Abstract
RV dysfunction associated with PH in the context of cardiac surgery is still a condition associated with significant morbidity and mortality. Multiple monitoring modalities including hemodynamic, cardiac and extra-cardiac ultrasound parameters help the clinician understands the importance of RV dysfunction and PH after CPB. In regard to this condition and its related burden of outcomes, the priorities of the clinician should be placed on early diagnosis, prevention and rapid institution of treatment to reverse the condition. An optimal approach to favour patient’s outcome would thus be one that includes different monitoring modalities and pharmacologic agents.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pulmonary hypertension
- Right ventricular dysfunction
- Cardiopulmonary bypass
- Preoperative pulmonary hypertension
- Right ventricular failure
- Transthoracic echocardiogram
- Intravenous vasodilators
- Inhalation therapy
-
1.
Right ventricular (RV) dysfunction after cardiopulmonary bypass (CPB) is a major complication in cardiac surgery with a mortality rate from 22 to 37%.
-
2.
Six most important causes of PH in cardiac surgery are: (1) left ventricular (LV) dysfunction, (2) lung injury during CPB, (3) protamine administration, (4) aortic or mitral patient prosthesis mismatch, (5) hypoxia, hypercapnia or pulmonary embolism, and (6) pulmonary disease.
-
3.
Echocardiography has a central role in the diagnosis of RVF, but it is important for the clinician to assess the hemodynamic impact of this finding to determine the best course of action.
-
4.
In patients with PH, it has been shown that MAP/MPAP ratio is not influenced by loading conditions and is an independent predictor of hemodynamic complication after cardiac surgery.
-
5.
Pulmonary vasodilators and Phosphodiesterase enzyme (PDE) type 3 inhibitors were strongly recommended to reduce pulmonary pressures and pulmonary vascular resistance (PVR) and improve RV performance.
-
6.
Inhaled therapies commonly used to treat PH in the cardiac surgical setting include inhaled nitric oxide, inhaled prostacyclin (iPGI2), inhaled iloprost, and inhaled PDE inhibitors such as milrinone.
Right ventricular (RV) dysfunction occurring just after cardiopulmonary bypass (CPB) is a major complication in cardiac surgery with a mortality rate from 22 to 37% [1, 2]. One of the major risk factors is preoperative pulmonary hypertension (PH) [3]. In the following chapter, we will define right ventricular failure (RVF) and review the critical role of cardiac and extracardiac ultrasound for its diagnosis and its consequences on extra-cardiac function. We will conclude by briefly describing our approach in managing this condition.
Prevalence and Outcome of Pulmonary Hypertension and Right Ventricular Dysfunction Post-Cardiopulmonary Bypass
Pulmonary hypertension is a hemodynamic problem that can result in RVF. It has a complex pathophysiology and is associated with increased morbidity and mortality. Although the true prevalence of PH in the general population is unknown, the prevalence of pulmonary arterial hypertension (PAH) (group 1 PH) has been estimated to range from five to 15 cases per million and is now believed to affect all age groups and both genders [4].
In the context of cardiac surgery, PH is frequently classified as pre-capillary or post-capillary. Pre-capillary PH is marked by changes limited to the arterial side of the pulmonary circulation, while post-capillary PH reflects changes within the pulmonary venous circulation, between the capillary bed and the left atrium. Since the most common form of PH encountered in the cardiac surgery patient is PH associated with left heart disease [5]; in this context, PH is typically post-capillary.
The PH in cardiac surgery has a complex etiology and may involve several mechanisms acting alone or in combination. We previously identified the six most important causes of PH in cardiac surgery, which may exist before the operation, or appear during or after the procedure: (1) left heart disease or left ventricular (LV) dysfunction; (2) lung injury during CPB; (3) protamine administration; (4) aortic or mitral patient prosthesis mismatch; (5) hypoxia, hypercapnia or pulmonary embolism; and (6) pulmonary disease [3].
Cardiac surgical patients with PH carry a higher risk for surgery than those without PH. Complications such as pneumonia, prolonged mechanical ventilation, renal failure, cardiac arrest, and multiple organ system failure occur more frequently with increasing mean pulmonary artery pressure (MPAP) [6]. In patients with severe PH, the incidence of major postoperative complications was previously reported at 32% [6].
Survival in the postoperative period is determined by the ability of the right heart to deal with the increased pulmonary pressure. Information on pulmonary hemodynamics and RV function is therefore essential to adapt the best approach for management. For this reason, standard hemodynamic monitoring techniques used during anesthesia and in the intensive care unit (ICU) are not sufficient for this high-risk cardiac population. Appropriate perioperative monitoring including, but not limited to, right heart catheterization, transesophageal echocardiography (TEE), and cerebral near-infrared spectroscopy (NIRS) are critical to provide guidance in the management of hemodynamic instability and RVF.
Definition of Right Ventricular Failure After Cardiopulmonary Bypass
There is no specific and uniform definition of RVF in the context of cardiac surgery. In two randomised trials in which prevention of RVF was the primary outcome, different definitions were used (Table 22.1) [1, 2]. Therefore in order to define RVF, hemodynamic, echocardiographic and pharmacologic elements must be present. For instance, similar echocardiographic features can be seen in RV dysfunction and RVF. However, the latter will be associated with reduced oxygen transport and typically reduced brain and systemic NIRS signals.
Cardiac Manifestations: Echocardiographic Evaluation
Guidelines unrelated to cardiac surgery addressing qualitative and quantitative transthoracic echocardiographic (TTE) evaluation and the abnormal RV function criteria in adults are available [7]. Transesophageal echocardiography RV views are similar to those obtained using TTE and a proposition of echocardiographic RV dysfunction for cardiac surgery was published in 2009 [8, 9]. Proposed echocardiographic RV dysfunction criteria are: (A) the presence of RV dilatation >2/3 of the LV in its transversal diameter; (B) RV fractional area change (RVFAC) <25% or ≥20% reduction compared to the pre-CPB evaluation; (C) a tricuspid annular plane systolic excursion (TAPSE) ≤16 mm, and (D) a systolic speed of the tissue Doppler tricuspid ring (S′) < 10 cm/s [8, 9]. The TEE and TTE RV evaluation is often challenging due to its complex crescent shape geometry that renders imaging of the inflow and outflow in the same two-dimensional (2D) plane difficult. Inward mechanical contraction of the RV is governed by superficial circumferential muscle fibers shortening whereas base-to-apex contraction results from inner longitudinal fibers. In comparison to LV, the base-to-apex shortening assumes a greater role in RV emptying. Views specific to RV should be used to evaluate its dimension and function with a qualitative and quantitative approach [8]. RV function can be evaluated through 2D volumetric and non-volumetric parameters, strain analysis and three-dimensional (3D) parameters.
Echocardiography is useful to characterize the consequences of PH on RV size and function. Specific conditions associated with PH and consequently RV dysfunction can be diagnosed with this imaging modality. Right ventricular hypertrophy is present if RV free wall exceeds 5 mm. RV remodeling and dilatation will increase the RV sphericity index defined by the ratio of RV end-diastolic mid-papillary diameter on RV end-diastolic longitudinal diameter.
2D Imaging Systolic Function Assessment
Right Ventricle Volumetric Assessment
Qualitative evaluation and estimation of the RV size is made by comparison with the LV. Normal RV is less than two-thirds of the LV; mildly enlarged RV is more than two-thirds but inferior to the LV; moderately enlarged RV is roughly equal to the LV size; and severely enlarged RV is superior to the LV [7]. The RV dysfunction is suspected when RV dilatation from volume overload produce septal flattening in diastole and when RV pressure overload produce septal flattening in both diastole and end-systole. Right ventricular diameter >42 mm at the base and >35 mm at the midlevel is diagnostic of RV dilatation [7]. Volumetric evaluation can be done with RVFAC defined as (end-diastolic area – end-systolic area)/end-diastolic area × 100. Diagnosis of RV dysfunction is made by RVFAC <35% and its severity can be described as mild, moderate, or severe for values of 25% to 35%, 18% to 25%, and ≤18%, respectively. However, RVFAC does not consider the RV outflow tract volume that corresponds to approximately 20% of the RV volume.
Right Ventricle Non-Volumetric Assessment
Global assessment of RV function can be done by RV myocardial performance index (RVMPI) using pulsed waved Doppler (PWD) or tissue Doppler imaging (TDI) at the lateral tricuspid annulus. It represents an estimate of both RV systolic and diastolic function. It is based on the relationship between ejection and non-ejection work of the heart. The RVMPI is defined as the ratio of isovolumetric time divided by ejection time (ET), or [(isovolumetric relaxation time (IVRT) + isovolumetric contraction time (IVCT))/(ET)]. Presence of RV dysfunction is characterized by a RVMPI >0.43 in PWD and >0.54 in TDI [10]. In valvular surgery, RVMPI is an independent predictor of difficult CPB weaning, mortality, circulatory failure, duration of hospitalization and ICU stay [11]. This parameter is load dependent and unreliable in situations where right atrial pressure is elevated and RR intervals are irregular such as atrial fibrillation because of reduced IVCT.
Regional assessment of RV function corresponds to TDI derived S′, RV acceleration during isovolumic contraction (RVIVA) and TAPSE. Interrogation of S′ by TDI <9.5 cm/s is a sign of RV dysfunction as it represents the basal RV free wall function. The RVIVA is also measured by TDI at the lateral tricuspid annulus. Right ventricular acceleration during isovolumic contraction is defined as the peak isovolumic myocardial velocity divided by time to peak velocity. This parameter is rate dependent and appears to be less load-dependent than RVMPI. Value <2.2 m/s2 is considered to be related to RV dysfunction. The RV function is commonly assessed by TAPSE as it represents an easily recognizable longitudinal movement on echocardiography. Typically measured in M-Mode and corrected for angulation of interrogation, TAPSE is defined as the total excursion of the tricuspid annulus from end-diastole to end-systole. Tricuspid annular plane systolic excursion <17 mm is suggestive of RV dysfunction. It has been correlated to RV ejection fraction (RVEF). However, it is important to note that TAPSE is angle and load dependent. Also, it reflects the longitudinal displacement of only a single segment of the complex RV 3D structure.
2D Imaging Diastolic Function Assessment
Presence of tricuspid regurgitation or irregular RR intervals renders the analysis of diastolic dysfunction difficult immediately after CPB weaning. However, in the absence of these conditions, gradation of diastolic RV function can be achieved through trans-tricuspid flow (TTF) and hepatic venous flow. Early and late filling waves velocities (E and A wave, respectively) and E deceleration time recorded by pulsed wave Doppler in the TTF and the lateral tricuspid annulus velocity during early filling (E′) recorded by TDI allows diastolic categorization of RV function. A tricuspid E/A ratio < 0.8 suggests impaired relaxation, a tricuspid E/A ratio of 0.8 to 2.1 with an E/E′ ratio > 6 or diastolic flow predominance in the hepatic veins suggests pseudonormal filling, and a tricuspid E/A ratio > 2.1 with a deceleration time < 120 ms suggests restrictive filling.
Strain Imaging
Right ventricular strain measurements are highly feasible and use an absolute cut-off value of > − 20% (or absolute value <20%) for both RV global longitudinal strain (RVGLS) and RV free wall strain (RVFWS) [12]. The RV strain is worsened after CBP but the clinical significance of that finding is unknown [13]. Preoperative TTE evaluation of the 2D RV longitudinal strain is a better predictor of mortality after cardiac surgery than RVFAC. Abnormal RVFAC (<35%) is associated to the greatest risk of postoperative mortality, probably because abnormal RVFAC reflects a severe and advanced RV dysfunction with both radial and longitudinal RV dysfunction. In patients with preserved RVFAC, RV speckle tracking appears as a sensitive method to identify early RV dysfunction [14]. RV strain imaging can represent an important prognostic value in the PH patient [15] and is associated with mortality after transcatheter aortic valve replacement [16]. Finally, both regional and global RV strain measurements are feasible with TEE during cardiac surgery [17].
3D Imaging
The use of 3D echocardiography to evaluate RV end-diastolic volume by identification of the minimal RV volume frame and end-systolic volume by maximal RV volume frame allows the determination of RVEF. Three-dimensional RVEF <45% is highly suggestive of RV dysfunction. End-diastolic volumes >87 mL/m2 for men and > 74 mL/m2 for women are indicative of RV enlargement. Intraoperative RVEF assessment with 3D TEE seems feasible and reproducible in patients with normal RV function and in patients with dilated RV without being excessively time consuming [18].
Echocardiographic Evaluation of Pulmonary Artery Pressure
Pulmonary artery pressure (PAP) can be estimated with the use of a Bernoulli equation when a pulmonary artery catheter is not used. The presence of tricuspid regurgitation makes it possible to estimate systolic pulmonary artery pressure (SPAP) using peak tricuspid regurgitation velocity. Diastolic pulmonary artery pressure (DPAP) can be estimated from the velocity at the end of the pulmonary regurgitation, and MPAP from the maximal velocity of the pulmonary regurgitation. Right atrial pressure must be added to these two previous measurements to obtain an adequate estimate. The MPAP can be estimated by the pulmonary artery acceleration time (AT) (MPAP = 79 − (0.45 × AT) and MPAP = 90 − (0.62 × AT) if AT <120 ms) or derived from the systolic and diastolic pressures (MPAP = (1/3(SPAP) + 2/3(DPAP)).
Extra-Cardiac Manifestations
Hemodynamic Impact of Right Ventricular Dysfunction
The progressive increase in central venous pressure (CVP) in patients with right heart failure has a detrimental impact on organ function. The cardio-renal syndrome, the cardio-intestinal syndrome and the cardio-hepatic syndrome have all been attributed to an inadequate tissue delivery of oxygen and nutriments stemming from a combination of decreased cardiac output and increased venous pressures [19,20,21,22,23]. While autonomic and hormonal autoregulation is able to compensate for a reduced cardiac index to maintain adequate blood flow until it falls below a critical threshold, the presence of elevated venous pressure creates a synergy where interstitial edema and a reduced arterio-venous gradient is providing the “second-hit” resulting in organ dysfunction. Venous congestion appears to be one of the most important factors leading to adverse outcomes in patients with heart failure.
While echocardiography has a central role in the diagnosis of RVF, it is important for the clinician to assess the hemodynamic impact of this finding to determine the best course of action. While a critical decrease in cardiac output resulting in hypotension is immediately clinically evident from the use of traditional monitoring techniques, the impact of venous congestion on organ function is very covert in its presentation. Novel tools are being investigated to provide insight about the hemodynamic impact of RVF.
Venous Pressures Monitoring
Absolute CVP measurements are routinely performed in the perioperative period. The elevation of CVP is associated with an increase in renal dysfunction in broad populations of patients with cardiovascular diseases and is associated with adverse outcomes in critically ill patients [24]. In the setting of cardiac surgery, absolute CVP measurements, six hours after surgery is associated with mortality and renal failure [25]. However, despite multiple sources reporting the association between CVP and outcomes, an absolute cut-off or “target value” to prevent complications has not been determined. In a study by Williams et al. in a large cohort of coronary artery bypass graft surgery patients, the elevation of risk with CVP was present even for values under 9 mmHg [25]. Consequently, other indices should be sought in order to better risk stratify patients. Perfusion pressure (mean arterial pressure (MAP)-CVP, or diastolic arterial pressure (DAP)-CVP) as a surrogate for the arterio-venous pressure gradients in end organs is also associated with renal failure in cardiac surgery patients, and may be a better predictor than absolute CVP values [26].
Additional pressure monitoring to be considered includes the appearance of the CVP and the RV pressure waveform during the cardiac cycle. This information is often readily available and could be used to detect RV dysfunction at the bedside. In the patient with a normal diastolic RV function, pressure inside the RV will remain low during the ventricular systole manifesting as a plateau on RV pressure monitoring (Fig. 22.1a). With increasing diastolic RV dysfunction, ventricular compliance during diastolic decrease and the waveform will have an oblique appearance (Fig. 22.1f) and, in very severe cases, a square-root pattern (Fig. 22.1k). During RV systole, the tricuspid annulus moves downward (TAPSE) which is partially responsible for the decrease in right atrial pressure indicated by the X descent (Fig. 22.1b). With systolic RV dysfunction, the reduction in the TAPSE leads to the disappearance of the X descent (Fig. 22.1g and l) and a prominent Y descent during diastole. Recognition of these patterns could be used by the astute clinician as possible warning signs of RVF in an unstable patient.
Features of acute right ventricular (RV) failure on (1) RV pressure monitoring (2) right atrial pressure monitoring (3) hepatic vein (HV) and portal vein (PV) transthoracic Doppler ultrasound and (4) renal arterio-venous Doppler ultrasound. Patterns in patients with normal RV function (a, b, c, d, e) and typical patterns commonly observed in patients with mild (f, g, h, i, j) and severe (k, l, m, n, o) RV dysfunction. Abbreviations: AR, atrial reversal; D, diastole; IVC, inferior vena cava; Ppa, pulmonary artery pressure; Prv, right ventricular pressure, S, systole. The numbers on the images correspond to the location of the images: 1, right ventricle; 2, right atrium; 3, liver; 3H, hepatic venous flow; 3P, portal venous flow; 4, renal venous flow. (Adapted from Amsalem et al. [27] and Beaubien-Souligny et al. [28])
Continuous RV pressure monitoring makes it possible to assess the hemodynamic effect of the pharmacological agent on RV function and is also a useful tool in the diagnosis of RV outflow tract obstruction (RVOTO). Excessive inotropic stimulation and afterload reduction may lead to RVOTO that can be instantaneously diagnosed with continuous pulmonary artery and RV pressure monitoring (Fig. 22.2). This condition is seen when the RV systolic pressure is at least 6 mmHg above the SPAP. Right ventricular outflow tract obstruction can be dynamic or mechanical and can be the source of important hemodynamic instability [30]. In this situation, similar to dynamic LV outflow tract obstruction associated with systolic anterior motion of the mitral valve, inotropic agents would be contraindicated. If the RVOTO is non-mechanical, volume and beta-blocking agents can be used. Hemodynamic instability from RVOTO can occur in presence of an anterior pneumothorax, during sternal closure and after lung transplantation. This condition is likely to be under-diagnosed in the perioperative period.
Right ventricular outflow tract (RVOT) obstruction. Mid-esophageal inflow-outflow views in (a–b) diastole and (c–d) systole show significant collapse of the RVOT during systole. (e) A 22 mmHg pressure gradient is present using combined right ventricular pressure (Prv) and pulmonary artery pressure (Ppa) waveforms. (f) The intraoperative aspect of the right ventricle (RV) shows a dimpling on the RVOT. Abbreviations: LA left atrium, LV left ventricle, RA right atrium. (With permission of Denault et al. [29])
The MAP/MPAP ratio helps the clinician to appreciate the importance of PH related to the cardiac condition. In a situation where there is rapid variation of loading conditions, MPAP can be underestimated as it can be reduced proportionally to the decrease in systemic pressures. In patients with PH, it has been shown that MAP/MPAP ratio is not influenced by loading conditions and is an independent predictor of hemodynamic complication after cardiac surgery [31,32,33,34,35]. Also, MAP/MPAP ratio correlates with the TTE interventricular septal curvature and is correlated to five-year mortality after aortic valve replacement [36].
Extra-Cardiac Ultrasound Assessment
By using point-of-care ultrasound, the clinician can investigate the hemodynamic impact of right heart failure in end-organs. Doppler ultrasound of the liver offers the possibility to assess flow in the portal and hepatic veins. These can provide an important insight into the severity of RVF and hepatic congestion and require only basic training in Doppler ultrasound. Hepatic venous flow can be used to evaluate RV systolic function based on the aspect of the Doppler signal pattern. Hepatic vein flow can be obtained using a phased array probe or a curved array probe in the sub-xiphoid or lateral chest regions. Normal hepatic flow is directed away from the liver and fluctuates during the cardiac cycle as shown in Fig. 22.1c. Systolic flow is usually of higher velocity than diastolic flow. This is due to downward motion of the tricuspid annulus during ventricular systole resulting in a rapid filling of the right atria. In patients with right heart failure, decreased TAPSE and/or tricuspid regurgitation during ventricular systole lead to a reduction of the velocity in systole and to a systolic-to-diastolic ratio less than one as shown in Fig. 22.1h [37]. In severe right heart failure or tricuspid regurgitation, the systolic wave (S) appears to be completely reversed with backward flow in the hepatic veins during systole (Fig. 22.1m).
Flow in the portal vein can be assessed using a phased array or a curved linear array probe positioned in a right mid-axillary coronal view. Portal vein imaging using TEE is accomplished in the transgastric position. A transverse (short axis) cut of the liver is obtained by turning the probe to the right side of the patient. A multiplane angle rotation of 90 to 110 degrees leads to a craniocaudal plane of the liver. The portal vein is usually within a few centimeters of the transducer and the inferior vena cava (IVC) is usually not included in the same 2D view. Venous flow through the portal vein is of low velocity (20 cm/s) because this circulation is isolated from the systemic circulation by the liver sinusoids and splanchnic capillary bed. Therefore, portal venous flow presents minimal variations through the cardiac cycle (Fig. 22.1d). Portal vein pulsatility index (PVPI) can be calculated as the ratio of maximal and minimal velocity [(Vmax – Vmin)/Vmax]. A PVPI of more than 50% can be considered abnormal and is called pulsatile portal flow (Fig. 22.1i and n).
An alternative approach to the portal vein flow evaluation is to assess splenic vein flow. The splenic vein is a direct tributary of the portal vein and thus its assessment could provide the same information. Assessment of splenic vein flow can also be done with TEE via the transgastric approach. This view can be obtained by turning the probe to the left side of the body and by performing a multiplane angle rotation of 90 degrees. This will bring the view close to the splenic hilum. In this view, venous blood will travel in the direction of the probe and the velocities measured during Doppler examination will be positive. An alternative view can be obtained. With the probe toward the posterior aspect of the body, the electronic rotation angle is maintained at 0 degree. From this position, the splenic vein is located anterior to the descending aorta. From this view, the venous flow will be travelling away from the probe and the Doppler signal produced will exhibit negative velocities. An association has been observed between PVPI values greater than 50 % and high right atrial pressure, moderate or greater tricuspid regurgitation, and RV dysfunction [38, 39]. More recently, PVPI has been linked to cardiorenal syndrome and acute kidney injury after cardiac surgery [40, 41]. Intraoperative PVPI ≥ 0.5 has been previously reported to be the most important predictor of postoperative complications after cardiac surgery by being superior to any hemodynamic, 2D and Doppler cardiac measurement [39]. An international multicenter study (NCT03656263) is currently exploring the clinical significance of portal hypertension after cardiac surgery.
Pulsatile blood flow is a sign of post-hepatic portal hypertension and has been studied as a sign of severity in patients with congestive heart failure. The presence of an abnormal portal pulsatility predicts increased CVP and worse functional class in heart failure patients. Venous congestion resulting from congestive heart failure begins with an elevation of the CVP and dilatation of the IVC and its main tributaries such as the hepatic veins. When the dilatation becomes severe, the venous compliance of the IVC is decreased and pressure is transduced through the hepatic sinusoids to the portal system. This results in a decrease in velocities in the portal system or, when severe, in a complete absence or reversal of portal flow. Doppler evaluation of the portal flow could be used as a marker of end-organ venous congestion. For the portal flow to be representative of central venous congestion, other causes of portal hypertension such as cirrhosis and portal thrombosis must be absent. A PF of more than 50% has also been reported in some individuals with low body mass index and normal cardiac function. However, other signs of elevated CVP such as IVC dilatation/non-collapsibility and abnormal hepatic vein flow waveform should support this finding.
The impact of elevated CVP on intra-renal hemodynamics can be assessed by Doppler ultrasound. In physiological conditions, blood flow in the interlobar veins is continuous during the cardiac cycle (Fig. 22.1e). With high CVP, venous flow transforms into a discontinuous biphasic pattern like the Doppler pattern seen in the hepatic veins (Fig. 22.1j). With severe right heart failure, venous flow transforms into a monophasic discontinuous pattern with flow being present only during diastole (Fig. 22.1o). Discontinuous flow in the interlobar renal veins can be linked to the CVP waveform during the cardiac cycle. As CVP increases and the IVC becomes non-compliant, the CVP waveform is transmitted deep into the renal parenchyma. Flow in the interlobar vein can be observed during the systolic and diastolic filling of the right atria (during the X and Y descent on CVP waveform). As right heart failure worsens, intra-renal venous flow becomes monophasic reflecting the predominance of the Y descent of the CVP waveform analogous to the variation in the S/D ratio in the hepatic vein waveform (decrease filling of the right atria during systole).
In Iida et al., the intra-renal venous flow pattern strongly correlated with death from cardiovascular disease and unplanned hospitalization for heart failure independent of renal resistance index, CVP and hemodynamic status including echocardiographic parameters [42]. In this study, patients with the monophasic discontinuous flow pattern also had lower estimated glomerular filtration rate (55 mL/min/1.73 m2) compared with continuous and biphasic flow (67 mL/min/1.73 m2) (p = 0.005). The biphasic and monophasic patterns have been associated with increased mortality in heart failure [43, 44] and postoperative renal failure in cardiac surgery [41, 45].
Integrating Ultrasound Assessment into Practice
Clinical assessment of RV function during surgery relies on the integration of multiple parameters, each having their own advantages and caveats. As such, patient management should not be based solely on findings from ultrasound assessment. Use of bedside ultrasound and venous waveform interpretation to enhance physical examination would rather provide the opportunity for early detection of the effects of venous congestion on end-organs. This assessment can be repeated to monitor the response to therapy.
Integrating the understanding of the pathophysiology of a clinical syndrome and ultrasonographic assessment, as described in this chapter, may help individualize the management of patients with acute or chronic right heart failure in order to avoid the adverse effects of venous congestion.
Treatment and Management
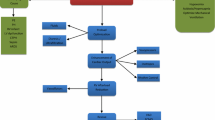
Treatment of patients with PH and RVF is particularly challenging. The choice of appropriate therapy depends on identifying the underlying cause and the hemodynamic effects of PH (Fig. 22.3). Despite the fact that the treatment of PAH has undergone an extraordinary evolution in recent years, there is currently no specific treatment for PH associated with lung disease and/or hypoxemia or secondary to left heart disease. As a result, drugs with proven efficacy in PAH are increasingly being used, in spite of the absence of clinical trials in support of this approach. Therefore, when managing a patient with PH in the ICU, treatment should primarily target the specific cause of PH and the resolution of RVF.
Bedside approach to acute right ventricular (RV) dysfunction in cardiac surgery. Abbreviations: CPB cardiopulmonary bypass, FRC functional residual capacity, HR heart rate, iPGI2 inhaled prostacyclin (epoprostenol), iMil inhaled milrinone, LV left ventricle, LVOT left ventricular outflow tract, NIRS near-infrared spectroscopy, PA pulmonary artery, RCA right coronary artery, RVOT right ventricular outflow tract, TEE transesophageal echocardiography, VAD ventricular assist device. (Adapted from Denault et al. [46])
Intravenous vasodilators have traditionally been used in managing PH in heart surgery, but they lack specificity for the pulmonary circulation and their systemic hypotensive effects necessitating additional vasopressor support often limit their use. This limitation highlights the need for selective pulmonary vasodilators for this cohort of patients, whereby nebulization of drugs could provide such localized effect with no hypotension.
Following a systematic review of the literature covering the period between 1980 and 2010, Price et al. [47] made recommendations regarding the management of pulmonary vascular and RV dysfunction in intensive care patients. Phosphodiesterase enzyme (PDE) type 3 inhibitors were strongly recommended to reduce pulmonary pressures and pulmonary vascular resistance (PVR) and improve RV performance. Furthermore, the use of pulmonary vasodilators was strongly recommended for reduction of PVR, improvement of cardiac output and oxygenation, and following cardiac surgery when PH and RV dysfunction are present. In addition, a strong recommendation was made for the administration of pulmonary vasodilators by inhalation rather than by intravenous route when systemic hypotension is expected.
Inhalation Therapy in Cardiac Surgery
Several PH-specific therapies have been approved for the treatment of PH. Although not currently approved for this indication, administration by nebulization of some of these agents is being increasingly investigated in adults and in cardiac surgery. Therefore, most of our understanding on the use of inhaled therapy is derived from small observational or single centre trials reported in the literature, and offers little clarity on the best therapeutic option for cardiac surgical patients with PH. Further larger randomized clinical studies are warranted in this field to provide much needed clinical practice guidelines. Inhaled therapies commonly used to treat PH in the cardiac surgical setting include inhaled nitric oxide, inhaled prostacyclin (iPGI2), inhaled iloprost, and inhaled PDE inhibitors such as milrinone.
When to Administer Inhaled Agents in Cardiac Surgery
Owing to their selective pulmonary vasodilator effect, inhaled agents are being increasingly used in high-risk patients during cardiac surgery for management of acute PH or RVF and to facilitate weaning from CPB. However, there is still no consensus on the appropriate timing of drug administration. Review of the literature reveals that inhaled agents are administered at different times throughout the perioperative period. In a study by Groves et al. [48] the impact of early versus late initiation of iPGI2 therapy was retrospectively investigated in 37 consecutive patients undergoing LV assist device placement. Inhaled prostacyclin was initiated at weaning from CPB in group I, whereas it was started shortly after induction of anesthesia and continued throughout and post-CPB in group II. Results show that iPGI2 reduces SPAP and MPAP in the postoperative period regardless of the timing of initiation. Early initiation of therapy reduced SPAP and MPAP more effectively during weaning from CPB, but this was associated with an increased blood loss in the immediate postoperative period. Similarly, Lamarche et al. [49] evaluated the impact and timing of administration of inhaled milrinone (iMil) on retrospective data from 73 high-risk patients undergoing cardiac surgery with CPB. Patients receiving iMil prior to CPB initiation had greater reduction in PAP after CPB and less emergency re-initiation of CPB after weaning compared to those with administration after CPB. No detectable side effects were linked to administration of the drug. These data suggest that administration of iPGI2 and iMil before CPB initiation may help weaning from CPB.
Systematic Review and Meta-Analysis of Inhaled Agents in Cardiac Surgery
In recent years, there has been a growing interest for using inhaled agents for the treatment of PH in cardiac surgery. The efficacy of these inhaled strategies, however, continues to be shown only through a limited number of small trials, case reports and series. We recently published a systematic review and meta-analysis comparing the efficacy of inhaled aerosolized agents with intravenously administered agents or placebo for the treatment and management of PH in patients undergoing cardiac surgery [50]. The purpose of this review was to summarize the state of the art in this field. Databases such as MEDLINE, CENTRAL, EMBASE, Web of Science, and clinicaltrials.gov were searched, which identified 2897 relevant citations. From those, 10 studies were included in the review and meta-analysis, comprising a total of 434 patients.
The primary outcome of the study was the incidence of mortality. Secondary outcomes were length of stay in hospital and in the ICU and evaluation of the hemodynamic profile. The meta-analysis revealed that inhaled aerosolized agents were associated with a significant decrease in PVR and a significant increase in MAP and RVEF when compared to intravenously administered agents. No significant hemodynamically meaningful differences were observed between inhaled agents and placebo. However, an increase in length of stay in the ICU was shown with the use of inhaled aerosolized agents compared to placebo.
This systematic review and meta-analysis showed that the administration of inhaled aerosolized vasodilators is associated with improved RV performance when compared to intravenously administered agents for the treatment of PH during cardiac surgery. This study, however, did not show any benefit on mortality, nor did it support any benefit compared to placebo on major outcomes. The limitations of this review were the limited number of studies published on this topic and the small size of the trials. This review shows that more studies are required in this area of research and that these should focus on clinically significant outcomes.
Intratracheal Milrinone
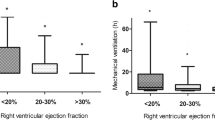
In acute RVF, the administration of inhaled agents is limited by the availability of a nebuliser and also their time-consuming administration. In such a situation when immediate treatment has to be initiated to avoid further cardiac deterioration and hemodynamic compromise, at the Montreal Heart Institute, we use intratracheal bolus administration of milrinone. Our experience over 12 years has been reported in patients with acute RVF. The success rate in avoiding the use of inotropic agents or return on CPB was 61.9% when intratracheal milrinone was given during CPB separation. Severely decreased left ventricular ejection fraction (<35% vs >50%), longer CPB duration and elevated postoperative fluid balance were found to be significant predictors of persistent RV failure despite intratracheal milrinone [51, 52]. Thus, direct intratracheal bolus administration of milrinone might offer a rapid and easily applicable alternative delivery mode for milrinone in acute RVF.
References
Denault AY, Pearl RG, Michler RE, Rao V, Tsui SS, Seitelberger R, et al. Tezosentan and right ventricular failure in patients with pulmonary hypertension undergoing cardiac surgery: the TACTICS trial. J Cardiothorac Vasc Anesth. 2013;27(6):1212–7.
Denault AY, Bussieres JS, Arellano R, Finegan B, Gavra P, Haddad F, et al. A multicentre randomized-controlled trial of inhaled milrinone in high-risk cardiac surgical patients. Can J Anesth. 2016;63(10):1140–53.
Denault A, Deschamps A, Tardif JC, Lambert J, Perrault L. Pulmonary hypertension in cardiac surgery. Curr Cardiol Rev. 2010;6(1):1–14.
Orem C. Epidemiology of pulmonary hypertension in the elderly. J Geriatric Cardiol. 2017;14(1):11–6.
Thunberg CA, Gaitan BD, Grewal A, Ramakrishna H, Stansbury LG, Grigore AM. Pulmonary hypertension in patients undergoing cardiac surgery: pathophysiology, perioperative management, and outcomes. J Cardiothorac Vasc Anesth. 2013;27(3):551–72.
Kennedy JL, LaPar DJ, Kern JA, Kron IL, Bergin JD, Kamath S, et al. Does the Society of Thoracic Surgeons risk score accurately predict operative mortality for patients with pulmonary hypertension? J Thorac Cardiovasc Surg. 2013;146(3):631–7.
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713; quiz 86–8.
Haddad F, Couture P, Tousignant C, Denault AY. The right ventricle in cardiac surgery, a perioperative perspective: I. Anatomy, physiology, and assessment. Anesth Analg. 2009;108(2):407–21.
Haddad F, Couture P, Tousignant C, Denault AY. The right ventricle in cardiac surgery, a perioperative perspective: II. Pathophysiology, clinical importance, and management. Anesth Analg. 2009;108(2):422–33.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14.
Haddad F, Denault AY, Couture P, Cartier R, Pellerin M, Levesque S, et al. Right ventricular myocardial performance index predicts perioperative mortality or circulatory failure in high-risk valvular surgery. J Am Soc Echocardiogr. 2007;20(9):1065–72.
Silverton N, Meineri M. Speckle tracking strain of the right ventricle: an emerging tool for intraoperative echocardiography. Anesth Analg. 2017;125(5):1475–8.
Duncan AE, Sarwar S, Kateby Kashy B, Sonny A, Sale S, Alfirevic A, et al. Early left and right ventricular response to aortic valve replacement. Anesth Analg. 2017;124(2):406–18.
Ternacle J, Berry M, Cognet T, Kloeckner M, Damy T, Monin JL, et al. Prognostic value of right ventricular two-dimensional global strain in patients referred for cardiac surgery. J Am Soc Echocardiogr. 2013;26(7):721–6.
Shukla M, Park JH, Thomas JD, Delgado V, Bax JJ, Kane GC, et al. Prognostic Value of Right Ventricular Strain Using Speckle-Tracking Echocardiography in Pulmonary Hypertension: A Systematic Review and Meta-analysis. Can J Cardiol. 2018;34(8):1069–78.
Medvedofsky D, Koifman E, Jarrett H, Miyoshi T, Rogers T, Ben-Dor I, et al. Association of Right Ventricular Longitudinal Strain with Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement. J Am Soc Echocardiogr. 2020;33(4):452–60.
Silverton NA, Lee JP, Morrissey CK, Tanner C, Zimmerman J. Regional Versus Global Measurements of Right Ventricular Strain Performed in the Operating Room With Transesophageal Echocardiography. J Cardiothorac Vasc Anesth. 2020;34(1):48–57.
Fusini L, Tamborini G, Gripari P, Maffessanti F, Mazzanti V, Muratori M, et al. Feasibility of intraoperative three-dimensional transesophageal echocardiography in the evaluation of right ventricular volumes and function in patients undergoing cardiac surgery. J Am Soc Echocardiogr. 2011;24(8):868–77.
Verbrugge FH, Dupont M, Steels P, Grieten L, Malbrain M, Tang WH, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol. 2013;62(6):485–95.
Hassan MO, Duarte R, Dix-Peek T, Vachiat A, Naidoo S, Dickens C, et al. Correlation between volume overload, chronic inflammation, and left ventricular dysfunction in chronic kidney disease patients. Clin Nephrol. 2016;86 (2016)(13):131–5.
Sundaram V, Fang JC. Gastrointestinal and Liver Issues in Heart Failure. Circulation. 2016;133(17):1696–703.
Valentova M, von Haehling S, Bauditz J, Doehner W, Ebner N, Bekfani T, et al. Intestinal congestion and right ventricular dysfunction: a link with appetite loss, inflammation, and cachexia in chronic heart failure. Eur Heart J. 2016;37(21):1684–91.
Fudim M, Hernandez AF, Felker GM. Role of Volume Redistribution in the Congestion of Heart Failure. J Am Heart Assoc. 2017;6(8).
Li DK, Wang XT, Liu DW. Association between elevated central venous pressure and outcomes in critically ill patients. Ann Intensive Care. 2017;7(1):83.
Williams JB, Peterson ED, Wojdyla D, Harskamp R, Southerland KW, Ferguson TB, et al. Central venous pressure after coronary artery bypass surgery: does it predict postoperative mortality or renal failure? J Crit Care. 2014;29(6):1006–10.
Saito S, Uchino S, Takinami M, Uezono S, Bellomo R. Postoperative blood pressure deficit and acute kidney injury progression in vasopressor-dependent cardiovascular surgery patients. Crit Care. 2016;20(1):74.
Amsallem M, Kuznetsova T, Hanneman K, Denault A, Haddad F. Right heart imaging in patients with heart failure: a tale of two ventricles. Curr Opin Cardiol. 2016;31(5):469–82.
Beaubien-Souligny W, Bouchard J, Desjardins G, Lamarche Y, Liszkowski M, Robillard P, et al. Extracardiac signs of fluid overload in the critically ill cardiac patient: a focused evaluation using bedside ultrasound. Can J Cardiol. 2017;33(1):88–100.
Denault A, Lamarche Y, Rochon A, Cogan J, Liszkowski M, Lebon JS, et al. Innovative approaches in the perioperative care of the cardiac surgical patient in the operating room and intensive care unit. Can J Cardiol. 2014;30(12 Suppl):S459–77.
Denault AY, Chaput M, Couture P, Hébert Y, Haddad F, Tardif JC. Dynamic right ventricular outflow tract obstruction in cardiac surgery. J Thorac Cardiovasc Surg. 2006;132(1):43–9.
Robitaille A, Denault AY, Couture P, Belisle S, Fortier A, Guertin MC, et al. Importance of relative pulmonary hypertension in cardiac surgery: the mean systemic-to-pulmonary artery pressure ratio. J Cardiothorac Vasc Anesth. 2006;20(3):331–9.
Bianco JC, Qizilbash B, Carrier M, Couture P, Fortier A, Tardif JC, et al. Is patient-prosthesis mismatch a perioperative predictor of long-term mortality after aortic valve replacement? J Cardiothorac Vasc Anesth. 2013;27(4):647–53.
Haddad F, Guihaire J, Skhiri M, Denault AY, Mercier O, Al-Halabi S, et al. Septal curvature is marker of hemodynamic, anatomical, and electromechanical ventricular interdependence in patients with pulmonary arterial hypertension. Echocardiography. 2014;31(6):699–707.
Rebel A, Nguyen D, Bauer B, Sloan PA, DiLorenzo A, Hassan ZU. Systemic-to-pulmonary artery pressure ratio as a predictor of patient outcome following liver transplantation. World J Hepatol. 2016;8(32):1384–91.
Bianco JC, Mc Loughlin S, Denault AY, Marenchino RG, Rojas JI, Bonofiglio FC. Heart Transplantation in Patients >60 Years: Importance of Relative Pulmonary Hypertension and Right Ventricular Failure on Midterm Survival. J Cardiothorac Vasc Anesth. 2018;32(1):32–40.
Haddad F, Elmi-Sarabi M, Fadel E, Mercier O, Denault AY. Pearls and pitfalls in managing right heart failure in cardiac surgery. Curr Opin Anaesthesiol. 2016;29(1):68–79.
Scheinfeld MH, Bilali A, Koenigsberg M. Understanding the spectral Doppler waveform of the hepatic veins in health and disease. Radiographics. 2009;29(7):2081–98.
Styczynski G, Milewska A, Marczewska M, Sobieraj P, Sobczynska M, Dabrowski M, et al. Echocardiographic Correlates of Abnormal Liver Tests in Patients with Exacerbation of Chronic Heart Failure. J Am Soc Echocardiog. 2016;29(2):132–9.
Eljaiek R, Cavayas YA, Rodrigue E, Desjardins G, Lamarche Y, Toupin F, et al. High postoperative portal venous flow pulsatility indicates right ventricular dysfunction and predicts complications in cardiac surgery patients. Br J Anaesth. 2019;122(2):206–14.
Beaubien-Souligny W, Eljaiek R, Fortier A, Lamarche Y, Liszkowski M, Bouchard J, et al. The Association Between Pulsatile Portal Flow and Acute Kidney Injury after Cardiac Surgery: A Retrospective Cohort Study. J Cardiothorac Vasc Anesth. 2018;32(4):1780–7.
Beaubien-Souligny W, Benkreira A, Robillard P, Bouabdallaoui N, Chasse M, Desjardins G, et al. Alterations in Portal Vein Flow and Intrarenal Venous Flow Are Associated With Acute Kidney Injury After Cardiac Surgery: A Prospective Observational Cohort Study. J Am Heart Assoc. 2018;7(19):e009961.
Iida N, Seo Y, Sai S, Machino-Ohtsuka T, Yamamoto M, Ishizu T, et al. Clinical implications of intrarenal hemodynamic evaluation by Doppler ultrasonography in heart failure. JACC Heart Fail. 2016;4(8):674–82.
Iida N, Seo Y, Sai S, Machino-Ohtsuka T, Yamamoto M, Ishizu T, et al. Clinical Implications of Intrarenal Hemodynamic Evaluation by Doppler Ultrasonography in Heart Failure. JACC Heart Fail. 2016;4(8):674–82.
Puzzovivo A, Monitillo F, Guida P, Leone M, Rizzo C, Grande D, et al. Renal Venous Pattern: A New Parameter for Predicting Prognosis in Heart Failure Outpatients. J Cardiovasc Dev Dis. 2018;5(4):52.
Beaubien-Souligny W, Denault AY. Real-Time Assessment of Renal Venous Flow by Transesophageal Echography During Cardiac Surgery. A&A practice. 2019;12(1):30–2.
Denault AY, Haddad F, Jacobsohn E, Deschamps A. Perioperative right ventricular dysfunction. Curr Opin Anaesthesiol. 2013;26(1):71–81.
Price LC, Wort SJ, Finney SJ, Marino PS, Brett SJ. Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care. 2010;14(5):R169.
Groves DS, Blum FE, Huffmyer JL, Kennedy JL, Ahmad HB, Durieux ME, et al. Effects of early inhaled epoprostenol therapy on pulmonary artery pressure and blood loss during LVAD placement. J Cardiothorac Vasc Anesth. 2014;28(3):652–60.
Lamarche Y, Perrault LP, Maltais S, Tetreault K, Lambert J, Denault AY. Preliminary experience with inhaled milrinone in cardiac surgery. Eur J Cardiothorac Surg. 2007;31(6):1081–7.
Elmi-Sarabi M, Deschamps A, Delisle S, Ased H, Haddad F, Lamarche Y, et al. Aerosolized vasodilators for the treatment of pulmonary hypertension in cardiac surgical patients: a systematic review and meta-analysis. Anesth Analg. 2017;125(2):393–402.
Gebhard CE, Desjardins G, Gebhard C, Gavra P, Denault AY. Intratracheal Milrinone Bolus Administration During Acute Right Ventricular Dysfunction After Cardiopulmonary Bypass. J Cardiothorac Vasc Anesth. 2017;31(2):489–96.
Gebhard CE, Rochon A, Cogan J, Ased H, Desjardins G, Deschamps A, et al. Acute Right Ventricular Failure in Cardiac Surgery During Cardiopulmonary Bypass Separation: A Retrospective Case Series of 12 Years’ Experience With Intratracheal Milrinone Administration. J Cardiothor Vasc An. 2019;33(3):651–60.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Couture, E.J., Elmi-Sarabi, M., Beaubien-Souligny, W., Denault, A. (2021). Pulmonary Hypertension and Right Ventricular Dysfunction Post-Cardiopulmonary Bypass. In: Cheng, D.C., Martin, J., David, T. (eds) Evidence-Based Practice in Perioperative Cardiac Anesthesia and Surgery. Springer, Cham. https://doi.org/10.1007/978-3-030-47887-2_22
Download citation
DOI: https://doi.org/10.1007/978-3-030-47887-2_22
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-47886-5
Online ISBN: 978-3-030-47887-2
eBook Packages: MedicineMedicine (R0)