Abstract
Synthetic signal transduction is an exciting new research field that applies supramolecular chemistry in a membrane environment to provide insight into the physical processes involved in natural signal transduction and to open new opportunities in synthetic biology, for example the integration of artificial signaling pathways into cells. Although it is still a developing field, we discuss a selection of recent stimuli-responsive supramolecular constructs that, when embedded in the phospholipid bilayer, can mimic aspects of the behavior of different natural signaling proteins, including ligand-gated ion channels, G-protein coupled receptors and receptor tyrosine kinases. The lipid bilayer plays a key part in these biomimetic systems, as this complex anisotropic environment provides challenges both when designing supramolecular systems that function in the bilayer and when analyzing the data they provide. Nonetheless these recent studies have provided key insights into how the bilayer affects binding to, the conformation of, and catalysis by membrane-embedded supramolecular constructs. If successful, these model systems promise to be key components for bottom-up synthetic biology, the creation of artificial cells and devices starting from molecular components.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The cell membrane is a semipermeable physical barrier that defines the cell, giving it a compartmentalized network of chemical reactions that do not freely interact with constituents of the extracellular environment. The major component of the cell membrane is a phospholipid bilayer, a noncovalent fluid assembly that is typically 3 to 4 nm thick and largely impermeable to hydrophilic molecules and small inorganic ions. This impermeability to ions gives the membrane a key role in energy storage, through the maintenance of transmembrane concentration gradients. The membrane is also home to a large number of biomolecules including proteins, carbohydrates and steroids, which have specific functions such as regulating interactions with the extracellular environment, biocatalysis and using concentration gradients to drive biosynthesis (Luckey 2008).
This barrier has to have controllable permeability, however, to allow specific molecules to enter and exit the cell according to the demands of cellular metabolism. Some of the molecules that translocate through the bilayer act as messengers, and the communication of information across the cell membrane is essential to coordinate biological events with other cells and adapt to changes in the external environment. Nature has developed many sophisticated mechanisms to sense, transduce and process external signals, before converting them into complex intracellular responses. The translocation of messenger molecules across the membrane can occur by diffusion through the hydrophobic core of the bilayer (alone or when complexed to carrier proteins) or by passage through a hydrophilic channel within a transmembrane protein (either passive or active transport). Alternatively, the messenger molecules may regulate the opening/closing (“gating”) of a channel protein to allow the transit of other molecules.
Instead of physical mass transfer, the binding of messenger molecules to receptors at the outer surface of the cells can initiate the transfer of information via transmembrane domains. Binding to a primary messenger can alter the conformation, orientation or position of the transmembrane domains, and these global changes are necessarily transmitted to the far side of the bilayer. This mechanism also allows for signal amplification, as binding to the primary messenger molecule outside the cell can initiate the catalytic production of a large number of secondary messengers inside the cell. Despite being some of the most intensely studied protein families in the proteome, the complexity of signal transduction processes involving large proteins within a self-assembled lipid matrix makes the unambiguous interpretation of biophysical data challenging.
Self-assembled artificial vesicles doped with synthetic molecules are widely used to construct simplified systems that mimic aspects of the behavior of cell membranes. Phospholipid vesicles reproduce some basic features of the cell membrane, an internal aqueous compartment surrounded by a semipermeable barrier, yet are less complex and simple to fabricate. Despite their apparent simplicity, the physical properties of these self-assembled soft materials, such as anisotropy, chirality, low fluidity, low permeability and variable thickness, make bilayers more complex environments than isotropic solvent. Several of these properties depend on bilayer composition and temperature, and can determine the behavior of any synthetic molecules within the bilayers (Pintre and Webb 2013). Nonetheless since the receipt and relay of messages is fundamental to the function of living organisms, a detailed understanding of the physical processes that produce these features is a key objective in biomimetic chemistry.
In this review we discuss a selection of recently published supramolecular approaches to mimicking signal transduction processes, with a focus on systems that are controlled by external stimuli, such as messenger molecules or light. There are other recent reviews both in this area (Bekus and Schrader 2020; Vanuytsel et al. 2019) and in other related contexts, such as synthetic ion channels (Zheng et al. 2021) and supramolecular chemistry in biomembranes (Webb 2013; Barba-Bon et al. 2020), which can provide useful alternative narratives. These recent reviews reflect rapidly growing interest in this area, an area that is inspired by the complexity and elegance of biological signaling processes.
2 Natural Transmembrane Signaling Mechanisms
2.1 Protein Ion Channels
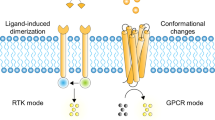
Natural ion channels regulate many key functions, such as the transmission of electric signals across the membranes of neurons and other excitable cells (Unwin 1989). Other important examples include the cystic fibrosis transmembrane conductance regulator (CFTR), which controls secretion and absorption in different types of epithelial tissue (Saint-Criq and Gray 2017). It is not surprising that unregulated function of ion channels gives rise to a series of diseases collectively known as channelopathies (e.g. epilepsy, myotonia, ataxia cardiac, arrhythmia and cystic fibrosis) (Ptáček 1997; Gadsby et al. 2006). Many protein ion channels have a relatively similar structure, with homologous domains within the membrane that delineate a central pore for the channel. Passage through these protein ion channels is usually “gated”, i.e. the channels are opened and closed by specific stimuli. Depending on the gating mechanism, natural ion channels can be classified as being voltage-gated, ligand-gated, light-gated or mechano-sensitive. Such gating is usually mediated by a conformational change rather than a change in the extent of self-association of multisubunit channels (Fig. 1a).
Schematic representations of a Ligand binding to an ion channel to allow passage of cations. b Ligand induced dimerization of receptor tyrosine kinases (RTKs). P = phosphate. c Ligand induced change in the conformational states of a G-protein coupled receptor (GPCR). d Ligand (steroid) translocation through a membrane and binding to a steroid hormone receptor (SHR)
Voltage-gated ion channels form a large family whose gating activity depends on changes in the potential difference across the membrane, and regulate the flux of Na+, Ca2+ and K+ across the cellular membrane. The structure of voltage-gated K+ ion channels has been elucidated in recent years, (Gulbis et al. 1999; Sands et al. 2005) and a proposed mode of action described. The canonical model describes voltage-gated K+ channels as transmembrane pores composed of four identical subunits around a central ion conduction pathway. Each subunit consists of six α-helices (S1–S6), with helices S5–S6 in the pore domain and transmembrane helices S1–S4 forming the voltage-sensing domain. The arginine-rich S4 helices are sensitive to membrane (de)polarization, and are proposed to slide or screw in the direction of the extracellular surface with subsequent relocation of the arginine residues to externally facing pockets. This overall conformational change results in channel opening and the diffusion of K+ ions down a concentration gradient.
Ligand-gated ion channels include cystic fibrosis transmembrane conductance regulator (CFTR, ATP-gated) and nicotinic acetylcholine receptors (nAChRs, acetylcholine-gated). The latter belongs to the pentameric ligand-gated ion channel (pLGICs) class, which are involved in processes where a neurotransmitter released by synapses induces the transmission of action potential between neurons or between a neuron and a muscle (Herrington and Arey 2014). Channels binding γ-aminobutyric acid (GABA), serotonin, glycine and glutamate also belong to this class of gated ion channels. The general structure comprises five subunits that form a pentameric channel. Each subunit contains a large extracellular domain with a characteristic disulfide bridge formed by a pair of cysteine residues. The structure of glutamate receptors is slightly different, with a large extracellular domain, an extracellular ligand binding domain and a characteristic transmembrane domain formed by two helical subunits that open/close the channels in a ‘clamshell’ fashion (Zhu et al. 2016).
Light-gated ion channels are extremely rare in nature, with channelrhodopsin the only example currently known (Nagel et al. 2002). Structurally similar to rhodopsin (see below), channelrhodopsin contains all-trans-retinal as the isomerizable chromophore. The chromophore domain is covalently linked to a seven-segment transmembrane domain, and upon absorption of blue light a configurational change from all-trans to 13-cis-retinal induces conformational change of the transmembrane domain with consequent opening of the pore.
Mechano-sensitive channels are a class of membrane-embedded proteins whose gating mechanism is dependent on external mechanical stimuli (Haswell et al. 2011). Senses of touch, hearing and balance involve the presence of mechano-sensitive channels, as well as the regulation of the cardiovascular system and osmotic homeostasis. Several models are proposed for the functioning of mechano-sensitive channels. One relies on the change of membrane tension caused by application of mechanical stress, which induces channel opening as a result of the transmitted force producing a conformational change in the membrane-embedded domains. Alternatively, the transmembrane channel can have an extracellular gating system connected to an extramembrane structural component (e.g. cytoskeleton or peptidoglycan). In this case movement of the extracellular component transfers mechanical force to the extracellular gating system, resulting in the regulation of the channel activity.
2.2 Receptor Tyrosine Kinases (RTKs)
Receptor Tyrosine Kinases (RTKs) are a large family containing important receptors such as the epidermal growth factor receptor (EGFR) and the insulin receptor (IR). RTKs play a crucial role in several cellular activities from cell growth to differentiation, migration and metabolism (Schlessinger 2000). RTKs are strongly associated with tyrosine kinases, enzymes which catalyze phosphoryl transfer to tyrosine residues from ATP.
The general structure of RTKs comprises an extracellular binding domain connected to a cytoplasmic domain by a single transmembrane helix (Fig. 1b). The activation mechanism relies on the binding of a ligand to the RTK extracellular binding domain. For instance, EGF binding induces dimerization of the RTK monomers, as a result of lateral movement of the transmembrane helices. Alternatively, the ligand can induce conformational change of a pre-formed inactive dimer, as in the case of insulin (Hubbard 2004). The active dimer induces the switch-on of the catalytic activity of tyrosine kinases and, hence, the activation of domains towards cytoplasmic enzymes that migrate to the membrane. The result is the onset of multiple signal cascades from a single extracellular binding event, providing the characteristic signal amplification properties showed by RTKs.
2.3 G-Protein Coupled Receptors (GPCRs)
G-Protein Coupled Receptors (GPCRs) are a family of seven-transmembrane domain receptors that associate with heterotrimeric proteins able to bind guanosine triphosphate (GTP), known as G-proteins. GPCRs are evolutionary conserved in many species and are important for the senses of light, smell and taste (Fig. 1c) (Tompa 2016). GPCRs respond to a large variety of external primary messengers, which activate multiple signal cascades. The binding of an agonist to an extracellular domain induces a subsequent conformational change in the transmembrane domains, producing a net change in the relative positions of some transmembrane domains. Thus, the binding event is transmitted from the cell exterior, through the membrane to activate a specific intracellular response. In the basal state, three subunits are associated (Gαβγ). Upon binding of the incoming ligand, Gα undergoes a conformational change, exchanges its bound GDP for GTP and dissociates from the βγ heterodimer. In this activated state, both Gα and Gβγ subunits can independently interact with effector proteins. For instance, transmembrane adenylyl cyclase binds the Gα subunit by means of its cytoplasmic catalytic domain (Tesmer et al. 1997). The domain undergoes a conformational change to activate the production of cyclic adenosine monophosphate (cAMP), which is involved in muscle relaxation. On the other hand, the Gβγ subunit has been found associated to voltage-dependent Ca2+ channels for the controlled release of neurotransmitters at synapses (De Waard et al. 1997).
Rhodopsin, which is involved in the sensing of light, is probably the most studied GPCR (Manglik and Kobilka 2014; Palczewski et al. 2000). Rhodopsin is bound to 11-cis-retinal, which upon illumination by light isomerizes to all-trans-retinal and induces a conformational change in the neighboring rhodopsin domain. The net change in rhodopsin shape allows binding and activation of the G-protein transducin. In this light-activated mode, it has been established that a single rhodopsin can activate hundreds of transducin proteins resulting in highly efficient amplification of the signal.
2.4 Steroid Hormone Receptors (SHRs)
The activation of signal proteins as a result of translocation of a messenger molecule is an alternative mechanism of signal transduction. This behavior is clearly elucidated by steroid hormone receptors (SHRs) (Klinge 2018; Griekspoor et al. 2007). SHRs are a large family of nuclear receptors that play a key role in physiological functions such as organ development and metabolite homeostasis. Alterations in these functions are involved several diseases, for instance breast cancer.
The general structure of SHRs comprises many independent functional domains. The DNA-binding domain is the most conserved among all SHRs with characteristic zinc-finger motifs. The ligand-binding domain is usually formed by twelve α-helices.
All SHRs share a similar mechanism of activation (Fig. 1d). Upon translocation to the cytoplasm by passive diffusion, the hormone binds the ligand-binding domain followed by a rapid relocation of the SHR to the nucleus. Here, SHRs undergo homologous dimerization form active species that are able to bind specific DNA regions, called Hormone Responsive Elements (HREs), through the zinc-finger motifs. This binding event induces recruitment of other co-factors which regulate gene transcription. Location to the nucleus is ligand- and concentration-dependent. Gene transcription can be modulated by receptor antagonists, proteasomes, and other signaling pathways.
3 Artificial Signal Transduction
3.1 Reversible Control over Synthetic Ion Channels
Much like the natural examples, the activity of artificial voltage-gated ion channels is regulated by the transmembrane potential. An early example was reported by Kobuke and co-workers (Kobuke et al. 1995). The system comprises ion pairs of tetra(butylene-1,4-glycol) monobutyl ether phosphate monoesters and di(octadecyl)dimethylammonium cations. Planar bilayer conductance (PBC) studies of these ion pairs revealed a positive voltage dependence of the opening and closing times, with the ion channels in an open state at higher voltages. It was suggested that the amphiphilic molecules self-assemble with a tail-to-tail orientation to form half-channels in each membrane leaflet. Upon application of a voltage, the half-channels move laterally to form supramolecular constructs with an uneven charge density at the two sides of the bilayer, resulting in a net dipole moment. The mechanism was further clarified by inserting asymmetric derivatives in a planar bilayer (Goto et al. 2001). These species bear a combination of carboxylate and phosphate groups whose different extent of deprotonation at slightly basic pH allowed voltage-gated ion channels with rectifying properties to form. The concept of asymmetry was further investigated by Fyles and co-workers, who reported voltage-gated ion channels formed by bis-macrocyclic bolaamphiphiles (Fyles et al. 1998). PBC studies showed a current-voltage response similar to that shown by the archetypical peptaibol alamethicin, which has well-studied ion channel activity (Woolley and Wallace 1992). Matile and co-workers proposed an alternative design based on rigid rod-like p-octiphenyl scaffolds able to completely span the bilayer (Sakai et al. 2001, 2003). A series of pendant azacrown moieties provided a unimolecular ion-conductive pathway. PBC and fluorescence-based studies, performed using both planar bilayers and synthetic vesicles, confirmed that the voltage-gated ion channel activity is closely related to the alignment of the axial rods upon application of a transmembrane potential. The ion channel activity was similar to that shown by α-helical natural antibiotics (Kobayashi et al. 2000). Hou and co-workers proposed an alternative strategy, using voltage to reversibly control the insertion of channel molecules within lipid bilayers (Fig. 2a) (Si et al. 2014). Inspired by the arginine-rich S4 domain of natural voltage-gated K+ channel (see above), Hou designed a series of arginine-rich pillar[5]arene transmembrane species (1a–1d). Similarly to its natural counterpart, these pillar[5]arene species can be reversibly inserted/removed from the bilayer by applying voltages of, respectively, −100 and +100 mV. Recently, Roh, Kim and co-workers showed that shape-resistant organic cages can give weakly voltage-gated channels (Benke et al. 2017). These cages, based on porphyrin units, are assembled by dynamic covalent chemistry and showed selectivity for anions. Successfully incorporated into cellular membranes, these porphyrin cages mediated iodide flux across the cell membrane.
Artificial ion channels with reversible control. a Voltage-gated ion channels. Adapted with permission from reference (Si et al. 2014). Copyright 2014 John Wiley and Sons. b Ligand-gated ion channels. Adapted with permission from reference (Kiwada et al. 2006). Copyright 2006 American Chemical Society. c Light-gated ion channels. Adapted with permission from reference (Zhou et al. 2017). Copyright 2017 Royal Society of Chemistry. d Mechano-sensitive ion channel. Adapted with permission from reference (Muraoka et al. 2017). Copyright 2017 American Chemical Society
The ligand-mediated formation of artificial ion channels has been very popular in the last two decades and many examples are now available (Talukdar et al. 2005). Nonetheless, achieving reversible ion channel activity by addition/removal of these ligands has proved to be more challenging. Futaki and co-workers designed an artificial channel peptide with an alamethicin segment connected through a flexible tetraglycine to a leucine zipper portion derived from the cFos protein (Fig. 2b) (Kiwada et al. 2006). The alamethicin portion inserts within the membrane. The extramembrane leucine zipper bears a pair of lysine side-chains functionalized with iminodiacetic acid (Ida), which are able to complex Fe(III) ions. In the absence of Fe(III) ions, the extramembrane leucine zipper adopts a helical conformation that promotes self-association. This conformation is destabilized upon complexation to Fe(III) ions, which allows a channel to open and is revealed by PBC studies as an increase in the channel current level. The ion channel opening was reversed upon the addition of excess EDTA, which complexes the Fe(III) ions and regenerates the helical conformation of the extramembrane segment; an elegant example of changes in conformation producing switching of channel activity. An assembly/disassembly approach to cation-mediated artificial ion channel formation was developed by Webb and co-workers, who created pyridyl-cholate conjugates able to be dimerized within the membrane by Pd(II) ions (Wilson and Webb 2008; Wilson et al. 2011). The activity of these self-assembled multimeric ion channels could be reversed by addition of hexathia-18-crown-6, which as strong chelator for Pd(II) ions dissociated the membrane-spanning complexes. More recently, Peters et al. showed that metal ion-gated artificial ion channels could also have antibiotic activity. They demonstrated the switch-on of ion channel activity by Cu(II) complexation to chelating octameric α-aminoisobutyric (Aib) foldamers (Peters et al. 2020). PBC and vesicle studies showed that complexation increased ionophoric activity, with X-ray crystallographic analysis supporting the formation of multimeric channels by the Cu(II) complexes as well as a direct interaction between anions and the foldamer backbone. Activity in vesicles could be reversed upon EDTA addition, and the increased activity of the Cu(II) complexes in vesicles corresponded with increased antibiotic activity.
Ion channel activity can be controlled by organic ligands instead of metal ions. Recently, Kinbara and co-workers illustrated this principle using synthetic chiral receptors able to bind 2-phenethylamine (PA) (Muraoka et al. 2014). The channel monomer is an amphiphile that adopts a bent conformation when embedded in artificial vesicles due to stacking of its diphenylacetylene units. Conductance studies of the vesicle-embedded systems in the presence of PA showed ion channel activity, which could be reversed after addition of β-cyclodextrin, a good host for PA (Rekharsky et al. 1995). Alternatively, organic ligands can be used in a blocking/unblocking approach to switching activity. An elegant example of an organic ligand-gated artificial ion channel has been recently reported by Nitschke and co-workers (Haynes et al. 2017). The channel is formed through the self-assembly of pyridyl ligands (L) around Zn(II). X-ray crystallography showed Zn10L15 pentagonal prisms with bromide accommodated in the channel-like cavity. PBC measurements of Zn10L15 showed channel behavior, while HPTS assays revealed selective transport of halide anions (Cl− < Br− < I−) over larger anions. Crucially, channel activity could be switched off by the addition of dodecyl sulfate, which was proposed to block the entrances and exits of the membrane-embedded channels. Calixarenes can also give efficient artificial ion channels, as reported by Li, Chen and co-workers (Hu et al. 2019). In this case, ion channel activity could be switched off by addition of methylene blue, a common cavity binder for calixarenes.
Unlike in the natural world, several examples of synthetic light-switchable ion channels are known. Schreiber and co-workers reported an example of a light-gated artificial ion channel in 1991 (Stankovic et al. 1991). They functionalized gramicidin monomers with the linker 3,3’-azobis(benzeneacetic acid) to give photoactive dimers. Photoisomerization and conductance studies of the azo-linked dimer in artificial vesicles in the dark demonstrated that unimolecular ion channels did not form as the molecules are in the inactive trans configuration. Photoactivation to the cis form produces a relative change of position of the gramicidin subunits, inducing alignment of their interior pores and ion channel activity. The cis/trans isomerization of azobenzene moieties is a versatile switch, and has also been exploited in channels reported by Woolley, (Lien et al. 1996) Kobuke, (Kobuke and Ohgoshi 2000) and Gin (Jog and Gin 2008). Alternatively, Hou, Liu and co-workers exploited the light-induced E/Z isomerization of the C=N bonds of acylhydrazone units to realize light-gated reversible ion channels (Fig. 2c) (Zhou et al. 2017). Acylhydrazone substituted crown ether triads stack on top of each other (2E) to give a self-assembled supramolecular transmembrane channel with selectivity for NH4+ and K+ ions. However, this ion transport could be switched off by irradiation with 320 nm UV light (to give 2z), then switched back on by 365 nm UV light.
Studies on mechano-sensitive artificial channels are rare, but an example has been reported by Kinbara and co-workers (Fig. 2d) (Muraoka et al. 2017). These artificial channels, formed by oligomers composed of alternating oligo(ethylene glycol) chains and 3,3’-dimethyl-5,5’-bis(phenylethynyl)-2,2’-bipyridine units (3) in membranes, mimic the domain structure of multipass transmembrane proteins. When embedded in synthetic bilayers, changes in membrane tension cause these amphiphiles to change the extent of stacking interactions between “domains”, which modulates ion transportation.
3.2 Mimics of TKRs
Instead of ligand-induced changes in conformation allowing a chemical message to pass through the bilayer, TKRs use ligand-induced changes in position in the membrane to relay a message. The formation of a termolecular (or higher order) active complex involving two or more membrane-embedded components makes this a mechanistically distinct process. The membrane phase state or extent of phase separation in the membrane should play a key role in these intermembrane aggregation processes. Furthermore, unlike ion channels, TKR-mediated signal transduction occurs without physical transport of molecules or ions across the membrane.
Hunter, Williams and co-workers were the first to explore these issues, by creating a simple mimic of TKR-mediated signal transduction (Fig. 3a) (Barton et al. 2002). In the place of the membrane-spanning domains of TKRs, a transmembrane section was formed by linking two molecules of cholenic acid through a tail-to-tail dialkyne bridge. The two termini were decorated with cysteine residues to give 6 and provide access to thiol-disulfide exchange chemistry, with the termini of one portion of dimer 6 transformed into a chromogenic disulfide (i.e. 6 to 4). Both 6 and 4 were embedded in artificial vesicles, then the system activated by tri(3-sulfonatophenyl)phosphane under slightly acidic conditions, a charged reducing agent that does not cross the lipid membrane. This phosphane reduces any external disulfides to the corresponding thiols (i.e. 4 to 5) but leaves the internal disulfides intact. Now ‘activated’, the external thiols are oxidized to disulfides, such as 5•6 by the addition of potassium ferricyanide (i.e. the external messenger, step [O] in Fig. 3a). This oxidative sensing event induces dimerization of the membrane-spanning bis(steroids). The external dimerization pulls the reactive groups at the interior of the membrane into close proximity, accelerating the release of the secondary messenger (the chromophore pyridine-2-thiol, 7) as this thiol/disulfide displacement becomes a fast intramolecular reaction.
Schematic representations of bio-inspired signal transduction based on dimerization of membrane-spanning systems within artificial bilayers. a Release of a secondary messenger as result of membrane-spanning dimerization (Barton et al. 2002). b Cooperativity of transmembrane spanning receptors (Dijkstra et al. 2007). c Complexation of adrenaline (red) inducing release of a secondary messenger (Schrader et al. 2006). d FRET induced by formation of ternary complex with DET (red) (Bernitzki and Schrader 2009)
In a following study, the membrane-spanning bis(steroid) unit was re-designed to demonstrate that this dimerization process shows cooperativity (Fig. 3b) (Dijkstra et al. 2007). The bis(steroid) core was labelled at the termini with fluorescent dansyl groups, with copper(II) ions acting as messengers that quench the fluorescence upon binding with the dansyl group. Due the formation of higher stoichiometry complexes, (Doyle et al. 2003) binding to Cu(II) induces aggregation of the membrane-spanning bis(steroid) molecules (Fig. 3b, left). Comparison with a control monomer that does not span the membrane revealed that affinity for Cu(II) was higher for the membrane-spanning dimers, as shown by stronger fluorescence quenching. This increase in affinity (i.e. high Kint, Fig. 3b, left ) was attributed to transmembrane cooperativity producing tighter binding to Cu(II) in the vesicle lumen. Unlike the thiol/disulfide system developed previously, this artificial signal event involves species at equilibrium; a messenger on one side of the membrane leads to cooperative binding on the opposite side of the membrane.
In the quest to develop systems able to recognize natural primary messengers, Schrader and co-workers modified bis(lithocholate) membrane-spanning molecules to introduce an alternative non-covalent approach for signal transduction (Schrader et al. 2006). They decorated the terminus facing the exterior of the bilayer with m-xylylene bisphosphonate dianion and aminomethylphenylboronic acid moieties (Fig. 3c); these two fragments were designed to form a ternary complex with adrenaline. In this way, an excess of adrenaline (the primary messenger) facilitated the dimerization of vesicle-embedded bis(steroid) lipids by forming a supramolecular complex (8•9). This external dimerization induced intermolecular cyclization at the vesicle interior as described above, with release of pyridine-2-thiol 7 as secondary messenger. However, the release of the secondary messenger could not be unambiguously proven because the detection system was not sufficiently specific and sensitive. This issue led to a re-design of the membrane-spanning systems to demonstrate that their non-covalent approach was indeed capable of signal transduction (Bernitzki and Schrader 2009). They developed two different unsymmetrical transmembrane units (10 and 11, Fig. 3d) in order to have the recognition system for the primary messenger at the vesicle exterior and a FRET couple at the interior. In this way, the formation of the ternary complex of the primary messenger facilitated the dimerization of the membrane-spanning systems, bringing the fluorescence dyes into sufficient proximity to observe FRET. Further studies demonstrated that the binding of the primary messenger, the resulting signal transmission, and subsequent release of the secondary messenger are strictly unidirectional. Importantly the effect of membrane fluidity on this intramembrane reaction was explored, with fluid DMPC bilayers providing a background reaction that is too high but gel-phase DPPC bilayers slowing the signaling reaction too much; a 3:1 mixture of the two was the optimized mixture (Bernitzki et al. 2012).
3.3 Mimics of GPCRs
Unlike mimics of RTKs, where bringing two transmembrane units together may be very sensitive to membrane fluidity and phase separation, mimics of GPCRs should combine binding of an external ligand with conformational change in a single embedded transmembrane molecule. To this end, Clayden, Webb and co-workers have explored an artificial transmembrane signaling strategy that employs dynamic foldamers with two interconverting conformations (Le Bailly and Clayden 2016). These synthetic receptors are 310 helical α-aminoisobutyric acid (Aib) oligopeptides able to alter their screw sense distribution in response to specific chemical or physical stimuli. The stereochemical information inherent in 310 helix handedness is transduced over distances commensurate with the foldamer length (rise per residue 0.194 nm), where it can be translated into specific outputs. These hydrophobic foldamers are soluble in several organic solvents and the Aib oligopeptide also acts as a hydrophobic anchor that promotes receptor insertion into the phospholipid bilayer; indeed natural Aib-rich peptaibols work as membrane-disrupting fungal antibiotics (Toniolo and Benedetti 1991). Aib oligopeptides of ≥4 residues adopt stable hydrogen-bonded 310 helical conformations with no inherent screw sense preference. The two helix conformations (M and P enantiomers) have a low energy barrier of inversion (ΔG‡ = 46 kJ mol−1 at −8 °C) (Solà et al. 2011) and interconvert on a timescale of submilliseconds at ambient temperature. Aib is achiral, so both P and M helices are present in equal proportion. However, a chiral amino acid at a terminus (typically the N-terminus) induces a preference for either the left- or the right-handed screw sense (Pengo et al. 1998).
To this Aib foldamer core, C-terminal spectroscopic probes can be added to report on changes in the relative populations of M and P helices. 1H, 13C and 19F NMR spectroscopic probes have been extensively used to quantify this helical excess (h.e., the fractional excess of P or M helices), including probes that are inherently achiral (e.g. the methylene protons of glycine) or chiral (e.g. Aib enantioselectively 13C labeled at a methyl group); the latter can identify which helical conformation predominates (Solà et al. 2011; Clayden et al. 2009; Solà et al. 2010; Pike et al. 2013). More recently, fluorescent probes have been developed, which can be employed in complex environments and can provide additional information, e.g. allowing visualization in bilayers by fluorescence microscopy. However, unlike NMR probes, a fluorescent probe needs to be chiral to allow changes in the P to M helical ratio to be detected using standard fluorescence spectroscopy. One example of this is a conformationally sensitive bis(pyrene) probe (Fig. 4b, blue) that exploited the high sensitivity of excimer emission to interpyrene distance. A series of Aib foldamers were attached at their C-terminus to the acetamide of (S,S)-1,2-bis(pyren-1'-yl)ethylenediamine. X-ray crystal structures indicated that one helix screw sense gave widely separated pyrene groups while the other had these groups close in space. Fluorescence emission mirrored these observations, with an increase in excimer/monomer (E/M) fluorescence emission ratio correlating with an increase in the proportion of P helix. Transfer of these receptors to a bilayer environment showed that this correlation of E/M with h.e. was maintained, although the spectroscopic changes were weaker and an M helix gave the higher E/M ratio. The latter effect was the reverse of that observed in organic solvent and was ascribed to changes in the reporter conformation (Lister et al. 2018). It was also found that phospholipid chirality had an influence on global conformation, although it is unclear how strong this influence is.
Schematic representations of approaches to bio-inspired signal transduction based on conformational change of membrane-spanning systems. a Light induces a conformational change in an azo-benzene (orange) substituted Aib foldamer 12 that bears a 19F ss-NMR reporter (green) (De Poli et al. 2016). b Binding to chiral carboxylate 14 (red) induces conformational change in a Aib foldamer 13 that bears a metal complex (grey) and a bis(pyrene) fluorescence reporter (blue) (Lister et al. 2017)
Several types of stimuli for responsive Aib foldamers were investigated in solution prior to membrane studies (Le Bailly and Clayden 2016). An important example had a boron-containing N-terminus that bound chiral diols. Competitive reversible binding to diols was demonstrated in methanol, including of adenosine, which is a natural signaling molecule for purinergic receptors. Successive displacement of diols with low association constants by more strongly coordinating diols of opposite configuration allowing switching of the helix between left- and right-handedness (Brown et al. 2013). Despite these promising observations, the diol/boronic acid interaction was found to be much weaker at a bilayer interface, which prevented development of these receptors into mimics of ligand-activated GPCRs.
Not all GPCRs are ligand activated, however; rhodopsin undergoes a conformational change in response to light-induced photoisomerization (Manglik and Kobilka 2014; Palczewski et al. 2000). This was the inspiration for photo-responsive GPCR mimic 12 (Fig. 4a) consisting of an Aib foldamer core, with an azobenzene-2-carboxamide capped L-valine residue at its N-terminus and a 19F NMR probe at the C-terminus (De Poli et al. 2016). Validation studies in methanol showed that E/Z photo-isomerization of the azobenzene altered the global conformation of the helix: azobenzene in its Z configuration gave no measurable preference for a P or an M helix, while the E configuration induced a preference for a left-handed helix. For membrane studies, a 2,2’-difluoroAib probe was used as lipid signals obscure most 1H and 13C NMR probe signals and the membrane environment broadens 1H NMR signals. The receptors were embedded in the membranes of multilamellar vesicles prepared from 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC, hydrophobic region ca. 2.88 nm thick). DOPC was hoped to provide a very fluid matrix, which would permit fast conformational interchange on the NMR timescale. The NMR experiments confirmed that the foldamers localized in the bilayer and the chirality of the DOPC had no significant influence on the screw sense of these helical compounds. Furthermore, linear dichroism showed that an Aib8 core favors a perpendicular orientation relative to the bilayer surface (Lizio et al. 2021). The change in population distribution between M and P helices upon light irradiation was monitored by solid-state magic angle spinning 19F NMR spectroscopy (19F ss-NMR). Using foldamers made of four Aib units, they showed azobenzene photo-isomerization (illumination at 365 nm for 5 min) in the membrane altered the helical conformation, going from 65% M population for the E isomer to an almost equal population of P and M helices for the Z isomer. Illumination at 455 nm switched the azobenzene back to an E configuration and restored the initial screw sense preference; multiple photo-switching cycles could be carried out. A longer foldamer made of eight Aib units (three helical turns, with a length commensurate with the hydrophobic region thickness) showed similar behavior in membranes, switching from a screw-sense preference to no preference and back. Thus, the system communicates conformational information along its several nanometer length within a membrane environment, although there was clearly weaker conformational induction. Solid-state NMR was shown to be an important analytical tool in the field, but also to have a number of practical disadvantages: it requires high concentrations of vesicles, conditions that can perturb the vesicle structure (spinning rate of 10 kHz) and long acquisition times that prevent real-time dynamic detection of conformational change.
The (S,S-BisPyrEt)NHAc reporter (Fig. 4b, blue) can overcome many of these signal reporting issues. An Aib8 oligopeptide with this fluorescent reporter at its C-terminus was equipped at its N-terminus with a water compatible, Cu(II)-containing binding pocket (Lister et al. 2017). This metallofoldamer was studied first in acetonitrile and then in the membranes of large unilamellar DOPC vesicles. In organic solvent, the addition of chiral carboxylate (either Boc-L-Pro or Boc-D-Pro 14) to the metallofoldamer induced circular dichroism signals of opposite sign at 239 nm, as binding to the messengers led to opposite propeller conformations around the binding pocket. Crucially, non-covalent binding of these messengers also led to a change in E/M emission ratio from the distant C-terminal reporter. These studies in organic solvent confirmed that local conformational preferences are relayed to the remote fluorescent probe through a global change in helical handedness. Furthermore, analysis of the binding data obtained showed that this reversible non-covalent binding was tight in acetonitrile, with estimated affinities of 106 M−1; much stronger than the diol/boronate interaction studied previously.
The artificial receptors were added to a suspension of DOPC vesicles and the migration into the phospholipid bilayers was monitored by fluorescence microscopy. Analysis of the spectral emission suggested that the C-terminal pyrenes were in a polar environment, perhaps close to the interface with water at the inner side of the membrane. The receptor-embedded vesicles were titrated with L-Pro or D-Pro carboxylates, and circular dichroism showed that the propeller induction at the binding site occurred with the same sense as in solution. Binding to the messenger molecules also induced a relay of conformational change along the receptor deep into the bilayer, transducing the stereochemical information in the messengers to the pyrene fluorophores positioned ~2.6 nm away. As found for the covalently controlled analogues (Lister et al. 2018), the effect of the ligands on the reporter emission spectra was opposite to that observed in acetonitrile, with D-Pro now increasing the excimer emission. Competitive binding between an ‘agonist’ and an ‘antagonist’ was also demonstrated: the addition of the messenger molecule Leu-enkephalin (a natural agonist for the μ- and δ-opioid receptors) resulted in an increase in E/M ratio, which could be inverted by competitive binding of L-BocPro. The work shows both the binding and conformation relay of GPCRs can be mimicked with synthetic molecules (albeit with weaker ligand affinities and a weaker relay), although further essential functions need to be added, such as signal amplification.
4 Translocation Approaches
Hunter, Williams and co-workers created a new type of transmembrane signal transduction, with transmission of information across vesicle bilayers without transfer of physical matter into the aqueous lumen, which although related to steroid translocation is largely orthogonal to known biological signaling processes. Importantly, this system is able to catalytically generate secondary messengers, a signal cascade that has analogy with biological transduction mechanisms. This work also illustrates how a purely synthetic approach can give access to signal transduction pathways beyond those found in biology.
Their strategy features the controlled translocation across a membrane of a synthetic transducer that is embedded within a bilayer but too short to span it (Fig. 5a) (Langton et al. 2017a). The transducer is equipped with two different functional groups. One is a sensor head that reads extracellular signals, and the second functional group is a pro-catalyst, either in its inactive form when buried within the bilayer or in its active form when protruding at the inner surface of the vesicle bilayer into the vesicle lumen. The motion of the transducer between the outer and the inner surface of the lipid bilayer is driven by extravesicular signals.
Schematic representations of approaches to bio-inspired signal transduction based on translocation of a catalytically active steroid. a A stimulus outside the vesicle controls the translocation of the steroid into the lumen, where it complexes to Zn(II) (black square) to make a catalytically active oximate that hydrolyses an internal ester (grey). R = Me for the generation of the HPTS fluorescent probe (green) only or R = 2-naphthyl for the co-generation of a surfactant that permeabilizes the bilayer (Langton et al. 2017a, b). b Translocation control exerted by protonation/deprotonation (Langton et al. 2017a). c Translocation control exerted by Cu(II) complexation/decomplexation (Langton et al. 2017c)
The transducer core is made of lithocholic acid; its planar structure is thought to favor a perpendicular orientation in the bilayer. The rigidity and length of the steroid core should allow only one of the functionalized termini to stick out into aqueous solution at any time. Vesicles were prepared from mixtures of the transducer with DOPC and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and encapsulated a pH 7 buffered aqueous solution containing zinc cations and a reactant. The choice of lipid is crucial, as this mixture should be fluid yet also maintain a transmembrane pH gradient. Although a statistical distribution of orientations within the lipid bilayer is obtained, only the transducers with sensor heads pointing outwards can respond to exterior chemical signals. The polarity of the sensor head switches by interacting with chemical signals from outside of the vesicle (Fig. 5b,c); the other end bears the non-polar pro-catalyst that becomes polar upon coordination to Zn(II) inside the vesicle. Control over molecular motion across the lipid bilayer exploits the preference of polar groups to sit in an aqueous phase and non-polar groups to be embedded in the bilayer core.
Initially N-alkylmorpholine (pKa 7.4) was chosen as a sensor head as changing the pH (between 7 and 9) outside the vesicles switched the polarity of the head group by protonation/deprotonation (Fig. 5b). For example, receipt of an external pH 9 signal decreases the polarity of the sensor heads through deprotonation; this switching from polar to non-polar gives transducers with two non-polar termini, which are free to diffuse across the bilayer. Now the pro-catalyst group (an oxime) can be activated by complexation to the zinc(II) cofactor in the aqueous lumen, to give a polar catalyst head that locates the transducer at the inner surface of the vesicle bilayer. This Zn(II) oximate in turn catalyzes the hydrolysis of a non-fluorescent ester inside the vesicle aqueous lumen to produce a fluorescent second messenger. Both the reactant and the hydrolysis product were chosen to be highly charged so they could not leak out of the vesicles, and the reaction inside the vesicles could be monitored by fluorescence microscopy. The position of the transducer is under equilibrium control, so loss of the chemical signal outside the vesicle reverses translocation of the transducer. A lower external pH switches off catalysis due to the affinity of the sensor head (amine) for the extra-vesicular signal (H+) being higher than that of the pro-catalyst for Zn(II). Multiple cycles of pH variation outside of the vesicles led to reversible switching of the hydrolysis reaction inside the vesicles, indicating that the controlled reversible translocation of molecular shuttles across a lipid bilayer is an effective signal transduction mechanism. The catalyzed hydrolysis inside the vesicles produced a large number of secondary messengers; such amplified output signal is reminiscent of amplification processes commonly exploited by biological signal transduction pathways, with binding of a single molecule generating a large number of output molecules. The system also cleverly exploits the high effective molarity of membrane-embedded reactive groups relative to the small volume of the vesicle lumen.
Subsequent studies used a different ester substrate, which was converted into a surfactant (2-naphthoic acid) upon hydrolysis (Langton et al. 2017b). A pH increase outside of the vesicle triggered the catalytic production of 2-naphthoic acid inside the vesicle by the Zn(II) oximate. The 2-naphthoic acid produced then permeabilized the vesicles without disrupting the membranes, which enabled the efflux of encapsulated calcein from vesicles and a fluorescence signal due to the relief of self-quenching. This cooperative chemical system demonstrated controlled release of cargo molecules as a response to a chemical signal.
The sensor head can also be varied, as exemplified by phenanthroline bearing transducers (Fig. 5c) (Langton et al. 2017c). For transducers of the correct orientation, the phenanthroline group coordinated to external Cu(II) ions to form a charged polar head at the vesicle outer surface. This Cu(II) messenger could be sequestered using EDTA, producing a polarity switch that released the transducer from the vesicle outer surface. The active catalyst then formed at the inner surface of the vesicle and once again catalyzed the hydrolysis of a non-fluorescent ester to form a fluorescent product. As in the previous example, transducers of the wrong orientation were inactive. The system also relies on the high affinity of phenanthroline for Cu(II) ions to prevent poisoning of the catalytic terminus by complexation to Cu(II) in the place of Zn(II). The intra-vesicular catalysis was repeatedly turned ON by addition of EDTA and OFF by addition of CuCl2 to the external solution.
Beyond switching the polarity of sensor heads to control transducer translocation, Williams and Hunter further advanced their dynamic signal transduction system by introducing biological molecular recognition (Ding et al. 2019). In one population of vesicles, transducers with desthiobiotin as the sensor head were embedded in the membrane, with complexation to external NeutrAvidin fixing transducers with the right orientation to the vesicle outer surface. The addition of a second population of vesicles that presented high affinity biotin signals at their surface led to NeutrAvidin sequestration, releasing the transducers from the outer surface of the first set of vesicles and activating intra-vesicular catalytic signaling. This ingenious inter-vesicle signaling system is reminiscent of some biological cell-to-cell signaling processes.
5 Future Perspectives
The development of supramolecular systems able to mimic aspects of biological signal transduction is still in an early stage, but several important design principles are becoming apparent. Although studies in isotropic organic solvent are a useful guide, the complex anisotropic environment of the bilayer necessitates additional important considerations when designing membrane-active systems and interpreting the data they provide. Bilayer fluidity is lower than many organic solvents and can vary by three orders of magnitude between bilayer types, (Pintre and Webb 2013) altering the rates of conformational change, lateral (across membrane) diffusion and transverse (through membrane) diffusion. The preferred orientation and location of any synthetic components added to the bilayer should be determined, for example by covalent labeling (Lin and London 2014) although linear dichroism looks to be a useful spectroscopic alternative (Lizio et al. 2021). A specific consideration for ligand-gated systems is the effect of the bilayer on any binding interactions between aqueous components (the ligand) and components that are embedded in the membrane (the synthetic receptor). The membrane can provide a significant steric barrier as well as much lower polarity compared to bulk aqueous solution, but on the other hand intramembrane interactions can benefit from the high effective molarities of membrane-bound components (Doyle et al. 2003). An often overlooked factor when using bilayers composed of natural phospholipids is the chirality of these molecules (sn phospholipids have an (R) configuration), and this can influence the conformation of membrane-bound components.
Several parts of the field stand out as needing further development, including increasing the number of systems able to catalytically generate second messengers on the far side of the membrane and the introduction of information-processing molecular machines into the bilayer; the molecular shuttle of Chen et al. indicates a pathway towards the latter (Chen et al. 2018). Finally, a link between a transmembrane information relay and out-of-equilibrium chemical networks in the vesicle membrane (Scheming et al. 1995) or lumen would be an exciting step towards mimicking the behavior of biochemical networks in cells.
Should these hurdles be overcome, the rewards will be significant. Artificial signal transduction would facilitate communication between compartments in artificial tissues (Villar et al. 2013; Booth et al. 2017) or even allow orthogonal signaling pathways to be introduced into cells, “short-circuiting” their signaling networks. For this ambitious goal to be realized, very robust signaling systems will have to be developed that can operate in the very complex and crowded milieu of the cell membrane. Synthetic signal transduction will be a key component needed for bottom-up synthetic biology, the creation of artificial cells and devices starting from molecular components. For example, artificial vesicles capable of maintaining (or even generating) transmembrane concentration gradients, controlling molecular ingress/egress and exchanging information across the bilayer could give stimuli-responsive dynamic cell-like systems with emergent features.
Abbreviations
- Aib:
-
α-amino-iso-butyric acid
- ATP:
-
adenosine triphosphate
- CFTR:
-
cystic fibrosis transmembrane conductance regulator
- DET:
-
diethylenetriamine
- DMPC:
-
1,2-dimyristoyl-sn-glycero-3-phosphocholine
- DNA:
-
deoxyribonucleic acid
- DOPC:
-
1,2-dioleoyl-sn-glycero-3-phosphocholine
- DOPE:
-
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
- DPPC:
-
1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- E/M :
-
excimer/monomer
- EDTA:
-
ethylenediaminetetraacetic acid
- EGFR:
-
epidermal growth factor receptor
- FRET:
-
Förster resonance energy transfer
- GABA:
-
γ-aminobutyric acid
- GDP:
-
guanosine diphosphate
- GPCR:
-
G-protein coupled receptor
- GTP:
-
guanosine triphosphate
- h.e.:
-
helical excess
- HPTS:
-
8-hydroxypyrene-1,3,6-trisulfonic acid
- HRE:
-
hormone responsive element
- IR:
-
insulin receptor
- nAChR:
-
nicotinic acetylcholine receptor
- NMR:
-
nuclear magnetic resonance
- PA:
-
2-phenethylamine
- PBC:
-
planar bilayer conductance
- pLGIC:
-
pentameric ligand-gated ion channel
- RTK:
-
receptor tyrosine kinase
- SHR:
-
steroid hormone receptor
- ss-NMR:
-
solid state nuclear magnetic resonance
References
Barba-Bon A, Nilam M, Hennig A (2020) Supramolecular chemistry in the biomembrane. ChemBioChem 21:886–910. https://doi.org/10.1002/cbic.201900646
Barton P, Hunter CA, Potter TJ, Webb SJ, Williams NH (2002) Transmembrane signalling. Angew Chem Int Ed 41:3878–3881. https://doi.org/10.1002/1521-3773(20021018)41:20<3878::AID-ANIE3878>3.0.CO;2-F
Bekus R, Schrader T (2020) Artificial signal transduction. ChemistryOpen 9:667–682. https://doi.org/10.1002/open.201900367
Benke BP, Aich P, Kim Y, Kim KL, Rohman R, Hong S, Hwang I-C, Lee EH, Roh JH, Kim K (2017) Iodide-selective synthetic ion channels based on shape-persistent organic cages. J Am Chem Soc 139:7432–7435. https://doi.org/10.1021/jacs.7b02708
Bernitzki K, Schrader T (2009) Entirely artificial signal transduction with a primary messenger. Angew Chem Int Ed 48:8001–8005. https://doi.org/10.1002/anie.200902973
Bernitzki K, Maue M, Schrader T (2012) Artificial signal transduction with primary and secondary messengers. Chem Eur J 18:13412–13417. https://doi.org/10.1002/chem.201200623
Booth MJ, Restrepo Schild V, Box SJ, Bayley H (2017) Light-patterning of synthetic tissues with single droplet resolution. Sci Rep 7:9315. https://doi.org/10.1038/s41598-017-09394-9
Brown RA, Diemer V, Webb SJ, Clayden J (2013) End-to-end conformational communication through a synthetic purinergic receptor by ligand-induced helicity switching. Nat Chem 5:853–860. https://doi.org/10.1038/nchem.1747
Chen S, Wang Y, Nie T, Bao C, Wang C, Xu T, Lin Q, Qu D-H, Gong X, Yang Y, Zhu L, Tian H (2018) An artificial molecular shuttle operates in lipid bilayers for ion transport. J Am Chem Soc 140:17992–17998. https://doi.org/10.1021/jacs.8b09580
Clayden J, Castellanos A, Solà J, Morris GA (2009) Quantifying end-to-end conformational communication of chirality through an achiral peptide chain. Angew Chem Int Ed 48:5962–5965. https://doi.org/10.1002/anie.200901892
De Poli M, Zawodny W, Quinonero O, Lorch M, Webb SJ, Clayden J (2016) Conformational photoswitching of a synthetic peptide foldamer bound within a phospholipid bilayer. Science 352:575–580. https://doi.org/10.1126/science.aad8352
De Waard M, Liu H, Walker D, Scott VES, Gurnett CA, Campbell KP (1997) Direct binding of G-protein βγ complex to voltage-dependent calcium channel. Nature 385:446–450. https://doi.org/10.1038/385446a0
Dijkstra HP, Hutchinson JJ, Hunter CA, Qin H, Tomas S, Webb SJ, Williams NH (2007) Transmission of binding information across lipid bilayers. Chem Eur J 13:7215–7222. https://doi.org/10.1002/chem.200601723
Ding Y, Williams NH, Hunter CA (2019) A synthetic vesicle-to-vesicle communication system. J Am Chem Soc 141:17847–17853. https://doi.org/10.1021/jacs.9b09102
Doyle EL, Hunter CA, Phillips HC, Webb SJ, Williams NH (2003) Cooperative binding at lipid bilayer membrane surfaces. J Am Chem Soc 125:4593–4599. https://doi.org/10.1021/ja021048a
Fyles TM, Loock D, Zhou X (1998) A voltage-gated ion channel based on a bis-macrocyclic bolaamphiphile. J Am Chem Soc 120:2997–3003. https://doi.org/10.1021/ja972648q
Gadsby DC, Vergani P, Csanády L (2006) The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature 440:477–483. https://doi.org/10.1038/nature04712
Goto C, Yamamura M, Satake A, Kobuke Y (2001) Artificial ion channels showing rectified current behavior. J Am Chem Soc 123:12152–12159. https://doi.org/10.1021/ja010761h
Griekspoor A, Zwart W, Neefjes J, Michalides R (2007) Visualizing the action of steroid hormone receptors in living cells. Nucl Recept Signal 5: https://doi.org/10.1621/nrs.05003
Gulbis JM, Mann S, Mackinnon R (1999) Voltage-dependent K+ channel β subunit. Cell 97:943–952. https://doi.org/10.1016/S0092-8674(00)80805-3
Haswell ES, Phillips R, Rees DC (2011) Mechanosensitive channels: what can they do and how do they do it? Structure 19:1356–1369. https://doi.org/10.1016/j.str.2011.09.005
Haynes CJE, Zhu J, Chimerel C, Hernández-Ainsa S, Riddell IA, Ronson TK, Keyser UF, Nitschke JR (2017) Blockable Zn10L15 ion channels through subcomponent self-assembly. Angew Chem Int Ed 56:15388–15392. https://doi.org/10.1002/anie.201709544
Herrington J, Arey BJ (2014) Conformational mechanisms of signaling bias of ion channels. In: Arey B (ed) Biased signaling in physiology, pharmacology and therapeutics. Elsevier, pp 173–207. https://doi.org/10.1016/B978-0-12-411460-9.00006-9
Hu X, Liu N, Yang H, Wu F, Chen X, Li C, Chen X (2019) A reversible ion transportation switch of ON-OFF-ON type by a ligand-gated calix[6]arene channel. Chem Commun 55:3008–3011. https://doi.org/10.1039/c9cc00732f
Hubbard SR (2004) Juxtamembrane autoinhibition in receptor tyrosine kinases. Nat Rev Mol Cell Biol 5:464–470. https://doi.org/10.1038/nrm1399
Jog PV, Gin MS (2008) A light-gated synthetic ion channel. Org Lett 10:3693–3696. https://doi.org/10.1021/ol8013045
Kiwada T, Sonomura K, Sugiura Y, Asami K, Futaki S (2006) Transmission of extramembrane conformational change into current: construction of metal-gated ion channel. J Am Chem Soc 128:6010–6011. https://doi.org/10.1021/ja060515b
Klinge CM (2018) Steroid hormone receptors and signal transduction processes. In: Belfiore A, LeRoith D. (eds) Principles of endocrinology and hormone action. Endocrinology. Springer, pp 187–232. https://doi.org/10.1007/978-3-319-44675-2_9
Kobayashi S, Takeshima K, Bae Park C, Chang Kim S, Matsuzaki K (2000) Interactions of the novel antimicrobial peptide Buforin 2 with lipid bilayers: proline as a translocation promoting factor. Biochemistry 39:8648–8654. https://doi.org/10.1021/bi0004549
Kobuke Y, Ohgoshi A (2000) Supramolecular ion channel containing trans-azobenzene for photocontrol of ionic fluxes. Colloids Surf A Physicochem Eng Asp 169:187–197. https://doi.org/10.1016/S0927-7757(00)00435-0
Kobuke Y, Ueda K, Sokabe M (1995) Totally synthetic voltage dependent ion channel. Chem Lett 24:435–436. https://doi.org/10.1246/cl.1995.435
Langton MJ, Keymeulen F, Ciaccia M, Williams NH, Hunter CA (2017a) Controlled membrane translocation provides a mechanism for signal transduction and amplification. Nat Chem 9:426–430. https://doi.org/10.1038/nchem.2678
Langton MJ, Scriven LM, Williams NH, Hunter CA (2017b) Triggered release from lipid bilayer vesicles by an artificial transmembrane signal transduction system. J Am Chem Soc 139:15768–15773. https://doi.org/10.1021/jacs.7b07747
Langton MJ, Williams NH, Hunter CA (2017c) Recognition-controlled membrane translocation for signal transduction across lipid bilayers. J Am Chem Soc 139:6461–6466. https://doi.org/10.1021/jacs.7b02345
Le Bailly BAF, Clayden J (2016) Dynamic foldamer chemistry. Chem Commun 52:4852–4863. https://doi.org/10.1039/C6CC00788K
Lien L, Jaikaran DCJ, Zhang Z, Woolley GA (1996) Photomodulated blocking of gramicidin ion channels. J Am Chem Soc 118:12222–12223. https://doi.org/10.1021/ja962217s
Lin Q, London E (2014) preparation of artificial plasma membrane mimicking vesicles with lipid asymmetry. PLoS ONE 9: https://doi.org/10.1371/journal.pone.0087903
Lister FGA, Le Bailly BAF, Webb SJ, Clayden J (2017) Ligand-modulated conformational switching in a fully synthetic membrane-bound receptor. Nat Chem 9:420–425. https://doi.org/10.1038/nchem.2736
Lister FGA, Eccles N, Pike SJ, Brown RA, Whitehead GFS, Raftery J, Webb SJ, Clayden J (2018) Bis-pyrene probes of foldamer conformation in solution and in phospholipid bilayers. Chem Sci 9:6860–6870. https://doi.org/10.1039/C8SC02532K
Lizio MG, Campana M, De Poli M, Jefferies DF, Cullen W, Andrushchenko V, Chmel NP, Bouř P, Khalid S, Clayden J, Blanch E, Rodger A, Webb SJ (2021, in press) Insight into mechanism of action and peptide-membrane interactions of Aib-rich peptides: multi-technique experimental and theoretical analysis. https://doi.org/10.1002/cbic.202000834
Luckey M (2008) Membrane structural biology with biochemical and biophysical foundations. Cambridge University Press, New York
Manglik A, Kobilka B (2014) The role of protein dynamics in GPCR function: insights from the β2AR and rhodopsin. Curr Opin Cell Biol 27:136–143. https://doi.org/10.1016/J.CEB.2014.01.008
Muraoka T, Endo T, Tabata KV, Noji H, Nagatoishi S, Tsumoto K, Li R, Kinbara K (2014) Reversible ion transportation switch by a ligand-gated synthetic supramolecular ion channel. J Am Chem Soc 136:15584–15595. https://doi.org/10.1021/ja5070312
Muraoka T, Umetsu K, Tabata KV, Hamada T, Noji H, Yamashita T, Kinbara K (2017) Mechano-sensitive synthetic ion channels. J Am Chem Soc 139:18016–18023. https://doi.org/10.1021/jacs.7b09515
Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E, Hegemann P (2002) Channelrhodopsin-1: a light-gated proton channel in green algae. Science 296:2395–2398. https://doi.org/10.1126/science.1072068
Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M (2000) Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289:739–745. https://doi.org/10.1126/science.289.5480.739
Pengo B, Formaggio F, Crisma M, Toniolo C, Bonora GM, Broxterman QB, Kamphius J, Saviano M, Iacovino R, Rossi F, Benedetti E (1998) Linear oligopeptides. Part 406.1 helical screw sense of peptide molecules: the pentapeptide system (Aib)4/L-Val[L-(αMe)Val] in solution. J Chem Soc, Perkin Trans 2:1651–1658. https://doi.org/10.1039/A800653I
Peters AD, Borsley S, della Sala F, Cairns-Gibson DF, Leonidou M, Clayden J, Whitehead GFS, Vitórica-Yrezábal IJ, Takano E, Burthem J, Cockroft SL, Webb SJ (2020) Switchable foldamer ion channels with antibacterial activity. Chem Sci. 11:7023-7030. https://doi.org/10.1039/D0SC02393K
Pike SJ, De Poli M, Zawodny W, Raftery J, Webb SJ, Clayden J (2013) Diastereotopic fluorine substituents as 19F NMR probes of screw-sense preference in helical foldamers. Org Biomol Chem 11:3168–3176. https://doi.org/10.1039/c3ob40463c
Pintre IC, Webb SJ (2013) Binding and reactivity at bilayer membranes. Adv Phys Org Chem 47:129–183. https://doi.org/10.1016/B978-0-12-407754-6.00003-X
Ptáček LJ (1997) Channelopathies: ion channel disorders of muscle as a paradigm for paroxysmal disorders of the nervous system. Neuromuscul Disord 7:250–255. https://doi.org/10.1016/S0960-8966(97)00046-1
Rekharsky MV, Goldberg RN, Schwarz FP, Tewari YB, Ross PD, Yamashoji Y, Inoue Y (1995) Thermodynamic and nuclear magnetic resonance study of the interactions of α- and β-cyclodextrin with model substances: phenethylamine, ephedrines, and related substances. J Am Chem Soc 117:8830–8840. https://doi.org/10.1021/ja00139a017
Saint-Criq V, Gray MA (2017) Role of CFTR in epithelial physiology. Cell Mol Life Sci 74:93–115. https://doi.org/10.1007/s00018-016-2391-y
Sakai N, Gerard D, Matile S (2001) Electrostatics of cell membrane recognition: structure and activity of neutral and cationic rigid push-pull rods in isoelectric, anionic, and polarized lipid bilayer membranes. J Am Chem Soc 123:2517–2524. https://doi.org/10.1021/ja003141
Sakai N, Houdebert D, Matile S (2003) Voltage-dependent formation of anion channels by synthetic rigid-rod push-pull β-barrels. Chem Eur J 9:223–232. https://doi.org/10.1002/chem.200390016
Sands Z, Grottesi A, Sansom MSP (2005) Voltage-gated ion channels. Curr Biol 15:R44–R47. https://doi.org/10.1016/j.cub.2004.12.050
Scheming APHJ, Lutje Spelberg JH, Driessen MCPF, Hauser MJB, Feiters MC, Nolte RJM (1995) Enzyme mimic displaying oscillatory behavior. oscillating reduction of Manganese(III) Porphyrin in a membrane-bound Cytochrome P-450 model system. J Am Chem Soc 117:12655–12656. https://doi.org/10.1021/ja00155a600
Schlessinger J (2000) Cell signaling by receptor tyrosine kinases. Cell 103:211–225. https://doi.org/10.1016/j.cell.2010.06.011
Schrader T, Maue M, Ellermann M (2006) Entirely artificial signal transduction with adrenaline. J Recept Signal Transduct 26:473–485. https://doi.org/10.1080/10799890600950545
Si W, Li ZT, Hou JL (2014) Voltage-driven reversible insertion into and leaving from a lipid bilayer: tuning transmembrane transport of artificial channels. Angew Chem Int Ed 53:4578–4581. https://doi.org/10.1002/anie.201311249
Solà J, Fletcher SP, Castellanos A, Clayden J (2010) Nanometer-range communication of stereochemical information by reversible switching of molecular helicity. Angew Chem Int Ed 49:6836–6839. https://doi.org/10.1002/anie.201001130
Solà J, Morris GA, Clayden J (2011) Measuring screw-sense preference in a helical oligomer by comparison of 13C NMR signal separation at slow and fast exchange. J Am Chem Soc 133:3712–3715. https://doi.org/10.1021/ja1097034
Stankovic CJ, Heinemann SH, Schreiber SL (1991) Photo-modulated ion channels based on covalently linked gramicidins. Biochim Biophys Acta 1061:163–170. https://doi.org/10.1016/0005-2736(91)90281-C
Talukdar P, Bollot G, Mareda J, Sakai N, Matile S (2005) Ligand-gated synthetic ion channels. Chem Eur J 11:6525–6532. https://doi.org/10.1002/chem.200500516
Tesmer JJG, Sunahara RK, Gilman AG, Sprang SR (1997) Crystal structure of the catalytic domains of adenylyl cyclase in a complex with GSα.GTPγS. Science 278:1907–1916. https://doi.org/10.1126/science.278.5345.1907
Tompa P (2016) The principle of conformational signaling. Chem Soc Rev 45:4252–4284. https://doi.org/10.1039/C6CS00011H
Toniolo C, Benedetti E (1991) The polypeptide 310-helix. Trends Biochem Sci 16:350–353. https://doi.org/10.1016/0968-0004(91)90142-I
Unwin N (1989) The structure of ion channels in membranes of excitable cells. Neuron 3:665–676. https://doi.org/10.1016/0896-6273(89)90235-3
Vanuytsel S, Carniello J, Wallace MI (2019) Artificial signal transduction across membranes. ChemBioChem 20:2569–2580. https://doi.org/10.1002/cbic.201900254
Villar G, Graham AD, Bayley H (2013) A tissue-like printed material. Science 340:48–52. https://doi.org/10.1126/science.1229495
Webb SJ (2013) Supramolecular approaches to combining membrane transport with adhesion. Acc Chem Res 46:2878–2887. https://doi.org/10.1021/ar400032c
Wilson CP, Webb SJ (2008) Palladium(II)-gated ion channels. Chem Commun 34:4007–4009. https://doi.org/10.1039/b809087d
Wilson CP, Boglio C, Ma L, Cockroft SL, Webb SJ (2011) Palladium(II)-mediated assembly of biotinylated ion channels. Chem Eur J 17:3465–3473. https://doi.org/10.1002/chem.201002031
Woolley GA, Wallace BA (1992) Membrane biology topical review model ion channels: Gramicidin and Alamethicin. J Membr Biol 129:109–136. https://doi.org/10.1007/BF00219508
Zheng S-P, Huang L-B, Sun Z, Barboiu M (2021) Self‐assembled artificial ion‐channels toward natural selection of functions. Angew Chem Int Ed 60:566–597. https://doi.org/10.1002/anie.201915287
Zhou Y, Chen Y, Zhu P-P, Si W, Hou J-L, Liu Y (2017) Reversible photo-gated transmembrane channel assembled from an acylhydrazone-containing crown ether triad. Chem Commun 53:3681–3684. https://doi.org/10.1039/c7cc01123g
Zhu S, Stein RA, Yoshioka C, Lee C-H, Goehring A, Mchaourab HS, Gouaux E (2016) Mechanism of NMDA receptor inhibition and activation. Cell 165:704–714. https://doi.org/10.1016/j.cell.2016.03.028
Acknowledgements
FDS, DPT and SJW thank the EPSRC (grant EP/P027067/1) for financial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
della Sala, F., Tilly, D.P., Webb, S.J. (2021). Approaches Towards Synthetic Signal Transduction in Phospholipid Bilayers. In: J.M. Abadie, M., Pinteala, M., Rotaru, A. (eds) New Trends in Macromolecular and Supramolecular Chemistry for Biological Applications. Springer, Cham. https://doi.org/10.1007/978-3-030-57456-7_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-57456-7_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-57455-0
Online ISBN: 978-3-030-57456-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)