Abstract
One of the most important challenges that pharmaceutical companies are presently facing is low bioavailability of drug, which is generally a result of poor aqueous drug solubility/dissolution rates; this may restrict the therapeutic efficiency of marketed drugs. The bioavailability of pharmaceuticals’ existing in a solid formulation strongly relies on the size, particle size distribution, and morphology of the particles. In recent years, the major approaches that have been put into practice to overcome poor drug solubility/dissolution rates are drug particle size reduction (i.e., micronization/nanonization). Numerous particle engineering techniques have been applied for this purpose, including spray-drying, freeze-drying, liquid anti-solvent crystallization or milling processes. These technologies present numerous drawbacks, for example, the difficulty of controlling particle size and particle size distribution, product degradation due to mechanical or thermal stresses, or the contamination of the particles with organic solvents or other toxic substances. Therefore, different alternative precipitation techniques are being explored. In recent years pharmaceutical processing using supercritical fluids, for the precipitation of pharmaceuticals and natural substances, has attracted great attention from the pharmaceutical industry. This is mostly attributable to the some well-known beneficial technological features of this method, as well as to other increasingly important subjects for the pharmaceutical industry, namely, their “green” sustainable, safe, and “environmentally friendly” intrinsic characteristics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The bioavailability of pharmaceuticals existing in a solid formulation strongly relies on the size, particle size distribution, and morphology of the particles. The particle precipitation into micro/nanoparticles has been an active research area for decades (Chattopadhyay and Gupta 2001a; Kalogiannis et al. 2005; Rehman et al. 2001; Velaga et al. 2002; Yeo and Lee 2004). The greatest prerequisite in the appliance of nanomaterials is its size along with morphology control which decides the potential application of the nanoparticles, as their properties differ notably with size. Because of this, there is an increasing interest in the development of well-organized micronization/nanonization technologies. Micro- and nanoparticles can be obtained by a variety of techniques. Conventional techniques counting spray drying , freeze-drying , solute recrystallization , interfacial polymerization , and milling processes present numerous problems such as excessive use of solvent, degradation of the product due to mechanical or thermal stresses, structural changes, formulation instability, low drug loading efficiency, and, mainly, broad particle size distribution (He et al. 2004; Chen et al. 2011). In some cases, the processing of particle formation is extended to achieve uniform size distribution by subsequent milling and sieving, which often give rise to the damage of sensitive biomolecules because of high shear forces (Ginty et al. 2005, 2006).
In addition, the majority of these processes usually depend on the use of a large number of organic solvents , which cause product damage, toxicity, inflammability, and biocompatibility problems, among others (Pasquali and Bettini 2008). Therefore these processes for particle formation may not be worthwhile. For this reason, different alternative precipitation methods are being explored (Martin and Cocero 2008). However, the application of supercritical fluids (SCFs) is an attractive alternative for this particle formation because it removes these drawbacks.
In the last few years, the supercritical fluid (SCF) technology has gained tremendous attention from investigators over the established pharmaceutical manufacturing strategies because of the environmentally benignant nature and economically promising character of SCFs (Kompella and Koushik 2001; Bałdyga et al. 2010; Chen et al. 2017). SCF technology has been commonly utilized for a variety of applications, for instance chromatography, extraction, material processing, and reaction. A most significant feature of particle formation from the SCF technique is the ability of manufacturing solids with unique morphology and small size.
The appliance of supercritical fluids (SCFs) for the precipitation of pharmaceuticals and natural substances has gained remarkable interest attributable to the extraordinary properties of these fluids (Bertucco and Vetter 2001). These SCFs have unique properties such as liquid-like density , gas-like viscosity , and larger diffusivities than those of typical liquids, resulting in higher mass transfer rate. These make them excellent solvents for various industrial developments. Additionally, by altering the experimental conditions like temperature and pressure, its solvent power, as well as selectivity, can be modified (Montes et al. 2019).
In the past few decades, this high-pressure technology has been commonly implemented for acquiring products because of the environmentally friendly nature and economically hopeful nature of SCFs (Hauthal 2001). In several SCF precipitation techniques , the need for organic solvents is totally eliminated, whereas in others a small quantity of organic solvent is employed, which can be totally removed from the product because of the high solubility of these solvents in SCF, as a result circumventing the contamination of the product (Shariati and Peters 2003; Jung and Perrut 2001; Reverchon 1999). SCFs take benefit of the benign solvents, that is, CO2 and water, to avoid the issues associated with the traditional strategies for precipitation of drug either alone or in combination with the biodegradable polymers (Kalani and Yunus 2011). For that reason, these SCFs act as an effectual replacement for organic solvents in producing pharmaceutical products (Hauthal 2001; Ginty et al. 2005). More frequently, SCFs as harmless solvents present substantial attention in pharmaceutical manufacturing processes due to their solvating power in sorting out the components and significant alterations in their physicochemical properties beyond the critical point (Kankala et al. 2017). Furthermore, additional advantages of SCFs consist of solubilizing ability and simplicity of recycling, among others. By modifying the critical pressure and temperature, the physical properties of SCFs specifically density, viscosity, solvency, and diffusivity that exist amid both liquid and gas can be simply changed during the processing of solutes (Pasquali and Bettini 2008; Kalani and Yunus 2011; Wu and Li 2008; Davies et al. 2008). In this context, SCFs such as water and solvents like acetone, CO2/ethanol mixture, chlorodifluoromethane, diethyl ether, nitrous oxide, propane, and trifluoromethane are operated at their equivalent supercritical conditions (Kankala et al. 2017; Davies et al. 2008; Reverchon and Adami 2006; Byrappa et al. 2008; Hakuta et al. 2003; Meziani et al. 2002; Warwick et al. 2002; Krober and Teipel 2002). The distinguishing characteristics of these SCFs, counting the critical parameters and other features, for instance, solubility, have been previously reported elsewhere (Perry 1997).
In all the SCFs existing, supercritical CO2 (SC-CO2) has greater focus from investigators because of its broad adaptableness, safety, cost-efficiency, and demanding gentle conditions for operation (temperature 304 K/31.1 °C and pressure 7.38 MPa/73.8 bar) under ambient conditions (Kankala et al. 2017). In addition, it ought to be distinguished that SC-CO2 is accepted as safe and sound by the US Food and Drug Administration in pharmaceutical production as it is harmless, nonreactive, nontoxic, nonpolluting, and nonflammable (Djerafi et al. 2015; Kalani and Yunus 2011; Kankala et al. 2017). Moreover, it presents numerous benefits which are greatly favorable for particle manufacturing, for example, low cohesive energy density, low polarizability per unit volume, and higher volatility, among others (Davies et al. 2008). Furthermore, the distinctive physical properties of SC-CO2, like density, diffusivity solvency, and viscosity, can be operated beyond its critical point by setting the temperature and pressure (Kankala et al. 2017). Growing demand for particle manufacturing of different active pharmaceutical ingredients (APIs) and study on their crystalline morphologies along with the target of solving the drawbacks of presently existing conventional techniques specifically particle damage as well as detriment of bioactivity by strong shear forces, different particle size distribution, and others have acquired great consideration of investigators to the SCF technology (Tomasko et al. 2003; Chen et al. 2011). Though the knowledge of implementing SCF for particle fabrication is still in their early years (46) (Huang et al. 2005). Numerous papers have been published in relation to the appliances of SCF on the fabrication of nanomaterials (Jung and Perrut 2001; Reverchon and Adami 2006; Martin and Cocero 2008).
2 Supercritical Fluid Technology
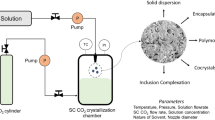
SCF technology is in exploit since the late nineteenth century as a means to know the natural mineralization , the actual momentum for this technique as a tool to handle a considerable number of materials started in the 1980s. With the discovery of Green Chemistry in the early 1990s, there was a rush in the acceptance of SCF technology. Green Chemistry is vital due to SCFs, mainly SC-CO2, and, to a more limited degree, SC-H2O is noticed as environment friendly more satisfactory substitute for the petroleum-based solvents , which are at present employed in the world’s chemical industries (DeSimone 2002). The undesirable effects of the residual solvents from both processing and environmental point of view have been acknowledged. Therefore, the intermediary processing methods for pharmaceutical products have restricted applications compared to the alternate methods of material processing like SCF technique. In the last decade SCF technology has observed a decisive growth in its application for manufacturing a choice of materials. SCF technology substitutes organic solvents in several chemical processes , counting chemical manufacturing , food processing like decaffeination of coffee beans, extraction, nanoparticle production, particles coating, polymer processing, recycling, waste treatment, etc. (Markocic et al. 2013; Wang and Chang 2015; Habulin et al. 2007; Skerget et al. 2011; Knez and Weidner 2003). Especially for the nanomaterial manufacturing for the advanced drug delivery and for drug formulation systems , SCF technique comes out as an option to the majority of the present techniques (Palakodaty et al. 2002). Contrary to the traditional particle fabrication techniques such as freeze-drying , spray-drying , and precipitation , where a large particle is initially produced and after that comminuted to the preferred size, SCF technique entails growing the particles in a controlled manner to achieve the desired morphology. The adverse effects instigating from the energy imparted to the system to achieve size reduction can thus be avoided (McHugh and Krukonis 1994; Cabanas and Poliakoff 2001). The particles once produced need not experience additional processing or handling and this characteristic putting SCF technology flexible to fabricate biomolecules and other sensitive molecules in their inhabitant pure state (Hamidreza et al. 2016; Chattopadhyay and Gupta 2002a). These techniques are rooted in a simple theory in which a drug and a polymer are co-precipitated together by means of the anti-solvent (non-solvent) properties of SC-CO2, since the majority polymers and drugs are not appropriate in SC-CO2. This process, however, is expected to be successful when polymer and drug molecules are able to form a solid solution. SCF technologies produce nanoparticles with solvent levels below 25 ppm. The advancements in SCF technology have promoted the manufacturing of new pharmaceuticals from small molecular drugs to biological macromolecules, for example, peptides, proteins, and nucleic acids (Tservistas et al. 2001). These growths have given birth to a new discipline, viz., pharmaceutical materials science, which deals with physical principles ordinary in materials science to confront in such areas as drug delivery, manufacture, and processing of nanoparticle systems for exploit in pharmaceutical applications (Elliot and Hancook 2006).
The benefits of SCF technology, for instance, are (i) quick one-step processing; (ii) mild operating temperature that has made SCFs an attractive technology particularly for heat-sensitive materials; (iii) facilitating the particle size to be reduced to such a great extent, which can be employed for aerosol drug delivery systems (Erkey 2009). The solubility of poorly water-soluble drugs can be improved, devoid of heating the substance, only with the aid of micronization induced by SC-CO2 (Lin and Jang 2008); (iv) SCF technology has the potential to replace the use of organic solvents that have been commonly used in the production of solid composite lipid/drug nanoparticles (Thote and Gupta 2005; Chattopadhyay et al. 2007).
The elevated pressure needed, higher maintenance cost, and prerequisite of the accessories/auxiliary types of equipment restrict the utilization of SCF technology for most of the pharmaceuticals. Thus, it gives the impression that this method cannot totally replace traditional methods as it is not appropriate for the processing of all pharmaceuticals (Girotra et al. 2013).
3 Supercritical Fluids
“Supercritical” is a condition of a substance beyond its critical temperature (TC) and critical pressure (PC) . A substance in its supercritical condition is characterized as supercritical fluid (SCF). The critical point stands for the highest temperature and pressure at which the substance can be present as a vapor and liquid in equilibrium (Sheth et al. 2012). At this condition, the fluid has inimitable properties , where it does not condense or evaporate to form a liquid or gas. A characteristic pressure-temperature phase diagram is shown in Fig. 6.1.
In the supercritical phase, there is no phase boundary amid the gas and the liquid phase . Briefly, it can act as if it is a liquid or a gas, but is actually neither (Sheth et al. 2012). The properties of SCF are in between that of liquid and gas. This Janus-faced nature of SCFs takes place from the reality that the liquid and gaseous phases join together and turn into identical at the critical point (Matsubara et al. 2010; Pinkston et al. 2004; Li and Hsieh 2008). The density of an SCF is similar to that of liquids, whereas its diffusivity and viscosity are similar to those of gases , as can be seen from Table 6.1. Additionally, the surface tension of an SCF is zero (Ollanketo et al. 2001). The “law of corresponding states” as given by van der Waals suggests that compounds act likewise below the same values of the reduced variables. This enables important comparison of different compounds under a variety of situations; however differences can be considerable in close proximity to the critical point (Saito 2013).
The densities of a substance in its supercritical region are either similar or near to that of the same substance in its liquid phase. This feature permits SCF to improve the solubility of poorly soluble drugs greater than the gaseous state could. The liquid-like density makes possible the strong solvent power of SCFs for a variety of solutes. The most fascinating features of SCFs are that their physical properties are very susceptible to temperature and pressure and there exists density inhomogeneity in the critical state. In a supercritical state, the density of the solvent around the solute can be much higher than that of the bulk, especially in the critical state of supercritical solvents, which is frequently described as a clustering (Yamini and Bahramifar 2000; Housaindokht and Bozorgmehr 2008; Knez et al. 2014; Ramsey et al. 2009; Sovilj et al. 2011; Yang and Zhong 2005). In contrast, the diffusivity and viscosity of SCFs are near to that of gas; which facilitates fast mass transfer or else diffusion of SCFs into materials as compared to that of the liquid states (Sheth et al. 2012).
Not each and every feature of SCFs are in between those of liquids and gases; properties like compressibility as well as heat capacity are notably elevated close to the critical stage than they are in liquids or gases. Though the properties of a substance may alter significantly with pressure in close proximity to the critical point, the majority of them display no discontinuity (Sovilj et al. 2011; Yang and Zhong 2005; Munshi and Bhaduri 2009; Dong et al. 2013; Cooper 2000). The alterations set up steadily, more willingly than with a sudden onset, when the situations move toward the critical point . SCFs are very much compressible, predominantly close to the critical point and their density, and therefore the solvation power can be cautiously regulated by little alterations in temperature and/or pressure (Yasuji et al. 2008; Mishima 2008). Even though these distinctive and balancing physical properties permit the progress of well-organized and adaptable methods, the SCFs are not worldwide “super-solvents.” Handfuls of drug substances are displaying solubility in SCFs with no addition of a co-solvent.
Though every gas possibly will achieve supercritical state beyond their critical stage , for several, tremendously higher pressure, as well as temperatures, might be necessary which may not be appropriate for pharmaceuticals. One must also consider the safety and affordability in addition to mild processing conditions, when choosing the SCF; for example, Xenon and Sulfur hexafluoride (when sufficiently purified) have low critical values, but remain too expensive for commercial use. Gases like nitrous oxide or ethane also has low critical values but they are able to produce explosive mixtures and are thus dangerous to handle. Trifluoromethane , a chemically inert and nonflammable compound, has low critical temperature and pressure and also low toxicity. Additionally, trifluoromethane has strong lasting dipole moments (1.56 D), which aid the solubilization of pharmaceutical materials. On the other hand, carbon dioxide (CO2) is the most favored SCF for the treating of heat-sensitive pharmaceuticals like biologicals. It has a low critical temperature (31.2 °C) and pressure (73.8 bars) and is nonhazardous, nonflammable, and environmentally harmless (Sheth et al. 2012).
The most commonly employed SCF is supercritical CO2 (SC-CO2) , which is economical and nonpolluting and whose critical parameters are easy to be achieved in industrial equipment. Regardless of the issues over the greenhouse consequence of CO2, it can be measured as an environmentally affable substitute to available organic solvents, in view of the fact that the CO2 utilized in this method is already recycled and so the net load on the environment is unaffected (Byrappa et al. 2008). Though in last few years, not only the materials handling is being carried out with SC-CO2, but also alcohols, ammonia, light hydrocarbons, and water have been suggested, along with the others, for nanomaterials manufacturing at supercritical states (Markocic et al. 2013; Wang and Chang 2015).
4 Supercritical Processes for Nanoparticles Manufacturing
SCFs have been suggested as a medium to manufacture nanomaterials. SCF precipitation processes can be categorized according to the function of the SCFs in the method. In fact, SCFs have been intended as solvents, solutes, anti-solvents, and reaction media. SCF can take action as a solvent, as in the rapid expansion of supercritical solutions (RESS) technique (Martin and Cocero 2008; Gosselin et al. 2003). It can act as a solute, as in the precipitation from gas-saturated solution (PGSS) technique (Fraile et al. 2013; Martin and Cocero 2008), and as an anti-solvent, in the supercritical anti-solvent (SAS) (Bertucco et al. 1996; Sacchetin et al. 2016) and gaseous anti-solvent (GAS) techniques (Yeo et al. 1993). It can act as a propeller, in the supercritical assisted atomization (SAA) technique (Martin and Cocero 2008; Reverchon 2002; Shen et al. 2014). It can also act as a reagent (Beckman 2004) and others like aerosol solvent extraction system (ASES) (Hakuta et al. 2003), precipitation with compressed anti-solvent (PCA) (Falk et al. 1997), supercritical anti-solvent with enhanced mass transfer (SAS-EM) (Chattopadhyay and Gupta 2002a), solution-enhanced dispersion by supercritical fluids (SEDS) (Chen et al. 2012a), and suspension-enhanced dispersion by supercritical fluids (SpEDS) (Chen et al. 2012b). Regardless of the variation in the actions, SCF behaves as a re-precipitation aid for quick, homogenous, as well as smooth nucleation of solutes (drug and/or polymer) in each and every above-mentioned technique proposed for particle manufacturing. Furthermore, the operating effectiveness of these techniques entirely depends upon the choice of a proper solvent and fine adjustment of the critical factors (Vemavarapu et al. 2005).
4.1 Particles from Gas-Saturated Solutions (PGSS)
Lots of drugs are either polar or exhibit higher molecular weights . It is not easy to solubilize these materials in CO2, which is a nonpolar solvent, still in a supercritical situation excluding the help of a co-solvent. Conversely, SC-CO2 has the aptitude to penetrate into organic substances, like polymers. When SC-CO2 penetrates into the polymer, it decreases the melting point and reduces its viscosity. These aspects are made exploit of in the PGSS technique (Sheth et al. 2012). The SCF is employed as a solute in the PGSS method (Kerc et al. 1999).
In the PGSS operations , the physical mixture of the drug and the polymer are initially subjected to SCF. It entails the melting of the substance to be treated, which afterward dissolves in SCF under pressure. In subsequent melting, the additional introduction of the SC-CO2 dissolves the mixture further and viscosity diminishes. This solution is, after that, atomized by the use of a nozzle and a pressure regulating valve into a receiver. Because of quick depressurization, the dissolved SCF gets away, as a result, the development of composite microparticles (Mishima 2008). For the reason that the solubility of compressed gases in liquids and solids such as polymers are generally elevated, and much greater than the solubility of such liquids and solids in the compressed gas state, the method exists in solubilizing SC-CO2 in melted or liquid-suspended materials, leading to a named gas-saturated solution/suspension that is again expanded through a nozzle with the development of solid particles or droplets. The materials subjected do not require solubility in SC-CO2, which is the most important benefit of this technique. This method can be employed with suspensions of active substrates in a polymer or other carrier substance which leads composite microspheres (Jung and Perrut 2001; Date and Patravale 2004; Palakodaty et al. 2002; Byrappa et al. 2008). This procedure is intended for fabricating particles of materials that absorb SCFs at higher concentrations like polyethylene, polyester, polyethylene glycol, poly (vinylpyrrolidone) (PVP), and polylactic acid (Sheth et al. 2012). This strategy is beneficial than other SCF processes because it requires a small amount of SCF (Hakuta et al. 2003; Kerc et al. 1999).
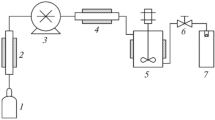
Figure 6.2 shows the fundamental apparatus employed in the PGSS processing of materials. The PGSS can be applicable to process inorganic powders to pharmaceutical compounds. Low handling cost and wide range of products that can be processed like liquid droplets or solid particles is the straightforwardness of this technique, which unlock wide opportunities for the development of PGSS system, not only for high-value substances but also possibly for merchandise, despite limits related to the complexity to observe particle size. A number of pharmaceuticals, for example albuterol sulfate, calcium antagonist drugs, cromolyn sodium, DL-alanine, glucose, glutathione, nifedipine, tobramycin, etc., and in addition lots of inorganic and organic compounds such as glycerides, metal oxides, plastic additives, pigments, phosphors, spinels, etc. have been urbanized with the PGSS technique (Jung and Perrut 2001; Date and Patravale 2004; Palakodaty et al. 2002; Byrappa et al. 2008).
Schematic representation of equipment set up for PGSS process (Sheth et al. 2012)
The benefits of the PGSS technique are similar to those of the RESS technique; it can be carried out without employing organic solvents and it generally needed low pressures as well as consumption of gas as compared to the RESS technique. The segregation of the ingredients when they move across the pressure drop is one of the major problems of the conventional PGSS technique. Particles of the drug and the polymer are produced independently, but the polymer microparticles comprising the drug could not be achieved. PGSS has been customized to conquer the agglomeration and nonuniform particle size distribution issues. Researchers suggested a system to defeat the separation difficulty, by an arrangement of two separate mixing compartments in the apparatus. In the first compartment, the drug and the polymer are mixed to homogeneity; let them melt in SC-CO2. This melt was then moved from the first compartment to the second one, where it was mixed with additional SCF, resulting in an extra fall in the viscosity of the melt. The mixture was at last sprayed and further expansion took place, resulting in the formation of uniform size of microparticles of the polymer-drug mix (Shekunov et al. 2006; Sheth et al. 2012).
4.2 Rapid Expansion of Supercritical Solutions (RESS)
The RESS technique is schematically illustrated in Fig. 6.3. This technique is employed when the solute like polymer, drug, or drug-polymer matrix freely dissolves in the SCF.
The RESS technique is accomplished by means of the saturation of the SCF with a drug or drug-polymer matrix, followed by depressurization of the solution by passing through a heated nozzle into a low-pressure vessel that creates quick nucleation of the drug or drug-polymer in the form of incredibly smaller particles that are collected from the gaseous stream. The morphology of the obtained solid material, amorphous or crystalline, depends upon the chemical constitution of the substance and on the RESS parameters like impact distance of the jet against a surface, temperature, pressure drop, nozzle geometry, etc. (Jung and Perrut 2001). The very quick discharge of the solute in the gaseous state is supposed to give surety of the fabrication of nanoparticles. This technique is most appealing because of the absence of organic solvents.
In designing this technique, the solubility of the substance plays a vital role in particle fabrication and processing as the majority of the pharmaceutical materials, for example, drugs, high molecular weight proteins, and polymers are polar in nature. In a few cases, little quantities of organic solvents are needed to get better the affinity of polar drug molecules (Mishima 2008). RESS technique is the simpler and an effectual technique in the SCF technology, but it is restricted in its appliance owing to its comparatively higher cost and poor solubility of polymers in non-polar SC-CO2. To tackle this problem, high amounts of SC-CO2 are favored at an industrial scale (Chen et al. 2011). Furthermore, the progressions in the RESS technique have been developed to beat certain drawbacks. A fascinating variant of the RESS technique is the rapid expansion of a supercritical solution into a liquid solvent (RESOLV) that is comprised of atomizing the supercritical solution into a liquid. Processing in this way, it should be feasible to reduce particle growth in the precipitator chamber, as a consequence enhancing the RESS technique performance. In addition, through interaction with the nucleating solid particles as well as the materials present in the liquid medium, a chemical reaction step can also be included. These types of alterations in the procedure reduce particle agglomeration in the expansion jet (Dalvi et al. 2013).
From the hypothetical point of analysis , the budding aspects of RESS are very fascinating, although the outcomes have not been predominantly in high quality in some cases. It is in many cases problematic to control the particle size of the precipitates. At some stage in the expansion step, the particles fuse in the supersonic free jet generated in the precipitation chamber, and, so, in several cases, needlelike particles have been achieved. The generation of tilting needles can be elucidated by the existence of electrostatic charges on the surface of the particles, provoked by the rapid relative motion among the particles and the gas contained in the expansion vessel (Reverchon et al. 1995).
For the production of nanoparticles, RESOLV configuration has been confirmed to be more successful, as the liquid that gets the expanding jet can inhibit the particle growth. To shield particles from agglomeration, a little quantity of stabilizing agent is added in the liquid.
The main drawback of RESS and RESOLV techniques is that both techniques are applicable only to those products which have a moderate solubility in the chosen SCF. Regrettably, a lot of solid materials with high molecular weight and polar bonds are a good candidate for fabrication of nanoparticles, displaying extremely low or negligible solubility in SC-CO2, and show a decreased solubility in lots of other substances which can be good candidates as SCF (Reverchon and Adami 2006). RESOLV technique has also the issue of the recovery of particles from the liquid solution employed to get better the performance of process: in this configuration, the technique is no more solventless (Reverchon and Adami 2006). A further modification of the RESOLV process consists of the use of water in SC-CO2 (w/c) microemulsion used as a modified supercritical solvent to dissolve AgNO3 (Sun et al. 2001).
One more modified technique is the rapid expansion of a supercritical solution with solid co-solvent (RESS-SC) , which results in nanoparticles. In processing, the additional co-solvent enhances the solubility of the APIs to a larger degree by circumventing superficial exposure among particles, which augments the surface area of contact to SCF, and ultimately, lyophilization can eliminate the co-solvent (Thakur and Gupta 2005). In spite of its progressions, RESS still has some restraints that are improved on by the changed SCF actions as anti-solvent in the reaction chamber .
4.3 Gas Anti-solvent Processes (GAS)
Gas anti-solvent (GAS) technique has been urbanized with the intention to attain nanoparticles of the hydrophobic materials which cannot be developed by the RESS technique because of their poor solubility in SCFs . The starting point of the GAS technique is derived from the fact that when a solution is expanded satisfactorily by a gas, the liquid state is no longer a choice of solvent for the solute and nucleation takes place. For instance, a GAS was employed to decrease the lower critical solution temperature of polymeric solutions to concentrate polymers (McHugh and Guckes 1985; Seckner et al. 1988). The solute to be undergoing micronization is present in a liquid solution; the SCF must be totally miscible with the liquid solvent, while the solute must be insoluble in the SCF. As a consequence, the liquid solution along with SCF brings the generation of a solution, causing supersaturation and precipitation of the solute. The generation of the liquid mix is incredibly quick caused by the improved mass transfer rates that distinguish SCFs, and accordingly, nanoparticles could be formed (Byrappa et al. 2008).
This method is most widely used for polymers as they are generally insoluble in SCFs. Saturation of the polar solvent, comprising a dissolved substrate, with SC-CO2, in this manner diminishing the solvent power of a polar solvent, resulting in the precipitation of the substrate is the fundamental theory of this system (Dehghani and Foster 2003). A ternary system consisting of polymer, liquid organic solvent, and gas as anti-solvent is employed in this method. The polymer first dissolved in a chosen organic solvent and then the gas is permitted to pass from a closed vessel. With the increase in pressure, the concentration of the gas so employed increases in the chamber and the polymer is precipitated out. As soon as the solvent is introduced in the method, phase separation among liquid-solid and liquid-liquid took place because they are shifted to higher temperatures (Jung and Perrut 2001). The quick expansion of SCFs through the nozzle of the vessel happens which is after that followed by a fall in temperature and pressure. The size of the particle produced by this technique mostly relies on the diameter of the nozzle and its length (Girotra et al. 2013).
The GAS technique has been fruitfully used for the formation of insulin in poly-l-lactide (PLLA) nanoparticles. The method produces nanoparticles with high encapsulation efficiency as well as high yield of nanoparticles of with the size range of 400–600 nm and preservation of greater than 80% of the insulin hypoglycemic activity and was as well capable of getting rid of extensive utilization of organic solvent (Elvassore et al. 2001).
4.4 Supercritical Anti-solvent Processes (SAS)
The SAS technique is intended for the compounds that have poor solubility in SCF. In this technique, organic solvents like acetone, dichloromethane (DCM), and dimethyl sulfoxide (DMSO) are used to dissolve the materials, and SCF act as a non-solvent to solute/API (Kalani and Yunus 2011). Briefly, in the SAS, the solute undergoing a micronization process is present in the liquid solution, and the SCF must be completely miscible with the liquid solvent, while the solute is ought to be insoluble in the SCF at the process arrangements. As a result, exposing the liquid solution with the SCF brings about the generation of a solution, forming supersaturation and subsequent precipitation of the solute. Because of the improved mass transfer rates, the formation of the liquid mix is very rapid that differentiates SCFs, and, accordingly, nanoparticles could be developed (Kalani and Yunus 2011). The SAS technique has been utilized by a number of researchers for the production of nanoparticles by using different process conditions, but the most important variations have relied upon the manner by which the process brings about, in a batch or a semicontinuous means (Reverchon 1999). In a batch operation (GAS), the precipitation chamber is first charged with a known quantity of the liquid solution, and, after that, the supercritical anti-solvent is introduced until the ultimate pressure is achieved. While in the semicontinuous operation (SAS), the liquid solutions, as well as the supercritical anti-solvent, are constantly added to the precipitation chamber in co-current or countercurrent manner. The liquid solution injection device as well plays a significant role (Dehghani and Foster 2003). The injector is specially planned to turn out liquid jet breakup and the creation of small-sized droplets to create a greater mass transfer surface among the liquid and the gaseous state. High-pressure vapor-liquid equilibria (VLEs) and mass transfer amid the liquid and the SCF, in addition, play a significant action in SAS. Predominantly, VLEs of the ternary system solute-solvent-SC anti-solvent and the location of the working point in SAS processing with regard to these VLEs can be influential for the victory of the procedure. The development of a single supercritical region is the key footstep for the winning fabrication of nanoparticles (Reverchon et al. 2003). At the last stage of the precipitation process, the washing step with pure supercritical anti-solvent is as well essential to circumvent the condensation of the liquid medium that otherwise showers on the precipitate transforming its distinctiveness . As a rule, SC-CO2 has been employed in this technique.
As the injection of the liquid solution is appropriately carried out, the boundaries of the SAS process are in the complexity of guessing VLE adjustments brought by the existence of solute on the binary liquid-SCF system. Extremely multifaceted phase behaviors can be developed. In the simpler situation, the change of the mixture critical point (MCP) in direction to the higher pressures can be found. In the condition of more raise in the MCP pressure, incredibly high pressures will be needed to attain a single-phase system and the successful manufacturing of nanoparticles achieved (Reverchon and De Marco 2004).
The production of extremely larger crystals is found in certain situations which are in relation to SAS precipitation from a liquid-rich phase because of an alteration of the shape and the degree of the miscibility gap (Reverchon 1999). When two phases are concurrently present, a variety of morphologies can be achieved that can be depending upon the precipitation from a liquid-rich state (crystals) and the SCF-rich state (amorphous particles); the comparative amounts of precipitates are correlated to the partition factor of the solute among the two phases.
The product of the SAS technique is completely related to the order of the introduction of solvent, SCF, and other substrates. Furthermore, parameters like the chemical composition of solute as well as an organic solvent, temperature, and pressure are essential to be optimized. SAS has achieved superior drug loading capacity as compared to that of the RESS technique, facilitating the production of fine particles (Mishima 2008). Latest progressions in SAS micronization methods consist of expanded liquid anti-solvent (ELS) (Prosapio et al. 2016) and the supercritical assisted injection in a liquid anti-solvent (SAILA) techniques (Campardelli et al. 2012); conversely, profound analysis on these techniques so far remains to be reported. The ELS is processed with SCF and an organic solvent at expanding liquidity states (Prosapio et al. 2016). SCF-assisted extraction of emulsions (SFEE) is another tailored SAS method (Della et al. 2010).
4.5 Aerosol Solvent Extraction System (ASES)
In ASES , formation of particles takes place at a higher anti-solvent-to-solvent proportion following spraying the drug/polymer solution into SCF through an atomization device. The mass transfer of SCF relies upon atomization efficiency, whereas mass transfer of solvent depends on the dispersing as well as the mixing of organic solvent and SCF. For loading high quantities of drugs, ASES is not an appropriate technique attributable to their more affinity for organic solvent, which finally decreases the loading quantity in the polymer following organic solvent extraction process (Mishima 2008).
This technique is very much related to the SAS technique . In the ASES, the solution is sprayed via an atomization nozzle as fine droplets into compressed CO2 (Bleich et al. 1993). The dissolution of the SCF into the liquid droplets is achieved by expansion of large volume, and, as a result, there is a drop in the liquid solvent power, inducing a sharp increase in the supersaturation in the liquid mix, and resulting into the generation of uniform-sized micro/nanoparticles. Briefly, the SCF is introduced to the top of the high-pressure chamber through a high-pressure pump. The material to be micronized in a solution form is added into the high-pressure chamber by means of a nozzle as soon as the system achieves a steady state. The liquid has to be introduced at a higher pressure than the chamber operating pressure to get tiny liquid droplets and the particles are brought together at the base of the chamber (Byrappa et al. 2008). The fluid mixture (SCF and the solvent) exits the vessel and flows to a depressurization tank where the pressure-temperature conditions allow gas-liquid separation. The pumping of the liquid has to be discontinued once the gathering of enough amounts of micro/nanoparticles and pure SCF goes on to run through the chamber to eliminate leftover solvent from the micro/nanoparticles (Byrappa et al. 2008; Hakuta et al. 2003).
The fundamental working theory of this system is the extraction characteristics of the SCFs. First, the drug and the polymer are dissolved into an organic solvent and then this solvent is atomized into the SC-CO2. The organic solvent is chosen in a manner ensuring that it is soluble in SC-CO2. The solvent is afterward extracted resulting into the development of micro/nanoparticles (Girotra et al. 2013).
In addition, a minor modification of the ASES technique is referred to as the precipitation with a compressed anti-solvent (PCA) technique, which successfully manufactures micro/nanoparticles with a narrow size distribution. This progression has been documented as a single-step method specially processed to precipitate proteins (Shoyele and Cawthorne 2006).
4.6 Supercritical Anti-solvent with Enhanced Mass Transfer (SAS-EM)
SAS-EM is a sophisticated SAS technique specifically designed to defeat the existing drawbacks of the SAS technique (Chattopadhyay and Gupta 2002a). In the SAS-EM technique, a vibrating ultrasonic processor has been employed for atomizing the solution jet into micro-droplets. Because of this modification, the working method produces higher turbulence, which ultimately improves the mixing process and consequently the mass transfer rate and forms smaller-sized particles (Langer and Vacanti 1993).
4.7 Solution-Enhanced Dispersion by Supercritical Fluids (SEDS)
One more up-gradation of the SAS technique is the SEDS technique which is particularly designed for single as well as binary compounds. In the SEDS technique, a specially designed coaxial nozzle has been utilized, which comprises two channels for a single compound and three channels for binary compounds.
The SEDS method is carried out at a lesser drying time in addition to improved mass transfer rates, which diminish the ASES procedure restraints. In a characteristic SEDS technique, the dispersed materials are atomized through a specifically designed co-axial nozzle for the control of the particle morphology (Mishima 2008). The fundamental theory is rooted in dispersing an aqueous solution, which comprises the biomaterials, along with SC-CO2 and a polar organic solvent in a three-channeled coaxial nozzle. Moreover, the SC-CO2 is employed to take out the aqueous part of the product. The organic solvent is acting as a precipitating agent as well as a modifier, making possible the non-polar CO2 to eliminate the water (Tservistas et al. 2001; Young et al. 1999). The dispersion in the jet at the nozzle exit allows the quick development of small-sized dry micro/nanoparticles (Byrappa et al. 2008). The mass transfer rate of SCF into the sprayed droplet decides the particle generation by the solvent transfer rate into the SCF stage. The higher the mass transfer rate facilitating, the quicker the nucleation, resulting into smaller particle sizes with not as much agglomeration (Shoyele and Cawthorne 2006).
In fact, a polymer processing with organic solvents is very easy to get with the SEDS technique owing to solubility issues. In addition, the continuous SEDS procedure has prolonged the shelf life of polymeric substances. In this method, water-soluble materials can as well be dealt with by means of introducing organic solvent via a co-axial three-compartment nozzle (Palakodaty et al. 1998). Identical to GAS, even SEDS has been comprehensively utilized for the micro/nanoparticles fabrication of a wide variety of organics, biopolymers, composites , etc. (Byrappa et al. 2008; Girotra et al. 2013).
4.8 Suspension-Enhanced Dispersion by Supercritical Fluids (SpEDS Process)
The SEDS technique has been specially modified into SpEDS technique to conquer its processing damage problems (Chen et al. 2012b). The operation of both techniques is mostly identical, except that SpEDS has an auxiliary injector arrangement for efficiently pumping the loaded suspension (Chen et al. 2012b). The SpEDS technique is specifically planned to achieve core-shell structured micro/nanoparticles with high drug encapsulation efficiency as well as longer sustained drug release features in contrast to other SCF-assisted co-precipitation techniques (Chen et al. 2012a, c).
4.9 Supercritical Assisted Atomization (SAA)
Supercritical assisted atomization (SAA) is the latest technique (Reverchon 2002) in which the SCF acts as an atomizing medium. The technique is anchored in the solubilization of SC-CO2 in the liquid solution produced by the solvent and the solid solute, followed by atomization of resulting solution using a thin wall nozzle. When SAA technique is accurately performed, two atomization procedures come to pass: the first one is the formation of primary droplets at the outlet of the nozzle by means of pneumatic atomization; the second one obliterates these droplets with the quick discharge of CO2 from the interior of the droplet known as decompressive atomization . Amorphous or crystalline particles have been formed, on the basis of the process temperatures as well as the chemical structures of the solid solute. By utilizing SAA technique, investigators have prepared polymethylmethacrylate nanoparticles using acetone as a solvent. In this method, they used 10 mg/mL polymethylmethacrylate in acetone, and they employed a mixing temperature 80 °C and a mixing pressure of 76 bar and produced nanoparticles with a mean diameter of 120 nm (Reverchon and Spada 2004). The major problem of this technique is that the smallest particles formed relay on the dimensions of the smallest droplets produced (one droplet-one particle method). These dimensions are related to the standard parameters that regulate droplet dimensions at some stage in atomization: surface tension, viscosity, and amount of SCF dissolved in the liquid.
5 Application of SCF for Production of Nanoparticles
Zinc acetate nanoparticles , a catalyst precursor, have been prepared by the SAS technique. Nanoparticles formed showing particle size in a range of 30–50 nm at the best processing temperature-pressure conditions (Reverchon et al. 1999). Chattopadhyay and Gupta produced fullerene (C60) nanoparticles from a solution of toluene. They have performed experiments in a SAS batch mode (injection in static SCF) and fullerene nanoparticles as small as 29–63 nm were achieved operating at different conditions (Chattopadhyay and Gupta 2000).
Reverchon and coworkers have been processed dextran, a bio-polymer using DMSO. The nanoparticles formed have a spherical morphology and a mean particle size ranging between 125 and 150 nm (Reverchon et al. 2000). Chattopadhyay and Gupta (2001b, 2002b) produced griseofulvin (antifungal, antibiotic) particles as low as 130 nm and lysozyme (enzyme) particles of about 190 nm using SAS-EM technique.
β-sitosterol nanoparticles of about 200 nm mean diameter have been prepared by Turk and coworkers using the RESS technique. They tested the method in SC-CO2 at different pre-expansion temperatures as well as pressures and found that for β-sitosterol the alteration of pre-expansion conditions does not lead to substantial differences in the diameters of nanoparticles. They also employed this technique for the manufacturing of griseofulvin nanoparticles using supercritical CHF3 (Turk et al. 2002).
Snavely et al. (2002) developed insulin nanoparticles using the SAS technique with the aid of an ultrasonic nozzle. They achieved powder consisting of physical aggregates of 50 nm spherical particles forming cobweb-like and sponge-like structures that can be de-agglomerated in smaller components. The development of cobweb structures has been described by other researchers and is most likely attributable to the impact, and the accidental coalescence of the nanoparticles of some polymers has also been formed. Nanometric lysozyme particles with the smallest mean diameter of 180 nm were also developed by Muhrer and Mazzotti (2003) using the GAS technique.
Cyclosporine nanoparticles have been developed by the rapid expansion from supercritical to aqueous solutions (RESAS) technique, and the effect of different stabilizes like nonionic surfactants, e.g. Tween 80, Pluronic F127, Myrtj 52, and phospholipid-based surfactant on the particle size, is also compared. Among all the nonionic surfactant, Tween 80 generates smaller particle sizes that range from 660 to 970 nm, while phospholipid-based surfactant generates cyclosporine nanoparticles with size that ranges between 200 and 300 nm, which is smaller than particles generated by Tween 80 at the same concentration of the surfactant and drug/surfactant ratio . This outcome is due to the aggregation of a large quantity of surfactant for phospholipid vesicle than that for micelle. In addition, the favored curvature of the surfactant is more encouraging for vesicle than micelle as the boundary with water is little curved for vesicle as compared to micelle. Also, vesicles are comparatively stable, so the enlargement of the drug particles by collision/coagulation is diminished (Young et al. 2003).
Sane et al. (2003) developed fluorinated tetraphenyl porphyrin spherical, agglomerated nanoparticles with mean particle sizes from 60 to 80 nm, at various pre-expansion temperatures using RESS technique. Pestov and coworkers have also employed the RESS technique for the production of solvent-free and photoreactive nanoparticles of 2, 5-distyrylpyrazine (DSP) monomer . They precipitated DSP from CHClF2 and they have also observed greater photoreactivity in the solid state as compared to micro-scale crystals (Pestov et al., 2003).
Using the RESS technique, Varshosaz et al. (2009) produced amorphous cefuroxime axetil nanoparticles. They studied the effect of formulation parameters, like the nozzle temperature (varying between 50 and 70 °C) and the extraction column temperatures (varying between 60 and 90 °C), on the particle size as well as morphology. Amorphous nanoparticles showing the mean size of 159 nm were achieved with 60 °C nozzle temperature and 90 °C column temperature. They also observed that when the temperatures of the nozzle as well as the extraction column were reduced to 50 °C and 75 °C, correspondingly, and the particle size was raised to 465 nm.
Pure naproxen nanoparticles were produced and naproxen nanoparticles coated with polylactic acid using the RESS technique. The researchers confirmed that the coating of polylactic acid stabilized the naproxen nanoparticles towards aggregation as well as coagulation (Gadermann et al. 2009).
Amoxicillin nanoparticles were developed by the SAS technique using N-methyl pyrrolidone and CO2 as solvent and anti-solvent, respectively, and the effect of primary drug concentration, the flow rate of drug solution, temperature, and pressure and nozzle diameter on particle size as well as size distribution have been studied. Investigators found that if the initial drug concentration increased, it results in bigger particle sizes with a wide particle size distribution. The outcome is that the higher the condensation rate from the higher drug concentration, the higher the super-saturation from higher drug concentration. Greater flow rate results in smaller particle sizes because of a greater degree of mixing. Spherical nanoparticles of amoxicillin with a mean size diameter of 216–505 nm were achieved by the SAS technique (Montes et al. 2010).
Digitoxin nanoparticles were formulated by the RESS technique, and the effects of processing parameters, for example flow rate, distance of spray, and pre-expansion temperature on the particle size, were studied. It was observed that the particle size of digitoxin particles reduces with the raise of flow rate as well as spray distance. In the earlier case, the residence time of droplets within the nozzle and in the expansion vessel is reduced by raising the flow rate. This reduces growth time for particle and resultant in smaller particle sizes. The latter case is the ensuing of two opposing phenomena. First, as the spraying distance is small, the residence time of droplets inside the expansion vessel lessens resulting in smaller particle sizes. In the contrary, small spraying distance leads to the coalescence of droplets because of lessening angles amid droplets. Conversely, particle size rises by raising the pre-expansion temperature. In every circumstance, the particle size of digitoxin was reduced from 0.2–8 μm to 68–458 nm (Atila et al. 2010).
5-Fluorouracil (5-FU) nanoparticles were formulated using the SAS technique to improve the physical characteristics of 5-FU to administer it directly to the respiratory tract. Different mixtures of methanol with dichloromethane, acetone, or ethanol were utilized for particle fabrication, and their effects on the physical characteristics of the final products were investigated. The experimental conditions for pressure in the range of 100 and 150 bars, a temperature of 40 °C, and a flow rate of 1 mL/min were kept. Regardless of variations in size, the particles did not differ in their morphology. The obtained nanoparticles were of a regular shape, somewhat spherical, and showed smooth surface, while the automatically milled particles exhibited less uniformity, showed surface irregularities and a wide particle size distribution, and appeared clustered. 5-FU nanoparticles prepared from methanol-dichloromethane 50:50 had a mean particle size of 248 nm (Kalantarian et al. 2010).
Raloxifene nanoparticles were formulated by the RESS technique, and the effect of extraction temperature, extraction pressure, and spray distance on the formation were investigated. It was observed that by raising extraction pressure from 10 to 18 MPa as well as spray distance from 5 to 10 cm, the particle size reduced. On the other hand, by raising extraction temperature from 40 to 60 °C, the particle size turned out to be smaller; however, a further raise in temperature to 80 °C reduces the particle size. The latter case can be clarified by the fact that higher temperature brings about a higher degree of supersaturation because the solubility increases at the elevated temperature. And this high level of supersaturation enhances the number of nuclei creation, which sequentially raises the likelihood of a collision and subsequently larger particle production. Raloxifene nanoparticles of 14.11 nm were achieved at the best possible condition of 50 °C temperature, 17.7 MPa extraction pressure, and 10 cm spraying distance (Keshavarz et al. 2012).
Beclometasone dipropionate nanoparticles were developed using the RESS technique, employing CO2 as a supercritical solvent. A full factorial two-level design was used so as to evaluate the processing parameters counting the pre-expansion temperature, extraction pressure, and fraction of co-solvent on the particle size and particle size distribution of beclometasone dipropionate nanoparticles. The mean sizes of beclometasone dipropionate nanoparticles were found in between 64.1 and 294 nm. Analysis of Variance (ANOVA) demonstrated that the extraction pressure was the most significant parameter and a high extraction pressure also plays a significant role for the development of small-sized particles, whereas increasing the pre-expansion temperature and weight fraction of co-solvent displays an increase in particle size. The RESS technique demonstrated as a talented process for the fabrication of beclometasone dipropionate nanoparticles that may well result in the enhancement of the drug’s physicochemical characteristics (Mohsen et al. 2015).
Cefquinome nanoparticles were prepared by using the SAS technique. By orthogonal experimentations, it was established that the concentration of the solution was the most important feature in this technique, followed by the feeding speed of solution, precipitation pressure, and precipitation temperature. For the moment, the best possible processing conditions of preparing cefquinome nanoparticles were 100 mg/mL concentration of the solution, 1.5 mL/min solution flow speed, 13Mpa processing pressure, and processing temperature 33 °C. A confirmatory trial was performed under this situation. It was observed that the look of particles was flakes and the mean diameter of particles processed was 710 nm. Furthermore, univariate analysis was carried out to study the influence of the decree of individual factors on particle size. Outcomes confirmed that the mean diameter enlarged with an increase in the concentration of the solution, however, lowered with an increase in the feeding speed of the solution. The consequence of both precipitation pressure as well as temperature on the mean diameter was comprehensive. When these two parameters increased, the mean diameter might show an extreme point. The SAS technique for preparing cefquinome nanoparticles grasps significant insinuations for the enhancement of the effectiveness of cefquinome and the growth of pharmaceutical developments (Xiao et al. 2015).
Spherical-shaped polycaprolactone nanoparticles were fruitfully produced by SCF extraction of emulsions. The competence of the SC-CO2 extraction was studied and related to that of solvent extraction at atmospheric pressure. The investigations have been done on the effects of operating parameters like the concentration of polymer (0.6–10% w/w in acetone), the concentration of surfactant (0.07 and 0.14% w/w), and polymer-to-surfactant weight ratio (1:1–16:1 w/w) on the surface morphology as well as particle size. Spherical polycaprolactone nanoparticles with average particle sizes between 190 and 350 nm were developed that mostly rely on the polymer concentration, which was the most significant parameter where an increase in the particle size was directly compared to total polymer concentration in the product. Polycaprolactone nanoparticles were characterized by dynamic light scattering technique and scanning electron microscopy technique. The results showed that SCF extraction of emulsions can be useful for the formulation of polycaprolactone nanoparticles without aggregation and in a relatively small period of just 1 h (Ajiboye et al. 2018).
6 Summary and Future Perspective
The nanoparticles for drug delivery manufactured using different SCFs present controllable particle size and high degrees of diffusivity in both drugs and synthetic polymers. The SCF technique has given a clean environmentally pleasant nanoparticle manufacturing technique as a substitute to the customary technique. SCF technique will aid to deal with the industry’s challenges to hit upon quicker, faster, and more cost-effective techniques to grow novel drug nanoparticles for sustained and controlled delivery without being affected by the harsh operating environments. The production manufacturing of nanoparticles with precise physicochemical characteristics is a major problem in the pharmaceutical industry. The problems one should deal with for victorious nanoparticle manufacturing is to opt for a successful SCF technique relating to temperature, pressure, mass transfer, and type of solvent utilized and phase behavior. Application of SCF technique to manufacture nanoparticles for drug delivery along with small particle size, improved flexibility, and enhanced rate of dissolution with SC-CO2 proposes a significant role for this technology in future drug delivery appliances and takes new prototypes in healthcare and getting better human health. A great number of SCF-based techniques are urbanized in current years. There is still potential to progress the available SCF techniques by improving processing conditions like physical and chemical parameters. Moreover, lots of new techniques can be urbanized by accepting the features of SCFs, the nature of the solute, and their interaction. For sure, SCF techniques are superior techniques over available and traditional techniques like milling/crushing for size reduction, soxhlet extraction, spray coating, impregnation by soaking, etc. Still, extensive investigation is required to put together it in realistic and at an industrial level. Hence, the overall perspective is very optimistic for future applications, and the healthiness of the future generation can be guaranteed with the help of the SCF technique. In this sense, nanoparticles manufacturing with SCF technology might see thrilling perspectives.
References
Ajiboye AL, Trivedi V, Mitchell JC. Preparation of polycaprolactone nanoparticles via supercritical carbon dioxide extraction of emulsions. Drug Deliv Transl Res. 2018;8:1790–6.
Atila C, Yildiz N, Caliml A. Particle size design of digitoxin in supercritical fluids. J Supercrit Fluids. 2010;51:404–11.
Bałdyga J, Kubicki D, Shekunov BY, Smith KB. Mixing effects on particle formation in supercritical fluids. Chem Eng Res Des. 2010;88:1131–41.
Beckman EJ. Supercritical and near-critical CO2 in green chemical synthesis and processing. J Supercrit Fluids. 2004;28:121–91.
Bertucco A, Vetter G, editors. High pressure process technology: fundamentals and applications, industrial chemistry library, vol. 9. 1st ed. Amsterdam: Elsevier; 2001.
Bertucco A, Pallado P, Benedetti L. Formation of biocompatible polymer microspheres for controlled drug delivery by a supercritical anti-solvent technique. Process Technol Proc. 1996;12:217–22.
Bleich J, Muller BW, Wabmus W. Aerosol solvent extraction system: a new microparticle production technique. Int J Pharm. 1993;97:111–7.
Byrappa K, Ohara S, Adschiri T. Nanoparticle’s synthesis using supercritical fluid technology – towards biomedical applications. Adv Drug Deliv Rev. 2008;60:299–327.
Cabanas A, Poliakoff M. The continuous hydrothermal synthesis of nano-particulate ferrites in near critical and supercritical water. J Mater Chem. 2001;11:1408–16.
Campardelli R, Adami R, Della Porta G, Reverchon E. Nanoparticle precipitation by supercritical assisted injection in a liquid anti-solvent. Chem Eng J. 2012;192:246–51.
Chattopadhyay P, Gupta RB. Supercritical CO2-based production of fullerene nanoparticles. Ind Eng Chem Res. 2000;39:2281–9.
Chattopadhyay P, Gupta RB. Production of antibiotic nanoparticles using supercritical CO2 as anti-solvent with enhanced mass transfer. Ind Eng Chem Res. 2001a;40:3530–9.
Chattopadhyay P, Gupta RB. Production of griseofulvin nanoparticles using supercritical CO2 anti-solvent with enhanced mass transfer. Int J Pharm. 2001b;228:19–31.
Chattopadhyay P, Gupta RB. Supercritical CO2 based production of magnetically responsive micro- and nanoparticles for drug targeting. Ind Eng Chem Res. 2002a;41:6049–58.
Chattopadhyay P, Gupta RB. Protein nanoparticles formation by supercritical anti-solvent with enhanced mass transfer. AICHE J. 2002b;48:235–44.
Chattopadhyay P, Shekunov BY, Yim D, Cipolla D, Boyd B, Farr S. Production of solid lipid nanoparticle suspensions using supercritical fluid extraction of emulsions (SFEE) for pulmonary delivery using the AERx system. Adv Drug Deliv Rev. 2007;59:444–53.
Chen AZ, Zhao Z, Wang SB, Li Y, Zhao C, Liu YG. A continuous RESS process to prepare PLA–PEG–PLA microparticles. J Supercrit Fluids. 2011;59:92–7.
Chen AZ, Wang GY, Wang SB, Feng JG, Liu YG, Kang YQ. Preparation of poly-(methyl vinyl ether-co-maleic anhydride) nanoparticles by solution-enhanced dispersion by supercritical CO2. Materials. 2012a;5:1841–52.
Chen AZ, Wang GY, Wang SB, Li L, Liu YG, Zhao C. Formation of methotrexate-PLLA-PEG-PLLA composite microspheres by microencapsulation through a process of suspension-enhanced dispersion by supercritical CO2. Int J Nanomedicine. 2012b;7:3013–22.
Chen AZ, Li L, Wang SB, Lin XF, Liu YG, Zhao C, et al. Study of Fe3O4-PLLA-PEG-PLLA magnetic microspheres based on supercritical CO2: preparation, physicochemical characterization, and drug loading investigation. J Supercrit Fluids. 2012c;67:139–48.
Chen BQ, Kankala RK, Chen AZ, Yang DZ, Cheng DZ, Jiang XX, et al. Investigation of silk fibroin nanoparticle-decorated poly(l-lactic acid) composite scaffolds for osteoblast growth and differentiation. Int J Nanomedicine. 2017;12:1877–90.
Cooper AI. Polymer synthesis and processing using supercritical carbon dioxide. J Mater Chem. 2000;10:207–14.
Dalvi SV, Azad MA, Dave R. Precipitation and stabilization of ultrafine particles of Fenofibrate in aqueous suspension by RESOLV. Powder Technol. 2013;236:75–84.
Date AA, Patravale VB. Current strategies for engineering drug nanoparticles. Curr Opin Colloid Interface Sci. 2004;9:222–35.
Davies OR, Lewis AL, Whitaker MJ, Tai H, Shakesheff KM, Howdle SM. Applications of supercritical CO2 in the fabrication of polymer systems for drug delivery and tissue engineering. Adv Drug Deliv Rev. 2008;60:373–87.
Dehghani F, Foster NR. Dense gas anti-solvent processes for pharmaceutical formulation. Curr Opin Solid State Mater Sci. 2003;7:363–9.
Della PG, Falco N, Reverchon E. NSAID drugs release from injectable microspheres produced by supercritical fluid emulsion extraction. J Pharm Sci. 2010;99:1484–99.
DeSimone JM. Practical approaches to green solvents. Science. 2002;297:799–803.
Djerafi R, Masmoudi Y, Crampon C, Meniai A, Badens E. Supercritical anti-solvent precipitation of ethyl cellulose. J Supercrit Fluids. 2015;105:92–8.
Dong Z, Li Y, Lin M, Li M. A study of the mechanism of enhancing oil recovery using supercritical carbon dioxide microemulsions. Pet Sci. 2013;10:91–6.
Elliot J, Hancook B. Pharmaceutical materials science: an active new frontier in materials research. MRS Bull. 2006;31:869–71.
Elvassore N, Bertucco A, Caliceti P. Production of insulin-loaded poly(ethylene glycol)/poly(l-lactide) (PEG/PLA) nanoparticles by gas anti-solvent techniques. J Pharm Sci. 2001;90:1628–36.
Erkey C. Preparation of metallic supported nanoparticles and films using supercritical fluid deposition. J Supercrit Fluids. 2009;47:517–22.
Falk R, Randolph TW, Meyer JD, Kelly RM, Manning MC. Controlled release of ionic compounds from poly (L-lactide) microspheres produced by precipitation with a compressed anti-solvent. J Control Release. 1997;44:77–85.
Fraile M, Martin A, Deodato D, Rodriguez-Rojo S, Nogueira ID, Simplicio AL, et al. Production of new hybrid systems for drug delivery by PGSS (particles from gas saturated solutions) process. J Supercrit Fluids. 2013;81:226–35.
Gadermann M, Kular S, Al-Marzouqi A, Signorell R. Formation of naproxen/polylactic acid nanoparticles by pulsed rapid expansion of supercritical solutions. Phys Chem Chem Phys. 2009;11:7861–8.
Ginty PJ, Whitaker MJ, Shakesheff KM, Howdle SM. Drug delivery goes supercritical. Mater Today. 2005;8:42–8.
Ginty PJ, Howard D, Rose FRAJ, Whitaker MJ, Barry JJA, Tighe P, et al. Mammalian cell survival and processing in supercritical CO2. Proc Natl Acad Sci. 2006;103:7426–31.
Girotra P, Singh SK, Nagpal K. Supercritical fluid technology: a promising approach in pharmaceutical research. Pharm Dev Technol. 2013;18:22–38.
Gosselin PM, Thibert R, Preda M, McMullen JN. Polymorphic properties of micronized carbamazepine produced by RESS. Int J Pharm. 2003;252:225–33.
Habulin M, Primozic M, Knez Z. Supercritical fluids as solvents for enzymatic reactions. Acta Chim Slov. 2007;54:667–77.
Hakuta Y, Hayashi H, Arai K. Fine particle formation using supercritical fluids. Curr Opin Solid State Mater Sci. 2003;7:341–51.
Hamidreza AB, Reza BS, Mahdi CS, Mahdi DY, Fatemeh EF, Hassan FH. Nanotechnology and supercritical fluids. J Fundam Appl Sci. 2016;8:839–59.
Hauthal WH. Advances with supercritical fluids. Chemosphere. 2001;43:123–35.
He WZ, Suo QL, Jiang ZH, Shan A, Hong HL. Precipitation of ephedrine by SEDS process using a specially designed prefilming atomizer. J Supercrit Fluids. 2004;31:101–10.
Housaindokht MR, Bozorgmehr MR. Calculation of solubility of methimazole, phenazopyridine and propranolol in supercritical carbon dioxide. J Supercrit Fluids. 2008;43:390–7.
Huang Z, Sun GB, Chiew YC, Kawi S. Formation of ultrafine aspirin particles through rapid expansion of supercritical solutions (RESS). Powder Technol. 2005;160:127–34.
Jung J, Perrut M. Particle design using supercritical fluids: literature and patent survey. J Supercrit Fluids. 2001;20:179–219.
Kalani M, Yunus R. Application of supercritical anti-solvent method in drug encapsulation: a review. Int J Nanomedicine. 2011;6:1429–42.
Kalantarian P, Najafabadi AR, Haririan I, Vatanara A, Yamini Y, Darabi M, et al. Preparation of 5-fluorouracil nanoparticles by supercritical anti-solvents for pulmonary delivery. Int J Nanomedicine. 2010;5:763–70.
Kalogiannis CG, Pavlidou E, Panayiotou CG. Production of amoxicillin microparticles by supercritical anti-solvent precipitation. Ind Eng Chem Res. 2005;44:9339–46.
Kankala RK, Zhang YS, Wang SB, Lee CH, Chen AZ. Supercritical fluid technology: an emphasis on drug delivery and related biomedical applications. Adv Healthc Mater. 2017;6:1700433.
Kerc J, Srcic S, Knez Z, Sencar-Bozic P. Micronization of drugs using supercritical carbon dioxide. Int J Pharm. 1999;182:33–9.
Keshavarz A, Karimi-Sabet J, Fattahi A, Golzary AA, Rafiee-Tehrani M, Dorkoosh FA. Preparation and characterization of raloxifene nanoparticles using Rapid Expansion of Supercritical Solution (RESS). J Supercrit Fluids. 2012;63:169–79.
Knez Z, Weidner E. Particle’s formation and particle design using supercritical fluids. Curr Opin Solid State Mater. 2003;7:353–61.
Knez Z, Markocic E, Leitgeb M, Primozic M, Hrncic MK, Skerget M. Industrial applications of supercritical fluids: a review. Energy. 2014;77:235–43.
Kompella UB, Koushik K. Preparation of drug delivery systems using supercritical fluid technology. Crit Rev Ther Drug Carrier Syst. 2001;18:173–99.
Krober H, Teipel U. Materials processing with supercritical anti-solvent precipitation: process parameters and morphology of tartaric acid. J Supercrit Fluids. 2002;22:229–35.
Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6.
Li F, Hsieh Y. Supercritical fluid chromatography-mass spectrometry for chemical analysis. J Sep Sci. 2008;31:1231–7.
Lin YC, Jang SM. Formation of micro-size drug particles with supercritical fluids. J Biotechnol. 2008;136S:S144–6.
Markocic E, Kramberger B, Bennekom JGV, Heeres HJ, Vos J, Knez Z. Glycerol reforming in supercritical water; a short review. Renew Sust Energ Rev. 2013;8:23–40.
Martin A, Cocero MJ. Micronization processes with supercritical fluids: fundamentals and mechanisms. Adv Drug Deliv Rev. 2008;60:339–50.
Matsubara A, Fukusaki E, Bamba T. Metabolite analysis by supercritical fluid chromatography. Bioanalysis. 2010;2:27–34.
McHugh MA, Guckes TL. Separating polymer solutions with supercritical Fluids. Macromolecules. 1985;18:674–80.
McHugh MA, Krukonis VJ. Supercritical fluid extraction: principles and practice. 2nd ed. Boston: Butterworth-Heinemann; 1994. p. 1–144.
Meziani MJ, Rollins HW, Allard LF, Sun YP. Protein-protected nanoparticles from rapid expansion of supercritical solution into aqueous solution. J Phys Chem B. 2002;106:11178–82.
Mishima K. Biodegradable particle formation for drug and gene delivery using supercritical fluid and dense gas. Adv Drug Deliv Rev. 2008;60:411–32.
Mohsen H, Alireza V, Reza Z. Formation and characterization of beclomethasone dipropionate nanoparticles using rapid expansion of supercritical solution. Adv Pharm Bull. 2015;5:343–9.
Montes A, Tenorio A, Gordillo MD, Pereyra C, Ossa EJM. Screening design of experiment applied to supercritical anti-solvent precipitation of amoxicillin: Exploring new miscible conditions. J Supercrit Fluids. 2010;51:399–403.
Montes A, Gordillo MD, Pereyra C, Martinez EJ. Particles formation using supercritical fluids. In: Nakajima H, editor. Mass transfer – advanced aspects. Europe: InTech Open; 2011. p. 461–80.
Montes A, Pereyra C, de la Ossa EJM. Mean aspects controlling supercritical CO2 precipitation processes. In: Alfredo I, editor. Heat and mass transfer – advances in science and technology applications. London: Intech Open; 2019. p. 1–15.
Muhrer G, Mazzotti M. Precipitation of lysozyme nanoparticles from dimethyl sulfoxide using carbon dioxide as anti-solvent. Biotechnol Prog. 2003;19:549–56.
Munshi P, Bhaduri S. Supercritical CO2: a twenty-first century solvent for the chemical industry. Curr Sci India. 2009;97:63–72.
Ollanketo M, Hartonen K, Riekkola M, Holm Y, Hiltunen R. Supercritical carbon dioxide extraction of lycopene in tomato skins. Eur Food Res Technol. 2001;212:561–5.
Palakodaty S, York P, Pritchard J. Supercritical fluid processing of materials from aqueous solutions: the application of SEDS to lactose as a model substance. Pharm Res. 1998;15:1835–43.
Palakodaty S, Sloan R, Kordikowski A. Pharmaceutical and biological materials processing with supercritical fluids. In: Sun YP, editor. Supercritical fluid technology in materials science and engineering. New York: Marcel Dekker Inc.; 2002. p. 439–90.
Pasquali I, Bettini R. Are pharmaceutics really going supercritical? Int J Pharm. 2008;364:176–87.
Perry RH. Perry’s chemical engineers’ handbook. 7th ed. New York: McGraw-Hill; 1997.
Pestov D, Levit N, Kessick R, Tepper G. Photosensitive 2,5- distyrylpyrazine particles produced from rapid expansion of supercritical solutions. Polymer. 2003;44:3177–83.
Pinkston JD, Stanton DT, Wen D. Elution and preliminary structure retention modeling of polar and ionic substances in supercritical fluid chromatography using volatile ammonium salts as mobile phase additives. J Sep Sci. 2004;27:115–23.
Prosapio V, Reverchon E, De Marco I. Polymers ultrafine particles for drug delivery systems precipitated by supercritical carbon dioxide+ organic solvent mixtures. Powder Technol. 2016;292:140–8.
Ramsey E, Sun Q, Zhang Z, Zhang C, Gou W. Mini-review: green sustainable processes using supercritical fluid carbon dioxide. J Environ Sci. 2009;21:720–6.
Rehman M, Shekunov BY, York P, Colthorpe P. Solubility and precipitation of nicotinic acid in supercritical carbon dioxide. J Pharm Sci. 2001;90:1570–82.
Reverchon E. Supercritical anti-solvent precipitation of micro- and nano-particles. J Supercrit Fluids. 1999;15:1–21.
Reverchon E. Supercritical-assisted atomization to produce micro and/or nanoparticles of controlled size and distribution. Ind Eng Chem Res. 2002;41:2405–11.
Reverchon E, Adami R. Nanomaterials and supercritical fluids. J Supercrit Fluids. 2006;37:1–22.
Reverchon E, De Marco I. Supercritical anti-solvent micronization of Cefonicid: thermodynamic interpretation of results. J Supercrit Fluids. 2004;31:207–15.
Reverchon E, Spada A. Crystalline microparticles of controlled size produced by supercritical-assisted atomization. Ind Eng Chem Res. 2004;43:1460–5.
Reverchon E, Porta GD, Taddeo R, Pallado P, Stassi A. Solubility and micronization of griseofulvin in supercritical CHF3. Ind Eng Chem Res. 1995;34:4087–91.
Reverchon E, Porta GD, Sannino D, Ciambelli P. Supercritical anti-solvent precipitation of nanoparticles of a zinc oxide precursor. Powder Technol. 1999;102:127–34.
Reverchon E, Porta GD, De Rosa I, Subra P, Letourneur D. Supercritical anti-solvent micronization of some biopolymers. J Supercrit Fluids. 2000;18:239–45.
Reverchon E, Caputo G, De Marco I. Role of phase behavior and atomization in the supercritical anti-solvent precipitation. Ind Eng Chem Res. 2003;42:6406–14.
Sacchetin PSC, Setti RF, Vieira e Rosa P de T, Moraes AM. Properties of PLA/PCL particles as vehicles for oral delivery of the androgen hormone 17α-methyltestosterone. Mater Sci Eng C. 2016;58:870–81.
Saito M. History of supercritical fluid chromatography: Instrumental development. J Bioeng. 2013;115:590–9.
Sane A, Taylor S, Sun YP, Thies MC. RESS for the preparation of fluorinated porphyrin nanoparticles. Chem Commun. 2003;21:2720–1.
Seckner AJ, McClellan AK, McHugh MA. High pressure solution behavior of the polymer-toluene-ethane system. AICHE J. 1988;34:9–14.
Shariati A, Peters CJ. Recent developments in particle design using supercritical fluids. Curr Opin Solid State Mater Sci. 2003;7:371–83.
Shekunov BY, Chattopadhyay P, Seitzinger JS. Method and apparatus for enhanced size reduction of particles using liquefaction of materials. US Patent 6,986,846, January 17, 2006. Accessed 20 Sept 2019.
Shen YB, Du Z, Wang Q, Guan YX, Yao SJ. Preparation of chitosan microparticles with diverse molecular weights using supercritical fluid assisted atomization introduced by hydrodynamic cavitation mixer. Powder Technol. 2014;254:416–24.
Sheth P, Sandhu H, Singhal D, Malick W, Shah N, Kislalioglu MS. Nanoparticles in the pharmaceutical industry and the use of supercritical fluid technologies for nanoparticle production. Curr Drug Deliv. 2012;9:269–84.
Shoyele SA, Cawthorne S. Particle engineering techniques for inhaled biopharmaceuticals. Adv Drug Deliv Rev. 2006;58:1009–29.
Skerget M, Knez Z, Hrncic MK. Solubility of solids in sub- and supercritical fluids: a review. J Chem Eng Data. 2011;56:694–719.
Smith RM. Supercritical fluid in separation science the dreams, the reality and the future. J Chromatogr A. 1999;856:83–115.
Snavely WK, Subramaniam B, Rajewski RA, Defelippis MR. Micronization of insulin from halogenated alcohol solution using supercritical carbon dioxide as an anti-solvent. J Pharm Sci. 2002;91:2026–39.
Sovilj MN, Nikolovski BG, Spasojevic MD. Critical review of supercritical fluid extraction of selected spice plant materials. Maced J Chem Chem Eng. 2011;30:197–220.
Sun YP, Atorngitjawat P, Meziani MJ. Preparation of silver nanoparticles via rapid expansion of water in carbon dioxide microemulsion into reductant solution. Langmuir. 2001;17:5707–10.
Thakur R, Gupta RB. Rapid expansion of supercritical solution with solid co solvent (RESS-SC) process: formation of griseofulvin nanoparticles. Ind Eng Chem Res. 2005;44:7380–7.
Thote AJ, Gupta RB. Formation of nanoparticles of a hydrophilic drug using supercritical carbon dioxide and microencapsulation for sustained release. Nanomedicine. 2005;1:85–90.
Tomasko DL, Li H, Liu D, Han X, Wingert MJ, Lee LJ, Koelling KW. A review of CO2 applications in the processing of polymers. Ind Eng Chem Res. 2003;42:6431–56.
Tservistas M, Levy MS, Lo-Yim JYA, O'Kennedy RD, York P, Humphrey GO, et al. The formation of plasmid DNA loaded pharmaceutical powders using SCF technology. Biotechnol Bioeng. 2001;72:12–8.
Turk M, Hils P, Helfgen B, Schaber K, Martin HJ, Wahl MA. Micronization of pharmaceutical substances by the rapid expansion of supercritical solutions (RESS): a promising method to improve bioavailability of poorly soluble pharmaceutical agents. J Supercrit Fluids. 2002;22:75–84.
Varshosaz J, Hassanzadeh F, Mahmoudzadeh M, Sadeghi A. Preparation of cefuroxime axetil nanoparticles by rapid expansion of supercritical fluid technology. Powder Technol. 2009;189:97–102.
Velaga SP, Ghaderi R, Carlfors J. Preparation and characterisation of hydrocortisone particles using a supercritical fluids extraction process. Int J Pharm. 2002;231:155–66.
Vemavarapu C, Mollan MJ, Lodaya M, Needham TE. Design and process aspects of laboratory scale SCF particle formation systems. Int J Pharm. 2005;292:1–16.
Wang TC, Chang PC. Measurement and correlation for the solid solubility of antioxidants sodium L-ascorbate and sodium erythorbate monohydrate in supercritical carbon dioxide. J Chem Eng Data. 2015;60:790–4.
Warwick B, Dehghani F, Foster NR, Biffin JR, Regtop HL. Micronization of copper indomethacin using gas anti-solvent processes. Ind Eng Chem Res. 2002;41:1993–2004.
Wu K, Li J. Precipitation of a biodegradable polymer using compressed carbon dioxide as anti-solvent. J Supercrit Fluids. 2008;46:211–6.
Xiao K, Wang W, Hu D, Hao Z, Qu Y, Liu Y. Preparation of cefquinome nanoparticles by using the supercritical anti-solvent process. J Nanomater. 2015;2015:Article ID 767945.
Yamini Y, Bahramifar N. Solubility of polycyclic aromatic hydrocarbons in supercritical carbon dioxide. J Chem Eng Data. 2000;45:53–6.
Yang H, Zhong C. Modeling of the solubility of aromatic compounds in supercritical carbon dioxide–co solvent systems using SAFT equation of state. J Supercrit Fluids. 2005;33:99–106.
Yasuji T, Takeuchi H, Kawashima Y. Particle design of poorly water-soluble drug substances using supercritical fluid technologies. Adv Drug Deliv Rev. 2008;60:388–98.
Yeo SD, Lee JC. Crystallization of sulfamethizole using the supercritical and liquid anti-solvent processes. J Supercrit Fluids. 2004;30:315–23.
Yeo SD, Lim GB, Debendetti PG, Bernstein H. Formation of microparticulate protein powder using a supercritical fluid anti-solvent. Biotechnol Bioeng. 1993;41:341–6.
Young TJ, Johnston KP, Mishima K, Tanaka H. Encapsulation of lysozyme in a biodegradable polymer by precipitation with a vapor-overliquid anti-solvent. J Pharm Sci. 1999;88:640–50.
Young TJ, Johnston KP, Pace GW, Mishra AK. Phospholipid-stabilized nanoparticles of cyclosporine a by rapid expansion from supercritical to aqueous solution. AAPS PharmSciTech. 2003;5:1–16.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Patel, J.K., Bhatia, D., Pathak, Y.V., Patel, A. (2021). The Use of Supercritical Fluid Technologies for Nanoparticle Production. In: Patel, J.K., Pathak, Y.V. (eds) Emerging Technologies for Nanoparticle Manufacturing. Springer, Cham. https://doi.org/10.1007/978-3-030-50703-9_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-50703-9_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-50702-2
Online ISBN: 978-3-030-50703-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)