Abstract
Diabetic retinopathy is an eye disease generated by long-standing diabetes, and it is one of the main causes of vision loss if not diagnosed and treated properly. Diabetic retinopathy consists of several types of lesions found in the retina of diabetic individuals. One of the important lesions of diabetic retinopathy is microaneurysms, which are small red dots that appear due to the local weakness of the capillary walls. This paper presents a novel automatic microaneurysms detection method, in retinal images by employing fuzzy image processing and deep learning. Firstly, the paper explores the existing systems of diabetic retinopathy screening, with a focus on the microaneurysms detection methods and deep learning classification. The proposed system consists of two parts, namely: image preprocessing with a combination of fuzzy image processing techniques, and also the microaneurysms classification using deep neural networks. This paper investigates the capability of a combination of different fuzzy image preprocessing techniques for the detection of microaneurysms in eye fundus images. In addition to the proposed microaneurysms detection system, the paper also highlights a novel dataset for the microaneurysms detection that includes the ground truth data. The purpose of the proposed automated microaneurysm detection with digital analysis of eye fundus images is to substitute current practice that is based on manual diagnosis and visual inspection, and eventually to contribute to producing a more reliable diabetic retinopathy screening system.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Diabetic Retinopathy
- Microaneurysms
- Fuzzy image processing
- Deep learning
- Colour fundus images
- Eye screening
1 Introduction
Diabetic Retinopathy (DR) is one of the major complications of diabetes mellitus, which causes blindness. In the early phases of the disease, diabetic retinopathy is asymptomatic, where diabetic patients are unaware of their diabetes condition and the retinopathy changes. Therefore, screening is necessary for an early detection of DR and to identify the group at risk of losing vision.
Diabetes mellitus is a growing problem, with high numbers of diabetics reported each year all over the world. A user/patient friendly and cost effective screening tool is required to take care of such large number of individuals at risk, without compromising the care standard. Early detection through the screening and effective risk management would help avoid complications of diabetes and eventually reduce the mortality. According to Mathers and Loncar [1], in the year of 2014, the number of diabetic people was 422 million. World Health Organization (WHO) reveals that diabetes is one of the major causes of blindness, in addition to heart attack, kidney failure, stroke and also lower limb amputation [2]. In addition, it is claimed in [2] that, recently, approximately 1.3 billion people live with vision impairment worldwide. Furthermore, people over 50 years form the majority with vision impairment and it is considered that 80% of all vision impairment cases are preventable [2]. Diabetic retinopathy is one of the leading reasons of vision impairment [2], besides glaucoma, cataract, uncorrected refractive errors, age-related macular degeneration, corneal opacity and trachoma.
Screening of diabetic retinopathy is one of the primary and important ways for preventing loss of vision. Cunha-Vaz [3] reports that there is an urgent need to create software with automatic methods to detect retinopathy, mainly by digital analysis of the retinal photographs. Therefore, the proposed image processing based system aims to be able to detect the presence of early stage diabetic retinopathy signs, which is microaneurysms, and direct those patients for further management, i.e., follow-up and appropriate treatment.
This paper focuses on the microaneurysms detection, one of the earliest and important features of diabetic retinopathy. Microaneurysms are small dots found on the retina, resulted from the ballooning out process of a weak capillary wall. Microaneurysms initially appear as small red dots, which may later become yellowish due to the related leakage, and which finally occlude [3]. According to Taylor and Batey [4], in a small number of cases, microaneurysms which are found outside the macular region should not disturb the sight of the patient, and they are detectable only via photography. However, the microaneurysms detection at an initial phase is vital, and it is the first step towards the diabetic retinopathy prevention. Figure 1 shows the fundus photograph image of a diabetic retinopathy eye showing microaneurysms signs.
Microaneurysm representation [5]
The detection of microaneurysms is considered a challenging task. According to the medical expert from the Department of Ophthalmology, Melaka Hospital, Malaysia, the counting of microaneurysms is very difficult because of the following issues: (a) In the case microaneurysms are present, there will be many of them indeed and this will produce an inaccurate counting. The counting process becomes even more complicated in the case of overlapping microaneurysms (b) Microaneurysms could be simply confused with blot haemorrhages, as they have almost the same size. Therefore, this chapter proposes a novel development of a microaneurysms detection system, by introducing fuzzy image processing approaches to overcome those challenges. The developed methodology uses a novel dataset collected from the Melaka Hospital, Malaysia.
This chapter is structured as follows. Section 2 presents some previous related research work on automatic methods for diabetic retinopathy detection, focusing on detection methods for microaneurysms. The section is divided into two sub-sections for better understanding, comprising the developed microaneurysms detection system and deep learning methods. Meanwhile, Sect. 3 defines in detail the newly developed dataset, which highlights the microaneurysms ground truth. Section 4 explains the proposed system for the detection of microaneurysms in eye fundus images by implementing fuzzy image processing techniques and deep learning. Finally, Sect. 5 concludes the proposed work and presents some future work.
2 Related Work
There are several previously developed systems reported in the literature that aimed for automatic diabetic retinopathy detection and diagnosis. Some are general detection systems for diabetic retinopathy, which classify the fundus image into two general detection classes, i.e., retinopathy absence and retinopathy presence [28,29,30,31,32,33,34,35]. Moreover, some other systems focus on the diabetic retinopathy features’ detection, namely microaneurysms, haemorrhages, exudates and others [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22, 36,37,38,39,40,41,42,43]. The automatic localisation of microaneurysms is still considered as a very challenging task, and, thus, further study is required in order to find appropriate methods for this purpose.
2.1 Microaneurysms Detection
Automatic detection methods aim to highlight the issues of manual screening, such as time consuming, low sensitivity, high cost and, most importantly, low human detection ability. Several systems for the detection of microaneurysms have been reported in the literature [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22], where various techniques and methods were proposed, which aim to create a reliable system. However, the reported microaneurysms detection systems have not implemented fuzzy processing during the preprocessing stage and deep learning for the classification.
In our earlier work, a basic system for general diabetic retinopathy detection has been proposed by implementing a combination of non-fuzzy techniques [23]. In addition, several individual systems for the automatic detection of microaneurysms in colour eye fundus images have also been reported in [24]. The first system proposed the automatic localisation of microaneurysms in colour eye fundus images using segmentation of blood vessels and extraction of some related features. The second system highlights an image processing techniques combination and circular Hough transform, while the third system performed the microaneurysms detection by implementing fuzzy image processing. The third developed system proposed the use of a method called circular Hough transform for the microaneurysms localisation, due to the ability of this method in detecting circular shapes. The third system which consists of two subsystems, where the first subsystem proposed image processing techniques combination and also circular Hough transform method, while the second system variant presented fuzzy image processing techniques for the detection of microaneurysms. Both systems were compared to investigate the ability of implemented fuzzy image processing techniques. From the obtained results, it was summarised that the fuzzy preprocessing techniques implementation enable improved contrast enhancement for eye fundus images, and it greatly supports in the detection of the microaneurysms. The development of the microaneurysms detection system was enhanced in the fourth developed system, based on the promising results of the fuzzy histogram equalisation technique implementation to detect microaneurysms in the third developed system. The implementation of fuzzy filtering and also fuzzy edge detection, individually, besides the fuzzy histogram equalisation for the automatic localisation of microaneurysms was proposed in [25]. The reported analysis shows that the fuzzy pre-processing techniques implementation enhanced the contrast as well as other improvements, like better segmentation and brightness for fundus images. It also indicates that the use of fuzzy image processing techniques plays a significant part in generating a better image quality and performance.
The capability of a combination of different fuzzy image processing techniques has been investigated later, for the detection of diabetic retinopathy and maculopathy in eye fundus images, in [26]. This time, the proposed system implements fuzzy techniques in the image pre-processing part, by using fuzzy filtering, followed by the fuzzy histogram equalisation and fuzzy edge detection. Firstly, the system classified images into two classes (DR present or not). Then, a more detailed classification was done by classifying into ten classes of retinopathy, which provides more details about the disease stage. In addition to the proposed system, we also proposed a new dataset, which contains 600 colour eye fundus images [27]. We enhanced the combination of different fuzzy image processing techniques capability for the diabetic retinopathy and maculopathy detection with the retinal structures segmentation in [27]. This paper presented a novel combination of diabetic retinopathy detection as well as maculopathy detection in eye fundus images by implementing fuzzy image processing. The maculopathy detection is vital, since maculopathy will eventually cause vision loss if the affected macula is not timely treated. The results showed that employing fuzzy image processing in addition to the extraction of retinal structures can help create a more reliable diabetic retinopathy screening system. Hence, the proposed system in this chapter continues our previous work and implements a fuzzy techniques combination for the image pre-processing part for the detection of microaneurysms.
2.2 Microaneurysms Detection
Deep learning is a popular area of machine learning, where a model learns to perform classification tasks directly from images, texts or sound. A traditional machine learning classification workflow starts with manually extracting relevant features from the input data and uses them for classification. On the other hand deep learning automatically extracts the necessary features from the input data, which eliminates the need for feature engineering. Deep neural networks utilise multiple nonlinear processing layers to learn a nested hierarchy of representations. However, deep learning models require large amounts of training data that is usually costly to acquire, especially in the medical field.
The most popular deep learning approach to image classification is deep convolutional neural networks (DCNNs). The DCNN architecture is well suited to process two-dimensional data, such as images, as it utilizes the convolution operation. Recently deep learning has been gaining a lot of attention, where the recent advances in deep learning help meet user expectations, which is crucial for some applications. Deep learning have been used extensively in many application areas, such as automated driving, aerospace and defense, industrial automation, text processing, and others. For example, in medical research, deep learning has been used to automatically detect cancer cells [46, 47] and in other medical image processing tasks.
Deep learning has been previously implemented in the screening of diabetic retinopathy [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. Automated diabetic retinopathy detection in eye fundus images employing deep learning have been proposed in [28,29,30,31,32,33,34,35]. These research works demonstrated and implemented different methods and structures, neural networks architectures, used different datasets, training and testing methods and grading, with various results and performances. In addition, some researchers proposed some image pre-processing techniques aiming to improve the quality of image and also the recognition of subtle features and, eventually, targeting to increase the classification accuracy. Among the pre-processing techniques proposed were resizing, normalisation, denoising, scaling, center, cropping, extraction of green channel and contrast enhancement, such as histogram equalisation and contrast limited adaptive histogram equalisation (CLAHE).
The research works demonstrated in [36,37,38,39,40,41,42,43] focus on a more specific detection of diabetic retinopathy signs, which is the detection of microneurysms. These research works presented the microaneurysms detection through the integration of a deep learning classification framework. Chudzik et al. [36] proposed a patch-based CNN for microaneurysms detection, with a network fine-tuning scheme called Interleaved Freezing. During the preprocessing phase, the green plane of the images are extracted followed by the application of the Otsu thresholding. Lam et al. [37] developed an automated method of localising microaneurysms and exudates with a limited number of training data, using five CNN models, i.e., AlexNet, VGG16, GoogleNet, ResNet and Inception-v3. The original images were cropped, normalised and classified using patch-based sliding-window approach.
Meanwhile, Hatanaka et al. [38] proposed the combination of three types of detectors, i.e., the double-ring filter, shape index based on the Hessian matrix and Gabor filter to conduct automated microaneurysms detection using deep CNNs. Dai et al. [39] developed an interleaved deep mining technique to cope with the unbalanced microaneurysms detection. Harangi et al. [40] proposed a deep learning-based approach for the detection of microaneurysms, with fusion of different individual deep CNN architectures. Shan and Li [41] implemented a Stacked Sparse Autoencoder (SSAE), as the automatic feature extractor, to learn from the image patches, and later fed into a Softmax Classifier (SMC) to categorize a patch into two classes, which are microaneurysms present or microaneurysms not present. In addition, microaneurysms detection in colour fundus images using a deep neural network with a dropout training procedure, using maxout activation function without preprocessing techniques or feature extraction, is presented by Haloi [42]. Tan and colleagues [43] proposed a 10-layer CNN to automatically segment and discriminate the diabetic retinopathy signs, such as exudates, haemorrhages and microaneurysms. The colour input image is normalised, and resulting areas that receive no light or little light will appear grey. Although the study shows that it is possible to get a single CNN to segment these diabetic retinopathy features on a wide range of fundus images with reasonable accuracy, however, the results show lower sensitivity for microaneurysms compared to the other two signs. This shows that the detection of microaneurysms is more difficult than the detection of other diabetic retinopathy signs.
It can be concluded that various approaches and different deep learning classifiers have been introduced to detect the microaneurysms. However, further study is required to find suitable techniques for the detection of microaneurysms, as the automated microaneurysms detection is still considered as a very challenging task. It can be concluded that within these previously reported diabetic retinopathy and microaneurysms detection systems using deep learning classification, fuzzy processing has not been implemented during the pre-processing stage. Therefore, the main impact of this chapter is proposing a combination of fuzzy image pre-processing techniques in detecting the microaneurysms with a deep learning classifier. The proposed developed approaches are evaluated with a new data set, and it offers an understanding into the appropriateness for utilisation in an automatic screening system for diabetic retinopathy.
3 Experimental Dataset
The newly developed dataset consists of a total of 600 colour fundus images from a 300 patient’s folder collected from the Eye Clinic, Department of Ophthalmology, Melaka Hospital, Malaysia. The original images, which are sized 3872 × 2592 pixels stored in JPEG format, provide high-quality details. The information of the developed dataset are presented in [26], in Sect. 3-“Proposed System” and in [27], in Sect. 3-“Experimental Datasets”. Initially, the 600 colour fundus images were classified by three experts from the Department of Ophthalmology, Melaka Hospital, Malaysia into ten retinopathy stages: No Diabetic Retinopathy (DR), Mild DR without maculopathy, Mild DR with maculopathy, Moderate DR without maculopathy, Moderate DR with maculopathy, Severe DR without maculopathy, Severe DR with maculopathy, Proliferative DR without maculopathy, Proliferative DR with maculopathy and Advanced Diabetic Eye Disease.
However, the mentioned dataset in [27] is focusing on the ten retinopathy classification of diabetic retinopathy and maculopathy detection. By using the same 600 images, the manual diagnosis was extended and it focused on the diagnosis of microaneurysms by the Consultant Ophthalmologists, Department of Ophthalmologist, Melaka Hospital, Malaysia. This is another contribution, as this research provides another novel dataset that focuses on the important sign of diabetic retinopathy, microaneurysms. The images have been classified into four stages: “No” microaneurysms detected, “Yes-mild” microaneurysms detected, “Yes-moderate/medium” and “Yes-severe” number of microaneurysms detected. Some examples of the images from the newly developed microaneurysms data set are shown in Fig. 2.
For the manual grading process, an excel file comprising the link to each eye fundus image and the microaneurysms classes, as presented in Fig. 3, was provided. As presented in Fig. 3, the microaneurysms presence first concerns two main classifications, i.e., “No” for microaneurysms not detected, and “Yes” for microaneurysms detected. There are another three sub-choices for the “Yes” choice, which are “Low”, “Moderate/Medium” and Severe”, according to an approximate number of microaneurysms estimated by the expert. The choice of these three cases is dependent on the threshold values for each class, which have been fixed in advance. If there are just a few microaneurysms detected, i.e., between one to ten, then the image is classified as “Low”. If more microaneurysms are detected, i.e., between 11 to 20, it is classified as “Moderate/Medium”. Finally if there are even more, i.e., more than 20, then the classification will be “Severe”.
The findings of the microaneurysms expert diagnosis are presented in Fig. 4. As a result of the expert grading, the total number of images in each class was as follows: not detected (“No”) class with 379 images, while the detected (“Yes”) can be divided into three categories: low (104), moderate/medium (62) and, finally, severe (49). There were six images unclassified and excluded from the expert manual diagnosis, due to the poor quality, and it resulted in a total of 594, instead of 600 images. Microaneurysms detection is challenging due to the small size of microaneurysms, therefore high quality images are required to ensure an accurate diagnosis. The ground truth delivered by the expert can be placed into two categories. Table 1 shows the original classification made by the expert, which is divided into four classes of microaneurysms detection. The second categorisation divides into two cases, which are microaneurysms absence “No” and microaneurysms presence (“Yes”), as presented in Table 2.
4 Proposed System
The proposed system implements a combination of fuzzy techniques for image pre-processing, such as fuzzy filtering and fuzzy histogram equalisation. In addition, the system implements the classification stage using deep learning. These techniques are implemented using the newly developed dataset for microaneurysms detection.
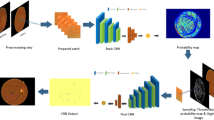
The proposed microaneurysms detection system has been developed using the Matlab R2018b environment. The system starts with the image acquisition process, where the system selects images from the folder for further processing. Next, the image pre-processing task takes place in order to improve the image quality. This task includes the implementation of the fuzzy image pre-processing techniques. Finally, in the classification phase, a deep learning classifier is trained using the generated features, in order to classify the images into their respective classes. Figure 5 shows the block diagram of the proposed system for automatic detection of microaneurysms for diabetic retinopathy screening and classification. The individual stages are discussed in more detail in the following sections.
4.1 Image Preprocessing
Image preprocessing takes place after the image acquisition process in order to improve the quality of the image. The present system utilizes the following image pre-processing techniques: resizing, greyscale, green channel extraction, fuzzy filtering, fuzzy histogram equalisation and fuzzy edge detection. Figure 6 shows the output image for different colour channels, meanwhile Fig. 7 shows the output image after fuzzy filtering, fuzzy histogram equalisation and the fuzzy edge detection process are performed.
4.1.1 Resizing
The original colour fundus images from the newly dataset were resized. The original images, which are of size 3872 × 2592 in JPEG format, provide high quality and good details. The resizing is performed for convenient use of the CNN. Most of previous research suggested that the images are cropped and resized to square number of pixels. However, in this proposed work, the fundus images are downsized proportional to the original image size, the new size being 242 × 162, in order to preserve the details of the original images.
4.1.2 Colour Channel Conversion
The second preprocessing technique is the conversion of the colour fundus image, which consists of the red, green and blue channels. The colour input images are converted into greyscale and green channel image. In our previous proposed systems [23,24,25,26], the colour fundus images were converted into a greyscale image. Due to the capability of the green channel previously presented in [27], the green channel extraction is used rather than other image colour conversion formats. The reason of the extraction of the green channel from the colour fundus images has been explained in [27]. The next pre-processing techniques are performed on the greyscale and green channel inverted images. Figure 6 shows the output image obtained after the different conversions are applied, i.e., greyscale conversion, red channel, and green channel, for comparison.
4.1.3 Fuzzy Image Pre-processing
After extracting the green channel from the original colour image, the filtering process needs to be implemented to improve the image quality or restore the digital image, which tends to have a variety of noise types. The poor photo quality may be due to the noise acquired during the acquisition or transmission process. Therefore, noise removal is required to enhance the image quality, and is important before any processing task. The proposed system implements the median filter with fuzzy techniques, described by Toh et al. [44], called the Fuzzy Switching Median (FSM) filter. The proposed technique was working well in removing noise. The technique has been used in [26, 27] for the diabetic retinopathy and maculopathy detection. The detailed explanations about the FSM filter implementation are presented in [25, 44].
Next, after filtering the image from noise, the fuzzy histogram equalisation is performed on the images, which aims to improve the image’s contrast. The colour fundus images are more challenging compared to the other types of fundus photography examination, which are angiography and red-free. Therefore, the implementation of the histogram equalisation by employing fuzzy techniques helps to enhance the contrast of the fundus images for better visualisation and detection. The technique called Brightness Preserving Dynamic Fuzzy Histogram Equalisation (BPDFHE) proposed by Sheet et al. [45] was found to work well on colour fundus images in [24,25,26,27], and has been chosen as a preprocessing technique in this proposed system as well. Detailed explanation about the proposed fuzzy histogram equalisation, including the comparison implementation with other histogram equalisation techniques, can be found in [24, 25].
The final fuzzy image processing technique performed is the fuzzy edge detection. By using membership functions, we are able to overcome the small intensity differences between two neighbouring pixels problem, by defining the degree with which the pixel belongs to an edge or a uniform region. A detailed explanation about the proposed fuzzy edge detection, including the membership functions for the inputs and outputs, is presented in [25].
4.2 Classification
A Deep CNN model is used for the classification phase. Figure 8 shows the structure of the proposed network architecture for the microaneurysms detection. The CNN architecture starts with an image input layer of the size (162 × 242 × 1), where the resized preprocessed images are inputted. Following that, the model has four convolutional layers, each followed by a rectified linear unit (ReLU), batch normalization and max pooling layers. The output of the final max pooling layer is then flattened before being inputted into the output layer which has two neurons. The final layer implements a Softmax function that is tasked with outputting the probabilities of the input image belonging to either of the two classes, microaneurysms presence or microaneurysms absence.
The final error for the model is calculated using the cross entropy function (C):
where N is the total number of images, K is 2 (the number of classes), \( t_{ij} \) is the indicator that sample \( i \) belongs to class \( j \), and \( y_{ij} \) is the model’s output for sample \( i \) for class \( j \). Stochastic gradient descent with momentum (SGDM) was used to train the model with an initial learning rate of 0.0001. 70% of the samples in the dataset are selected randomly to form the training set, while the remaining 30% is used for testing. Each of the models was trained for 100 epochs with a batch size of 128.
4.3 Results
The performance analysis summary of the proposed system, which employs different techniques (System Variants I-V), is presented in Table 3. For System Variant I, there is no image processing implemented, except the input image resizing process, while System Variant II proposed the implementation of fuzzy image processing techniques, such as fuzzy filtering and fuzzy histogram equalisation, besides the greyscale conversion. System Variant III implemented greyscale conversion, fuzzy filtering, fuzzy histogram equalisation and fuzzy edge detection. The extraction of green channel, fuzzy filtering and fuzzy histogram equalisation are implemented in System Variant IV. System Variant V proposed the fuzzy filtering, fuzzy histogram equalisation and fuzzy edge detection including the green channel extraction. A total of 594 images, which consist of 379 images from the “microaneurysms not detected” class and 215 images from the “microaneurysms detected” class are involved in the classification stage. For the validation phase, the network is trained to predict the labels of the validation data and the final validation accuracy is calculated. Accuracy means the fraction of examples that the network predicts correctly. All systems are trained and validated on the same neural network architecture. The validation accuracy for System I is 66.29%, and the validation accuracy for System II is 69.66%. System III and System IV generated validation accuracies of 69.10%, while System V obtained a validation accuracy of 68.54%.
The experimental results show that the trained network is able to identify and classify for the two classes, which are microaneurysms presence and microaneurysms absence. It can be observed that there is a small increment in the validation accuracy between the no image pre-processsing (System I) and the pre-processing techniques-based systems (System II, System III, System IV, System V), although the largest accuracy difference (between 66.29% and 69.66%) is small (3.37%). However, it can be concluded that the pre-processing techniques, i.e., fuzzy filtering, fuzzy histogram equalisation and fuzzy edge detection, help increase the accuracy and produce better results. The accuracy difference could be higher if the proposed system is trained and validated with more input data. Moreover, the results show that it is possible to segment the diabetic retinopathy features on a small range of fundus images with reasonable accuracy. In addition, some changes and improvements on the network architecture, for example, the number of layers and the layer parameters, have been performed in order to produce better results. Although the accuracy generated is in the medium range (i.e., not that good), it is considered acceptable due to some limitations, including the total number of images available.
5 Conclusions and Future Work
An automatic system for the detection of microaneurysms in colour fundus images using fuzzy image pre-processing techniques and deep learning has been developed in this paper. Although the detection of microneurysms is challenging due to the special characteristics of the microaneurysms, it can be concluded that the proposed techniques were able to improve to some degree the contrast and eventually improve the system performance for the automated microaneurysms detection system.
The system can be enhanced by implementing different other combinations of pre-processing techniques including those based on fuzzy approaches. The system can be further extended by implementing retinal structures extraction, such as optic disc and vessel segmentation for image preprocessing to produce a more reliable microaneurysms detection system. The system will also be extended to get more details on the microaneurysms classification, as proposed by the first categorisation of the novel dataset (Table 1), namely to classify into no microaneurysms presence, low, moderate/medium and severe cases. Further explorations on deep learning architectures for classification will be performed in order to increase the microaneurysms detection accuracy. Deep learning implementations are considered challenging when dealing with small or medium size datasets, as it is our case. Other convolutional neural networks, such as AlexNet, GoogLeNet, ResNet VCG16, VCG19, Inception-v3 and Inceptionrestnet-v2, could be used and validated with the proposed system. An accurate system could be used to help the diabetic retinopathy screening team to perform better and more efficient screening. The proposed developed system could be a benchmark for the development of other retinopathy signs’ detection system, such as for exudates, haemorrhages and neovascularisation. Besides presenting the novel development of an automatic detection for microaneurysms, this paper also introducing a new dataset of microaneurysms, which would be useful to researchers and practitioners working in the diabetic retinopathy screening field.
Deep Learning approaches have great potential for automatic detection of diabetic retinopathy, but the limitations of such approaches are exactly in the “automatic” thus “black-box” behaviour. Due to raising legal issues such automatic approaches become difficult to use in the future, consequently the field of explainable AI [48] becomes more and more important. Explainable AI develops methods for making such deep learning approaches transparent. However, for diagnostic and educational purposes there is a need to go beyond explainable AI; For example, to reach a level of explainable medicine there is a crucial need for causability. Causability [49] is different from Causality [50] but closely connected. Causability provides measurements for the quality of explanations produced by explainable AI methods and to enable the medical professional to understand why an algorithm came up with a certain result, or why a result had a certain error rate. This calls for contextual understanding which can be fostered by bringing a human-in-the-loop [51], which adds the component of human expertise to AI processes.
References
Mathers, C.D., Loncar, D.: Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 3(11), e442 (2006)
World of Organization. http://www.who.int/en/news-room/fact-sheets/detail/blindness-and-visual-impairment. Accessed 20 Mar 2019
Cunha-vaz, J.: Diabetic Retinopathy. World Scientific Publishing Co Pte Ltd, Singapore (2010)
Taylor, R., Batey, D.: Handbook of Retinal Screening in Diabetes: Diagnosis and Management. Wiley, Chichester (2012)
Ministry of Health Malaysia Diabetic Retinopathy Screening Team: Handbook guide to diabetic retinopathy screening-Module 5-2012. Ministry of Health Malaysia, Putrajaya (2012)
Adal, K.M., Ali, S., Sidibe, D., Karnowski, T., Chaum, E., Meriaudeau, F.: Automated detection of microaneurysms using robust blob descriptors. SPIE Medical Imaging-Computer Aided Diagnosis, 8670-22 (2013)
Adal, K.M., Sidibe, D., Ali, S., Chaum, E., Karnowski, T.P., Meriaudeau, F.: Automated detection of microaneurysms using scale-adapted blob analysis and semi-supervised learning. Comput. Methods Programs Biomed. 114, 1–10 (2014)
Akram, M.U., Khalid, S., Tariq, A., Khan, S.A., Azam, F.: Detection and classification of retinal lesions for grading of diabetic retinopathy. Comput. Biol. Med. 45, 161–171 (2014)
Akram, M.U., Khalid, S., Khan, S.A.: Identification and classification of microaneurysms for aerly detection of diabetic retinopathy. Pattern Recogn. 46, 107–116 (2012)
Alipour, S.H., Rabbani, H., Akhlaghi, M.R.: Diabetic retinopathy grading by digital curvelet transform. Comput. Math. Med. 2020 (2012). Article 761901. https://doi.org/10.1155/2012/761901
Antal, B., Hajdu, A.: Improving microaneurysm detection in color fundus images by using context-aware approaches. Comput. Med. Imaging Graph. 37, 403–408 (2013)
Aravind, C., Ponnibala, M., Vijayachitra, S.: Automatic detection of microaneurysms and classification of diabetic retinopathy images using SVM technique. In: IJCA Proceedings on International Conference on Innovations in Intelligent Instrumentation, Optimization and Electrical Sciences ICIIIOES, vol. 11, pp. 18–22 (2013)
Hatanaka, Y., Inoue, T., Okumura, S., Muramatsu, C., Fujita, H.: Automated microaneurysm detection method based on double-ring filter and feature analysis in retinal fundus images. In: Soda, P. (eds.) Proceedings of the 25th International Symposium on Computer-Based Medical Systems, CBMS, USA, pp. 1–4. IEEE (2012)
Kose, C., Sevik, U., Ikibas, C., Erdol, H.: Simple methods for segmentation and measurement of diabetic retinopathy lesions in retinal fundus images. Comput. Methods Programs Biomed. 107, 274–293 (2012)
Lichode, R.V., Kulkarni, P.S.: Automatic diagnosis of diabetic retinopathy by hybrid multilayer feed forward neural network. Int. J. Sci. Eng. Technol. Res. (IJSETR) 2(9), 1727–1733 (2013)
Prakash, J., Sumanthi, K.: Detection and classification of microaneurysms for diabetic retinopathy. Int. J. Eng. Res. Appl. 4, 31–36 (2013)
Punnolil, A.: A novel approach for diagnosis and severity grading of diabetic maculopathy. In: Proceedings of the 2013 International Conference on Advances in Computing, Communications and Informatics, New York, pp. 1230–1235. IEEE (2013)
Saleh, M.D., Eswaran, C.: An automated decision-support system for non-proliferative diabetic retinopathy disease based on Mas and HAs detection. Comput. Methods Programs Biomed. 108, 186–196 (2012)
Selvathi, D., Prakash, N.B., Balagopal, N.: Automated detection of diabetic retinopathy for early diagnosis using feature extraction and support vector machine. Int. J. Emerg. Technol. Adv. Eng. 2(11), 762–767 (2012)
Sopharak, A., Uyyanonvara, B., Barman, S.: Automated microaneurysm detection algorithms applied to diabetic retinopathy retinal images. Maejo Int. J. Sci. Technol. 7(2), 294–314 (2013)
Sujithkumar, S.B., Vipula, S.: Automatic detection of diabetic retinopathy in non-dilated RGB retinal fundus images. Int. J. Comput. Appl. 47(19), 26–32 (2012)
Sundhar, C., Archana, D.: Automatic screening of fundus images for detection of diabetic retinopathy. Int. J. Commun. Comput. Technol. 2(1), 100–105 (2014)
Rahim, S.S., Palade, V., Shuttleworth, J., Jayne, C.: Automatic screening and classification of diabetic retinopathy fundus images. In: Mladenov, V., Jayne, C., Iliadis, L. (eds.) EANN 2014. CCIS, vol. 459, pp. 113–122. Springer, Cham (2014). https://doi.org/10.1007/978-3-319-11071-4_11
Rahim, S.S., Jayne, C., Palade, V., Shuttleworth, J.: Automatic detection of microaneurysms in colour fundus images for diabetic retinopathy screening. J. Neural Comput. Appl. 521, 1–16 (2015)
Rahim, S.S., Palade, V., Shuttleworth, J., Jayne, C., Omar, R.N.R.: Automatic detection of microaneurysms for diabetic retinopathy screening using fuzzy image processing. In: Iliadis, L., Jayne, C. (eds.) EANN 2015. CCIS, vol. 517, pp. 69–79. Springer, Cham (2015). https://doi.org/10.1007/978-3-319-23983-5_7
Rahim, S.S., Palade, V., Jayne, C., Holzinger, A., Shuttleworth, J.: Detection of diabetic retinopathy and maculopathy in eye fundus images using fuzzy image processing. In: Guo, Y., Friston, K., Aldo, F., Hill, S., Peng, H. (eds.) BIH 2015. LNCS (LNAI), vol. 9250, pp. 379–388. Springer, Cham (2015). https://doi.org/10.1007/978-3-319-23344-4_37
Rahim, S.S., Palade, V., Shuttleworth, J., Jayne, C.: Automatic screening and classification of diabetic retinopathy and maculopathy using fuzzy image processing. Brain Inf. 3(4), 249–267 (2016). https://doi.org/10.1007/s40708-016-0045-3
Lam, C., Yi, D., Guo, M., Lindsey, T.: Automated detection of diabetic retinopathy using deep learning. In: AMIA Joint Summits on Translational Science Proceedings. AMIA Joint Summits on Translational Science 2017, pp. 147–155 (2018)
Voets, M., Mollersen, K., Bongo, L.A.: Replication study: development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs (2018). https://arxiv.org/pdf/1803.04337.pdf
Xu, K., Feng, D., Mi, H.: Deep convolutional neural network-based early automated detection of diabetic retinopathy using fundus image. Molecules 22(12), 1–7 (2017)
Rakhlin, A.: Diabetic retinopathy detection through integration of deep learning classification framework (2017). https://www.biorxiv.org/content/biorxiv/early/2018/06/19/225508.full.pdf
Gulshan, V., Peng, L., Coram, M., et al.: Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 316, 1–9 (2016)
Rajanna, A.R., Aryafar, K., Ramchandran, R., Sisson, C., Shokoufandeh, A., Ptucha, R.: Neural networks with manifold learning for diabetic retinopathy detection. In: Proceedings of IEEE Western NY Image & Signal Processing Workshop (2016). https://arxiv.org/pdf/1612.03961.pdf
Pratt, H., Coenen, F., Broadbent, D.M., Harding, S.P., Zheng, Y.: Convolutional neural networks for diabetic retinopathy. Procedia Comput. Sci. 90, 1–6 (2016)
Ghosh, R., Ghosh, K., Maitra, S.: Automatic detection and classification of diabetic retinopathy stages using CNN. In: 4th International Conference on Signal Processing and Integrated Networks (SPIN), USA, pp. 550–554. IEEE (2017)
Chudzik, P., Majumdar, S., Caliva, F., Al-Diri, B., Hunter, A.: Microaneurysm detection using deep learning and interleaved freezing. In: Proceedings of SPIE 10574, Medical Imaging 2018: Image Processing 1057411, pp. 1–9 (2018)
Lam, C., Yu, C., Huang, L., Rubin, D.: Retinal lesion detection with deep learning using image patches. Invest. Ophthalmol. Vis. Sci. 59(1), 590–596 (2018)
Hatanaka, Y., Ogohara, K., Sunayama, W., Miyashita, M., Muramatsu, C., Fujita, H.: Automatic microaneurysms detection on retinal images using deep convolution neural network. In: International Workshop on Advanced Image Technology (IWAIT), pp. 1–2 (2018)
Dai, L., et al.: Clinical report guided retinal microaneurysm detection with multi-sieving deep learning. IEEE Trans. Med. Imaging 37(5), 1149–1161 (2018)
Harangi, B., Toth, J., Hajdu, A.: Fusion of deep convolutional neural networks for microaneurysm detection in color fundus images. In: 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), pp. 3705–3708 (2018)
Shan, J., Li, L.: A deep learning method for microaneurysm detection in fundus images. In: 2016 IEEE First International Conference on Connected Health: Applications, Systems and Engineering Technologies (CHASE), pp. 357–358 (2016)
Haloi, M.: Improved microaneurysm detection using deep neural network (2016). https://arxiv.org/pdf/1505.04424.pdf
Tan, J.H., et al.: Automated segmentation of exudates, haemorrhages, microaneurysms using single convolutional neural network. Inf. Sci. 420, 66–76 (2017)
Toh, K.K.V., Mat Isa, N.A.: Noise adaptive fuzzy switching median filter for salt-and-pepper noise reduction. IEEE Signal Process. Lett. 17(3), 281–284 (2010)
Sheet, D., Garud, H., Suveer, A., Mahadevappa, M., Chatterjee, J.: Brightness preserving dynamic fuzzy histogram equalization. IEEE Trans. Consum. Electron. 56(4), 2475–2480 (2010)
Hu, Z., Tan, J., Wang, Z., Zhang, K., Zhang, L., Sun, Q.: Deep learning for image-based cancer detection and diagnosis – a survey. Pattern Recogn. 83, 134–149 (2018)
Couture, H.D., et al.: Image analysis with deep learning to predict breast cancer grade, ER status, histologic subtype, and intrinsic subtype. NPJ Breast Cancer 30, 1–8 (2018)
Holzinger, A., Kieseberg, P., Weippl, E., Tjoa A.M.: Current advances, trends and challenges of machine learning and knowledge extraction: from machine learning to explainable AI. In: Holzinger, A., Kieseberg, P., Tjoa A., Weippl E. (eds) Machine Learning and Knowledge Extraction, CD-MAKE 2018. LNCS, vol. 11015, pp. 1–8. Springer, Cham (2018). https://doi.org/10.1007/978-3-319-99740-7_1
Holzinger, A., Langs, G., Denk, H., Zatloukal, K., Mueller, H.: causability and explainability of artificial intelligence in medicine. Wiley Interdisc. Rev. Data Min. Knowl. Discovery, 9(4) (2019). https://doi.org/10.1002/widm.1312
Pearl, J.: Causality: Models, Reasoning, and Inference (2nd Ed.). Cambridge, Cambridge University Press (2009)
Holzinger, A.: Interactive machine learning for health informatics: when do we need the human-in-the-loop?. Brain Inf. 3(2), 119–131. https://doi.org/10.1007/s40708-016-0042-6. Springer Nature
Acknowledgements
This project is part of a postdoctoral research currently being carried out at the Faculty of Engineering, Environment and Computing, Coventry University, United Kingdom. The deepest gratitude and thanks go to the Universiti Teknikal Malaysia Melaka (UTeM) for sponsoring this postdoctoral research. The authors are thankful to the Ministry of Health Malaysia and the Melaka Hospital, Malaysia, for providing the database of retinal images and also for the manual grading done by the experts.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rahim, S.S., Palade, V., Almakky, I., Holzinger, A. (2020). Fuzzy Image Processing and Deep Learning for Microaneurysms Detection. In: Holzinger, A., Goebel, R., Mengel, M., Müller, H. (eds) Artificial Intelligence and Machine Learning for Digital Pathology. Lecture Notes in Computer Science(), vol 12090. Springer, Cham. https://doi.org/10.1007/978-3-030-50402-1_19
Download citation
DOI: https://doi.org/10.1007/978-3-030-50402-1_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-50401-4
Online ISBN: 978-3-030-50402-1
eBook Packages: Computer ScienceComputer Science (R0)