Abstract
At the 1971 meeting North American Society for Bat Research (NASBR) in Albuquerque, Don Wilson and James S. Findley presented “Randomness in Bat Homing.” The central tenet of their paper was that homing ability in bats could be explained by chance alone or by some sort of random search [Wilson and Findley (Am Nat 106:418–424, 1972)]. In this retrospective, we assess the knowledge gained from, but also the limitations of, older studies on bat homing and review the advances in our understanding of homing and navigation in bats. Although we have learned much over the last half-century about the orientation and navigational abilities of bats, particularly our understanding of cues and spatial orientation, we still do not know if bats are capable of true navigation nor how they learn to do so. Partly because of technological advances, the study of homing has expanded from bats’ ability to return to roosts after being displaced short distances to determining how bats navigate and find destinations during long-distance seasonal migrations. We advocate for expansion of the study of navigation to include inter-seasonal movements and tropical areas and highlight the need to apply new knowledge of movement and navigation to the conservation of bats.
Homing refers to an animal’s ability to return to a known goal (e.g. a nest, roost, or den) after being displaced. The ability to home to familiar sites is both a fascination for the general public and the subject of intense study by scientists aiming to understand the mechanisms that govern it. The study of homing in various taxa, such as birds, insects, sea turtles, and salmonids, made significant advances during the past half-century leading to amazing discoveries about the capabilities of animals to navigate and the cues they use to do so. Despite these findings, an increase in technologies available to study small animals, and an increased interest in the topic of movement ecology, understanding of homing in bats has lagged behind other taxa (Holland 2019). Here we review the study of homing in bats over the last 50 years and argue for its continued importance, not only to understand the specifics of how bats navigate across the landscape, but also for the importance of applying this knowledge to improve conservation outcomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Homing? What’s That?
Before moving forward, some definitions are needed:
-
1.
Home range is the area used by an individual for its normal activities, such as roosting and foraging. For bats in temperate areas, it has been applied conventionally to areas used during summer.
-
2.
Familiar area is the geographic region an animal uses over the course of a year. For non-migratory animals, familiar areas may be small, consisting of just their home ranges, but for migratory animals, familiar areas may be quite large. In temperate areas, the familiar area consists of summer and winter home ranges and the space used to move between them during spring and autumn migration (Leffler et al. 1979).
-
3.
Homing, in the simplest sense, is the ability of an animal to find its way home after being displaced. Displacement can occur within or outside an animal’s familiar area.
-
4.
Orientation is the ability of an animal to determine the differences between directions and select a specific direction to move toward. It is often referred to as a “compass.”
-
5.
Familiar area navigation is the ability to return to a specific location from an area where the animal has been before (i.e. from within a familiar area), presumably based on landmarks and/or spatial memory of recognizable features.
-
6.
True navigation is the ability to return to a location from an area where the animal has never been before (i.e. from an unfamiliar area) based only on cues detected at the site of displacement. True navigation requires both a “map” (the ability of an animal to determine its position in space relative to its goal) and a “compass” (orientation abilities).
2 Starting from Home
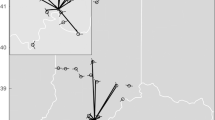
At the 1971 meeting of the North American Society for Bat Research (NASBR) in Albuquerque, Don Wilson and James S. Findley presented, and subsequently published, “Randomness in Bat Homing.” The central tenets of their paper were that “Investigations to date have been unable to demonstrate whether the mechanism of bat homing is: (1) some innate homing mechanism or navigational abilities such as many species must possess for migration; or (2) randomness, that is, returning to a familiar area by chance alone or by some sort of random search” (Wilson and Findley 1972: 418–419). They concluded that the most parsimonious explanation for homing ability in bats is randomness. If bats are taken away from their roosting area, some proportion will find their way back through chance alone, not through any natural navigational ability. If this is the case, returns will decrease with increasing displacement distances and these data could be used to calculate an expected return percentage (Fig. 11.1) and an expected return curve (Fig. 11.2). To test for homing ability, they compared their conceptual model to rates of return to roosts for bats displaced different distances for individuals of two neotropical species, the black myotis (Myotis nigricans) and greater spear-nosed bat (Phyllostomus hastatus).
The probability of a bat returning to familiar area (x) when released at R is Ø/360. From Wilson and Findley (1972)
Expected returns of bats displaced various multiples of familiar area radius. E = expected; M = Myotis nigrans; P = Phyllostomus hastatus. Numbers following M and P are actual distances (in kilometers) for various releases. From Wilson and Findley (1972)
Interest in homing in bats was at its peak in the early days of NASBR. Since then, we have accumulated additional evidence demonstrating the homing abilities of bats, such as fidelity of individual bats to their summer activity areas and specific roost sites (e.g. Lewis 1995). However, research into bats’ ability to return to familiar areas and how they do it has slowed in recent decades (Table 11.1). We contend this is due partly to changes in both terminology and technology. Fifty years ago, radio-telemetry was in its infancy. Williams and Williams (1967) used 7-g transmitters to study homing in greater spear-nosed bats. But most researchers of the time did not have access to radio transmitters or study species capable of carrying such large payloads. As a result, displacement experiments were used to understand the radius of animal’s home range that, by default, was assumed to be circular and centered on the roost. In the decades since the first NASBR, decreases in the size and cost of transmitters have resulted in more studies aimed at characterizing home range size and habitat composition. Concomitantly there has been a shift, at least in terminology, from homing studies to home-range studies. Emphasis has shifted to understanding sensory systems (e.g. vision) and extrinsic factors (e.g. magnetic fields) that guide orientation and navigation. As a result, we are not much farther ahead on understanding the extent of bats’ homing abilities than Wilson and Findley were in 1972. Why? Is it that navigation research flourished in other taxa (Mouritsen 2018) and bat researchers assumed that if all these other taxa exhibit true navigation, bats must be able to navigate as well?
We have learned a great deal about the complex orientation and navigational abilities of bats in the 50 years since Wilson and Findley (1972) posited that homing in bats occurs by random chance, but we still have not demonstrated the limits of homing ability in bats or if bats are capable of “true navigation.” Based on what we know from other taxa, particularly migratory birds, it would be remarkable if bats did not show similar abilities to correct for displacements outside their familiar area or to exhibit “true navigation” but, even using modern techniques, this has not been demonstrated conclusively. Nevertheless, given the divergent evolutionary pathways that may have led to migration and navigation in bats versus birds, it is important to continue to evaluate bats in a comparative context.
We contend that much of the early work to understand homing abilities in bats remains unfinished, limiting our ability to understand fully the movement capabilities of bats and the mechanisms used to complete these movements. Although Wilson and Findley (1972) concluded that displaced bats returned to their roost via random searching, it was less than a decade later when Leffler et al. (1979:201) stated, “Bats obviously can navigate successfully within their familiar area, perhaps by use of memorized landmarks.” Since then, there have been numerous examples of the sophisticated spatial memory bats possess, but there has been little work describing the details, cues, or mechanisms of how bats learn and remember specific sites or landmarks within their familiar areas. Further, we emphasize that the familiar areas of bats are not simply where they roost and forage during pup-rearing or hibernation seasons. Familiar areas include not only summering and wintering areas but critically important migration routes and mating grounds.
3 Limitations of Previous Homing Studies
With no other way to determine home range size of bats, the first researchers investigated homing by capturing bats at their roost, moving them some distance away, releasing them, and then determining whether they returned (Davis 1966). The rationale for these early studies was that the proportion of bats that returned during a displacement experiment was correlated with the homing ability, and thus home range size, of the species. With the benefit of hindsight and modern technologies, we can now appreciate the limitations of this approach for determining the homing ability of bats.
First, and likely foremost, in most cases bats were not displaced far enough outside their home ranges or familiar areas to establish homing distances reliably. They could have found their way back using landmarks and spatial memory or random searching, as Wilson and Findley (1972) suggested. In most cases, bats were displaced <50 km from their roost, but researchers of the time had no way to know nightly foraging distances or distances traveled during seasonal migration. For example, Leffler et al. (1979) displaced little brown bats (Myotis lucifugus) up to 161 km, but we now know this species can move up to 464 km from one summer roost to another (Norquay et al. 2013). Long-tailed bats (Chalinolobis tuberculatus) in New Zealand were displaced a maximum distance of 20 km (Guilbert et al. 2007) and homed to their roost within 3 days. However, long-tailed bats can travel over 35 km throughout a night (O’Donnell 2001). In general, home ranges and familiar areas of bats are much larger than initially thought, as we are discovering with new technology. For example, Egyptian fruit bats, while having a confined foraging area around a home cave, appear capable of recognizing large landmarks when transported over 100 km away, suggesting an overall larger familiar area compared to their foraging area (Tsoar et al. 2011).
Our ability to determine homing ability or true navigation is also confounded by how frequently and readily bats switch roosts. The concept of intra-seasonal roost switching was virtually non-existent 50 years ago, but it is now well known that many species of bats, particularly those roosting in trees and rock crevices, but also in buildings, switch roosts regularly (Lewis 1995). However, most displacement studies did not conduct intensive repeat surveys of the roost to determine if bats returned eventually. For example, Wason (1978) displaced individuals from four insectivorous species various distances in India, but only surveyed for returns up to 3 days post-displacement. More recent displacement studies that used automated detection of bats with radio-transmitters generally report higher rates of return (Guilbert et al. 2007; Holland et al. 2010). Hence, roost switching and limitations in the ability to detect returning animals may be alternative explanations for what was considered previously to be a failure to home.
4 Inter-seasonal Movements
Most experimental work with homing (e.g. displacement studies) has been conducted within the maternity season, and at least in studies involving temperate species, bats were not displaced far relative to their inter-seasonal movements. For example, we know that home ranges change in size depending on season and length of day and reproductive status (Frafjord 2013), possibly confounding homing studies done in a single season (i.e. maternity, migration, or hibernation). We contend that the definition of familiar areas must be expanded to include all areas in which an animal is active, and likely revisits annually, throughout its full annual cycle. Evidence of inter-seasonal movements has been derived largely from recoveries of bats that move between summer and winter roosts in temperate species (Hutterer et al. 2005). But new technologies are allowing insights into the familiar areas experienced by bats at other times of year. Miniature global positioning system (GPS) tags revealed that the autumn familiar area of a male hoary bat (Lasiurus cinereus) in northern California included two sites 70 km apart and that it used both in two separate years (TJW, unpublished data). Another hoary bat appeared to wander a minimum of 1000 km during a single month in autumn (Weller et al. 2016). However, the final trajectory of its movement and its ultimate recapture at the original capture site suggest the animal was navigating rather than wandering, perhaps with the aid of a large-scale spatial map. Regardless of how animals are finding their way between seasonal activity areas, the greater distances covered suggests that the cues used by bats during inter-seasonal movements differ from those used to re-locate roosts within a season.
5 Cues Used for Orientation and Navigation
To orient and navigate, animals integrate multiple sensory signals over varying scales, likely in a hierarchical fashion. Locating a roost within a home range may rely on vision, echolocation, passive listening, and/or olfaction. At the other end of the spatial spectrum, bats may rely on magnetic field maps and calibration of their compass via solar or celestial cues to make longer distance seasonal movements.
At the largest scale, the earth’s magnetic field is the most likely mechanism for orientation and navigation. The magnetic field represents a reliable source of directional and locational information that animals can use as a compass and/or as a map (Wiltschko and Wiltschko 2005). The magnetic vector (i.e. the direction the magnetic field is pointing) can provide directional information and be used to calibrate the compass mechanism. The levels of magnetic intensity and inclination, vary with physical location and can be used to create a map (Fig. 11.3; Wiltschko and Wiltschko 2005).
(a) The Earth’s magnetic field inclination angles are the angles formed between the field lines and the Earth. At the magnetic equator, field lines are parallel to the Earth’s surface. The field lines become progressively steeper as one travels north toward the magnetic pole, where the field lines are directed straight down into the Earth and the inclination angle is 90°. (b) An isodynamic chart of the Earth’s magnetic intensity, which tends to decrease from the poles to the equator. Maps from https://www.ngdc.noaa.gov/geomag/WMM/image.shtml
Studies of other taxa have demonstrated that information gathered from the earth’s magnetic field can be combined with additional cues to navigate. For example, both sea turtles and salmon used a combination of chemical and physical cues (e.g. wave and tidal patterns) to guide these animals to and from natal areas while close to shore, but used a magnetic map and compass to navigate at a larger scale in the open ocean (Lohmann and Lohmann 2019). The compass system consists of several interacting signals, but primacy among them appeared to vary depending on the system and study. Similarly, bats use a variety of cues to calibrate directionality relative to the magnetic field (Holland 2019). For example, the greater mouse-eared bat (Myotis myotis) used polarized light at sunset (Greif et al. 2014), and soprano pipistrelles (Pipistrellus pygmaeus) used the position of the solar disk (Lindecke et al. 2019). One experiment, using pulse re-magnetization (Holland et al. 2008), suggested that bats detect the magnetic field using magnetite in magnetoreceptor cells. In birds, these magnetite-containing magnetoreceptor cells have been implicated in a magnetic map for navigation in unfamiliar areas (Holland and Helm 2013; Munro et al. 1997;). However, Holland et al. (2008) could not distinguish between an effect on a map or a compass or determine where the magnetoreceptor cells were located in bats. The magnetic compass of bats also appears to be polarity based (Wang et al. 2007), unlike the magnetic compass of birds that is based on the angle of magnetic inclination, highlighting differences between these divergent groups.
Although bats perceive and use numerous signals for orientation and navigation at varying scales, research on the sensory systems of bats has focused predominantly on echolocation while detailed information on the use of non-auditory cues and other perceptual abilities of bats is lacking (Holland 2019). For example, olfactory cues are important for navigation in birds (Gagliardo 2013), but this sensory modality remains underexplored in bats, particularly in non-frugivorous species. Although many insectivorous bats are highly specialized for echolocation, it seems unlikely that they are using echolocation to navigate from outside their familiar area (Griffin 1970).
There are many reasons to dismiss the use of echolocation for large-scale navigation. Low-frequency sounds travel longer distances than sounds with high frequencies because they are attenuated less by the atmosphere and therefore should be more useful for long-distance navigation. However, the frequencies used by most echolocating species (>10 kHz) have operating ranges of only tens of meters (Griffin 1970; Kick 1982). Besides, echolocation relies on the return of echoes, and if a bat is flying high and away from objects, the animal would not receive echoes in return. Even if bats were using echolocation to navigate, it would need to be used in conjunction with spatial memory and context (i.e. a bat could receive an echo from a tree, but the animal would need to know the specific tree and the position of that tree relative to others) to use it as a navigational aid.
Although we have known for over 50 years that vision is important for homing in bats (e.g. Williams et al. 1966), we are just beginning to understand the specifics of how they use it. Bats are capable of perceiving stars (Childs and Buchler 1981), exploiting post-sunset glow (Buchler and Childs 1982; Holland et al. 2010), and using geographical landmarks and linear structures (Furmankiewicz and Kucharska 2009; Williams et al. 1966) for orientation and navigation. Visual cues apparently take precedence over audio cues and/or echolocation when light is sufficient (Eklöf et al. 2002; Orbach and Fenton 2010). Migratory bats have better visual acuity compared to non-migratory bats (Eklöf et al. 2014), perhaps to see distant features such as stars and post-sunset glow or landscape features in low light. Vision is relied upon heavily for homing in the Egyptian fruit bat (Rousettus aegyptiacus; Tsoar et al. 2011). However, when removed from the familiar area with no availability of familiar visual signals, Egyptian fruit bats, while initially disoriented, were able to home to their familiar area and roost (Tsoar et al. 2011), suggesting reliance on an additional mechanism for navigation or well-developed spatial memory. Additional work to understand the use of vision by bats for navigation is needed.
6 Remembering the Way
Bats possess excellent spatial memory, which they use extensively while foraging (Barchi et al. 2013; Carter et al. 2010). Spatial memory may also aid in orientation and navigation during migration (Geva-Sagiv et al. 2015), as in other mammalian migrants, such as mule deer (Odocoileus hemionus; Merkle et al. 2019) and blue whales (Balaenoptera musculus; Abrahms et al. 2019). Examples of long-distance movements (>100 km) between roosts in different seasons suggest that bats also possess spatial memory over large spatial scales (Norquay et al. 2013; Rodrigues and Palmeirim 2007), but we do not know how they develop this memory nor how bats select a route and destination for migratory movements during their first year. It does not appear that migration routes are socially transmitted from mothers to young in either hoary bats or silver-haired bats (Lasionycteris noctivagans; Baerwald and Barclay 2016). We need studies that explicitly quantify spatial memory in bats: what features do bats use as landmarks and how do they learn and remember them? Early studies will likely be most productive if completed during a single season, when movement distances are typically limited. Understanding how bats develop and use a mental “map” of their surroundings during a single season will complement studies of the cues and mechanisms used by bats for true navigation. We can then work on combining these information streams to determine how bats might develop and use spatial maps that encompass their full annual cycle.
7 Integration of Information During Seasonal Movements
Bats may indeed use random, exploratory movements to a certain degree, as Wilson and Findley (1972) suggested. It could be that an individual uses a combination of innate and extrinsic cues to start moving in a suitable direction, but the path it follows is likely not linear nor precise. If the path leads to an endpoint that meets the bat’s needs (e.g. new roost, profitable foraging area, suitable hibernacula), then the individual remembers this route and incorporates it into the animal’s decision-making process in future years. By this process, a young bat may develop its first spatial memory for migratory movements. Some individual hoary bats in northern California have exhibited inter-annual fidelity to capture sites during autumn (Weller et al. 2016). This fidelity suggests that bats remember and seek specific destinations during their inter-seasonal movements, perhaps in addition to their ultimate winter destination. Are these sites initially discovered by chance, as Wilson and Findley (1972) suggested for intra-seasonal homing at much smaller spatial scales, or are bats using other behavioural cues to locate these sites in the first place? While birds appear to have an innate ability for orientation and navigation through a magnetic compass (Wiltschko and Gwinner 1974), recent research suggests that migratory bats learn to orient and navigate enroute during the first migration (Lindecke et al. 2019).
Bats are flexible in their use of seasonal movements (Rodrigues and Palmeirim 2007), indicating that inter-seasonal movements are not dictated entirely by innate cues, but instead governed by the incorporation of contemporaneous assessment of environmental conditions. For example, if food remains in one part of its range, an individual may decide to delay migration (Richter and Cumming 2006), or if roost temperatures remain suitable in one area, the risks of long-distance movement may not be worthwhile to justify migration (Rodrigues and Palmeirim 2007). Bats also time migratory movements to coincide with favourable weather conditions (Cryan and Brown 2007). In other words, not only do bats possess a large-scale spatial map but they also integrate temporal and local environmental information into decisions about when and whether to migrate.
8 Homing: The Next Generation
After a half-century of research, it is clear that bats have the ability for orientation and navigation, but we do not yet understand the scope of those abilities for true navigation or the scale at which bats can return “home” successfully. We have learned much about the sensory modalities and cues that bats have at their disposal, but this has not paid dividends in terms of empirical understanding of homing distances or paths. How do we get at this? Vanishing bearings from translocations (i.e. the direction toward which animals orient following release at an unknown location) have been used to determine the ability of bats to orient (Lindecke et al. 2019) and the mechanisms by which they do so (Holland et al. 2008). These methods can provide invaluable insights into the “compass” side of “map and compass” navigation, but they may not be informative about bats’ ability to return home from an unfamiliar area, which is a crucial assessment of navigation ability (Gagliardo 2013). It seems logical that within a seasonal home range bats would rely on landmarks and spatial memory for homing rather than magnetic fields or celestial cues that are more suited to long-distance movements. However, the details of how bats develop and use their cognitive maps, and at what scale, are almost completely unknown.
Ideally, we could track the full path of a displaced bat to determine its endpoint and efficiency (minimization of travel between displacement and goal). Doing so requires technology such as active nocturnal radio-tracking via aircraft and GPS technology that can generate detailed movement tracks over short periods. Currently, both of these technologies have limitations. For aircraft tracking, we are limited by cost and the availability of pilots trained for nighttime telemetry. Satellite telemetry has been used to study movements of bats in excess of 450 g for durations approaching a full year and with locational accuracy in the hundreds of meters (Breed et al. 2010). GPS with capabilities for download via mobile phone networks offers improved accuracy and shorter intervals between locations but are limited to use on bats with masses >500 g (Oleksy et al. 2019). More recently, miniature GPS technology has been used on smaller bats (>15 g; Weller et al. 2016). Miniature GPS units capture and store dozens of locations and can be attached so as to obtain information over multiple months. The primary drawback to miniature GPS is that tags must be recovered to obtain the data; hence miniature GPS is useful in situations where bats return to roosts accessible to humans or where biologists are willing to expend extraordinary effort to recapture free-flying bats. As these technologies advance, they should be extremely useful for understanding heretofore unknowable movement patterns of bats and for use in precise route tracking in displacement experiments.
Although many of the earliest homing experiments were conducted on tropical species (Williams et al. 1966; Wilson and Findley 1972), work with tropical species has waned in recent decades. Given the wealth of bat diversity in tropical regions, this is a missed opportunity to understand the full range of navigational capabilities and strategies in bats. For example, migration is well known in temperate species, but latitudinal and elevational seasonal movements also occur in tropical species (Arnone et al. 2016), however, such migrations are poorly documented. Seasonal movements of tropical bats and the cues used to guide them are underexplored topics deserving of additional attention in future years.
9 So What? Conservation Implications of Bat Movements
We need to understand the extent of the homing and navigational ability of bats more than ever because many of the most pressing conservation issues facing bats are directly related to bat movement. For example, one of the biggest threats to migratory bats is fatalities at wind-energy facilities. Most fatalities worldwide are of bats migrating or dispersing during autumn (Barclay et al. 2017). Fatality rates vary considerably within and among regions, but it is not clear these deaths are correlated with migratory routes used by bats, primarily because we do not know if bats use clearly defined migratory routes. If bats are using clearly defined migration corridors based on predictable features, then these areas could potentially be avoided for wind-energy development.
At least some migratory individuals do not follow clearly defined routes, but rather, seem to “wander” (e.g. hoary bats; Weller et al. 2016 and silver-haired bats; McGuire 2019). These circuitous movements complicate the narrative that seasonal migrations are driven simply by the response to innate cues. Instead, “wandering” suggests that these species rely heavily on a map that consists of much more than the starting and endpoints of migration. These movements also suggest that bats may make decisions about travel direction and areas to visit using real-time decisions informed by weather, prey densities, and internal assessment of trade-offs between energetic and reproductive needs versus their need to reach their destination. Seemingly erratic movements increase the complexity of defining migratory routes, even for single species of bats, and highlight the challenge of incorporating migratory routes into conservation measures.
In many parts of the world, fruit bats in the genera Pteropus and Eidolon are reservoirs of zoonotic diseases and viruses, such as Hendra, Nipah and Ebola, which result in deadly disease when they spillover to humans (Breed et al. 2010). Fruit bats can travel long distances during nightly foraging bouts and seasonal migrations, sometimes crossing international borders in the process. Determination of the likely geographic scale and patterns of movement of bats could be helpful for predicting the transfer of viruses within regions and mobilizing disease response actions to protect human health. Because many of the implicated species are large-bodied, they can be tracked using GPS technologies that will allow us to understand their movements in greater detail than is currently possible with smaller species. For example, precise reconstructions of movement routes will facilitate our understanding of the extent to which these bats use navigational cues from the landscape.
The study of homing in bats began 50 years ago as fundamental research to determine the homing abilities of bats in small local areas. Since then, our focus shifted to understanding the sensory basis of orientation, and our knowledge of this topic has increased greatly. We now understand that homing ability in bats is not achieved by random searching, however, the central question of how bats find home within and between seasons remains a mystery. Due to technological advances, both in the ability to study bats in the wild and to assess their orientation abilities under controlled conditions, we are no longer limited to asking questions about how bats orient or navigate within small areas. Rather, we can ask bigger questions about how bats move among their seasonal home ranges and how they find their way in new and unfamiliar areas. Inter-seasonal migrations expand our appreciation of the scale and complexity of bat movements, as well as increasing the challenges of trying to learn about them.
We emphasize that most knowledge of homing and movement in bats has come from temperate species, despite this group comprising a small proportion of bat diversity worldwide. We encourage comparative approaches for the study of homing and movement between temperate and tropical species to enhance our understanding of the full range of capabilities and strategies used by bats. We note, too, that technological advances (Chapter 14, this volume) have allowed us to expand the spectrum of possibilities of what we can learn about bat movement and homing and expect that careful applications of these technologies in future years will advance our understanding of bats movements at multiple spatial scales. As knowledge increases about the navigational abilities of bats within and among habitats during different times of year, it will be critically important to apply that knowledge to bat conservation efforts.
References
Abrahms B, Hazen EL, Aikens EO et al (2019) Memory and resource tracking drive blue whale migrations. Proc Natl Acad Sci 116(12):5582–5587
Arnone IS, Trajano E, Pulchério-Leite A et al (2016) Long-distance movement by a great fruit-eating bat, Artibeus lituratus (Olfers, 1818), in southeastern Brazil (Chiroptera, Phyllostomidae): evidence for migration in Neotropical bats? Biota Neotrop 16(1)
Baerwald E, Barclay R (2016) Are migratory behaviours of bats socially transmitted? R Soc Open Sci 3(4):150658
Barchi JR, Knowles JM, Simmons JA (2013) Spatial memory and stereotypy of flight paths by big brown bats in cluttered surroundings. J Exp Biol 216(6):1053–1063
Barclay RMR, Baerwald EF, Rydell J (2017) Bats. In: Perrow M (ed) Wildlife and wind farms - conflicts and solutions, volume 1: onshore: potential effects. Pelagic Publishing, Exeter
Breed AC, Field HE, Smith CS et al (2010) Bats without borders: long-distance movements and implications for disease risk management. EcoHealth 7(2):204–212
Buchler ER, Childs SB (1982) Use of the post-sunset glow as an orientation cue by big brown bats (Eptesicus fuscus). J Mammal 63(2):243–247
Carter GG, Ratcliffe JM, Galef BG (2010) Flower bats (Glossophaga soricina) and fruit bats (Carollia perspicillata) rely on spatial cues over shapes and scents when relocating food. PLoS One 5(5):e10808
Childs SB, Buchler ER (1981) Perception of simulated stars by Eptesicus fuscus (Vespertilionidae): a potential navigation mechanism. Anim Behav 29:1029–1035
Cryan PM, Brown AC (2007) Migration of bats past a remote island offers clues toward the problem of bat fatalities at wind turbines. Biol Conserv 139(1–2):1–11
Davis R (1966) Homing performance and homing ability in bats. Ecol Monogr 36(3):201–237
Eklöf J, Svensson AM, Rydell J (2002) Northern bats, Eptesicus nilssonii, use vision but not flutter-detection when searching for prey in clutter. Oikos 99(2):347–351
Eklöf J, Šuba J, Petersons G et al (2014) Visual acuity and eye size in five European bat species in relation to foraging and migration strategies. Environ Exp Biol 12(1):01–06
Frafjord K (2013) Influence of night length on home range size in the northern bat Eptesicus nilssonii. Mamm Biol 78(3):205–211
Furmankiewicz J, Kucharska M (2009) Migration of bats along a large river valley in southwestern Poland. J Mammal 90(6):1310–1317
Gagliardo A (2013) Forty years of olfactory navigation in birds. J Exp Biol 216(12):2165–2171
Geva-Sagiv M, Las L, Yovel Y et al (2015) Spatial cognition in bats and rats: from sensory acquisition to multiscale maps and navigation. Nat Rev Neurosci 16(2):94
Greif S, Borissov I, Yovel Y et al (2014) A functional role of the sky’s polarization pattern for orientation in the greater mouse-eared bat. Nat Commun 5:4488
Griffin DR (1970) Migrations and homing of bats. In: Wimsatt WA (ed) Biology of bats, vol 1. Academic Press, New York, pp 233–264
Guilbert J, Walker M, Greif S et al (2007) Evidence of homing following translocation of long-tailed bats (Chalinolobus tuberculatus) at grand canyon cave, New Zealand. NZ J Zool 34(3):239–246
Holland RA (2019) Orientation, navigation and homing in bats. Encycl Anim Behav 3:611–621
Holland RA, Helm B (2013) A strong magnetic pulse affects the precision of departure direction of naturally migrating adult but not juvenile birds. J Royal Soc Interface 10(81):20121047
Holland RA, Kirschvink JL, Doak TG et al (2008) Bats use magnetite to detect the earth’s magnetic field. PLoS One 3(2):e1676
Holland RA, Borissov I, Siemers BM (2010) A nocturnal mammal, the greater mouse-eared bat, calibrates a magnetic compass by the sun. P Natl Acad Sci 107(15):6941–6945
Hutterer R, Ivanova T, Meyer-Cords C et al (2005) Bat migrations in Europe. A review of banding data and literature. Vol 28. Federal agency for nature conservation, Bonn, Germany
Kick SA (1982) Target-detection by the echolocating bat, Eptesicus fuscus. J Comp Physiol A 145:431–435
Leffler JW, Leffler LT, Hall JS (1979) Effects of familiar area on the homing ability of the little brown bat, Myotis lucifugus. J Mammal 60(1):201–204
Lewis SE (1995) Roost fidelity of bats: a review. J Mammal 76:481–496
Lindecke O, Elksne A, Holland RA et al (2019) Experienced migratory bats integrate the sun’s position at dusk for navigation at night. Curr Biol 29(8):1369–1373
Lohmann KJ, Lohmann CM (2019) There and back again: natal homing by magnetic navigation in sea turtles and salmon. J Exp Biol 222(Suppl 1):jeb184077
McGuire LP (2019) Migratory wanderings: bat migration at the regional scale. Bat Res News 60(1):46–47
Merkle JA, Sawyer H, Monteith KL et al (2019) Spatial memory shapes migration and its benefits: evidence from a large herbivore. Ecol Lett 22:1797–1805
Mouritsen H (2018) Long-distance navigation and magnetoreception in migratory animals. Nature 558(7708):50–59
Munro U, Munro J, Phillips J et al (1997) Evidence for a magnetite-based navigational “map” in birds. Naturwissenschaften 84(1):26–28
Norquay KJ, Martinez-Nuñez F, Dubois JE et al (2013) Long-distance movements of little brown bats (Myotis lucifugus). J Mammal 94(2):506–515
O’Donnell CF (2001) Home range and use of space by Chalinolobus tuberculatus, a temperate rainforest bat from New Zealand. J Zool (Lond) 253(2):253–264
Oleksy RZ, Ayady CL, Tatayah V et al (2019) The movement ecology of the Mauritian flying fox (Pteropus niger): a long-term study using solar-powered GSM/GPS tags. Mov Ecol 7(1):12
Orbach DN, Fenton B (2010) Vision impairs the abilities of bats to avoid colliding with stationary obstacles. PLoS One 5(11):e13912
Richter HV, Cumming GS (2006) Food availability and annual migration of the straw-colored fruit bat (Eidolon helvum). J Zool (Lond) 268(1):35–44
Rodrigues L, Palmeirim JM (2007) Migratory behaviour of the Schreiber’s bat: when, where and why do cave bats migrate in a Mediterranean region? J Zool (Lond) 274:116–125
Tsoar A, Nathan R, Bartan Y et al (2011) Large-scale navigational map in a mammal. Proc Natl Acad Sci 108(37):E718–E724
Wang Y, Pan Y, Parsons S et al (2007) Bats respond to polarity of a magnetic field. Pro Roy Soc B 274:2901–2905
Wason A (1978) Observations on homing ability of some insectivorous bats. Zeitschrift für Säugetierkunde 46(5):331–332
Weller TJ, Castle KT, Liechti F et al (2016) First direct evidence of long-distance seasonal movements and hibernation in a migratory bat. Sci Rep 6:34585
Williams TC, Williams JM (1967) Radio tracking of homing bats. Science 155(3768):1435–1436
Williams TC, Williams JM, Griffin DR (1966) The homing ability of the neotropical bat Phyllostomus hastatus, with evidence for visual orientation. Anim Behav 14(4):468–473
Wilson DE, Findley JS (1972) Randomness in bat homing. Am Nat 106(949):418–424
Wiltschko W, Gwinner E (1974) Evidence for an innate magnetic compass in garden warblers. Naturwissenschaften 61(9):406–406
Wiltschko W, Wiltschko R (2005) Magnetic orientation and magnetoreception in birds and other animals. J Comp Physiol A 191:675–693
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 North American Society for Bat Research (NASBR)
About this chapter
Cite this chapter
Baerwald, E.F., Weller, T.J., Green, D.M., Holland, R.A. (2021). There and Back Again: Homing in Bats Revisited. In: Lim, B.K., et al. 50 Years of Bat Research. Fascinating Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-54727-1_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-54727-1_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-54726-4
Online ISBN: 978-3-030-54727-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)