Abstract
Japanese encephalitis is a serious infection of the brain caused by Japanese encephalitis virus (JEV). The virus is endemic in 24 countries located in Asia and the Western Pacific where it is transmitted to humans by mosquitoes. Asymptomatic infection is common. Symptomatic disease involving the central nervous system, however, is associated with substantial morbidity and mortality, particularly among young children. Before the widespread availability of safe and effective vaccines to prevent JEV infection, tens of thousands of cases of Japanese encephalitis were reported annually from Asia. In the decade spanning 1965 to 1975, more than one million cases were reported from China alone. Countries successful in achieving and maintaining high immunization coverage rates in young children have experienced declines in disease incidence from more than 20 to less than 1 case per 100,000 children each year. The combined efforts of robust childhood vaccination programs, increased urbanization, and improved agricultural practices have led to the virtual elimination of human disease in Japan, South Korea, and Taiwan despite an ongoing enzootic presence of JEV in pigs and aquatic birds.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Japanese Encephalitis Virus Infection
Etiology
Japanese encephalitis virus (JEV) is an enveloped, positive sense, single-stranded RNA member of the Flaviviridae family that is transmitted to humans by mosquitoes . Five genotypes, I through V, are recognized based on the nucleotide sequence of the envelope gene. Between 1870 and the mid-1990s, most infections were caused by genotype III. Infections caused by JEV genotype I have predominated during the last two decades. The recent emergence of genotype V in parts of China, Malaysia, and South Korea is an important reminder that more than one genotype can circulate simultaneously. JEV is the cause of Japanese encephalitis, a life-threatening infection of the brain parenchyma , but most human infections are mild or completely asymptomatic. Severe clinical illness is more common in children than in adults, occurring in approximately 1 of every 250 infections.
Global Epidemiology of Japanese Encephalitis Virus
The first case of Japanese encephalitis was documented in Japan in 1871. Infections caused by JEV remained largely restricted to temperate areas of Japan, Korea, Taiwan, and China during the first half of twentieth century but subsequently spread west to India, Bangladesh, Sri Lanka, and Nepal and south to most of Southeast Asia. During the 1990s, the range of infection spread deeper throughout the Western Pacific region to Australia and Saipan, the largest of the Northern Mariana Islands. Presently, the distribution of JEV includes 24 countries, representing approximately 60% of the world’s population (Fig. 17.1). The combined number of infections from China and India alone accounts for more than 85% of all JEV infections reported. The expanding geographic distribution of JEV observed over the last 50 years is likely due to a variety of factors including population shifts and changes in ecology, agricultural practices , animal husbandry , and migratory patterns of birds .
Japanese encephalitis primarily affects children living in endemic regions where the overall mean prevalence of infection in children less than 15 years of age is estimated at 5.4 per 100,000 population. Regionally, in parts of China and North Korea, disease prevalence has been reported as high as 12.6 per 100,000 population. During the last decade, JEV is estimated to have caused approximately 65,000 symptomatic infections and more than 13,000 deaths each year. The majority of adults who reside in JEV endemic areas are naturally immune to disease from infections they had during childhood.
Disease incidence changes from year to year and varies widely both across and within endemic countries. Major outbreaks occur every 2–15 years across endemic regions, occurring primarily in rural agricultural areas, often in association with the common practice of flood irrigation used to farm rice.
The risk for JEV infection in US travelers to endemic regions of Asia is generally quite low but varies by destination, duration and season of travel, living accommodations while abroad, and the types of activities planned during the visit(s).
The overall incidence of JEV infection among travelers to Asia is less than 1 case per 1 million travelers. The incidence increases to approximately 10 cases per 100,000 people per year in those traveling to areas with active transmission who stay for prolonged periods of time. The incidence of disease further increases for those engaged in extensive outdoor activities, particularly at night. Japanese encephalitis should be suspected in any traveler returning from Asia or the Western Pacific with evidence of a central nervous system infection such as encephalitis, meningoencephalitis, aseptic meningitis, or acute flaccid paralysis.
Transmission
JEV is transmitted to humans by infected mosquitoes. The key competent vectors are Culex genus mosquitos , primarily C. tritaeniorhynchus. Female C. tritaeniorhynchus mosquitoes bite during the evening and nighttime, being most active just after sunset.
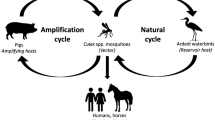
C. tritaeniorhynchus larva can be found in flooded rice fields, marshes, and other stagnant water sources. At least 30 other mosquito species can transmit JEV including C. annulirostris, C. fuscocephala, and others belonging to the genera Culiseta, Ochlerotatus, Aedes, Anopheles, and Mansonia. In temperate regions of the Northern Hemisphere, the greatest mosquito density is found between the months of June and November. Virus is maintained in an enzootic cycle between mosquitoes and principal vertebrates, amplifying in pigs and/or aquatic birds such as egrets and herons. Domestic and agricultural pigs develop high JEV titers and long-lasting viremia from natural infection.
Infected humans do not usually develop a sufficient level or duration of viremia to infect feeding mosquitoes. Humans are dead-end hosts, so infected travelers pose little or no risk of transmitting virus when they arrive at home.
Clinical Presentation
Symptomatic JEV infection most commonly presents as acute viral meningoencephalitis. The illness begins with the abrupt onset of high fever, rigors, headache, weakness, vomiting, and diarrhea. Despite the neurotropic nature of the virus, gastrointestinal complaints are the most common presenting symptoms in children. As the illness progresses, the infected person develops mental status changes and focal neurologic deficits such as hemiplegia, tetraplegia, and/or cranial nerve palsies. Seizures are very common, especially in children. A classic presentation includes development of a parkinsonian-like syndrome with mask-like facies, tremor, cogwheel rigidity, and choreoathetoid movements. JEV can also present as a polio-like illness with acute-onset, asymmetrical flaccid paralysis.
Common complications of encephalitis include life-threatening conditions such as status epilepticus, aspiration pneumonia, increased intracranial pressure, brain hypoxia, and brainstem herniation. Between 30% and 50% of patients who survive JEV encephalitis suffer permanent neurologic, psychiatric, and/or cognitive deficits. The case-fatality rate is highest in young children where it exceeds 50%. Those who survive infection from any of the five JEV genotypes develop lifelong immunity against all genotypes.
Management
Antiviral therapy is not available. Mild to moderate infections are managed at home with supportive care to address the symptom complex. Adequate fluids to maintain hydration and over-the-counter pain relievers and fever reducers help to relieve minor symptoms. Those with signs of central nervous system involvement require hospitalization and aggressive supportive care to monitor for and treat complications.
Prevention: Japanese Encephalitis Vaccines
In areas endemic for JEV, human vaccination is prioritized over the vaccination of pigs and efforts to control mosquito populations. All 15 of the JEV vaccine formulations currently in use are based on virus genotype III. Three of these vaccines are WHO prequalified, and one is licensed in the United States. Available vaccines can be grouped into four categories (Table 17.1): inactivated whole virus derived from mouse brain, inactivated whole virus derived from cell culture, live attenuated virus, and live attenuated recombinant-chimeric vaccine. First developed in the mid-1930s, mouse brain-derived, inactivated whole virus vaccines were used exclusively until the late 1980s. In 2015, WHO recommended that mouse brain-derived vaccine be replaced by newer generation vaccines with better reactogenicity profiles , cheaper costs, and reduced dosing requirements. CD.JEVAX, the only live attenuated JEV vaccine formulation available for international use, was licensed in China in 1988. Cell culture-derived inactivated whole virus vaccine formulations (IXIARO, JESPECT, JEEV) first emerged a decade later in 1998. IXIARO was licensed in the United States and Europe in 2009, JESPECT was licensed in Australia and New Zealand in 2009, and JEEV was licensed in India in 2012. Live attenuated recombinant/chimeric vaccines (IMOJEV, JE-CV, ChimeriVax-JE) were first licensed in Australia in 2010.
The WHO recommends JE immunization in regions where humans live near environments that support the enzootic cycle of JEV transmission. Vaccine recommendations include one-time catch-up campaigns in target populations as defined by the local disease epidemiology, most typically in children under 15 years of age, followed by incorporation of the vaccine into the routine childhood immunization schedule .
Vaccines Available in the United States
The Vero cell culture-derived, inactivated whole virus product, IXIARO, is the only JEV vaccine formulation currently approved and available for use in the United States. IXIARO is manufactured by Valneva Scotland, Ltd. It was first licensed in the United States in 2009 for use in individuals 17 years and older based on immunogenicity trials. Licensing approval was extended to 2 months of age in 2013.
Immunizing Antigen
Genotype III , JEV strain SA14-14-2 is propagated in Vero cells. Multiple harvests are pooled, clarified, and concentrated and then further purified by sucrose density gradient centrifugation following treatment with protamine sulfate. Purified virus is inactivated with formaldehyde and then adjusted to the desired concentration of 6 antigen units/0.5 mL dose. Aluminum hydroxide is added as an adjuvant.
Additives and Excipients
Vaccine additives and excipients include phosphate-buffered saline, <100 ng/mL bovine albumin, <200 pg/mL host cell DNA, <100 ng/mL host cell protein, <200 ppm sodium metabisulfite, <1 μg/mL protamine sulfate, <200 ppm formaldehyde, and 250 mcg of aluminum hydroxide. The vaccine is preservative-free and does not contain natural rubber latex.
Vaccine Recommendations
Vaccine is recommended for travelers to endemic areas after consideration of the exact travel destination, travel duration, planned activities, accommodations, and season.
Dosing recommendations for the primary vaccine series vary according to age (Table 17.2).
Travelers should plan to receive the second dose of the primary series at least 1 week before planned travel. Individuals with ongoing exposure, and those at risk for reexposure, should receive a booster dose of vaccine if a year or more has elapsed since their primary vaccine series was completed. In addition to travelers to endemic regions, JEV vaccine is recommended for laboratory workers with a high risk of exposure to JEV.
Vaccine Contraindications
Contraindications to receiving JEV vaccine include any previous life-threatening allergic reactions from a previous dose or a known severe allergy to any vaccine component, especially protamine sulfate, which is known to cause hypersensitivity reactions in some people. Moderately or severely ill individuals should postpone immunization until after they recover.
Warnings and Precautions
Warnings and precautions for JEV vaccine include use during pregnancy where the risk of infection must outweigh the risk of immunization. Use in immunocompromised patients may result in a diminished immunological response to vaccine. The safety and efficacy have not been established in infants less than 2 months of age. Seroprotection rates following the primary series are significantly lower in adults 65 years and older compared to all other age groups.
Common Side Effects
During a clinical trial performed in the Philippines, the most common adverse events seen in infants aged 2 to 12 months were fever (>20%), injection site redness (>15%), irritability (>15%), and diarrhea (>10%). Children between 1 and 3 years of age had similar rates of fever (13–20%) but lower rates of injection site redness (3–6%). Other adverse events included diarrhea (5–7%), influenza-like symptoms (4–8%), irritability (3–8%), loss of appetite (3–6%), vomiting (3–4%), rash (1–4%), excessive fatigue (1–3%), and headache (1%). Similar adverse reactions were experienced by children 3–12 years of age with lower frequency. Adolescents 12 to <18 years old experienced pain (7–15%), tenderness (5–10%), redness (1–4%), or swelling (<1%) at the injection site. Between 3% and 5% developed fever or headache. Serious adverse reactions were rare across all groups. Approximately 1% of children less than 3 years of age experienced a febrile seizure ranging from 2 days to >5 months after receiving a dose of IXIARO. No temporal clustering related to the timing of vaccination was observed.
Estimated Effectiveness or Efficacy from Clinical Vaccine Trials
Vaccine efficacy data are not available from clinical trials. IXIARO vaccine was licensed in the United States based on immunogenicity studies demonstrating development of protective neutralizing antibody. For clinical trial purposes, a 50% plaque-reduction neutralization antibody test (PRNT50) result showing a titer of ≥1:10 was used as the surrogate for protective immunity. Titers measured 28 days after completing the primary series showed 100% seroprotection in children 2 months to 17 years old and 96% seroprotection in adults. In adults ≥65 years, titers measured 42 days after completing the primary series showed 65% seroprotection.
Japanese encephalitis causes substantial morbidity and mortality across endemic regions of the world, disproportionately affecting children. Survivors of the infection are often left with serious lifelong neurologic and cognitive deficits . The infection is vaccine-preventable starting as early as 2 months of age. Vaccine should also be considered for those who plan to travel to endemic regions, particularly during the known seasonal periods of disease transmission.
References and Suggested Reading
World Health Organization
https://www.who.int/news-room/fact-sheets/detail/japanese-encephalitis
https://www.who.int/immunization/diseases/japanese_encephalitis/en/
U.S. Centers for Disease Control and Prevention
Vaccine Information Sheet
Ixiaro Package Insert
https://www.fda.gov/vaccines-blood-biologics/vaccines/ixiaro
Connor B, Bunn WB. The changing epidemiology of Japanese encephalitis and new data: the implications for new recommendations for Japanese encephalitis vaccine. Trop Dis Travel Med Vaccines. 2017;3:14. https://doi.org/10.1186/s40794-017-0057-x.
Heffelfinger JD, Li X, Batmunkh N, et al. Japanese encephalitis: surveillance and immunization in Asia and Western Pacific, 2016. WHO Weekly Epidemiological Record. 2017;92:321–32.
Hegde NR, Gore MM. Japanese encephalitis vaccines: immunogenicity, protective efficacy, effectiveness, and impact on burden of disease. Hum Vaccin Immunother. 2017;13:1320–37.
Hills SL, Walter EB, Atmar RL, et al. Japanese encephalitis vaccine: recommendations of the advisory committee on immunization practices. MMWR. 2019;68:1–33.
Japanese encephalitis vaccines. WHO position paper. WHO Weekly Epidemiological Record. 2015;90:69–88.
McNaughton H, Singh A, Khan SA. An outbreak of Japanese encephalitis in a non-endemic region of Northeast India. J R Coll Physicians Edinb. 2018;48:25–9.
Muniaraj M, Rajamannar V. Impact of SA 14-14-2 vaccination on the occurrence of Japanese encephalitis in India. Hum Vaccin Immunother. 2019;15:834–40.
Oliveira ARS, Piaggio J, Cohnstaedt LW, et al. Introduction of the Japanese encephalitis virus (JEV) in the United States-a quality risk assessment. Transbound Emerg Dis. 2019;66:1558–74.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bonville, C., Domachowske, J. (2021). Japanese Encephalitis. In: Domachowske, J., Suryadevara, M. (eds) Vaccines. Springer, Cham. https://doi.org/10.1007/978-3-030-58414-6_17
Download citation
DOI: https://doi.org/10.1007/978-3-030-58414-6_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-58413-9
Online ISBN: 978-3-030-58414-6
eBook Packages: MedicineMedicine (R0)