Abstract
Streams and rivers are critical components of Arctic watersheds, functioning as corridors for the movement of water, carbon, and other solutes from headwater streams to larger rivers, estuaries, and the Arctic Ocean. Recent climate change in the Arctic has altered stream and river discharge, temperature, and biogeochemical processes. In this chapter, we summarize the state of research linking watershed hydrology and biogeochemical cycling in Arctic rivers, and how these processes are changing in response to changing climate and disturbance regimes (e.g., permafrost thaw, wildfire). The chapter is divided into three main sections. First, we examine hydrologic controls on stream and river chemistry, including the roles of spring snowmelt and subsurface hydrology as mediated by permafrost characteristics. Second, we summarize recent findings from the literature that describe biogeochemical processes in Arctic rivers, with particular focus on the cycling of organic carbon, nitrogen and phosphorus species, and a suite of trace elements. Third, we identify uncertainties and current gaps in our knowledge of biogeochemical processes in Arctic rivers and recommend steps forward to address these uncertainties.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
The Arctic region is characterized by extreme climatologic and physiographic conditions not found elsewhere on Earth (Hinzman et al. 2005). Air temperatures can vary as much as 70 °C between winter and summer and sunlight shifts from total darkness to continuous sunlight. Air temperatures in the Arctic are warming at a faster rate than temperate and tropical regions at lower latitudes and much research is focused on the responses of Arctic systems to this rapid warming and associated changing seasonality (Larsen et al. 2014). Of particular concern is the response of Arctic ecosystems to changing disturbance regimes, including changing hydrology and increasing river discharge, wildfire frequency and severity, and permafrost thaw (Turetsky et al. 2011; Schuur et al. 2015; Walvoord and Kurylyk 2016). Warming and disturbance can alter biogeochemical cycles in both terrestrial and aquatic ecosystems. Recent research has focused on biogeochemical feedbacks from terrestrial ecosystems to the atmosphere and climate system (e.g., Schuur et al. 2015), but there is also considerable interest in the response of aquatic ecosystems to changing conditions in the Arctic, including streams and rivers draining high-latitude watersheds (e.g., Vonk et al. 2015a).
Arctic rivers and streams are important corridors for the movement of water, carbon (C), other nutrients, and a variety of other solutes from headwater streams to larger rivers, estuaries, and ultimately, the Arctic Ocean. The chemical composition of streams and rivers in the Arctic varies considerably across space and time, as dictated by a variety of factors including landscape characteristics (vegetation, lithology), watershed hydrology, latitudes, and in-stream processes (Guo et al. 2004a; Neff et al. 2006; Holmes et al. 2012; Douglas et al. 2013; Pokrovsky et al. 2016, 2018). Arctic river chemistry typically exhibits strong seasonal patterns, with significant differences observed between the peak flows of spring snowmelt and base flow conditions during summer and winter (e.g., Finlay et al. 2006; Guo et al. 2012). Recent work has documented long-term changes in Arctic stream and river chemistry over the past 40–50 years, indicating climate and landscape change may be altering the concentration and flux of various solutes, although specific pathways and mechanisms are not well understood and could be seemingly contradictory (Frey and Smith 2005; Striegl et al. 2005; Toohey et al. 2016; Lung et al. 2018). Given the rapid pace of change in the Arctic, many uncertainties remain regarding the dynamics and trajectory of river chemistry and biogeochemical processes (McGuire et al. 2009).
In this chapter, we summarize riverine sources and transport of chemical species and their biogeochemical processes in Arctic watersheds with a focus on the major biogeochemical compounds of greatest impact in watershed processes. These include dissolved organic carbon (DOC), nutrients (nitrogen (N) and phosphorus (P)), dissolved silica, and selected trace elements (iron, strontium, uranium, and mercury). We review recent findings from the literature that document how the cycling of these constituents are changing in response to climate change and disturbance in the Arctic and identify knowledge gaps that limit our ability to project how river biogeochemical cycles will be affected in a warmer future.
2 Hydrologic Controls on Arctic River Biogeochemistry
2.1 Spring Melt Runoff Processes as Major Drivers of Arctic Watershed Biogeochemistry
One unique aspect of Arctic watersheds is the spring snowmelt runoff (freshet). In a short time window in May or June (often in less than 2 weeks), much of the yearly surface water discharge runs from the land through river channels to the sea (Kane et al. 1989; McNamara et al. 1997, 2008; Bowling et al. 2003; Holmes et al. 2012). A white, snow-covered landscape is converted rapidly to a brown and eventually green one (Fig. 11.1). During the spring snowmelt period, discharge is often at peak yearly values and there is a significant dilution of river solutes, yet stream water concentrations of dissolved organic C (DOC) and other nutrients like ammonium (NH4+), phosphate (PO43−), and nitrate (NO3−) reach their highest levels of the year due to hydrologic “flushing” (e.g., Hornberger et al. 1994; Finlay et al. 2006; Raymond et al. 2007; Cai et al. 2008a, b; Guo et al. 2004a, 2012; Douglas et al. 2013).
Photographs of a small watershed near Utqiaġvik, Alaska, representing the major seasons and seasonal transitions in Arctic watersheds. Most of the Arctic is snow-covered for up to 8 months of the year. The spring melt period includes a dramatic flux of surface water and biogeochemical compounds that is active for only a few weeks.
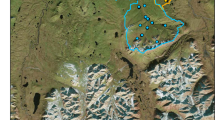
The snowmelt period is dynamic across a range of spatial scales. For instance, at the macro-scale, the timing of peak snowmelt varies longitudinally within a watershed, with the lower reaches of the river snow-free by mid-June and starting to Greenup, while headwater reaches are still snow-covered and just beginning to melt (Fig. 11.2). As a result, the timing of melt-driven changes in stream and river chemistry varies within an individual watershed. At the micro-scale, carbon (C), nutrients, major elements, and trace metals in the snowpack become constituents in meltwater. An “ionic pulse” of solutes moves downward into the pool at the base of the snowpack (Tranter et al. 1986; Rember and Trefry 2004; Douglas et al. 2017). Elevated solute concentrations before snowmelt are partially caused by ion exclusion from the quasi-liquid water layer, a disordered microthin layer on snow grain surfaces (Dominé and Shepson 2002). As snow and ice crystals approach 0 °C the snow grains start to metamorphose into rounded melt grain clusters. Larger molecules or ions (like sulfate (SO42−) and some metals) present as impurities on the snow or ice crystal surface undergo preferential elution compared to smaller ions like chloride (Cragin and McGilvary 1995). As a consequence, DOC, SO42−, NO3−, lead (Pb), and mercury (Hg) concentrations in the early pulse of snowmelt water that moves across the landscape typically exceed bulk snowpack values (Williams and Melack 1991; Douglas et al. 2017). Though vegetation, soils, and atmospheric deposition are the dominant sources of nutrients, major ions, and trace metals in river waters (Rember and Trefry 2004; Cai et al. 2008a; Barker et al. 2014) the snowpack is a source of some chemical species (e.g., Pb, SO42−; Douglas and Sturm 2004).
A series of photos, taken within 2 days of one another, exhibiting the different scales and the delayed timing of spring melt across a broad geographical range. Each blue box represents a hydrologic source to the larger system it feeds. Note that in the upstream mountains and on hillslopes snowmelt is just starting while lower elevation small streams are beginning to flow and reach a peak discharge and, at the regional scale, the Yukon River (large background photo) is snow and ice-free and has initiated Greenup.
In most Arctic locations, the surface soils of the seasonally thawed active layer are frozen during spring melt, which limits infiltration of snowmelt water into surface soils depending on the previous year’s soil moisture and snow water equivalent (Gray et al. 2001), in addition to a variety of other factors including the presence of preferential pathways (Stähl et al. 2004), soil aggregates (Koren et al. 1999), and abundance of air-filled macropores (Niu and Yang 2006). Where permafrost is present, soils and bedrock can be frozen to a depth of up to 300 m. As such, groundwater discharge to surface waters is negligible during snowmelt and the nutrient load in runoff is predominantly controlled by the vegetation cover and soil type (Judd and Kling 2002; Hobbie and Gough 2004). Decaying plant litter and shallow organic-soil horizons at the base of the snowpack are the major sources of DOC and nutrients to river flows during this period (Fig. 11.3). Snowmelt has been found to contain up to 60% of the annual DOC export from major Arctic rivers (Guo et al. 2007; Raymond et al. 2007). This C, leached from vegetation and surface organic-soil layers, is of the modern age (Neff et al. 2006; Guo and Macdonald 2006) and close to a third of the DOC pool may be biologically labile (Holmes et al. 2008) and over 90% of chromophoric DOM can be photochemically degraded (e.g., Cory et al. 2014) although only a small fraction of soil organic C can be leached out during permafrost thaw (Xu et al. 2009; Gao et al. 2018).
Photos of Imnavait Creek north of the Brooks Range in the Alaskan Arctic during peak spring melt flow (left) and in early fall (right). Note the dark brown color of the water during spring melt which is attributable to the heavy load of particulate organic C, decayed vegetation, and mineral particles. For most of the year, this watershed is a small trickle roughly a few meters wide (fall photo).
2.2 Seasonally Frozen Ground and Permafrost as Controls on Arctic River Biogeochemistry
As noted above, many Arctic watersheds are underlain by permafrost, earth material such as soil, organic matter, or bedrock that has remained below 0 °C for at least two consecutive years. In most cases, permafrost has remained frozen for hundreds to thousands of years. However, recent warming in the Arctic has caused permafrost to warm and thaw (Jorgenson et al. 2006; Romanovsky et al. 2010). This has important consequences for watershed hydrology and the chemical composition of rivers (Frey and McClelland 2009; Walvoord et al. 2012). Permafrost properties, including thermal and hydraulic characteristics, are important for controlling depth and velocity of subsurface flow paths that contribute to streamflow. The active layer is the shallow surface vegetation and soil above the permafrost that thaws and refreezes on an annual basis (Fig. 11.4). Seasonally thawing of the active layer governs depths of subsurface flow paths during the summer (i.e., the supra-permafrost aquifer; Lamontagne-Halle et al. 2018) and consequently influences leaching and mobilization of solutes from soil horizons.
The surface vegetation (dark brown mass) in an 80-cm-long SIPRE core collected in early spring. Note the lighter colored material toward the base of the core. This region, comprised of small (1–2 cm) layers of transition zone ice, is the boundary between the seasonally thawed active layer and the top of the near-surface permafrost.
In the continuous permafrost zone (>90% of the land area underlain by permafrost), groundwater discharge from deep, sub-permafrost aquifers to surface waters is limited (Fig. 11.5). In this area, the permafrost is hundreds of meters thick. By contrast, in the discontinuous permafrost zone to the south (50–90% of the land area underlain by permafrost) the permafrost is warmer and less thick (10–100 m), groundwater discharge is higher and can comprise a larger fraction of river discharge (Walvoord and Striegl 2007). Within the discontinuous zone, slope and aspect can drive the spatial extent of permafrost with north-facing slopes and valley bottoms underlain by permafrost and south-facing slopes and ridge tops are often permafrost-free. Headwater catchments that vary with permafrost extent also exhibit striking variations in discharge patterns and chemical composition (Maclean et al. 1999). The hydrologic mixing of waters from shallow supra-permafrost and deep sub-permafrost aquifers is a key determinant of spatial and temporal variability in stream water chemistry (O’Donnell et al. 2012, 2016). Thermokarst, or the subsidence of the ground surface following the thawing of ice-rich permafrost (Loranty et al. 2018), can also alter stream and river chemistry (Bowden et al. 2008). However, yields and fluxes of C, nutrients, and trace elements via lateral transport from soils to streams, rivers, and the Arctic Ocean during permafrost thaw and coastal erosion are poorly understood although laboratory studies have mimicked the permafrost–water interactions and quantified their yields and fluxes of C and nutrients (Dou et al. 2008; Xu et al. 2009; Gao et al. 2018). Much uncertainty exists regarding the effects of permafrost thaw on river chemistry under a warming climate (Liao and Zhuang 2017). Recent findings on this topic are highlighted below.

(adapted from Walvoord and Kurylyk 2016)
A conceptual model of subsurface hydrologic change associated with thawing permafrost
3 Major Chemical Species/Components in the Arctic Stream and River Waters
3.1 Dissolved Organic Carbon
DOC is a critical component of aquatic ecosystems and the Arctic C cycle as it functions to regulate water quality and nutrient availability and serves as a substrate for microbially mediated reactions (Cole et al. 2007; Tranvik et al. 2009). DOC can also influence photochemical reactions and interactions with trace metals and organic pollutants, altering their fate, transport, and bioavailability/toxicity (Chin et al. 1997; Aiken et al. 2011; Philippe and Schaumann 2014). Increases in DOC concentration have contributed to the browning of surface waters in some high-latitude regions (Roulet and Moore 2006), affecting the photic zone and rates of primary productivity. In the Arctic, DOC in rivers is sensitive to watershed hydrology (Striegl et al. 2005; O’Donnell et al. 2010, 2012), permafrost presence (Maclean et al. 1999; Frey and McClelland 2009; Olefeldt et al. 2014), active layer thickness (O’Donnell et al. 2016; Harms et al. 2016), wetland extent, and permafrost soil properties (O’Donnell et al. 2016).
Soils of the northern circumpolar permafrost region store approximately 1300 petagrams (Pg) of organic C (Hugelius et al. 2014). As a result, high-latitude rivers tend to have higher DOC concentrations (Finlay et al. 2006) than temperate or tropical rivers (Hope et al. 1994). Regardless of sources, the flux of C in Arctic rivers represents an important component of the high-latitude C budget (McGuire et al. 2009), and the magnitude of this flux is expected to change under projected warming and disturbance scenarios (Frey and Smith 2005; Striegl et al. 2005; Kicklighter et al. 2013). Moreover, inland waters can release C to the atmosphere through microbial and photochemical processes (Kling et al. 1991; Cory et al. 2014), which can function as a positive feedback to the climate system. By incorporating DOC into terrestrial ecosystem and earth system models, it is possible to better constrain C sources and sinks in the Arctic and globally (Kicklighter et al. 2013; Wu et al. 2014).
DOC in rivers is chemically complex, reflecting a broad gradient of compounds ranging in molecular size, structure, and decomposability/reactivity (Xu and Guo 2017). Scientists have developed a variety of analytical techniques to characterize the composition and reactivity of dissolved organic matter (DOM), including bulk measurements of concentration (DOC, dissolved organic N (DON)), optical measurements (ultraviolet (UV)–visible absorbance and fluorescence), chemical fractionation, and high-resolution mass spectroscopy (e.g., FTICR-MS) among others. In the Arctic rivers, DOM composition varies across space and time, as determined by hydrology and landscape features. Most rivers are dominated by hydrophobic organic acids (e.g., humic and fulvic acids) during the open-water period, which tends to be highly aromatic compounds derived from organic-rich wetland soils (Striegl et al. 2005; Spencer et al. 2008; O’Donnell et al. 2010). During winter or in groundwater-dominated systems, DOM is comprised primarily of low-molecular weight aliphatic compounds and tends to more N-rich (O’Donnell et al. 2012). DOM composition reflects and integrates processing in both the terrestrial (e.g., microbial transformation, sorption to mineral soils) and aquatic ecosystems (e.g., photo-oxidation), and is closely linked to its bio-lability (Holmes et al. 2008). In general, DOM lability is higher in high-latitude rivers than other biomes and tends to decrease with increasing stream size or order (Vonk et al. 2015b). In addition, DOM liability is highly related to chemical composition, molecular size, and specific degradation pathways (e.g., photochemical vs biological degradation; Xu and Guo 2018).
In the Arctic, the majority of riverine DOC is derived from terrestrial sources (Wauthy et al. 2018). The decomposition of plant litter, organic soils, and mineral-associated soil organic matter can drive production of DOC, which is then, transported from terrestrial to aquatic ecosystems through runoff and subsurface flow paths. Recent work has also documented the importance of permafrost thaw on the delivery of ancient DOC to river networks (e.g., Spencer et al. 2015). However, DOC transported in Arctic rivers is mostly contemporary, especially the high-molecular weight (>1 lDa) DOC fraction (Benner et al. 2004; Guo and Macdonald 2006; Raymond et al. 2007) or much younger than both particulate organic C (POC) pool and river sediment (Guo et al. 2007). This indicates riverine DOC derived from old permafrost soil is limited although pre-aged POC derived from permafrost has been measured in Arctic rivers (Guo et al. 2004a, b, 2007). In addition, the 14C ages among DOC, POC, and river sediment changed across different rivers and sampling season, and the relative contributions of DOC from modern primary production and ancient soil organic C also varied with rivers (Fig. 11.6). Young DOC but extremely old POC observed in Arctic rivers are consistent with the experimental results that only a small fraction (<2%) of permafrost TOC is water-soluble and can be released into the DOC pool during permafrost interactions with aquatic environments (Guo et al. 2007; Dou et al. 2008; Xu et al. 2009; Gao et al. 2018).
Changes in ∆14C values (or 14C ages) among DOC, POC and river sedimentary organic carbon pool in the Mackenzie, Sag (Sagavanirktok) and Yukon Rivers (left panel) and relative contributions of DOC from modern primary production and ancient soil organic C in different Arctic rivers, including the Mackenzie, Sagavanirktok (Sag), and Yukon Rivers (right panel, from Guo et al. 2007)
Given the large stores of permafrost OC in the Arctic terrestrial ecosystem, warming and thawing could potentially release significant amounts of DOC into Arctic waterways should permafrost soils be thawed and flushed into aquatic environments even though only a small fraction of total soil organic C can be released into water-extractable phase (Xu et al. 2009; Gao et al. 2018). Within the soil-derived DOC pool, the aromatic components can be readily decomposed photochemically (Cory et al. 2014) while bio-labile organic acids (e.g., acetate, Ewing et al. 2015) and other carbohydrates and protein-like components can be rapidly degraded microbially regardless of their radiocarbon (14C) ages (Vonk et al. 2013, 2015b; Xu and Guo 2018; Gao et al. 2018). Evidence from 14C measurements indicates permafrost-derived old DOC is not an important component of the DOC pool in higher order rivers (Guo and Macdonald 2006; Guo et al. 2007; Raymond et al. 2007; O’Donnell et al. 2014; Aiken et al. 2014). This is consistent with the consistently low DOC yields of soil-OC in different aged permafrost (up to 16,000 years BP) from northern Alaska (Guo et al. 2007; Dou et al. 2008; Xu et al. 2009; Gao et al. 2018). Therefore, DOC in Arctic rivers should be mostly derived from surface soil and contemporary terrestrial primary production enhanced by warming, and autochthonous sources of DOC to rivers, include stream periphyton, are generally a minor component of DOC pool in Arctic watersheds (e.g., Mann et al. 2015). However, the contribution of autochthonous DOC to Arctic rivers can be greatly underestimated due to its 14C reservoir effect or old dissolved inorganic C (DIC) 14C ages (Guo et al., unpublished data).
DOC concentration and DOM composition vary across watersheds that differ with respect to landscape and subsurface properties. In the Yukon River basin of Alaska and Canada, DOC concentration and flux vary among watersheds according to watershed hydrogeologic characteristics and source water contribution to streamflow (Striegl et al. 2007; O’Donnell et al. 2010). Blackwater streams (i.e., streams dominated by hydrologic and C inputs from wetlands) have high DOC concentrations relative to glacially fed or groundwater-dominated streams. Further, blackwater streams tend to have higher concentrations of aromatic DOM (i.e., high specific ultraviolet absorbance, or SUVA254; Weishaar et al. 2003) compared to glacially fed or groundwater-dominated streams which tend to have higher concentrations of low-molecular weight organic acids and aliphatic compounds. Permafrost thermal state (e.g., ground temperatures, rates and timing of seasonal thaw, and active layer thickness) and hydraulic properties (e.g., parent material, ground ice content) also influence spatial patterns of DOC in streams and rivers (Olefeldt et al. 2014; Harms et al. 2016; O’Donnell et al. 2016; Fig. 11.7). More generally, watershed properties that convey water through runoff and shallow subsurface flow paths (supra-permafrost flow; Fig. 11.5) typically yield larger concentrations of DOC in surface waters. Alternately, watersheds that allow for more groundwater discharge from sub-permafrost aquifers typically yield low concentrations of DOC in surface waters.
Arctic rivers drain watersheds that vary with respect to soil and bedrock characteristics and permafrost mineralogy and ground-ice content. In the Alaskan Arctic, watersheds are mostly commonly underlain by ice-poor bedrock (top left), coarse-grained glacial till (top middle), ice-rich, fine-grained glaciolacustrine deposits (top left), ice-rich Pleistocene loess (yedoma; lower left), sand (lower middle), and volcanic deposits (lower right). These watershed properties strongly influence the hydrology and chemical composition of streams and rivers (O’Donnell et al. 2016)
DOC concentration and flux are highly sensitive to recent warming in high-latitude regions. Numerous measurements and studies have focused on the flux of C in large Arctic rivers and estuaries (Dittmar and Kattner 2003; Raymond et al. 2007; Holmes et al. 2008; Spencer et al. 2008; Guo et al. 2012), given the importance of these riverine fluxes as components of regional-scale terrestrial budgets (McGuire et al. 2010; Kicklighter et al. 2013) and the large C stores in permafrost (Hugelius et al. 2014). In the Yukon River basin discharge-normalized DOC flux has decreased over the past 40 years (Striegl et al. 2005), likely due to permafrost thaw and associated increases in groundwater discharge to streamflow (Walvoord & Striegl 2007). Thawing of permafrost exposes more mineral soils to weathering and provides the potential for extensive physical stabilization (i.e., adsorption) and retention of DOC by soils, reducing the flux to rivers (Kawahigashi et al. 2004, 2006). On the other hand, warming could also increase primary production (e.g., higher plants) in Arctic terrestrial ecosystems, enhancing the release of contemporary DOC to Arctic rivers since river DOC has been found mostly contemporary in all major Arctic rivers (e.g., Benner et al. 2004; Guo and Macdonald 2006; Raymond et al. 2007). Total yearly DOC export has also declined in the much smaller Kuparuk River in northern Alaska, likely due to the decreases in discharge during the spring snowmelt period (McClelland et al. 2007) or possibly due to the increases in baseflow (e.g., Walvoord and Striegl 2007; O’Donnell et al. 2012). However, DOC flux has increased by nearly 40% in the much larger Mackenzie River in northwest Canada, with much of the change occurring during autumn and winter flow (Tank et al. 2016). This appears to be influenced by permafrost and hydrological conditions and higher rates of plant production, but the exact mechanisms driving this change are still unclear. The different responses to the two terrain types to climate warming and associated disturbances may be driven by geomorphologic characteristics. Model simulations indicate that DOC loading from terrestrial ecosystems to Arctic rivers has increased in recent decades due to the increased water yields (Kicklighter et al. 2013). These seemingly contradictory observations and conclusions between different studies on the increased and decreased DOC fluxes remain to be verified. Future changes in riverine DOC flux will likely depend on the balance of factors that promote DOC production, loss, and hydrologic transport.
Once in a stream or river DOC can undergo in-stream processing which modifies lateral C fluxes from terrestrial ecosystems to the Arctic Ocean. In the northern permafrost region, DOC concentrations vary considerably across the continuum of soils, streams, and larger rivers (O’Donnell et al., unpublished data; Fig. 11.8). DOM composition strongly determines its fate and reactivity along the river reaches. While aromatic and humic-like DOM components are preferentially decomposed through photochemical pathways, carbohydrates and protein-like DOM components are somewhat resistant to photochemical degradation but preferentially utilized by microorganisms (Cory et al. 2014; Xu et al. 2018; Xu and Guo 2018). For example, recent work by Cory et al. (2014) has documented the importance of photo-oxidation of CDOM and DOC by sunlight in the water column of Arctic streams. Complete oxidation of DOC by sunlight and microorganisms results in the release of CO2 from surface waters to the atmosphere, whereas partial oxidation results in the chemical transformation of the DOC pool prior to transport to downstream ecosystems. In many cases, rates of photo-oxidation outpace rates of DOC mineralization by aquatic microbial communities depending on the DOM composition. Further, the chemical alteration of DOC by sunlight can stimulate microbial mineralization of the residual DOC pool through the production of more bio-labile compounds (Ward et al. 2017). River ecosystems can also function to retain DOC, reducing the flux of C to downstream ecosystems or to the atmosphere. Some evidence suggests that benthic biofilm communities in streams and rivers can function to retain DOC through adsorption processes (Battin et al. 2016).
3.2 Major Nutrients: Nitrogen and Phosphorus
N and P are essential nutrients that can govern C-cycle processes, such as rates of primary production and microbial process rates in terrestrial and aquatic ecosystems (Elser et al. 1990; Vitousek and Howarth 1991; Heimann and Reichstein 2008). N occurs in various forms in stream ecosystems, including particulate N, dissolved organic N (DON, e.g., amino acids, proteins, etc.), and dissolved inorganic N (DIN: NO3−, nitrite (NO2−), and NH4+). N-containing compounds can cycle between these various forms through microbially mediated reactions, which are typically controlled by reduction–oxidation (redox) state and availability (Hedin et al. 1998). N can also be assimilated by aquatic vegetation, including benthic algae, moss, phytoplankton, and macrophytes. While P typically limits primary productivity in aquatic systems, increased availability of inorganic N can directly limit or co-limit primary production in some systems (Grimm and Fisher 1986). Some microbial processes, such as nitrification (conversion of NH4+ to NO3−) and denitrification (conversion of NO3− to nitrous oxide (N2O) and dinitrogen gas (N2)) can result in the loss of N from the system to the atmosphere. N2O is a greenhouse gas, and emission of N2O from aquatic ecosystems can function as positive feedback to the climate system (Beaulieu et al. 2010).
In addition, the abundance, chemical speciation, and export fluxes of nutrients from rivers can be linked to changes in permafrost dynamics, vegetation/land cover, hydrological conditions, and human disturbance in river basins and changes in the ecosystem. Humans have dramatically altered the global N cycle (Vitousek et al. 1997), primarily through N fertilization of agricultural lands and through altering rates of atmospheric deposition. In undisturbed regions of the Arctic, the primary inputs of N to watersheds are through fixation of atmospheric N2 by N-fixing organisms (e.g., boreal feather mosses) and atmospheric deposition. While terrestrial ecosystems retain a significant fraction of these N inputs, some are released to streams and rivers (Vitousek and Reiners 1975).
Some research indicates that boreal watersheds are losing N, as fluvial N fluxes exceed watershed inputs (Jones et al. 2005). Compared to other forms of N, NO3− is highly mobile and can be readily transported via runoff and subsurface flow paths to surface waters, and is often the dominant form of DIN and even TDN in high-latitude streams and rivers (Petrone et al. 2006). Indeed, in the Chena River of interior Alaska, DIN accounted for 60 ± 15% of the total N while DON and particulate-N comprised 30 ± 8 and 10 ± 9%, respectively (Cai et al. 2008b). However, in the upper Yukon River, DON is the predominant N species, followed by particulate N and then DIN (Guo et al. 2004a, b). The same is true for the lower Yukon River with DON being the major N species (Guo et al. 2012). The predominance of DON in Arctic river waters is consistent with those derived from the leaching of different-aged permafrost soils in northern Alaska (Gao et al. 2018). Under warmer climatic conditions, increased mineralization of soil organic N pools may also increase production and transport of inorganic N to streams and rivers. Still, there are large uncertainties with respect to the fate of N in high-latitude watersheds in response to warming and disturbance (e.g., permafrost thaw; Frey and McClelland 2009). Sources of uncertainty include changing magnitude and seasonality of runoff and river discharge, the release of permafrost nitrogen, and rates of soil and in-stream N processing.
Wildfire, permafrost thaw, and erosional processes are three primary disturbance processes that may alter stream and river chemistry. Wildfires reduce terrestrial N stocks, but also temporarily reduce N retention by vegetation and soil microbial communities. Evidence from the boreal region indicates that wildfire may increase NO3− concentrations in headwater streams (Betts and Jones 2009). Wildfire can accelerate rates of permafrost thaw (O’Donnell et al. 2011), and thaw waters are typically enriched with DIN (Ewing et al. 2015). Recently, Ping et al (2011) reported that total N fluxes through coastal erosion along the Alaska Beaufort Sea coastal line could reach 7.76 ± 1.31 109 g-N/year during 1950–2000. On the other hand, based on laboratory leaching experiments, different aged permafrost with TN contents ranging from 500 to 12,000 µg-TN can produce soluble DIN, including NO3−, NO −,2 and NH4+, varying from 12 to 41 µg-N/g-soil, and DON between 8 and 116 µg-N/g-soil (Gao et al. 2018). Yields of DON from soil TN were only 0.9–3.4% while yields of DIN from TN varied from 0.3 to 2.3% depending on specific soil (Gao et al. 2018). Nevertheless, the overall TDN released from soil can be enormous should permafrost thaw. Within the soil TDN pool, DON is the dominant N species followed by NH4 and NO3 and NO2.
There are three primary sources of P to stream ecosystems, including soil/mineral leaching, anthropogenic input, and atmospheric deposition. Erosion and sedimentation through changes in watershed land use or disturbance can load P into aquatic systems. In soils, most P is found in particulate forms where organic or inorganic P complexes with mineral particles, such as iron (Fe), aluminum (Al), or manganese (Mn) hydroxides (Schlesinger and Bernhardt 2013). Permafrost soil leaching experiments show that yields of PO43− from soil ranged from 0.6 to 6 µg-P/g-soil or 0.12 to 1.2% of the total P in soil, while yields of dissolved organic P (DOP) were 1.1–8.1 µg-P/g-soil or 1.2–2.9% of total organic P (Gao et al. 2018). Decomposition of soil organic matter by microbes can also release P, both as DOP and mineralized forms (e.g., PO43−). Weathering of parent materials, such as limestone or volcanic deposits, can also contribute P to stream ecosystems. P cycling in streams is controlled by both abiotic processes (e.g., sorption–desorption reactions between surface water and sediments) and biological processes (decomposition of organic matter, assimilation, and immobilization in microbial biomass; Hendricks and White 2000). In most Arctic streams, P is the primary limiting nutrient governing primary production (Peterson et al. 1985). For example, long-term P fertilization of the Kuparuk River near the Toolik Field Station in northern Alaska has altered stream ecosystem function, leading to enhanced moss and algal productivity, invertebrate production, and fish production (Slavik et al. 2004). In many Arctic streams and rivers, N and P concentrations are very low, and in many cases below detection limits (e.g., O’Donnell et al. 2015). However, given that permafrost soils store large amounts of N and P (Harden et al. 2012; Ewing et al. 2015), warming and disturbance will likely increase both N and P inputs to streams from soils (e.g., Frey et al. 2007; Bowden et al. 2008) although yields of soluble N and P from permafrost are generally low (Gao et al. 2018), driving bottom-up effects on food web dynamics due to the overall large store in the northern high-latitude regions.
Overall, studies of the detailed chemical speciation of nutrients for Arctic rivers are somewhat limited. In contrast to rivers with significant anthropogenic influences such as the Mississippi, where PO43− is the predominant P species (Cai and Guo 2009), >80–90% of total P was measured in the >0.4 µm particulate phase in the upper Yukon River, followed by DOP, leaving PO43− mostly below the detection limit (Guo et al. 2004a, b). Similarly, particulate organic P is also the predominant P species in the Lower Yukon River (Guo et al. 2012). In the Chena River, particulate P is the dominant P species, especially during the spring freshet, comprising on average 74 ± 10% of the total P while DIP and DOP comprised 19 ± 9% and 7 ± 4% of the total P, respectively (Cai et al. 2008b; Fig. 11.9). A low abundance of dissolved P species in Arctic rivers is likely derived from the high surface reactivity of P or high values of partition coefficient between dissolved and particulate phases (e.g., Lin and Guo 2016). More studies are needed to better understand the transformation of P between dissolved and particulate, and between inorganic and organic P species during soil leaching and transport from land to waterways.
3.3 Dissolved Silica
Silicon (Si) is the second most abundant element in the Earth’s crust and a critical nutrient for diatoms in aquatic environments (De La Rocha et al. 1998), in addition to being essential to plant productivity and C cycling (Cornelis et al. 2011). Dissolved Si (DSi) exists in river waters as a result of weathering of Si-containing solid phases. In some Arctic rivers, dissolved silica responds differently than other solutes to permafrost dynamics in Arctic river systems. For example, Guo et al. (2004a, b) showed that thawing of the active layer and thus leaching of deeper soil horizon and riverbank erosion gave rise to higher orthosilicic acid (Si(OH)4) and DIC but lower DOC, DOP, and PO43− concentrations. Controls on DSi and nutrient exports (when normalized to watershed size) are also different across constituents. For instance, DSi fluxes are primarily governed by latitude (or temperature) and chemical weathering, whereas both N and P fluxes are regulated largely by anthropogenic influences or human disturbances (Guo et al. 2004a, b). In comparison with DOC and DIC, the concentration of DSi have been shown to be negatively correlated with DOC concentration, but positively correlated to conductivity and DIC, indicating that Si(OH)4 had a similar source function as DIC but different from DOC and other nutrients, especially during the snowmelt and early ice opening in the Yukon River Basin (Guo et al. 2004a, b).
DSi, as a proxy for silicate mineral weathering, can be used to identify the relative sources of major ions and trace metals in watersheds. As the seasonal thaw expands downward in a given summer thaw season the deeper thawed soils are increasingly comprised of a material that is only exposed to chemical or physical weathering for months or even days per year (Keller et al. 2007, 2010; Douglas et al. 2013; Barker et al. 2014; Fig. 11.10). At the local scale, this is also associated with an oxidation/reduction front that moves downward into the thawed soil horizon over time. This oxidation/reduction front has major ramifications for the fate and transport of trace elements and nutrients in permafrost watersheds because solubility is often controlled largely by oxidation state and pH (Kimbrough et al. 1999; Barker et al. 2014). Silicate minerals can also be exposed to weathering when the thaw front reaches formerly frozen permafrost soil mineral horizons, leading to the direct release of DSi species into soil solution and local surface waters. Any future expanse of the active layer and degradation of permafrost is expected to impact concentrations of DSi in Arctic river systems (Guo et al. 2004a, b; Holmes et al. 2012; Pokrovsky et al. 2013).
Photographs identifying oxidation and reduction zones in permafrost in the Imnavait Creek watershed north of the Brooks Range in the Alaskan Arctic. Left: the edge of a tundra pond where surface conditions are oxidizing but anaerobic, low pH, and photochemically limited reducing conditions are likely to present at the base of the water column and in the pond sediments. Right: a cross section of a pit excavated in mid-summer with a jackhammer. The seasonal thaw at this time was roughly 45 cm. Note the zone of oxidized Fe just above the seasonal thaw line. Also, note the reduced Fe (grey and gleyed) from 50 to 80 cm depth. This is likely active layer soils that were frozen at the time of the picture that denote a reducing environment.
Element cycling in the Arctic is expected to change as a result of increasing air temperatures (Alfredsson et al. 2016) through the expanse of the active layer, degradation of permafrost, and altering of vegetation and snow cover (Tape et al. 2006; Pokrovsky et al. 2013; Vaughan 2013). These changes will likely yield increased concentrations of dissolved Si in Arctic surface waters and the enrichment of the light Si stable isotope (Pokrovsky et al. 2013). Eventually, the formation of new wetlands and expansion of existing wetlands is expected to occur with increasing air temperatures, which have been shown to increase biological fixation and reduce the export of DSi (Sannel and Kuhry 2011). Any decrease in the amount of DSi that is transported to the Arctic Ocean as a result of riverine export will likely result in disturbances to marine biological life and C cycling (Alfredsson et al. 2016). Therefore, the effect of climate change on Arctic terrestrial systems with respect to DSi transport is complex and may yield varying feedback responses.
3.4 Trace Metals
Metals comprise a major fraction of soil, surface water, and sediment on Earth and play a significant role in hydro-biogeochemical reactions in the environment. In rivers, metals partition between phases and the fate, transport, mobility, and speciation of metals are controlled by a variety of site-specific environmental parameters, predominantly pH, oxidation state, temperature, soil moisture, hydraulic conductivity, soil type, dissolved oxygen, presence of organic materials/clay minerals, wind, and the presence/concentrations of other competing elements (Loretta and Li 2001; Knechtenhofer et al. 2003; Dinis and Fiúza 2006; Pachana et al. 2010; Liao et al. 2017). A summary of key biogeochemical weathering processes and their associated impact on watersheds is summarized in Table 11.1. In Arctic ecosystems, there are additional landscape-scale controls on the reaction chemistry of metals in rivers (e.g., extreme temperature variations, varying oxidation–reduction conditions seasonally in the subsurface (Fig. 11.10), and the presence of permafrost and seasonally thawed surface soils).
Increasing air temperatures, permafrost thaw, and projected shifting of permafrost boundaries have the potential to affect the fate and transport of metals to surface waters as a result of the unique chemical reactions that occur in the subsurface. Understanding metal reactions directly at the permafrost-active layer boundary can be complicated because there often exist sharp redox transitions, surface charge gradients, and phase changes (liquid/ice). In the near-surface environment, metals (particularly Fe and Mn) can have multiple oxidation states and thus act as electron donors or acceptors depending on local geochemical conditions. This can impact microbial communities and C speciation/cycling but C cycle processes can, in turn, control metals fate and transport (Pokrovsky and Schott 2002; Page et al. 2013; Stolpe et al. 2013; Salvadό et al. 2015; Emerson et al. 2015; Trusiak et al. 2018). Additionally, seasonal changes and annual freeze–thaw cycles inherent to Arctic freshwater processes influences metal transport to surface waters (Barker et al. 2014; Pokrovsky et al. 2016) and metal cycling in Arctic thaw ponds (Loiko et al. 2017; Pokrovsky et al. 2018; Raudina et al. 2018). As such, the chemical environment of metals in Arctic rivers tends to be highly heterogeneous, dependent on a variety of site-specific factors, and constantly undergoing a transformation. In an attempt to decrease the complexities of measuring permafrost-active layer dynamics, metals (e.g., strontium (Sr), Fe, Mn, and Al) have been employed as geochemical tracers to infer changes within Arctic watersheds and scale to the landscape watershed scale (Rember and Trefry 2004; Keller et al. 2010; Barker et al. 2014; Lehn et al. 2017).
Riverine sediment environments are a unique chemical setting for trace metal studies, particularly in Arctic ecosystems. Riverine sediments are composed of fine-grained materials that offer excellent surface capacity for metals to chemically and physically sorb or bind, including clay minerals (Ilgen and Trainor 2011; Cai et al. 2014), colloids (Pachana et al. 2010), organic matter (humic and fulvic acids; Sklodowski et al. 2006; Clemens and Ma 2016), Fe oxides (Yin et al. 2016), and Mn oxides (Gunawardana et al. 2015). Unlike surface water and soil systems, sediment environments tend to be more reducing and contain lesser amounts of dissolved oxygen (Morford and Emerson 1999). As a result, metal speciation, mobility, and accumulation are affected by deposition, burial, interaction, and re-suspension in aquatic sediment systems. In particular, metal(loid)s have been shown to accumulate in reducing environments (Kimbrough et al. 1999; Barker et al. 2014; Ilgen et al. 2014) and sediments have been shown to stabilize reduced metals (Miao et al. 2006). Mobilization of sediment as a result of natural disruption events (i.e., snowmelt, wind, rain events, increased turbidity), can result in the transport of metals to the water column and subsequent migration downstream (Kaplan et al. 1995; Pachana et al. 2010). As a result, riverine fate and transport of metals are often controlled by sediment acting as a source, a sink, and a reaction surface for environmental systems. This can have both positive and negative effects on Arctic ecosystems since certain metals (e.g., copper, chromium, and zinc) provide essential nutrients for in-country foods (Horowitz and Elrick 1987; Kabata-Pendias 2010), while others (i.e., mercury (Hg) and cadmium) are toxic even in trace amounts (Schuster et al. 2018). Within the context of Arctic ecosystem processes outlined above, we discuss the role and response of metals in Arctic C cycle processes. We focus on Fe, Sr, uranium (U), and Hg because they represent a ubiquitous element in earth’s crust (Fe), a tracer used to distinguish between silicate and carbonate weathering reactions in soils (Sr), a long-lived radioactive element (U), and a toxic metal of concern because it bioaccumulates in the environment (Hg).
3.4.1 Iron and the Arctic Carbon Cycle
Fe is the most abundant element on Earth and the fourth most abundant element in the Earth’s crust, by mass. As such, Fe has significant importance to life and plays an integral role in biogeochemical reactions. Fe exists in multiple valence states, ranging from -2 to +6, but in the near-surface, it is found primarily as divalent Fe (Fe(II)) or trivalent Fe ((Fe(III)). Fe(II) and Fe(III) readily form complexes with other species, which can act as catalysts in biogeochemical reactions, sinks for organic C, and facilitate electron transfer (Williams and Scherer 2004; Lalonde et al. 2012; Frey and Reed 2012; Page et al. 2013; Trusiak et al. 2018). Fe redox cycling is complex and is intricately tied to the presence of organic C and microbial activity and can vary even within one watershed since reduced Fe tends to accumulate at the permafrost-active layer boundary and oxidized Fe found generally in near-surface environments (Barker et al. 2014; Fig. 11.10).
In the Arctic, the C cycle is a significantly important process due to the abundance of C stored currently frozen in permafrost (Tarnocai et al. 2009; Hugelius et al. 2014) and the risk of CO2 and CH4 release if air temperatures continue to increase and permafrost thaws and degrades (Vincent et al. 2017). Fe plays a role in the overall Arctic C cycle, contributing both positive and negative permafrost-C feedbacks to the climate. Fe acts as a sink for organic C due to its reactivity and large surface area (Salvadó et al. 2015), thereby stabilizing C in soil and decreasing the potential for degradation and release of CO2 (Lalonde et al. 2012; Yang et al. 2017). However, various microbial communities active in Arctic soil systems have been shown to use Fe(II) or Fe(III) as energy sources depending on the community composition (Emerson et al. 2015), leading to substantial positive feedback to climate change by the direct release of CO2 and potentially CH4 into the atmosphere (Yang et al. 2017). Additionally, two recent studies have focused on the chemical oxidation of DOC mediated by reduced Fe in anoxic soil waters in the Arctic through the production of hydroxyl radical (•OH), which is a highly reactive oxidant (Page et al. 2013; Trusiak et al. 2018). Therefore, the presence of Fe in soils and soil waters contributes to both the production and consumption of CO2. A simplified schematic showing the overall role of Fe in the Arctic C cycle is shown in Fig. 11.11. Fe can act as a sink for organic C, energy source for microbial reduction and oxidation, and contribute to the formation of •OH thereby oxidizing organic C. Investigating Fe redox cycling is critical to understanding the fate of C in the Arctic, particularly with projected warming and thawing of permafrost soils.
A simplified schematic diagram showing the roles of Fe in the C cycle of Arctic soils and soil pore waters a CO2 is consumed during microbial oxidation of Fe, b CO2 is produced during microbial reduction of Fe, c Fe can act as a sink with organic C through precipitation, complexation, and sorption reactions, and d reduced Fe plays a role in the formation of hydroxyl radical in anoxic soil waters, which is a highly reactive oxidant for C or metals oxidation
The chemical speciation of Fe and thus its fate and transport from streams/rivers to the Arctic Ocean are also related to permafrost and C dynamics. Stolpe et al. (2013) have shown that during spring freshet when DOM and Fe abundance is the highest, dissolved Fe is mostly complexed with DOM and present almost exclusively in the form of nanocolloids or the <4 nm size fractions in small Alaska rivers. In contrast, during late summer/early fall when DOM is low in abundance, the molecular size spectra of dissolved Fe show two peaks: one associated with nanocolloids derived from the complexation of Fe with and DOM, and the other with large sized colloids ranging from 10 to 50 nm derived from the formation of Fe oxide/hydroxide which could be removed through coagulation from the dissolved phase. Similar metal size spectra were also observed for Cr and other trace elements in small Alaska rivers based on the analysis using flow field-flow fraction coupled online with ICP-MS (Stolpe et al. 2013). In addition, Pokrovsky et al (2018) found freeze–thaw cycles of Arctic thaw ponds remove colloidal metals and generate low-molecular weight organic matter in boreal rivers in Siberia.
3.4.2 Sr and U Isotopes as Geochemical Tracers
The use of isotopes as environmental tracers for biogeochemical processes in the Arctic has become increasingly popular in recent years to determine trace element dynamics, quantify variations between varied source materials, monitor active layer deepening, and characterize seasonal changes in geochemical processes in watersheds. Since these processes are also the main drivers of C-cycle processes in soils and watersheds isotope studies can be used to infer C cycle processes. Specifically, radiogenic isotopes of Sr (87Sr and 86Sr; Guo et al. 2004b; Bagard et al. 2013; Douglas et al. 2013; Stevenson et al. 2016, 2018; Lehn et al. 2017) and U (238U and 234U) (Koch et al. 2013; Ewing et al. 2015; 2016; Hindshaw et al. 2018) have been applied to understand seasonal geochemical weathering reactions and rates in permafrost environments. Radiogenic Sr ratios are a function of their source in the near-surface environment resulting from weathering of primary materials and, as such, they are useful for predicting preferential weathering and hydrological flow paths (Stevenson et al. 2016; Keller et al. 2010; Douglas et al. 2013). Conversely, U isotope activity ratios (234U/238U) tend to be a function of water residence times (e.g., groundwater versus surface water) and studies have shown that longer water residence times translate to higher 234U/238U activity ratios. Permafrost systems can complicate this measurement because uranium isotopic enrichment can also be affected by ice residence time in addition to water residence time (Hindshaw et al. 2018). A study by Ewing et al. (2015) showed that 234U/238U ratios were higher for waters sourced from older permafrost thawing compared to younger permafrost. This established U isotope ratios as tracers for identifying the likely sources of soil and organic materials in watersheds.
3.4.3 Mercury Bioaccumulation and Release in the Arctic
Hg is a naturally occurring toxic element that is of increasing concern in Arctic ecosystems due to the large stocks of Hg currently stored in the northern permafrost region, and the sensitivity of permafrost Hg to thaw and mobilization (Ci et al. 2018; Schuster et al. 2018). Hg can bioaccumulate in terrestrial and aquatic food webs and has been found in foods across the Arctic region (Jæger et al. 2009). Hg can accumulate in Arctic snow and is present in snowmelt runoff due to the long-range atmospheric transport, oxidation, and subsequent deposition onto the snow surface (Douglas and Sturm 2004; Douglas et al. 2005, 2017; Driscoll et al. 2013; Agnan et al. 2018). The interaction of Hg and organic matter can drive the production of methylmercury (MeHg), which is a neurotoxin that strongly bioaccumulates and biomagnifies in nature (Schartup et al. 2013). MeHg is highly mobile in aquatic systems and is relatively lipid-soluble, making it an ideal species to accumulate in bio (Rice et al. 2014). Since MeHg production, retention, and mobilization are largely controlled by the presence of organic species in soils or water Hg is intimately associated with the C cycle.
Projected future permafrost degradation, particularly the dramatic loss of near-surface permafrost over the next decades (Pastick et al. 2015), will expose soil organic C that is currently bound to Hg and stored frozen. Once exposed, microbial activity can decompose organic matter, releasing CO2 in the process. Any Hg bound to the soil organic matter is also expected to be released into the environment during permafrost thaw and organic matter liberation (Schuster et al. 2018). Once released, the Hg can interact with both DOM and soil organic matter and form MeHg in watersheds (French et al. 2014). Similar observations in Arctic and sub-Arctic permafrost thaw ponds have already been reported by MacMillan et al. (2015) showing that high MeHg concentrations were correlated with high inputs of organic matter and microbial activity. Organic C affects Hg cycling and bioaccumulation in the environment and permafrost degradation is expected to impact the fate of Hg. However, the magnitude of that impact on the environment remains to be determined and further work is needed to quantify potential human health concerns.
4 Conclusions and Knowledge Gaps
Despite the growing body of literature documenting the response of Arctic stream and river biogeochemistry to warming and disturbance, there are still many challenges and uncertainties for the scientific community. In this chapter, we discuss some of these uncertainties and directions for future research, including (1) thermokarst effects (2) changing seasonality, (3) altered watershed hydrology, and (4) timing and magnitude of changes across Arctic landscapes.
The effects of thermokarst and thermal erosional processes on stream and river biogeochemistry represent a critical uncertainty for Arctic researchers. Much of the uncertainty regarding thermokarst is due to the complex, three-dimensional nature of the problem, and the difficulty in predicting the initiation and extent of thermokarst features across the landscape. Whereas many land surface models can now simulate one-dimensional permafrost thaw via active layer thickening, measuring and modeling thermokarst represents a true challenge to the scientific community. Thermokarst can develop across a variety of landscape types and in a variety of modes, including thaw slumps, slope failures, pits, scarps, gullies, and expanding lake margins. Some evidence indicates thermokarst and thermal erosion features may function as a large source of C and nutrients to streams. Other studies have documented that permafrost degradation via thermokarst can enhance riverine fluxes of water, sediment, organic C, nutrients, and trace metals. Still, the persistence and spatial extent of these thermokarst impacts are not clear and require more long-term monitoring and intensive research to better constrain.
As noted above, Arctic streams and rivers exhibit strong seasonal patterns with respect to flows and chemical composition. Under a warming Arctic, changing seasonality represents another key uncertainty that could impact hydrologic and biogeochemical processes in stream and river ecosystems. Evidence indicates many components of northern watersheds are already changing, including the timing and magnitude of snowmelt, the extent of the ice-free season, increase groundwater discharge to surface water (Lamontagne-Halle et al. 2018). In some cases, perennial streams may shift to ephemeral states in response to changing water balance and longer open-water seasons. Tracking the impacts of changing seasonality on river biogeochemical processes will take a considerable, multidisciplinary effort, linking remote sensing, field-based, and modeling approaches.
Changing watershed and subsurface hydrology in Arctic regions represents another key uncertainty and an important area of current and future research. The timing and magnitude of precipitation are likely to change, which may alter runoff to surface water and thawing rates in near-surface soils. Warming and disturbance (wildfire, permafrost thaw) can dramatically alter watershed hydrology and the delivery of water and solutes to stream and river channels. Spatial differences in permafrost characteristics across watersheds, including ground ice content, hydraulic properties, and lithology, all influence the hydrologic and biogeochemical response to warming and thawing. Recent advances in airborne and ground-based geophysical techniques have improved characterizations of subsurface properties in permafrost landscapes. Groundwater models have also been improved in recent years through the incorporation of freeze–thaw processes. Still, much work is still needed to establish conceptual and quantitative linkages between changing hydrologic conditions, permafrost dynamics, and biogeochemical responses in surface waters.
Predicting the timing and magnitude of change represents yet another key uncertainty and knowledge gap for Arctic river systems. The response of the biogeochemistry of C species, nutrients, and trace elements in Arctic aquatic ecosystems to warming and permafrost thaw should have different extents and time-dependent features, and thus different impacts on ecosystems. In addition, changes in river biogeochemistry are also distinct across Arctic rivers that draining different watershed types (O’Donnell et al. 2016; Lung et al. 2018). Therefore, comprehensive time-series observations in different watersheds across different latitudes are needed to better understand the evolution in C, nutrients, and trace elements biogeochemistry in Arctic rivers in a changing climate. Further, a more process-based understanding of biogeochemical processes that span the terrestrial–aquatic interface will strengthen our understanding of the mechanisms driving change. Large-scale studies of river biogeochemistry and the seasonality of flows and fluxes form the basis for statistical models of the export of DOC, other nutrients, major elements, and trace metals, but for predictions, we need physically based models, and these require an understanding of snow and chemical processes that occur at a much smaller scales.
References
Agnan Y, Douglas TA, Helmig D et al (2018) Mercury in the Arctic tundra snowpack: temporal and spatial concentration patterns and trace gas exchanges. Cryosphere 12:1939–1956
Aiken GR, Hsu-Kim H, Ryan JN (2011) Influence of dissolved organic matter on the environmental fate of metals, nanoparticles, and colloids. Environ Sci Technol 45:3196–3201
Aiken GR, Spencer RG, Striegl RG et al (2014) Influences of glacier melt and permafrost thaw on the age of dissolved organic carbon in the Yukon River basin. Glob Biogeochem Cycles 28(5):525–37
Alfredsson H, Clymans W, Hugelius G et al (2016) Estimated storage of amorphous silica in soils of the circum-Arctic tundra region. Glob Biogeochem Cycles 30:479–500
Bagard M-L, Schmitt A-D, Chabauc F et al (2013) Biogeochemistry of stable Ca and radiogenic Sr isotopes in a larch-covered permafrost-dominated watershed of Central Siberia. Geochim Cosmochim Acta 114(1):169–187
Barker AJ, Douglas TA, Jacobson AD et al (2014) Late season mobilization of trace metals in two small Alaskan arctic watersheds as a proxy for landscape scale permafrost active layer dynamics. Chem Geol 381:180–93
Battin TJ, Besemer K, Bengtsson MM et al (2016) The ecology and biogeochemistry of stream biofilms. Nat Rev Microbiol 14(4):251
Beaulieu JJ, Tank JL, Hamilton SK et al (2010) Nitrous oxide emission from denitrification in stream and river networks. P Natl Acad Sci USA 15:201011464
Benner R, Benitez‐Nelson B, Kaiser K et al (2004) Export of young terrigenous dissolved organic carbon from rivers to the Arctic Ocean. Geophys Res Lett 31(5). https://doi.org/10.1029/2003gl019251
Betts EF, Jones JB Jr (2009) Impact of wildfire on stream nutrient chemistry and ecosystem metabolism in boreal forest catchments of interior Alaska. Arct Antarct Alp Res 41(4):407–417
Bowden WB, Gooseff MN, Balser A et al (2008) Sediment and nutrient delivery from thermokarst features in the foothills of the North Slope, Alaska: potential impacts on headwater stream ecosystems. J Geophys Res-Biogeosci 113:G2. https://doi.org/10.1029/2007JG000470
Bowling LC, Kane DL, Gieck RE et al (2003) The role of surface storage in a low-gradient Arctic watershed. Water Resour Res 39(4). https://doi.org/10.1029/2002wr001466
Cai Y, Guo L, Douglas TA (2008a) Temporal variations in organic carbon species and fluxes from the Chena River, Alaska. Limnol Ocean 53(4):1408–1419
Cai Y, Guo L, Douglas T et al (2008b) Seasonal variations in nutrient concentrations and speciation in the Chena River, Alaska. J Geophys Res-Biogeosci 113(G030035). https://doi.org/10.1029/2008jg000733
Cai Y, Guo L (2009) Abundance and variation of colloidal organic phosphorus in riverine, estuarine, and coastal waters in the northern Gulf of Mexico. Limnol Oceanogr 54. https://doi.org/10.4319/lo.2009.54.4.1393
Cai L, Tong M, Wang X et al (2014) Influence of clay particles on the transport and retention of titanium dioxide nanoparticles in quartz sand. Environ Sci Technol 48(13):7323–7332
Chin Y-P, Aiken GR, Danielsen KM (1997) Binding of pyrene to aquatic and commercial humic substances: the role of molecular weight and aromaticity. Environ Sci Technol 31:1630–1635. https://doi.org/10.1021/es960404k
Ci Z, Peng F, Xue X et al (2018) Temperature sensitivity of gaseous elemental mercury in the active layer of the Qinghai-Tibet Plateau permafrost. Environ Pollut 238:508–515
Clemens S, Ma JF (2016) Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu Rev Plant Biol 67:489–512
Cole JJ, Prairie YT, Caraco NF et al (2007) Plumbing the global carbon cycle: integrating inland waters into the terrestrial carbon budget. Ecosystems 10:172–185
Cornelis J-T, Delvaux B, Georg RB et al (2011) Tracing the origin of dissolved silicon transferred from various soil-plant systems towards rivers: a review. Biogeosciences 8:89–112
Cory RM, Ward CP, Crump BC et al (2014) Sunlight controls water column processing of carbon in arctic fresh waters. Science 345:925–928. https://doi.org/10.1126/science.1253119
Cragin JH, McGilvary R (1995) Can inorganic chemical species volatilize from snow? Biogeochemistry of seasonally snow-covered catchments. IAHS publ. no. 228
De La Rocha CL, Brzezinski MA, DeNiro MJ et al (1998) Silicon-isotope composition of diatoms as an indicator of past oceanic change. Nature 395:680–683
Dinis M, Fiúza A (2006) Modeling the transport and fate of contaminants in the environment: soil, water and air. In: Simeonov L, Chirila E (eds) Chemicals as intentional and accidental global environmental threats. Springer Publishing, New York, pp 469–476
Dittmar T, Kattner G (2003) The biogeochemistry of the river and shelf ecosystem of the Arctic Ocean: a review. Mar Chem 83:103–120. https://doi.org/10.1016/S0304-4203(03)00105-1
Dominé F, Shepson PB (2002) Air-snow interactions and atmospheric chemistry. Science 297:1506–1510. https://doi.org/10.1126/science.1074610
Dou F, Ping CL, Guo L et al (2008) Estimating the impact of seawater on the production of soil water-extractable organic carbon during coastal erosion. J Environ Qual 37:2368–2374. https://doi.org/10.2134/jeq2007.0403
Douglas TA, Sturm M (2004) Arctic haze, mercury and the chemical composition of snow across northwestern Alaska. Atmos Environ 38(6):805–20
Douglas TA, Sturm M, Simpson WR et al (2005) Elevated mercury measured in snow and frost flowers near Arctic sea ice leads. Geophys Res, Lett, p 32
Douglas TA, Blum JD, Guo L et al (2013) Hydrogeochemistry of seasonal flow regimes in the Chena River, a subarctic watershed draining discontinuous permafrost in interior Alaska (USA). Chem Geol 335:48–62
Douglas TA, Sturm M, Blum JD et al (2017) Pulse of mercury and major ions in snowmelt runoff from a small arctic Alaska watershed. Environ Sci Technol 51(19):11145–55
Driscoll CT, Mason RP, Chan HM et al (2013) Mercury as a global pollutant: sources, pathways, and effects. Environ Sci Technol 47:4967–4983
Elser JJ, Marzolf ER, Goldman CR (1990) Phosphorus and nitrogen limitation of phytoplankton growth in the freshwaters of North America: a review and critique of experimental enrichments. Can J Fish Aquat Sci 47(7):1468–1477
Emerson D, Scott J, Benes J et al (2015) Microbial iron oxidation in the Arctic tundra and the implications for biogeochemical cycling. Appl Environ Microb 8:AEM-02832
Ewing SA, O’Donnell JA, Aiken GR et al (2015) Long-term anoxia and release of ancient, labile carbon upon thaw of Pleistocene permafrost. Geophys Res Lett 42(24):10–730
Ewing SA, Paces JB, O’Donnell JA et al (2016) Uranium isotopes and dissolved organic carbon in loess permafrost: modeling the age of ancient ice. Geochim Cosmochim Acta 152:143–165
Finlay J, Neff J, Zimov S, Davydova A, Davydov S (2006) Snowmelt dominance of DOC in high-latitude watersheds: implications for characterization and flux of river DOC. Geophys Res Lett 33(l10401)
French TD, Houben AJ, Desforges J-PW et al (2014) Dissolved organic carbon thresholds affect mercury bioaccumulation in Arctic Lakes. Environ Sci Technol 48:3162–3168
Frey KE, Smith LC (2005) Amplified carbon release from vast West Siberian peatlands by 2100. Geophys Res Lett 32(9)
Frey KE, McClelland JW (2009) Impacts of permafrost degradation on arctic river biogeochemistry. Hydrol Process 23(1):169–182
Frey PA, Reed GH (2012) The ubiquity of iron. ACS Chem Biol 7:1477–1481
Frey KE, McClelland JW, Holmes RM et al (2007) Impacts of climate warming and permafrost thaw on the riverine transport of nitrogen and phosphorus to the Kara Sea. J Geophys Res-Biogeosci 112(G4)
Gao L, Zhou Z, Reyes A, Guo L (2018) Yields and characterization of dissolved organic matter from different-aged soil in northern Alaska. J Geophys Res Biogeosci 123(7):2035–2052. https://doi.org/10.1029/2018JG004408
Gray DM, Toth B, Zhao L et al (2001) Estimating areal snowmelt infiltration into frozen soils. Hydrol Process 15:3095–3111
Grimm NB, Fisher SG (1986) Nitrogen limitation in a Sonoran Desert stream. J North Am Benthol Soc 5(1):2–15
Gunawardana C, Egodawatta P, Goonetilleke A (2015) Adsorption and mobility of metals in build-up on road surfaces. Chemosphere 119:1391–8
Guo L, Macdonald RW (2006) Source and transport of terrigenous organic matter in the upper Yukon River: evidence from isotope (δ13C, Δ14C, and δ15 N) composition of dissolved, colloidal, and particulate phases. Glob Biogeochem Cycles 20(2):GB2011. https://doi.org/10.1029/2005gb002593
Guo L, Zhang JZ, Guéguen C (2004a) Speciation and fluxes of nutrients (N, P, Si) from the upper Yukon River. Glob Biogeochem Cycles 18(1):GB1038. https://doi.org/10.1029/2003gb002152
Guo L, Semiletov I, Gustafsson Ö et al (2004b) Characterization of Siberian Arctic coastal sediments: Implications for terrestrial organic carbon export. Glob Biogeochem Cycles 18(1):GB1036. https://doi.org/10.1029/2003gb002087
Guo L, Ping CL, Macdonald RW (2007) Mobilization pathways of organic carbon from permafrost to arctic rivers in a changing climate. Geophys Res Lett 34(13):L13603. https://doi.org/10.1029/2007GL030689
Guo L, Cai Y, Belzile C, Macdonald RW (2012) Sources and export fluxes of inorganic and organic carbon and nutrient species from the seasonally ice-covered Yukon River. Biogeochemistry 107(1–3):187–206
Harden JW, Koven CD, Ping CL et al (2012) Field information links permafrost carbon to physical vulnerabilities of thawing. Geophys Res Lett 39(15)
Harms TK, Edmonds JW, Genet H et al (2016) Catchment influence on nitrate and dissolved organic matter in Alaskan streams along a latitudinal gradient. J Geophys Res-Biogeosci 121:350–369. https://doi.org/10.1002/2015JG003201
Hedin LO, von Fischer JC, Ostrom NE et al (1998) Thermodynamic constraints on nitrogen transformations and other biogeochemical processes at soil–stream interfaces. Ecology 79(2):684–703
Heimann M, Reichstein M (2008) Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 451(7176):289
Hendricks SP, White DS (2000) Stream and groundwater influences on phosphorus biogeochemistry. In: Jones JB, Mulholland PJ (eds) Streams and ground waters. Academic Press, San Diego, pp 221–235
Hindshaw RS, Aciego SM and Tipper ET (2018) Li and U isotopes as a potential tool for monitoring active layer deepening in permafrost dominated catchments. Front. Earth Sci 6(102):17
Hinzman LD, Bettez ND, Bolton WR et al (2005) Evidence and implications of recent climate change in northern Alaska and other arctic regions. Clim Chang 72:251–298
Hobbie SE, Gough L (2004) Litter decomposition in moist acidic and non-acidic tundra with different glacial histories. Oecologia 140:113–124
Holmes RM, McClelland JW, Raymond PA et al (2008) Lability of DOC transported by Alaskan rivers to the Arctic Ocean. Geophys Res Lett 35(3). https://doi.org/10.1029/2007gl032837
Holmes RM, McClelland JW, Peterson BJ et al (2012) Seasonal and annual fluxes of nutrients and organic matter from large rivers to the arctic ocean and surrounding seas. Estuaries Coasts 35(2):369–382
Hope D, Billett MF, Cresser MS (1994) A review of the export of carbon in river water: fluxes and processes. Environ Pollut 84:301–324. https://doi.org/10.1016/0269-7491(94)90142-2
Hornberger GM, Bencala KE, McKnight DM (1994) Hydrological controls on dissolved organic carbon during snowmelt in the Snake River near Montezuma, Colorado. Biogeochemistry 25:147–165
Horowitz AJ, Elrick KA (1987) The relation of stream sediment surface area, grain size and composition to trace element chemistry. Appl Geochem 2(4):437–51
Hugelius G, Strauss J, Zubrzycki S et al (2014) Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps. Biogeosciences 11:6573–6593. https://doi.org/10.5194/bg-11-6573-2014
Ilgen AG, Trainor TP (2011) Sb (III) and Sb (V) sorption onto Al-rich phases: hydrous Al oxide and the clay minerals kaolinite KGa-1b and oxidized and reduced nontronite NAu-1. Environ Sci Technol 46(2):843–51
Ilgen AG, Majs F, Barker AJ, Douglas TA, Trainor TP (2014) Oxidation and mobilization of metallic antimony in aqueous systems with simulated groundwater. Geochim Cosmochim Acta 1(132):16–30
Jæger I, Hop H, Gabrielsen GW (2009) Biomagnification of mercury in selected species from an Arctic marine food web in Svalbard. Sci Total Environ 407(16):4744–51
Jones JB Jr, Petrone KC, Finlay JC et al (2005) Nitrogen loss from watersheds of interior Alaska underlain with discontinuous permafrost. Geophys Res Lett 32(2)
Jorgenson MT, Shur YL, Pullman ER (2006) Abrupt increase in permafrost degradation in Arctic Alaska. Geophys Res Lett 33:L02503. https://doi.org/10.1029/2005GL024960
Judd KE, Kling GW (2002) Production and export of dissolved C in arctic tundra mesocosms: the roles of vegetation and water flow. Biogeochemistry 60:213–234
Kabata-Pendias A (2010) Trace elements in soils and plants, 4th edn. Taylor & Francis, Boca Raton
Kane DL, Hinzman LD, Benson CS, Everett KR (1989) Hydrology of Imnaviat Creek, an arctic watershed. Ecography 12:262–269. https://doi.org/10.1111/j.1600-0587.1989.tb00845.x
Kaplan DI, Bertsch PM, Adriano DC (1995) Facilitated transport of contaminant metals through an acidified aquifer. Groundwater 33(5):708–17
Kawahigashi M, Kaiser K, Kalbitz K et al (2004) Dissolved organic matter in small streams along a gradient from discontinuous to continuous permafrost. Glob Chang Biol 10(9):1576–86
Kawahigashi M, Kaiser K, Rodionov A et al (2006) Sorption of dissolved organic matter by mineral soils of the Siberian forest tundra. Glob Change Biol 12(10):1868–77
Keller K, Blum JD, Kling GW (2007) Geochemistry of soils and streams on surfaces of varying ages in Arctic Alaska. Arct Antarct Alp Res 39:84–98
Keller K, Blum JD, Kling GW (2010) Stream geochemistry as an indicator of increasing permafrost thaw depth in an arctic watershed. Chem Geol 273(1–2):76–81
Kicklighter DW, Hayes DJ, McClelland JW et al (2013) Insights and issues with simulating terrestrial DOC loading of Arctic river networks. Ecol Appl 23:1817–1836. https://doi.org/10.1890/11-1050.1
Kimbrough DE, Cohen Y, Winer AM et al (1999) A critical assessment of chromium in the environment. Crit Rev Environ Sci Tech 29(1):1–46
Kling GW, Kipphut GW, Miller MC (1991) Arctic lakes and streams as gas conduits to the atmosphere: implications for tundra carbon budgets. Science 251(4991):298–301
Knechtenhofer L, Xifra I, Scheinost AC et al (2003) Fate of heavy metals in a strongly acidic shooting range-soil: small-scale metal distribution and its relation to preferential water flow. J Plant Nutr Soil Sci 166:84–92
Koch JC, Ewing SA, Striegl R et al (2013) Rapid runoff via shallow throughflow and deeper preferential flow in a boreal catchment underlain by frozen silt (Alaska, USA). Hydrogeol J 21(1):93–106
Koren V, Schaake J, Mitchell K et al (1999) A parameterization of snowpack and frozen ground intended for NCEP weather and climate models. J Geophys Res 104(D16):19569–19585
Lalonde K, Mucci A, Ouellet A et al (2012) Preservation of organic matter in sediments promoted by iron. Nature 483(7388):198–200
Lamontagne-Halle P, McKenzie JM, Kurylyk BL et al (2018) Changing groundwater discharge dynamics in permafrost regions. Environ Res Lett 13:084017
Larsen JN, Anisimov OA, Constable A et al (2014) Polar regions. In: Climate change 2014, impacts, adaptation, and vulnerability, part B: regional aspects. Contribution of working group II to the fifth asessment report of the intergovernmental panel on climate change, pp 1567–1612
Lehn GO, Jacobson AD, Douglas TA et al (2017) Constraining seasonal active layer dynamics and chemical weathering reactions occurring in North Slope Alaskan watersheds with major ion and isotope (δ34SSO4, δ13CDIC, 87Sr/86Sr, δ44/40Ca, and δ44/42Ca) measurements. Geochim Cosmochim Acta 217:399–420
Liao C, Zhuang Q (2017) Quantifying the role of permafrost distribution in groundwater and surface water interactions using a three-dimensional hydrological model. Arct Antarct Alp Res 49:81–100
Liao P, Li W, Jiang Y et al (2017) Formation, aggregation, and deposition dynamics of NOM-iron colloids at anoxic–oxic interfaces. Environ Sci Technol 12235–12245
Lin P, Guo L (2016) Dynamic changes in the abundance and chemical speciation of dissolved and particulate phosphorus across the river-lake interface in southwest Lake Michigan. Limnol Oceanogr 61:771–789
Loiko SV, Pokrovsky OS, Raudina TV et al (2017) Abrupt permafrost collapse enhances organic carbon, CO2, nutrient and metal release into surface waters. Chem Geol 471:153–65
Loranty M et al (2018) Changing ecosystem influences on soil thermal regimes in northern high-latitude permafrost regions. Biogeosciences 15:5287–5313
Loretta YL, Li F (2001) Heavy metal sorption and hydraulic conductivity studies using three types of bentonite admixes. J Environ Eng 10:420–429
Lung JYSLY, Tank SE, Spence C et al (2018) Seasonal and geographic variation in dissolved carbon biogeochemistry of rivers draining to the Canadian Arctic Ocean and Hudson Bay. J Geophys Res Biogeosci. https://doi.org/10.1029/2018JG004659
Maclean R, Oswood MW, Irons JG et al (1999) The effect of permafrost on stream biogeochemistry: a case study of two streams in the Alaskan (USA) taiga. Biogeochemistry 47:239–267
MacMillan GA, Girard C, Chételat J et al (2015) High methylmercury in Arctic and subarctic ponds is related to nutrient levels in the warming eastern Canadian Arctic. Environ Sci Technol 49:7743–7753
Mann PJ, Eglinton TI, McIntyre CP et al (2015) Utilization of ancient permafrost carbon in headwaters of Arctic fluvial networks. Nat Commun 6:7856
McClelland JW, Stieglitz M, Pan F et al (2007) Recent changes in nitrate and dissolved organic carbon export from the upper Kuparuk River, North Slope, Alaska. J Geophys Res Biogeosci 112(G4). https://doi.org/10.1029/2006jg000371
McGuire AD, Anderson L, Christensen TR et al (2009) Sensitivity of the carbon cycle in the Arctic to climate change (review). Ecol Monogr 79(4):523–555
McGuire AD, Hayes DJ, Kicklighter DW et al (2010) An analysis of the carbon balance of the Arctic Basin from 1997 to 2006. Tellus B 62:455–474. https://doi.org/10.1111/j.1600-0889.2010.00497.x
McNamara JP, Kane DL, Hinzman DL (1997) Hydrograph separations in an arctic watershed using mixing model and graphical techniques. Water Resour Res 33:1707–1719. https://doi.org/10.1029/97WR01033
McNamara JP, Kane DL, Hobbie JE, Kling GW (2008) Hydrologic and biogeochemical controls on the spatial and temporal patterns of nitrogen and phosphorus in the Kuparuk River, arctic Alaska. Hydrol Process 22(17):3294–3309
Miao S, DeLaune RD, Jugsujinda A (2006) Influence of sediment redox conditions on release/solubility of metals and nutrients in a Louisiana Mississippi River deltaic plain freshwater lake. Sci Total Environ 371(1–3):334–43
Morford JL, Emerson S (1999) The geochemistry of redox sensitive trace metals in sediments. Geochim Cosmochim Acta 63(11–12):1735–50
Neff JC, Finlay JC, Zimov SA et al (2006) Seasonal changes in the age and structure of dissolved organic carbon in Siberian rivers and streams. Geophys Res Lett 33. https://doi.org/10.1029/2006GL028222
Niu G-Y, Yang Z-L (2006) Effects of frozen soil on snowmelt runoff and soil water storage at a continental scale. J Hydrometeorol 7(5):937–952
O’Donnell JA, Aiken GR, Kane ES, Jones JB (2010) Source water controls on the character and origin of dissolved organic matter in streams of the Yukon River basin, Alaska. J Geophys Res: Biogeosci 115(G3). https://doi.org/10.1029/2009jg001153
O’Donnell JA, Harden JW, McGuire AD et al (2011) The effect of fire and permafrost interactions on soil carbon accumulation in an upland black spruce ecosystem of interior Alaska: implications for post-thaw carbon loss. Glob Chang Biol 17(3):1461–74
O’Donnell JA, Aiken GR, Walvoord MA, Butler KD (2012) Dissolved organic matter composition of winter flow in the Yukon River basin: implications of permafrost thaw and increased groundwater discharge. Global Biogeochem Cycles 26(4). https://doi.org/10.1029/2012gb004341
O’Donnell JA, Aiken GR, Walvoord MA et al (2014) Using dissolved organic matter age and composition to detect permafrost thaw in boreal watersheds of interior Alaska. J Geophys Res-Biogeosci 119:2155–2170. https://doi.org/10.1002/2014JG002695
O’Donnell JA, Aiken GR, Butler KD et al (2015) Chemical composition of rivers in Alaska’s Arctic network, 2013–2014. Natural Resource Data Series, NPS/ARCN/NRDS – 2015/809. National Park Service. Fort Collins, Colorado. Published Report-2222958
O’Donnell JA, Aiken GR, Swanson DK et al (2016) Dissolved organic matter composition of Arctic rivers: linking permafrost and parent material to riverine carbon. Glob Biogeochem Cycles 30:1811–1826. https://doi.org/10.1002/2016GB005482
Olefeldt D, Persson A, Turetsky MR (2014) Influence of the permafrost boundary on dissolved organic matter characteristics in rivers within the boreal and taiga plains of western Canada. Environ Res Lett 9:035005
Pachana K, Wattanakornsiri A, Nanuam J (2010) Heavy metal transport and fate in the environmental compartments. NU Int J Sci 7(1):1–1
Page SE, Kling GW, Sander M et al (2013) Dark formation of hydroxyl radical in Arctic soil and surface waters. Environ Sci Technol 47(22):12860–7
Pastick NJ, Jorgenson MT, Wylie BK, Nield SJ, Johnson KD, Finley AO (2015) Distribution of near-surface permafrost in Alaska: Estimates of present and future conditions. Remote Sens Environ 168:301–15
Peterson BJ, Hobbie JE, Hershey AE et al (1985) Transformation of a tundra river from heterotrophy to autotrophy by addition of phosphorus. Science 229:1383–1386. https://doi.org/10.1126/science.229.4720.1383
Petrone KC, Jones JB, Hinzman LD et al (2006) Seasonal export of carbon, nitrogen, and major solutes from Alaskan catchments with discontinuous permafrost. J Geophys Res-Biogeosci 111(G2)
Philippe A, Schaumann GE (2014) Interactions of dissolved organic matter with natural and engineered inorganic colloids: a review. Environ Sci Technol 48:8946–8962
Ping C.-L, Michaelson GJ, Guo L et al (2011) Soil carbon and material flux across the eroding coastline of the Beaufort Sea, Alaska. J Geophys Res: Biogeosci 116(G02004). https://doi.org/10.1029/2010jg001588
Pokrovsky OS, Schott J (2002) Iron colloids/organic matter associated transport of major and trace elements in small boreal rivers and their estuaries (NW Russia). Chem Geol 190:141–179
Pokrovsky OS, Reynolds BC, Prokushkin AS et al (2013) Silicon isotope variations in Central Siberian rivers during basalt weathering in permafrost-dominated larch forests. Chem Geol 355:103–116
Pokrovsky OS, Manasypov RM, Loiko SV et al (2016) Trace element transport in western Siberian rivers across a permafrost gradient. Biogeosciences 13(6):1877–900
Pokrovsky OS, Karlsson J, Giesler R (2018) Freeze-thaw cycles of Arctic thaw ponds remove colloidal metals and generate low-molecular-weight organic matter. Biogeochemistry 137(3):321–336
Raudina TV, Loiko SV, Lim A et al (2018) Permafrost thaw and climate warming may decrease the CO 2, carbon, and metal concentration in peat soil waters of the Western Siberia Lowland. Sci Total Environ 634:1004–23
Raymond PA, McClelland JW, Holmes RM et al (2007) Flux and age of dissolved organic carbon exported to the Arctic Ocean: a carbon isotopic study of the five largest rivers. Global Biogeochem Cycles 21. https://doi.org/10.1029/2007GB002934
Rember RD, Trefry JH (2004) Increased concentrations of dissolved trace metals and organic carbon during snowmelt in rivers of the Alaskan Arctic. Geochim Cosmochim Acta 68(3):477–489
Rice KM, Walker EM Jr, Wu M et al (2014) Environmental mercury and its toxic effects. J Prev Med Public Health 47(2):74–83
Romanovsky VE, Smith SL, Christiansen HH (2010) Permafrost thermal state in the polar Northern Hemisphere during the international polar year 2007–2009: a synthesis. Permafrost Periglac 21:106–116. https://doi.org/10.1002/ppp.689
Roulet N, Moore TR (2006) Environmental chemistry: browning the waters. Nature 444:283–284
Salvadó JA, Tesi T, Andersson A et al (2015) Organic carbon remobilized from thawing permafrost is resequestered by reactive iron on the Eurasian Arctic Shelf. Geophys Res Lett 42(19):8122–30
Sannel ABK, Kuhry P (2011) Warming induced destabilization of peat plateau/thermokarst lake complexes. J Geophys Res 116:G03035
Schartup AT, Mason RP, Balcom PH et al (2013) Methylmercury production in estuarine sediments: role of organic matter. Environ Sci Technol 47:695–700
Schlesinger WH, Bernhardt ES (2013) Biogeochemistry: an analysis of global change. Academic Press, Cambridge
Schuster PF, Schaefer KM, Aiken GR et al (2018) Permafrost stores a globally significant amount of mercury. Geophys Res Lett 45(3):1463–71
Schuur EAG, McGuire AD, Schadel C et al (2015) Climate change and the permafrost carbon feedback. Nature 520:171–179
Skłodowski P, Maciejewska A, Kwiatkowska J (2006) The effect of organic matter from brown coal on bioavailability of heavy metals in contaminated soils. Nato science series SS IV Ear, vol 3, pp 299–307
Slavik K, Peterson BJ, Deegan LA et al (2004) Long-term responses of the Kuparuk River ecosystem to phosphorus fertilization. Ecology 85(4):939–54
Spencer RG, Aiken GR, Wickland KP et al (2008) Seasonal and spatial variability in dissolved organic matter quantity and composition from the Yukon River basin, Alaska. Glob Biogeochem Cycles 22(4). https://doi.org/10.1029/2008gb003231
Spencer RG, Mann PJ, Dittmar T et al (2015) Detecting the signature of permafrost thaw in Arctic rivers. Geophys Res Lett 42(8):2830–5
Stähl M, Bayard D, Wydler H et al (2004) Snowmelt infiltration into alpine soils visualized by dye tracer technique. Arct Anarct Alp Res 36:128–135
Stevenson EI, Aciego SM, Chutcharavan P et al (2016) Insights into combined radiogenic and stable strontium isotopes as tracers for weathering processes in subglacial environments. Chem Geol 429:33–43
Stevenson R, Pearce CR, Rosa E et al (2018) Weathering processes, catchment geology and river management impacts on radiogenic (87 Sr/86 Sr) and stable (δ88/86 Sr) strontium isotope compositions of Canadian boreal rivers. Chem Geol 486:50–60
Stolpe B, Guo L, Shiller AM, Aiken GR (2013) Abundance, size distributions and trace-element binding of organic and iron-rich nanocolloids in Alaskan rivers, as revealed by field-flow fractionation and ICP-MS. Geochim Cosmochim Acta 105:221–239
Striegl RG, Aiken GR, Dornblaser MM et al (2005) A decrease in discharge-normalized DOC export by the Yukon River during summer through autumn. Geophys Res Lett 32(21):L21413
Striegl RG, Dornblaser MM, Aiken GR et al (2007) Carbon export and cycling by the Yukon, Tanana, and Porcupine rivers, Alaska, 2001–2005. Water Resour Res 43(2). https://doi.org/10.1029/2006wr005201
Tank SE, Striegl RG, McClelland JW et al (2016) Multi-decadal increases in dissolved organic carbon and alkalinity flux from the Mackenzie drainage basin to the Arctic Ocean. Environ Res Lett 11(5):054015
Tape K, Sturm M, Racine C (2006) The evidence for shrub expansion in Northern Alaska and the Pan-Arctic. Glob Chang Biol 12:686–702
Tarnocai C, Canadell JG, Schuur EAG et al (2009) Soil organic carbon pools in the northern circumpolar permafrost region. Glob Biogeochem Cycles 23:1–11
Toohey RC, Herman-Mercer NM, Schuster PF et al (2016) Multidecadal increases in the Yukon River Basin of chemical fluxes as indicators of changing flowpaths, groundwater, and permafrost. Geophys Res Lett 43:12120–12130. https://doi.org/10.1002/2016GL070817
Tranter M, Brimblecombe P, Davies TD et al (1986) The composition of snowfall, snowpack and meltwater in the Scottish highlands-evidence for preferential elution. Atmos Environ 20:517–525
Tranvik LJ, Downing JA, Cotner JB et al (2009) Lakes and reservoirs as regulators of carbon cycling and climate. Limnol Oceanogr 54:2298–2314
Trusiak A, Treibergs LA, Kling GW et al (2018) The role of iron and reactive oxygen species in the production of CO2 in arctic soil waters. Geochim Cosmochim Acta 224:80–95
Turetsky MR, Kane ES, Harden JW et al (2011) Recent acceleration of biomass burning and carbon losses in Alaskan forests and peatlands. Nat Geosci 4:27–31
Vaughan DG (2013), Observations: cryosphere. In: Stocker TF et al (eds) Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge Univ. Press, Cambridge
Vincent WF, Lemay M, Allard M (2017) Arctic permafrost landscapes in transition: towards an integrated Earth system approach. Arct Sci 3:39–64
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13(2):87–115
Vitousek PM, Reiners WA (1975) Ecosystem succession and nutrient retention: a hypothesis. Bioscience 25(6):376–81
Vitousek PM, Aber JD, Howarth RW et al (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol App 7(3):737–50
Vonk JE, Mann PJ, Davydov S et al (2013) High biolability of ancient permafrost carbon upon thaw. Geophys Res Lett 40:2689–2693. https://doi.org/10.1002/grl.50348
Vonk JE, Tank SE, Bowden WB et al (2015a) Reviews and syntheses: effects of permafrost thaw on arctic aquatic ecosystems. Biogeosciences 23:7129–7167
Vonk JE, Tank SE, Mann PJ et al (2015b) Biodegradability of dissolved organic carbon in permafrost soils and aquatic systems: a meta-analysis. Biogeosciences 12:6915–6930. https://doi.org/10.5194/bg-12-6915-2015
Walvoord MA, Striegl RG (2007) Increased groundwater to stream discharge from permafrost thawing in the Yukon River basin: potential impacts on lateral export of carbon and nitrogen. Geophys Res Lett 34. https://doi.org/10.1029/2007GL030216
Walvoord MA, Kurylyk BL (2016) Hydrologic impacts of thawing permafrost—a review. Vadose Zone J 15:vzj2016.01.0010
Walvoord MA, Voss CI, Wellman TP (2012) Influence of permafrost distribution on groundwater flow in the context of climate-driven permafrost thaw: example from Yukon Flats Basin, Alaska, United States. Water Resour Res 48:7524. https://doi.org/10.1029/2011W011595
Ward CP, Nalven SG, Crump BC et al (2017) Photochemical alteration of organic carbon draining permafrost soils shifts microbial metabolic pathways and stimulates respiration. Nat Commun 8(1):772
Wauthy M, Rautio M, Christoffersen KS et al (2018) Increasing dominance of terrigenous organic matter in circumpolar freshwaters due to permafrost thaw. Limnol Oceanogr Lett 3(3):186–98
Weishaar JL, Aiken GR, Bergamaschi BA et al (2003) Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol 37(20):4702–8
Williams MW, Melack JM (1991) Solute chemistry of snowmelt and runoff in an Alpine Basin, Sierra Nevada. Water Resour Res 27:1575–1588. https://doi.org/10.1029/90WR02774
Williams AGB, Scherer MM (2004) Spectroscopic evidence for Fe(II)-Fe(III) electron transfer at the iron oxide-water interface. Environ Sci Technol 38(18):4782–4790
Wu HC, Peng TR, Moore D et al (2014) Modeling dissolved organic carbon in temperate forest soils: TRIPLEX-DOC model development and validation. Geosci Model Dev 7:867–881
Xu HC, Guo L (2017) Molecular size-dependent abundance and chemical composition of dissolved organic matter in river, lake and sea waters. Water Res 117:115–126. https://doi.org/10.1016/j/waters.2017.04.006
Xu HC, Guo L (2018) Intriguing changes in molecular size and composition of dissolved organic matter induced by microbial degradation and self-assembly. Water Res 135:187–194. https://doi.org/10.1016/j.watres.2018.02.016
Xu C, Guo L, Dou F, Ping CL (2009) Potential DOC production from size-fractionated Arctic tundra soils. Cold Reg Sci Technol 55:141–150. https://doi.org/10.1016/j.coldregions.2008.08.001
Xu H, Guan D, Lin H, Guo L (2018) Contrasting effects of photochemical and microbial degradation on Cu(II) binding with fluorescent DOM from different origins. Environ Pollut 239:205–214
Yang Z, Yang S, Van Nostrand JD et al (2017) Microbial community and functional gene changes in Arctic tundra soils in a microcosm warming experiment. Front Microbiol 8:1741
Yin H, Tan N, Liu C et al (2016) The associations of heavy metals with crystalline iron oxides in the polluted soils around the mining areas in Guangdong Province, China. Chemosphere 161:181–9
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
O’Donnell, J., Douglas, T., Barker, A., Guo, L. (2021). Changing Biogeochemical Cycles of Organic Carbon, Nitrogen, Phosphorus, and Trace Elements in Arctic Rivers. In: Yang, D., Kane, D.L. (eds) Arctic Hydrology, Permafrost and Ecosystems. Springer, Cham. https://doi.org/10.1007/978-3-030-50930-9_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-50930-9_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-50928-6
Online ISBN: 978-3-030-50930-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)