Abstract
This chapter presents the outlook for the field of cold atmospheric plasma applications in cancer therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Over the last decade, CAP is shown to demonstrate a strong potential modality in cancer therapy. The key driver involves CAP’s ability to differentiate between cancer and normal cells in vitro and the demonstrated potential to reduce tumor size in vivo. Multiple plasma sources and various diagnostic tools have been developed to monitor reactive oxygen and nitrogen species in plasma, gas, and liquid phases.
As an evolving research field, the mechanism by which CAP affects cancer has been further elucidated. This includes such observations as the CAP modulated immune response, the cancer cell instantaneous generation of unusually high μM-levels of H2O2 during the direct CAP treatment on the cells cultured in vitro, and CAP as a sensitizer of chemotherapy. The latter potentially supports the idea of CAP as a new modality in conjunction with chemotherapy and radiation. In addition, evidence that CAP activation leads to cell sensitivity to plasma activated media allows us to formulate possible mechanism of plasma medicine; namely, fast activation of cells by direct plasma action followed by RONS interaction with cells including transport across the cell membrane. Recent data has also demonstrated that the slow deactivation process is an enabler for the strong effect of RONS on cells.
The unique nature of plasma can be profoundly utilized in an adaptive therapeutic platform. Some aspects of such a system include plasma chemistry modulation, discharge mode control, and a model predictive control-based feedback system. This biomedical approach based on adaptive cold atmospheric plasma could potentially revolutionize therapy by introducing personalized treatment. Consequently, the medical treatment could be tailored to meet peculiarities associated with the patient and the tumor’s genetic makeup.
An even more intriguing potential use of CAP can be associated with the plasma self-organization phenomena. As a dynamic system, plasma independent of its type or composition tends to evolve towards a state of equilibrium or an attractor. Such tendencies lead to reducing the uncertainty about the system’s state, and therefore to reduction of the system’s statistical entropy. Self-organization in plasmas can be described as a process of spontaneous transition from a homogeneous stable state to a regular pattern in a spatially extended system or transition between different patterns.
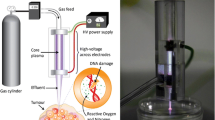
To this end, some recent observations of CAP self-organization near normal and cancer cells provide evidence of the potential for self-adaptive plasma cancer therapy [1]. The protocol of the double-cell (cancer and normal cells) test procedure and key result are shown in Fig. 13.1. For the setup, a plastic cell barrier was designed to separate the two cell colonies during the incubation. One normal cell line and another cancer cell line were seeded in the two wells of the barrier. After the incubation, the barrier was removed creating a small gap between the two cell colonies. This gap represents the boundary between the two cell colonies.
In vitro cell culture for observing the self-organization patterns of CAPJ. (a) The cell preparation procedure of double-cell test. The 3D model of the cell barrier. The E6/E7/hTERT cell colony (left) and U87MG cell colony (right) in 20 mL of DMEM medium. (b) A side-view example of normal cells (E6/E7) the negative-x region and cancer cells (U87MG) at positive-x region with a grounded copper plate beneath the dish. (c) The brightness summary of the side-view self-organization patterns that taking average brightness from y = 0.15 mm to y = 0.35 mm. One can see plasma shift towards the cancer cells. Li et al., Ref. [1]
The observed SOP is a luminous CAPJ-target contact region. The brightness distribution of SOP indicates where the CAPJ propagates. Figure 13.1b shows a side-view example of the CAPJ directed at the boundary between normal brain cell (E6/E7) and cancer brain cell (U87MG) colonies. The contact spot appeared as a bright ellipse. For comparison, the contact spot brightness distribution for the pure DMEM case is symmetric as shown in Fig. 13.1c. In contrast, the contacted spot was inclined to the right (positive x side) where the U87MG colony was seeded, while E6/E7 colony located at the negative x side. The boundary of these two cell colonies was located at x = 0 mm. This result might also explain the stronger killing effect of U87MG when compared with E6/E7/hTERT with a metal plate beneath the dish, which had been reported earlier.
In light of recent observations of the possible electromagnetic nature of the plasma effect, it is clear that the plasma therapeutic effect is complicated and involves various chemical and physical pathways [2]. Thus, systematic study of mechanisms of plasma action on cancer and normal cells is paramount for further progress. To this end, it is also important to understand the limitations of plasma’s potential in cancer therapy including understanding which types of cancers are more or less affected/resistant to CAP.
In order to effectively take the CAP technology to the next level of cancer treatment (i.e., standard of care treatment), a strong campaign must ensue to surpass the barriers to reach a true proof of clinical efficacy. With the various techniques by which CAP can be delivered, we must first determine the ideal delivery modality at the bench top. The next major hurdle involves incorporating CAP in the treatment of various tumor subtypes in a large animal model. This data can then be utilized to justify human clinical trials. Traditionally these trials serve to validate clinical equipoise with existing treatments. With effective pre-clinical work, we look to show how CAP can go beyond this low validation bar. We look to show true efficacy such that treatment with CAP improves patient survival by synergistically increasing the efficacy of radiation and chemotherapy. With persistence and dedication to defining the mechanism by which CAP selectively affects cancer cells, this goal can certainly be realized.
References
L. Lin, D. Yan, E. Gjika, J.H. Sherman, M. Keidar, Atmospheric plasma meets cell: plasma tailoring by living cells. ACS Appl. Mater. Interfaces 11(34), 30621–30630 (2019)
Dayun Yan, Qihui, Wang, Manish Adhikari, Alisa Malyavko, Li Lin Denis Zolotukhin, Xiaoliang Yao, Megan Kirschner, Jonathan Sherman, and Michael Keidar, A Physically Triggered Cell Death via Transbarrier Cold Atmospheric Plasma Cancer Treatment, ACS Applied Materials & Interfaces, 2020, https://doi.org/10.1021/acsami.0c06500
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Keidar, M., Sherman, J.H. (2020). Outlook. In: Keidar, M. (eds) Plasma Cancer Therapy. Springer Series on Atomic, Optical, and Plasma Physics, vol 115. Springer, Cham. https://doi.org/10.1007/978-3-030-49966-2_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-49966-2_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-49965-5
Online ISBN: 978-3-030-49966-2
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)