Abstract
The aim of this review chapter is to give a concise overview of clinical applications of plasma energy and future prospects for this promising modality.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Plasma Medicine: A Novel Application of Plasma Energy
Plasma medicine is a dynamic emerging field that combines physics and biomedicine. The understanding that plasma can be used with medical devices and instrumentation has been well established, but only recently has plasma energy been translated directly into biomedical applications. Physicians and life scientists are beginning to expand the use of physical plasma and for the first time are gaining a sense of how plasma interacts with living tissue [1]. Currently, there are three types of plasma sources, which are utilized in a multitude of applications in clinical medicine including discharges, plasma jets, and corona discharges [2]. Recently, there have been numerous studies that have expanded the use of these plasma applications to a multitude of disciplines within clinical medicine. The aim of this review chapter is to give a concise overview of clinical applications of plasma energy and future prospects for this promising modality.
12.2 Plasma Energy Enhancing Coagulation and Hemostasis
Plasma energy has not only expanded the boundaries of surgical specialties but also shown great promise in coagulation, hemostasis, and tissue devitalization. Argon plasma coagulation (APC) is a technique that utilizes argon gas, which is ignited into a plasma to conduct current directly to the grounded tissue resulting in coagulation necrosis [3].
The thermal energy that is produced is capable of denaturing proteins and evaporates water (Chua, Santacruz, & Gildea, 2011), resulting in the destruction of tissue and ultimately coagulation [4]. There are an increasing number of manufacturers that are employing APC as a coagulation agent in surgical interventions for indications such as hemostasis and tissue devitalization. Procedures involving treatment of ulcers, blood vessels, and tumors are especially at risk for being complicated by hemorrhage. APC has also been shown to be an effective hemostatic agent in procedures involving breast surgery and urologic surgery [5]. In addition, APC has been utilized to shrink tumors and obstructive tissues through the effects of coagulation and desiccation. Numerous studies have illustrated the utilization of APC to devitalize tissues, specifically in stomach cancer, airway obstructions, and bladder tumors [5]. Furthermore, nonthermal plasma can be used as an agent to cauterize the blood and achieve coagulation without damaging surrounding tissue [6].
Plasma coagulation was initially applied in the setting of endoscopy within the digestive tract [7]. Recent studies have also shown the utilization of plasma coagulation as an effective treatment option of early gastric cancer with intramucosal invasion [8]. APC can be directly used as an agent in hemostasis, and in these settings can directly cauterize superficial surfaces.
The mechanism of argon plasma coagulation resulting in tissue necrosis and coagulation allows for direct application within the field of pulmonology. Often, vascular tumors located within the airway can be complicated by bleeding. Plasma is an effective treatment option and has shown multiple advantages over laser including the ability to coagulate the lesion in view of the bronchoscope and the capability to debulk tissue [4, 9, 10].
It is evident that the ability of cold plasma to coagulate blood efficiently and sterilize tissue allows it to be a very useful modality for surgical subspecialties. Specifically, numerous applications of cold plasma are plausible with the potential to control bleeding within organs and during endoscopic procedures.
12.3 Regenerative Medicine: Nonthermal Plasma and Cellular Proliferation
The interaction of nonthermal plasma and living cells is a novel field of study. Nonthermal plasma has been applied in a variety of disciplines as a medical treatment and its applications within biomedical engineering are now increasing. Recent studies have been especially interested in investigating the ability of nonthermal plasma to increase the proliferation of vascular endothelial cells [11,12,13]. Kalghatgi et al. results illustrated the effects of nonthermal plasma on the vasculature [14]. Specifically, their results indicated that cellular proliferation following the application of nonthermal plasma is tied to the production of reactive oxygen species. These preliminary data are highly promising and show that nonthermal plasma treatment has enormous potential in angiogenesis.
Furthermore, recent studies by Liu et al. showed that the application of plasma to murine fibroblast cells was able to induce a significant increase in proliferation [15]. Interestingly, their results showed that with an increased treatment duration of nonthermal plasma, there was increased production of reaction oxygen species during this period. The mechanism by which nonthermal plasma induces proliferation of fibroblasts has not yet been elucidated and is currently under investigation.
Another field of study with the potential to be advanced through the application of nonthermal plasma involves synthetic implants. Subcutaneous synthetic biologic implants are utilized in a multitude of surgical subspecialties to reconstruct patient anatomy following abdominal, breast, or facial surgery procedures [16]. Currently, increased risk of infection or rejection of the foreign synthetic material of the implant contributes to patient morbidity and mortality. It has been postulated by Griffin et al. that the demise of the subcutaneous synthetic implant within the patient is due to suboptimal angiogenesis and integration with the nearby surrounding tissue. Their results indicated that after 3 months, plasma surface modification utilizing argon is a cost-effective modality that greatly enhances the integration of the synthetic implant with the surrounding tissue via the augmentation of angiogenesis.
Further studies by this research team also illustrated the efficacy of argon plasma surface modification in conjunction with adipose-derived stem cells to augment vascularization and tissue formation [17]. This combination is especially promising and could potentially increase the survival of tissue implants. These findings could have a profound impact on regenerative medicine, tissue integration, and angiogenesis. Future research aims to apply argon surface modification to various clinically approved biomedical substances to optimize function. It is especially intriguing that the physical chemistry principles of plasma surface modification highlighted in this study can be translated to a multitude of other surfaces in biomedicine, with an enormous potential to advance applications within surgery that require extended use of a subcutaneous synthetic implant.
12.4 Plasma: A Substitute for Antibacterial Treatment?
A multitude of methods has been developed over the past decades to sterilize contaminated media including autoclaving, incineration, and radiation [18]. Current antimicrobial research is focused on developing a novel methodology to improve sterilization. Irradiation by an electron beam was first investigated in 1996 by Laroussi et al [18]. Their initial results indicated that discharge plasma can be generated at atmospheric pressure and exposure for several minutes can eliminate microorganisms that are thriving in the exposed medium. Since their initial findings, numerous other groups have investigated utilizing single plasma modalities in order to eliminate microorganisms [19, 20]. As mentioned previously, these plasma agents lead to the production of molecular species including OH and NOx, which are increasingly reactive agents and play a major role in the sterilization process [20]. Recent studies have also utilized low-temperature physical plasma as a means to counter pathogen and biofilm resistance. Ermolaeva et al. utilized nonthermal plasma to eliminate pathogenic bacteria from biofilms and wound surfaces [21]. This modality demonstrated particular efficacy against Pseudomonas aeruginosa and Staphylococcus aureus, which are commonly implicated in biofilms and complicate chronic wound infections. Two minutes of cold atmospheric plasma treatment have shown efficacy against bacteria including Escherichia coli, group A Streptococcus, Methicillin-resistant Staphylococcus aureus (MRSA), and Pseudomonas aeruginosa [22]. In addition, studies have illustrated the effect of CAP on various fungal strains and a significant reduction in bacteria [23]. These findings pave the way for future applications of cold atmospheric plasma, which could have a greater role in addressing wound infections and decontamination procedures.
At the same time, despite the advances in using single plasma agents in the sterilization process, there are numerous obstacles that this modality faces and have prevented the technique from being widely utilized. The key issue is that the current modalities utilized in disinfection are simply much more cost-effective [19]. This has relegated the use of plasma agents to fields such as endoscopy where they serve a unique niche in the decontamination process. Hot plasmas are also used to disinfect the surgical equipment [21]. It is evident that plasma has enormous potential particularly against multiple-antibiotic resistant and pathogenic bacteria. In addition, plasma has the advantage of being painless and minimizing contamination of the environment [21]. Furthermore, optimizing plasma for clinical applications of decontamination and sterilization will continue to require the collaborative efforts of physicists, biologists, and healthcare professionals in order to surmount the current obstacles of time and cost-effectiveness and expand the use of plasma energy.
12.5 Dermatological Applications of Cold Atmospheric Plasma
The understanding that cold atmospheric plasma has applications as an antimicrobial agent and microbial inactivation has led to applications within dermatology. The human skin is a dynamic surface and is often complicated by infectious microorganisms and disease processes, which can complicate wound healing [1, 24]. Atopic dermatitis, the most common form of eczema, is an inflammatory skin disease that affects one-fifth of all individuals at some point in their life [25]. A case study in 2008 showed a significant reduction in itch after daily application of CAP treatment [26]. Another study by Isbary et al. illustrated that the application of cold atmospheric plasma can lead to a significant pain reduction in patients with a chronic postoperative ear infection [27]. The preliminary data of these studies highlight that cold atmospheric plasma has a beneficial impact in atopic eczema, and within specific applications can also relieve itch and pain.
In addition, Helmke et al. studied the effect of cold atmospheric plasma on pH of the hydrolipid film of the skin [28]. Researchers utilized a DBD plasma source to treat lipid films with CAP and observed a significant decrease in pH values. The decreased pH would lead to decreased growth of pathogens and optimize wound healing [28]. Another recent research team showed the efficacy of CAP on acne scar treatment [29]. Ten patients received a single treatment with CAP and 30% experienced an improvement in their acne scar. Given the established antimicrobial effect of CAP, treatment with plasma could have enormous potential in standard of acne care in the future [24].
Actinic keratosis is an erythematous, scaly skin lesion, which portends an increased risk of squamous cell carcinoma and other skin malignancies [30]. Wirtz et al. results showed that cold atmospheric plasma has a positive impact on the healing of actinic keratosis [31]. Specifically, CAP treatment administered twice a week had a favorable outcome in all seven patients in the study [32]. These preliminary results indicate that CAP has enormous potential in the field of dermato-oncology as research continues to further elucidate the beneficial effects of cold atmospheric plasma on precancerous lesions [24].
Another major focus of the application of plasma sources has shifted to addressing wound healing and treatment of skin disease. It is evident that CAP, and the generation of plasma, involves the production of multiple molecular species. Multiple attributes of CAP can promote wound healing including UV radiation and the production of ROS, generation of nitric oxide, and angiogenesis, which is a byproduct of electric current [24]. A recent study illustrated that CAP treatment led to increased angiogenesis, resulting in accelerated in vivo wound healing and increased cellular proliferation [33]. Until recently, CAP has been largely characterized by in vitro microbiology; as in vivo studies rapidly expand such as the aforementioned study, we will be able to gain a better sense of the therapeutic potential of CAP.
Plasma medicine is gradually being adapted into clinical applications and incorporated into medical devices. It is evident that for a multitude of skin diseases, application of CAP is already a standard of care [24]. There are an increasing number of studies that are investigating patient outcomes following delivery of CAP through such devices.
One such study utilizing devices administering CAP enrolled 50 patients to study the positive impact of CAP on ulcers. Twenty-five individuals received treatment with cold atmospheric plasma delivered with a bioplasma jet device [34]. Their results showed a positive impact of CAP on healing of pressure ulcers. Another study by Ulrich et al. investigated the impact of CAP on the healing of chronic leg ulcers. Their results showed that delivery of CAP via a plasma source kINPen MED had a similar reduction in bacterial load compared to octenidine, an antiseptic, treatment [35].
Furthermore, cold atmospheric plasma is currently involved in three clinical trials within Germany investigating ulcer treatment [36]. In 2013, two sources of CAP got a CE marking as a medical device [1].
12.6 Cold Atmospheric Plasma in Oncology
CAP has both physical and chemical properties, which lend itself being selective toward cancer cells [37]. As treatments move toward targeted therapies with decreased systemic effects, CAP emerges as a promising modality. Two clinical trials have investigated the palliative effects of CAP on head and neck cancers [37]. As previously mentioned, CAP is an appropriate modality for this pathology due to its capability of treating ulcerations, applications within wound healing, and ability to induce coagulation necrosis.
Plasma is an ionized gas that is conventionally generated under high-temperature conditions. The advent of cold plasmas led to the discovery that CAP is capable of selectively killing cancer cells in both in vitro and in vivo experiments. Utilization of CAP allows for the application of another state of matter for tumor ablation and suggests a possible shift in the current paradigm of cancer treatment moving forward [14]. Metelmann et al. investigated the efficacy of cold plasma treatment for head and neck cancer. It is well established that CAP has antitumor effects on a multitude of cell lines and models. Metelmann et al. were the first to demonstrate the ability of CAP to not just impact the tumor surface but lead to lasting partial remission in the setting of inoperable head and neck cancer patients. Researchers investigated six patients who had squamous cell carcinoma of the oropharynx and were suffering from open infected ulcerations who underwent treatment with a jet plasma source. Their results indicated that following treatment with CAP patients had a reduction in odor and pain levels as well as an increase in social function. Furthermore, two patients experienced a significant response to the treatment and experienced tumor reduction, specifically partial remission for 9 months.
Metelmann et al.’s clinical trial highlights the clinical relevance and potential CAP therapy could have in cancer treatments going forward [38].
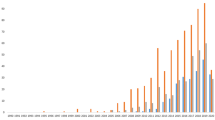
In one clinical trial described in Ref. [38], 5 of the patients died between 1 month and 12 months of CAP-treatment. One patient was still under control and was receiving treatment (by the time of publication) for more than 9 months (as shown in Fig. 12.1). It was concluded that result described in Fig. 12.1 corresponds to expectations: With regard to the very limited literature available, the median survival time of patients with advanced cancer of oropharynx just under palliative treatment amounts to 7.5 months.
Survival time, taken from Ref. [38] with permissions
Researchers also went one step further to investigate the use of CAP as a treatment modality in palliative cancer. As discussed above, individuals with head and neck cancer can often experience superinfection of the chronic wound. Seebauer et al. applied CAP therapy directly to a superinfected necrotic tumor. Results showed that CAP treatment led to a decrease in wound inflammation, odor, and colonization due to bacteria. Interestingly, the tumor had been reduced to nearly one-quarter of the original size prior to application of CAP. Furthermore, histological examination investigating cytology highlighted an increased number of apoptotic tumor cells and augmented immune system effects. Ultimately, these results highlight that CAP is a valuable treatment modality in palliative cancer care that targeted multiple facets in addition to the known antimicrobial effects [39].
The molecular underpinnings that allow cold atmospheric plasma to have an impact on cancer cells are currently unknown. It is postulated that reactive oxygen species, ionized particles, and charges contribute greatly to the mechanism of plasma driven cell death [40].
Additional research teams have investigated the utility of CAP treatment in advanced head and neck cancers and ulcerations which were contaminated. Schuster et al. were interested in determining the properties of CAP that make it highly effective in palliative cancer treatment with minimal side effects. Ulcerations in oral carcinoma can often become infected and these patients are known to have poor outcomes [41]. This study entailed a retrospective analysis of 20 patients who underwent CAP treatment for decontamination and specifically reviewed the various side effects experienced in these patients due to treatment with CAP. The authors reported that there were no, mild, or moderate unwanted outcomes due to the application of CAP. Furthermore, the researchers postulated as to the etiology of the biochemical basis, which leads CAP to not have the severe side effects as observed. Given the abundant data, and our prior discussion involving the abundant production of ROS due to CAP, this raises the question as to why CAP does not have deleterious side effects due to off-target toxicity of ROS. Schuster et al. proposed a model in which CAP does not directly have anti-microbial or antitumor effects, but instead has the ability to facilitate a specific effect on target tissue due to singlet oxygen turning on a switch mechanism. This targeted action leads to the activation of ROS/RNS-dependent apoptosis only in tumor cells. Ultimately, there is unique specificity in the target tissue in which plasma can turn on the switch by a singlet oxygen, but this does not occur in off-target sites. Since the singlet oxygen cannot turn on the switch mechanism in healthy tissue, there is no harm and off-target toxicity [41].
The clinical use of this promising modality in various other fields of oncology is currently under investigation. Countless in vitro experiments with CAP have paved the way for promising in vivo studies which are only now being translated into clinical trials as described above. CAP-induced tumor mass reduction is a promising finding that will be further investigated in the coming years. Currently, the FDA has given approval for 20 patients to undergo CAP treatment as an adjunct to the treatment protocol for their solid cancerous tumors (https://www.businesswire.com/news/home/20190731005521/en/USMI-JCRI-ABTS-Receive-FDA-Approval-Conduct-U.S.) [42]. Gaining further insight into CAP’s tolerable side effect profile in addition to its effectiveness as a selective agent in anticancer treatment modality will pave the way for additional clinical trials [43]. Additional studies are required to ascertain the therapeutic effect of CAP in various other cancers.
References
T. von Woedtke, H.-R. Metelmann, K.-D. Weltmann, Clinical plasma medicine: state and perspectives of in vivo application of cold atmospheric plasma. Contrib. Plasma Physics 54, 104–117 (2014)
T. von Woedtke, S. Reuter, K. Masur, K.-D. Weltmann, Plasmas for medicine. Phys. Rep. 530, 291–320 (2013)
A. Chua, J.F. Santacruz, T.R. Gildea, Chap. 29. Pulmonary complications of cancer therapy and central airway obstruction, in Supportive Oncology, (Saunders, Philadelphia, 2011), pp. 309–325
F.D. Sheski, D.J. Feller-Kopman, G. Finlay, Bronchoscopic argon plasma coagulation in the management of airway disease in adults, 1
M. Zenker, Argon plasma coagulation. GMS Krankenhaushygiene Interdisziplinär. 3, Doc15 (2008)
S. Kalghatgi, Applications of non thermal atmospheric pressure plasma in medicine, in Plasma Assisted Decontamination of Biological and Chemical Agents, ed. by S. Güçeri, A. Fridman, K. Gibson, C. Haas, (Springer, Dordrecht, 2008)
G. Farin, K.E. Grund, Technology of argon plasma coagulation with particular regard to endoscopic applications. Endosc. Surg. Allied Technol. 2, 71–77 (1994)
T. Sagawa, T. Takayama, T. Oku, T. Hayashi, H. Ota, T. Okamoto, et al., Argon plasma coagulation for successful treatment of early gastric cancer with intramucosal invasion. Gut 52, 334–339 (2003)
C. Crosta, L. Spaggiari, A. De Stefano, G. Fiori, D. Ravizza, U. Pastorino, Endoscopic argon plasma coagulation for palliative treatment of malignant airway obstructions: early results in 47 cases. Lung Cancer 33, 75–80 (2001)
G. Reichle, L. Freitag, H.J. Kullmann, R. Prenzel, H.N. Macha, G. Farin, Argon plasma coagulation in bronchology: a new method—alternative or complementary? Pneumologie 54, 508–516 (2000)
K.P. Arjunan, G. Friedman, A. Fridman, A.M. Clyne, Non-thermal dielectric barrier discharge plasma induces angiogenesis through reactive oxygen species. J. R. Soc. Interface 9, 147–157 (2012)
S. Kalghatgi, G. Friedman, A. Fridman, A.M. Clyne, Endothelial cell proliferation is enhanced by low dose non-thermal plasma through fibroblast growth factor-2 release. Ann. Biomed. Eng. 38, 748–757 (2010)
C. Kobayashi, T. Komachi, T. Kishimoto, T. Hirata, A. Mori, Effect of neoangiogenesis using micro-spot atmospheric pressure plasma, 1044, 2012
M. Keidar, R. Walk, A. Shashurin, P. Srinivasan, A. Sandler, S. Dasgupta, et al., Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer 105, 1295–1301 (2011)
J. Liu, G. Xu, X. Shi, G. Zhang, Low temperature plasma promoting fibroblast proliferation by activating the NF-κB pathway and increasing cyclinD1 expression. Sci. Rep. 7, 11698 (2017)
M. Griffin, R. Palgrave, V.G. Baldovino-Medrano, P. Butler, M.D. Kalaskar, Argon plasma improves the tissue integration and angiogenesis of subcutaneous implants by modifying surface chemistry and topography. Int. J. Nanomedicine 18, 6123–6141 (2018)
M.F. Griffin, N. Naderi, D.M. Kalaskar, A.M. Seifalian, P.E. Butler, Argon plasma surface modification promotes the therapeutic angiogenesis and tissue formation of tissue-engineered scaffolds in vivo by adipose-derived stem cells. Stem Cell Res Ther 10, 110 (2019)
M. Laroussi, Sterilization of contaminated matter with an atmospheric pressure plasma. IEEE Tran. Plasma Sci. 24, 1188–1191 (1996)
J. Ehlbeck, U. Schnabel, M. Polak, J. Winter, T. von Woedtke, R. Brandenburg, et al., Low temperature atmospheric pressure plasma sources for microbial decontamination. J. Phys. D 44, 013002 (2010)
M. Laroussi, F. Leipold, Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 233, 81–86 (2004)
S.A. Ermolaeva, A.F. Varfolomeev, M.Y. Chernukha, D.S. Yurov, M.M. Vasiliev, A.A. Kaminskaya, et al., Bactericidal effects of non-thermal argon plasma in vitro, in biofilms and in the animal model of infected wounds. J. Med. Microbiol. 60, 75–83 (2011)
G. Daeschlein, S. Scholz, A. Arnold, S. von Podewils, H. Haase, S. Emmert, et al., In vitro susceptibility of important skin and wound pathogens against low temperature atmospheric pressure plasma jet (APPJ) and dielectric barrier discharge plasma (DBD). Plasma Process. Polym. 9, 380–389 (2012)
G. Daeschlein, S. Scholz, T. von Woedtke, M. Niggemeier, E. Kindel, R. Brandenburg, et al., In vitro killing of clinical fungal strains by low-temperature atmospheric-pressure plasma jet. IEEE Trans. Plasma Sci. 39, 815–821 (2011)
T. Bernhardt, M.L. Semmler, M. Schäfer, S. Bekeschus, S. Emmert, L. Boeckmann, Plasma medicine: applications of cold atmospheric pressure plasma in dermatology. Oxidative Med. Cell. Longev. 2019, 10 (2019)
S.F. Thomsen, Atopic dermatitis: natural history, diagnosis, and treatment. ISRN Allergy 2014, 354250 (2014)
N. Mertens, A. Goppold, S. Emmert, W. Vioel, Dielectric barrier discharge plasma-A powerful tool for medical applications. In 20th International Conference of the Society for Medical Innovation and Technology (SMIT) (2008)
G. Isbary, T. Shimizu, J.L. Zimmermann, H.M. Thomas, G.E. Morfill, W. Stolz, Cold atmospheric plasma for local infection control and subsequent pain reduction in a patient with chronic post-operative ear infection. New Microbes New Infect. 1, 41–43 (2013)
A. Helmke, D. Hoffmeiste, N. Mertens, S. Emmert, J. Schuette, W. Vioel, The acidification of lipid film surfaces by non-thermal DBD at atmospheric pressure in air. New J. Phys. 11, 115025 (2009)
C. Chutsirimongkol, D. Boonyawan, N. Polnikorn, W. Techawatthanawisan, T. Kundilokchai, Non-thermal plasma for acne and aesthetic skin improvement. Plasma Med. 4, 79–88 (2014)
A. Dodds, A. Chia, S. Shumack, Actinic keratosis: rationale and management. Dermatol. Ther. 4, 11–31 (2014)
M. Wirtz, I. Stoffels, J. Dissemond, D. Schadendorf, A. Roesch, Actinic keratoses treated with cold atmospheric plasma. J. Eur. Acad. Dermatol. Venereol. 32, e37–e39 (2018)
R.H. Rosen, A.K. Gupta, S.K. Tyring, Dual mechanism of action of ingenol mebutate gel for topical treatment of actinic keratoses: rapid lesion necrosis followed by lesion-specific immune response. J. Am. Acad. Dermatol. 66, 486–493 (2012)
C. Duchesne, S. Banzet, J. Lataillade, A. Rousseau, N. Frescaline, Cold atmospheric plasma modulates endothelial nitric oxide synthase signalling and enhances burn wound neovascularisation. J. Pathol. 249, 368–380 (2019)
A. Chuangsuwanich, T. Assadamongkol, D. Boonyawan, The healing effect of low-temperature atmospheric-pressure plasma in pressure ulcer: a randomized controlled trial. Int J Low Extrem Wounds 15, 313–319 (2016)
C. Ulrich, F. Kluschke, A. Patzelt, S. Vandersee, V.A. Czaika, H. Richter, et al., Clinical use of cold atmospheric pressure argon plasma in chronic leg ulcers: a pilot study. J. Wound Care 24, 196 (2015)., 198-200, 202
F. Brehmer, H.A. Haenssle, G. Daeschlein, R. Ahmed, S. Pfeiffer, A. Görlitz, et al., Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (PlasmaDerm(®) VU-2010): results of a monocentric, two-armed, open, prospective, randomized and controlled trial (NCT01415622). J. Eur. Acad. Dermatol. Venereol. 29, 148–155 (2015)
A. Dubuc, P. Monsarrat, F. Virard, N. Merbahi, J. Sarrette, S. Laurencin-Dalicieux, et al., Use of cold-atmospheric plasma in oncology: a concise systematic review. Thera. Adv. Med. Oncol. 10, 175883591878647 (2018)
Metelmann H-, Seebauer C, Miller V, Fridman A, Bauer G, Graves DB, et al: Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clin. Plasma Med. 9:6-13, 2018
C. Seebauer, S. Kindler, T. von Woedtke, H. Metelmann, Physical plasma in palliative cancer care: introduction and perspectives. New Horiz. Clin. Case Rep. 1, 28 (2017)
A. Lin, N. Chernets, J. Han, Y. Alicea, D. Dobrynin, G. Fridman, et al., Non-equilibrium dielectric barrier discharge treatment of mesenchymal stem cells: charges and reactive oxygen species play the major role in cell death. Plasma Process. Polym. 12, 1117–1127 (2015)
M. Schuster, R. Rutkowski, A. Hauschild, R. Shojaei, T. von Woedtke, A. Rana, et al., Side effects in cold plasma treatment of advanced oral cancer—clinical data and biological interpretation. Clin. Plasma Med. 10, 9–15 (2018)
J. Canady, B. Trink, J.H. Sherman, M. Keidar, GWU-USMI plasma medicine research program, 5th international workshop on plasma for cancer treatment. Clin. Plasma Med. 9, 3 (2018)
H.-R. Metelmann, T. von Woedtke, K.-D. Weltmann, Comprehensive Clinical Plasma Medicine: Cold Physical Plasma for Medical Application (Springer, Cham, 2018), pp. 185–195
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Almeida, N.D., Sack, K., Sherman, J.H. (2020). Clinical Applications of Cold Atmospheric Plasma. In: Keidar, M. (eds) Plasma Cancer Therapy. Springer Series on Atomic, Optical, and Plasma Physics, vol 115. Springer, Cham. https://doi.org/10.1007/978-3-030-49966-2_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-49966-2_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-49965-5
Online ISBN: 978-3-030-49966-2
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)