Abstract
Core symptoms of psychosis include delusions, hallucinations, motor symptoms, and cognitive impairments. The cholinergic system has been increasingly implied in the pathophysiology of psychotic disorders. PET and SPECT imaging can be useful tools to increase our insight in the role of the neurotransmitter acetylcholine in psychosis. In this chapter we will first globally describe cholinergic neurotransmission and the function of the nicotinic and muscarinic receptors. Second, we will provide an overview of PET and SPECT studies examining the cholinergic system in psychosis. Finally, we will briefly discuss the results of these studies as well as future directions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Psychosis spectrum disorders, schizophrenia being the most severe form, are highly disabling psychiatric disorders characterized by positive (delusions, hallucinations), negative (blunted affect, anhedonia), motor, and cognitive symptoms. The prevalence of psychotic disorders is estimated at approximately 3% in the Western population, (Perälä et al. 2007) and the World Health Organization (WHO) estimates the direct costs of schizophrenia alone in Western countries at 1.6–2.6% of total healthcare expenditures (Chong et al. 2016). Current pharmacological treatments reduce positive symptoms, but do not target negative and cognitive symptoms and are accompanied by (severe) side effects (e.g., parkinsonism). However, alleviation of only positive symptoms often does not lead to functional recovery. Research suggests that, with current treatment, less than 15% of patients fully recover in terms of daily/social functioning (Remington et al. 2016). Therefore, there is an urgent need for treatment of negative and cognitive symptoms. However, the biological mechanisms underlying these symptoms remain largely unknown. A growing body of evidence implicate the cholinergic system in the pathology of psychosis, in particular cognitive symptoms (Carruthers et al. 2015; Raedler et al. 2007). Numerous studies have shown that receptor antagonist compounds targeting the central cholinergic system, such as scopolamine, can induce learning and memory problems, whereas cholinergic receptor agonists as well as acetylcholinesterase inhibitors (AChE-Is) can enhance these functions (Everitt and Robbins 1997; Fibiger 1991). Therefore, it has been suggested that development of pharmacological treatment targeting the cholinergic system could improve cognition in psychosis. However, in order to develop effective medication, more insight is necessary into the precise role of the cholinergic system in psychotic disorders. Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) are highly useful tools as these imaging techniques allow for chemical characterization of the cholinergic system in vivo. Although choline containing compounds can be measured with magnetic resonance spectroscopy (MRS), at present no other noninvasive neuroimaging techniques are available.
2 Cholinergic Neurotransmission
Acetylcholine (Ach) is the first discovered neurotransmitter and plays a role in many central nervous system (CNS) functions, including motor function, sleep, learning, and memory (Sofuoglu and Mooney 2009). Ach is synthesized in the presynaptic nerve terminal from a reaction between acetyl-coenzyme A and choline (Cho) and catalyzed by choline acetyltransferase (ChAT), an enzyme primarily expressed by cholinergic neurons, and stored in the synaptic vesicles (Sofuoglu and Mooney 2009; Sarter and Parikh 2005). Ach is released into the synaptic cleft where it is degraded into the inactive metabolites Cho and acetate by acetylcholinesterase (AChE) (Sarter and Parikh 2005). Subsequently, Cho is transported back into the axon terminal for synthesis of more Ach (Sarter and Parikh 2005). Ach binds to two major classes of cholinergic receptors: nicotinic (ionotropic) and muscarinic (metabotropic) receptors.
2.1 Nicotinic Receptors
Nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channel (inotropic) receptors with fast responses and excitatory effects (Sofuoglu and Mooney 2009; Dani and Bertrand 2007). Nicotinic receptors are expressed both in the peripheral nervous system (PNS) and the CNS and composed of ligand binding subunits, α2–α10 (Jones et al. 2012), and structural subunits, β2–β4 (Gu 2002). Nicotinic AChRs are localized at post-, pre-, peri-, and extra-synaptic sites of cholinergic and other neurons (Dani and Bertrand 2007). Presynaptically located nAChRs regulate release of endogenous ACh (Sofuoglu and Mooney 2009; Jones et al. 2012), whereas postsynaptic nAChRs contribute a small minority of fast excitatory transmission, and non-synaptic nAChRs influence neuronal excitability, thereby modulating multiple neurotransmitter systems (Dani and Bertrand 2007). Their ability to modulate activity-dependent events underlies nAChR involvement in fundamental aspects of synaptic plasticity, attention, learning, and memory (Dani and Bertrand 2007; Albuquerque et al. 1997). Disruptions in central nicotinic modulated cholinergic transmission have been implicated in a variety of disorders including schizophrenia (Dani and Bertrand 2007; Jones et al. 2012).
2.2 Muscarinic Receptors
Muscarinic acetylcholine receptors (mAChRs) are G-protein-coupled (metabotropic) cholinergic receptors that can have either excitatory or inhibitory effects and have a longer onset latency (Gu 2002). Muscarinic AChRs are expressed both in the CNS and PNS and account for approximately 90% of all cholinergic receptors (Sofuoglu and Mooney 2009). At least five different subtypes can be distinguished, labeled M1–M5 (Bymaster et al. 2003), that can be divided into two classes based on their G-protein-coupling mechanism: M1, M3, and M5 versus M2 and M4 (Caulfield and Birdsall 1998; Eglen 2006; Ryan et al. 2019). The first group, referred to as the “M1-like” subtypes are located postsynaptically and have excitatory downstream effects, whereas the second group, referred to as the “M2-like” subtypes, are located pre- and postsynaptically and have predominantly inhibitory effects (Jones et al. 2012; Ryan et al. 2019). In addition to a “classic” agonist binding site, muscarinic receptors also have allosteric binding sites enabling the modulation of agonist activation. Of all mAChRs, the M1 receptor has the highest expression rate in the CNS, in particular in the cortex, hippocampus, and striatum, and is increasingly implicated in cognitive processes (Eglen 2006) and considered a potential target for treatment of cognitive symptoms in psychiatric and neurological disorders including schizophrenia.
3 Cholinergic System in Psychosis
The cholinergic system has been broadly related to neurodegenerative disorders characterized by cognitive decline and/or motor symptoms including Alzheimer’s disease (AD) and Parkinson’s disease (PD), partly because of its close relation with the dopaminergic system (McCluskey et al. 2019). Since psychotic disorders are also associated with cognitive decline, the cholinergic system became of interest for these disorders as well. Nevertheless, to date, studies examining the cholinergic system in patients with a psychotic disorder are limited, and most of the evidence is derived from preclinical and post-mortem studies.
3.1 Nicotinic PET/SPECT Imaging in Psychosis
3.1.1 Post-Mortem Studies
Most evidence for changes in nAChRs availability in psychotic patients comes from post-mortem studies. Several studies have demonstrated decreased expression of nicotinic receptors in schizophrenia (Vingerhoets et al. 2019a; Court et al. 1999; Freedman et al. 1995; Marutle et al. 2001). Freedman et al. (Freedman et al. 1995) used both [125I]-α-bungarotoxin (α-BTX), an α-neurotoxin with a binding site at nicotinic α7, α8, and α9 subunits (Court et al. 1999; Chen and Patrick 1997), and [3H]-cytisine, a nicotinic agonist with high affinity predominantly for the α4β2 subunits, to assess nicotinic receptor binding in schizophrenia. They report a decrease in the number of nicotinic receptors in hippocampal brain tissue of eight patients with schizophrenia compared to eight age-matched controls. In line with these results, Court et al. (1999) found reduced [125I]-α-BTX binding in the thalamus of schizophrenia patients compared to controls. Contrary, the authors did not find a reduction of [3H]-nicotine binding. Similarly, using [125I]-α-BTX, Guan et al. (1999) found a decrease of nicotinic α7 subunits in the frontal cortex of schizophrenia patients. Finally, the laminar distribution of nicotinic receptors were examined in 12 post-mortem brains of schizophrenia patients using [125I]-α-BTX, [3H]-cytisine, and [3H]-epibatidine (high affinity for α3 and α4 subunits) (Marutle et al. 2001). Patients with schizophrenia had fewer [125I]-α-BTX binding sites in the cingulate cortex compared to smoking control subjects, but not when compared to all control subjects. In the orbitofrontal and temporal cortex, results did not reach significance, although a trend for decreased [125I]-α-BTX binding in the orbitofrontal cortex and increased [125I]-α-BTX binding in the temporal cortex was found. Moreover, [3H]-cytisine binding was increased in the cingulate cortex of schizophrenia patients compared to the total sample of controls as well as only the smoking controls. In the same region, higher [3H]-cytisine binding was observed in all layers with the exception of layer 1 in schizophrenia compared to controls that smoked.
3.1.2 In Vivo Studies
Nicotine is considered the most addictive component of tobacco (Brody et al. 2006). NAChRs in the brain mediate nicotine’s action, of which those containing α4β2 are the most abundant in the brain, have the highest affinity for nicotine, and are instrumental in mediating nicotine’s reinforcing properties (D’Souza et al. 2012). Human post-mortem studies in “healthy” tobacco users demonstrated an increase in nicotinic binding sites (Benwell et al. 1988). In vivo, an upregulation of the nACh α4β2 receptor subtype has been reported in healthy smokers compared to nonsmokers using 2-[18F]fluoro-3-(2(S)azetidinylmethoxy)pyridine (2-[18F]F-A-85380) positron emission tomography (PET) (Wüllner et al. 2008). The vast majority of patients with a psychotic disorder smoke tobacco (Myles et al. 2012; Lucatch et al. 2018), and most patients use tobacco excessively (Barnes et al. 2006; Vingerhoets et al. 2019b), suggesting altered nAChR binding in these patients. Indeed, thalamic 2-[18F]F-A-85380 binding potential nondisplaceable (BPND) was found to be lower in tobacco using patients with paranoid schizophrenia and one tobacco using healthy control subject compared to four nonsmoking healthy controls (Brašić et al. 2012). It must be noted though that all tobacco-using participants had smoked shortly before scanning. Therefore, it is likely that many of the nAChRs were already occupied by nicotine.
The SPECT ligand [(123)I]-5-iodo-3-[2(S)-azetidinylmethoxy]pyridine) ([123I]5-IA-85380) has been used for in vivo quantification of the nicotinic receptors with high affinity for β2 subunits. Using this tracer, earlier post-mortem findings of lower β2*-nicotinic receptor availability in tobacco using schizophrenia patients compared to smokers without schizophrenia were confirmed in the frontal cortex, parietal cortex, and thalamus (D’Souza et al. 2012). Moreover, β2*-nicotinic receptor availability was inversely correlated with negative symptoms. Elaborating on these findings, β2*-nicotinic receptor availability was compared between tobacco and non-tobacco-using patients with schizophrenia as well as healthy controls matched for smoking, age, and sex (Esterlis et al. 2014). The total sample of schizophrenia patients displayed lower β2*-nicotinic receptor availability relative to the total control group, and, overall, nonsmokers had lower β2*-nicotinic receptor availability compared to smokers. Interestingly, there was no smoking by diagnosis interaction effect, but smoking schizophrenia patients had higher β2*-nicotinic receptor availability opposed to nonsmoking schizophrenia patients. Higher β2*-nicotinic receptor availability was associated with less negative symptoms and better executive control, and chronic use of antipsychotics did not appear to be related to β2*-nicotinic receptor availability.
Another nAChR subtype strongly associated with tobacco use is the α7 receptor which consists entirely of α7 subunits (Lucatch et al. 2018). Of all the nicotinic receptors, this subtype has been studied most frequently in relation with psychotic disorders, and like the nACh α4β2 receptor, this subtype has been linked to excessive tobacco use in schizophrenia (Tregellas and Wylie 2019). Because of its relatively low expression rate in the human brain, it has been proven difficult to develop PET/SPECT tracers to image this receptor subtype in the brain (Marutle et al. 2001; Coughlin et al. 2019). Recently, quantification of the nicotinic α7 receptor has been feasible using the PET tracer [18F]JHU82132; 3-(1,4-diazabicyclo[3.2.2]nonan-4-yl)-6-[18F]fluorodibenzo[b,d]thiophene 5,5-dioxide ([18F]ASEM) (Wong et al. 2014). Using [18F]ASEM PET, Wong et al. (Wong et al. 2006) found reduced binding in the cingulate cortex, frontal cortex, and hippocampus in five out of six schizophrenia patients compared to controls (Wong et al. 2018). Moreover, a small pilot study in nonsmoking patients with recent-onset psychosis showed lower [18F]ASEM binding in the hippocampus than control subjects, in particular those patients with non-affective psychosis (Coughlin et al. 2018). In addition, lower [18F]ASEM binding was associated with worse verbal memory and processing speed, implying a role of het nicotinic α7 receptor in cognitive deficits in psychotic disorders.
3.2 Muscarinic PET/SPECT Imaging in Psychosis
3.2.1 Post-Mortem Studies
Accumulating evidence suggests that altered or deficient muscarinic signaling underlies the clinical symptoms of psychosis, including cognitive deficits (Carruthers et al. 2015; Ryan et al. 2019; Vingerhoets et al. 2019a; Bakker et al. 2018). Evidence for involvement of the cholinergic muscarinic receptors in psychosis has mainly been gained from studying CNS tissue obtained post-mortem (Raedler et al. 2007). These post-mortem studies have yielded mixed results. An early study using the nonselective tracer [3H](R)-3-quinuclidinylbenzilate ([3H]QNB) reported reduced levels of muscarinic receptor binding in the frontal cortex of schizophrenia patients compared to control subjects (Bennett et al. 1979). In a different study also using [3H]QNB, an increased number of muscarinic receptors were found in the orbitofrontal (OFC) and medial frontal cortex in medicated patients with schizophrenia compared to controls (Watanabe et al. 1983). Contrary, unmedicated patients did not differ from controls, suggesting an effect of long-term antipsychotic use. However, this discrepancy in findings could also be associated with the nonselectiveness of [3H]QNB. Later studies have reported differences using more selective ligands such as [3H]pirenzepine and found a significant decrease of muscarinic M1 and M4 receptors in the striatum (Dean et al. 1996; Crook et al. 1999), hippocampus (Crook et al. 2000), and PFC (Crook et al. 2001; Dean et al. 2002) of schizophrenia patients but not in the parietal cortex (Dean et al. 2002). In addition, these changes in M1 receptor density appear to be characteristic for schizophrenia since no changes were found in bipolar and depressive disorders (Zavitsanou et al. 2004). However, other studies did find altered numbers of M2 and M3 receptors in schizophrenia (Raedler et al. 2007; Zavitsanou et al. 2004; Scarr et al. 2006).
A more recent post-mortem study using [3H]pirenzepine reported a decrease of muscarinic M1 receptors up to 74% in the dorsolateral prefrontal cortex (DLPFC) in subgroup of patients with schizophrenia comprising approximately 25% of the sample (Scarr et al. 2009). The authors labeled this subgroup “muscarinic receptor-deficit schizophrenia” (MRDS) and hypothesized that this may be a subgroup of patients displaying more severe cognitive symptoms, given the role of M1 receptors in several cognitive functions, although data of cognitive function were not available. Interestingly, this so-called MRDS subgroup did not differ from other schizophrenia patients in terms of clinical characteristics including gender, age, duration of illness, or any particular drug treatment (Scarr et al. 2009).
3.2.2 In Vivo Studies
The number of in vivo studies examining changes in muscarinic receptor density in psychosis is still limited. Nevertheless, these studies support the post-mortem findings of reduced M1 receptor density in patients with a psychotic disorder. In a [123I]IQNB SPECT study by Raedler et al. (Raedler et al. 2003a), patients with schizophrenia who were medication-free at time of scanning displayed decreased muscarinic receptor availability in the cortex and basal ganglia. Muscarinic receptor occupancy was decreased up to 35% in schizophrenia patients compared to matched control subjects in this sample of 12 patients. Unfortunately, [123I]IQNB binds with very high affinity to all subtypes of the muscarinic receptors (Raedler et al. 2007). Therefore, it is unknown whether this decreased availability reflects predominantly a reduction in M1 receptors.
SPECT imaging was also used to examine the effects of antipsychotic medication on the cholinergic muscarinic system. Raedler et al. (Raedler et al. 2000) used [123I]IQNB SPECT to study the effects of the antipsychotic olanzapine at a daily dose of 5 and 20 mg, respectively, on the cholinergic muscarinic receptors in the cortex, striatum, thalamus, and the pons of schizophrenia patients. At both a low and high dosage of olanzapine, [123I]IQNB binding was significantly lower compared to [123I]IQNB binding in medication-free subjects in all brain regions except the striatum. Moreover, in these same brain areas, [123I]IQNB binding was significantly lower at a dose of 20 mg compared to 5 mg indicating that olanzapine blocks muscarinic receptors in vivo significantly, possibly explaining the low incidence of anticholinergic side effects. In a different study by Raedler et al. (Raedler et al. 2003b), and also using [123I]IQNB SPECT, the impact of low to moderate dosages of the antipsychotic clozapine on muscarinic receptors in the basal ganglia, cortex, thalamus, and pons was examined. Compared to medication-free psychotic subjects, [123I]IQNB binding was lower in all examined brain areas, and decreases in binding were observed with increasing dose. In a separate study, the authors compared the previously reported effects of olanzapine and clozapine directly and concluded that clozapine treatment resulted in a stronger muscarinic receptor blockade than olanzapine (Raedler 2007).
The effects of antipsychotic treatment on cholinergic muscarinic receptors were also examined with the SPECT ligand [123I]-iododexetimide ([123I]IDEX). This ligand predominantly binds to the M1 subtype, although it also has relatively high affinity for the M4 subtype, but not for M2,3,5 receptors (Bakker et al. 2015). In this study by Lavalaye and co-workers (Lavalaye et al. 2001), [123I]IDEX binding in the cortex and striatum of patients stabilized on the antipsychotics olanzapine or risperidone was compared directly as well as to healthy control subjects. Patients treated with olanzapine displayed lower binding ratios in both the cortex and striatum than patients treated with risperidone as well as control subjects, reflecting higher levels of muscarinic receptor occupancy by olanzapine. Moreover, patients treated with risperidone also showed lower binding ratios compared to controls, but this was only significant in the striatum.
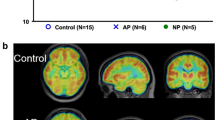
In a more recent study also using [123I]IDEX SPECT imaging, Bakker et al. (Bakker et al. 2018), found a link between lower M1 receptor binding in the DLPFC and poorer performance on a verbal learning and memory task. Moreover, lower M1 binding in the hippocampus was associated with worse delayed recognition of verbal information. In addition, higher negative symptom severity was associated with lower M1 binding in the hippocampus. Since in this study no healthy control subjects were included, it remains unknown whether these findings are specific for patients with a psychotic disorder. Indeed, administration of a muscarinic M1 antagonist produced cognitive impairments in subjects with a psychotic disorder (Vingerhoets et al. 2017; Veselinović et al. 2015). Figure 15.1 displays [123I]IDEX binding in the brain of a patient with a psychotic disorder.
Adapted from Bakker et al. (Bakker et al. 2018). The left panel displays [123I]IDEX binding in a subject with a psychotic disorder demonstrating high cortical binding and no binding in white matter and cerebrospinal fluid. The right panel shows the same [123I]IDEX SPECT scan co-registered to the subjects’ own structural T1 MRI scan. Darker areas are overlay white matter tracts, ventricles, and cerebellum indicating no [123I]IDEX binding in these areas
4 Conclusion and Future Directions
To summarize, cholinergic alterations are increasingly linked to psychosis. Both post-mortem and in vivo PET and SPECT studies provided evidence for altered nicotinic and muscarinic receptors in psychosis, suggesting that reductions of cholinergic receptors, in particular nicotinic α4β2 and α7 as well as muscarinic M1 receptors, could be identifiable biomarkers for psychotic disorders. These results are supported by findings of altered brain choline concentrations in MRS studies (Kirtaş et al. 2016; Bustillo et al. 2002, 2014; Plitman et al. 2016) and pharmacological (challenge) studies reporting memory deficits after administration of anticholinergics in psychotic patients (Vingerhoets et al. 2017; Veselinović et al. 2015). Overall, this may suggest that a subgroup of psychotic patients may benefit from cholinergic pharmacological treatment. However, results of pharmacological interventions targeting the cholinergic system have been mixed. For example, AChE-Is have overall not yielded much positive results (Vingerhoets et al. 2013; Santos et al. 2018), although some studies have found improvement in speed of processing after AChE-I add-on treatment in schizophrenia (Santos et al. 2018). These limited beneficial effects of AChE-Is are likely due to the fact that AChE-Is may not affect AChR function directly. Contrary, monotherapy with xanomeline, a muscarinic M1 agonist, has shown to improve both positive and negative as well as cognitive symptoms in patients with schizophrenia (Shekhar et al. 2008). This may suggest that selective targeting of cholinergic receptors may be a more successful strategy than increasing ACh levels per se. Nevertheless, muscarinic drugs cause a variety of PNS-related side effects (particularly related to the gastrointestinal tract), possibly because lack of selectivity for the M1 subtype, and currently no M1 agonists are approved for treatment of psychosis. Since PET and SPECT have the potency to identify chemical signatures, these techniques may be useful tools for identification of biomarkers and examining medication response. Moreover, PET and SPECT have the potential to become useful tools in stratification of patients in order to inform treatment decisions, if validated further (Coughlin et al. 2019). In particular patients with first-episode psychosis may benefit from methods allowing identification of personalized effective treatments. At present, finding an effective treatment can be a search which burdens affected (young) patients by unnecessary prolongation of symptoms and unwanted side effects. Moreover, this could lead to nonadherence of future treatment. Stratification of psychotic patients can guide personalized medicine which is beneficial for patients, their environment, and the broader society.
4.1 Cholinergic Transporter Imaging
In addition to targeting nicotinic and muscarinic cholinergic receptors, there are developments to target the vesicular acetylcholine transporter (VAChT). VAChT activity is distinct from the therapeutic site of AChE-I and has been described as a more pure indication of presynaptic cholinergic terminal density compared to other cholinergic targets (McCluskey et al. 2019; Bohnen et al. 2018). Although [123I]iodobenzovesamicol ([123I]IBVM) SPECT has been available for imaging VAChT for decades (Kuhl et al. 1994), no studies using this technique to examine patients with psychotic disorders have been performed. In recent years, new PET tracers have been developed to study VAChT. One of these tracers is [18F]fluoroethoxybenzovesamicol ([18F]FEOBV). As compared to the SPECT tracer [123I]IBVM, this PET tracer offers the possibility to in vivo examine smaller brain regions (Petrou et al. 2014). Although at present, no [18F]FEOBV PET studies have been performed in psychotic disorders, decreased [18F]FEOBV binding has recently been demonstrated in cortical and subcortical brain areas involved in cognition functioning in disorders characterized by cognitive deficits, namely, dementia with Lewy bodies and AD (Nejad-Davarani et al. 2019; Aghourian et al. 2017). Therefore, and considering the increasing evidence of involvement of the cholinergic system in psychotic disorder and efforts of developing cholinergic treatments for these disorders, [18F]FEOBV PET may be a useful tool to increase our knowledge of cholinergic abnormalities in psychosis. Potentially, it can be used to stratify patients and guide clinical decision-making in the future.
5 Concluding Remarks
To conclude, both post-mortem and in vivo SPECT and PET studies demonstrated cholinergic alterations in patients with psychotic disorders which may be linked to negative and cognitive symptoms. Therefore, a subgroup of patients could possibly benefit from cholinergic treatment. Cholinergic PET and SPECT imaging can potentially be used to stratify patients with cholinergic abnormalities in the future in order to guide clinical decision-making and personalized medicine and to optimize treatment outcome for psychotic patients.
Abbreviations
- Ach:
-
Acetylcholine
- AcCoa:
-
Acetyl-coenzyme A
- AChE:
-
Acetylcholinesterase
- AChE-Is:
-
Acetylcholinesterase inhibitors
- AD:
-
Alzheimer’s disease
- α-BTX:
-
α-Bungarotoxin
- BPND:
-
Binding potential nondisplaceable
- ChAT:
-
Choline acetyltransferase
- Cho:
-
Choline
- CNS:
-
Central nervous system
- DLPFC:
-
Dorsolateral prefrontal cortex
- [18F]FEOBV:
-
[18F]fluoroethoxybenzovesamicol
- [18F]ASEM:
-
[18F]-JHU82132; 3-(1,4-diazabicyclo[3.2.2]nonan-4-yl)-6-18F]fluorodibenzo[b,d]thiophene 5,5-dioxide)
- 2-[18F]F-A-85380:
-
2-[18F]fluoro-3-(2(S)azetidinylmethoxy)pyridine
- [3H]QNB:
-
[3H](R)-3-quinuclidinylbenzilate
- [123I]-IDEX:
-
[123I]-iododexetimide
- [123I]-IBVM:
-
[123I]-iodobenzovesamicol
- [123I]5-IA-85380:
-
[(123)I]-5-iodo-3-[2(S)-azetidinylmethoxy]pyridine)
- mAChRs:
-
Muscarinic acetylcholine receptors
- MRS:
-
Magnetic resonance spectroscopy
- nAChRs:
-
Nicotinic acetylcholine receptors
- OFC:
-
Orbitofrontal cortex
- PD:
-
Parkinson’s disease
- PET:
-
Positron emission tomography
- PNS:
-
Peripheral nervous system
- SPECT:
-
Single-photon emission computed tomography
- VAChT:
-
Vesicular acetylcholine transporter
References
Aghourian M, Legault-Denis C, Soucy J-P, Rosa-Neto P, Gauthier S, Kostikov A et al (2017) Quantification of brain cholinergic denervation in Alzheimer’s disease using PET imaging with [18F]-FEOBV. Mol Psychiatry 22:1531–1538
Albuquerque EX, Alkondon M, Pereira EF, Castro NG, Schrattenholz A, Barbosa CT et al (1997) Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J Pharmacol Exp Ther 280:1117–1136
Bakker G, Vingerhoets WA, van Wieringen J-P, de Bruin K, Eersels J, de Jong J et al (2015) 123I-Iododexetimide Preferentially Binds to the Muscarinic Receptor Subtype M1 In Vivo. J Nucl Med 56:317–322
Bakker G, Vingerhoets C, Boucherie D, Caan M, Bloemen O, Eersels J et al (2018) Relationship between muscarinic M1 receptor binding and cognition in medication-free subjects with psychosis. NeuroImage Clin 18:713–719
Barnes TRE, Mutsatsa SH, Hutton SB, Watt HC, Joyce EM (2006) Comorbid substance use and age at onset of schizophrenia. Br J Psychiatry 188:237–242
Bennett JP, Enna SJ, Bylund DB, Gillin JC, Wyatt RJ, Snyder SH (1979) Neurotransmitter receptors in frontal cortex of schizophrenics. Arch Gen Psychiatry 36:927
Benwell MEM, Balfour DJK, Anderson JM (1988) Evidence that tobacco smoking increases the density of (-)-[ 3 H]nicotine binding sites in human brain. J Neurochem 50:1243–1247
Bohnen NI, Grothe MJ, Ray NJ, Müller MLTM, Teipel SJ (2018) Recent advances in cholinergic imaging and cognitive decline—revisiting the cholinergic hypothesis of dementia. Curr Geriatr Rep 7:1–11
Brašić JR, Cascella N, Kumar A, Zhou Y, Hilton J, Raymont V et al (2012) Positron emission tomography experience with 2-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[18F]FA) in the living human brain of smokers with paranoid schizophrenia. Synapse 66:352–368
Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D et al (2006) Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry 63:907–915
Bustillo JR, Rowland LM, Lauriello J, Petropoulos H, Hammond R, Hart B et al (2002) High choline concentrations in the caudate nucleus in antipsychotic-naive patients with schizophrenia. Am J Psychiatry 159:130–133
Bustillo JR, Chen H, Jones T, Lemke N, Abbott C, Qualls C et al (2014) Increased glutamine in patients undergoing long-term treatment for schizophrenia: a proton magnetic resonance spectroscopy study at 3 T. JAMA Psychiatry 71:265–272
Bymaster FP, McKinzie DL, Felder CC, Wess J (2003) Use of M1–M5 muscarinic receptor knockout mice as novel tools to delineate the physiological roles of the muscarinic cholinergic system. Neurochem Res 28:437–442
Carruthers SP, Gurvich CT, Rossell SL (2015) The muscarinic system, cognition and schizophrenia. Neurosci Biobehav Rev 55:393–402
Caulfield MP, Birdsall NJ (1998) International union of pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 50:279–290
Chen D, Patrick JW (1997) The alpha-bungarotoxin-binding nicotinic acetylcholine receptor from rat brain contains only the alpha7 subunit. J Biol Chem 272:24,024–24,029
Chong HY, Teoh SL, DB-C W, Kotirum S, Chiou C-F, Chaiyakunapruk N (2016) Global economic burden of schizophrenia: a systematic review. Neuropsychiatr Dis Treat 12:357–373
Coughlin J, Du Y, Crawford JL, Rubin LH, Behnam Azad B, Lesniak WG et al (2018) The availability of the α7 nicotinic acetylcholine receptor in recent-onset psychosis: a study using 18F-ASEM PET. J Nucl Med 60:241–243
Coughlin JM, Horti AG, Pomper MG (2019) Opportunities in precision psychiatry using PET neuroimaging in psychosis. Neurobiol Dis 131:104,428
Court J, Spurden D, Lloyd S, McKeith I, Ballard C, Cairns N et al (1999) Neuronal nicotinic receptors in dementia with lewy bodies and schizophrenia: α-bungarotoxin and nicotine binding in the thalamus. J Neurochem 73:1590–1597
Crook JM, Dean B, Pavey G, Copolov D (1999) The binding of [3H]AF-DX 384 is reduced in the caudate-putamen of subjects with schizophrenia. Life Sci 64:1761–1771
Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B (2000) Decreased muscarinic receptor binding in subjects with schizophrenia: a study of the human hippocampal formation. Biol Psychiatry 48:381–388
Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B (2001) Low muscarinic receptor binding in prefrontal cortex from subjects with schizophrenia: a study of brodmann’s areas 8, 9, 10, and 46 and the effects of neuroleptic drug treatment. Am J Psychiatry 158:918–925
D’Souza DC, Esterlis I, Carbuto M, Krasenics M, Seibyl J, Bois F et al (2012) Lower β2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia. Am J Psychiatry 169:326–334
Dani JA, Bertrand D (2007) Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol 47:699–729
Dean B, Crook JM, Opeskin K, Hill C, Keks N, Copolov DL (1996) The density of muscarinic M1 receptors is decreased in the caudate-putamen of subjects with schizophrenia. Mol Psychiatry 1:54–58
Dean B, Mcleod M, Keriakous D, Mckenzie J, Scarr E (2002) Decreased muscarinic 1 receptors in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry 7:1083–1091
Eglen RM (2006) Muscarinic receptor subtypes in neuronal and non-neuronal cholinergic function. Auton Autacoid Pharmacol 26:219–233
Esterlis I, Ranganathan M, Bois F, Pittman B, Picciotto MR, Shearer L et al (2014) In vivo evidence for β2 nicotinic acetylcholine receptor subunit upregulation in smokers as compared with nonsmokers with schizophrenia. Biol Psychiatry 76:495–502
Everitt BJ, Robbins TW (1997) Central cholinerig systems. Annu Rev Psychol 48:649–684
Fibiger HC (1991) Cholinergic mechanisms in learning, memory and dementia: a review of recent evidence. Trends Neurosci 14:220–223
Freedman R, Hall M, Adler LE, Leonard S (1995) Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry 38:22–33
Gu Q (2002) Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience 111:815–835
Guan ZZ, Zhang X, Blennow K, Nordberg A (1999) Decreased protein level of nicotinic receptor α7 subunit in the frontal cortex from schizophrenic brain. Neuroreport 10:1779–1782
Jones CK, Byun N, Bubser M (2012) Muscarinic and nicotinic acetylcholine receptor agonists and allosteric modulators for the treatment of schizophrenia. Neuropsychopharmacology 37:16–42
Kirtaş D, Karadağ RF, Balci Şengül MC, Kiroğlu Y (2016) 1H-magnetic resonance spectroscopy in first episode and chronic schizophrenia patients. Turkish J Med Sci 46:862–871
Kuhl DE, Koeppe RA, Fessler JA, Minoshima S, Ackermann RJ, Carey JE et al (1994) In vivo mapping of cholinergic neurons in the human brain using SPECT and IBVM. J Nucl Med 35:405–410
Lavalaye J, Booij J, Linszen D, Reneman L, van Royen E (2001) Higher occupancy of muscarinic receptors by olanzapine than risperidone in patients with schizophrenia. Psychopharmacology (Berl) 156:53–57
Lucatch AM, Lowe DJE, Clark RC, Kozak K, George TP (2018) Neurobiological determinants of tobacco smoking in schizophrenia. Front Psychiatry 9:672
Marutle A, Zhang X, Court J, Piggott M, Johnson M, Perry R et al (2001) Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. J Chem Neuroanat 22:115–126
McCluskey SP, Plisson C, Rabiner EA, Howes O (2019) Advances in CNS PET: the state-of-the-art for new imaging targets for pathophysiology and drug development. Eur J Nucl Med Mol Imaging 47:1–39
Myles N, Newall HD, Curtis J, Nielssen O, Shiers D, Large M (2012) Tobacco use before, at, and after first-episode psychosis. J Clin Psychiatry 73:468–475
Nejad-Davarani S, Koeppe RA, Albin RL, Frey KA, Müller MLTM, Bohnen NI (2019) Quantification of brain cholinergic denervation in dementia with Lewy bodies using PET imaging with [18F]-FEOBV. Mol Psychiatry 24:322–327
Perälä J, Suvisaari J, Saarni SI, Kuoppasalmi K, Isometsä E, Pirkola S et al (2007) Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry 64:19–28
Petrou M, Frey KA, Kilbourn MR, Scott PJH, Raffel DM, Bohnen NI et al (2014) In vivo imaging of human cholinergic nerve terminals with (-)-5-(18)F-fluoroethoxybenzovesamicol: biodistribution, dosimetry, and tracer kinetic analyses. J Nucl Med. 55:396–404
Plitman E, de la Fuente-Sandoval C, Reyes-Madrigal F, Chavez S, Gómez-Cruz G, León-Ortiz P et al (2016) Elevated myo-inositol, choline, and glutamate levels in the associative striatum of antipsychotic-naive patients with first-episode psychosis: a proton magnetic resonance spectroscopy study with implications for glial dysfunction. Schizophr Bull 42:415–424
Raedler TJ (2007) Comparison of the in-vivo muscarinic cholinergic receptor availability in patients treated with clozapine and olanzapine. Int J Neuropsychopharmacol 10:275–280
Raedler T, Knable MB, Jones DW, Lafargue T, Urbina RA, Egan MF et al (2000) In vivo olanzapine occupancy of muscarinic acetylcholine receptors in patients with schizophrenia. Neuropsychopharmacology 23:56–68
Raedler TJ, Knable MB, Jones DW, Urbina RA, Gorey JG, Lee KS et al (2003a) In vivo determination of muscarinic acetylcholine receptor availability in schizophrenia. Am J Psychiatry 160:118–127
Raedler TJ, Knable MB, Jones DW, Urbina RA, Egan MF, Weinberger DR (2003b) Central muscarinic acetylcholine receptor availability in patients treated with clozapine. Neuropsychopharmacology 28:1531–1537
Raedler T, Bymaster F, Tandon R, Copolov D, Dean B (2007) Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry 12:232–246
Remington G, Foussias G, Fervaha G, Agid O, Takeuchi H, Lee J et al (2016) Treating negative symptoms in schizophrenia: an update. Curr Treat Options Psychiatry 3:133–150
Ryan AE, Mowry BJ, Kesby JP, Scott JG, Greer JM (2019) Is there a role for antibodies targeting muscarinic acetylcholine receptors in the pathogenesis of schizophrenia? Aust New Zeal J Psychiatry 53:1059–1069
Santos B, González-Fraile E, Zabala A, Guillén V, Rueda JR, Ballesteros J (2018) Cognitive improvement of acetylcholinesterase inhibitors in schizophrenia. J Psychopharmacol 32:1155–1166
Sarter M, Parikh V (2005) Choline transporters, cholinergic transmission and cognition. Nat Rev Neurosci 6:48–56
Scarr E, Keriakous D, Crossland N, Dean B (2006) No change in cortical muscarinic M2, M3 receptors or [35S]GTPγS binding in schizophrenia. Life Sci 78:1231–1237
Scarr E, Cowie TF, Kanellakis S, Sundram S, Pantelis C, Dean B (2009) Decreased cortical muscarinic receptors define a subgroup of subjects with schizophrenia. Mol Psychiatry 14:1017–1023
Shekhar A, Potter WZ, Lightfoot J, Lienemann J, Dubé S, Mallinckrodt C et al (2008) Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry 165:1033–1039
Sofuoglu M, Mooney M (2009) Cholinergic functioning in stimulant addiction: implications for medications development. CNS Drugs 23:939–952
Tregellas J, Wylie K (2019) Alpha7 nicotinic receptors as therapeutic targets in schizophrenia. Nicotine Tob Res 21:349–356
Veselinović T, Vernaleken I, Janouschek H, Kellermann T, Paulzen M, Cumming P et al (2015) Effects of anticholinergic challenge on psychopathology and cognition in drug-free patients with schizophrenia and healthy volunteers. Psychopharmacology 232:1607–1617
Vingerhoets WAM, Bloemen OJN, Bakker G, van Amelsvoort TAMJ (2013) Pharmacological interventions for the MATRICS cognitive domains in schizophrenia: what’s the evidence? Front Psychiatry 4:157
Vingerhoets C, Bakker G, van Dijk J, Bloemen OJN, Wang Y, Chan RCK et al (2017) The effect of the muscarinic M1 receptor antagonist biperiden on cognition in medication free subjects with psychosis. Eur Neuropsychopharmacol 27:854–864
Vingerhoets C, Bakker G, Schrantee A, Van Der PM, OJN B, Reneman L et al (2019a) Influence of muscarinic M 1 receptor antagonism on brain choline levels and functional connectivity in medication-free subjects with psychosis: a placebo controlled, cross-over study. Psychiatry Res Neuroimaging 290:5–13
Vingerhoets C, van Oudenaren MJF, Bloemen OJN, Boot E, van Duin EDA, Evers LJM et al (2019b) Low prevalence of substance use in people with 22q11.2 deletion syndrome. Br J Psychiatry 215:661–667
Watanabe S, Nishikawa T, Takashima M, Toru M (1983) Increased muscarinic cholinergic receptors in prefrontal cortices of medicated schizophrenics. Life Sci 33:2187–2196
Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A et al (2006) Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology 31:2716–2727
Wong DF, Kuwabara H, Pomper M, Holt DP, Brasic JR, George N et al (2014) Human brain imaging of α7 nAChR with [18F]ASEM: a new PET radiotracer for neuropsychiatry and determination of drug occupancy. Mol Imaging Biol 16:730–738
Wong DF, Kuwabara H, Horti AG, Roberts JM, Nandi A, Cascella N et al (2018) Brain PET imaging of α7-nAChR with [18F]ASEM: reproducibility, occupancy, receptor density, and changes in schizophrenia. Int J Neuropsychopharmacol 21:656–667
Wüllner U, Gündisch D, Herzog H, Minnerop M, Joe A, Warnecke M et al (2008) Smoking upregulates α4β2* nicotinic acetylcholine receptors in the human brain. Neurosci Lett 430:34–37
Zavitsanou K, Katerina Z, Katsifis A, Andrew K, Mattner F, Filomena M et al (2004) Investigation of m1/m4 muscarinic receptors in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression disorder. Neuropsychopharmacology 29:619–625
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Vingerhoets, C., Booij, J., van Amelsvoort, T. (2021). Acetylcholine Imaging in Psychosis. In: Dierckx, R.A., Otte, A., de Vries, E.F.J., van Waarde, A., Sommer, I.E. (eds) PET and SPECT in Psychiatry. Springer, Cham. https://doi.org/10.1007/978-3-030-57231-0_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-57231-0_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-57230-3
Online ISBN: 978-3-030-57231-0
eBook Packages: MedicineMedicine (R0)