Abstract
Contrast-enhanced ultrasound (CEUS) is being increasingly recognized as an important method of imaging a variety of adult malignancies including in the liver, breast, prostate and kidneys. Although most applications have been pursued in adults, children are the ideal patient population for CEUS for numerous reasons including avoidance of exposure to ionizing radiation and sedation. In 2016, the US Federal Drug Administration approved the first ultrasound contrast agent for use in imaging liver lesions in both adults and children. While paediatric CEUS has a more established and widespread use in Europe, this approval has ushered in a new era for the growth and development of CEUS in children in the United States, where there is a burgeoning interest in this modality among paediatric radiologists. In this chapter, we discuss applications of CEUS in paediatric oncology for diagnosis, as a problem-solving tool and its role in guidance of oncologic procedures in interventional radiology, with additional discussion of future directions of CEUS in paediatric oncology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
18.1 Introduction
Ultrasound (US) has many positive attributes in pediatric imaging; it is non-invasive, portable, provides Doppler capabilities for vascular assessment, does not require sedation, and, most importantly, does not expose the child to the potentially harmful effects of ionizing radiation. The avoidance of radiation and sedation is of heightened concern in pediatric oncology because these children undergo innumerable imaging examinations at diagnosis, for staging, to monitor treatment response, to assess acute and chronic treatment-related complications, and to assess for tumor recurrence after completion of therapy. A recent study showed an association between radiation exposure from computed tomography (CT) imaging and an increased risk of developing brain tumors and leukemia in children [1]. Those investigators reported that brain tumor risk was comparable to observed risk estimates for brain tumors following childhood radiation exposure in Japanese nuclear blast survivors. These findings underscore the importance of minimizing radiation exposure in children whenever possible.
In children with cancer, US is often the first-line imaging modality to identify and localize pathology in the abdomen, pelvis, and extremities. However, B-mode US has recognized limitations and further imaging with CT, magnetic resonance (MR), and nuclear medicine imaging is required for diagnosis and staging. The addition of a contrast agent to US imaging offers the opportunity to improve lesion conspicuity, better characterize lesions, distinguish benign from malignant features, and improve diagnostic confidence. The current role of contrast-enhanced ultrasound (CEUS) in pediatric oncology is to guide interventional procedures and for use as a problem-solving tool at the time of diagnosis or when complications arise during and after therapy. In some circumstances, CEUS could replace CT or MR imaging, which exposes the patient to radiation and sedation, adds cost, can create anxiety, and usually necessitates the administration of an intravenous contrast agent. An added benefit of CEUS is that ultrasound contrast agents (UCA) are not metabolized by the kidneys and can be safely administered to patients with renal insufficiency. Additionally, rates of adverse reactions to UCA are low, similar to that of gadolinium agents for MR imaging. With the recent US FDA approval of a UCA for children, coupled with an increasing emphasis on medical cost containment and radiation and anesthesia reduction, the role of this important alternative imaging modality is expanding in pediatric clinical practice. In this chapter, we illustrate the value of CEUS in pediatric oncology, specifically in the diagnosis and management of pediatric malignancies, in assessing complications of therapy and in guiding interventional procedures. The potential role of CEUS in assessing tumor response to therapy and as a treatment modality is also presented.
18.2 CEUS of Pediatric Solid Tumors
In the pediatric oncology patient setting, CEUS has tremendous potential for diagnostic problem-solving, given its excellent safety and cost profile compared to CT and MR imaging, both of which utilize contrast agents that may be contraindicated in patients with renal insufficiency. Compared to CT, major advantages of CEUS are its lack of ionizing radiation, excellent temporal resolution and the ability to easily image during all phases of contrast enhancement. Compared to MR imaging, the major advantages of CEUS are increased accessibility, faster examination, lower cost, and the ability to perform the examination without sedation. In young patients (usually between 6 months and 7 years), sedation is almost always necessary to provide MR images that are of diagnostic quality, and general anesthesia would be required to obtain sequences that require breath holds. Anesthesia or sedation medications carry a risk of adverse events in this patient population, and this may be avoided with CEUS.
The use of CT or MR imaging contrast agents also poses potential risks for pediatric oncology patients, as renal excretion of contrast agents may be impaired due to concomitant use of chemotherapeutic agents, and this may increase the risk of further injury or nephrogenic systemic fibrosis. The deposition of gadolinium in the body has also been described, although there is currently no known clinical significance. Finally, these patients usually undergo multiple imaging evaluations during the course of diagnosis, treatment, and disease surveillance, and repeat imaging may compound the aforementioned risks [2, 3].
There are limitations to CEUS imaging in the pediatric oncology setting. Most importantly, tumor staging is not possible with CEUS. Although CEUS can confidently diagnose malignancy, once a diagnosis of malignancy has been made, further evaluation with CT and/or MR imaging for tumor staging is mandatory. In patients who have multiple, non-adjacent lesions with different appearances (and therefore potentially several different coexistent types of lesions), multi-phase CT and/or MR imaging should be considered to allow easier characterization of each lesion. It should be noted that although evaluation of only up to two lesions is possible in the arterial phase at CEUS, scanning through the entire organ is possible in the delayed phase of contrast enhancement, which enables limited evaluation of additional lesions.
In the pediatric oncology setting, CEUS may be most helpful to determine if a newly detected liver lesion is benign or malignant [4]. Specifically, although there are various appearances of different malignancies in the arterial phase, liver lesions that do not retain contrast compared to the background liver parenchyma in the delayed phase may be confidently diagnosed as malignant with high specificity [5, 6]. Conversely, liver lesions that retain contrast on the delayed phase of CEUS may confidently be diagnosed as benign, with sensitivity of up to 98% and negative predictive value of 100% in pediatric patients [4]. Specific examples of the use of CEUS to characterize different types of pediatric liver tumors are detailed below, as is the use of CEUS to characterize lesions in other organs.
18.3 Malignant Liver Lesions
18.3.1 Hepatoblastoma
Hepatoblastoma is the most common primary pediatric liver tumor and is usually diagnosed within the first 3 years of life [7]. Although many associated conditions have been described, including Beckwith–Wiedemann syndrome, very low birth weight and premature infants, most cases of hepatoblastoma are sporadic [8,9,10]. Histologically, hepatoblastoma is composed either entirely of epithelial cells or a mixture of epithelial and mesenchymal cells. There are several subtypes of hepatoblastoma, including fetal, embryonal, pleomorphic epithelial, small cell undifferentiated, and cholangioblastic. Tumors with more histologically mature cell lines usually have a better prognosis than tumors with undifferentiated or immature cell lines [11]. Hepatoblastoma staging relies on the PRE-Treatment EXTent of Tumor (PRETEXT) staging system to standardize the imaging evaluation and to stratify tumors that may be surgically resectable and those which are unresectable. The PRETEXT group (I, II, III, or IV) is based on the number of contiguous tumor-free liver sections. Several annotation factors were recently updated, which define areas of extrahepatic involvement including tumor involvement of the inferior vena cava, hepatic veins, and portal veins [12, 13].
Contrast-enhanced ultrasound can be used to risk stratify patients who have a liver lesion into malignant or non-malignant categories. Although the appearance of hepatoblastoma at CEUS is not well described [14], like other non-hepatocellular primary liver tumors, the washout phase of contrast enhancement is likely most helpful to confirm a diagnosis of malignancy (Fig. 18.1). No characteristic arterial phase or portal venous appearance has yet been described for hepatoblastoma. Once a diagnosis of hepatoblastoma is suspected or confirmed, further evaluation with CT or MR imaging is required for appropriate PRETEXT staging of the tumor beyond the liver. Chest CT is required to evaluate the lungs, which is the most common site of hepatoblastoma metastasis [13]. CEUS may be beneficial for evaluating vascular involvement of the tumor, if questions remain after either CT or MR imaging, as it may demonstrate enhancement of tumor thrombus with good temporal and spatial resolution.
A 2-year-old male with a hepatoblastoma. (a) Coronal contrast-enhanced CT demonstrates the pedunculated primary tumor arising from the inferior right hepatic lobe (arrow). (b) Contrast-enhanced ultrasound obtained at 11 s demonstrates hypo-enhancement of the mass in the arterial phase. (c) The lesion demonstrates early portal venous peripheral washout at 28 s
18.3.2 Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is the second most common primary pediatric liver tumor after hepatoblastoma, and usually affects children 10–14 years of age. In distinction to hepatoblastoma, HCC is rarely encountered in children less than 5 years of age [15]. Unlike in adults, the majority (almost 70%) of cases of HCC in pediatric patients occur in patients with no underlying liver disease [16]. However, cirrhosis increases the risk of developing HCC. Causes of cirrhosis in children include Alagille syndrome, glycogen storage diseases, Hepatitis B and C, progressive familial intrahepatic cholestasis (PFIC) types 2 and 3, Wilson disease, biliary atresia, Fanconi syndrome, and tyrosinemia [17]. Also in distinction to HCC in adults, pediatric HCC usually presents with larger tumor size, more advanced disease, and higher rates of both locoregional and distant metastatic disease at presentation [18]. However, pediatric HCC usually has a better response to chemotherapy compared to adults. Additionally, whereas adults must often meet specific criteria for liver transplantation, children with unresectable HCC may be treated by liver transplantation regardless the size of the tumor or number of tumor lesions in the liver, as long as there is no vascular invasion or extrahepatic disease [17, 19].
Fibrolamellar HCC is considered a distinct entity from HCC. Of all types of HCC encountered under the age of 20 years, fibrolamellar accounts for almost 30% [20, 21]. Serum alpha fetoprotein level is almost always normal, and there is typically no underlying hepatocellular disease in these patients. Unless resectable, fibrolamellar HCC has a poor prognosis. Another variant tumor of HCC is the recently described hepatocellular malignant neoplasm not otherwise specified (NOS), formerly referred to as transitional liver cell tumor because of the admixture of both hepatocellular and hepatoblastoma histopathologic components [22]. These tumors have been described to have highly elevated serum alpha fetoprotein levels and worse outcomes than traditional hepatoblastoma [22, 23].
The CEUS appearance of HCC in children has not yet been well described. However, the appearance is likely to be similar to that described in adults. In adults, HCC classically demonstrates early arterial enhancement, with late-phase subtle washout [24]. In contradistinction, metastatic liver tumors and non-HCC primary liver tumors have much more prominent and earlier washout than HCC.
18.3.3 Liver Metastases
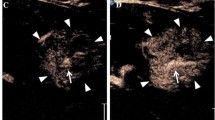
Metastasis is the most frequently encountered pediatric liver neoplasm and the most common pediatric tumors to metastasize to the liver include neuroblastoma, Wilms tumor, and lymphoma [15]. Identification of a primary malignancy aids in diagnosing a focal liver lesion as a metastasis, particularly at initial diagnosis. However, it should be noted that almost 20% of children treated for a solid malignancy develop focal liver lesions after therapy [25] and they are often discovered on routine surveillance imaging raising concern for tumor recurrence. In such cases, CEUS provides a rapid, low-risk, low cost, and highly accurate method of distinguishing benign from malignant etiologies. This approach allows almost immediate feedback to the physician and parent/care giver, thus alleviating anxiety and avoiding the need for additional imaging and delays in treatment. Liver metastases are characterized by early, marked washout of the UCA, normally by 1 min after injection (Fig. 18.2) [24].
A 20-year-old male with desmoplastic small round cell tumor. (a) Axial contrast-enhanced CT shows a liver lesion (arrow) that was suspicious for metastatic disease. (b) Contrast-enhanced ultrasound split screen image of the liver lesion (arrows) in the early arterial phase demonstrates patchy iso-enhancement compared to surrounding normal liver. (c) In the early portal venous phase, the lesion (arrow) demonstrates early washout. (d) In the late portal venous phase the lesion (arrow) shows clear washout compared to surrounding normal liver. Early washout, such as seen here, is typical of metastatic disease in the liver
18.4 Benign Liver Lesions
18.4.1 Hemangioma
Infantile hepatic hemangioma is the most common benign liver mass in children [26,27,28]. Infantile hemangiomas are true vascular neoplasms that develop within a few weeks to months of life and are not present at birth. These lesions demonstrate GLUT1 staining on pathology. The CEUS appearance of infantile hemangioma is not well reported, however, the CEUS enhancement pattern is expected to follow that seen on other modalities, including early peripheral arterial enhancement, enhancement through the portal venous phase, and complete iso-enhancement in the delayed phase, with minimal subtle washout possible (Fig. 18.3) [5, 29]. The primary differential considerations for infantile hemangiomas are metastases and multifocal hepatoblastoma, which will demonstrate early (<1 min) and pronounced washout. Marked washout has not been described with infantile hepatic hemangioma at CEUS.
A 4-month-old female with multiple cutaneous infantile hemangiomas. (a) Contrast-enhanced ultrasound images obtained in the early arterial phase at 10 s, showing two (arrows) rapidly enhancing lesions (arrows), with centripetal flow. (b) Contrast-enhanced ultrasound images in the later arterial phase demonstrates the complete centripetal fill-in (arrows), compatible with infantile hepatic hemangiomas
Congenital hepatic hemangiomas are usually solitary lesions. Unlike infantile hepatic hemangioma, congenital hepatic hemangiomas are present, and fully proliferated, at birth. These lesions are GLUT1 negative and most commonly spontaneously involute by 1 year of life [30]. Like infantile hemangioma, the CEUS appearance of congenital hemangioma is not well described. Limited reports describe these lesions as heterogeneously enhancing in the arterial phase with either complete or incomplete fill-in on delayed phases. Areas of contrast fill-in will have sustained enhancement in delayed phases [5]. The primary differential consideration for congenital hemangioma is hepatoblastoma, which is expected to have early contrast washout, an appearance not described with congenital hemangioma.
18.4.2 Focal Nodular Hyperplasia
Focal nodular hyperplasia (FNH) is a regenerative mass and is typically asymptomatic. Focal nodular hyperplasia is the second most common benign liver lesion in older children and young adults, but is very uncommon in young children. The exception is that FNH is frequently encountered in childhood cancer survivors [25]. Ultrasound is often used for screening and follow-up of patients with a known primary malignancy, but because FNH has a non-specific conventional grayscale US appearance, additional imaging is necessary for characterization. The major advantage of CEUS over CT and MR imaging is that FNH can be confidently diagnosed with CEUS the same day a new liver lesion is discovered, which is not always possible with CT and/or MR imaging [31]. This rapid and definitive diagnosis of FNH with CEUS offers the additional benefit of decreasing patient and parental anxiety about the unknown etiology of a new liver lesion in a patient who is at risk for metastatic disease [25, 32].
At CEUS, FNH are hypervascular lesions that have a stellate appearance of early arterial enhancement (Fig. 18.4). A tortuous feeding artery may additionally be seen. An FNH typically has complete or incomplete centrifugal pattern contrast fill-in during the portal venous phase, with a central scar possible, as seen with other modalities. An FNH has sustained enhancement about 90% of the time, again unlike metastases which will have early and noticeable washout [33,34,35,36].
A 10-year-old female with non-alcoholic fatty liver disease. (a) Contrast-enhanced ultrasound early arterial phase images obtained at 12 s, showing initial spoke wheel pattern of arterial enhancement to the central aspect of the lesion (arrows). (b) In the later arterial phase, at 15 s, the spoke-wheel pattern is more established with the beginnings of diffuse lesion enhancement (arrows), diagnostic of focal nodular hyperplasia. The lesion will remain hyperenhancing to background liver in all vascular phases
18.5 Benign and Malignant Renal Lesions
Intravenous CEUS for characterization of renal lesions is much less well described than hepatic lesions. However, CEUS may aid in the diagnosis of certain renal lesions.
18.5.1 Complex Renal Cysts
Simple renal cysts, including those with an imperceptible wall, posterior acoustic enhancement, and no internal septations, can usually be easily diagnosed with grayscale ultrasound. This is particularly true in pediatric patients, in whom a smaller body habitus allows visualization of the kidneys with higher resolution than in adults. However, when adequate visualization of simple and complex renal cysts is not possible, CEUS may be helpful for additional evaluation. Specifically, CEUS can demonstrate the presence of internal septations with greater sensitivity than CT and can more easily demonstrate the thin or nodular character of septations (Fig. 18.5) [37]. When present, thick nodular septations raise concern for possible malignancy and additional imaging may be necessary.
18.5.2 Renal Tumors
Primary renal tumors encountered in childhood, including Wilms tumor, rhabdoid tumor, and clear cell sarcoma, have not yet been described to have a characteristic appearance on CEUS. Currently, CEUS for renal tumors is often reserved for problem-solving and surgical planning, including better delineation of vascular involvement.
18.5.3 Renal Pseudotumor
Pseudotumor, including a hypertrophied column of Bertin or dromedary hump, is often easily diagnosed with grayscale and color Doppler US. However, CEUS may increase diagnostic confidence and provide better reassurance that a contour abnormality is not neoplastic. On CEUS, these lesions follow the enhancement appearance of the background renal parenchyma throughout all contrast phases, unlike a true renal tumor [38].
18.6 CEUS in Pediatric Oncologic Interventions
Interventional oncology involves application of interventional radiology techniques to the diagnosis and treatment of cancer and relies on image guidance to increase the efficacy and precision of targeted minimally invasive therapy. Broadly, the role of interventional radiology in the care of cancer can be subdivided into three categories:
-
(a)
Interventions for diagnosis: biopsy (tumors of soft tissue and bone, and marrow), lumbar puncture.
-
(b)
Interventions for supportive care: venous access (temporary and long term), drainage procedures (pleural and peritoneal fluid, abscesses), additional interventions for less common complications such as venous thrombosis.
-
(c)
Interventions to treat the primary tumor or palliate metastasis: tumor ablation, tumor embolization.
Interventions in the first two categories are commonly applied in pediatrics. However, the third category, while an established pillar of cancer therapy in adults, is only recently gaining recognition in pediatric oncology.
Ultrasound has been established as a core modality in pediatric image guidance because of the advantages it offers over other modalities (multiplanar imaging, excellent temporal and good spatial resolution, portability, lack of ionizing radiation) previously described in this chapter. Contrast-enhanced ultrasound adds to these advantages, by improving contrast resolution for procedure guidance, given the lower contrast resolution of grayscale US relative to CT or MR imaging. Contrast-enhanced ultrasound can play in guiding procedures in pediatric oncology, with a focus on biopsy and tumor ablation, which are among the best-supported indications for the use of CEUS in interventional radiology [39]. These procedures involve intravascular administration of the UCA, which enables the visualization of perfusion at the microvascular level, and thus provides a reliable assessment of tumor vascularity and viability. Technical aspects of CEUS in interventional radiology, as well as applications of CEUS in the above-noted procedures for supportive care, are discussed in Chap. 20.
18.6.1 Biopsy
Ultrasound contrast agents are sometimes useful to increase the conspicuity of small lesions in the liver. In children, this is often in the setting of a concern for metastasis, as primary liver neoplasms, while less common in children, are frequently large and readily visible on sonography at presentation. More frequently, CEUS is used to determine the optimum site of biopsy within the tumor, as enhancement indicates regions of intact microvasculature, suggestive of areas of most tumor viability and least necrosis (Fig. 18.6). Biopsy of non-necrotic regions should increase diagnostic yield, and the need for high-viability may be particularly salient if next-generation sequencing for characterization of signal transduction defects is desired. In this regard, CEUS can shorten the procedure, as it can increase the confidence of adequate sampling, thereby decreasing the number of passes the interventionist would make, and also decrease the need for frozen section analysis during the procedure. If there is concern for significant post-procedure hemorrhage, this can be assessed by CEUS of the region of biopsy, often faster and with less logistical complexity than CT [39]. The excellent depiction of vasculature allows the easy detection of pseudoaneurysms and active extravasation [40].
A 19-year-old male with a mass in the left iliac fossa. (a) A left-sided mass is demonstrated on an 18F-fluorodeoxyglucose positron emission tomography (FDG-PET)-CT scan, as avid uptake (arrow). A biopsy of the superficial component yielded only necrotic tissue. (b) On the CEUS examination, the lesion shows enhancement of only the deep component alongside the bone (arrows). This was targeted for biopsy, and on histology was a deposit of Hodgkin lymphoma
Contrast-enhanced ultrasound may also be used to guide decisions during the procedure, and this entails applying diagnostic principles of CEUS, particularly of liver lesions, described in Chap. 8 and in the prior section of this chapter. It is, therefore, important that the CEUS technique applied in interventional radiology be consistent with that used for diagnostic applications. As children who undergo interventional procedures have venous access established and are under sedation or anesthesia, diagnostic characterization of a lesion can be performed in a manner that is more controlled and convenient for the radiologist. One illustrative scenario is that a lesion may be demonstrated to be benign in the interventional suite, therefore obviating the need for biopsy and sedation (Fig. 18.7).
A 6-year-old male with history of neuroblastoma. (a) Grayscale US demonstrating multiple echogenic lesions (arrows), and the patient was referred for a biopsy. (b) The CEUS of the liver performed by the interventionist just prior to biopsy, which showed no enhancement of the lesion (arrows) in the arterial phase. (c) In the portal venous phase, the lesions (arrows) are isoenhancing with the remainder of the liver, consistent with focal fat infiltration and no biopsy was performed
18.6.2 Tumor Ablation
Tumor ablation involves the application of probes that induce thermal injury (radiofrequency ablation, microwave ablation, cryoablation, high-intensity focused ultrasound) or the injection of chemicals (ethanol ablation) in the tumor. For technical success, the ablation procedure must precisely treat the tumor and a surrounding margin of normal tissue; it is also important to avoid injury to surrounding organs. The most common application of CEUS in this realm is intra-procedural feedback: tumor vascularity is assessed before and after ablation. A lack of contrast enhancement provides strong confirmation of adequacy of treatment (Figs. 18.8 and 18.9), and residual perfusion of the tumor would indicate that further ablation is necessary. For this purpose, CEUS provides superior spatial and temporal resolution of tissue perfusion than CT or MR imaging, as perfusion can be visualized in real-time and with a small field-of-view. In adults undergoing ablation of hepatocellular carcinoma, the absence of perfusion after ablation has shown to be highly predictive of therapeutic success, and residual tumor detection has been shown to be at least commensurate with other imaging modalities [39]. It should be noted that the immediate post-ablation assessment of tissue perfusion is best suited for cryoablation, as collection of gas in the treatment area from tissue vaporization will hinder US assessment immediately after radiofrequency or microwave ablation [39]. In the latter settings, CEUS assessment may have to be performed shortly after the procedure. Selective trans-arterial embolization of tumors, most frequently in the liver, is an established procedure in interventional oncology, and specific subsets include “bland” particle embolization, chemoembolization, embolization with drug-eluting beads, and radioembolization with beta-emitting particles. Tumor embolization is much less frequently performed in children, largely due to the different spectrum of pediatric cancers and efficacy of conventional treatment, but is occasionally indicated. During trans-arterial embolization of liver tumors, intra-procedural CEUS with arterial administration of a UCA has been used to guide embolization (confirm appropriate sub-selectivity prior to embolization) and to determine adequacy of treatment, similar to tumor ablation [41].
An 8-year-old male with history of a right paraspinal rhabdomyosarcoma. (a) A lobulated metastatic recurrence (arrow) in the right posterior supraclavicular soft tissues on axial single T2-weighted MR image. (b) On the 18FDG PET, there is uptake in the mass (arrow). (c) On the corresponding transverse, arterial phase CEUS shows enhancement of the lesion (arrows). (d) The corresponding sagittal CEUS 3 months after percutaneous cryoablation of the metastatic lesion (arrows) show no residual uptake or contrast enhancement
A 16-year-old female with relapsed papillary cell thyroid cancer. (a) A lymph node with a metastatic deposit shown on the grayscale US (arrow). (b) The corresponding CEUS in arterial phase (arrow) showing arterial enhancement, with patchy areas of non-enhancement, early washout occurred later in the study. (c) Grayscale image during the US-guided ethanol ablation of the metastasis (arrow); needle shown with arrowheads. (d) The CEUS of the lesion (arrow) immediately after the procedure showing no residual enhancement, consistent with complete ablation. (Case courtesy of Dr. Fernando Escobar)
18.7 Future Directions of CEUS in Oncology
The role of CEUS in oncology is rapidly evolving and there are a wide variety of potential applications in the management of adult and pediatric oncology patients. Because pediatric malignancies are relatively rare, much of the clinical research in this area is occurring in the adult population. However, the principles can be easily applied to the pediatric population which is the ideal population for the use of ultrasound in general. Adult clinical investigators have reported the value of CEUS in distinguishing benign from malignant thyroid nodules, endometrial hyperplasia from neoplasms, benign from malignant soft tissue masses, low from high-grade bladder carcinoma, benign from malignant lymph nodes, benign prostatic hypertrophy from prostate carcinoma and to monitor response to therapy in breast cancer, liver metastases, and liver tumors treated with trans-arterial chemoembolization and radiofrequency ablation [42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64]. Clearly, there is considerable interest in the development of CEUS to diagnose malignancies and assess treatment response in the oncology population.
Angiogenesis (the development of new blood vessels) is essential for tumor development, growth, and metastasis and accurate imaging and quantitation of tumor vascularity is an important area of investigation [65, 66]. Contrast-enhanced ultrasound has unique attributes that make it more appealing for measuring tumor blood flow than other imaging modalities and it is emerging as a reliable method of quantitating tumor vascularity and assessing response of a variety of adult malignancies [67,68,69,70,71,72,73,74,75,76]. Because UCAs remain in the vascular space, the pharmacodynamics is less complex than those for CT and MR contrast agents that freely diffuse across the vascular membrane. Additionally, CEUS is less expensive than contrast-enhanced CT and MR imaging, can be performed at the bedside, does not require sedation, and, most importantly in the pediatric population, does not expose the patient to the harmful effects of ionizing radiation. With the use of contrast-specific software, several perfusion parameters can be quantitated. These include peak enhancement intensity, rise time, mean transit time, and area under the curve. These parameters can be quantitated at baseline (before initiation of therapy) and then remeasured at specific time points during therapy to assess change. The baseline values or the change between baseline and follow-up time points, may provide unique insight into the biological behavior of tumors that could ultimately be predictive of patient outcome.
The role of CEUS in oncology is also expanding beyond diagnosis and treatment response into molecular imaging and targeted therapy. Several methods of UCA-mediated drug delivery are under investigation in pre-clinical and clinical trials, including for direct and indirect drug delivery and development of nano-scaled UCAs. By applying an ultrasound pulse, a UCA can be destroyed to create micro-jets or be excited to physically interact with the vascular wall and create pores in the vascular membrane. This approach results in enhanced vessel permeability allowing co-administered drugs to extravasate into the tumor interstitial space (indirect drug delivery). Alternatively, the UCA shell itself can be loaded with a drug that is released during microbubble destruction and then extravasates, through US-mediated, permeabilization of the vascular membrane (direct drug delivery). However, with these techniques, it is difficult to achieve high enough doses of the therapeutic agent. Nanobubble, nanoparticle, and nanodroplet UCA are capable of passing through the damaged endothelium of tumor vessels and accumulate in the extracellular space without the need to enhance vessel permeability. Once in the extracellular space they can be manipulated to cause tissue cavitation and release drugs directly into the tumor. This approach could be especially advantageous in treating brain tumors because the nanoparticles could pass through the blood–brain barrier. Although some of these agents have a short shelf life and handling difficulties, they provide promising future clinical directions and exciting research opportunities [77].
18.8 Conclusions
Contrast-enhanced ultrasound is especially well suited for pediatric use because the contrast agents are safe in children, do not require prior laboratory testing or sedation, the equipment is accessible, and, importantly, does not expose the patient to the harmful effects of ionizing radiation. The latter benefit is particularly relevant to the pediatric cancer population because these children undergo innumerable imaging examinations during diagnosis and staging, throughout treatment, and during surveillance after the completion of therapy. We have presented information to promote the use of CEUS in pediatric oncology patients and have described current clinical applications including distinguishing benign from malignant liver and renal masses, guidance of interventional procedures, and the assessment of tumor ablation. There is ongoing research investigating the value of CEUS to quantitatively assess the effect of cancer therapy and as a therapeutic tool in oncology. These developments will likely significantly expand the role and increase the impact of CEUS in the management of pediatric oncology patients in the near future.
References
Berrington de Gonzalez A, Salotti JA, McHugh K, Little MP, Harbron RW, Lee C, et al. Relationship between pediatric CT scans and subsequent risk of leukemia and brain tumors: assessment of the impact of underlying conditions. Br J Cancer. 2016;114(4):388–94.
Ruggiero A, Ferrara P, Attina G, Rizzo D, Riccardi R. Renal toxicity and chemotherapy in children with cancer. Br J Clin Pharmacol. 2017;83(12):2605–14.
Latham GJ, Greenberg RS. Anesthetic considerations for the pediatric oncology patient—part 2: systems-based approach to anesthesia. Paediatr Anaesth. 2010;20(5):396–420.
Jacob J, Deganello A, Sellars ME, Hadzic N, Sidhu PS. Contrast enhanced ultrasound (CEUS) characterization of grey-scale sonographic indeterminate focal liver lesions in pediatric practice. Ultraschall Med (Stuttgart, Germany: 1980). 2013;34(6):529–40.
Anupindi SA, Biko DM, Ntoulia A, Poznick L, Morgan TA, Darge K, et al. Contrast-enhanced US assessment of focal liver lesions in children. Radiographics. 2017;37(6):1632–47.
Sidhu PS, Cantisani V, Deganello A, Dietrich CF, Duran C, Franke D, et al. Role of contrast-enhanced ultrasound (CEUS) in pediatric practice: an EFSUMB position statement. Ultraschall Med (Stuttgart, Germany: 1980). 2017;38(1):33–43.
Czauderna P, Lopez-Terrada D, Hiyama E, Haberle B, Malogolowkin MH, Meyers RL. Hepatoblastoma state of the art: pathology, genetics, risk stratification, and chemotherapy. Curr Opin Paediatr. 2014;26(1):19–28.
Shuman C, Beckwith JB, Weksberg R. Beckwith-Wiedemann syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, et al., editors. GeneReviews®. Seattle, WA: University of Washington; 1993.
Spector LG, Birch J. The epidemiology of hepatoblastoma. Paediatr Blood Cancer. 2012;59(5):776–9.
Trobaugh-Lotrario AD, Lopez-Terrada D, Li P, Feusner JH. Hepatoblastoma in patients with molecularly proven familial adenomatous polyposis: clinical characteristics and rationale for surveillance screening. Paediatr Blood Cancer. 2018;65(8):e27103.
Sharma D, Subbarao G, Saxena R. Hepatoblastoma. Semin Diagn Pathol. 2017;34(2):192–200.
Roebuck DJ, Aronson D, Clapuyt P, Czauderna P, de Ville de Goyet J, Gauthier F, et al. 2005 PRETEXT: a revised staging system for primary malignant liver tumors of childhood developed by the SIOPEL group. Paediatr Radiol. 2007;37(2):123–32; quiz 249–50.
Towbin AJ, Meyers RL, Woodley H, Miyazaki O, Weldon CB, Morland B, et al. 2017 PRETEXT: radiologic staging system for primary hepatic malignancies of childhood revised for the pediatric hepatic international tumor trial (PHITT). Paediatr Radiol. 2018;48(4):536–54.
McCarville MB. Contrast-enhanced sonography in pediatrics. Paediatr Radiol. 2011;41(Suppl 1):S238–42.
Chung EM, Lattin GE Jr, Cube R, Lewis RB, Marichal-Hernandez C, Shawhan R, et al. From the archives of the AFIP: Pediatric liver masses: radiologic-pathologic correlation. Part 2. Malignant tumors. Radiographics. 2011;31(2):483–507.
Czauderna P, Mackinlay G, Perilongo G, Brown J, Shafford E, Aronson D, et al. Hepatocellular carcinoma in children: results of the first prospective study of the International Society of Pediatric Oncology group. J Clin Oncol. 2002;20(12):2798–804.
Walther A, Tiao G. Approach to pediatric hepatocellular carcinoma. Clin Liver Dis. 2013;2(5):219–22.
Lau CS, Mahendraraj K, Chamberlain RS. Hepatocellular carcinoma in the pediatric population: a population based clinical outcomes study involving 257 patients from the surveillance, epidemiology, and end result (SEER) database (1973–2011). HPB Surg. 2015;2015:670728.
Elshamy M, Aucejo F, Menon KV, Eghtesad B. Hepatocellular carcinoma beyond Milan criteria: management and transplant selection criteria. World J Hepatol. 2016;8(21):874–80.
Lopez-Terrada D, Alaggio R, de Davila MT, Czauderna P, Hiyama E, Katzenstein H, et al. Towards an international pediatric liver tumor consensus classification: proceedings of the Los Angeles COG liver tumors symposium. Mod Pathol. 2014;27(3):472–91.
Weeda VB, Murawski M, McCabe AJ, Maibach R, Brugieres L, Roebuck D, et al. Fibrolamellar variant of hepatocellular carcinoma does not have a better survival than conventional hepatocellular carcinoma—results and treatment recommendations from the Childhood Liver Tumor Strategy Group (SIOPEL) experience. Eur J Cancer (Oxford, England: 1990). 2013;49(12):2698–704.
Prokurat A, Kluge P, Kosciesza A, Perek D, Kappeler A, Zimmermann A. Transitional liver cell tumors (TLCT) in older children and adolescents: a novel group of aggressive hepatic tumors expressing beta-catenin. Med Paediatr Oncol. 2002;39(5):510–8.
Zhou S, Venkatramani R, Gupta S, Wang K, Stein JE, Wang L, et al. Hepatocellular malignant neoplasm, NOS: a clinicopathological study of 11 cases from a single institution. Histopathology. 2017;71(5):813–22.
Burrowes DP, Medellin A, Harris AC, Milot L, Wilson SR. Contrast-enhanced US approach to the diagnosis of focal liver masses. Radiographics. 2017;37(5):1388–400.
Smith EA, Salisbury S, Martin R, Towbin AJ. Incidence and etiology of new liver lesions in pediatric patients previously treated for malignancy. AJR Am J Roentgenol. 2012;199(1):186–91.
Chung EM, Cube R, Lewis RB, Conran RM. From the archives of the AFIP: Pediatric liver masses: radiologic-pathologic correlation part 1. Benign tumors. Radiographics. 2010;30(3):801–26.
Merrow AC, Gupta A, Patel MN, Adams DM. 2014 Revised classification of vascular lesions from the International Society for the study of vascular anomalies: radiologic-pathologic update. Radiographics. 2016;36(5):1494–516.
McLean CK, Squires JH, Reyes-Mugica M, McCormick A, Mahmood B. Hepatic vascular tumors in the neonate: angiosarcoma. J Paediatr. 2018;193:245–248.e1.
Stenzel M. Intravenous contrast-enhanced sonography in children and adolescents—a single center experience. J Ultrasonogr. 2013;13(53):133–44.
Christison-Lagay ER, Burrows PE, Alomari A, Dubois J, Kozakewich HP, Lane TS, et al. Hepatic hemangiomas: subtype classification and development of a clinical practice algorithm and registry. J Paediatr Surg. 2007;42(1):62–7; discussion 7–8.
Rumack CLD. Diagnostic ultrasound. Philadelphia, PA: Elsevier Health Sciences; 2017.
Masetti R, Colecchia A, Rondelli R, Martoni A, Vendemini F, Biagi C, et al. Benign hepatic nodular lesions after treatment for childhood cancer. J Paediatr Gastroenterol Nutr. 2013;56(2):151–5.
Dietrich CF, Schuessler G, Trojan J, Fellbaum C, Ignee A. Differentiation of focal nodular hyperplasia and hepatocellular adenoma by contrast-enhanced ultrasound. Br J Radiol. 2005;78(932):704–7.
Strobel D, Seitz K, Blank W, Schuler A, Dietrich CF, von Herbay A, et al. Tumor-specific vascularization pattern of liver metastasis, hepatocellular carcinoma, hemangioma and focal nodular hyperplasia in the differential diagnosis of 1,349 liver lesions in contrast-enhanced ultrasound (CEUS). Ultraschall Med (Stuttgart, Germany: 1980). 2009;30(4):376–82.
Ungermann L, Elias P, Zizka J, Ryska P, Klzo L. Focal nodular hyperplasia: spoke-wheel arterial pattern and other signs on dynamic contrast-enhanced ultrasonography. Eur J Radiol. 2007;63(2):290–4.
Kim TK, Jang HJ, Burns PN, Murphy-Lavallee J, Wilson SR. Focal nodular hyperplasia and hepatic adenoma: differentiation with low-mechanical-index contrast-enhanced sonography. AJR Am J Roentgenol. 2008;190(1):58–66.
Kapur JOH. Contrast-enhanced ultrasound of kidneys in children with renal failure. J Med Ultrasound. 2015;23(2):86–97.
Sparchez Z, Radu P, Sparchez M, Crisan N, Kacso G, Petrut B. Contrast enhanced ultrasound of renal masses. A reappraisal of EFSUMB recommendations and possible emerging applications. Med Ultrason. 2015;17(2):219–26.
Nolsoe CP, Nolsoe AB, Klubien J, Pommergaard HC, Rosenberg J, Meloni MF, et al. Use of ultrasound contrast agents in relation to percutaneous interventional procedures: a systematic review and pictorial essay. J Ultrasound Med. 2018;37(6):1305–24.
Huang DY, Yusuf GT, Daneshi M, Ramnarine R, Deganello A, Sellars ME, et al. Contrast-enhanced ultrasound (CEUS) in abdominal intervention. Abdom Radiol (New York). 2018;43(4):960–76.
Lekht I, Nayyar M, Luu B, Guichet PL, Ho J, Ter-Oganesyan R, et al. Intra-arterial contrast-enhanced ultrasound (IA CEUS) for localization of hepatocellular carcinoma (HCC) supply during transarterial chemoembolization (TACE): a case series. Abdom Radiol (New York). 2017;42(5):1400–7.
D’Onofrio M, Crosara S, De Robertis R, Canestrini S, Cantisani V, Morana G, et al. Malignant focal liver lesions at contrast-enhanced ultrasonography and magnetic resonance with hepatospecific contrast agent. Ultrasound (Leeds, England). 2014;22(2):91–8.
Quaia E, De Paoli L, Angileri R, Cabibbo B, Cova MA. Indeterminate solid hepatic lesions identified on non-diagnostic contrast-enhanced computed tomography: assessment of the additional diagnostic value of contrast-enhanced ultrasound in the non-cirrhotic liver. Eur J Radiol. 2014;83(3):456–62.
Trillaud H, Bruel JM, Valette PJ, Vilgrain V, Schmutz G, Oyen R, et al. Characterization of focal liver lesions with SonoVue-enhanced sonography: international multicenter-study in comparison to CT and MRI. World J Gastroenterol. 2009;15(30):3748–56.
Zhang Y, Luo YK, Zhang MB, Li J, Li J, Tang J. Diagnostic accuracy of contrast-enhanced ultrasound enhancement patterns for thyroid nodules. Med Sci Monit. 2016;22:4755–64.
Defortescu G, Cornu JN, Bejar S, Giwerc A, Gobet F, Werquin C, et al. Diagnostic performance of contrast-enhanced ultrasonography and magnetic resonance imaging for the assessment of complex renal cysts: a prospective study. Int J Urol. 2017;24(3):184–9.
Wei SP, Xu CL, Zhang Q, Zhang QR, Zhao YE, Huang PF, et al. Contrast-enhanced ultrasound for differentiating benign from malignant solid small renal masses: comparison with contrast-enhanced CT. Abdom Radiol (New York). 2017;42(8):2135–45.
Sanz E, Hevia V, Gomez V, Alvarez S, Fabuel JJ, Martinez L, et al. Renal complex cystic masses: usefulness of contrast-enhanced ultrasound (CEUS) in their assessment and its agreement with computed tomography. Curr Urol Rep. 2016;17(12):89.
Barr RG, Peterson C, Hindi A. Evaluation of indeterminate renal masses with contrast-enhanced US: a diagnostic performance study. Radiology. 2014;271(1):133–42.
Edenberg J, Gloersen K, Osman HA, Dimmen M, Berg GV. The role of contrast-enhanced ultrasound in the classification of CT-indeterminate renal lesions. Scand J Urol. 2016;50(6):445–51.
Putz FJ, Erlmeier A, Wiesinger I, Verloh N, Stroszczynski C, Banas B, et al. Contrast-enhanced ultrasound (CEUS) in renal imaging at an interdisciplinary ultrasound Centre: possibilities of dynamic microvascularisation and perfusion. Clin Hemorheol Microcirc. 2017;66(4):293–302.
Liu Y, Xu Y, Cheng W, Liu X. Quantitative contrast-enhanced ultrasonography for the differential diagnosis of endometrial hyperplasia and endometrial neoplasms. Oncol Lett. 2016;12(5):3763–70.
Gruber L, Loizides A, Luger AK, Glodny B, Moser P, Henninger B, et al. Soft-tissue tumor contrast enhancement patterns: diagnostic value and comparison between ultrasound and MRI. AJR Am J Roentgenol. 2017;208(2):393–401.
Guo S, Xu P, Zhou A, Wang G, Chen W, Mei J, et al. Contrast-enhanced ultrasound differentiation between low- and high-grade bladder urothelial carcinoma and correlation with tumor microvessel density. J Ultrasound Med. 2017;36(11):2287–97.
Cantisani V, Bertolotto M, Weskott HP, Romanini L, Grazhdani H, Passamonti M, et al. Growing indications for CEUS: the kidney, testis, lymph nodes, thyroid, prostate, and small bowel. Eur J Radiol. 2015;84(9):1675–84.
Knieling F, Strobel D, Rompel O, Zapke M, Menendez-Castro C, Wolfel M, et al. Spectrum, applicability and diagnostic capacity of contrast-enhanced ultrasound in pediatric patients and young adults after intravenous application—a retrospective trial. Ultraschall Med (Stuttgart, Germany: 1980). 2016;37(6):619–26.
Seitz K, Strobel D. A milestone: approval of CEUS for diagnostic liver imaging in adults and children in the USA. Ultraschall Med (Stuttgart, Germany: 1980). 2016;37(3):229–32.
Seitz K, Strobel D, Bernatik T, Blank W, Friedrich-Rust M, Herbay A, et al. Contrast-enhanced ultrasound (CEUS) for the characterization of focal liver lesions—prospective comparison in clinical practice: CEUS vs. CT (DEGUM multicenter trial). Parts of this manuscript were presented at the Ultrasound Dreilandertreffen 2008, Davos. Ultraschall Med (Stuttgart, Germany: 1980). 2009;30(4):383–9.
Mori N, Mugikura S, Takahashi S, Ito K, Takasawa C, Li L, et al. Quantitative analysis of contrast-enhanced ultrasound imaging in invasive breast Cancer: a novel technique to obtain histopathologic information of microvessel density. Ultrasound Med Biol. 2017;43(3):607–14.
Tai CJ, Huang MT, Wu CH, Tai CJ, Shi YC, Chang CC, et al. Contrast-enhanced ultrasound and computed tomography assessment of hepatocellular carcinoma after transcatheter arterial chemo-embolization: a systematic review. J Gastroint Liver Dis. 2016;25(4):499–507.
Ishii T, Numata K, Hao Y, Doba N, Hara K, Kondo M, et al. Evaluation of hepatocellular carcinoma tumor vascularity using contrast-enhanced ultrasonography as a predictor for local recurrence following radiofrequency ablation. Eur J Radiol. 2017;89:234–41.
Bartolotta TV, Taibbi A, Picone D, Anastasi A, Midiri M, Lagalla R. Detection of liver metastases in cancer patients with geographic fatty infiltration of the liver: the added value of contrast-enhanced sonography. Ultrasonography (Seoul, Korea). 2017;36(2):160–9.
Pandey P, Lewis H, Pandey A, Schmidt C, Dillhoff M, Kamel IR, et al. Updates in hepatic oncology imaging. Surg Oncol. 2017;26(2):195–206.
Granata V, Fusco R, Catalano O, Avallone A, Palaia R, Botti G, et al. Diagnostic accuracy of magnetic resonance, computed tomography and contrast enhanced ultrasound in radiological multimodality assessment of peribiliary liver metastases. PLoS One. 2017;12(6):e0179951.
Maj E, Papiernik D, Wietrzyk J. Antiangiogenic cancer treatment: the great discovery and greater complexity (review). Int J Oncol. 2016;49(5):1773–84.
Hendry SA, Farnsworth RH, Solomon B, Achen MG, Stacker SA, Fox SB. The role of the tumor vasculature in the host immune response: implications for therapeutic strategies targeting the tumor microenvironment. Front Immunol. 2016;7:621.
Lassau N, Bonastre J, Kind M, Vilgrain V, Lacroix J, Cuinet M, et al. Validation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: the French multicenter support for innovative and expensive techniques study. Investig Radiol. 2014;49(12):794–800.
Atri M, Hudson JM, Sinaei M, Williams R, Milot L, Moshonov H, et al. Impact of acquisition method and region of interest placement on inter-observer agreement and measurement of tumor response to targeted therapy using dynamic contrast-enhanced ultrasound. Ultrasound Med Biol. 2016;42(3):763–8.
Amioka A, Masumoto N, Gouda N, Kajitani K, Shigematsu H, Emi A, et al. Ability of contrast-enhanced ultrasonography to determine clinical responses of breast cancer to neoadjuvant chemotherapy. Jpn J Clin Oncol. 2016;46(4):303–9.
Saracco A, Szabo BK, Tanczos E, Bergh J, Hatschek T. Contrast-enhanced ultrasound (CEUS) in assessing early response among patients with invasive breast cancer undergoing neoadjuvant chemotherapy. Acta Radiol (Stockholm, Sweden: 1987). 2017;58(4):394–402.
Matsui S, Kudo M, Kitano M, Asakuma Y. Evaluation of the response to chemotherapy in advanced gastric cancer by contrast-enhanced harmonic EUS. Hepato-Gastroenterology. 2015;62(139):595–8.
Peng C, Liu LZ, Zheng W, Xie YJ, Xiong YH, Li AH, et al. Can quantitative contrast-enhanced ultrasonography predict cervical tumor response to neoadjuvant chemotherapy? Eur J Radiol. 2016;85(11):2111–8.
Jia WR, Tang L, Wang DB, Chai WM, Fei XC, He JR, et al. Three-dimensional contrast-enhanced ultrasound in response assessment for breast Cancer: a comparison with dynamic contrast-enhanced magnetic resonance imaging and pathology. Sci Rep. 2016;6:33832.
Ohno N, Miyati T, Yamashita M, Narikawa M. Quantitative assessment of tissue perfusion in hepatocellular carcinoma using perflubutane dynamic contrast-enhanced ultrasonography: a preliminary study. Diagnostics (Basel, Switzerland). 2015;5(2):210–8.
Mogensen MB, Hansen ML, Henriksen BM, Axelsen T, Vainer B, Osterlind K, et al. Dynamic contrast-enhanced ultrasound of colorectal liver metastases as an imaging modality for early response prediction to chemotherapy. Diagnostics (Basel, Switzerland). 2017;7(2):35.
Wu Z, Yang X, Chen L, Wang Z, Shi Y, Mao H, et al. Anti-angiogenic therapy with contrast-enhanced ultrasound in colorectal cancer patients with liver metastasis. Medicine. 2017;96(20):e6731.
Guvener N, Appold L, de Lorenzi F, Golombek SK, Rizzo LY, Lammers T, et al. Recent advances in ultrasound-based diagnosis and therapy with micro- and nanometer-sized formulations. Methods (San Diego, CA). 2017;130:4–13.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Squires, J., Srinivasan, A., McCarville, M.B. (2021). Contrast-Enhanced Ultrasound in Childhood Oncology. In: Sidhu, P., Sellars, M., Deganello, A. (eds) Contrast-Enhanced Ultrasound in Pediatric Imaging. Springer, Cham. https://doi.org/10.1007/978-3-030-49691-3_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-49691-3_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-49690-6
Online ISBN: 978-3-030-49691-3
eBook Packages: MedicineMedicine (R0)