diagnostic modality. It is also reasonable to consider stress imaging as the initial modality in women who have poor exercise capacity or an abnormal stress EKG [113,114,115,116]. In the WOMEN trial, women with intermediate to high pretest risk, use of myocardial perfusion imaging (MPI) along with ETT was associated with higher accuracy to detect obstructive CAD [103].
Radionuclide single-photon emission computed tomography
Several studies have reported gender-based differences in the diagnostic accuracy of SPECT MPI. The diagnostic accuracy has been reported to be lower in women than men [117,118,119]. There are multiple reasons for this which include lower exercise capacity, higher prevalence of single-vessel disease, and anterior wall attenuation artifacts from breast tissue. Women also have a smaller heart size which often results in a blurred image affecting the sensitivity of the test [120]. Women also have higher left ventricular ejection fraction (LVEF) [121] leading to higher normal limits of transient ischemic dilation ratio [122]. The use of attenuation correction significantly improves the specificity of SPECT MPI, especially in women with high probability of breast tissue attenuation but does not typically affect the sensitivity of SPECT MPI [123, 124]. Another method to increase accuracy in women is using gender-based normal limits and software interpretation [125]. It is currently recommended by American Society of Nuclear Cardiology (ASNC) to use gender-based normal limits for reporting of LVEF and volumes [104].
The prognostic value SPECT MPI in women has also been debated. However, in a large meta-analysis, women with normal SPECT MPI had prognosis comparable to men with 99% event-free survival over 36 months [126]. A normal study with both normal SPECT MPI and stress EKG has excellent prognosis regarding CAD death or MI [127]. On the other hand, abnormal studies are associated with increases in adverse CAD events and worse prognosis in women [128]. Left ventricular volumes and LVEF on SPECT MPI also provide an added prognostic value in predicting CAD death or MI. Other studies have confirmed the excellent prognostic value of SPECT MPI in women, including elderly women [130] and women of diverse racial and ethnic subsets [131]. It is also important to note that some women have a normal SPECT with ischemic EKG changes [132]; these patients have worse outcomes with more cardiovascular events despite normal perfusion pattern [133].
Positron emission tomography
The use of PET has been associated with improved diagnostic accuracy compared with SPECT [134, 135]. The use of PET has provided multiple advantages over SPECT including depth-independent attenuation correction, higher spatial and temporal resolution, lower radiation dose with radiopharmaceuticals that have short half-lives. PET also provides the ability to perform absolute quantification of myocardial perfusion [136]. These advantages render PET preferable in women as it negates the higher risk of both false positive and false negative studies given breast tissue attenuation, and amplified partial volume effects in small left ventricles, respectively. The ability to measure absolute myocardial blood flow is specifically relevant in women who have higher incidence of microvascular ischemia that is increasingly recognized to be associated with significant cardiovascular morbidity and mortality [137,138,139].
Regarding prognosis, a normal PET scan carries an excellent prognosis with 0.4% annual event rate [140] An abnormal PET study is associated with worse prognosis with more perfusion defects being associated with a graded increase in risk [141]. The prognostic value was found to be similar in men and women in the PET Prognosis Multicenter Registry sex-specific sub-analysis [142].
Stress echocardiography
Similar to stress MPI, stress echocardiography significantly improves the specificity and sensitivity for CAD diagnosis. Echocardiography has its limitations in women which include the variability in acoustic windows with breast tissue and being operator-dependent, which affects the ability to capture images at maximal stress. The diagnostic accuracy of stress echocardiography is generally comparable in men and women [143] The echocardiographic evidence of ischemia on pharmacologic stress echocardiography was found to be the only independent predictor of cardiovascular events in 456 women in the study by Cortigiani et al. [144]. Despite its limitations, stress echocardiography is preferred in pregnant and young women due to the absence of ionizing radiation needed for the test.
-
(C)
CT coronary angiography
CT coronary angiography (CTCA) has evolved as a reliable gatekeeper of invasive coronary angiogram. A negative CT study reliably excluded obstructive CAD with a high degree of certainty in two independent studies [145, 146]. In patients at low to intermediate risk of CAD, CTCA is highly accurate in excluding the presence of obstructive disease [147], and the accuracy of CTCA is similar in men and women [147,148,149]. With advancing techniques and the development of CT-derived fractional flow reserve, future application of CTCA might improve outcomes in women suspected to have CAD. The main limitations of CTCA in women include radiation exposure and lower positive predictive value leading to increased downstream testing with invasive testing.
-
(D)
Coronary angiography in women
Coronary angiography remains the gold-standard for diagnosis of CAD. In women, a limitation of coronary angiography is the higher prevalence of non-obstructive CAD [150]. Significant obstructive lesion may not be identified in the cardiac catheterization laboratory unless coronary flow reserve is measured [151]. Women generally have smaller left ventricles and small coronary artery sizes. The smaller size along with breast tissue attenuation leads to partial inability to assess smaller mid and distal coronary segments [152, 153]. Moreover, interpretation of the severity of lesions might be different between men and women due to the difference in the area of myocardium supplied. In the FAME trial sub-study, angiographic lesions with similar severity in men and women were less likely to be ischemia-producing in women [154].
The use of fractional flow reserve (FFR) assessment of undetermined lesions have been evolving because of the superiority over angiography-guided PCI [155] However, using similar FFR values to guide therapy in women and men have been debated. In 2014, a study by Lin et al. in 1,090 patients, women who underwent FFR-guided PCI had less favorable long-term outcomes compared with men [156]. This study raised the issue to consider gender-based FFR-guided treatment protocols. More studies are required to further characterize the best women-specific FFR-guided treatment strategy.
The gender-related differences in utilization of various diagnostic modalities for CAD are summarized in Table 1.3.
Summary
Women have a significant burden of coronary atherosclerosis. This burden increases in post-menopausal years and is ascribed to a decline in estrogen levels; however, the precise etiology is not known. In addition to the atherosclerotic burden, women tend to have a higher prevalence of microvascular angina. Women generally present late and with atypical symptoms of ischemia and hence diagnostic strategies are employed late. There are also variabilities in the use and result of diagnostic strategies in men and women. Reperfusion strategies are used less often in women resulting in adverse outcomes. It is likely that better recognition of these unique gender differences in CAD will lead to improved outcomes in women.
References
Kumar A, Cannon CP (2009) Acute coronary syndromes: diagnosis and management, part I. Mayo Clin Proc 84:917–938
Benjamin EJ, Blaha MJ, Chiuve SE et al (2017) Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 135:e146–e603
Lloyd-Jones D, Adams RJ, Brown TM et al (2010) Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 121:e46–e215
Yang X-P, Reckelhoff JF (2011) Estrogen, hormonal replacement therapy and cardiovascular disease. Curr Opin Nephrol Hypertens 20:133
Mehta JL, McSweeney J (2018) Gender differences in the pathogenesis and management of heart disease. Springer, Berlin
Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL (1991) Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men?: the Rancho Bernardo Study. JAMA 265:627–631
Geer EB, Shen W (2009) Gender differences in insulin resistance, body composition, and energy balance. Gend Med 6:60–75
Varlamov O, Bethea CL, Roberts CT Jr (2015) Sex-specific differences in lipid and glucose metabolism. Front Endocrinol 5:241
Salas-Salvadó J, Granada M, Bulló M et al (2007) Plasma adiponectin distribution in a Mediterranean population and its association with cardiovascular risk factors and metabolic syndrome. Metabolism 56:1486–1492
Yamauchi T, Kamon J, Waki H et al (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7:941
Moreira GC, Cipullo JP, Ciorlia LAS et al (2014) Prevalence of metabolic syndrome: association with risk factors and cardiovascular complications in an urban population. PLoS ONE 9:e105056
Park E, Kim J (2015) Gender-and age-specific prevalence of metabolic syndrome among Korean adults: analysis of the fifth Korean National Health and Nutrition Examination Survey. J Cardiovasc Nurs 30:256–266
Vishram JK, Borglykke A, Andreasen AH, et al (2014) Impact of age and gender on the prevalence and prognostic importance of the metabolic syndrome and its components in Europeans. The MORGAM Prospective Cohort Project. PloS one 9:e107294
Ogden CL, Carroll MD, Curtin LR et al (2006) Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295:1549–1555
Shaw LJ, Bairey Merz CN, Azziz R et al (2008) Withdrawn: postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health—National heart, lung, and blood institute sponsored women’s ischemia syndrome evaluation. J Clin Endocrinol Metab 93:1276–1284
Vaccarino V, Badimon L, Corti R et al (2010) Ischaemic heart disease in women: are there sex differences in pathophysiology and risk factors? Position paper from the working group on coronary pathophysiology and microcirculation of the European society of cardiology. Cardiovasc Res 90:9–17
Wang X, Magkos F, Mittendorfer B (2011) Sex differences in lipid and lipoprotein metabolism: it’s not just about sex hormones. J Clin Endocrinol Metab 96:885–893
Magkos F, Mittendorfer B (2009) Gender differences in lipid metabolism and the effect of obesity. Obstet Gynecol Clin North Am 36:245–265
Freedman DS, Otvos JD, Jeyarajah EJ et al (2004) Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: the Framingham Study. Clin Chem 50:1189–1200
Mora S, Szklo M, Otvos JD et al (2007) LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 192:211–217
Manolio TA, Pearson TA, Wenger NK et al (1992) Cholesterol and heart disease in older persons and women review of an NHLBI workshop. Ann Epidemiol 2:161–176
Shai I, Rimm EB, Hankinson SE et al (2004) Multivariate assessment of lipid parameters as predictors of coronary heart disease among postmenopausal women: potential implications for clinical guidelines. Circulation 110:2824–2830
Hokanson JE, Austin MA (1996) Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a metaanalysis of population-based prospective studies. J Cardiovasc Risk 3:213–219
Handberg EM, Eastwood J-A, Eteiba W et al (2013) Clinical implications of the Women’s Ischemia Syndrome Evaluation: inter-relationships between symptoms, psychosocial factors and cardiovascular outcomes. Womens Health 9:479–490
Moller-Leimkuhler AM (2010) Higher comorbidity of depression and cardiovascular disease in women: a biopsychosocial perspective. World J Biol Psychiatry 11:922–933
Huxley RR, Woodward M (2011) Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet 378:1297–1305
Willett WC, Green A, Stampfer MJ et al (1987) Relative and absolute excess risks of coronary heart disease among women who smoke cigarettes. N Engl J Med 317:1303–1309
Kawachi I, Colditz GA, Stampfer MJ et al (1993) Smoking cessation in relation to total mortality rates in women: a prospective cohort study. Ann Intern Med 119:992–1000
Yusuf S, Hawken S, Ounpuu S et al (2004) Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 364:937–952
Mallik S, Spertus JA, Reid KJ et al (2006) Depressive symptoms after acute myocardial infarction: evidence for highest rates in younger women. Arch Intern Med 166:876–883
Wassertheil-Smoller S, Shumaker S, Ockene J, et al (2004) Depression and cardiovascular sequelae in postmenopausal women. The Women's Health Initiative (WHI). Arch Intern Med 164:289–98
Rutledge T, Reis SE, Olson MB et al (2006) Depression symptom severity and reported treatment history in the prediction of cardiac risk in women with suspected myocardial ischemia: the NHLBI-sponsored WISE study. Arch Gen Psychiatry 63:874–880
Whang W, Kubzansky LD, Kawachi I et al (2009) Depression and risk of sudden cardiac death and coronary heart disease in women: results from the Nurses’ Health Study. J Am Coll Cardiol 53:950–958
Roest AM, Martens EJ, de Jonge P, Denollet J (2010) Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol 56:38–46
Orth-Gomer K, Wamala SP, Horsten M et al (2000) Marital stress worsens prognosis in women with coronary heart disease: the stockholm female coronary risk study. JAMA 284:3008–3014
Wang HX, Leineweber C, Kirkeeide R, et al (2007) Psychosocial stress and atherosclerosis: family and work stress accelerate progression of coronary disease in women: the stockholm female coronary angiography study. J Int Med 261:245–254
Gallo LC, Troxel WM, Kuller LH et al (2003) Marital status, marital quality, and atherosclerotic burden in postmenopausal women. Psychosom Med 65:952–962
Moore JX, Chaudhary N, Akinyemiju T (2017) Peer reviewed: metabolic syndrome prevalence by race/ethnicity and sex in the United States, national health and nutrition examination survey, 1988–2012. Prev Chronic Dis 14
Grundy SM, Cleeman JI, Daniels SR et al (2005) Diagnosis and management of the metabolic syndrome: an American Heart Association/National heart, lung, and blood institute scientific statement. Circulation 112:2735–2752
Grundy SM (2008) Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol 28:629–636
Pucci G, Alcidi R, Tap L et al (2017) Sex-and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: A review of the literature. Pharmacol Res 120:34–42
Wenger NK (2012) Women and coronary heart disease: a century after Herrick: understudied, underdiagnosed, and undertreated. Circulation 126:604–611
Regitz-Zagrosek V, Oertelt-Prigione S, Prescott E et al (2015) Gender in cardiovascular diseases: impact on clinical manifestations, management, and outcomes. Eur Heart J 37:24–34
Cherry COB, Serieux E, Didier M, et al. Prevalence of risk factors for the metabolic syndrome in the middle income Caribbean nation of st. Lucia. Adv Prev Med. 2014;2014.
Heer T, Schiele R, Schneider S et al (2002) Gender differences in acute myocardial infarction in the era of reperfusion (the MITRA registry). Am J Cardiol 89:511–517
Berger JS, Elliott L, Gallup D et al (2009) Sex differences in mortality following acute coronary syndromes. JAMA 302:874–882
Rosengren A, Wallentin L, K Gitt A, et al (2004) Sex, age, and clinical presentation of acute coronary syndromes. Eur Heart J 25:663–670
Kardys I, Vliegenthart R, Oudkerk M et al (2007) The female advantage in cardiovascular disease: do vascular beds contribute equally? Am J Epidimiol 166:403–412
Cameron AJ, Shaw JE, Zimmet PZ (2004) The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am 33:351–375
Aguilar M, Bhuket T, Torres S et al (2015) Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 313:1973–1974
Dallongeville J, Cottel D, Ferrières J et al (2005) Household income is associated with the risk of metabolic syndrome in a sex-specific manner. Diabetes Care 28:409–415
Ong KL, Tso AW, Lam KS, Cheung BM (2008) Gender difference in blood pressure control and cardiovascular risk factors in Americans with diagnosed hypertension. Hypertension 51:1142–1148
Regitz-Zagrosek V, Lehmkuhl E, Weickert MO (2006) Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol 95:136–147
Wong RJ (2015) Trends in prevalence of the metabolic syndrome—reply. JAMA 314:950–951
Lovre D, Mauvais-Jarvis F (2015) Trends in prevalence of the metabolic syndrome. JAMA 314:950–950
Lidfeldt J, Nyberg P, Nerbrand C, et al (2003) Socio‐demographic and psychosocial factors are associated with features of the metabolic syndrome. The Women's Health in the Lund Area (WHILA) study. Diabetes Obes Metab 5:106–112
Nugent L, Mehta PK, Merz CNB (2011) Gender and microvascular angina. J Thromb Thrombolysis 31:37–46
Lanza GA, Crea F (2010) Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation 121:2317–2325
James PA, Oparil S, Carter BL et al (2014) 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA 311:507–520
O’Donnell E, Floras JS, Harvey PJ (2014) Estrogen status and the renin angiotensin aldosterone system. Am J Physiol Regul Integr Comp Physiol 307:R498–R500
Vitale C, Fini M, Speziale G, Chierchia S (2010) Gender differences in the cardiovascular effects of sex hormones. Fundam Clin Pharmacol 24:675–685
Yamauchi T, Kamon J, Waki H, et al (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7:941–946
Ding EL, Song Y, Malik VS, Liu S (2006) Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 295:1288–1299
Freedman DS, Otvos JD, Jeyarajah EJ et al (1998) Relation of lipoprotein subclasses as measured by proton nuclear magnetic resonance spectroscopy to coronary artery disease. Arterioscler Thromb Vasc Biol 18:1046–1053
Johnson JL, Slentz CA, Duscha BD et al (2004) Gender and racial differences in lipoprotein subclass distributions: the STRRIDE study. Atherosclerosis 176:371–377
Vanzetto G, Berger-Coz E, Barone-Rochette G et al (2009) Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg 35:250–254
Roura G, Ariza-Sole A, Rodriguez-Caballero IF et al (2016) Noninvasive follow-up of patients with spontaneous coronary artery dissection with CT angiography. JACC Cardiovasc Imaging 9:896–897
Saw J, Aymong E, Mancini GB et al (2014) Nonatherosclerotic coronary artery disease in young women. Can J Cardial 30:814–819
Henkin S, Negrotto SM, Tweet MS et al (2016) Spontaneous coronary artery dissection and its association with heritable connective tissue disorders. Heart 102:876–881
Alfonso F, Bastante T (2014) Spontaneous coronary artery dissection: novel diagnostic insights from large series of patients. Circ Cardiovasc Interv 7:638–641
Tweet MS, Gulati R, Hayes SN (2015) What Clinicians Should Know Alphabout Spontaneous Coronary Artery Dissection. Mayo Clin Proc 90:1125–1130
Saw J, Ricci D, Starovoytov A et al (2013) Spontaneous coronary artery dissection: prevalence of predisposing conditions including fibromuscular dysplasia in a tertiary center cohort. JACC Cardiovasc Interv 6:44–52
Vijayaraghavan R, Verma S, Gupta N, Saw J (2014) Pregnancy-related spontaneous coronary artery dissection. Circulation 130:1915–1920
Mahmoud AN, Taduru SS, Mentias A et al (2018) Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv 11:80–90
Mozaffarian D, Benjamin EJ, Go AS et al (2016) Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 133:e38-360
Ann SH, De Jin C, Singh GB et al (2016) Gender differences in plaque characteristics of culprit lesions in patients with ST elevation myocardial infarction. Heart Vessels 31:1767–1775
Yahagi K, Davis HR, Arbustini E, Virmani R (2015) Sex differences in coronary artery disease: pathological observations. Atherosclerosis 239:260–267
Dreyer RP, Wang Y, Strait KM et al (2015) Gender differences in the trajectory of recovery in health status among young patients with acute myocardial infarction: results from the variation in recovery: role of gender on outcomes of young AMI patients (VIRGO) study. Circulation 131:1971–1980
Lansky AJ, Ng VG, Maehara A et al (2012) Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc Imaging 5:S62-72
Schwartz RS, Burke A, Farb A et al (2009) Microemboli and microvascular obstruction in acute coronary thrombosis and sudden coronary death: relation to epicardial plaque histopathology. J Am Coll Cardiol 54:2167–2173
White SJ, Newby AC, Johnson TW (2016) Endothelial erosion of plaques as a substrate for coronary thrombosis. Thromb Haemost 115:509–519
Gulati M, Shaw LJ, Bairey Merz CN (2012) Myocardial ischemia in women: lessons from the NHLBI WISE study. Clin Cardiol 35:141–148
Chandrasekhar J, Mehran R (2016) Sex-Based Differences in Acute Coronary Syndromes: Insights From Invasive and Noninvasive Coronary Technologies. JACC Cardiovasc Imaging 9:451–464
Mendelsohn ME, Karas RH (2005) Molecular and cellular basis of cardiovascular gender differences. Science 308:1583–1587
Edwards DP (2005) Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol 67:335–376
Rubanyi GM, Kauser K, Johns A (2002) Role of estrogen receptors in the vascular system. Vascul Pharmacol 38:81–88
Mieres JH, Shaw LJ, Arai A et al (2005) Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention. American Heart Association. Circulation 111:682–696
Crilly MA, Bundred PE, Leckey LC, Johnstone FC (2008) Gender bias in the clinical management of women with angina: another look at the Yentl syndrome. J Womens Health 17:331–342
Khan NA, Daskalopoulou SS, Karp I et al (2013) Sex differences in acute coronary syndrome symptom presentation in young patients. JAMA Intern Med 173:1863–1871
Meischke H, Larsen MP, Eisenberg MS (1998) Gender differences in reported symptoms for acute myocardial infarction: impact on prehospital delay time interval. Am J Emerg Med 16:363–366
Rosenfeld AG, Lindauer A, Darney BG (2005) Understanding treatment-seeking delay in women with acute myocardial infarction: descriptions of decision-making patterns. Am J Crit Care 14:285–293
Oertelt-Prigione S, Seeland U, Kendel F et al (2015) Cardiovascular risk factor distribution and subjective risk estimation in urban women–The BEFRI Study: a randomized cross-sectional study. BMC Med 13:52
Nguyen HL, Saczynski JS, Gore JM, Goldberg RJ (2010) Age and sex differences in duration of prehospital delay in patients with acute myocardial infarction: a systematic review. Circ Cardiovasc Qual Outcomes 3:82–92
Shaw LJ, Mieres JH, Hendel RH, Boden WE et al (2011) Comparative effectiveness of exercise electrocardiography with or without myocardial perfusion single photon emission computed tomography in women with suspected coronary artery disease: results from the What Is the Optimal Method for Ischemia Evaluation in Women (WOMEN) trial. Circulation 124:1239–1249
Johnson BD, Shaw LJ, Pepine CJ, Reis SE et al (2006) Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI-sponsored Women’s Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J 27:1408–1415
Figueras J, Domingo E, Ferreira I et al (2012) Persistent angina pectoris, cardiac mortality and myocardial infarction during a 12 year follow-up in 273 variant angina patients without significant fixed coronary stenosis. Am J Cardiol 110:1249–1255
Brorsson B, Bernstein SJ, Brook RH, Werkö L (2002) Quality of life of patients with chronic stable angina before and four years after coronary revascularisation compared with a normal population. Heart 87:140–145
Jespersen L, Hvelplund A, Abildstrøm SZ et al (2011) Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Euro Heart J 33:734–744
Canto JG, Rogers WJ, Goldberg RJ et al (2012) Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA 307:813–822
Daly C, Clemens F, Lopez Sendon JL et al (2006) CLINICAL PERSPECTIVE. Circulation 113:490–498
Boccia A, Damiani G, D'Errico MM, et al. Age-and sex-related utilisation of cardiac procedures and interventions: a multicentric study in Italy. Int J Cardiol
Hendel RC, Berman DS, Di Carli MF et al (2009) ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine Endorsed by the American College of Emergency Physicians. J Am Coll Cardiol 53:2201–2229
Kwok Y, Kim C, Grady D et al (1999) Meta-analysis of exercise testing to detect coronary artery disease in women. Am J Cardiol 83:660–666
Taqueti VR, Dorbala S, Wolinsky D et al (2017) Myocardial perfusion imaging in women for the evaluation of stable ischemic heart disease—state-of-the-evidence and clinical recommendations. J Nucl Cardiol 24:1402–1426
Gibbons RJ, Balady GJ, Bricker JT et al (2002) ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol 40:1531–1540
Kohli P, Gulati M (2010) Exercise stress testing in women: going back to the basics. Circulation 122:2570–2580
Hlatky MA, Pryor DB, Harrell FE et al (1984) Factors affecting sensitivity and specificity of exercise electrocardiography: multivariable analysis. Am J Med 77:64–71
Morise AP, Beto R (1997) The specificity of exercise electrocardiography in women grouped by estrogen status. Int J Cardiol 60:55–65
Grzybowski A, Puchalski W, Zieba B et al (2008) How to improve noninvasive coronary artery disease diagnostics in premenopausal women?: The influence of menstrual cycle on ST depression, left ventricle contractility, and chest pain observed during exercise echocardiography in women with angina and normal coronary angiogram. Am Heart J 156:964
Barolsky SM, Gilbert CA, Faruqui A et al (1979) Differences in electrocardiographic response to exercise of women and men: a non-Bayesian factor. Circulation 60:1021–1027
Fitzgerald BT, Scalia WM, Scalia GM (2019) Female False Positive Exercise Stress ECG Testing-Fact Versus Fiction. Heart Lung Circ 28:735–741
Sharma S, Mehta PK, Arsanjani R et al (2018) False-positive stress testing: Does endothelial vascular dysfunction contribute to ST-segment depression in women? a Pilot Study. Clin Cardiol 41:1044–1048
Iskandar A, Limone B, Parker MW et al (2013) Gender differences in the diagnostic accuracy of SPECT myocardial perfusion imaging: a bivariate meta-analysis. J Nucl Cardiol 20:53–63
Murthy VL, Naya M, Taqueti VR et al (2014) Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 129:2518–2527
Greenwood JP, Motwani M, Maredia N et al (2014) Comparison of cardiovascular magnetic resonance and single-photon emission computed tomography in women with suspected coronary artery disease from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease (CE-MARC) Trial Circulation 129:1129–1138
Jug B, Gupta M, Papazian J et al (2012) Diagnostic performance of 64-slice multidetector coronary computed tomographic angiography in women. J Nucl Cardiol 19:1154–1161
Mullani NA, Caras D, Ahn C et al (2000) Fewer women than men have positive SPECT and PET cardiac findings among patients with no history of heart disease. J Nucl Med 41:263–268
Iskandrian AE, Heo J, Nallamothu N (1997) Detection of coronary artery disease in women with use of stress single-photon emission computed tomography myocardial perfusion imaging. J Nucl Cardiol 4:329–335
Hansen CL, Crabbe D, Rubin S (1996) Lower diagnostic accuracy of thallium-201 SPECT myocardial perfusion imaging in women: an effect of smaller chamber size. J Am Coll Cardiol 28:1214–1219
Taillefer R, DePuey EG, Udelson JE et al (1997) Comparative diagnostic accuracy of Tl-201 and Tc-99m sestamibi SPECT imaging (perfusion and ECG-gated SPECT) in detecting coronary artery disease in women. J Am Coll Cardiol 29:69–77
Sharir T, Kang X, Shaw LJ et al (2006) Prognostic value of poststress left ventricular volume and ejection fraction by gated myocardial perfusion SPECT in women and men: gender-related differences in normal limits and outcomes. J Nucl Cardiol 13:495–506
Rivero A, Santana C, Folks RD et al (2006) Attenuation correction reveals gender-related differences in the normal values of transient ischemic dilation index in rest-exercise stress sestamibi myocardial perfusion imaging. J Nucl Cardiol 13:338–344
Sharma P, Patel CD, Karunanithi S et al (2012) Comparative Accuracy of CT Attenuation-Corrected and Non–Attenuation-Corrected SPECT Myocardial Perfusion Imaging. Clin Nucl Med 37:332–338
Masood Y, Liu Y-H, DePuey G et al (2005) Clinical validation of SPECT attenuation correction using x-ray computed tomography–derived attenuation maps: multicenter clinical trial with angiographic correlation. J Nucl Cardiol 13:676–686
Van Train KF, Maddahi J, Berman DS et al (1990) Quantitative analysis of tomographic stress thallium-201 myocardial scintigrams: a multicenter trial. J Nucl Med 31:1168–1179
Metz LD, Beattie M, Hom R et al (2007) The prognostic value of normal exercise myocardial perfusion imaging and exercise echocardiography: a meta-analysis. J Am Coll Cardiol. 49:227–237
Shaw LJ, Hage FG, Berman DS et al (2012) Prognosis in the era of comparative effectiveness research: Where is nuclear cardiology now and where should it be? J Nucl Cardiol 19:1026–1043
Hachamovitch R, Berman DS, Kiat H et al (1996) Effective risk stratification using exercise myocardial perfusion SPECT in women: gender-related differences in prognostic nuclear testing. J Am Coll Cardiol 28:34–44
Sharir T, Germano G, Kavanagh PB et al (1999) Incremental prognostic value of post-stress left ventricular ejection fraction and volume by gated myocardial perfusion single photon emission computed tomography. Circulation 100:1035–1042
Valeti US, Miller TD, Hodge DO, Gibbons RJ (2005) Exercise single-photon emission computed tomography provides effective risk stratification of elderly men and elderly women. Circulation 111:1771–1776
Cerci MSJ, Cerci JJ, Cerci RJ, Neto CCP et al (2011) Myocardial perfusion imaging is a strong predictor of death in women. JACC Cardiovasc Imaging 4:880–888
Abbott BG, Afshar M, Berger AK, Frans J (2003) Prognostic significance of ischemic electrocardiographic changes during adenosine infusion in patients with normal myocardial perfusion imaging. J Nucl Cardiol 10:9–16
Klodas E, Miller TD, Christian TF et al (2003) Prognostic significance of ischemic electrocardiographic changes during vasodilator stress testing in patients with normal SPECT images. J Nucl Cardiol 10:4–8
Parker MW, Iskandar A, Limone B et al (2012) Diagnostic accuracy of cardiac positron emission tomography versus single photon emission computed tomography for coronary artery disease: a bivariate meta-analysis. Circ Cardiovasc Imaging 5:700–707
Mc Ardle BA, Dowsley TF, Wells GA, Beanlands RS (2012) Does rubidium-82 PET have superior accuracy to SPECT perfusion imaging for the diagnosis of obstructive coronary disease?: A systematic review and meta-analysis. J Am Coll Cardiol 60:1828–1837
Dorbala S, Di Carli MF (2014) Cardiac PET perfusion: prognosis, risk stratification, and clinical management. Semin Nucl Med 44:344–357
Merz CNB, Shaw LJ, Reis SE et al (2006) Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol 47:S21–S29
Shaw LJ, Merz CNB, Pepine CJ et al (2006) Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol 47:S4–S20
Taqueti VR, Shaw LJ, Cook NR et al (2017) Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation 135:566–577
Yoshinaga K, Chow BJ, Williams K et al (2006) What is the prognostic value of myocardial perfusion imaging using rubidium-82 positron emission tomography? J Am Coll Cardiol 48:1029–1039
Dorbala S, Di Carli MF, Beanlands RS et al (2013) Prognostic value of stress myocardial perfusion positron emission tomography: results from a multicenter observational registry. J Am Coll Cardiol 61:176–184
Kay J, Dorbala S, Goyal A et al (2013) Influence of sex on risk stratification with stress myocardial perfusion Rb-82 positron emission tomography: results from the PET (Positron Emission Tomography) Prognosis Multicenter Registry. J Am Coll Cardiol 62:1866–1876
Roger VL, Pellikka PA, Bell MR et al (1997) Sex and test verification bias: impact on the diagnostic value of exercise echocardiography. Circulation 95:405–410
Cortigiani L, Dodi C, Paolini EA et al (1998) Prognostic value of pharmacological stress echocardiography in women with chest pain and unknown coronary artery disease. J Am Coll Cardiol 32:1975–1981
Chow BJ, Abraham A, Wells GA et al (2009) Diagnostic accuracy and impact of computed tomographic coronary angiography on utilization of invasive coronary angiography. Circ Cardiovasc Imaging 2:16–23
Meijboom WB, Meijs MF, Schuijf JD et al (2008) Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 52:2135–2144
Dharampal AS, Papadopoulou SL, Rossi A et al (2012) Computed tomography coronary angiography accuracy in women and men at low to intermediate risk of coronary artery disease. Eur Radiol 22:2415–2423
Mieres JH, Gulati M, Bairey Merz N et al (2014) Role of noninvasive testing in the clinical evaluation of women with suspected ischemic heart disease: a consensus statement from the American Heart Association. Circulation 130:350–379
Pundziute G, Schuijf JD, Jukema JW et al (2008) Gender influence on the diagnostic accuracy of 64-slice multislice computed tomography coronary angiography for detection of obstructive coronary artery disease. Heart 94:48–52
Jespersen L, Hvelplund A, Abildstrom SZ et al (2012) Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 33:734–744
Kern MJ, Lerman A, Bech J-W et al (2006) Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: a scientific statement from the American Heart Association committee on diagnostic and interventional cardiac catheterization. Council Clin Cardiol Circ 114:1321–1341
Solimene MC (2010) Coronary heart disease in women: a challenge for the 21st century. Clinics 65:99–106
Nevsky G, Jacobs JE, Lim RP et al (2011) Sex-specific normalized reference values of heart and great vessel dimensions in cardiac CT angiography. Am J Roentgenol 196:788–794
Kim H-S, Tonino PA, De Bruyne B et al (2012) The impact of sex differences on fractional flow reserve–guided percutaneous coronary intervention: a FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) substudy. JACC Cardiovasc Interv 5:1037–1042
Tonino PA, De Bruyne B, Pijls NH et al (2009) Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 360:213–224
Li J, Rihal CS, Matsuo Y et al (2013) Sex-related differences in fractional flow reserve–guided treatment. Circ Cardiovasc Interv. 6:662–670
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Saad, M., Megaly, M., Romeo, F., Mehta, J.L. (2020). Gender-Related Differences in the Pathogenesis and Diagnosis of Ischemic Heart Disease. In: Ostadal, B., Dhalla, N.S. (eds) Sex Differences in Heart Disease. Advances in Biochemistry in Health and Disease, vol 21. Springer, Cham. https://doi.org/10.1007/978-3-030-58677-5_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-58677-5_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-58676-8
Online ISBN: 978-3-030-58677-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)