Abstract
In recent years, an increasing number of investigations has demonstrated the therapeutic potential of molecules targeting the endocannabinoid system. Cannabinoids of endogenous, phytogenic, and synthetic nature have been assessed in a wide variety of disease models ranging from neurological to metabolic disorders. Even though very few compounds of this type have already reached the market, numerous preclinical and clinical studies suggest that cannabinoids are suitable drugs for the clinical management of diverse pathologies.
In this chapter, we will provide an overview of the endocannabinoid system under certain physiopathological conditions, with a focus on neurological, oncologic, and metabolic disorders. Cannabinoids evaluated as potential therapeutic agents in experimental models with an emphasis in the most successful chemical entities and their perspectives towards the clinic will be discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Components from the plant Cannabis Sativa as well as synthetic derivatives developed by academic and industry researchers have been extensively studied as therapeutics in the past few decades. However, very few have successfully entered the clinical scenario, thus far. Numerous ongoing investigations are trying to decipher the potential of these chemical entities in the treatment of a wide variety of diseases.
A growing number of preclinical studies published in the last years highlight the therapeutic actions of these compounds in different experimental models. Therefore, medical efforts and patient hopes are quite high for the development of cannabinoids as pharmacological agents for metabolic, neurological, or oncologic diseases among others. Presumably, in the near future, this field will greatly benefit patients with otherwise difficult to treat disorders. It is noteworthy that in June 2018, the U.S. Food and Drug Administration approved the non-psychoactive phytocannabinoid cannabidiol (CBD, commercialized as Epidiolex®) for the treatment of seizures in children with Lennox–Gastaut and Dravet syndromes (Devinsky et al. 2018, 2019).
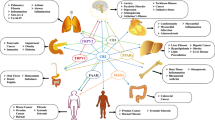
Cannabinoids are molecules that target the endocannabinoid system (ECS), which are involved in the regulation of numerous physiological and pathological processes. These compounds may bind or modulate one or various receptors that are part of ECS. Thus far, two G-protein-coupled receptors (GPCRs) have been identified as the two major cannabinoid receptors CB1 and CB2. CB1 is mostly found in the central nervous system, while CB2 is predominantly in the immune system among other organs and tissues (Matsuda et al. 1990; Herkenham et al. 1991; Demuth and Molleman 2006). Their endogenous ligands (endocannabinoids) and the enzymes implicated in their biosynthesis and degradation [(fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL)] are also part of this intricate system (Mechoulam et al. 1995, 1996; Beltramo et al. 1997; Fu et al. 2011; Marsicano and Chaouloff 2011). Whether additional cannabinoid receptors are part of the ECS still instigates a strong debate (Morales and Reggio 2017). Recent studies have shown that several cannabinoid ligands bind to the receptor GPR55 (Morales and Jagerovic 2016) and GPR18 (McHugh et al. 2010), supporting the idea that they may play an important role in ECS. Moreover, there is extensive evidence indicating that ECS also interacts with a number of established non-CB1, non-CB2 GPCRs, ion channels, and nuclear receptors (Pertwee et al. 2010; Morales et al. 2017; Morales and Reggio 2017).
4.1.1 Cannabinoid Classifications
Cannabinoid classifications have been established according to their pharmacology, their molecular structure, or their origin. Attending to the last criterion, cannabinergic compounds can be classified as endogenous (endocannabinoids), phytogenic (phytocannabinoids), and synthetic compounds.
4.2 Endocannabinoids
Endocannabinoids are endogenous lipidic molecules that bind to the cannabinoid receptors mediating retrograde neurotransmission (Wilson and Nicoll 2001). This family of compounds is formed by eicosanoids derived from arachidonic acid and other polyunsaturated fatty acids. Anandamide (AEA) and 2-arachidonoylglycerol (2-AG, Fig. 4.1) are the first endocannabinoids discovered and are most abundant in the human brain (Basavarajappa 2007). AEA partially activates both cannabinoid receptors CB1 and CB2, whereas 2-AG fully activates both of them. (Di Marzo et al. 1994; Stella et al. 1997). Other endocannabinoids identified include 2-arachidonoylglyceryl ether (noladin ether, 2-AGE), O-arachidonoyl ethanolamine (virodhamine), and N-arachidonoyl-dopamine (NADA) (Fig. 4.1).
The endocannabinoid tone is sustained by enzymes that synthesize and degrade these eicosanoids. Due to the physiopathological implication of this machinery, diverse drug discovery approaches have explored the modulation of the endocannabinoid tone. Strategies such as inhibition of degrading enzymes, positive allosteric modulation of CB1 and/or CB2, and development of endocannabinoid mimetics with a lower affinity towards metabolic enzymes have shown promising results in preclinical models (Pertwee 2005; Di Marzo 2018). Medicinal chemistry programs have developed synthetic analogs of endocannabinoids with structural modifications at key positions following the aforementioned strategies. Instances of this approach are ACEA (arachidonyl-2′-chloroethylamide) or ACPA (arachidonylcyclopropylamide, Fig. 4.1), analogs of AEA with improved CB1 affinity (Hillard et al. 1999). (R)-(+)-Methanandamide (Met-AEA, Fig. 4.1), a methylated AEA derivative, displays the same functional profile at the cannabinoid receptors while being longer-lived because it is more difficult for FAAH to metabolize.
4.3 Phytocannabinoids
To date, over 120 cannabinoids, termed “phytocannabinoids”, have been isolated from the Cannabis plant. These compounds bear a benzone-1,3-diol or a benzopyran ring and a hydrophobic alkyl chain. Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD, Fig. 4.1) are the most abundant cannabinoids in the plant and the most widely studied. Other phytocannabinoids include cannabinol (CBN), cannabigerol (CBG), and cannabichromene (CBC) (Fig. 4.1).
Phytocannabinoids exhibit different activities at the cannabinoid receptors CB1 and CB2 (Morales and Reggio 2017). Δ9-THC has been consistently shown to activate CB1 and CB2 with similar potency. Many of the therapeutic effects as well as the psychotropic outcomes of Cannabis Sativa are due to this phytocannabinoid. The non-psychoactive plant derivative CBD has also shown pharmacological potential in a wide range of pathologies (Mechoulam et al. 2007). Its functional profile at ECS is quite complex and is currently being investigated by diverse research groups (Morales and Reggio 2019) (Fig. 4.2).
Synthetic cannabinoid derivatives have been developed in the search for improved therapeutics and often trying to dissociate CB1 and CB2 activity. Structure-activity relationship studies of phytocannabinoid analogs have helped to understand the molecular requirements for cannabinoid activity. Derivatization at pharmacophoric positions including the alkyl lipophilic chain, the phenolic, and the pyran ring has resulted in compounds with a cannabinoid selective profile. Widely studied synthetic phytocannabinoid derivatives include CP55,940, HU210, JWH133, and HU308 (Fig. 4.3). CP55,940 and HU210 are very potent CB1/CB2 agonists, whereas the deoxy and the methoxy-Δ9-THC derivatives JWH133 and HU308 are CB2 agonists with significant selectivity over CB1 (Huffman 2000). The only structural modification of Δ9-THC that has led to an approved drug, thus far, is nabilone (Fig. 4.3).
4.4 Synthetic Cannabinoids
The therapeutic relevance of ECS has prompted the identification of numerous synthetic cannabinoid scaffolds. Strategies for the development of cannabimimetic compounds include the design of drugs that selectively activate or block CB1 or CB2, molecules that can act as allosteric modulators or biased agonists of these receptors, inhibitors of the metabolic enzymes FAAH or MAGL, as well as the development of compounds acting at peripheral cannabinoid receptors (Morales and Jagerovic 2020). These synthetic cannabinoids aim to provide optimized therapeutic effects and pharmacokinetical profile, while reducing undesirable side actions.
As we will describe in the following sections, numerous synthetic compounds have been used as pharmacological tools or therapeutic agents in different disease models.
The best-known compounds of this synthetic family involve aminoalkyindoles, such as R-(+)-WIN55,212–2 (D’Ambra et al. 1992) and JWH-015 (Fig. 4.4), CB1/CB2 and CB2 agonists, respectively; arylpyrazoles, such as SR141716A (rimonabant) (Rinaldi-Carmona et al. 1994) or AM251 (Fig. 4.4), CB1 antagonist/inverse agonists; or indole-2-carboxamides such as ORG27569 (Fig. 4.4), identified as the first CB1 allosteric modulator (Price et al. 2005).
In the following sections, we will describe the ECS upregulation in diverse pathologies to provide an overview of the chemical entities evaluated in experimental disease models. Their potential for further drug development or their progress towards the clinic will be also discussed.
4.5 Cannabinoids in Neuromodulation
ECS has a crucial role in mediating and modulating physiological responses in the central nervous system (CNS). ECS has been shown to be involved in synaptic plasticity and homeostatic processes in the brain. Therefore, it is not surprising that numerous reports have proved the dysregulation of cannabinoid receptor expression under specific neurological disorders providing a therapeutic scenario for the use of cannabinoids.
CB1 is one of the most abundant GPCRs in CNS, its expression is found particularly high in the basal ganglia, neocortex, hippocampus, and cerebellum CNS (Herkenham et al. 1991; Marsicano and Kuner 2008). The CB1 receptors are highly present at the presynaptic and axonal compartments, and thus their function is tightly associated with synaptic activity (Straiker and Mackie 2005). The activation of these receptors has been found to positively affect inwardly rectifying potassium channel conductance, while triggering a decrease in the N-type and P/Q-type voltage-operated calcium channel conductance and to reduce endocannabinoid production. This cascade of events leads to a decrease of neurotransmitter release at excitatory and inhibitory synapses conferring to CB1 the ability to modulate neurotransmission (Katona et al. 1999; Blázquez et al. 2011). Numerous investigations have demonstrated that the CB1 receptors exhibit neuroprotective effects against excitotoxicity induced by diverse stimuli (Marsicano et al. 2003). Therefore, multiple pathophysiological events, ranging from neurodegenerative disorders to memory deficits, have been associated with their actions (Kano et al. 2009; Di Marzo et al. 2015).
Moreover, the CB2 receptors, although initially thought to be peripherally restricted, have been found in particular brain regions offering a very promising therapeutic approach in certain neurological diseases. At a central level, the expression of these receptors is enhanced upon inflammation being mainly localized in the microglia (Fernández-Ruiz et al. 2015). Since neuroinflammatory alterations are associated with several neurological pathologies, the CB2 receptor agonists offer a promising therapeutic approach for the treatment of these disorders (Roche and Finn 2010; Navarro et al. 2016).
4.5.1 Cannabinoids in Epilepsy
Epilepsy is characterized by an imbalance between excitatory and inhibitory neurotransmitter release and abnormal neuronal electrical activity. Even though, antiepileptic drugs have been shown to limit seizures, over 30% of patients remain pharmacoresistant (Kwan et al. 2011). In this scenario, increasing research demonstrates that the exogenous modulation of ECS offers a promising and effective option for the treatment of refractory epilepsy (Rosenberg et al. 2015; Billakota et al. 2019). Although, the exact molecular mechanisms are still under investigation, the anticonvulsant potential of cannabinoids is supported by their neuromodulatory effects and their ability to inhibit hyperexcitability (Rosenberg et al. 2015).
Diverse phytocannabinoids, including Δ9-THC, Δ9-THCA (Δ9-tetrahydrocannabinolic acid, Fig. 4.5), Δ9-THCV (Δ9-tetrahydrocannabivarin, Fig. 4.5), CBD, and CBDV (cannabidivarin, Fig. 4.5), have shown anticonvulsant effects in different experimental models of seizures. Whereas, very few studies have been reported for the use of Δ9-THCA, Δ9-THCV, and CBDV, abundant data support the potential use of Δ9-THC and CBD for the treatment of epilepsy (Gaston and Friedman 2017).
Most studies have supported the anticonvulsant potential of Δ9-THC, however, some experiments have revealed mixed or no effects (Rosenberg et al. 2015). Among cannabinoids, the non-psychoactive phytocannabinoid, CBD is currently the best hope for the treatment of refractory epileptic seizures. Its potent anticonvulsant actions have been widely demonstrated in in vitro and in vivo human studies leading to CBD’s approval for the management of seizures in children with Lennox–Gastaut and Dravet syndromes (Devinsky et al. 2018, 2019). Placebo-controlled clinical trials revealed that CBD is well-tolerated and does not present side effects on CNS or vital signs (Bergamaschi et al. 2011; Friedman et al. 2019).
The proposed mechanisms of CBD anti-epileptogenic actions include the activation of TRPV1 channels (Bisogno et al. 2001), blockage of T-type voltage-gated calcium channels (VGCC) (Ibeas Bih et al. 2015), and modulation of GPCRs including the cannabinoid receptors CB1 and CB2 (Wallace et al. 2001, 2002), GPR55, the adenosine receptors A1 and A2 (Gaston and Friedman 2017), and the serotonin receptors 5-HT1A and 5-HT2A (Sourbron et al. 2016).
Synthetic cannabinoids have also been tested in preclinical seizures models (Rosenberg et al. 2015). FAAH inhibitors such as URB597 and AM404 (Fig. 4.6) did not exert significant anticonvulsant actions in animal models. Likewise, the CB1 antagonists, including SR141716A and AM251 (Fig. 4.4), were not successful in the assessed models. CB1 agonists, such as WIN55,212–2 (Fig. 4.4) and ACEA (Fig. 4.1), showed anti-seizure effects, although proconvulsive effects were reported in a low percentage of cases (Rosenberg et al. 2015). In fact, one study suggested that the CB1 agonists may exhibit proconvulsant effects at high doses via TRPV1 activation (Manna and Umathe 2012).
In summary, the activation of ECS exerts anti-epileptic effects whereas inhibition of the endogenous cannabinoid machinery does not prevent seizures in reported epilepsy models.
4.5.2 Cannabinoids in Alzheimer’s Disease
Alzheimer’s disease (AD) is a neurodegenerative disorder that is defined by the progressive deterioration of cognition and memory caused by the formation of β-amyloid plaques and neurofibrillary tangles. Alteration of ECS has been identified in animal models and human postmortem samples in the AD brain, especially in the hippocampus and cerebral cortex brain regions severely affected by this disease. AD patients experience a loss of the neuronal CB1 receptors (Ramírez et al. 2005), while significant upregulation of the CB2 receptors in microglial cells has been extensively reported (Benito et al. 2003; Aso and Ferrer 2016; López et al. 2018). Additionally, increased 2-AG and elevation of FAAH enzymes have also been associated with the progression of AD pathogenesis (Benito et al. 2003).
The enhanced 2-AG levels along with the increased CB2 receptors expression in microglial cells have been proposed to exert protective effects against β-amyloid-induced neuroinflammation and neuronal injury (Benito et al. 2003; López et al. 2018). However, the CB1 receptor downregulation in the hippocampus and basal ganglia may contribute to the destructive inflammatory process experienced by the AD patients (Ramírez et al. 2005). Increased FAAH activity in astrocytes has been associated with the formation of more arachidonic acid, which eventually leads to pro-inflammatory effects.
The exogenous modulation of ECS has shown promising results in preclinical AD models. On the one hand, CB1 activation has been reported to prevent amyloid β-induced neurotoxicity in vitro (Milton 2002; Benito et al. 2003; Ramírez et al. 2005) and to improve memory deficits and cognitive impairment in diverse animal models (Van Der Stelt et al. 2006; Haghani et al. 2012; Aso et al. 2012). Moreover, the activation of the CB2 receptors has been reported to attenuate the inflammation associated with AD modulating Aβ aberrant processing (Aso and Ferrer 2016). On the other hand, the inhibition of the endocannabinoid enzymes, FAAH and MAGL, has also been proposed as a potential therapeutic strategy for AD (Benito et al. 2012).
Among the cannabinoids tested in AD experimental models, the most promising results come from the phytocannabinoids Δ9-THC, CBD, or combinations of both (commercialized as Sativex®) (Fernández-Ruiz et al. 2015). These molecules, and the Δ9-THC synthetic derivative nabilone (Fig. 4.3), have been shown to counteract specific pathological hallmarks of AD, such as tau and β-amyloid aggregation, leading to cognitive and behavioral improvements. The few clinical trials performed so far confirmed the results observed in the animal models of the disease.Footnote 1 However, more controlled trials are needed to evaluate the efficacy of cannabinoids in the management of the different stages of this neurodegenerative disease.
Synthetic cannabinoids with diverse pharmacological profiles have also been tested in AD preclinical models. For instance, CB2 agonists, such as the naphthoylindole, JWH-015 (Fig. 4.4), or the phytocannabinoid derivatives, JWH-133 (Fig. 4.3), and HU-308 (Fig. 4.3), have been shown to reduce plaque aggregation, thereby exerting anti-inflammatory effects (Aso and Ferrer 2016). Likewise, CB1/CB2 mixed agonists including WIN55,212–2 (Fig. 4.4) and HU-210 (Fig. 4.3) have been demonstrated to have the ability to reduce pro-inflammatory markers and improve cognitive performance in the AD models (Ramírez et al. 2005; Martín-Moreno et al. 2011). Although, more studies need to confirm these effects, endocannabinoid reuptake inhibitors, such as VDM11 (Fig. 4.7) or MAGL inhibitors such as JLZ184 (Fig. 4.7), can decrease amyloid neurotoxicity (Van Der Stelt et al. 2006; Chen et al. 2012).
It has been extensively demonstrated that the pleiotropic activity of cannabinoids can target several crucial processes associated with AD. This includes neuroinflammation, β-amyloid and tau aberrant processing, excitotoxicity, or oxidative stress. In a multifactorial disease, such as AD, this offers a promising strategy. Hopefully, results from more clinical trials will shed additional light into this research such that AD patients worldwide can soon benefit from cannabinoid therapy.
4.5.3 Cannabinoids in Parkinson’s Disease
Parkinson’s disease (PD) is a long-term degenerative disorder that mainly affects motor coordination, although non-motor symptoms also appear with the progression of the disease. One of the main pathological hallmarks of PD is cell death in the basal ganglia, especially of dopaminergic neurons.
As in the previously mentioned neurological disorders, ECS has been shown to be abnormally regulated in this pathology. For instance, the upregulation of the CB1 receptors has been shown in the basal ganglia of experimental models of PD (Stampanoni Bassi et al. 2017). Moreover, a loss of the neuronal CB2 receptors was detected in the postmortem tissues of PD patients due to the degeneration of nigrostriatal dopaminergic neurons (García et al. 2015).
Pharmacological cannabinoid strategies to manage PD include activation of CB2, to control inflammatory events, and blockage of CB1 receptors, to improve akinesia and reduce motor inhibition. Since one of the main characteristics of PD is high oxidative stress, the experiments reported so far in the PD models have been focused on the use of antioxidant phytocannabinoids. The evaluation of Δ9-THC (Lastres-Becker et al. 2005), CBD (Lastres-Becker et al. 2005; García-Arencibia et al. 2007; García et al. 2011), and Δ9-THCV (García et al. 2011) in animal models revealed their ability to reduce parkinsonian motor symptoms. In fact, clinical trials to assess the potential of CBD, nabilone, or Cannabis oils in the PD motor and non-motor symptoms are currently ongoing.Footnote 2
Synthetic cannabinoids such as the potent CB1/CB2 receptor agonists WIN55,212–2 (Price et al. 2009; More and Choi 2015) and CP55,940 (Jimenez-Del-Rio et al. 2008) or the AEA synthetic derivative AM404 (García-Arencibia et al. 2007) have been shown to provide neuroprotection in the PD models.
Even though further clinical research is required, the knowledge gained in this field and ongoing clinical efforts point towards a cannabinoid-based neuroprotection for the treatment of PD.
As thoroughly reviewed by others, cannabinoids have been shown to impact many other neurological disease models, such as multiple sclerosis (MS), traumatic brain injury (TBI) or amyotrophic lateral sclerosis (ALS), as well as mental disorders including schizophrenia, anxiety, or depression (Kendall and Yudowski 2017; Aymerich et al. 2018; Ibarra-Lecue et al. 2018; Friedman et al. 2019). Moreover, symptoms associated with these diseases can also be treated with cannabinoid-based medicines, for instance, Sativex® is used for the symptomatic relief of pain and spasticity in adults suffering from MS (Giacoppo et al. 2017).
Even though much more research needs to be conducted, the modulation of ECS is a great therapeutic opportunity for the treatment of several neuropsychiatric and neurodegenerative disorders.
4.6 Cannabinoids in Cancer
The ability of Cannabis to prevent nausea and vomiting, stimulate appetite, and reduce pain has been widely demonstrated. Therefore, cannabinoids have been successfully used in the treatment of specific cancer chemotherapy side effects (Abrams and Guzman 2015).
A few decades ago, dronabinol (Marinol®) and nabilone (Cesamet®) were approved to treat emesis and nausea induced by antitumor agents (Tramèr et al. 2001). However, they are only prescribed in certain countries upon failure of conventional anti-emetics (Sharkey et al. 2014).
Extensive research has demonstrated the palliative potential of cannabinoids for cancer patients. For instance, Δ9-THC acts as an appetite stimulant increasing food intake in rodents. Clinical trials confirmed this orexigenic effect in the management of cancer anorexia (Jatoi et al. 2002; Berry and Mechoulam 2002; Walsh et al. 2003). Moreover, the ability of cannabinoids in reducing chemotherapy-induced pain has also been reported. Δ9-THC and synthetic analogs have shown to act as potent analgesic drugs in diverse clinical trials highlighting their beneficial role in the treatment of cancer pain (Campbell et al. 2001; Iversen and Chapman 2002; Mantyh et al. 2002). Actually, Sativex® can be currently prescribed in certain countries to reduce pain in adults with advanced tumors (Pertwee 2009; Fallon et al. 2017).
Preclinical data indicate that peripheral neuropathies associated with cancer treatment can also be ameliorated upon cannabinoid administration (Guindon et al. 2014). Synthetic agonists such as the aminoalkylindole WIN55,212–2, diminishes mechanical and cold allodynia in rodent models of paclitaxel (Pascual et al. 2005), vincristine (Rahn et al. 2007), and cisplatin-evoked neuropathy (Vera et al. 2007). Moreover, CBD is able to reduce doxorubicin-induced cardiomyopathies (Hao et al. 2015) and cisplatin-induced nephrotoxicity (Pan et al. 2009).
Besides their palliative potential, cannabinoids have exhibited antitumor effects in numerous in vitro and in vivo experimental models of cancer (Guzmán 2003; Chakravarti et al. 2014; Velasco et al. 2016). Since the early 2000s, a growing body of research has evidenced the potential of cannabinoids in the reduction of tumor growth and progression in diverse cancer models (Galve-Roperh et al. 2000; Guzmán et al. 2002; Guzmán 2003; Carracedo et al. 2006; Sarfaraz et al. 2008; Velasco et al. 2012).
ECS alterations have also been detected in cancer physiopathology. Abnormal expression of the ECS components in neoplasms compared with healthy tissues has been detected (Guzmán 2003; Caffarel et al. 2006; Malfitano et al. 2011; Velasco et al. 2012). These data can be tumor type-specific and therefore, studies need to determine how ECS is regulated in each cancer type (Malfitano et al. 2011; Velasco et al. 2016). In specific cancer types, such as glioblastoma (Schley et al. 2009) or specific breast tumors (Qamri et al. 2009; Caffarel et al. 2010), increased CB2 receptor levels have been shown. Other tumors, including gastric carcinoma (Miyato et al. 2009) or rhabdomyosarcoma (Oesch et al. 2009) are characterized by the overexpression of the CB1 receptor. Upregulated expression of both CB1 and CB2 has also been detected in acute myeloid leukemia (Joseph et al. 2004) malignant astrocytomas (Stella 2010), pancreatic cancer (Carracedo et al. 2006), and hepatocellular carcinoma (Giuliano et al. 2009) among others. Levels of endocannabinoids, AEA and 2-AG, have also been shown to differ between cancer cells and their normal counterparts in specific tumors (Bifulco et al. 2006). Upregulation of the putative cannabinoid receptor, GPR55, has also been observed in cells of diverse cancer types including breast adenocarcinoma, squamous skin cell carcinoma, or gliomas (Oka et al. 2010; Andradas et al. 2011; Leyva-Illades and Demorrow 2013; Pérez-Gómez et al. 2013). GPR55 expression has been shown to correlate with proliferation and thus, it has been proposed as a novel oncology biomarker with a potential prognostic value (Henstridge et al. 2011). Expression of GPR55-CB2 heterodimers has also been reported in human tumors (Moreno et al. 2014; Balenga et al. 2014).
Even if further research is required to clarify the intricate role of this complex system in the course of oncological processes, there is no doubt that cannabinoids are useful drugs for the management of cancer and related symptoms.
As in previously described diseases, thus far, preclinical and clinical studies on cannabinoids as antitumor agents have been mainly focused on understanding the mechanism of action of Δ9-THC and CBD (Pellati et al. 2018; Hinz and Ramer 2019). Δ9-THC has shown antiproliferative effects in diverse cancer types including glioblastoma, prostate, breast, colon, pancreatic, lymphoma, or lung among others (Fowler 2015; Fraguas-Sánchez et al. 2016). Mechanisms of this antitumor action include the CB receptor-dependent and independent pathways (Powles et al. 2005). Moreover, CBD has been widely proved to reduce tumor growth via proapoptotic actions in numerous cancer cell lines (Hinz and Ramer 2019). The anticancer effects of CBD have been suggested to be mediated by several targets, including COX-2, 5-LOX, PPARγ, TRPV2, mTOR, and the p38 MAPK pathway (Ligresti 2006; Hinz and Ramer 2019). Clinical trials are trying to unravel the antitumor potential of phytocannabinoids (such as Δ9-THC) alone or in combination with benchmark chemotherapeutic agents in different types of cancer. Guzmán et al. developed the first clinical trial to further explore the antitumor actions of cannabinoids in cancer patients. This pilot trial investigated the effects of Δ9-THC on nine patients with recurrent glioblastoma multiforme. The preliminary results attained from this study suggest a reduction in tumor growth upon Δ9-THC administration (Guzmán et al. 2006). Ongoing clinical trials are trying to decipher the potential antitumor role of cannabinoids.Footnote 3
Even if phytocannabinoids are in the forefront towards the clinic, many other cannabinoids with antitumor properties have been reported in the literature (Morales and Jagerovic 2019). For instance, the well-known aminoalkylindole WIN55,212–2 is able to decrease cell proliferation and migration in models of different cancer types, hepatocellular carcinoma (Xu et al. 2015), neuroblastoma (Müller et al. 2017), myeloma (Barbado et al. 2017), renal carcinoma (Khan et al. 2018), prostate (Morell et al. 2016), or gastric cancer (Xian et al. 2016) among them.
Moreover, it is worth highlighting the anticancer potential of cannabinoid quinones. Oxidized derivatives of phytocannabinoids cannabidiol (HU-331, Fig. 4.8), Δ8-THC (HU-336, Fig. 4.8) and cannabinol (HU-345, Fig. 4.8) were effective in reducing tumor growth in mice cancer models (Kogan et al. 2004). However, their biological activity was attributed to their quinone structure independently of their cannabinoid character, since they do not modulate the cannabinoid receptors (Kogan et al. 2006, 2007). Para- and ortho- quinones of chromenopyrazoles were also reported as antitumor agents (Morales et al. 2013, 2015). These compounds were able to reduce cancer proliferation through mechanisms that involve the cannabinoid receptors. For instance, para-quinones PM49 (Fig. 4.8) was able to reduce prostate cancer in vitro and in vivo (Morales et al. 2013). 1,4-naphthoquinone derivatives, such as 3a (Fig. 4.8), have also been reported to inhibit tumor proliferation. GPR55 has been proposed as the target through which they exhibit their antitumor effects (Badolato et al. 2019).
Currently, the use of cannabinoids is limited to the management of chemotherapy-induced side effects. Nevertheless, the aforementioned preclinical data clearly evidence the antitumor potential of cannabinoids. Hopefully, further clinical data can soon confirm the therapeutic potential of cannabinoids in the treatment of cancer.
4.7 Cannabinoids in Metabolic Disorders
ECS has been recognized to play a crucial role in the regulation of metabolic events, particularly in energy balance, food intake, and lipid metabolism (Scherma et al. 2014; Williams et al. 2015). This system has shown dysregulation in metabolic pathologies including obesity. For instance, the increased levels of circulating endocannabinoids (Blüher et al. 2006; Matias et al. 2006) and upregulation of the CB1 receptors have been observed in obese rodents and human obesity (Murdolo et al. 2007; Pagano et al. 2007). In this disorder, ECS dysregulation has been reported, not only in CNS but also at the peripheral level, in diverse organs including the pancreas, liver, and adipose tissues.
It is well-known that ECS activation induces orexigenic effects (Rossi et al. 2018), therefore, the inhibition of the CB1 receptors has been considered as a potential strategy for the management of obesity and metabolic syndrome. In fact, the CB1 antagonist/inverse agonist rimonabant (SR141716A, Fig. 4.4, commercialized as Acomplia®), was approved in certain European countries in 2006 for the management of obesity (Després et al. 2006). The anti-obesity effects of this drug were accompanied by the undesired effects such as depression, anxiety, headache, and suicidal thoughts forcing its withdrawal from the clinic, a couple of years later. Numerous research projects from academia and the pharmaceutical industry were centered on the development of CB1 receptor antagonists, however, the psychiatric side effects of rimonabant led to a significant decrease in the continuation of this approach (Serrano et al. 2012; Silvestri and Di Marzo 2012; Sharma et al. 2015; Yadav and Murumkar 2018; Amato et al. 2019).
Other pharmacological strategies targeting ECS, but without severe psychiatric side effects, have been attempted. Peripherally restricted CB1 antagonists, such as URB447 and AM6545 (Fig. 4.9), have shown promising results in the control of fat intake and obesity (DiPatrizio et al. 2011; Argueta and DiPatrizio 2017).
Moreover, molecules acting preferentially via the CB2 receptors have shown efficacy in a rat model of alcoholic hepatic steatosis by decreasing the liver/body weight ratio and hepatic triglyceride content (Lotersztajn et al. 2008, 2011). The inhibitors of the enzymes involved in the degradation of endocannabinoids, such as FAAH inhibitors, has also shown potential for the regulation of energy balance (Balsevich et al. 2018). However, this approach should be taken with caution, since the FAAH inhibitor BIA 10–2474 (Fig. 4.9) caused severe neurotoxicity in a phase I clinical trial probably due to off-target effects (Van Esbroeck et al. 2017).
Despite the clinical failures obtained so far, ECS still represents a very promising pharmacological target to treat metabolic disorders.
4.8 Conclusions
It has been widely demonstrated that compounds targeting ECS, particularly CB1 and/or CB2, have therapeutic potential for the clinical management of numerous diseases. These include neurological disorders, metabolic pathologies, cancer, or symptoms such as inflammatory and neuropathic pain. However, just a few of these diseases can be treated with cannabinoid-based medicines currently (Table 4.1).
Even though CB1/CB2 agonists are in the forefront of clinical research for neuroprotection or cancer treatment, there is an increasing interest in exploiting novel pharmacological approaches (Picone and Kendall 2015). CB2 selective agonists or peripherally restricted CB1/CB2 agonists exhibit a promising therapeutic potential for treating various pathologies, while avoiding the adverse psychotropic effects related to the modulation of CB1 in the brain (Dhopeshwarkar and Mackie 2014). CB1 and/or CB2 antagonists or inverse agonists, as well as, allosteric cannabinoid ligands are also emerging and may prove useful in the treatment of certain diseases (Picone and Kendall 2015; Vemuri and Makriyannis 2015). Biased cannabinoid agonists can also fine-tune the therapeutic effects, while minimizing side effects associated with other receptor pathways (Morales et al. 2018; Al-zoubi et al. 2019). Even though phytocannabinoids are way closer to the bedside, some of the aforementioned synthetic cannabinoids may provide advantages in the treatment of specific pathologies. Nonetheless, more preclinical and especially clinical research needs to be done in this field.
Notes
- 1.
Clinical trials: THC in Alzheimer Disease - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=Alzheimer+Disease&term=THC&cntry=&state=&city=&dist=. Accessed 7 Oct 2019.
- 2.
Clinical trials: cannabinoids in Parkinson Disease-ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=Parkinson+Disease&term=cannabis&cntry=&state=&city=&dist=. Accessed 3 Oct 2019.
- 3.
Clinical trials: cannabinoids in Cancer-ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=Cancer&term=cannabinoid&cntry=&state=&city=&dist= Accessed 29 June 2020.
References
Abrams DI, Guzman M (2015) Cannabis in cancer care. Clin Pharmacol Ther 97:575–586. https://doi.org/10.1002/cpt.108
Al-zoubi R, Morales P, Reggio PH (2019) Structural insights into CB1 receptor biased signaling. Int J Mol Sci 20:1837. https://doi.org/10.3390/ijms20081837
Amato G, Khan NS, Maitra R (2019) A patent update on cannabinoid receptor 1 antagonists (2015-2018). Expert Opin Ther Pat 29:261–269. https://doi.org/10.1080/13543776.2019.1597851
Andradas C, Caffarel MM, Pérez-Gómez E et al (2011) The orphan G protein-coupled receptor GPR55 promotes cancer cell proliferation via ERK. Oncogene 30:245–252. https://doi.org/10.1038/onc.2010.402
Argueta DA, DiPatrizio NV (2017) Peripheral endocannabinoid signaling controls hyperphagia in western diet-induced obesity. Physiol Behav 171:32–39. https://doi.org/10.1016/j.physbeh.2016.12.044
Aso E, Ferrer I (2016) CB2 cannabinoid receptor as potential target against Alzheimer’s disease. Front Neurosci 10:1–10. https://doi.org/10.3389/fnins.2016.00243
Aso E, Palomer E, Juvés S et al (2012) CB1 agonist ACEA protects neurons and reduces the cognitive impairment of AβPP/PS1 mice. J Alzheimers Dis 30:439–459. https://doi.org/10.3233/JAD-2012-111862
Aymerich MS, Aso E, Abellanas MA et al (2018) Cannabinoid pharmacology/therapeutics in chronic degenerative disorders affecting the central nervous system. Biochem Pharmacol 157:67–84. https://doi.org/10.1016/j.bcp.2018.08.016
Badolato M, Carullo G, Caroleo MC et al (2019) Discovery of 1,4-Naphthoquinones as a new class of Antiproliferative agents targeting GPR55. ACS Med Chem Lett acsmedchemlett 10(4):402–406. https://doi.org/10.1021/acsmedchemlett.8b00333
Balenga NA, Martínez-Pinilla E, Kargl J et al (2014) Heteromerization of GPR55 and cannabinoid CB2 receptors modulates signaling. Br J Pharmacol 171:5387–5406. https://doi.org/10.1111/bph.12850
Balsevich G, Sticht M, Bowles NP et al (2018) Role for fatty acid amide hydrolase (faah) in the leptin-mediated effects on feeding and energy balance. Proc Natl Acad Sci U S A 115:7605–7610. https://doi.org/10.1073/pnas.1802251115
Barbado MV, Medrano M, Caballero-Velázquez T et al (2017) Cannabinoid derivatives exert a potent anti-myeloma activity both in vitro and in vivo. Int J Cancer 140:674–685. https://doi.org/10.1002/ijc.30483
Basavarajappa BS (2007) Neuropharmacology of the endocannabinoid signaling system-molecular mechanisms, biological actions and synaptic plasticity. Curr Neuropharmacol 5:81–97. https://doi.org/10.2174/157015907780866910
Beltramo M, Stella N, Calignano A et al (1997) Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science 277:1094–1097. https://doi.org/10.1126/science.277.5329.1094
Benito C, Núñez E, Tolón RM et al (2003) Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in Neuritic plaque-associated glia in Alzheimer’s disease brains. J Neurosci 23:11136–11141. https://doi.org/10.1523/JNEUROSCI.23-35-11136.2003
Benito C, Tolõn RM, Castillo AI et al (2012) β-Amyloid exacerbates inflammation in astrocytes lacking fatty acid amide hydrolase through a mechanism involving PPAR-α, PPAR-γ and TRPV1, but not CB1 or CB2 receptors. Br J Pharmacol 166:1474–1489. https://doi.org/10.1111/j.1476-5381.2012.01889.x
Bergamaschi MM, Queiroz RHC, Zuardi AW, Crippa JAS (2011) Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf 6:237–249
Berry EM, Mechoulam R (2002) Tetrahydrocannabinol and endocannabinoids in feeding and appetite. Pharmacol Ther 95:185–190. https://doi.org/10.1016/S0163-7258(02)00257-7
Bifulco M, Laezza C, Pisanti S, Gazzerro P (2006) Cannabinoids and cancer: pros and cons of an antitumour strategy. Br J Pharmacol 148:123–135. https://doi.org/10.1038/sj.bjp.0706632
Billakota S, Devinsky O, Marsh E (2019) Cannabinoid therapy in epilepsy. Curr Opin Neurol 32:220–226. https://doi.org/10.1097/WCO.0000000000000660
Bisogno T, Hanuš L, De Petrocellis L et al (2001) Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 134:845–852. https://doi.org/10.1038/sj.bjp.0704327
Blázquez C, Chiarlone A, Sagredo O et al (2011) Loss of striatal type 1 cannabinoid receptors is a key pathogenic factor in Huntington’s disease. Brain 134:119–136. https://doi.org/10.1093/brain/awq278
Blüher M, Engeli S, Klöting N et al (2006) Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes 55:3053–3060. https://doi.org/10.2337/db06-0812
Caffarel MM, Andradas C, Mira E et al (2010) Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition. Mol Cancer 9:196–206. https://doi.org/10.1186/1476-4598-9-196
Caffarel MM, Sarrió D, Palacios J et al (2006) Delta9-tetrahydrocannabinol inhibits cell cycle progression in human breast cancer cells through Cdc2 regulation. Cancer Res 66:6615–6621. https://doi.org/10.1158/0008-5472.CAN-05-4566
Campbell FA, Tramèr MR, Carroll D et al (2001) Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ 323:13–16
Carracedo A, Gironella M, Lorente M et al (2006) Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress-related genes. Cancer Res 66:6748–6755. https://doi.org/10.1158/0008-5472.CAN-06-0169
Chakravarti B, Ravi J, Ganju RK (2014) Cannabinoids as therapeutic agents in cancer : current status and future implications. Oncotarget 5:5852–5872
Chen R, Zhang J, Wu Y et al (2012) Monoacylglycerol lipase is a therapeutic target for Alzheimer’s disease. Cell Rep 2:1329–1339. https://doi.org/10.1016/j.celrep.2012.09.030
D’Ambra TE, Estep KG, Bell MR et al (1992) Conformationally restrained analogs of pravadoline: nanomolar potent, enantioselective, (aminoalkyl)indole agonists of the cannabinoid receptor. J Med Chem 35:124–135. https://doi.org/10.1021/jm00079a016
Demuth DG, Molleman A (2006) Cannabinoid signalling. Life Sci 78:549–563. https://doi.org/10.1016/j.lfs.2005.05.055
Després J-P, Lemieux I, Alméras N (2006) Contribution of CB1 blockade to the management of high-risk abdominal obesity. Int J Obes (Lond) 30(Suppl 1):S44–S52. https://doi.org/10.1038/sj.ijo.0803278
Devinsky O, Nabbout R, Miller I et al (2019) Long-term cannabidiol treatment in patients with Dravet syndrome: an open-label extension trial. Epilepsia 60:294–302. https://doi.org/10.1111/epi.14628
Devinsky O, Patel AD, Cross JH et al (2018) Effect of Cannabidiol on drop seizures in the Lennox–Gastaut syndrome. N Engl J Med 378:1888–1897. https://doi.org/10.1056/NEJMoa1714631
Dhopeshwarkar A, Mackie K (2014) CB2 cannabinoid receptors as a therapeutic target - what does the future hold? Mol Pharmacol 86:430–437. https://doi.org/10.1124/mol.114.094649
Di Marzo V (2018) New approaches and challenges to targeting the endocannabinoid system. Nat Rev Drug Discov 17(9):623–639. https://doi.org/10.1038/nrd.2018.115
Di Marzo V, Fontana A, Cadas H et al (1994) Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 372:686–691. https://doi.org/10.1038/372686a0
Di Marzo V, Stella N, Zimmer A (2015) Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci 16:30–42. https://doi.org/10.1530/ERC-14-0411.Persistent
DiPatrizio NV, Astarita G, Schwartz G et al (2011) Endocannabinoid signal in the gut controls dietary fat intake. Proc Natl Acad Sci U S A 108:12904–12908. https://doi.org/10.1073/pnas.1104675108
Fallon MT, Lux EA, Mcquade R et al (2017) Sativex oromucosal spray as adjunctive therapy in advanced cancer patients with chronic pain unalleviated by optimized opioid therapy : two double-blind , randomized, placebo-controlled phase 3 studies. Br J Pain 11:119–133. https://doi.org/10.1177/2049463717710042
Fernández-Ruiz J, Romero J, Ramos JA (2015) Endocannabinoids and neurodegenerative disorders: Parkinson’s disease, Huntington’s chorea, Alzheimer’s disease, and others. In: Pertwee RG (ed) Endocannabinoids. Springer International Publishing, Cham, pp 233–259
Fowler CJ (2015) Delta9-tetrahydrocannabinol and cannabidiol as potential curative agents for cancer: a critical examination of the preclinical literature. Clin Pharmacol Ther 97:587–596. https://doi.org/10.1002/cpt.84
Fraguas-Sánchez AI, Fernández-Carballido A, Torres-Suárez AI (2016) Phyto-, endo- and synthetic cannabinoids: promising chemotherapeutic agents in the treatment of breast and prostate carcinomas. Expert Opin Investig Drugs 25:1311–1323. https://doi.org/10.1080/13543784.2016.1236913
Friedman D, French JA, Maccarrone M (2019) Safety, efficacy, and mechanisms of action of cannabinoids in neurological disorders. Lancet Neurol 18:504–512. https://doi.org/10.1016/S1474-4422(19)30032-8
Fu J, Bottegoni G, Sasso O et al (2011) A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat Neurosci 15:64–69. https://doi.org/10.1038/nn.2986
Galve-Roperh I, Sánchez C, Cortés ML et al (2000) Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat Med 6:313–319. https://doi.org/10.1038/73171
García MC, Cinquina V, Palomo-Garo C et al (2015) Identification of CB2 receptors in human nigral neurons that degenerate in Parkinson’s disease. Neurosci Lett 587:1–4. https://doi.org/10.1016/j.neulet.2014.12.003
García C, Palomo-Garo C, García-Arencibia M et al (2011) Symptom-relieving and neuroprotective effects of the phytocannabinoid Δ 9-THCV in animal models of Parkinson’s disease. Br J Pharmacol 163:1495–1506. https://doi.org/10.1111/j.1476-5381.2011.01278.x
García-Arencibia M, González S, de Lago E et al (2007) Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson’s disease: importance of antioxidant and cannabinoid receptor-independent properties. Brain Res 1134:162–170. https://doi.org/10.1016/j.brainres.2006.11.063
Gaston TE, Friedman D (2017) Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav 70:313–318. https://doi.org/10.1016/j.yebeh.2016.11.016
Giacoppo S, Bramanti P, Mazzon E (2017) Sativex in the management of multiple sclerosis-related spasticity: an overview of the last decade of clinical evaluation. Mult Scler Relat Disord 17:22–31. https://doi.org/10.1016/j.msard.2017.06.015
Giuliano M, Pellerito O, Portanova P et al (2009) Apoptosis induced in HepG2 cells by the synthetic cannabinoid WIN: involvement of the transcription factor PPARgamma. Biochimie 91:457–465. https://doi.org/10.1016/j.biochi.2008.11.003
Guindon J, Lai Y, Takacs SM et al (2014) Alterations in endocannabinoid tone following chemotherapy- induced peripheral neuropathy: effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following. Pharmacol Res 67:94–109. https://doi.org/10.1016/j.phrs.2012.10.013.Alterations
Guzmán M (2003) Cannabinoids: potential anticancer agents. Nat Rev Cancer 3:745–755. https://doi.org/10.1038/nrc1188
Guzmán M, Duarte MJ, Blázquez C et al (2006) A pilot clinical study of Delta9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br J Cancer 95:197–203. https://doi.org/10.1038/sj.bjc.6603236
Guzmán M, Sánchez C, Galve-Roperh I (2002) Cannabinoids and cell fate. Pharmacol Ther 95:175–184. https://doi.org/10.1016/S0163-7258(02)00256-5
Haghani M, Shabani M, Javan M et al (2012) CB1 cannabinoid receptor activation rescues amyloid ß-induced alterations in behaviour and intrinsic electrophysiological properties of rat hippocampal CA1 pyramidal Neurones. Cell Physiol Biochem 29:391–406. https://doi.org/10.1159/000338494
Hao E, Mukhopadhyay P, Cao Z et al (2015) Cannabidiol protects against doxorubicin-induced cardiomyopathy by modulating mitochondrial function and biogenesis. Mol Med 21:38–45. https://doi.org/10.2119/molmed.2014.00261
Henstridge CM, Balenga NAB, Kargl J et al (2011) Minireview: recent developments in the physiology and pathology of the lysophosphatidylinositol-sensitive receptor GPR55. Mol Endocrinol 25:1835–1848. https://doi.org/10.1210/me.2011-1197
Herkenham M, Lynn AB, Johnson MR et al (1991) Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 11:563–583
Hill A, Mercier M, Hill T et al (2012) Cannabidivarin is anticonvulsant in mouse and rat. Br J Pharmacol 167:1629–1642. https://doi.org/10.1111/j.1476-5381.2012.02207.x
Hill AJ, Weston SE, Jones NA et al (2010) Δ9-Tetrahydrocannabivarin suppresses in vitro epileptiform and in vivo seizure activity in adult rats. Epilepsia 51:1522–1532. https://doi.org/10.1111/j.1528-1167.2010.02523.x
Hillard CJ, Manna S, Greenberg MJ et al (1999) Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1). J Pharmacol Exp Ther 289:1427–1433
Hinz B, Ramer R (2019) Anti-tumour actions of cannabinoids. Br J Pharmacol 176:1384–1394. https://doi.org/10.1111/bph.14426
Huffman JW (2000) The search for selective ligands for the CB2 receptor. Curr Pharm Des 6:1323–1337
Ibarra-Lecue I, Pilar-Cuéllar F, Muguruza C et al (2018) The endocannabinoid system in mental disorders: evidence from human brain studies. Biochem Pharmacol 157:97–107. https://doi.org/10.1016/j.bcp.2018.07.009
Ibeas Bih C, Chen T, Nunn AVW et al (2015) Molecular targets of Cannabidiol in neurological disorders. Neurotherapeutics 12(4):699–730. https://doi.org/10.1007/s13311-015-0377-3
Iversen L, Chapman V (2002) Cannabinoids: a real prospect for pain relief? Curr Opin Pharmacol 2:50–55
Jatoi A, Windschitl HE, Loprinzi CL et al (2002) Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a north central cancer treatment group study. J Clin Oncol 20:567–573
Jimenez-Del-Rio M, Daza-Restrepo A, Velez-Pardo C (2008) The cannabinoid CP55,940 prolongs survival and improves locomotor activity in Drosophila melanogaster against paraquat: implications in Parkinson’s disease. Neurosci Res 61:404–411. https://doi.org/10.1016/j.neures.2008.04.011
Joseph J, Niggemann B, Zaenker KS, Entschladen F (2004) Anandamide is an endogenous inhibitor for the migration of tumor cells and T lymphocytes. Cancer Immunol Immunother 53:723–728. https://doi.org/10.1007/s00262-004-0509-9
Kano M, Ohno-Shosaku T, Hashimotodani Y et al (2009) Endocannabinoid-mediated control of synaptic transmission. Physiol Rev 89:309–380. https://doi.org/10.1152/physrev.00019.2008
Katona I, Sperlágh B, Sík A et al (1999) Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci 19:4544–4558
Kendall DA, Yudowski GA (2017) Cannabinoid receptors in the central nervous system: their signaling and roles in disease. Front Cell Neurosci 10:294. https://doi.org/10.3389/fncel.2016.00294
Khan MI, Soboci AA, Brodaczewska KK et al (2018) Involvement of the CB 2 cannabinoid receptor in cell growth inhibition and G0 / G1 cell cycle arrest via the cannabinoid agonist WIN 55, 212 – 2 in renal cell carcinoma. BMC Cancer 18:583
Kogan NM, Bl C, Alvarez L et al (2006) A cannabinoid Quinone inhibits angiogenesis by targeting vascular endothelial cells. Mol Pharmacol 70:51–59. https://doi.org/10.1124/mol.105.021089.cardiotoxic
Kogan NM, Rabinowitz R, Levi P et al (2004) Synthesis and antitumor activity of Quinonoid derivatives of cannabinoids. J Med Chem 47:3800–3806
Kogan NM, Schlesinger M, Peters M et al (2007) A cannabinoid anticancer quinone, HU-331, is more potent and less cardiotoxic than doxorubicin: a comparative in vivo study. J Pharmacol Exp Ther 322:646–653
Kwan P, Schachter SC, Brodie MJ (2011) Drug-Resistant Epilepsy. N Engl J Med 365:919–926. https://doi.org/10.1056/NEJMra1004418
Lastres-Becker I, Molina-Holgado F, Ramos JA et al (2005) Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson’s disease. Neurobiol Dis 19:96–107. https://doi.org/10.1016/j.nbd.2004.11.009
Leyva-Illades D, Demorrow S (2013) Orphan G protein receptor GPR55 as an emerging target in cancer therapy and management. Cancer Manag Res 5:147–155. https://doi.org/10.2147/CMAR.S35175
Ligresti A (2006) Antitumor activity of plant cannabinoids with emphasis on the effect of Cannabidiol on human breast carcinoma. J Pharmacol Exp Ther 318:1375–1387. https://doi.org/10.1124/jpet.106.105247
López A, Aparicio N, Pazos MR et al (2018) Cannabinoid CB2 receptors in the mouse brain: relevance for Alzheimer’s disease. J Neuroinflammation 15:158. https://doi.org/10.1186/s12974-018-1174-9
Lotersztajn S, Teixeira-Clerc F, Julien B et al (2008) CB2 receptors as new therapeutic targets for liver diseases. Br J Pharmacol 153:286–289. https://doi.org/10.1038/sj.bjp.0707511
Lotersztajn S, Teixeira-Clerc F, Mallat A, Louvet A (2011) Selective CB2 receptor agonists for use in the prevention or treatment of alcoholic liver disease
Malfitano AM, Ciaglia E, Gangemi G et al (2011) Update on the endocannabinoid system as an anticancer target. Expert Opin Ther Targets 15:297–308. https://doi.org/10.1517/14728222.2011.553606
Manna SSS, Umathe SN (2012) Involvement of transient receptor potential vanilloid type 1 channels in the pro-convulsant effect of anandamide in pentylenetetrazole-induced seizures. Epilepsy Res 100:113–124. https://doi.org/10.1016/j.eplepsyres.2012.02.003
Mantyh PW, Clohisy DR, Koltzenburg M, Hunt SP (2002) Molecular mechanisms of cancer pain. Nat Rev Cancer 2:201–209. https://doi.org/10.1038/nrc747
Marsicano G, Chaouloff F (2011) Moving bliss: a new anandamide transporter. Nat Neurosci 15:5–6. https://doi.org/10.1038/nn.3011
Marsicano G, Goodenough S, Monory K et al (2003) CB1 cannabinoid receptors and on-demand defense against Excitotoxicity. Science (80-) 302:84–88. https://doi.org/10.1126/science.1088208
Marsicano G, Kuner R (2008) Anatomical distribution of receptors, ligands and enzymes in the brain and in the spinal cord: circuitries and neurochemistry. In: Cannabinoids and the brain. Springer US, Boston, MA, pp 161–201
Martín-Moreno AM, Reigada D, Ramírez BG et al (2011) Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo : relevance to Alzheimer ’ s disease. Mol Pharmacol 79:964–973. https://doi.org/10.1124/mol.111.071290.Alzheimer
Matias I, Gonthier MP, Orlando P et al (2006) Regulation, function, and dysregulation of endocannabinoids in models of adipose and β-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab 91:3171–3180. https://doi.org/10.1210/jc.2005-2679
Matsuda LA, Lolait SJ, Brownstein MJ et al (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561–564. https://doi.org/10.1038/346561a0
McHugh D, Hu SSJ, Rimmerman N et al (2010) N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci 11:44–56. https://doi.org/10.1186/1471-2202-11-44
Mechoulam R, Ben Shabat S, Hanus L et al (1996) Endogenous cannabinoid ligands--chemical and biological studies. J Lipid Mediat Cell Signal 14:45–49
Mechoulam R, Ben-Shabat S, Hanus L et al (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50:83–90. https://doi.org/10.1016/0006-2952(95)00109-D
Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO (2007) Cannabidiol--recent advances. Chem Biodivers 4:1678–1692. https://doi.org/10.1002/cbdv.200790147
Milton NGN (2002) Anandamide and noladin ether prevent neurotoxicity of the human amyloid-β peptide. Neurosci Lett 332:127–130. https://doi.org/10.1016/S0304-3940(02)00936-9
Miyato H, Kitayama J, Yamashita H et al (2009) Pharmacological synergism between cannabinoids and paclitaxel in gastric cancer cell lines. J Surg Res 155:40–47. https://doi.org/10.1016/j.jss.2008.06.045
Morales P, Blasco-Benito S, Andradas C et al (2015) Selective, nontoxic CB2 cannabinoid o-quinone with in vivo activity against triple-negative breast cancer. J Med Chem 58:2256–2264. https://doi.org/10.1021/acs.jmedchem.5b00078
Morales P, Goya P, Jagerovic N (2018) Emerging strategies targeting CB2 cannabinoid receptor: biased agonism and allosterism. Biochem Pharmacol 157:8–17. https://doi.org/10.1016/j.bcp.2018.07.031
Morales P, Hurst DP, Reggio PH (2017) Molecular targets of the Phytocannabinoids: a complex picture. Prog Chem Org Nat Prod 103:103–131. https://doi.org/10.1007/978-3-319-45541-9_4
Morales P, Jagerovic N (2016) Advances towards the discovery of GPR55 ligands. Curr Med Chem 23:2087–2100
Morales P, Jagerovic N (2019) Antitumor cannabinoid Chemotypes: structural insights. Front Pharmacol 10:621. https://doi.org/10.3389/fphar.2019.00621
Morales P, Jagerovic N (2020) Novel approaches and current challenges with targeting the endocannabinoid system. Expert Opin Drug Discov 00:1–14. https://doi.org/10.1080/17460441.2020.1752178
Morales P, Reggio PH (2017) An update on non-CB1, non-CB2 cannabinoid related G-protein-coupled receptors. Cannabis Cannabinoid Res 2:265–273. https://doi.org/10.1089/can.2017.0036
Morales P, Reggio PH (2019) CBD: a new Hope? ACS Med Chem Lett 10:694–695. https://doi.org/10.1021/acsmedchemlett.9b00127
Morales P, Vara D, Goméz-Cañas M et al (2013) Synthetic cannabinoid quinones: preparation, in vitro antiproliferative effects and in vivo prostate antitumor activity. Eur J Med Chem 70:111–119
More SV, Choi D-K (2015) Promising cannabinoid-based therapies for Parkinson’s disease: motor symptoms to neuroprotection. Mol Neurodegener 10:17. https://doi.org/10.1186/s13024-015-0012-0
Morell C, Bort A, Vara D et al (2016) The cannabinoid WIN 55,212-2 prevents neuroendocrine differentiation of LNCaP prostate cancer cells. Prostate Cancer Prostatic Dis 19:248–257. https://doi.org/10.1038/pcan.2016.19
Moreno E, Andradas C, Medrano M et al (2014) Targeting CB2-GPR55 receptor Heteromers modulates cancer cell signaling. J Biol Chem 289:21960–21972. https://doi.org/10.1074/jbc.M114.561761
Müller L, Radtke A, Decker J et al (2017) The synthetic cannabinoid WIN 55,212-2 elicits death in human cancer cell lines. Anticancer Res 37:6341–6345
Murdolo G, Kempf K, Hammarstedt A et al (2007) Insulin differentially modulates the peripheral endocannabinoid system in human subcutaneous abdominal adipose tissue from lean and obese individuals. J Endocrinol Investig 30:RC17–RC21. https://doi.org/10.1007/BF03347440
Navarro G, Morales P, Rodríguez-Cueto C et al (2016) Targeting cannabinoid CB2 receptors in the central nervous system. Medicinal chemistry approaches with focus on neurodegenerative disorders. Front Neurosci 10:1–11. https://doi.org/10.3389/fnins.2016.00406
Oesch S, Walter D, Wachtel M et al (2009) Cannabinoid receptor 1 is a potential drug target for treatment of translocation-positive rhabdomyosarcoma. Mol Cancer Ther 8:1838–1845. https://doi.org/10.1158/1535-7163.MCT-08-1147
Oka S, Kimura S, Toshida T et al (2010) Lysophosphatidylinositol induces rapid phosphorylation of p38 mitogen-activated protein kinase and activating transcription factor 2 in HEK293 cells expressing GPR55 and IM-9 lymphoblastoid cells. J Biochem 147:671–678. https://doi.org/10.1093/jb/mvp208
Pagano C, Pilon C, Calcagno A et al (2007) The endogenous cannabinoid system stimulates glucose uptake in human fat cells via phosphatidylinositol 3-kinase and calcium-dependent mechanisms. J Clin Endocrinol Metab 92:4810–4819. https://doi.org/10.1210/jc.2007-0768
Pan H, Mukhopadhyay P, Rajesh M et al (2009) Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J Pharmacol Exp Ther 328:708–714. https://doi.org/10.1124/jpet.108.147181
Pascual D, Goicoechea C, Suardíaz M, Martín MI (2005) A cannabinoid agonist, WIN 55,212-2, reduces neuropathic nociception induced by paclitaxel in rats. Pain 118:23–34. https://doi.org/10.1016/j.pain.2005.07.008
Pellati F, Borgonetti V, Brighenti V et al (2018) Cannabis sativa L. and nonpsychoactive cannabinoids: their chemistry and role against oxidative stress, inflammation, and cancer. Biomed Res Int 2018:1–15. https://doi.org/10.1155/2018/1691428
Pérez-Gómez E, Andradas C, Flores JM et al (2013) The orphan receptor GPR55 drives skin carcinogenesis and is upregulated in human squamous cell carcinomas. Oncogene 32:2534–2542. https://doi.org/10.1038/onc.2012.278
Pertwee RG (2005) The therapeutic potential of drugs that target cannabinoid receptors or modulate the tissue levels or actions of endocannabinoids. AAPS J 7:E625–E654. https://doi.org/10.1208/aapsj070364
Pertwee RG (2009) Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol 156:397–411. https://doi.org/10.1111/j.1476-5381.2008.00048.x
Pertwee RG, Howlett AC, Abood ME et al (2010) International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev 62:588–631. https://doi.org/10.1124/pr.110.003004
Picone RP, Kendall D (2015) Minireview: from the bench, toward the clinic: therapeutic opportunities for cannabinoid receptor modulation. Mol Endocrinol 29:801–813. https://doi.org/10.1210/me.2015-1062
Powles T, Te Poele R, Shamash J et al (2005) Cannabis-induced cytotoxicity in leukemic cell lines: the role of the cannabinoid receptors and the MAPK pathway. Blood 105:1214–1221. https://doi.org/10.1182/blood-2004-03-1182
Price MR, Baillie GL, Thomas A et al (2005) Allosteric modulation of the cannabinoid CB1 receptor. Mol Pharmacol 68:1484–1495. https://doi.org/10.1124/mol.105.016162.view
Price DA, Martinez AA, Seillier A et al (2009) WIN55, 212-2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Eur J Neurosci 29:2177–2186. https://doi.org/10.1111/j.1460-9568.2009.06764.x
Qamri Z, Preet A, Nasser MW et al (2009) Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol Cancer Ther 8:3117–3129. https://doi.org/10.1158/1535-7163.MCT-09-0448
Rahn EJ, Makriyannis A, Hohmann AG (2007) Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. Br J Pharmacol 152:765–777. https://doi.org/10.1038/sj.bjp.0707333
Ramírez BG, Blázquez C, Gómez Del Pulgar T et al (2005) Prevention of Alzheimer’s disease pathology by cannabinoids: Neuroprotection mediated by blockade of microglial activation. J Neurosci 25:1904–1913. https://doi.org/10.1523/JNEUROSCI.4540-04.2005
Rinaldi-Carmona M, Barth F, Héaulme M et al (1994) SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 350:240–244
Roche M, Finn DP (2010) Brain CB2 receptors: implications for neuropsychiatric disorders. Pharmaceuticals 3:2517–2533. https://doi.org/10.3390/ph3082517
Rosenberg EC, Tsien RW, Whalley BJ, Devinsky O (2015) Cannabinoids and epilepsy. Neurotherapeutics 12(4):747–768. https://doi.org/10.1007/s13311-015-0375-5
Rossi F, Punzo F, Umano GR et al (2018) Role of cannabinoids in obesity. Int J Mol Sci 19:2690. https://doi.org/10.3390/ijms19092690
Sarfaraz S, Adhami VM, Syed DN et al (2008) Cannabinoids for cancer treatment: progress and promise. Cancer Res 68:339–342. https://doi.org/10.1158/0008-5472.CAN-07-2785
Scherma M, Fattore L, Paola Castelli M et al (2014) The role of the Endocannabinoid system in eating disorders: neurochemical and Behavioural preclinical evidence. Curr Pharm Des 20:2089–2099
Schley M, Ständer S, Kerner J et al (2009) Predominant CB2 receptor expression in endothelial cells of glioblastoma in humans. Brain Res Bull 79:333–337. https://doi.org/10.1016/j.brainresbull.2009.01.011
Serrano A, Pavon FJ, Suarez J et al (2012) Obesity and the Endocannabinoid system: is there still a future for CB1 antagonists in obesity? Curr Obes Rep 1:216–228. https://doi.org/10.1007/s13679-012-0031-x
Sharkey KA, Darmani NA, Parker LA (2014) Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur J Pharmacol 722:134–146. https://doi.org/10.1016/j.ejphar.2013.09.068
Sharma MK, Murumkar PR, Barmade MA et al (2015) A comprehensive patents review on cannabinoid 1 receptor antagonists as antiobesity agents. Expert Opin Ther Pat 25:1–24. https://doi.org/10.1517/13543776.2015.1064898
Silvestri C, Di Marzo V (2012) Second generation CB1 receptor blockers and other inhibitors of peripheral endocannabinoid overactivity and the rationale of their use against metabolic disorders. Expert Opin Investig Drugs 21:1309–1322. https://doi.org/10.1517/13543784.2012.704019
Sourbron J, Schneider H, Kecskés A et al (2016) Serotonergic modulation as effective treatment for Dravet syndrome in a Zebrafish mutant model. ACS Chem Neurosci 7:588–598. https://doi.org/10.1021/acschemneuro.5b00342
Stampanoni Bassi M, Sancesario A, Morace R et al (2017) Cannabinoids in Parkinson’s disease. Cannabis Cannabinoid Res 2:21–29. https://doi.org/10.1089/can.2017.0002
Stella N (2010) Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 58:1017–1030. https://doi.org/10.1002/glia.20983
Stella N, Schweitzer P, Piomelli D (1997) A second endogenous cannabinoid that modulates long-term potentiation. Nature 388:773–778. https://doi.org/10.1038/42015
Straiker A, Mackie K (2005) Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol 569:501–517. https://doi.org/10.1113/jphysiol.2005.091918
Tramèr MR, Carroll D, Campbell FA et al (2001) Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. Br Med J 323:16–21
Van Der Stelt M, Mazzola C, Esposito G et al (2006) Endocannabinoids and β-amyloid-induced neurotoxicity in vivo: effect of pharmacological elevation of endocannabinoid levels. Cell Mol Life Sci 63:1410–1424. https://doi.org/10.1007/s00018-006-6037-3
Van Esbroeck ACM, Janssen APA, Cognetta AB et al (2017) Activity-based protein profiling reveals off-target proteins of the FAAH inhibitor BIA 10-2474. Science (80-) 356:1084–1087. https://doi.org/10.1126/science.aaf7497
Velasco G, Hernández-Tiedra S, Dávila D, Lorente M (2016) The use of cannabinoids as anticancer agents. Prog Neuro-Psychopharmacol Biol Psychiatry 64:259–266. https://doi.org/10.1016/j.pnpbp.2015.05.010
Velasco G, Sánchez C, Guzmán M (2012) Towards the use of cannabinoids as antitumour agents. Nat Rev Cancer 12:436–444. https://doi.org/10.1038/nrc3247
Vemuri VK, Makriyannis A (2015) Medicinal chemistry of cannabinoids. Clin Pharmacol Ther 97:553–558. https://doi.org/10.1111/cpt.115
Vera G, Chiarlone A, Cabezos PA et al (2007) WIN 55,212-2 prevents mechanical allodynia but not alterations in feeding behaviour induced by chronic cisplatin in the rat. Life Sci 81:468–479. https://doi.org/10.1016/j.lfs.2007.06.012
Wallace MJ, Martin BR, DeLorenzo RJ (2002) Evidence for a physiological role of endocannabinoids in the modulation of seizure threshold and severity. Eur J Pharmacol 452:295–301. https://doi.org/10.1016/S0014-2999(02)02331-2
Wallace MJ, Wiley JL, Martin BR, DeLorenzo RJ (2001) Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur J Pharmacol 428:51–57. https://doi.org/10.1016/S0014-2999(01)01243-2
Walsh D, Nelson KA, Mahmoud FA (2003) Established and potential therapeutic applications of cannabinoids in oncology. Support Care Cancer 11:137–143. https://doi.org/10.1007/s00520-002-0387-7
Williams CM, Whalley BJ, McCabe C (2015) Cannabinoids and appetite (dys)regulation. In: Fattore L (ed) Cannabinoids in neurologic and mental disease. Elsevier, pp 315–339
Wilson RI, Nicoll RA (2001) Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410:588–592. https://doi.org/10.1038/35069076
Xian X, Huang L, Zhang B et al (2016) WIN 55,212-2 inhibits the epithelial Mesenchymal transition of gastric cancer cells via COX-2 signals. Cell Physiol Biochem 39:2149–2157. https://doi.org/10.1159/000447910
Xu D, Wang J, Zhou Z et al (2015) Cannabinoid WIN55, 212-2 induces cell cycle arrest and inhibits the proliferation and migration of human BEL7402 hepatocellular carcinoma cells. Mol Med Rep 12:7963–7970. https://doi.org/10.3892/mmr.2015.4477
Yadav MR, Murumkar PR (2018) Advances in patented CB1 receptor antagonists for obesity. Pharm Pat Anal 7:169–173. https://doi.org/10.4155/ppa-2018-0020
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Morales, P., Reggio, P.H. (2021). Emerging Roles of Cannabinoids and Synthetic Cannabinoids in Clinical Experimental Models. In: Murillo-Rodriguez, E., Pandi-Perumal, S.R., Monti, J.M. (eds) Cannabinoids and Neuropsychiatric Disorders. Advances in Experimental Medicine and Biology, vol 1264. Springer, Cham. https://doi.org/10.1007/978-3-030-57369-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-57369-0_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-57368-3
Online ISBN: 978-3-030-57369-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)