Abstract
Symptoms of constipation, abdominal pain and bloating are common among scleroderma patients. Differentiating between the underlying etiological factors and proposing a successful treatment strategy can be challenging. The following chapter deals with the management of these symptoms, both from a diagnostic and a therapeutic point. A clinical case and algorithms are also included.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Systemic sclerosis
- Scleroderma
- Gastrointestinal involvement
- Gastroparesis
- Small intestinal bacterial overgrowth
- Constipation
- Intestinal pseudo-obstruction
- Dysmotility
Introduction
GI involvement occurs in more than 90% of patients in systemic sclerosis (SSc). Any part of the GI tract can be involved, and the disease is progressive in nature [1]. Patients may be asymptomatic or present with a range of GI symptoms attributed to different etiologies. Bloating and abdominal pain are common among scleroderma patients. Gastric involvement manifesting as gastroparesis is reported in 38–50% of SSc patients [2] and small intestinal participation occurs in 40–70%. Colonic involvement leading to constipation is observed in 20–50% of scleroderma cases [3]. Symptoms of abdominal distension, pain and constipation can be a major source of poor quality of life and their management can prove challenging [4].
Clinical Case
VK is a 37-year-old patient of afro-caribbean origin, diagnosed with diffuse systemic sclerosis and dermatomyositis overlap. She has severe upper and lower GI involvement. She presented to the gastroenterology scleroderma clinic with symptoms of severe bloating, central abdominal pain, heartburn, dysphagia, constipation alternating with diarrhoea and weight loss.
VK has undergone several investigations to assess GI involvement. A barium swallow revealed a dilated esophagus, a sliding hiatus hernia and a patulous LES. A gastric emptying study confirmed gastroparesis. Upper GI endoscopy showed ulcerative esophagitis, and no gastric outlet obstruction. Colonoscopy was normal. A barium meal and follow-through pointed to the characteristic “accordion-like” appearance of the small bowel, with dilation of the distal duodenum and proximal jejunum. In addition, slow transit times were noted. A hydrogen breath test was positive for small intestinal bacterial overgrowth (SIBO).
VK’s treatment in terms of her GI symptoms is mainly symptomatic. She is on dual acid suppression therapy and she uses metoclopramide (previously domperidone) to aid with gastric emptying and intestinal motility. She has been on cyclical antibiotic courses for SIBO (rifaximin, doxycycline and ciprofloxacin). She is on a longstanding combination of laxatives (movicol, bisacodyl, senna), but lately symptoms improved following the addition of prucalopride to her treatment.
Constipation
Constipation has been reported in up to 50% of scleroderma patients. In practice, fewer patients may complain of decreased bowel frequency and symptoms may not appear until later in the disease process.
Pathophysiology
Colonic involvement is common in GI scleroderma. There is initially patchy muscle atrophy which is followed by fibrosis, mainly affecting the circular muscle layer. The predominant atrophic changes, in combination with hypomotility, impaired peristalsis and loss of gastro-colic reflex, lead to slow-transit times observed in scleroderma patients [5].
Diagnosis
Constipation is most commonly due to dysmolity and slow transit, but may also be the result of functional anorectal outlet obstruction or side effects of medication (e.g. opiates, iron supplements). Metabolic causes, e.g. hypothyroidism and diabetes mellitus, should be considered in the initial assessment. Detailed history, clinical assessment and investigations should aim to establish diagnosis and tailor treatment accordingly. Patients may complain of decreased bowel frequency, abdominal pain and discomfort, hard stool, straining, sense of incomplete bowel emptying, bloating and the need to use manual manoeuvres. Symptoms are usually longstanding and there is gradual deterioration.
Acute onset constipation, or presence of red flags, should be urgently investigated to exclude mechanical obstruction, malignancy or acute colonic pseudo-obstruction, which is a medical emergency requiring hospital care [6]. Other severe but rare complications are volvulus, colonic stenosis and stercoral ulceration.
Investigations
Slow transit constipation can be demonstrated with the use of radiopaque markers [7]. Plain abdominal radiographs and CT scans can be used to exclude mechanical obstruction, assess for colonic dilation and the presence of faecal impaction or wide-mouthed diverticula at the antimesenteric colonic border. Endoscopic assessment is recommended to exclude malignancy and endoluminal pathology, and should be considered in scleroderma patients with recent change in bowel habits.
Treatment
Dietary modifications can be initially adopted, however they have limited effect in slow transit constipation and high-fibre diets may exacerbate symptoms, particularly in the presence of gastroparesis and SIBO. The same applies for bulk-forming laxatives. Stimulant (e.g. bisacodyl, senna, lubiprostone) and osmotic (e.g. macrogol, lactulose) laxatives, and stool softeners form the mainstay of constipation management. They are usually more effective in the initial stages of the disease and combinations of different agents are usually employed, particularly in the long-term setting [8]. Pro-kinetic agents, like metoclopramide and domperidone, have been shown to have some effect on colonic motility [9]. Prucalopride is a 5-HT4 receptor agonist that promotes colonic transit and it has been used successfully in cases of SSc [10]. It is currently approved for patients failing laxative treatment.
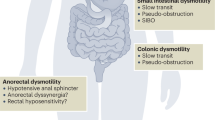
In addition to the pharmacologic management of constipation, other techniques such as biofeedback may be useful [3]. Surgery should be reserved as a last resort for emergency cases, as it carries a high risk of complications and is of limited therapeutic value. Limiting medications that promote constipation should be considered as part of the overall management, alongside correction of possible metabolic defects (e.g. hypercalcemia, hypothyroidism). Assessment of anorectal function should be considered. Figure 15.1 provides an algorithm for the overall management of constipation in SSc.
Algorithm for management of constipation (Ref. [3])
Bloating and Abdominal Pain
The main causes of abdominal pain and bloating in scleroderma patients are gastroparesis, SIBO and small intestinal pseudo-obstruction. Colonic pseudo-obstruction and true mechanical obstruction (e.g. tumour, volvulus) are rarer causes of abdominal pain and distention that have a more acute onset and require prompt medical attention and in-hospital care.
Pathophysiology
Smooth muscle atrophy followed by fibrosis and anatomic and neurovascular changes in the GI tract are characteristic processes of GI scleroderma. The dysmotility together with autonomic dysfunction contribute to impaired gastric accommodation and compliance, resulting in gastroparesis [11, 12]. In the small intestine, hypomotility, loss of Migrating Motor Complexes (MMC), the presence of diverticula and chronic PPI use, lead to stasis of intestinal contents and migration of colonic bacteria in the small intestine, creating ideal conditions for bacterial overgrowth [5]. Intestinal pseudo-obstruction is a result of the established dysmotility in combination with the uncoordinated contractile activity and failure to produce a successful propagatory movement of intestinal contents to the colon, in the absence of mechanical obstruction.
Diagnosis
Gastroparesis is reported in up to 50% of patients with SSc. Apart from abdominal pain and bloating, symptoms include early satiety, postprandial fullness, nausea, vomiting, exacerbation of reflux symptoms. Gastric outlet obstruction should be initially ruled out. Co-morbidities (e.g. diabetes mellitus) and medications that contribute to delayed gastric emptying (e.g. opiates, anti-cholinergics) should be considered and the presence of Helicobacter Pylori infection may exacerbate symptoms [6].
SIBO is reported in 43–55% of SSc patients [13, 14]. Patients complain of abdominal distention, pain, excessive flatulence, nausea, vomiting, early satiety, diarrhoea , and in more severe cases malabsorption and weight loss become evident. There is a marked symptomatic overlap between SIBO and gastroparesis, and in some cases chronic intestinal pseudo-obstruction (CIPO) (obstructive symptoms for >6 months), making the diagnostic distinction challenging. CIPO presents with very similar symptoms as SIBO, however it is much less frequent, with one centre reporting a 3.9% prevalence [15].
Acutely presenting or rapidly deteriorating abdominal pain and distention should prompt for immediate investigations to exclude mechanical obstruction and volvulus.
Investigations
Investigating abdominal pain and bloating in scleroderma should be initially tailored by history and clinical findings, and it should be remembered that symptoms may be also attributed to non-scleroderma causes. Gastroparesis is diagnosed with a scintigraphic gastric emptying study [16]. Upper GI endoscopy helps ruling out gastric outlet obstruction and assessing for H.Pylori.
The gold-standard for the diagnosis of SIBO is culture of jejunal aspirates collected during upper GI endoscopy. The test however has limitations and is not widely used in clinical practice. The glucose and lactulose hydrogen breath tests are non-invasive methods, that rely on the metabolism of ingested carbohydrates by intestinal bacteria to produce hydrogen and methane [17, 18, 19]. Fecal calprotectin has been found to increase in scleroderma patients with SIBO [20]. Although it cannot confirm diagnosis, it can be used as a tool to identify patients for further testing, and also assess treatment outcomes. In the same content, a low vitamin B12 level and a raised serum folate and/or vitamin K should raise clinical suspicion for SIBO. Bacteria in the small intestine consume vitamin B12 and produce folic acid and vitamin K, providing an indirect indication for further assessment [5, 21].
Imaging modalities should be employed to exclude mechanical obstruction and assess for CIPO. A plain abdominal radiograph may reveal dilated small bowel loops, with or without air-fluid levels. The small bowel loops may have the characteristic “accordion-like” appearance (on contrast studies), owing to the tightly packed volvulae convinantes. Cross-sectional imaging helps differentiate between CIPO and true mechanical obstruction. In CIPO, there is intestinal dilatation, in the absence of a transition point. Transient intussusceptions may also be observed and are associated with chronic abdominal distention and pain.
Treatment
Dietary and lifestyle modifications can be employed as a first approach in the management of gastroparesis. Low-residue and low-fat diets, in combination with small frequent meals and avoidance of recumbinant position following food ingestion, may provide some symptomatic control [22]. Prokinetic agents work in early disease stages, before muscle atrophy and fibrosis are well-established. Metoclopromide, domperidone and erythromycin have been used successfully in scleroderma patients, however potential side effects should be borne in mind [6, 23]. Enteral and parenteral nutrition may be the only options in advanced disease.
Management of SIBO relies in the empirical use of antibiotics. Antibiotics commonly used include ciprofloxacin, metronidazole, doxycycline, co-amoxiclav, norfloxacin, tetracycline and rifaximin [24, 25]. Cyclical courses of different compounds may be needed to achieve eradication and minimize resistance. Side effects, safety, effectiveness and availability usually determine the choice of antibiotics. In advanced disease states, continual rotating courses may be needed. Probiotics have been shown to provide some symptomatic relief, however there are no clear recommendations regarding their use [26].
Treatment of CIPO is conservative, comprising bowel rest, IV hydration, bowel decompression and use of prokinetic agents. Metoclopromide and domperidone may increase small intestinal motility. Octreotide has been successfully used in scleroderma patients to improve intestinal motility, however side effects and cost limit its long-term use [27, 28]. Surgery carries a high risk in these patients and should only be reserved for emergency cases (e.g. in bowel perforation, volvulus). Figure 15.2 provides an algorithm for the management of abdominal pain and distension in SSc.
Algorithm for management of bloating and abdominal pain (Ref. [3])
References
Kumar S, Singh J, Rattan S, DiMarino J, Cohen S, Jimenez SA. Review article: pathogenesis and clinical manifestations of gastrointestinal systemic sclerosis. Aliment Pharmacol Ther. 2017;45(7):883–98.
Savarino E, Mei F, Parodi A, Ghio M, Furnari M, Gentile A, et al. Gastrointestinal motility disorder assessment in systemic sclerosis. Rheumatology. 2013;52(6):1095–100.
Hansi N, Thoua N, Carulli M, Chakravarty K, Lal S, Smyth A, et al. Consensus best practice pathway of the UK scleroderma study group: gastrointestinal manifestations of systemic sclerosis. Clin Exp Rheumatol. 2014;32(6Suppl 86):S-214-21.
Khanna D, Nagaraja V, Gladue H, Chey W, Pimentel M, Frech T. Measuring response in the gastrointestinal tract in systemic sclerosis. Curr Opin Rheumatol. 2013;25(6):00–6. doi: 10.1097/01
Harrison E, Murray C, Lal S. Small and large intestinal involvement and nutritional issues. In: Varga J, Denton CP, Wigley FM, Allanore Y, Kuwana M, editors. Scleroderma: from pathogenesis to comprehensive management. New York: Springer; 2017. p. 443–58.
Shreiner AB, Murray C, Denton C, Khanna D. Gastrointestinal manifestations in systemic sclerosis. J Scleroderma Relat Disord. 2016;1(3):247–56. https://doi.org/10.5301/jsrd.5000214.
Basilico G, Barbera R, Vanoli M, Bianchi P. Anorectal dysfunction and delayed colonic transit in patients with progressive systemic sclerosis. Dig Dis Sci. 1993;38:1525–9.
Wald A. Is chronic use of stimulant laxatives harmful to the colon? J Clin Gastroenterol. 2003;36(5):386–9.
Emmanuel AV, Tack J, Quigley EM, Talley NJ. Pharmacological management of constipation. Neurogastroenterol Motil. 2009;21(Suppl 2):41–54. https://doi.org/10.1111/j.1365-2982.
Boeckxstaens GE, Bartelsman JF, Lauwers L, Tytgat GN. Treatment of GI dysmotility in scleroderma with the new enterokinetic agent prucalopride. Am J Gastroenterol. 2002;97:194–7.
Iovino P, Valentini G, Ciacci C, De Luca A, Tremolaterra F, Sabbatini F, et al. Proximal stomach function in systemic sclerosis: relationship with autonomic nerve function. Dig Dis Sci. 2001;46(4):723–30.
Savarino E, Furnari M, de Bortoli N, Martinucci I, Bodini G, Ghio M, et al. Gastrointestinal involvement in systemic sclerosis. Presse Med. 2014;43(10 Pt 2):e279–91. https://doi.org/10.1016/j.lpm.2014.03.029.
Marie I, Ducrotte P, Denis P, Menard JF, Levesque H. Small intestinal bacterial overgrowth in systemic sclerosis. Rheumatology. 2009;48(10):1314–9. https://doi.org/10.1093/rheumatology/kep226.
Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, et al. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010;16(24):2978–90.
Muangchan C, Canadian Scleroderma Research Group, Baron M, Pope J. The 15% rule in scleroderma: the frequency of severe organ complications in systemic sclerosis. A systematic review. J Rheumatol. 2013;40(9):1545–56. https://doi.org/10.3899/jrheum.121380.
Nagaraja V, McMahan ZH, Getzug T, Khanna D. Management of gastrointestinal involvement in scleroderma. Curr Treatm Opt Rheumatol. 2015;1(1):82–105.
Romagnuolo J, Schiller D, Bailey RJ. Using breath tests wisely in a gastroenterology practice: an evidence-based review of indications and pitfalls in interpretation. Am J Gastroenterol. 2002;97(5):1113–26.
Khoshini R, Dai SC, Lezcano S, Pimentel M. A systematic review of diagnostic tests for small intestinal bacterial overgrowth. Dig Dis Sci. 2008;53(6):1443–54.
Braun-Moscovici Y, Braum M, Khanna D, Balbir-Gurman A, Furst DE. What tests should you use to assess small intestinal bacterial overgrowth in systemic sclerosis? Clin Exp Rheumatol. 2015;33(4 Suppl 91):S117–22.
Marie I, Leroi AM, Menard JF, Levesque H, Quillard M, Ducrotte P. Fecal calprotectin in systemic sclerosis and review of the literature. Autoimmun Rev. 2015;14(6):547–54. https://doi.org/10.1016/j.autrev.2015.01.018.
Kaye SA, Lim SG, Taylor M, Patel S, Gillespie S, et al. Small bowel bacterial overgrowth in systemic sclerosis: detection using direct and indirect methods and treatment outcome. Br J Rheumatol. 1995;34:265–9.
Frech TM, Mar D. Gastrointestinal and hepatic disease in systemic sclerosis. Rheum Dis Clin N Am. 2018;44(1):15–28.
McFarlane IM, Bharma MS, Kreps A, Iqbal S, Al-Ani F, Saladini-Aponte C, et al. Gastrointestinal manifestations of systemic sclerosis. Rheumatology. 2018;8(1). pii: 235 https://doi.org/10.4172/2161-1149.1000235.
Baron M, Bernier P, Cote LF, Delegge MH, Falovitch G, Friedman G, et al. Screening and therapy for malnutrition and related gastro-intestinal disorders in systemic sclerosis: recommendations of a north American expert panel. Clin Exp Rheumatol. 2010;28(2 Suppl 58):S42–6.
Gatta L, Scarpignato C. Systematic review with meta-analysis: rifaximin is effective and safe for the treatment of small instestine bacterial overgrowth. Aliment Pharmacol Ther. 2017;45(5):604–6016.
Frech TM, Khanna D, Maranian P, Frech EJ, Sawitzke AD, Murtaugh MA. Probiotics for the treatment of systemic sclerosis-associated gastrointestinal bloating/ distention. Clin Exp Rheumatol. 2011;29(2 Suppl 65):S22–5.
Nikou GC, Toumpanakis C, Katsiari C, Charalambopoulos D, Sfikakis PP. Treatment of small intestinal disease in systemic sclerosis with octreotide: a prospective study in seven patients. J Clin Rheumatol. 2007;13(3):119–23.
Perlemuter G, Cacoub P, Chaussade S, Wechsler B, Couturier D, Piette JC. Octreotide treatment of chronic intestinal pseudoobstruction secondary to connective tissue diseases. Arthritis Rheum. 1999;42(7):1545–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chatzinikolaou, SL., Murray, C. (2021). Constipation, Bloating and Abdominal Pain. In: Matucci-Cerinic, M., Denton, C.P. (eds) Practical Management of Systemic Sclerosis in Clinical Practice. In Clinical Practice. Springer, Cham. https://doi.org/10.1007/978-3-030-53736-4_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-53736-4_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-53735-7
Online ISBN: 978-3-030-53736-4
eBook Packages: MedicineMedicine (R0)