Abstract
Stroke is one of the leading causes of disability and death around the world. As effectiveness of treatments for this pathology continues to advance, the number of people living with the sequelae is increasing as well. The current treatments, intravenous tissue plasminogen activator (tPA) and mechanical thrombectomy have improved the survival of patients in the acute setting by restoring blood flow through the brain vasculature. However, due to various complicating factors (timing of presentation, capabilities of medical centers, comorbidities of patient), not all patients receive these therapies, and for those that do, many have incurred brain damage prior to revascularization. Preclinical research over the past few decades has shown that in animal models of stroke, stem cell therapy can improve recovery and functional outcome in the post-ischemic brain. Although proven successful in preclinical trials, the clinical application of stem cells in stroke remains an area of active research to determine the effectiveness and safety profile in humans. However, studies are encouraging, and although there are many questions that remain to be answered, stem cell therapy for stroke appears to be a promising treatment for patients suffering from this devastating neurological disorder.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Stroke is a leading cause of death and disability worldwide. In recent years, the mortality rate of ischemic stroke has sharply decreased as a result of significant treatment advances. However, the incidence of this disease has not declined at the same pace; therefore, there exists a growing stroke burden in terms of disability and economic costs, as more patients are living with the physical sequelae of stroke [23].
Ischemic stroke comprises over 80% of the total number of strokes and occurs when blood supply to the brain is interrupted. This phenomenon is typically caused by thrombosis within a blood vessel, which interrupts normal blood flow supplying a region of brain tissue. Underlying medical conditions such as hypertension, atrial fibrillation, and diabetes, among others, all increase the risk of ischemic stroke. Acute strokes, if brought to medical attention quickly, can be treated by administration of tissue plasminogen activator (tPA) to dissolve the blood clot in the vessel and/or by direct removal of the clot through mechanical thrombectomy. Unfortunately, many patients do not receive these interventions as they do not reach medical attention within the required time frame, there is ambiguity of timing of symptom onset, or the medical center to which they present is not capable of performing the more advanced, invasive therapy of clot retrieval. As expected, these patients tend to have significant disability post-stroke. Of the patients that are fully revascularized (the blood clot is fully removed from the vessel and normal flow is restored), many still have significant disability as a result of the damage the brain endures prior to restoration of blood flow [38].
Common disabilities from stroke include hemiparesis or hemiplegia (weakness or paralysis of one side of the body) contralateral to the side of the stroke, problems with attention, learning, judgment, and/or memory. Damage to the dominant side of the brain (most often the left side) can cause significant difficulties with speech and language. For patients with the largest territory strokes, a decreased level of consciousness and a coma-like state may require the placement of tracheostomies and permanent feeding tubes and permanent placement in long-term nursing facilities.
Although advances in acute stroke care and post-stroke neurorehabilitation have proven effective in improving some neurological function, no approved therapies currently exist to reliably reverse residual deficits in stroke patients. Significant research efforts are focused on developing new approaches to restore damaged brain tissue and improve function in this patient population. One of the most promising avenues of current research is in cell-based therapies, specifically administration of stem cells to the post-stroke brain. This therapeutic approach is attractive due to stem cells multipotent, neuroprotective, and immunomodulatory potential. Preclinical and clinical studies are promising, with evidence of functional improvement in animal models and patients. Although a fast-growing area of investigation, many questions regarding the safety, efficacy, and appropriate clinical application in humans remain. This chapter reviews the most common stem cell-based therapies being investigated for ischemic stroke, describes the recent preclinical and clinical studies being performed, and discusses the current and future applications of this therapy for treatment of stroke patients.
15.2 Stem Cell-Based Therapies for Ischemic Stroke
Ischemic stroke causes extensive damage to multiple types of brain cells, as well as neuronal and vascular networks. Current treatments are effective in restoring blood flow through undamaged blood vessels; however, they are not effective at reversing the damage incurred during the period of ischemia before revascularization. Without further medical intervention, many stroke patients are able to gain some degree of functional recovery over time, suggesting that innate compensatory plasticity or remodeling may occur in the post-ischemic brain. Stem cell-based approaches gained momentum with the objective of enhancing this assumed endogenous repair mechanism or, as initially hypothesized, to replace injured cells in the ischemic core. However, current studies are beginning to suggest that several different mechanisms play a role in the neuroregeneration seen with stem cell therapies, including acting in a neuroprotective manner and by inducing angiogenesis, neurogenesis, and axonal sprouting [26], among others.
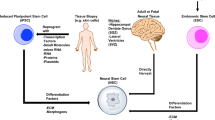
Over the past decade, cell-based regenerative therapies have been developed using different types of stem/progenitor cells in the attempt to restore lost brain tissue and function. Several types of stem cells have been investigated for use in this therapy, mainly mesenchymal (MSC), neural (NSC), embryonic (ESC), and induced pluripotent stem cells (iPSCs) . Although data on these stem cell-based therapies in animal models and human patients are conflicting, many studies suggest an important role for cellular-based therapy for stroke.
15.2.1 Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are pluripotent progenitor cells that give rise to a variety of tissues, including muscle, bone, cartilage, and adipose. The most widely studied source of MSCs in stroke therapy is bone marrow-derived cells, as it was hypothesized early on that these MSCs could differentiate into brain cells. Studies have confirmed that bone marrow-derived mesenchymal stem cells (BMSCs) are capable of differentiating into neural cell lineages and have been shown to express neuronal and glial markers upon differentiation [26, 40, 53]. BMSCs promote synaptogenesis, stimulate nerve regeneration , and improve motor function in animal models of ischemia [29, 40]. Furthermore, it is hypothesized that the more important mechanisms underlying the functional recovery observed is due to the effect of transplanted cells on neuroprotection, stimulation of regeneration, expression of cytokines and/or growth factors, and angiogenesis, rather than direct integration of the transplanted cells into damaged host networks [40].
15.2.2 Neural Stem Cells
Neural stem cells (NSCs) persist in the adult brain in the subgranular zone of the dentate gyrus of the hippocampus and subventricular zone of the third ventricle. They are multipotent cells that give rise to neurons and glial cells. In rodent models of hypoxia, NSCs are able to establish functional connections with innate neurons, develop into mature neurons and glial cells, and demonstrate some functional recovery in the animals observed [30, 52]. Furthermore, neuroimaging studies in rats have shown reduction of infarct volume which corresponds to improvements in behavioral testing [49]. These effects have been shown to occur through several mechanisms, including neuronal replacement, modulation of synaptic plasticity, enhanced neuroprotection, changes in inflammatory mediated processes, and stimulation of angiogenesis.
Although NSCs have shown promise for stroke therapy , their use has significant limitations in human therapies. Human stem cells are needed for implantation into stroke patients, and the main source of these cells is from fetal tissue. In addition to a limited availability of cells, this approach raises important and difficult ethical issues, which has impacted the ability to translate this research to human application. Studies have shown successful in vitro propagation of human fetal NSCs for an extended period of time with successful implantation and differentiation into the ischemic cortex of rats [18], requiring fewer human cells; regardless, much debate still surrounds the use of fetal tissue for medical therapies.
Interestingly, some of the functional recovery observed in post-stroke patients may be, in part, due to stimulation of innate adult NSCs after ischemic stroke. A few studies in rodents, primates, and humans suggest generation of new neurons from persistent host NSCs [4, 32, 54, 55] and hypothesize that they may play an important role in the post-ischemic brain. The functional significance of this neurogenesis remains unclear; however, these findings raise new possibilities for development of stem cell stimulation therapies and even potential transplantation of adult NSCs in stroke patients.
15.2.3 Embryonic Stem Cells
Embryonic stem cells are pluripotent cells derived from blastocysts 4–5 days after fertilization. These cells are valuable in that they are capable of unlimited and undifferentiated proliferation [51]. They readily differentiate into neuronal and glial elements, including astrocytes and oligodendrocytes. In vivo studies have shown that implanted rodent ESCs survive, migrate into ischemic tissue and can restore synaptic connections with improvement in behavioral deficits [11, 50, 58].
Similar to NSCs, however, production and use of ESCs raise challenging issues. Isolation of human ESCs requires destruction of a blastocyst and therefore again raises ethical issues as discussed above; however, cells can be captured from unused fertilized embryos from in vitro fertilization procedures and maintained in culture for use, potentially lessening the controversy surrounding their origination. However, as a result of their robust ability to propagate and transform, this risk of tumor formation after implantation is high [42]. One method developed to decrease this risk is the pre-differentiation of ESCs into NPCs that are restricted to neural cell lines upon differentiation, which has some efficacy once implanted into rodent models [17].
15.2.4 Induced Pluripotent Stem Cells
An important and recent advancement in this field has been the development of human-induced pluripotent stem cells (iPSCs) . This technology allows pluripotent cells to be created by reprogramming a host patient’s own somatic cells, which are easily obtained from a blood sample or connective tissue biopsy. The use of a patient’s own tissue sample for this therapy alleviates much of the ethical concerns surrounding the previously discussed stem cell-based treatments. Using specific transcriptional factors, cells can be reprogrammed [48] and induced to form specific cell types, including induced pluripotent stem cell-derived neural stem cells (iNSCs) [28]. As a distinct advantage, these cells exhibit the properties of ESCs and NSC and are less likely to undergo immune system rejection [3].
Given their capacity for proliferation and differentiation , tumorigenicity is a significant concern with iPSCs. To address this issue, researchers have developed induced pluripotent stem cell-derived neural stem cells (iNSCs), which retain their ability to differentiate into neural cells, with significantly decreased tumorigenicity compared to iPSCs. These iNSCs are showing promise in neuroprotection and regeneration after stroke [12]. A recent paper using an ischemic pig stroke model demonstrated reduced white matter, cerebral perfusion, and metabolism changes on magnetic resonance imaging (MRI) in animals implanted with iNSC implantation after induced stroke. The implanted cells differentiated into neurons, astrocytes, and oligodendrocytes, demonstrated long-term integration, promoted decreased microglial activation, and stimulated neurogenesis [7, 36]. Other studies using rodent models demonstrate similar findings [39, 41, 56].
15.3 Stem Cell Therapy Mechanisms of Action
The mechanism by which transplanted stem cells exert therapeutic effects is an area of active research. Initially, the hypothesis was that the engrafted cells would replace the lost cells in the ischemic core of the infarcted brain tissue and restore function. However, as research in this area continues to shed light on the interactions of these stem cells and the damaged brain, it is becoming clear that the interactions are much more complicated than initially imagined.
Neuronal replacement remains one of the main focuses of stem cell-based therapies, and many studies demonstrate synaptic connections between host and implanted cells, as well as functional integration of grafted neurons. The establishment of axial projections and synaptic connections in unaffected animal models has been demonstrated in multiple studies. Steinbeck et al. recently demonstrated that after implantation of ESCs into the motor cortex of the normal adult rodent brain, axons of donor neurons extended via the external and internal capsule to the cervical spinal cord and through the corpus callosum into the contralateral cortex, where they made functional synaptic connections with host neurons [47]. A recent study in a rodent stroke model using iPSCs showed motor improvement after transplantation of cells into stroke-damaged cortex. The cells differentiated into mature neurons, sent axonal projections to unaffected brain tissue, and exhibited appropriate electrophysiological and synaptic input signals from host neurons [39].
Interestingly, Oki et al. argue that the initial motor improvements observed in their animal model post-transplantation was likely due to increased vascular endothelial growth factor (VEGF) levels and resulting improvement in endogenous plasticity rather than neuronal replacement. Further studies corroborate VEGF as an important growth factor, stimulating neovascularization, enhanced integrity of the blood-brain barrier, axonal plasticity, and suppression of the inflammatory response, glial scar formation, and neuronal apoptosis [2, 5, 16, 27].
Preclinical studies of NSC implantation support the immunomodulatory effects of stem cells as a significant mechanism contributing to the beneficial effects seen with this therapy. In the ischemic brain, activation of microglia, the resident immune cells of the brain, causes a robust inflammatory response. Minutes after the onset of ischemia, many pro-inflammatory cytokines are produced. These cytokines also induce opening of the blood-brain barrier and infiltration of peripheral macrophages, further exacerbating the immune response and resulting injury [14]. NSCs dampen this inflammatory response by the release of neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and glial-derived neurotrophic factor [40]. Minimizing the immune response in the post-stroke brain has been shown to correlate with decreased infarct volume and improved functional recovery [14, 40].
Although most evidence for stem cell-mediated effects come from studies of direct implantation of cells into the post-infarcted brain, a few studies have found NSC culture-conditioned media alone may provide a neuroprotective effect and resulting behavioral improvements in animals [19, 57, 61]. Webb et al. demonstrated extracellular vesicles from NSC conditioned media were sufficient to alter the immune response, reduce lesion size, and improve motor outcomes in mice [57]. The advantage of NSC extracellular vesicle-based therapy is largely due to their limited tumorigenicity and enhanced biodistribution. However, further data are needed to determine if this type of acellular therapy will be effective in stroke patients.
15.4 Stem Cell Therapy in Experimental Stroke Models
Preclinical research on cell therapy for stroke began in the 1980s, when Mampalam et al. demonstrated the ability to graft cells from a fetal rat cortex to be successfully implanted into the ischemic cortex of an adult rat [37]. The study not only showed survival of the fetal cells but also demonstrated integration of these cells into the damaged host brain. As animal studies advanced, further evidence suggested implanted cells survived and integrated into ischemic brain tissue and, in some cases, stimulated anatomical reconstruction and behavioral recovery in the post-stroke brain.
Early on in this field, given their capacity to differentiate into a variety of neural cell types, the use of NCS and ESC quickly gained momentum. However, advancement to clinical application slowed after the recognition that allogenic transplantation (i.e., implanting stem cells from the same species) is safer and likely more effective . Difficulties in obtaining human-derived (fetal and embryonic) cells, confounded by the ethical challenges surrounding their harvesting, significantly slowed translation of preclinical research to patient trials. The late 1990s ban placed on the use of federal funds for research on embryonic tissue further discouraged the translation of preclinical advancements to clinical studies using human-derived embryonic and neural stem cells.
Given these constraints, adult stem cells became the focus of most studies, and specifically bone marrow-derived MSCs emerged as the commonly used adult source of these cells. BMSCs promote synaptogenesis, stimulate nerve regeneration, and improve motor function in animal models of ischemia [29, 40]. The use of MSCs has been widely studied in stroke, and although these studies vary in the source (human, rodent), route of administration (intracerebral, intraarterial, intrathecal), and timing of introduction (in relationship to the stroke onset), most of the published data show some positive effect on infarct volume or behavioral testing, or at the molecular level with changes associated with positive neurological benefits [62].
The most recent and significant advancement in the stem cell field has been the development of iPSC technology. Although in its infancy, there is significant focus on using iPSC as well as neuronal stem cells derived from iPSCs (iNSC) for stroke therapies. This technology appears to be a promising alternative that provides cells with the differentiation capability of NCS/ESC, with fewer ethical issues and minimal difficulty with harvesting. Recent studies have found implantation of iPSCs/iNSCs into infarcted tissue leads to reduction in infarct volume and improvement in functional recovery in animal models. Some studies attribute these effects to these cells’ ability to differentiate into adult stem cells after implantation [31, 56]. Human iPSCs implanted into ischemic cortex of rodents also show differentiation to neurons of different subtypes and exhibit electrophysiological properties of mature neurons [39]. Studies in a pig ischemic stroke model using iPSCs and iNSC have demonstrated decreased immune response, enhanced neuroprotection, increased neurogenesis, and functional recovery in treated animals [6, 7, 36].
15.4.1 Preclinical Models
Most preclinical studies on stroke pathology and treatment have relied on small animal models, specifically using rodents, given the ease of use and cost-effectiveness of these models. Although much of the field has advanced using these small animals, there has been difficulty translating novel therapeutics to the development of beneficial clinical treatments. Some propose this translational gap can be better addressed by a greater emphasis on the use of large animal models, specifically pigs, and sheep, and nonhuman primates. These animals have large gyrencephalic brains, which are more similar to the structure of human brains compared to the small lissencephalic brains of rodents. The lack of gyri and sulci in the brains of mice and rats, and therefore more simplified cortical structure and functional organization, as well as vascular anatomy, likely confounds the response of these animals to ischemic stroke and their response to the studied therapeutics. Larger animal models with more human-like brain structures are now being used more readily and often are becoming a key step of verification prior to introduction of novel therapies into humans [46]. Used in combination with initial studies in rodents, a greater use of large animal models will likely contribute to advancing therapeutic interventions and better predicting which therapies will likely have a clinical impact prior to testing in humans.
15.5 Clinical Studies on Stem Cell Implantation
Although hundreds of preclinical studies have been published over the past few decades showing positive results for stem cell-based therapies in animal models, many unique challenges exist in the translation of this therapy into human studies. In addition to the high cost and extended timelines for such studies, the harvesting and/or production of stem cells remains difficult and limited. Furthermore, preclinical studies have not successfully answered important questions including ideal route of administration and most effective source of stem cells to be used; therefore, these questions are being addressed by the clinical trials, in addition to safety and effectiveness. Importantly, although initial studies support the safety profile of this therapy, the potential adverse outcomes, specifically tumor formation and/or immune rejection, are significant and must be addressed [34].
Most clinical studies involve MSCs due to their well-studied and beneficial effects in in vitro and in vivo models. Initial trials using bone marrow MSCs (BMSCs) have also proven safe for human use. The first pilot study to introduce MSCs in stroke patients was in 2005 by Bang et al., and although a small study, they found improved functional recovery in the treated group [8]. The InVeST trial, which did not show a beneficial treatment effect, provided valuable evidence that intravenous infusion of BMSCs is safe and well tolerated in humans [43]. Similarly, the MASTERS trial, a phase 2, randomized, double-blinded, placebo-controlled study, showed no dose-limiting toxicity but also showed no significant improvement after 90 days [24]. The first clinical trial to investigate the intracranial implantation of neural stem cells, called PISCES, also supported the safety profile of stem cell transplants in patients and, furthermore, showed some neurological improvement in treated patients [33]. More recent studies being published continue to show safety and improvement in neurological function in patients [38].
A recent systematic review and meta-analysis of the literature regarding stem cell transplantation in patients after brain ischemia determined the therapy significantly improved neurological deficits and quality of life without serious adverse events [13]. However, although initial data are encouraging, larger studies are still needed to further investigate the safety and effectiveness of this treatment before it becomes widely used in the clinical setting.
15.6 Endogenous Stem Cell Therapy
Although most studies looking at stem cells in stroke are focused on implanting cells into the post-stroke brain, a few groups are exploring options to take advantage of the capacity of the adult brain for self-repair. Neurogenesis in the adult brain is located mainly in the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus, as well as the olfactory bulb [20]. Pathological processes, such as ischemia, that cause neuronal death have been found to stimulate new neuronal formation in these areas. Arvidsson et al. found that these newly developed endogenous neurons from the SVZ are able to migrate into damaged striatum in rodent models. However, they also noted that the majority of the new neurons died within 2 weeks after stroke, likely indicating an unfavorable environment to support these new cells in the post-ischemic brain [4] . In two studies, Tonchev et al. demonstrated increased proliferation of neuronal progenitor cells in the dentate gyrus, subventricular zone, and hippocampus in post-ischemic monkey brains [54, 55]. These results were translated to the human brain by Jin et al., when they demonstrated similar findings in human brain biopsies of ischemic strokes [32]. Immunohistochemical staining of these specimens showed new neurons with a migratory phenotype in the ischemic penumbra. They also demonstrated that in the stroke brain tissue, new neurons tended to cluster near blood vessels, suggesting vascular endothelial cells promote neurogenesis [32]. However, it appears from these studies that the number of newly generated neurons is low and does not represent a large enough population of cells to induce a significant therapeutic response. Factors that stimulate production as well as induce a supportive and protective brain environment for growth and survival of these cells are an active area of research. Granulocyte-colony stimulating factor (G-CSF) is one of these mechanisms and has been shown to reduce infarct volume in experimental models of ischemia, act as a neuroprotective mechanism by reducing glutamate release, inflammation, and apoptosis activation, among others. Administration of G-CSF immediately after middle cerebral artery occlusion in a rodent model immediately after and in the subacute period both showed increase in proliferation of endogenous stem cells in the post-stroke brain [1]. Studies are also investigating the effect of transplanted NSCs on endogenous neurogenic behavior, with some evidence suggesting enhancement of neurogenesis through secretion of neurotrophic and regenerative growth factors [6].
15.7 Important Considerations for Stem Cell-Based Therapies
Although much of the data from completed and ongoing clinical trials appear promising, most of the trials remain small, and many questions remain unanswered. Specifically, the most effective stem cell type, ideal route of administration, and timing of administration are active areas of research. Furthermore, important considerations with this therapy also include consideration of potential adverse events, including malignant potential and immunogenicity, as well as beneficial adjuvant treatments that may enhance the therapeutic effect of this therapy.
15.7.1 Selection of Stem Cell Type
Of the main types of stem cells investigated for use in stroke therapy, MSCs have been the most widely studied, in both preclinical and clinical trials. Given the ethical and sourcing issues of ESCs and NSCs, research using these cell types has lagged behind MSCs, and with the introduction of iPSCs, much of this research is being replaced given the advantages of this new technology. iPSCs have shown promise and ease of use, especially with the ability to differentiate them into iNSCs prior to transplantation. As the field advances and new technologies are introduced, a standardized type and readily available source will likely be developed for use in clinical therapies.
15.7.2 Route of Administration
Different routes of stem cell administration have been used, with the most common being intravascular (venous and arterial) and intraparenchymal routes [34]. Less common routes investigated include intracerebroventricular, intracisternal, intrathecal, and intraperitoneal routes [59]. The intravascular route has been mostly used for administration of MSCs. It is the least invasive and allows the introduction of a large number of cells. However, the delivery is non-specific, and although cells are able to migrate into ischemic regions, the number that were deposited in the brain was significantly less than the number of injected cells, and cells have been found to distribute into multiple other peripheral organs [15, 35]. Newer technologies are addressing this issue using mechanisms such as magnets and fibrin glue to target cells to the ischemic brain regions [14, 45]. Song et al. (2015) demonstrated that intravenously delivered, magnetically targeted NSCs accumulated in the ischemic brain and correlated to decreased infarct size compared to non-targeted NSCs, suggesting that targeting cells into the damaged tissue may be advantageous.
Intraparenchymal transplantation is more invasive and requires injecting a cell suspension directly into the brain tissue. However, this route allows precise control over cell placement and avoids the issue of cells distributing into peripheral organs. Some studies suggest better functional improvement with this technique [25]. Further consideration must also be given to the timing of cell therapy in determining the most effective route of administration. In subacute and chronic strokes, the blood-brain barrier is less permeable than in acute stroke [22], which would likely render intravenous therapies less effective. Although different routes have been investigated and compared, no optimal route of administration has yet been determined [44, 59], and there is little evidence for a specific route having a positive effect on patient outcome.
15.7.3 Malignant Potential
One of the most important considerations regarding the use of stem cells for any therapy is the potential for the cells to undergo malignant transformation and allow tumor formation in the host. Overall MSCs appear to be safe upon implantation into animal models and humans; however, several studies have shown the potential for tumorigenic transformation of iPSCs. Teratomas were found to develop in mice brains after implantation, and it is suggested that specific transcription factors contribute to the tumorigenic potential of iPSCs. The presence of undifferentiated cells may trigger tumor formation [21, 38, 60]. Given the ability for iPSCs to be transformed further into multipotent cells, researchers have found that differentiating them into neural stem cells (iNSCs) decreases the tumorigenicity significantly while maintaining their therapeutic potential [12].
15.7.4 Adjuvant Therapies
Biomaterials are being investigated for enhancing delivery of cells and improving post-implantation survival. This technology provides a scaffold for transplanted cells, as well as growth factors and other biochemical signals that could stimulate tissue restoration and neuronal differentiation. Although preclinical studies suggest improved survival and differentiation of implanted cells, it remains unclear if there is any benefit in functional outcome using this technology, and further studies are need to understand what adverse effects may be caused by introduction of these biomaterials in humans [9].
15.7.5 Neurorehabilitation
Neurorehabilitation is an important component of post-stroke therapy and can significantly improve the neurological function of patients. This is important to consider when designing clinical trials. Many initial studies, especially preclinical, did not take into account the potential benefits of patient improvement with rehabilitation. However, more attention is being drawn to this issue, and it is being encouraged in the literature and at the recent Stem Cell Therapeutics as an Emerging Paradigm for Stroke (STEP3) meeting to include rehabilitation therapy as part of clinical trials using stem cell therapy [10].
15.8 Conclusion
Much of the data from preclinical and clinical trials appears promising for stem cell therapy in stroke patients. However, it is important to acknowledge that most trials are, in general, small cohorts of patients, and therefore results should be interpreted conservatively. Larger and more conclusive studies are needed to show clear patient benefit before stem cell-based therapies become widely used clinically. Furthermore, as a recently developed technology, the transition from preclinical to clinical trials for iPSCs has not occurred. Many questions remain regarding their potential benefit for stroke treatment in a clinical setting. Stem cell therapy is a promising technology that continues to advance and will likely offer new treatment paradigms in the future.
Bibliography
Abe K, Yamashita T, Takizawa S, Kuroda S, Kinouchi H, Kawahara N. Stem cell therapy for cerebral ischemia: from basic science to clinical applications. J Cereb Blood Flow Metab. 2012;32(7):1317–31.
Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, Manley NC, Pereira MP, Sheikh LA, McMillan EL, Schaar BT, Svendsen CN, Bliss TM, Steinberg GK. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain J Neurol. 2011;134(Pt 6):1777–89.
Araki R, Uda M, Hoki Y, Sunayama M, Nakamura M, Ando S, Sugiura M, Ideno H, Shimada A, Nifuji A, Abe M. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494(7435):100–4.
Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–70.
Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, Kilic E, Kilic U, Salani G, Brambilla E, West MJ, Comi G, Martino G, Hermann DM. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain J Neurol. 2009;132(Pt 8):2239–51.
Baker EW, Kinder HA, West FD. Neural stem cell therapy for stroke: a multimechanistic approach to restoring neurological function. Brain Behav. 2019;9(3):e01214.
Baker EW, Platt SR, Lau VW, Grace HE, Holmes SP, Wang L, Duberstein KJ, Howerth EW, Kinder HA, Stice SL, Hess DC, Mao H, West FD. Induced pluripotent stem cell-derived neural stem cell therapy enhances recovery in an ischemic stroke Pig Model. Sci Rep. 2017;7(1):10075.
Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57(6):874–82.
Boltze J, Modo MM, Mays RW, Taguchi A, Jolkkonen J, Savitz SI, STEPS 4 Consortium. Stem cells as an emerging paradigm in stroke 4: advancing and accelerating preclinical research. Stroke. 2019;50(11):3299–306.
Borlongan CV, Jolkkonen J, Detante O. The future of stem cell therapy for stroke rehabilitation. Future Neurol. 2015;10:313.
Bühnemann C, Scholz A, Bernreuther C, Malik CY, Braun H, Schachner M, Reymann KG, Dihné M. Neuronal differentiation of transplanted embryonic stem cell-derived precursors in stroke lesions of adult rats. Brain J Neurol. 2006;129(Pt 12):3238–48.
Chang D-J, Lee N, Park I-H, Choi C, Jeon I, Kwon J, Oh S-H, Shin DA, Do JT, Lee DR, Lee H, Moon H, Hong KS, Daley GQ, Song J. Therapeutic potential of human induced pluripotent stem cells in experimental stroke. Cell Transplant. 2013;22(8):1427–40.
Chen L, Zhang G, Khan AA, Guo X, Gu Y. Clinical efficacy and meta-analysis of stem cell therapies for patients with brain ischemia. Stem Cells Int. 2016;2016:1–8.
Chen S-J, Chang C-M, Tsai S-K, Chang Y-L, Chou S-J, Huang S-S, Tai L-K, Chen Y-C, Ku H-H, Li H-Y, Chiou S-H. Functional improvement of focal cerebral ischemia injury by subdural transplantation of induced pluripotent stem cells with fibrin glue. Stem Cells Dev. 2010;19(11):1757–67.
Chu K, Kim M, Chae S-H, Jeong S-W, Kang K-S, Jung K-H, Kim J, Kim Y-J, Kang L, Kim SU, Yoon B-W. Distribution and in situ proliferation patterns of intravenously injected immortalized human neural stem-like cells in rats with focal cerebral ischemia. Neurosci Res. 2004;50(4):459–65.
Daadi MM, Davis AS, Arac A, Li Z, Maag A-L, Bhatnagar R, Jiang K, Sun G, Wu JC, Steinberg GK. Human neural stem cell grafts modify microglial response and enhance axonal sprouting in neonatal hypoxic-ischemic brain injury. Stroke. 2010;41(3):516–23.
Daadi MM, Maag A-L, Steinberg GK. Adherent self-renewable human embryonic stem cell-derived neural stem cell line: functional engraftment in experimental stroke model. PLoS One. 2008;3(2):e1644.
Darsalia V, Kallur T, Kokaia Z. Survival, migration and neuronal differentiation of human fetal striatal and cortical neural stem cells grafted in stroke-damaged rat striatum. Eur J Neurosci. 2007;26(3):605–14.
Delaloy C, Liu L, Lee J-A, Su H, Shen F, Yang G-Y, Young WL, Ivey KN, Gao F-B. MicroRNA-9 coordinates proliferation and migration of human embryonic stem cell-derived neural progenitors. Cell Stem Cell. 2010;6(4):323–35.
Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–7.
Fu W, Wang SJ, Zhou GD, Liu W, Cao Y, Zhang WJ. Residual undifferentiated cells during differentiation of induced pluripotent stem cells in vitro and in vivo. Stem Cells Dev. 2012;21(4):521–9.
Garbuzova-Davis S, Haller E, Williams SN, Haim ED, Tajiri N, Hernandez-Ontiveros DG, Frisina-Deyo A, Boffeli SM, Sanberg PR, Borlongan CV. Compromised blood-brain barrier competence in remote brain areas in ischemic stroke rats at chronic stage. J Comp Neurol. 2014;522:3120.
GBD 2016 Stroke Collaborators 2019. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study. Lancet Neurol. 2016;18(5):439–58.
Hess DC, Wechsler LR, Clark WM, Savitz SI, Ford GA, Chiu D, Yavagal DR, Uchino K, Liebeskind DS, Auchus AP, Sen S, Sila CA, Vest JD, Mays RW. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16(5):360–8.
Hicks AU, Lappalainen RS, Narkilahti S, Suuronen R, Corbett D, Sivenius J, Hovatta O, Jolkkonen J. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: cell survival and functional recovery. Eur J Neurosci. 2009;29(3):562–74.
Honmou O, Onodera R, Sasaki M, Waxman SG, Kocsis JD. Mesenchymal stem cells: therapeutic outlook for stroke. Trends Mol Med. 2012;18(5):292–7.
Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, Shamloo M, Hamilton SA, Jiang K, Huhn S, Palmer TD, Bliss TM, Steinberg GK. Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells. 2011;29(2):274–85.
Hu B-Y, Weick JP, Yu J, Ma L-X, Zhang X-Q, Thomson JA, Zhang S-C. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A. 2010;107(9):4335–40.
Huang W, Mo X, Qin C, Zheng J, Liang Z, Zhang C. Transplantation of differentiated bone marrow stromal cells promotes motor functional recovery in rats with stroke. Neurol Res. 2013;35(3):320–8.
Ishibashi S, Sakaguchi M, Kuroiwa T, Yamasaki M, Kanemura Y, Shizuko I, Shimazaki T, Onodera M, Okano H, Mizusawa H. Human neural stem/progenitor cells, expanded in long-term neurosphere culture, promote functional recovery after focal ischemia in Mongolian gerbils. J Neurosci Res. 2004;78(2):215–23.
Jiang M, Lv L, Ji H, Yang X, Zhu W, Cai L, Gu X, Chai C, Huang S, Sun J, Dong Q. Induction of pluripotent stem cells transplantation therapy for ischemic stroke. Mol Cell Biochem. 2011;354(1–2):67–75.
Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103(35):13198–202.
Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, McConnachie A, Santosh C, Bath PM, Dunn L, Muir KW. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet. 2016;388(10046):787–96.
Krause M, Phan TG, Ma H, Sobey CG, Lim R. Cell-based therapies for stroke: are we there yet? Front Neurol. 2019;10:656.
Lappalainen RS, Narkilahti S, Huhtala T, Liimatainen T, Suuronen T, Närvänen A, Suuronen R, Hovatta O, Jolkkonen J. The SPECT imaging shows the accumulation of neural progenitor cells into internal organs after systemic administration in middle cerebral artery occlusion rats. Neurosci Lett. 2008;440(3):246–50.
Lau VW, Platt SR, Grace HE, Baker EW, West FD. Human iNPC therapy leads to improvement in functional neurologic outcomes in a pig ischemic stroke model. Brain Behav. 2018;8(5):e00972.
Mampalam TJ, Gonzalez MF, Weinstein P, Sharp FR. Neuronal changes in fetal cortex transplanted to ischemic adult rat cortex. J Neurosurg. 1988;69(6):904–12.
Marei HE, Hasan A, Rizzi R, Althani A, Afifi N, Cenciarelli C, Caceci T, Shuaib A. Potential of stem cell-based therapy for ischemic stroke. Front Neurol. 2018;9:34.
Oki K, Tatarishvili J, Wood J, Koch P, Wattananit S, Mine Y, Monni E, Tornero D, Ahlenius H, Ladewig J, Brüstle O, Lindvall O, Kokaia Z. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells. 2012;30(6):1120–33.
Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40(7):609–19.
Polentes J, Jendelova P, Cailleret M, Braun H, Romanyuk N, Tropel P, Brenot M, Itier V, Seminatore C, Baldauf K, Turnovcova K, Jirak D, Teletin M, Côme J, Tournois J, Reymann K, Sykova E, Viville S, Onteniente B. Human induced pluripotent stem cells improve stroke outcome and reduce secondary degeneration in the recipient brain. Cell Transplant. 2012;21(12):2587–602.
Pomper MG, Hammond H, Yu X, Ye Z, Foss CA, Lin DD, Fox JJ, Cheng L. Serial imaging of human embryonic stem-cell engraftment and teratoma formation in live mouse models. Cell Res. 2009;19(3):370–9.
Prasad K, Sharma A, Garg A, Mohanty S, Bhatnagar S, Johri S, Singh KK, Nair V, Sarkar RS, Gorthi SP, Hassan KM, Prabhakar S, Marwaha N, Khandelwal N, Misra UK, Kalita J, Nityanand S, InveST Study Group. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: a multicentric, randomized trial. Stroke. 2014;45(12):3618–24.
Rodríguez-Frutos B, Otero-Ortega L, Gutiérrez-Fernández M, Fuentes B, Ramos-Cejudo J, Díez-Tejedor E. Stem cell therapy and administration routes after stroke. Transl Stroke Res. 2016;7(5):378–87.
Song M, Kim Y-J, Kim Y, Roh J, Kim SU, Yoon B-W. Using a neodymium magnet to target delivery of ferumoxide-labeled human neural stem cells in a rat model of focal cerebral ischemia. Hum Gene Ther. 2010;21(5):603–10.
Sorby-Adams AJ, Vink R, Turner RJ. Large animal models of stroke and traumatic brain injury as translational tools. Am J Physiol Regul Integr Comp Physiol. 2018;315(2):R165–90.
Steinbeck JA, Koch P, Derouiche A, Brüstle O. Human embryonic stem cell-derived neurons establish region-specific, long-range projections in the adult brain. Cell Mol Life Sci. 2012;69(3):461–70.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Takahashi K, Yasuhara T, Shingo T, Muraoka K, Kameda M, Takeuchi A, Yano A, Kurozumi K, Agari T, Miyoshi Y, Kinugasa K, Date I. Embryonic neural stem cells transplanted in middle cerebral artery occlusion model of rats demonstrated potent therapeutic effects, compared to adult neural stem cells. Brain Res. 2008;1234:172–82.
Theus MH, Wei L, Cui L, Francis K, Hu X, Keogh C, Yu SP. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210(2):656–70.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7.
Toda H, Takahashi J, Iwakami N, Kimura T, Hoki S, Mozumi-Kitamura K, Ono S, Hashimoto N. Grafting neural stem cells improved the impaired spatial recognition in ischemic rats. Neurosci Lett. 2001;316(1):9–12.
Tohill M, Mantovani C, Wiberg M, Terenghi G. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci Lett. 2004;362(3):200–3.
Tonchev AB, Yamashima T, Zhao L, Okano H. Differential proliferative response in the postischemic hippocampus, temporal cortex, and olfactory bulb of young adult macaque monkeys. Glia. 2003a;42(3):209–24.
Tonchev AB, Yamashima T, Zhao L, Okano HJ, Okano H. Proliferation of neural and neuronal progenitors after global brain ischemia in young adult macaque monkeys. Mol Cell Neurosci. 2003b;23(2):292–301.
Tornero D, Wattananit S, Grønning Madsen M, Koch P, Wood J, Tatarishvili J, Mine Y, Ge R, Monni E, Devaraju K, Hevner RF, Brüstle O, Lindvall O, Kokaia Z. Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain J Neurol. 2013;136(Pt 12):3561–77.
Webb RL, Kaiser EE, Scoville SL, Thompson TA, Fatima S, Pandya C, Sriram K, Swetenburg RL, Vaibhav K, Arbab AS, Baban B, Dhandapani KM, Hess DC, Hoda MN, Stice SL. Human neural stem cell extracellular vesicles improve tissue and functional recovery in the murine thromboembolic stroke model. Transl Stroke Res. 2018;9(5):530–9.
Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110(3):385–97.
Willing AE, Shahaduzzaman M. Delivery routes for cell therapy in stroke. In: Jolkkonen J, Walczak P, editors. Cell-based therapies in stroke. Vienna: Springer Vienna; 2013. p. 15–28.
Yamashita T, Kawai H, Tian F, Ohta Y, Abe K. Tumorigenic development of induced pluripotent stem cells in ischemic mouse brain. Cell Transplant. 2011;20(6):883–91.
Yang H, Wang C, Chen H, Li L, Ma S, Wang H, Fu Y, Qu T. Neural stem cell-conditioned medium ameliorated cerebral ischemia-reperfusion injury in rats. Stem Cells Int. 2018;2018:4659159.
Zheng H, Zhang B, Chhatbar PY, Dong Y, Alawieh A, Lowe F, Hu X, Feng W. Mesenchymal stem cell therapy in stroke: a systematic review of literature in pre-clinical and clinical research. Cell Transplant. 2018;27(12):1723–30.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Robert, S.M., Matouk, C. (2021). Stem Cell Therapy for Stroke. In: Navarro, T.P., Minchillo Lopes, L.L.N., Dardik, A. (eds) Stem Cell Therapy for Vascular Diseases. Springer, Cham. https://doi.org/10.1007/978-3-030-56954-9_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-56954-9_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-56953-2
Online ISBN: 978-3-030-56954-9
eBook Packages: MedicineMedicine (R0)