Abstract
The Red Sea is host to one of the world’s largest reef systems where invertebrates make up the majority of the diversity. Of those marine invertebrates, those that host microscopic algae or zooxanthellae are of the utmost importance in climate change research. Giant clams are photosymbiotic organisms and are protected under Appendix II of CITES. However, overfishing, habitat destruction, and climate change threaten their populations. The Red Sea is home to 3 of the 12 species of giant clams, Tridacna maxima, Tridacna squamosa, and Tridacna squamosina. Tridacna squamosina has the smallest geographical range, limited to the northern Red Sea, and is only present in low abundance. Overfishing in the past and present has been the main contributor to the loss of species in the Red Sea and globally. Climate change also threatens this symbiotic relationship between the giant clam and zooxanthellae in the family Symbiodiniaceae. Giant clams in the Red Sea have only been found to harbor Symbiodinium type A1 as their dominant type. However, as genetic techniques improve, the classification system of Symbiodiniaceae will improve and allow for better detection of diverse species within a host, which will allow for better understanding of the future of these organisms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction to the Red Sea
The Red Sea lies between Africa (on the West) and the Arabian Peninsula (on the East). The Gulf of Aqaba and the Gulf of Suez create narrow connections to the Mediterranean Sea in the North and the Bab-el-Mandeb strait connects to the Gulf of Aden in the South (see Fig. 1). Although the Red Sea is located on the northernmost boundary of the tropics, it hosts one of the largest coral reef systems in the world (Berumen et al. 2013). It is home to a diverse array of marine organisms, some of which are still waiting to be discovered, and many of which are endemic (Berumen et al. 2013; DiBattista et al. 2016). The Red Sea is considered to be an extreme environment due to its high sea surface temperatures (SST) and higher than normal salinity (Ngugi et al. 2012; Raitsos et al. 2013). Parts of the reef ecosystem here regularly experience water temperatures as high as 33 °C in the summer months with trends showing a continuous increase of the maximum SST (Chaidez et al. 2017). The salinity of the Red Sea, although differs slightly across latitudes, is approximately 37–40 psu (practical salinity units) as measured by the NOAA National Virtual Ocean Data System. The arid environment of the Arabian Peninsula causes high evaporation over the Red Sea, leaving behind a high salt content in the marine environment. The lack of heavy rainfall and access to other bodies of water add to the high salinity of the Red Sea (Raitsos et al. 2013). With these oceanographic parameters, marine organisms have adapted to this unique environment, which has created an abundance of endemic species (DiBattista et al. 2016). The Red Sea, with its high SST, has been seen as a living laboratory for reef systems. We may see Red Sea temperatures present in other reef systems in the future as the climate continues to warm (see Voolstra et al. 2015). The importance of studying the marine organisms in the Red Sea is evident especially when looking to find heat tolerant reef species. However, the Red Sea reef is overall an understudied region when compared to the Great Barrier Reef and the Caribbean. Furthermore, of all studies from the Red Sea, the majority are from the Gulf of Aqaba, which makes up less than 2% of the entire region (Berumen et al. 2013). Representing the most understudied organisms of the Red Sea and of marine organisms in general are the invertebrates. However, as they constitute the majority of the diversity in the ocean, and more specifically in coral reef ecosystems, they are of the utmost importance.
-
1.
Giant Clam Diversity
One of the most conspicuous and unique invertebrates present in the reef system of the Red Sea and reefs around the world is the giant clam. Bivalves in the family of Tridacnidae are the largest living bivalves with some species reaching up to a meter or more in length (Neo et al. 2017). There are twelve species of giant clams in the family Tridacnidae, two of which are in the genus Hippopus and ten of which are in the genus Tridacna (Neo et al. 2017) (Table 1). The diverse color morphologies of these clams have allowed for uncertainty in species descriptions that are only resolved by genetic analysis. Due to this, the species list of giant clams has and will continue to be modified (see Richter et al. 2008; Huber and Eschner 2010; Penny and Willan 2014; Su et al. 2014; Borsa et al. 2015a, b; Neo et al. 2017). Giant clams are spread throughout the Pacific and Indian Oceans with the northernmost boundary in the Red Sea (see Table 1). As they are brightly colored and easily accessible from reef tops, these bivalves have received interest from fishermen and researchers alike (Fig. 2).
The morphological diversity of giant clam species gives rise to the possibility of inaccurate species distinctions. However, the most recent species described have been cryptic species, which were separated from previously described species by molecular genetics techniques (see Richter et al. 2008; Borsa et al. 2015a, b; Su et al. 2014). Many species diversity studies have utilized genetic markers to resolve putative species distinctions among morphologically ambiguous organisms (see Palumbi 1997; Knowlton 2000; Warner et al. 2015; Johnson et al. 2015). For example, Tridacna noae was believed to be conspecific with Tridacna maxima based on basic morphology. However, with the use of genetic markers (16 s rRNA, 18 s rRNA, 28 s rRNA, and COI), two genes revealed separation between Tridacna maxima and the cryptic species Tridacna noae. Genetic results encouraged a more thorough morphological examination, which recognized small differences among shell shape and color pattern and allowed for an accurate description of the new species (Su et al. 2014). There is a possibility that there are more cryptic species of giant clams, and genetic techniques have been the most recent to resolve these distinctions accurately (see Richter et al. 2008; Naguit 2009; Huelsken et al. 2013; Su et al. 2014; Pappas et al. 2017). Genetic techniques can also give very detailed information on the evolution of species’ populations, movement ecology, and genetic diversity between individuals (see Ayala et al. 1973; Benzie and Williams 1997; Kochzius and Nuryanto 2008; Hui et al. 2017). Studies of giant clams in the Red Sea that utilized genetic markers provide phylogenetic trees to show the separation among species. Those studies show that three species are present in the Red Sea, Tridacna maxima, Tridacna squamosa, and Tridacna costata (later synonymized with Tridacna squamosina (Huber and Eschner 2010) (Richter et al. 2008; Pappas et al. 2017)). Genetic techniques prove to be useful when describing the diversity in the algal partners of giant clams as well (see Carlos et al. 1999; Baillie et al. 2000; DeBoer et al. 2012; Lee et al. 2015; Pappas et al. 2017).
-
2.
Giant Clam Symbiosis
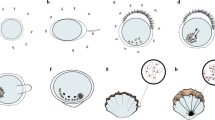
Giant clams are unique bivalves due to their symbiotic relationship with microscopic algae or zooxanthellae in the family Symbiodiniaceae. This relationship is similar to corals in that the host animal’s major food source comes from the photosynthate of the algal partner and is the main contributor to the clams’ giant size (Yonge 1975; Klumpp and Lucas 1994). This relationship has both changed the anatomy and habitat of the giant clam from that of any other bivalve (Fitt et al. 1986). For photosynthesis to be possible, giant clams live on shallow reef tops with their mantle tissue exposed upwards to receive sunlight (see Fig. 3). The umbo of the giant clam faces downwards and the shells are partially open through which the enlarged mantle tissue extends (Fig. 4a, c).
Anatomical sketch of a giant clam. Aerial view (a, b) and side view (c, d) show both organs and zooxanthellae tubule system. Adapted from Norton et al. 1995
The Symbiodiniaceae cells are stored in tubules that extend from the clam’s stomach to the mantle tissue (Fig. 4b, d). To obtain these cells, the clam will ingest them from the water column through the incurrent siphon where they will enter the stomach. From the stomach, the clam transports Symbiodiniaceae cells through the tubule system from the primary tubules to the secondary tubules to the tertiary tubules (see Fig. 4d). The Symbiodiniaceae cells are stacked in tertiary tubules perpendicular to the surface of the mantle tissue (Mansour 1946; Norton and Jones 1992). Specialized reflective cells called iridocytes in the mantle tissue act as mirrors and reflect light to stacked Symbiodiniaceae in the tubules to allow for the most efficient photosynthesis (Nishi 2001; Sweeney et al. 2012; Holt et al. 2014). A full description on the morphology of the giant clam due to the relationship with zooxanthellae can be found in literature (see Copland and Lucas 1988; Norton and Jones 1992; Venn et al. 2008).
-
3.
Diversity of Symbiodiniaceae
Previously, the algae that form symbioses with marine invertebrates have been classified into a cladal system (A through I) (see Pochon and Gates, 2010) of one genus, Symbiodinium. However, it has been discovered that the genetic diversity within and among these clades is greater than the genetic diversity seen in other dinoflagellate genera, families, and sometimes orders (Rowan and Powers 1992; Stern et al. 2010, 2012). To better capture the diversity of these algae and to better identify potential species and functional diversity, LaJeunesse et al. (2018) have proposed a new classification system which has created each clade into a genus (currently 6 defined), and each type into a species all belonging to the family Symbiodiniaceae. This system better exemplifies the diversity within the previous clade system. For example, species in both Cladocopium, previously known as Clade C, and Durusdinium, previously known as Clade D, are extremely diverse in ecological function, especially in thermal stress tolerance (LaJeunesse et al. 2010, 2014; Hume et al. 2015). Further, in just the genus Symbiodinium, previously known as Clade A, there have been 18 diverse types or species described with more being discovered (see LaJeunesse et al. 2015). Some types of Symbiodiniaceae within the genus Symbiodinium exhibit entirely different characteristics and ecological functions. Symbiodinium of type A1, A3, and A4 have been found to be essential partners of reef building corals (Baums et al. 2014), anemones, jellyfish, and zooanthids (LaJeunesse 2002; Finney et al. 2010; Lee et al. 2015). However, another species, Symbiodiniaceae necroappetens, has been described as an opportunistic and parasitic symbiont that becomes more abundant in bleached or diseased tissue of host animals (LaJeunesse et al. 2014). Although these types are of the same genus, they have different functions, which further emphasizes the need for a more precise classification system.
Identification of differences in Symbiodiniaceae genera and species are strongly supported by genetic differences. The most common gene region used to distinguish differences in Symbiodiniaceae is the ITS region, or the internal transcribed spacer region (LaJeunesse 2001). With current genetic technologies, scientists are able to determine Symbiodiniaceae down to type or species within a genus and can identify multiple species within an individual host animal (see Rowan et al. 1997; Baker 2001; Glynn et al. 2001; Pochon et al. 2001; Van Oppen 2001; LaJeunesse 2001; 2002; LaJeunesse et al. 2003; Jones et al. 2008). Further, studies examine the ability of host animals to shift or shuffle Symbiodiniaceae species when different species are better suited for the environment (see Baker et al. 2004; Baker and Romanski 2007; Jones et al. 2008; Stat and Gates 2011; Kemp et al. 2014; Davies et al. 2017).
Giant clams have been found to harbor Symbiodiniaceae in the genera Symbiodinium, Cladocopium, and Durusdinium (formerly Clades A, C, and D) (Baillie et al. 2000; DeBoer et al. 2012; Ikeda et al. 2017; Pappas et al. 2017; LaJeunesse et al. 2018). Giant clams in the Red Sea have only been found to harbor Symbiodinium, specifically type A1, as the dominant symbiont (Pappas et al. 2017). However, further research on Symbiodiniaceae diversity in giant clams of the Red Sea show that, although Symbiodinium may dominate, there are other background genera present in all giant clam hosts (Pappas unpublished). Changes in the dominant species type did occur when clams underwent thermal stress in a bleaching experiment, which may indicate potential for shifting to tolerate natural bleaching events or other changes in the environment (Pappas unpublished). As genetic technology progresses, the diversity of the family will most likely be greater than expected (see LaJeunesse et al. 2018). The new information on diversity and functional ecology of certain types of Symbiodiniaceae will be essential for understanding the potential of host animals to survive through increasing SSTs due to climate change.
-
4.
Red Sea Species
The Red Sea hosts three species of giant clams in the genus Tridacna: Tridacna maxima, Tridacna squamosa, and Tridacna squamosina (see Richter et al. 2008; Pappas et al. 2017). The major difference between members of the giant clam group are size, shell shape, and depth. Tridacna maxima grows to approximately 30 to 40 cm in length and has about four to five rounded shell scutes on each side (Fig. 2a). Further, Tridacna maxima has been found in shallower depths than Tridacna squamosa, which can also be used to help identify the species in situ. Tridacna squamosa, also known as the fluted clam, can grow up to 40 cm in length with large, leaf-like fluted scutes that distinguish it from Tridacna maxima (Fig. 2b). Tridacna squamosina is the smallest of the three giant clams in the Red Sea, only measuring up to 32 cm in length. The shell shape of Tridacna squamosina is distinguishable by the zigzag pattern and more angular scutes (Fig. 2c). Tridacna squamosina occupies the shallowest of depths, making them easy to remove from reef tops (reviewed in Neo et al. 2017).
The most common of these species present in the Red Sea and globally is Tridacna maxima (Roa-Quiaoit 2005; Richter et al. 2008; bin Othman et al. 2010; Mekawy and Madkour 2012; Pappas et al. 2017). Tridacna squamosina has the smallest geographical range in the Red Sea; it is only found in the Northern Red Sea in very low abundance (Richter et al. 2008). Tridacna squamosina occupies the shallowest reef tops, no deeper than 2 meters. Making up greater than 80% of the fossil record but less than 2% of the living stock, Richter et al. (2008) suggests that Tridacna squamosina was heavily overfished earlier in history, which has led to its present threatened status. In the Red Sea, Tridacna maxima have been found to occupy water depths between 2 and 10 m, while Tridacna squamosa can be found at depths between 5 and 20 m (Pappas et al. 2017). Although some species live at deeper depths, all tridacnids are threatened by human harvesting (see Lucas 1994; Gladstone 2000; Ashworth et al. 2004; Van Wynsberge et al. 2016). This is clearly a major threat to the populations of giant clams in the Red Sea and globally as some species may go extinct as a result of human collection and consumption (Richter et al. 2008; Larson 2016).
-
5.
Giant Clam Conservation
All giant clams are protected under Appendix II of CITES (2017), which indicates that, although not necessarily faced with extinction, proper management and conservation practices need to be in place so as to not threaten their populations. The IUCN Red List of Threatened Species (2017) includes eight giant clam species with four listed as vulnerable. Further, seven giant clam species are candidates for the Endangered Species Act (ESA, 2017) protection, which prohibits trade, commerce, and habitat destruction. Despite being protected, threats such as overfishing, habitat destruction, and climate change still exist (reviewed in Mies 2019; Neo et al. 2017). Giant clams have been fished for their meat and decorative purposes in the Indo-Pacific and the Red Sea throughout history (Bodoy 1984; Lewis et al. 1988; Heslinga 1996; Venkatesan 2010; Nijman et al. 2015). Aside from this direct human impact on giant clam populations, the more complicated threat to these bivalves is climate change (Pappas et al. 2017; Neo et al. 2017).
-
6.
Climate Change and Bleaching
It is already evident that climate change is a threat to the symbiotic relationship between zooxanthellae and their host animal; increasing SST has already devastated many reefs around the world (Hughes et al. 2017). As water becomes hotter than the optimal temperature range of these algae, they are expelled from the clam’s tissue. This phenomenon called bleaching is commonly seen in corals but can occur in any animal that hosts zooxanthellae (Fig. 5) (see Fromont and Garson 1999; Leggat et al. 2003; Perez et al. 2001; Venn et al. 2008; McGill and Pomory 2008; Ziegler et al. 2014). Once the major food source is gone, the clam may be able to feed itself by filter feeding, but will not be able to fully sustain itself this way (Klumpp et al. 1992; Klumpp and Lucas 1994). If sea surface temperatures remain high, the clam may not recover from bleaching and will starve (see Norton et al. 1995; Addessi 2001; Leggat et al. 2003; Pappas unpublished data). Although the Red Sea has not experienced many devastating bleaching events (Osman et al. 2018), temperatures are continuing to rise in this region and will have major impacts on the giant clam populations of the Red Sea in the near future (Chaidez et al. 2017; Pappas unpublished data).
Clade A, the genus Symbiodinium found in Red Sea clams has been described as a heat tolerant and opportunistic clade in studies focusing on corals and sea anemones (Rowan et al. 1997; Toller et al. 2001; Venn et al. 2008). The extreme temperatures of the Red Sea may have influenced the abundance of this genus in symbiotic organisms in this region. Symbiodiniaceae genera and species may be the key to understanding the ability of photosymbiotic organisms to tolerate or recover from bleaching events, but more research is needed to understand the benefits of the genus Symbiodinium in giant clams of the Red Sea.
Although this chapter has focused on the species diversity of both the giant clam and its associated Symbiodiniaceae in the Red Sea, due to the Red Sea being an understudied region in the field of marine biodiversity as compared to other regions, this chapter may not be complete. As species evolve and go extinct, the diversity of these organisms will change in the future and further research may provide missing links in our understanding of the diversity of these bivalves in this region. However, the conspicuousness of the giant clam and the drive for Symbiodiniaceae research has produced many thorough studies of these animals in this region (see Pappas et al. 2017). Further, as the new classification system for Symbiodiniaceae continues to be used, a more accurate description of the species present in the tridacnids of the Red Sea may allow for a better understanding in their ability to tolerate such an extreme environment.
References
Addessi L (2001) Giant clam bleaching in the lagoon of Takapoto atoll (French Polynesia). Coral Reefs 19(3):220–220
Ashworth JS, Ormond RF, Sturrock HT (2004) Effects of reef-top gathering and fishing on invertebrate abundance across take and no-take zones. J Exp Mar Biol Ecol 303(2):221–242
Ayala FJ, Hedgecock D, Zumwalt GS, Valentine JW (1973) Genetic variation in Tridacna maxima, an ecological analog of some unsuccessful evolutionary lineages. Evolution 27(2):177–191
Baillie B, Belda-Baillie C, Silvestre V, Sison M, Gomez A, Gomez E, Monje V (2000) Genetic variation in Symbiodiniaceae isolates from giant clams based on random-amplified-polymorphic DNA (RAPD) patterns. Mar Biol 136(5):829–836
Baker AC (2001) Reef corals bleach to survive change. Nature 411(6839):765–766
Baker AC, Romanski AM (2007) Multiple symbiotic partnerships are common in scleractinian corals, but not in octocorals: comment on Goulet (2006). Mar Ecol Prog Ser 335:237–242
Baker AC, Starger CJ, McClanahan TR, Glynn PW (2004) Coral reefs: corals’ adaptive response to climate change. Nature 430(7001):741
Baums IB, Devlin-Durante MK, LaJeunesse TC (2014) New insights into the dynamics between reef corals and their associated dinoflagellate endosymbionts from population genetic studies. Mol Ecol 23(17):4203–4215
Benzie JA, Williams ST (1997) Genetic structure of giant clam (tridacna maxima) populations in the West Pacific is not consistent with dispersal by present-day ocean currents. Evolution 51(3):768–783
Berumen ML, Hoey A, Bass WH, Bouwmeester J, Catania D, Cochran JE, Khalil MT, Miyake S, Mughal M, Spät JL (2013) The status of coral reef ecology research in the Red Sea. Coral Reefs 32(3):737–748
bin Othman AS, Goh GH, Todd PA (2010) The distribution and status of giant clams (family Tridacnidae)-a short review. Raffles Bull Zool 58(1):103–111
Bodoy A (1984) Assessment of human impact on giant clams, Tridacna maxima, near Jeddah, Saudi Arabia. In: Symposium on coral reefs environment of the Red Sea
Borsa P, Fauvelot C, Andréfouët S, Chai T-T, Kubo H, Liu L-L (2015a) On the validity of Noah’s giant clam Tridacna noae (Röding, 1798) and its synonymy with Ningaloo giant clam Tridacna ningaloo Penny & Willan, 2014. Raffles Bulletin of Zoology 63:484–489
Borsa P, Fauvelot C, Tiavouane J, Grulois D, Wabnitz C, Naguit MA, Andrefouet S (2015b) Distribution of Noah’s giant clam, Tridacna noae. Mar Biodivers 45(2):339–344
Carlos AA, Baillie BK, Kawachi M, Maruyama T (1999) Phylogenetic position of Symbiodiniaceae (Dinophyceae) isolates from tridacnids (Bivalvia), cardiids (Bivalvia), a sponge (Porifera), a soft coral (Anthozoa), and a free-living strain. J Phycol 35(5):1054–1062
Chaidez V, Dreano D, Agusti S, Duarte CM, Hoteit I (2017) Decadal trends in Red Sea maximum surface temperature. Sci Rep 7(1):8144
CITES (2017) Appendix II of CITES. Version 04.10.17
Copland JW, Lucas JS (1988) Australian Centre for International Agricultural Research Canberra. In: Giant clams in Asia and the Pacific, vol 9
Davies SW, Ries JB, Marchetti A, Granzotti R, Castillo KD (2017) Symbiodiniaceae functional diversity and clade specificity under global change stressors. bioRxiv:190413
de Lamarck JBDM (1822) Histoire naturelle des animaux sans vertébres. Verdiére
DeBoer TS, Baker AC, Erdmann MV, Ambariyanto JPR, Barber PH (2012) Patterns of Symbiodiniaceae distribution in three giant clam species across the biodiverse Bird’s Head region of Indonesia. Mar Ecol Prog Ser 444:117–132
DiBattista JD, Howard Choat J, Gaither MR, Hobbs JPA, Lozano-Cortés DF, Myers RF, Paulay G, Rocha LA, Toonen RJ, Westneat MW (2016) On the origin of endemic species in the Red Sea. J Biogeogr 43(1):13–30
ESA (2017) Candidate species petition under NOAA and NMFS. 82 FR 28946
Finney JC, Pettay DT, Sampayo EM, Warner ME, Oxenford HA, LaJeunesse TC (2010) The relative significance of host–habitat, depth, and geography on the ecology, endemism, and speciation of coral endosymbionts in the genus Symbiodiniaceae. Microb Ecol 60(1):250–263
Fitt WK, Fisher CR, Trench RK (1986) Contribution of the symbiotic dinoflagellate Symbiodiniaceae microadriaticum to the nutrition, growth and survival of larval and juvenile tridacnid clams. Aquaculture 55(1):5–22
Fromont J, Garson M (1999) Sponge bleaching on the West and East coasts of Australia. Coral Reefs 18(4):340–340
Gladstone W (2000) The ecological and social basis for management of a Red Sea marine-protected area. Ocean Coast Manag 43(12):1015–1032
Glynn PW, Maté JL, Baker AC, Calderón MO (2001) Coral bleaching and mortality in Panama and Ecuador during the 1997–1998 El Niþo–Southern Oscillation event: spatial/temporal patterns and comparisons with the 1982–1983 event. Bull Mar Sci 69(1):79–109
Heslinga G (1996) Clams to cash: how to make and sell giant clam shell products, vol 125. Center for Tropical and Subtropical Aquaculture, Waimanalo, Hawaii
Holt AL, Vahidinia S, Gagnon YL, Morse DE, Sweeney AM (2014) Photosymbiotic giant clams are transformers of solar flux. J R Soc Interface 11(101):20140678
Huber M, Eschner A (2010) Tridacna (Chametrachea) costata ROA-QUIAOIT, KOCHZIUS, JANTZEN, AL-ZIBDAH & RICHTER from the Red Sea, a junior synonym of Tridacna squamosina STURANY, 1899 (Bivalvia, Tridacnidae). Annalen des Naturhistorischen Museums in Wien. Serie B für Botanik und Zoologie:153–162
Huelsken T, Keyse J, Liggins L, Penny S, Treml EA, Riginos C (2013) A novel widespread cryptic species and phylogeographic patterns within several giant clam species (Cardiidae: Tridacna) from the Indo-Pacific Ocean. PLoS One 8(11):e80858
Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R (2017) Global warming and recurrent mass bleaching of corals. Nature 543(7645):373
Hui M, Nuryanto A, Kochzius M (2017) Concordance of microsatellite and mitochondrial DNA markers in detecting genetic population structure in the boring giant clam Tridacna crocea across the Indo-Malay Archipelago. Mar Ecol 38(1)
Hume BC, D'Angelo C, Smith EG, Stevens JR, Burt J, Wiedenmann J (2015) Symbiodiniaceae thermophilum sp. nov., a thermotolerant symbiotic alga prevalent in corals of the world's hottest sea, the Persian/Arabian Gulf. Sci Rep 5:8562
Ikeda S, Yamashita H, Kondo SN, Inoue K, Morishima SY, Koike K (2017) Zooxanthellal genetic varieties in giant clams are partially determined by species-intrinsic and growth-related characteristics. PLoS One 12(2):0172285
IUCN (2017) The IUCN Red List of Threatened Species. Version 2017–3
Johnson SB, Warén A, Tunnicliffe V, Dover CV, Wheat CG, Schultz TF, Vrijenhoek RC (2015) Molecular taxonomy and naming of five cryptic species of Alviniconcha snails (Gastropoda: Abyssochrysoidea) from hydrothermal vents. Syst Biodivers 13(3):278–295
Jones AM, Berkelmans R, van Oppen MJ, Mieog JC, Sinclair W (2008) A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proc R Soc Lond B Biol Sci 275(1641):1359–1365
Kemp DW, Hernandez-Pech X, Iglesias-Prieto R, Fitt WK, Schmidt GW (2014) Community dynamics and physiology of Symbiodiniaceae spp. before, during, and after a coral bleaching event. Limnol Oceanogr 59(3):788–797
Klumpp D, Bayne B, Hawkins A (1992) Nutrition of the giant clam Tridacna gigas (L.) I. Contribution of filter feeding and photosynthates to respiration and growth. J Exp Mar Biol Ecol 155(1):105–122
Klumpp D, Lucas J (1994) Nutritional ecology of the giant clams Tridacna tevoroa and Tridacna derasa from Tonga: influence of light on filter-feeding and photosynthesis. Mar Ecol Prog Ser:147–156
Knowlton N (2000) Molecular genetic analyses of species boundaries in the sea. In: Marine genetics. Springer, pp, pp 73–90
Kochzius M, Nuryanto A (2008) Strong genetic population structure in the boring giant clam, Tridacna crocea, across the Indo-Malay Archipelago: implications related to evolutionary processes and connectivity. Mol Ecol 17(17):3775–3787
Ladd HS (1934) Geology of Viti Levu, Fiji. Bernice P. Bishop Museum Bulletin 119
LaJeunesse TC (2001) Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the ITS region: in search of a “species” level marker. J Phys 37(5):866–880
LaJeunesse TC (2002) Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar Biol 141(2):387–400
LaJeunesse TC, Loh WK, Van Woesik R, Hoegh-Guldberg O, Schmidt GW, Fitt WK (2003) Low symbiont diversity in southern Great Barrier Reef corals, relative to those of the Caribbean. Limnol Oceanogr 48(5):2046–2054
LaJeunesse TC, Lee SY, Gil-Agudelo DL, Knowlton N, Jeong HJ (2015) Symbiodiniaceae necroappetens sp. nov. (Dinophyceae): an opportunist ‘zooxanthella’ found in bleached and diseased tissues of Caribbean reef corals. Eur J Phycol 50(2):223–238
LaJeunesse TC, Pettay DT, Sampayo EM, Phongsuwan N, Brown B, Obura DO, Hoegh-Guldberg O, Fitt WK (2010) Long-standing environmental conditions, geographic isolation and host–symbiont specificity influence the relative ecological dominance and genetic diversification of coral endosymbionts in the genus Symbiodiniaceae. J Biogeogr 37(5):785–800
LaJeunesse TC, Wham DC, Pettay DT, Parkinson JE, Keshavmurthy S, Chen CA (2014) Ecologically differentiated stress-tolerant endosymbionts in the dinoflagellate genus Symbiodinium (Dinophyceae) Clade D are different species. Phycologia 53(4):305–319
LaJeunesse TC, Parkinson JE, Gabrielson PW, Jeong HJ, Reimer JD, Voolstra CR, Santos SR (2018) Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr Biol 28(16):2570–2580
Larson C (2016) Shell trade pushes giant clams to the brink. American Association for the Advancement of Science 351(6271):323–324
Lee SY, Jeong HJ, Kang NS, Jang TY, Jang SH, Lajeunesse TC (2015) Symbiodiniaceae tridacnidorum sp. nov., a dinoflagellate common to Indo-Pacific giant clams, and a revised morphological description of Symbiodiniaceae microadriaticum Freudenthal, emended Trench & Blank. Eur J Phycol 50(2):155–172
Leggat W, Buck BH, Grice A, Yellowlees D (2003) The impact of bleaching on the metabolic contribution of dinoflagellate symbionts to their giant clam host. Plant Cell Environ 26(12):1951–1961
Lewis A, Adams T, Ledua E (1988) Fiji's giant clam stocks- A review of their distribution, abundance, exploitation and management. ACIAR Monograph Series 98:66–72
Linnaeus C (1758) Systema Naturae per Regna tria Naturae, secundum Classes, Ordines, Genera Species, cum Characteribus, Differentiis, Synonymis, Locis. Tomus I. Editio decima. Holmiae: Laur. Salvius:824
Lucas JS (1994) The biology, exploitation, and mariculture of giant clams (Tridacnidae). Rev Fish Sci 2(3):181–223
Mansour K (1946) Communication between the dorsal edge of the mantle and the stomach of Tridacna. Nature 157(3999):844
McGill CJ, Pomory CM (2008) Effects of bleaching and nutrient supplementation on wet weight in the jellyfish Cassiopea xamachana (Bigelow)(Cnidaria: Scyphozoa). Mar Freshw Behav Physiol 41(3):179–189
Mekawy MS, Madkour HA (2012) Studies on the Indo-Pacific Tridacnidae (Tridacna maxima) from the Northern Red Sea, Egypt. Int J Geosci 3(05):1089
Mies M (2019) Evolution, diversity, distribution and the endangered future of the giant clam–Symbiodiniaceae association. Coral Reefs 38(6):1067–1084
Monsecour K (2016) A new species of giant clam (Bivalvia: Cardiidae) from the Western Indian Ocean. Conchylia 46:1–4
Naguit MRA (2009) CHARACTERIZATION OF THE SIPHONAL MANTLE OF TRIDACNA CROCEA. Threshold 4
Neo ML, Wabnitz CC, Braley RD, Heslinga GA, Favoulet C, Van Wynsberge S, Andréfouët S, Waters C, Tan ASH (2017) Giant clams (Bivalvia: Cardiidae: Tridacninae): a comprehensive update of species and their distribution, current threats and conservation status. Oceanogr Mar Biol Annu Rev 55:87–288
Ngugi DK, Antunes A, Brune A, Stingl U (2012) Biogeography of pelagic bacterioplankton across an antagonistic temperature–salinity gradient in the Red Sea. Mol Ecol 21(2):388–405
Nijman V, Spaan D, Nekaris KA-I (2015) Large-scale trade in legally protected marine mollusc shells from Java and Bali, Indonesia. PLoS One 10(12):e0140593
Nishi E (2001) Fine structure and distribution of iridophores in the photo-symbiotic bivalve subfamily Fraginae (Cardioidea). Veliger 44(1):54–65
Norton JH, Jones GW (1992) The giant clam: an anatomical and histological atlas. The giant clam: an anatomical and histological atlas
Norton J, Prior H, Baillie B, Yellowlees D (1995) Atrophy of the zooxanthellal tubular system in bleached giant clams Tridacna gigas. J Invertebr Pathol 66(3):307–310
Osman EO, Smith DJ, Ziegler M, Kürten B, Conrad C, El-Haddad KM, Voolstra CR, Suggett DJ (2018) Thermal refugia against coral bleaching throughout the northern Red Sea. Glob Chang Biol 24(2)
Palumbi S (1997) Molecular biogeography of the Pacific. Coral Reefs 16(1):S47–S52
Pappas MK, He S, Hardenstine RS, Kanee H, Berumen ML (2017) Genetic diversity of giant clams (Tridacna spp.) and their associated Symbiodiniaceae in the central Red Sea. Mar Biodivers 47(4):1209–1222
Penny SS, Willan RC (2014) Description of a new species of giant clam (Bivalvia: Tridacnidae) from Ningaloo Reef, Western Australia. Molluscan Research 34(3):201–211
Perez SF, Cook CB, Brooks WR (2001) The role of symbiotic dinoflagellates in the temperature-induced bleaching response of the subtropical sea anemone Aiptasia pallida. J Exp Mar Biol Ecol 256(1):1–14
Pochon X, Gates RD (2010) A new Symbiodinium clade (Dinophyceae) from soritid foraminifera in Hawai’i. Mol phylogenet Evol 56(1):492–497
Pochon X, Pawlowski J, Zaninetti L, Rowan R (2001) High genetic diversity and relative specificity among Symbiodinium-like endosymbiotic dinoflagellates in soritid foraminiferans. Mar Biol 139(6):1069–1078
Raitsos DE, Pradhan Y, Brewin RJ, Stenchikov G, Hoteit I (2013) Remote sensing the phytoplankton seasonal succession of the Red Sea. PLoS One 8(6):e64909
Richter C, Roa-Quiaoit H, Jantzen C, Al-Zibdah M, Kochzius M (2008) Collapse of a new living species of giant clam in the Red Sea. Curr Biol 18(17):1349–1354
Roa-Quiaoit HAF (2005) In: Red Sea. PhD, Universitat Bremen (ed) The ecology and culture of giant clams (Tridacnidae) in the Jordanian sector of the Gulf of Aqaba
Rowan R, Powers DA (1992) Ribosomal RNA sequences and the diversity of symbiotic dinoflagellates (zooxanthellae). Proc Natl Acad Sci 89(8):3639–3643
Rowan R, Knowlton N, Baker A, Jara J (1997) Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388(6639):265–269
Sirenko BI, Scarlato OA (1991) Tridacna rosewateri sp. n., a new species of giant clam from the Indian Ocean. La Conchiglia 22(261):4–9
Stat M, Gates RD (2011) Clade D Symbiodiniaceae in scleractinian corals: a “nugget” of hope, a selfish opportunist, an ominous sign, or all of the above? Journal of Marine Biology 2011
Stern RF, Andersen RA, Jameson I, Küpper FC, Coffroth MA, Vaulot D, Kasai F (2012) Evaluating the ribosomal internal transcribed spacer (ITS) as a candidate dinoflagellate barcode marker. PLoS One 7(8):e42780
Stern RF, Horak A, Andrew RL, Coffroth MA, Andersen RA, Küpper FC, Brand J (2010) Environmental barcoding reveals massive dinoflagellate diversity in marine environments. PLoS One 5(11):e13991
Su Y, Hung J-H, Kubo H, Liu L-L (2014) Tridacna noae (Röding, 1798)–a valid giant clam species separated from Tridacna maxima (Röding, 1798) by morphological and genetic data. Raffles Bulletin of Zoology 62
Sweeney A, Holt A, Gagnon Y, Morse D (2012) Giant clam iridocytes optimize photosynthetic symbiosis. In: Annual Meeting of the Society-for-Integrative-and-Comparative-Biology (SICB). OXFORD UNIV PRESS INC, pp E171–E171
Toller WW, Rowan R, Knowlton N (2001) Zooxanthellae of the Montastraea annularis species complex: patterns of distribution of four taxa of Symbiodiniaceae on different reefs and across depths. Biol Bull 201(3):348–359
van Oppen MJ, Palstra FP, Piquet AMT, Miller DJ (2001) Patterns of coral–dinoflagellate associations in Acropora: significance of local availability and physiology of Symbiodinium strains and host–symbiont selectivity. Proc R Soc London, Ser B Biol Sci 268(1478):1759–1767
Van Wynsberge S, Andréfouët S, Gaertner-Mazouni N, Wabnitz CC, Gilbert A, Remoissenet G, Payri C, Fauvelot C (2016) Drivers of density for the exploited giant clam Tridacna maxima: a meta-analysis. Fish Fish 17(3):567–584
Venkatesan V (2010) Marine ornamental molluscs. National Training Programme on Marine Ornamental Fish Culture, pp 27–32
Venn A, Loram J, Douglas A (2008) Photosynthetic symbioses in animals. J Exp Bot 59(5):1069–1080
Voolstra CR, Miller DJ, Ragan MA, Hoffmann A, Hoegh-Guldberg O, Bourne D, Ball E, Ying H, Foret S, Takahashi S (2015) The ReFuGe 2020 Consortium—using “omics” approaches to explore the adaptability and resilience of coral holobionts to environmental change. Front Mar Sci 2:68
Warner PA, Oppen MJ, Willis BL (2015) Unexpected cryptic species diversity in the widespread coral Seriatopora hystrix masks spatial-genetic patterns of connectivity. Mol Ecol 24(12):2993–3008
Yonge C (1975) Giant clams. Sci Am 232(4):96–105
Ziegler M, FitzPatrick SK, Burghardt I, Liberatore KL, Leffler AJ, Takacs-Vesbach C, Shepherd U (2014) Thermal stress response in a dinoflagellate-bearing nudibranch and the octocoral on which it feeds. Coral Reefs 33(4):1085–1099
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pappas, M. (2021). The Diversity of the Giant Clams and Their Associated Symbiodiniaceae Algae in the Red Sea. In: Jawad, L.A. (eds) The Arabian Seas: Biodiversity, Environmental Challenges and Conservation Measures. Springer, Cham. https://doi.org/10.1007/978-3-030-51506-5_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-51506-5_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-51505-8
Online ISBN: 978-3-030-51506-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)