Abstract
Haematopoietic stem cell transplantation (HSCT) is a potentially curative treatment option for several non-malignant diseases. Continuous progress in the field has led to broader indications for HSCT, increasing the number of patients (including children) being treated, and leading to a better overall long-term outcome. This chapter focuses on particularities of counselling in these patients, describes the gonadotoxicity of HSCT and disease-specific side effects such as iron overload in thalassemia major patients, toxicity of hydroxycarbamide in sickle cell disease, as well as comorbidities of these mostly hereditary and chronic diseases. In most HSCT in non-malignant diseases, there is enough time to perform fertility preservation procedures such as cryopreservation of gametes and gonadal tissue as well as GnRH agonists in females.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bone marrow failure
- Fertility preservation
- FertiPROTEKT
- Gene therapy
- Immunodeficiency
- Iron overload
- Non-malignant diseases
- Sickle cell disease
- Stem cell transplantation
- Thalassemia
Introduction
Haematopoietic stem cell transplantation (HSCT) is an established and often the only curative treatment for serious haematological diseases. The number of stem cell transplantations for non-malignant diseases has therefore continuously increased over the last decades and has led to better overall survival of patients with congenital and acquired non-malignant diseases [1,2,3]. Advances in transplantation medicine and improved possibilities for diagnosis and thus indications for HSCT are responsible for this trend. With this development, topics such as quality of life and long-term consequences of HSCT are increasingly common, because the conditioning required for HSCT leads to infertility in 80–100% of patients, depending on the conditioning protocol used [4, 5].
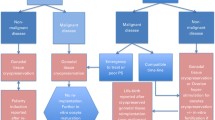
Many studies highlight the importance of fertility for the quality of life of long-term surviving patients. The frequency of patients wishing to have a child after HSCT corresponds with that of the normal population [6]. Many HSCTs in patients with non-malignant diseases are diagnosed in early childhood, i.e. long before puberty. Fortunately, advances in reproductive medicine are opening new perspectives, especially for prepubertal patients, through the use of fertility-preserving measures [7]. An overview of the most common non-malignant diseases that can be treated by HSCT is shown in Table 1.
Treatment Gonadotoxicity
Hematopoietic Stem Cell Transplantation
Gonadotoxic effects of chemotherapy and radiotherapy are well known [8,9,10]. Alkylating drugs in particular lead to partial or complete damage of gonadal function in both sexes, with the possible loss of germ cells or a shortened reproductive phase in affected girls and women [10, 11].
The risk of infertility after HSCT depends on the underlying disease, a reduced ovarian reserve even before the start of treatment, previous treatments, the conditioning drugs used and the age of the patient at the time of HSCT [5, 8, 10].
Only 1–5% of all patients who have undergone stem cell transplantation have children. However, there is little published data from adults who underwent transplantation as children or adolescents [5, 10]. Some studies report higher residual fertility when HSCT is performed at a younger age [8].
Regarding the regimes used, cyclophosphamide monotherapy showed the lowest gonadotoxic effect [11]. However, this protocol is only used in patients with severe aplastic anaemia. Patients who received busulfan-based protocols or total body irradiation (TBI) had a birth rate of <1% [5, 10,11,12].
Paediatric conditioning protocols are often myeloablative (87%); however, there is a trend to achieve conditioning with reduced intensity or toxicity (from 8% in 2000 to 16% in 2015) [4]. These protocols are preferably used in non-malignant settings. Further long-term studies are necessary to prove the suspected reduced gonadotoxic effect of these protocols [13, 14]. Two pregnancies were recently reported in a woman treated with a reduced intensity protocol at the age of 19 years [15].
Due to the lack of data on fertility after conditioning with reduced toxicity, all patients receiving HSCT after conditioning should currently be advised on fertility preservation measures.
Gene Therapy
In 2018, the first marketing authorisation application for gene therapy for patients with thalassaemia major was submitted to the European Medicines Agency (EMA). The EMA recommends approval of the new gene therapy for thalassaemia patients and the application is expected to be implemented in 2020.
Immunodeficiencies, metabolic disorders and cystic fibrosis are also among the diseases that are potentially eligible for gene therapy. Before retransfusion of the autologous genetically modified stem cells, conditioning, currently mostly with busulfan, is necessary. These patients should also be advised on the gonadotoxic effect of conditioning and fertility-preserving measures should be offered.
Advice on Fertility Preservation Measures
In 2016, experts from the Paediatric Diseases Working Party (PDWP) of the European Society for Blood and Marrow Transplantation (EBMT) published recommendations for advice and implementation of fertility-preserving measures in children and adolescents undergoing planned HSCT [3]. All patients of prepubertal or postpubertal age should be offered advice on the options available to them for fertility preservation. Counselling should include information on the risk of fertility impairment as a result of planned HSCT in relation to the underlying disease, age, pre-treatment and other comorbidities. At the request of patients or their parents, an individualised concept for maintaining fertility should be an integral part of the treatments.
During the consultation, attention should be drawn to the possibility of inheriting the underlying disease, especially in the case of autosomal dominant or x-linked inherited diseases. It is essential that patients are informed about the possible inheritance of these defective genes to their offspring, even though the disease has been clinically cured after HSCT. Testing of the partner and consultation prior to initiating infertility treatment is essential. The implementation of fertility-preserving measures in mentally retarded patients should be discussed and considered in detail with the parents.
The currently still experimental character of fertility preservation methods in prepubertal girls should be pointed out. However, there are now first case reports of successful pregnancies after retransplantation of prepubertal ovarian tissue, so that this method may lose its experimental character in the coming years [7].
Restrictions on Fertility Preservation Measures in Special Situations
Patients with Thrombocytopenia/Neutropenia
Patients who have thrombocytopenia or neutropenia due to their underlying disease have an increased risk of bleeding or infection when gonadal tissue or oocytes are removed. Preoperative preventive measures, such as necessary substitutions, should be taken accordingly.
Patients with Sickle Cell Disease (SCD)
Many patients with SCD receive hydroxycarbamide (HC) as long-term treatment. Hydroxycarbamide is indicated for the prevention of recurrent painful vaso-occlusive crises including acute chest syndrome in adults, adolescents and children over 2 years of age with symptomatic SCD [16]. In a randomised, placebo-controlled trial, the BABY HUG study has shown that hydroxycarbamide administration also reduces the incidence of complications in previously asymptomatic infants [17].
Many guidelines recommend the early use of HC. Therefore, an increased proportion of SCD patients who received previous HC before allogeneis HSCT is to be expected. Boys and men, regardless of whether they were treated with HC, have reduced spermatogenesis. There is a current lack of prospective studies on the negative influence of HC on fertility in girls and women [18]. However, a retrospective study showed a reduction in AMH concentration after HC therapy alone, and a negative influence on the ovarian reserve must currently be assumed [19].
Based on current data, it is not recommended that HC therapy be discontinued before fertility preservation measures are implemented, and a washout phase prior to conception should be considered for later transplantation of ovarian tissue obtained under HC therapy. Erythrocytes of patients with SCD have a shortened survival time and can lead to recurrent vascular occlusion crises and thromboses due to endothelial damage.
Cryopreservation of oocytes (see chapter “Cryopreservation of Unfertilized and Fertilized Oocytes”) is an established procedure which requires hormonal stimulation (see chapter “Ovarian Stimulation to Collect Oocytes”). This can lead to complications in SCD patients, such as severe pain crises due to increased estrogen levels during stimulation and the occurrence of thromboses, especially acute thoracic syndrome or central nervous system infarctions. Case reports on complications after stimulation for oocyte collection have been published [20,21,22].
Some European stem cell transplant centres therefore prefer the removal and cryopreservation of ovarian tissue (personal communication) (see chapters “Removal of Ovarian Tissue” and “Transportation, Cryopreservation and Storage of Ovarian Tissue”) as a fertility-preserving measure in pre- as well as post-pubertal SCD patients, in order to avoid hormonal stimulation.
Due to the lack of data and the possible serious complications associated with hormonal stimulation, we recommend not using hormonal stimulation and only offering these patients removal of ovarian tissue.
Recommendations regarding the perioperative procedure in SCD patients should be followed [23] for the removal of ovarian tissue (see chapter “Removal of Ovarian Tissue”).
Patients with Iron Overload
Patients who receive regular red blood cell transfusions may develop endocrinopathies such as primary or secondary hypogonadism as a result of transfusion-related iron overload. Haemosiderosis-related endocrinopathies are more common in patients with thalassemia than with SCD [24]. Transfusion-dependent thalassaemia patients have ovulation disorders and hypogonadism in 30–80% of cases [25]. These changes are dependent, for example, on genotype and the start and duration of iron overload as a result of non-compliant chelation therapy [26]. The extent of iron-related damage to the pituitary gland and/or ovarian tissue can only be estimated approximately by ultrasound determination of the antral follicle count (AFC) and AMH concentration. If the ovarian reserve is demonstrably limited due to iron overload (AMH and AFC reduced), neither ovarian stimulation nor cryopreservation of ovarian tissue is appropriate.
Other Patients
Both ovarian stimulation and cryopreservation of ovarian tissue can be considered in all other postpubertal patients. In non-malignant situations, multiple stimulation cycles to achieve the desired number of cryopreserved oocytes may be possible.
Practical Approach
While possible fertility-preserving measures may be limited by the time frame available in patients with oncological diseases (e.g. myelodysplastic syndrome, MDS), patients with non-malignant diseases usually have sufficient time for a detailed consultation and implementation of fertility-preserving therapies (even repeated ovarian stimulation, if the underlying disease allows). An exception may be children with severe combined immunodeficiency (SCID) or haemophagocytic lymphohistiocytosis (HLH), whose diagnosis may only be made during a life-threatening and intensive exacerbation of the disease. In these exceptional cases, fertility-preserving measures should be avoided after risk assessment, but the parents should nevertheless be advised and involved in the decision-making process.
Fertility preservation measures can often be carried out during other planned surgical interventions, such as the implantation of an indwelling catheter or the removal of an autologous bone marrow reserve (autologous back-up).
References
European Society for Blood and Marrow Transplantation. Annual report, 2017. Leiden: EBMT; 2017.
Passweg JR, Baldomero H, Peters C, Gaspar HB, Cesaro S, Dreger P, Duarte RF, Falkenburg JH, Farge-Bancel D, Gennery A, Halter J, Kröger N, Lanza F, Marsh J, Mohty M, Sureda A, Velardi A, Madrigal A. European Society for Blood and Marrow Transplantation EBMT. Hematopoietic SCT in Europe: data and trends in 2012 with special consideration of pediatric transplantation. Bone Marrow Transplant. 2014;49(6):744–50. https://doi.org/10.1038/bmt.2014.55.
Dalle JH, Lucchini G, Balduzzi A, Ifversen M, Jahnukainen K, Macklon KT, Ahler A, Jarisch A, Ansari M, Beohou E, Bresters D, Corbacioglu S, Dalissier A, Diaz de Heredia Rubio C, Diesch T, Gibson B, Klingebiel T, Lankester A, Lawitschka A, Moffat R, Peters C, Poirot C, Saenger N, Sedlacek P, Trigoso E, Vettenranta K, Wachowiak J, Willasch A, von Wolff M, Yaniv I, Yesilipek A, Bader P. State-of-the-art fertility preservation in children and adolescents undergoing haematopoietic stem cell transplantation: a report on the expert meeting of the Paediatric Diseases Working Party (PDWP) of the European Society for Blood and Marrow Transplantation (EBMT) in Baden, Austria, 29-30 September 2015. Bone Marrow Transplant. 2017;52(7):1029–35. https://doi.org/10.1038/bmt.2017.21.
Balduzzi A, Dalle JH, Jahnukainen K, von Wolff M, Lucchini G, Ifversen M, Macklon KT, Poirot C, Diesch T, Jarisch A, Bresters D, Yaniv I, Gibson B, Willasch AM, Fadini R, Ferrari L, Lawitschka A, Ahler A, Sänger N, Corbacioglu S, Ansari M, Moffat R, Dalissier A, Beohou E, Sedlacek P, Lankester A, De Heredia Rubio CD, Vettenranta K, Wachowiak J, Yesilipek A, Trigoso E, Klingebiel T, Peters C, Bader P. Fertility preservation issues in pediatric hematopoietic stem cell transplantation: practical approaches from the consensus of the pediatric diseases working party of the EBMT and the international BFM study group. Bone Marrow Transplant. 2017;52(10):1406–15. https://doi.org/10.1038/bmt.2017.147.
Borgmann-Staudt A, Rendtorff R, Reinmuth S, Hohmann C, Keil T, Schuster FR, Holter W, Ehlert K, Keslova P, Lawitschka A, Jarisch A, Strauss G. Fertility after allogeneic haematopoietic stem cell transplantation in childhood and adolescence. 2011; https://doi.org/10.1038/bmt.2011.78.
Zynda A, Reinmuth S, Pfitzer C, Hohmann C, Keil T, Borgmann-Staudt A. Childhood leukemia and its impact on graduation and having children: results from a national survey. Leuk Lymphoma. 2012;53(12):2419–22. https://doi.org/10.3109/10428194.2012.688965.
Matthews SJ, Picton H, Ernst E, Andersen CY. Successful pregnancy in a woman previously suffering from β-thalassemia following transplantation of ovarian tissue cryopreserved before puberty. Minerva Ginecol. 2018;70(4):432–5. https://doi.org/10.23736/S0026-4784.18.04240-5.
Dvorak CC, Gracia CR, Sanders JE, Cheng EY, Scott Baker K, Pulsipher MA, Petryk A. NCI, NHLBI/PBMTC first international conference on late effects after pediatric hematopoietic cell transplantation: endocrine challenges-thyroid dysfunction, growth impairment, bone health, & reproductive risks. Biol Blood Marrow Transplant. 2011;17:1725–38.
Chow EJ, Stratton KL, Leisenring WM, Oeffinger KC, Sklar CA, Donaldson SS, Ginsberg JP, Kenney LB, Levine JM, Robison LL, Shnorhavorian M, Stovall M, Armstrong GT, Green DM. (2016). Pregnancy after chemotherapy in male and female survivors of childhood cancer treated between 1970 and 1999: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2016 May;17(5):567-576. oi: https://doi.org/10.1016/S1470-2045(16)00086-3.
Pfitzer C, Orawa H, Balcerek M, Langer T, Dirksen U, Keslova P, Zubarovskaya N, Schuster FR, Jarisch A, Strauss G, Borgmann-Staudt A. Dynamics of fertility impairment and recovery after allogeneic haematopoietic stem cell transplantation in childhood and adolescence: results from a longitudinal study. J Cancer Res Clin Oncol. 2015;141(1):135–42. https://doi.org/10.1007/s00432-014-1781-5.
Sanders JE, Hawley J, Levy W, Gooley T, Buckner CD, Deeg HJ, Doney K, Storb R, Sullivan K, Witherspoon R, Appelbaum FR. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood. 1996;87:3045–52.
Vatanen A, Wilhelmsson M, Borgström B, Gustafsson B, Taskinen M, Saarinen-Pihkala UM, Winiarski J, Jahnukainen K. Ovarian function after allogeneic hematopoietic stem cell transplantation in childhood and adolescence. Eur J Endocrinol. 2014;170(2):211–8. https://doi.org/10.1530/EJE-13-0694.
Assouline E, Crocchiolo R, Prebet T, Broussais F, Coso D, Gamerre M, Vey N, Blaise D, Courbiere B. Impact of reduced-intensity conditioning allogeneic stem cell transplantation on women’s fertility. Clin Lymphoma Myeloma Leuk. 2013;13(6):704–10. https://doi.org/10.1016/j.clml.2013.05.014.
Panasiuk A, Nussey S, Veys P, Amrolia P, Rao K, Krawczuk-Rybak M, Leiper A. Gonadal function and fertility after stem cell transplantation in childhood: comparison of a reduced intensity conditioning regimen containing melphalan with a myeloablative regimen containing busulfan. Br J Haematol. 2015;170(5):719–26. https://doi.org/10.1111/bjh.13497.
Faraci M, Matthes-Martin S, Lanino E, Morreale G, Ferretti M, Giardino S, Micalizzi C, Balduzzi A. Two pregnancies shortly after transplantation with reduced intensity conditioning in chronic myeloid leukemia. Pediatr Transplant. 2016;20(1):158–61. https://doi.org/10.1111/petr.12630.
Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, Jordan L, Lanzkron SM, Lottenberg R, Savage WJ, Tanabe PJ, Ware RE, Murad MH, Goldsmith JC, Ortiz E, Fulwood R, Horton A, John-Sowah J. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033–48. https://doi.org/10.1001/jama.2014.10517.
Wang WC, Ware RE, Miller ST, Iyer RV, Casella JF, Minniti CP, Rana S, Thornburg CD, Rogers ZR, Kalpatthi RV, Barredo JC, Brown RC, Sarnaik SA, Howard TH, Wynn LW, Kutlar A, Armstrong FD, Files BA, Goldsmith JC, Waclawiw MA, Huang X, Thompson BW. BABY HUG investigators. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet. 2011;377(9778):1663–72. https://doi.org/10.1016/S0140-6736(11)60355-3.
McGann PT, Ware RE. Hydroxyurea therapy for sickle cell anemia. Expert Opin Drug Saf. 2015;14(11):1749–58. https://doi.org/10.1517/14740338.2015.1088827.
Elchuri SV, Williamson RS, Clark Brown R, Haight AE, Spencer JB, Buchanan I, Hassen-Schilling L, Brown MR, Mertens AC, Meacham LR. The effects of hydroxyurea and bone marrow transplant on anti-Müllerian hormone (AMH) levels in females with sickle cell anemia. Blood Cells Mol Dis. 2015;55(1):56–61. https://doi.org/10.1016/j.bcmd.2015.03.012.
Matthews M, Pollack R. (2017). Acute pain crisis in a patient with sickle cell disease undergoing ovarian simulation for fertility preservation prior to curative stem cell transplantation: case report and literature review. J Assist Reprod Genet. Nov;34(11):1445-1448.doi: https://doi.org/10.1007/s10815-017-1008-1.
Dovey S, Krishnamurti L, Sanfilippo J, Gunawardena S, Mclendon P, Campbell M, Alway S, Efymow B, Gracia C. Oocyte cryopreservation in a patient with sickle cell disease prior to hematopoietic stem cell transplantation: first report. J Assist Reprod Genet. 2012;29(3):265–9. https://doi.org/10.1007/s10815-011-9698-2.
Smith-Whitley K. Reproductive issues in sickle cell disease. Blood. 2014;124(24):3538–43. https://doi.org/10.1182/blood-2014-07-577619.
Schyrr F, Dolci M, Nydegger M, Canellini G, Andreu-Ullrich H, Joseph JM, Diezi M, Cachat F, Rizzi M, Renella R. Perioperative care of children with sickle cell disease: a systematic review and clinical recommendations. Am J Hematol. 2019;95(1):78–96. https://doi.org/10.1002/ajh.25626.
Fung EB, Harmatz PR, Lee PD, Milet M, Bellevue R, Jeng MR, Kalinyak KA, Hudes M, Bhatia S, Vichinsky EP, Multi-Centre Study of Iron Overload Research Group. Increased prevalence of iron-overload associated endocrinopathy in thalassaemia versus sickle-cell disease. Br J Haematol. 2006;135:574–82.
Carlberg KT, Singer ST, Vichinsky EP. Fertility and pregnancy in women with transfusion-dependent thalassemia. Hematol Oncol Clin North Am. 2018;32(2):297–315. https://doi.org/10.1016/j.hoc.2017.11.004.
Skordis N, Gourni M, Kanaris C, Toumba M, Kleanthous M, Karatzia N, Pavlides N, Angastiniotis M. The impact of iron overload and genotype on gonadal function in women with thalassaemia major. Pediatr Endocrinol Rev. 2004;2(Suppl 2):292–5.
Steward CG, Jarisch A. Haemopoietic stem cell transplantation for genetic disorders. Arch Dis Child. 2005;90:1259–63.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jarisch, A., Germeyer, A. (2020). Non-Malignant Diseases Requiring Stem Cell Transplantation. In: von Wolff, M., Nawroth, F. (eds) Fertility Preservation in Oncological and Non-Oncological Diseases. Springer, Cham. https://doi.org/10.1007/978-3-030-47568-0_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-47568-0_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-47567-3
Online ISBN: 978-3-030-47568-0
eBook Packages: MedicineMedicine (R0)