Abstract
Calcific aortic valve disease (CAVD) is a leading cause of death. Due to insufficient understanding of the molecular mechanisms that govern the disease, the current therapeutic options lack medical therapies and are limited to aortic valve (AV) replacement. AV calcification occurs preferentially in the fibrosa side of the valve, which is exposed to unstable hemodynamic conditions. In contrast, the ventricularis side is exposed to more stable hemodynamic conditions and is relatively protected from the disease, suggesting the role of biomechanical forces in CAVD. Recent studies have shown the role and mechanisms of biomechanical forces on regulation of AV biology and CAVD pathophysiology. Here, we review the molecular mechanisms by which biomechanical forces regulate mechanosensitive genes, especially microRNAs, and their roles in CAVD. Expression of these microRNAs can be easily manipulated, leading to effective inhibition of CAVD. These mechanosensitive microRNAs could be used for detection and treatment of CAVD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Calcific aortic valve disease (CAVD) is a leading underlying cause of mortality among the aging population and represents a growing burden in developed countries [1]. Although it was originally thought to be a degenerative disease, aortic valve (AV) calcification is now known to be an active process predominantly led by endothelial dysfunction and osteogenic differentiation of valvular interstitial cells (VICs) , leading to additional cardiovascular events [2].
CAVD is estimated to occur in 25–30% of adults aged 65 or older and is associated with a 50% increased risk of myocardial infarction [3,4,5,6]. CAVD ranges from thickening and hardening of the AV leaflets (sclerosis) to narrowing of the AV area due to impaired motion of calcified leaflets (stenosis) [2]. The only currently accepted therapeutic option for CAVD is valve replacement [7, 8].This is in large part due to the lack of sufficient mechanistic insights into CAVD. Recent studies by numerous investigators have been addressing this critical gap. Here, we will review the role of biomechanical forces on AV biology and pathophysiology with specific focus on mechanosensitive microRNAs (miRNAs) and their therapeutic potentials.
Development of CAVD begins with subclinical inflammation in the AV endothelium, followed by thickening due to cellular infiltration and extracellular matrix (ECM) remodeling, exacerbated by cytokines released by various AV cells including valvular endothelial cells (VECs) , VICs, and immune cells [4, 9,10,11,12]. These cytokines are hypothesized to also promote osteogenic differentiation of VICs, which is characterized by an upregulation of bone-related transcription factors, intermediates, and proteins, such as alkaline phosphatase (ALP), bone morphogenic proteins, osteocalcin, osteopontin, and RUNX2 [13,14,15].
It is important to note that calcification occurs preferentially on one side of the AV, namely, the fibrosa side. This layer of the AV faces the aorta and is exposed to complex and highly variable hemodynamic conditions [16]. Additionally, regions of the AV that experience relatively elevated axial stretch, such as near the aortic root, are more susceptible to calcification [17]. In this chapter we discuss the role of these biomechanical forces with a focus on mechanosensitive miRNAs and their target genes, which may be used as novel therapeutic approaches for treating and preventing CAVD.
Aortic Valve Structure and Biomechanical Forces
The AV regulates blood flow from the left ventricle to the aorta, supplying the systemic vasculature with oxygenated blood [2]. During systole, the AV is open, allowing blood to flow out of the contracted left ventricle at a peak velocity of approximately 1 m/s in physiological conditions [18]. In mild stenotic conditions , this peak velocity can increase to nearly 3 m/s, and, in severe stenosis, it can surpass 4 m/s. During diastole, the ventricle relaxes, and the AV closes due to the difference in pressures between the ventricle and the aorta. In physiological conditions, the transvalvular pressure ranges from 80 to 120 mmHg; however, in pathological conditions, it can reach up to 180 mmHg [19], leading to further AV dysfunction.
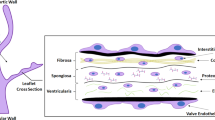
The AV leaflets, each less than 1 mm in thickness, are composed of three separate, complex layers, the fibrosa, spongiosa , and ventricularis (Fig. 6.1a). These layers dictate the mechanical function of the tissue and control its behavior:
-
1.
Fibrosa: This layer faces the aorta and is comprised of a VEC monolayer, directly in contact with blood, and VICs below impregnated within ECM. This layer is mostly composed of type I and type III collagen aligned in a circumferential manner and a small amount of elastin fibers [20]. This composition allows the AV to withstand high mechanical loads.
-
2.
Spongiosa: This layer is located in the middle of the AV leaflet. It contains most of the VICs and is comprised predominantly of glycosaminoglycans and proteoglycans [21]. Its main extracellular component, however, is hyaluronan, which can hold large amounts of water and serve as a shock absorber throughout the cardiac cycle [22].
-
3.
Ventricularis: This layer faces the left ventricle and is comprised of VECs and VICs [5]. Its ECM contains some collagen, but its composition is mostly elastin fibers aligned radially [21, 23]. This composition allows the ventricularis to withstand compression during systole and ensure the AV opens and closes consistently [22].
Structure and biomechanical forces in the aortic valve. (a) The fibrosa side, which faces the aorta, is composed of valve endothelial cells (VECs) and valve interstitial cells (VICs). Its extracellular matrix (ECM) is composed primarily of collagen fibers with some sparse elastin fibers. The spongiosa contains most of the VICs as well as glycosaminoglycans and some elastin fibers. The ventricularis side, which faces the left ventricle, is mostly composed of elastin fibers and contains some VICs as well as VECs. (b) During systole, the aortic valve (AV) experiences bending strain: the ventricularis is stretched, whereas the fibrosa is compressed. Blood flow across the ventricularis during ejection applies pulsatile, unidirectional shear stress on the VECs. During diastole, pressure is applied on the AV as it relaxes. Axial stretch prevents regurgitation by creating a tight seal in the radial and circumferential directions. The fibrosa experiences complex, time-varying, non-stable flow conditions, resulting in low, oscillatory shear stress.
The ever-present movement of the AV creates a dynamic mechanical environment whose understanding is critical for studying the pathobiological processes that regulate CAVD. The main forces exerted on the AV during the cardiac cycle are pressure, bending strain, axial stretch, and shear stress (Fig. 6.1b).
-
1.
Pressure: During diastole, the ventricular pressure drops, whereas the aortic pressure remains stable, closing the AV. In physiological conditions, the transvalvular pressure (difference between diastolic aortic and ventricular pressures) ranges from 80 to 120 mmHg [24]. In hypertensive patients, this pressure can exceed 180 mmHg [19]. The SMART study found a correlation between systolic blood pressure and cardiovascular calcification [25], suggesting a potential link between blood pressure and associated biomechanical forces on AV pathobiology. Other studies have also found links between increased systemic pressure and CAVD [26,27,28,29]; however, its effects on the underlying molecular mechanisms of the disease remain unclear.
-
2.
Bending strain: During systole, the concave fibrosa layer compresses, while the convex ventricularis layer extends under tension [30]. It is hypothesized that increased bending strain can induce more rapid and extensive calcification [31]. This was observed in patients with bicuspid aortic valves (BAV) . Several groups have found that the BAVs experience higher bending strains and patients can develop symptomatic CAVD nearly 20 years prior to tricuspid aortic valve (TAV) patients [32,33,34,35,36]. BAV is the most common congenital heart defect, with a prevalence of around 2%. The altered biomechanical conditions associated with BAV are considered as an important cause of the accelerated and aggressive disease phenotype and have been used as an important CAVD model system to understand its mechanisms [37].
-
3.
Axial stretch: A crucial step during the cardiac cycle is having the AV leaflets create a tight seal during diastole to prevent blood from regurgitating back into the left ventricle [30]. Axial stretch occurs in two directions: circumferential and radial. In humans, the AV leaflets deform more in the circumferential direction than the radial direction [38]. The region of maximum tension is located where the leaflet attaches to the aortic root (hinge region), which is also the region that tends to calcify first in CAVD [17]. A vicious cycle is generated when increased axial stretch leads to AV dysfunction, exacerbating the levels of stretch of the AV.
-
4.
Shear stress: This force is exerted on the AV by blood flow parallel to the endothelial surface. The fibrosa experiences complex, time-varying, non-stable flow conditions (d-flow) including oscillatory shear stress, while the ventricularis is exposed to a pulsatile but relatively stable flow (s-flow) conditions including unidirectional shear stress [16, 39]. These different shear profiles correlate with the preferential calcification pattern of the fibrosa [40,41,42]. Interestingly , in the AV, the non-coronary cusp (lacking a coronary ostium) experiences lower magnitude shear stress than the left and right coronary cusps and usually calcifies first [39]. Additional studies have also found that BAVs experience lower shear stress and more d-flow than TAVs, further implicating the role of shear stress in CAVD [43].
Mechanosensing in the Aortic Valve
Cells in the AV, such as VECs and VICs, sense various biomechanical forces through a variety of molecules and subcellular structures known as mechanosensors [44]. Most knowledge on mechanosensory mechanisms has been gained by studying vascular ECs. These include cell surface proteins (ion channels PIEZO1 and PIEZO2, glycocalyx, G protein-coupled receptors, G proteins, Notch1, and protein kinase receptors T kinase receptor and ST kinase receptor), subcellular structures (primary cilia and caveolae), cell-cell junction (PECAM-1, VE-cadherin, VEGFR2 mechanosensory complex), integrins, and intracellular actin cytoskeleton [45] (Fig. 6.2).Although these endothelial mechanosensors may serve similar functions in VECs and VICs, they remain to be validated. Here, we highlight a few of the major mechanosensors in the endothelium.
Mechanosensors in the aortic valve. Integrins bind to the actin cytoskeleton and to extracellular matrix to sense the mechanical environment surrounding the cell and transmit the information to the cellular interior. G protein-coupled receptors bind to extracellular ligands and activate downstream signaling (black arrows). The glycocalyx is a glycoprotein network that traps ions and molecules in the blood, allowing interactions with cellular membrane channels and receptors. PECAM-1 forms a mechanosensory complex with VE-cadherin and VEGF receptor II at the cell junctions, where biomechanical forces are recognized and transmitted to the cellular interior. Primary cilia and caveolae are subcellular structures that have been identified as mechanosensors in the vascular endothelium. Their function as mechanosensors in the aortic valve (AV) has not been determined. Surface proteins, such as PIEZO1/2, NOTCH1, T kinase receptor, and ST kinase receptor, have also been identified as mechanosensors in vascular endothelial cells; however, their function in the AV is not fully understood
Integrins are transmembrane adhesion receptors that have traditionally been found to bind ligands at the cell surface, within the ECM, and in the cytoplasm [46]. Integrins transduce signals from the outer environment to the cellular interior. Integrins have also been recognized as sensors of the mechanical environment that surrounds the cell, resulting in intracellular signal transduction pathway changes [47]. Furthermore, integrins receive intracellular signals that can regulate their ligand-binding affinity, enabling a tunable communication between the cell membrane and the rest of the cell [48]. Overall, integrins play a key role in cell adhesion, migration, proliferation, and cell survival, dictated by cues from the extracellular environment along with intracellular signaling.
Transmission of signals by integrins is dependent on their binding to the cytoskeleton [49]. Integrins co-localize with other cytoskeletal proteins near the cell surface, forming complexes known as focal adhesions [50, 51]. These complexes act as mediators between mechanical transduction among the integrins, external stimuli, and the cell [52]. Focal adhesions have the capability of sensing forces such as shear stress and tension [53,54,55]. Focal adhesions are also responsible for signaling changes in the cellular phenotype in response to mechanical and other cues. These can include signals for cell remodeling, proliferation, apoptosis, migration, and angiogenesis [56,57,58,59].
G protein-coupled receptors (GPCR) also bind to extracellular ligands to activate downstream signaling via G protein-activated signal transduction pathways [60]. GPCR are sensitive to changes in flow across the endothelium [61]. Although their role in AV biology is not clear, GPCR have been shown to become activated under certain mechanical stimuli, and their function has been well documented in the context of other diseases [62, 63].
ECs express proteoglycans and glycoproteins conjugated with long carbohydrate chains called glycosaminoglycans, on their cell surface [64, 65]. This glycoprotein network, called the glycocalyx, provides a physical barrier between the cells and the blood, where ions and other molecules found in the blood are trapped, allowing interactions between them and membrane channels and receptors [66, 67]. It has been shown that, under d-flow, the glycocalyx changes shape and its function is impaired [68, 69]. In the AV, the glycocalyx has been found to bind low-density lipoprotein and immunoglobulins more tightly in animals fed a high-cholesterol diet, particularly in the fibrosa [70, 71]. Higher affinity for these molecules can lead to increased infiltration and lesion formation, providing a link between mechanosensors and AV regions susceptible to calcification [69].
PECAM-1 has been extensively characterized as a mechanosensor located in vascular endothelial junctions [72, 73]. PECAM-1 forms a mechanosensory complex along with VE-cadherin and VEGF receptor II (VEGFR2) in the endothelial cell junction, where it recognizes shear stress and stretch and transmits the signal to intracellular biochemical responses such as intracellular calcium influx [74,75,76]. The mechanosensory response from this PECAM-1/VE-cadherin/VEGFR2 complex leads to activation of various signaling pathways, including PI3K, Akt, and eNOS [77,78,79,80,81,82,83]. Although it is known to play a role in adhesion and cell migration [84], its effect on AV biology and dysfunction has not been fully characterized.
In vascular ECs, primary cilia serve as mechanosensors [85].These membrane-bound structures protrude into the vessel lumen and bend during blood flow, leading to calcium influx via mechanosensitive channels and intracellular signal transduction pathway [86,87,88,89]. Abnormal ciliary length has been associated with altered blood flow sensing [90]. Their function in the AV was reported in the context of AV development [91], but definitive evidence supporting their role as AV mechanosensors is lacking at present.
PIEZO1 is a mechanically activated ion channel, serving as a mechanosensor of shear stress in vascular ECs [92]. Activation of PIEZO1 has been linked to atherogenic processes such as inflammation, angiogenesis, vascular formation and remodeling, and ATP release leading to nitric oxide production and regulation of vascular tone [93,94,95,96]. In the AV, PIEZO1 regulates AV development and outflow tract formation [97, 98]; however, its role as a mechanosensor in the AV has not yet been elucidated.
miRNAs in the Aortic Valve
We recently reviewed the role of shear-sensitive genes and signaling pathways in flow-mediated AV biology and disease [99]; therefore, this chapter will focus on discussing the role of miRNAs.
The miRNAs are small (18–22), non-coding nucleotide sequences that bind to the 3′ untranslated region of their target genes, leading to that gene’s degradation or inhibition of translation [100]. The roles of protective and pathological miRNAs have been well documented in many cardiovascular diseases, including atherosclerosis, heart failure, diabetes, and hypertension [101,102,103,104,105].
To identify miRNAs involved in CAVD, human AV leaflets obtained from patients with various AV calcification and stenoses were compared to controls by using miRNA array studies. These studies revealed many miRNAs differentially expressed in calcified AVs compared to the non-diseased controls [106,107,108,109,110], the underlying mechanisms, and potential target genes of each miRNA in AV calcification [111,112,113,114,115,116,117]. Additional array studies identified miRNAs differentially expressed in BAV leaflets in comparison to the TAVs [118, 119].
Most of the miRNAs studied in the context of CAVD have been implicated in the regulation of osteogenic differentiation of VICs (Table 6.1 and Fig. 6.3). The majority of miRNAs that are differentially regulated in CAVD patients seem to be downregulated and play anti-calcific roles; however, some miRNAs such as miR-29b, miR-34a, miR-92a, and miR-181b are increased in CAVD leaflets compared to controls and play pro-calcific roles. We will first discuss those miRNAs with undefined mechanosensitivity, followed by shear- and stretch-sensitive miRNAs.
miRNAs involved in CAVD. Various miRNA microarray studies have been conducted to identify miRNAs differentially expressed between calcified vs. non-calcified aortic valves (AVs), bicuspid vs. tricuspid AVs, fibrosa vs. ventricularis, and d-flow vs. s-flow. Although most identified miRNAs have undefined mechanosensitivity (blue), some have been validated as shear-sensitive (yellow) and stretch-sensitive (orange)
miRNAs with Undefined Mechanosensitivity in the Aortic Valve
miR-29b is increased during osteoblast differentiation of human VICs [116], suggesting it as a pro-calcific miRNA. miR-29b modulates osteoblastic differentiation by downregulatingTGF-β3 and upregulating expression of wnt, β-catenin, Runx2, and Smad3. It is unknown at present if miR-29b is mechanosensitive.
miR-34a is a pro-calcific miRNA and is increased in CAVD leaflets and in VICs treated with osteogenic stimulus [117]. miR-34a directly targets Notch1 and increases calcification signals by upregulating Runx2. While it was shown to be shear-sensitive in HUVECs [120], it remains to be validated if miR-34a is also shear-sensitive in AV ECs.
miR-92a is overexpressed in human calcified BAV leaflets compared to the TAV, suggesting its role as a potential biomarker of CAVD [118]. While miR-92a expression is well-known to be increased by d-flow conditions in vascular ECs, its flow-dependent expression in AV ECs and whether it induces pro-calcific responses in AV remain to be validated. In vascular ECs, miR-92a directly targets KLF-2 and KLF-4, which are some of the most well-characterized mechanosensitive and anti-atherogenic genes [121,122,123].
miR-138 is decreased in calcified AVs and inhibits osteogenesis of VICs [124]. Expression of miR-138 was reduced in leaflets from CAVD patients compared to non-calcified AVs. Overexpression of miR-138 prevented osteogenic differentiation of VICs, while miR-138 inhibitor enhanced the osteogenic differentiation by targeting FOXC1.
miR-204 expression is downregulated in calcified human AVs and by BMP-2 treatment in VICs [125]. miR-204 inhibited osteoblastic differentiation of VICs by targeting Runx2.
miR-30b also regulates osteogenic differentiation of VICs [126]. Transfection of VICs with miR-30b reduced expression of Runx2, Smad1, and Caspase-3 and inhibited ALP activity.
miR-449c-5p expression is reduced in CAVD leaflets compared with non-calcified AVs. miR-449c-5p targets Smad4, thereby preventing osteogenic differentiation of VICs [115].
miR-638 also plays a role in VIC osteogenic differentiation by directly targeting Sp7 [127].
miR-141 regulates BMP-2-dependent AV calcification [128]. miR-141 expression was decreased in human BAV tissue compared to the TAV. Furthermore, in porcine VICs, overexpression of miR-141 attenuated TGF-β-induced activation, BMP-2 signaling, and ALP activity.
miR-195 is downregulated in BAV leaflets compared to the TAV and promotes AV calcification [129]. Treatment of VICs with miR-195 inhibitor (anti-miR-195) increased expression of Smad7, BMP-2, and Runx2, activity of MMP-2, and calcification. miR-195 was shown to directly target Smad7.
Shear-Dependent miRNAs in AV
Numerous miRNAs are regulated by shear stress in VECs in vitro and in vivo. We identified miRNAs that are regulated in a shear stress- and AV leaflet side-dependent manner (ventricularis vs. fibrosa side) using both human AV ECs and porcine AV (PAV) leaflets [130, 131].
To better understand shear-dependent and side-dependent miRNAs in human AVECs (HAVECs) , we carried out miRNA microarrays using total RNA from human fibrosa-side ECs and ventricularis-side ECs exposed to d-flow or s-flow [130]. This study revealed that expression of 30 and 3 miRNAs is regulated in a shear- and side-dependent manner, respectively. Furthermore, studies using endothelial-enriched RNAs from healthy PAVs showed additional side-dependent miRNAs that are differentially expressed in the fibrosa and ventricularis sides [131]. Given the preferential development of AV calcification in the fibrosa side (exposed to pro-calcific biomechanical forces) compared to the ventricularis side (exposed to anti-calcific biomechanical forces), these side-dependent miRNAs may represent the effect of different mechanical force environments on miRNA expression patterns. This study identified 7 miRNAs (miR-100, miR-130a, miR-181a/b, miR-199a-3p, miR-199a-5p, and miR-214) significantly overexpressed in the fibrosa side compared to the ventricularis side. Some of these miRNAs were characterized for their roles in AV function.
miR-486-5p is one of the most shear-responsive miRNAs, and its expression is upregulated under s-flow in vitro and on the ventricularis of PAVs [132]. Overexpression of miR-486-5p in HAVECs was found to decrease expression of Efna1 and Prnd, which are involved in cell migration and early apoptosis.
miR-181b is a pro-calcific miRNA that is upregulated in the fibrosa and in response to d-flow compared to s-flow [114]. We found that miR-181b directly targets tissue inhibitor of metalloproteinase-3 (TIMP3), which manages uncontrolled ECM degradation by inhibiting matrix metalloproteinases (MMPs). Silencing of miR-181b leads to decreased MMP activity in HAVECs.
miR-214 is overexpressed in the fibrosa of PAVs compared to the ventricularis and in response to d-flow compared to s-flow [131]. Expression of miR-214 under d-flow was attenuated using anti-miR-214. Silencing of miR-214 also resulted in increased TGF-β1 protein expression; however, there was no effect on calcification.
miR-483-3p is a highly shear-sensitive miRNA expressed in HAVECs and inhibits AV calcification by regulating the hypoxia-inducible factor 1α (HIF1α) pathway [133]. miR-483-3p is upregulated in the ventricularis side compared to the fibrosa in PAV leaflets and in response to s-flow vs. d-flow in HAVECs. Overexpression of miR-483-3p in HAVECs leads to decreased inflammation and decreased endothelial-mesenchymal transition (EndMT). Mechanistically, miR-483-3p directly targets ubiquitin-conjugating enzyme 2c (Ube2c), which itself is upregulated in d-flow. UBE2C silences von Hippel-Lindau protein (pVHL), a known repressor of HIF1α [134], and its downstream target genes. Furthermore, treatment of PAVs with miR-483-3p mimic and HIF1α inhibitor prevented calcification of PAV leaflets. Consistent with the ex vivo and in vitro results, immunohistochemical staining studies of human AV leaflets demonstrated that the fibrosa side overexpressed markers of calcification (Alizarin red and RUNX2), inflammation (VCAM1), EndMT (TWIST1), HIF1α, and UBE2C compared to the ventricularis side, while pVHL expression was higher in the ventricularis side. These findings suggest a potential of miR-483-3p mimic and HIF1α inhibitors as potential therapeutics for CAVD.
Stretch-Dependent miRNAs in AV
miR-148-3p expression is downregulated by stretching (14%) compared to a static condition in VICs [112] and may serve an anti-calcific function. The downregulation of miR-148-3p led to upregulation of IKBKB, NF-κB signaling, and inflammatory responses in VICs compared to static conditions.
miR-214 is another stretch-dependent miRNA, decreasing by hyper-stretching (15%) relative to physiological stretch (10%) in PAV leaflets [135]. The study suggested that miR-214 may serve an anti-calcific role in the AV by directly targeting activating transcription factor 4 (ATF4), which is implicated in endoplasmic reticulum stress and linked to cardiovascular calcification.
Future Perspectives in Calcific Aortic Valve Disease Research and Treatment
Here, we summarized the AV structures, hemodynamics, and mechanosensors, as well as the recently discovered miRNAs that are implicated in CAVD pathophysiology. Our focus was on discussing the roles of these miRNAs in AV calcification and the roles of biomechanical forces on miRNA expression. Due to the predominant development of AV calcification in the fibrosa side (exposed to d-flow) and in the leaflet hinge regions (exposed to high stretch and strain), it is critical to define the molecular mechanisms such as the role of miRNAs by which these biomechanical forces regulate CAVD pathophysiology. Given the relative convenience and specificity of miRNA mimics (gain of function) and inhibitors such as anti-miRs and antagomiRs (loss of function) to manipulate their expression level and in vivo efficacies, targeting pro-calcific or anti-calcific miRNAs represents an exciting opportunity to develop novel therapeutics to prevent and reduce CAVD. Further, these miRNAs are easily detectable and quantified in blood samples, making them ideal as biomarkers to detect CAVD at an early stage. Despite these potential therapeutic candidates, there are several limitations to overcome. First, although numerous studies have emerged recently, there is still significant paucity in our molecular and cellular understanding of CAVD pathophysiology. Second, the role of mechanosensors and mechanotransduction pathways has been studied mostly in vascular ECs, while this knowledge is relatively lacking in valvular cells. Therefore, the mechanobiological understanding of AV cells needs to be significantly expanded. Third, there continues to be a significant gap in understanding the role of numerous miRNAs in pathophysiological mechanisms of CAVD. While there are many miRNAs that have been better characterized in other cell types, tissues, and disease contexts, the role and mechanisms of each miRNA in AV cells are not necessarily the same and should be validated. Last, technical limitations in AV-targeted delivery of miRNA therapeutics, such as miRNA mimics , anti-miRs, or antagomiRs, should be addressed. Successful delivery of these miRNA therapeutics in an AV-limited manner would overcome concerns regarding their potential off-target effects in other tissues, improving their safety and efficacy as therapeutics.
In summary, the AV is a complex and dynamic structure which is subject to ever-present biomechanical forces. The field of AV mechanics and biomechanical stress-mediated signaling has advanced greatly over the past decade; however, there is still no viable therapeutic for CAVD aside from AV open heart surgery or transcatheter valve replacement. In the future, targeting the mechanosensitive factors, such as miRNAs, discussed here could provide better options for the management of CAVD.
Abbreviations
- ALP:
-
Alkaline phosphatase
- AV:
-
Aortic valve
- BAV:
-
Bicuspid aortic valve
- CAVD:
-
Calcific aortic valve disease
- D-Flow:
-
Disturbed flow
- ECM:
-
Extracellular matrix
- GPCR:
-
G protein-coupled receptor
- miRNA:
-
microRNA
- S-Flow:
-
Stable flow
- TAV:
-
Tricuspid aortic valve
- VEC:
-
Valvular endothelial cell
- VIC:
-
Valvular interstitial cell
References
Benjamin EJ, et al. Heart disease and stroke Statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528.
Rajamannan NM, et al. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: calcific aortic valve disease-2011 update. Circulation. 2011;124(16):1783–91.
Hsu SY, et al. Aortic valve sclerosis is an echocardiographic indicator of significant coronary disease in patients undergoing diagnostic coronary angiography. Int J Clin Pract. 2005;59(1):72–7.
Otto CM, et al. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341(3):142–7.
Leopold JA. Cellular mechanisms of aortic valve calcification. Circ Cardiovasc Interv. 2012;5(4):605–14.
Mohler ER, et al. Development and progression of aortic valve stenosis: atherosclerosis risk factors–a causal relationship? A clinical morphologic study. Clin Cardiol. 1991;14(12):995–9.
Muneretto C, et al. A comparison of conventional surgery, transcatheter aortic valve replacement, and sutureless valves in “real-world” patients with aortic stenosis and intermediate- to high-risk profile. J Thorac Cardiovasc Surg. 2015;150(6):1570–7.. discussion 1577–9
Dasi LP, et al. On the mechanics of Transcatheter aortic valve replacement. Ann Biomed Eng. 2017;45(2):310–31.
Czarny MJ, Resar JR. Diagnosis and management of valvular aortic stenosis. Clin Med Insights Cardiol. 2014;8(Suppl 1):15–24.
Otto CM, et al. Characterization of the early lesion of 'degenerative' valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90(2):844–53.
Wallby L, et al. T lymphocyte infiltration in non-rheumatic aortic stenosis: a comparative descriptive study between tricuspid and bicuspid aortic valves. Heart. 2002;88(4):348–51.
Olsson M, et al. Accumulation of T lymphocytes and expression of interleukin-2 receptors in nonrheumatic stenotic aortic valves. J Am Coll Cardiol. 1994;23(5):1162–70.
Kaden JJ, et al. Interleukin-1 beta promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis. 2003;170(2):205–11.
Rajamannan NM, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107(17):2181–4.
Jian B, et al. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg. 2003;75(2):457–65.. discussion 465–6
Richards J, et al. Side-specific endothelial-dependent regulation of aortic valve calcification: interplay of hemodynamics and nitric oxide signaling. Am J Pathol. 2013;182(5):1922–31.
Singh R, et al. Age-related changes in the aortic valve affect leaflet stress distributions: implications for aortic valve degeneration. J Heart Valve Dis. 2008;17(3):290–8.. discussion 299
Lancellotti P. Grading aortic stenosis severity when the flow modifies the gradient valve area correlation. Cardiovasc Diagn Ther. 2012;2(1):6–9.
Then KL, Rankin JA. Hypertension: a review for clinicians. Nurs Clin North Am. 2004;39(4):793–814.
Sacks MS, Schoen FJ, Mayer JE. Bioengineering challenges for heart valve tissue engineering. Annu Rev Biomed Eng. 2009;11:289–313.
Wiltz D, et al. Extracellular matrix organization, structure, and function. In: Aikawa E, editor. Calcific Aortic Valve Disease. Rijeka, Croatia: InTech; 2013, pp. 3–30.
Schoen FJ. Aortic valve structure-function correlations: role of elastic fibers no longer a stretch of the imagination. J Heart Valve Dis. 1997;6(1):1–6.
Chen JH, Simmons CA. Cell-matrix interactions in the pathobiology of calcific aortic valve disease: critical roles for matricellular, matricrine, and matrix mechanics cues. Circ Res. 2011;108(12):1510–24.
Parnell A, Swanevelder J. High transvalvular pressure gradients on intraoperative transesophageal echocardiography after aortic valve replacement: what does it mean? HSR Proc Intensive Care Cardiovasc Anesth. 2009;1(4):7–18.
Takx RA, et al. The interdependence between cardiovascular calcifications in different arterial beds and vascular risk factors in patients at high cardiovascular risk. Atherosclerosis. 2015;238(1):140–6.
Iwata S, et al. Higher ambulatory blood pressure is associated with aortic valve calcification in the elderly: a population-based study. Hypertension. 2013;61(1):55–60.
Bermejo J. The effects of hypertension on aortic valve stenosis. Heart. 2005;91(3):280–2.
Ivanovic B, Tadic M, Dincic D. The effects of arterial hypertension on aortic valve stenosis. Vojnosanit Pregl. 2010;67(7):588–92.
Kaden JJ, Haghi D. Hypertension in aortic valve stenosis--a Trojan horse. Eur Heart J. 2008;29(16):1934–5.
Chester AH, et al. The living aortic valve: from molecules to function. Glob Cardiol Sci Pract. 2014;2014(1):52–77.
Arjunon S, et al. Aortic valve: mechanical environment and mechanobiology. Ann Biomed Eng. 2013;41(7):1331–46.
Beppu S, et al. Rapidity of progression of aortic stenosis in patients with congenital bicuspid aortic valves. Am J Cardiol. 1993;71(4):322–7.
Stewart BF, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular health study. J Am Coll Cardiol. 1997;29(3):630–4.
Boon A, et al. Cardiac valve calcification: characteristics of patients with calcification of the mitral annulus or aortic valve. Heart. 1997;78(5):472–4.
Katayama S, et al. Bicuspid aortic valves undergo excessive strain during opening: a simulation study. J Thorac Cardiovasc Surg. 2013;145(6):1570–6.
Conti CA, et al. Biomechanical implications of the congenital bicuspid aortic valve: a finite element study of aortic root function from in vivo data. J Thorac Cardiovasc Surg. 2010;140(4):890–6, 896.e1–2.
Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol. 2010;55(25):2789–800.
Martin C, Sun W. Biomechanical characterization of aortic valve tissue in humans and common animal models. J Biomed Mater Res A. 2012;100(6):1591–9.
Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111(24):3316–26.
Ankeny RF, et al. Preferential activation of SMAD1/5/8 on the fibrosa endothelium in calcified human aortic valves--association with low BMP antagonists and SMAD6. PLoS One. 2011;6(6):e20969.
Agmon Y, et al. Aortic valve sclerosis and aortic atherosclerosis: different manifestations of the same disease? Insights from a population-based study. J Am Coll Cardiol. 2001;38(3):827–34.
Weston MW, LaBorde DV, Yoganathan AP. Estimation of the shear stress on the surface of an aortic valve leaflet. Ann Biomed Eng. 1999;27(4):572–9.
Barker AJ, et al. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging. 2012;5(4):457–66.
Tarbell JM, et al. Fluid mechanics, arterial disease, and gene expression. Annu Rev Fluid Mech. 2014;46:591–614.
Demos C, Tamargo I, Jo H. Biomechanical regulation of endothelial function in atherosclerosis. In: Ohayon J, Finet G, Pettigrew R, editors. Biomechanics of coronary atherosclerotic plaque: from model to patient. Cambridge, MA: Academic Press; 2020, pp. 3–50.
Yang B, Rizzo V. Shear stress activates eNOS at the endothelial apical surface through beta1 containing Integrins and Caveolae. Cell Mol Bioeng. 2013;6(3):346–54.
Zhao F, et al. Roles for GP IIb/IIIa and alphavbeta3 integrins in MDA-MB-231 cell invasion and shear flow-induced cancer cell mechanotransduction. Cancer Lett. 2014;344(1):62–73.
Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8(5):215.
Ziegler WH, et al. Integrin connections to the cytoskeleton through Talin and vinculin. Biochem Soc Trans. 2008;36(Pt 2):235–9.
Parsons JT, et al. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene. 2000;19(49):5606–13.
Ciobanasu C, Faivre B, Le Clainche C. Integrating actin dynamics, mechanotransduction and integrin activation: the multiple functions of actin binding proteins in focal adhesions. Eur J Cell Biol. 2013;92(10–11):339–48.
Zebda N, Dubrovskyi O, Birukov KG. Focal adhesion kinase regulation of mechanotransduction and its impact on endothelial cell functions. Microvasc Res. 2012;83(1):71–81.
Lehoux S, et al. Differential regulation of vascular focal adhesion kinase by steady stretch and pulsatility. Circulation. 2005;111(5):643–9.
Li S, et al. The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc Natl Acad Sci U S A. 2002;99(6):3546–51.
Hirakawa M, et al. Sequential activation of RhoA and FAK/paxillin leads to ATP release and actin reorganization in human endothelium. J Physiol. 2004;558(Pt 2):479–88.
Hsu HJ, et al. Stretch-induced stress fiber remodeling and the activations of JNK and ERK depend on mechanical strain rate, but not FAK. PLoS One. 2010;5(8):e12470.
Sokabe M, et al. Mechanotransduction and intracellular signaling mechanisms of stretch-induced remodeling in endothelial cells. Heart Vessels. 1997; Suppl 12:191–3.
Wu CC, et al. Directional shear flow and Rho activation prevent the endothelial cell apoptosis induced by micropatterned anisotropic geometry. Proc Natl Acad Sci U S A. 2007;104(4):1254–9.
Wang JG, et al. Uniaxial cyclic stretch induces focal adhesion kinase (FAK) tyrosine phosphorylation followed by mitogen-activated protein kinase (MAPK) activation. Biochem Biophys Res Commun. 2001;288(2):356–61.
Katritch V, Cherezov V, Stevens RC. Structure-function of the G protein-coupled receptor superfamily. Annu Rev Pharmacol Toxicol. 2013;53:531–56.
Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci U S A. 2006;103(42):15463–8.
Cuerrier CM, et al. Effect of thrombin and bradykinin on endothelial cell mechanical properties monitored through membrane deformation. J Mol Recognit. 2009;22(5):389–96.
Christopoulos A. Advances in G protein-coupled receptor allostery: from function to structure. Mol Pharmacol. 2014;86(5):463–78.
Becker BF, Chappell D, Jacob M. Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Basic Res Cardiol. 2010;105(6):687–701.
Alphonsus CS, Rodseth RN. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia. 2014;69(7):777–84.
Afratis N, et al. Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J. 2012;279(7):1177–97.
Mehta D, Ravindran K, Kuebler WM. Novel regulators of endothelial barrier function. Am J Physiol Lung Cell Mol Physiol. 2014;307(12):L924–35.
Kolarova H, et al. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediat Inflamm. 2014;2014:694312.
Chien S. Molecular and mechanical bases of focal lipid accumulation in arterial wall. Prog Biophys Mol Biol. 2003;83(2):131–51.
Sarphie TG. Interactions of IgG and beta-VLDL with aortic valve endothelium from hypercholesterolemic rabbits. Atherosclerosis. 1987;68(3):199–212.
Sarphie TG. A cytochemical study of the surface properties of aortic and mitral valve endothelium from hypercholesterolemic rabbits. Exp Mol Pathol. 1986;44(3):281–96.
Kobayashi H, Boelte KC, Lin PC. Endothelial cell adhesion molecules and cancer progression. Curr Med Chem. 2007;14(4):377–86.
Szmitko PE, et al. New markers of inflammation and endothelial cell activation: Part I. Circulation. 2003;108(16):1917–23.
Tzima E, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437(7057):426–31.
Chiu YJ, McBeath E, Fujiwara K. Mechanotransduction in an extracted cell model: Fyn drives stretch- and flow-elicited PECAM-1 phosphorylation. J Cell Biol. 2008;182(4):753–63.
Collins C, et al. Localized tensional forces on PECAM-1 elicit a global mechanotransduction response via the integrin-RhoA pathway. Curr Biol. 2012;22(22):2087–94.
Coon BG, et al. Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. J Cell Biol. 2015;208(7):975–86.
Komarova YA, et al. Protein interactions at endothelial junctions and signaling mechanisms regulating endothelial permeability. Circ Res. 2017;120(1):179–206.
Russell-Puleri S, et al. Fluid shear stress induces upregulation of COX-2 and PGI2 release in endothelial cells via a pathway involving PECAM-1, PI3K, FAK, and p38. Am J Physiol Heart Circ Physiol. 2017;312(3):H485–h500.
Fleming I, et al. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci. 2005;118(Pt 18):4103–11.
Qin WD, et al. Low shear stress induced HMGB1 translocation and release via PECAM-1/PARP-1 pathway to induce inflammation response. PLoS One. 2015;10(3):e0120586.
Conway DE, et al. VE-cadherin phosphorylation regulates endothelial fluid shear stress responses through the polarity protein LGN. Curr Biol. 2017;27(14):2219–25.e5.
Pamukcu B, Lip GY, Shantsila E. The nuclear factor–kappa B pathway in atherosclerosis: a potential therapeutic target for atherothrombotic vascular disease. Thromb Res. 2011;128(2):117–23.
Privratsky JR, Newman PJ. PECAM-1: regulator of endothelial junctional integrity. Cell Tissue Res. 2014;355(3):607–19.
Jin X, et al. Cilioplasm is a cellular compartment for calcium signaling in response to mechanical and chemical stimuli. Cell Mol Life Sci. 2014;71(11):2165–78.
Luu VZ, et al. Role of endothelial primary cilia as fluid mechanosensors on vascular health. Atherosclerosis. 2018;275:196–204.
Nauli SM, et al. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117(9):1161–71.
Nauli SM, et al. Non-motile primary cilia as fluid shear stress mechanosensors. Methods Enzymol. 2013;525:1–20.
Pala R, et al. The roles of primary cilia in cardiovascular diseases. Cell. 2018:7(12).
Spasic M, Jacobs CR. Lengthening primary cilia enhances cellular mechanosensitivity. Eur Cell Mater. 2017;33:158–68.
Toomer KA, et al. A role for primary cilia in aortic valve development and disease. Dev Dyn. 2017;246(8):625–34.
Li J, et al. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515(7526):279–82.
Albarran-Juarez J, et al. Piezo1 and Gq/G11 promote endothelial inflammation depending on flow pattern and integrin activation. J Exp Med. 2018;215(10):2655–72.
Kang H, et al. Piezo1 mediates angiogenesis through activation of MT1-MMP signaling. Am J Physiol Cell Physiol. 2019;316(1):C92–c103.
Ranade SS, et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci U S A. 2014;111(28):10347–52.
Wang S, et al. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J Clin Invest. 2016;126(12):4527–36.
Faucherre A, et al. Piezo1 is required for outflow tract and aortic valve development. J Mol Cell Cardiol. 2019;3(143):51–62.
Duchemin AL, Vignes H, Vermot J. Mechanically activated piezo channels modulate outflow tract valve development through the Yap1 and Klf2-Notch signaling axis. Elife. 2019;16(8):e44706.
Fernandez Esmerats J, Heath J, Jo H. Shear-sensitive genes in aortic valve endothelium. Antioxid Redox Signal. 2016;25(7):401–14.
Guo H, et al. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466(7308):835–40.
Nazari-Jahantigh M, et al. MicroRNA-specific regulatory mechanisms in atherosclerosis. J Mol Cell Cardiol. 2015;89(Pt A):35–41.
Kumar S, et al. Role of flow-sensitive microRNAs in endothelial dysfunction and atherosclerosis: mechanosensitive athero-miRs. Arterioscler Thromb Vasc Biol. 2014;34(10):2206–16.
Kalozoumi G, Yacoub M, Sanoudou D. MicroRNAs in heart failure: small molecules with major impact. Glob Cardiol Sci Pract. 2014;2014(2):79–102.
Moura J, Borsheim E, Carvalho E. The role of MicroRNAs in diabetic complications-special emphasis on wound healing. Genes (Basel). 2014;5(4):926–56.
Neves VJ, et al. Exercise training in hypertension: role of microRNAs. World J Cardiol. 2014;6(8):713–27.
Wang H, et al. MicroRNA expression signature in human calcific aortic valve disease. Biomed Res Int. 2017;2017:4820275.
Nigam V, et al. Altered microRNAs in bicuspid aortic valve: a comparison between stenotic and insufficient valves. J Heart Valve Dis. 2010;19(4):459–65.
Takahashi K, et al. Dysregulation of ossification-related miRNAs in circulating osteogenic progenitor cells obtained from patients with aortic stenosis. Clin Sci (Lond). 2016;130(13):1115–24.
Ni WJ, Ma DH, Leng XM. NcRNAs in CAVD: a review of recent studies. J Cardiovasc Pharmacol. 2018;
Fiedler J, et al. Identification of miR-143 as a major contributor for human stenotic aortic valve disease. J Cardiovasc Transl Res. 2019;12(5):447–58.
Ohukainen P, et al. MicroRNA-125b and chemokine CCL4 expression are associated with calcific aortic valve disease. Ann Med. 2015;47(5):423–9.
Patel V, et al. The stretch responsive microRNA miR-148a-3p is a novel repressor of IKBKB, NF-kappaB signaling, and inflammatory gene expression in human aortic valve cells. FASEB J. 2015;29(5):1859–68.
Song R, et al. Altered microRNA expression is responsible for the pro-osteogenic phenotype of interstitial cells in calcified human aortic valves. J Am Heart Assoc. 2017;6(4):1–17.
Heath JM, et al. Mechanosensitive microRNA-181b regulates aortic valve endothelial matrix degradation by targeting TIMP3. Cardiovasc Eng Technol. 2018;9(2):141–50.
Xu R, et al. MicroRNA-449c-5p inhibits osteogenic differentiation of human VICs through Smad4-mediated pathway. Sci Rep. 2017;7(1):8740.
Fang M, et al. Mir-29b promotes human aortic valve interstitial cell calcification via inhibiting TGF-beta3 through activation of wnt3/beta-catenin/Smad3 signaling. J Cell Biochem. 2018;119(7):5175–85.
Toshima T, et al. Therapeutic inhibition of microRNA-34a ameliorates aortic valve calcification via modulation of Notch1-Runx2 signaling. Cardiovasc Res. 2019;
Nader J, et al. miR-92a: a novel potential biomarker of rapid aortic valve calcification. J Heart Valve Dis. 2017;26(3):327–33.
Sabatino J, et al. MicroRNAs fingerprint of bicuspid aortic valve. J Mol Cell Cardiol. 2019;134:98–106.
Fan W, et al. Shear-sensitive microRNA-34a modulates flow-dependent regulation of endothelial inflammation. J Cell Sci. 2015;128(1):70–80.
Boon RA, Hergenreider E, Dimmeler S. Atheroprotective mechanisms of shear stress-regulated microRNAs. Thromb Haemost. 2012;108(4):616–20.
Bonauer A, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324(5935):1710–3.
Wu W, et al. Flow-dependent regulation of Kruppel-like factor 2 is mediated by MicroRNA-92a. Circulation. 2011;124(5):633–41.
Lu P, Yin B, Liu L. MicroRNA-138 suppresses osteoblastic differentiation of Valvular interstitial cells in degenerative calcific aortic valve disease. Int Heart J. 2019;60(1):136–44.
Wang Y, et al. MicroRNA-204 targets Runx2 to attenuate BMP-2-induced osteoblast differentiation of human aortic valve interstitial cells. J Cardiovasc Pharmacol. 2015;66(1):63–71.
Zhang M, et al. MicroRNA-30b is a multifunctional regulator of aortic valve interstitial cells. J Thorac Cardiovasc Surg. 2014;147(3):1073–1080.e2.
Jiao W, et al. MicroRNA-638 inhibits human aortic valve interstitial cell calcification by targeting Sp7. J Cell Mol Med. 2019;23(8):5292–302.
Yanagawa B, et al. miRNA-141 is a novel regulator of BMP-2-mediated calcification in aortic stenosis. J Thorac Cardiovasc Surg. 2012;144(1):256–62.
Du J, et al. Downregulated MicroRNA-195 in the bicuspid aortic valve promotes calcification of valve interstitial cells via targeting SMAD7. Cell Physiol Biochem. 2017;44(3):884–96.
Holliday CJ, et al. Discovery of shear- and side-specific mRNAs and miRNAs in human aortic valvular endothelial cells. Am J Physiol Heart Circ Physiol. 2011;301(3):H856–67.
Rathan S, et al. Identification of side- and shear-dependent microRNAs regulating porcine aortic valve pathogenesis. Sci Rep. 2016;6:25397.
Holliday-Ankeny CJ, et al. The function of shear-responsive and side-dependent microRNA-486-5p in aortic valve endothelium. Cardiovas Patholo $V 22, 2013(3).
Fernandez Esmerats J, et al. Disturbed flow increases UBE2C (ubiquitin E2 ligase C) via loss of miR-483-3p, inducing aortic valve calcification by the pVHL (von Hippel-Lindau protein) and HIF-1alpha (hypoxia-inducible factor-1alpha) pathway in endothelial cells. Arterioscler Thromb Vasc Biol. 2019;39(3):467–81.
Maxwell PH, Pugh CW, Ratcliffe PJ. The pVHL-hIF-1 system. A key mediator of oxygen homeostasis. Adv Exp Med Biol. 2001;502:365–76.
Salim MT, et al. miR-214 is stretch-sensitive in aortic valve and inhibits aortic valve calcification. Ann Biomed Eng. 2019;47(4):1106–15.
Acknowledgments
This work was supported by funding from NIH R01 grants HL095070, HL114772, HL113451 to HJ.
Sources of Funding
NIH R01 grants HL095070, HL114772, HL113451, and HHSN268201000043C to HJ.
NV is supported by the Cell and Tissue Engineering NIH Biotechnology Training Grant (T32 GM-008433).
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Villa-Roel, N., Ryu, K., Jo, H. (2020). Role of Biomechanical Stress and Mechanosensitive miRNAs in Calcific Aortic Valve Disease. In: Aikawa, E., Hutcheson, J. (eds) Cardiovascular Calcification and Bone Mineralization. Contemporary Cardiology. Humana, Cham. https://doi.org/10.1007/978-3-030-46725-8_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-46725-8_6
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-46724-1
Online ISBN: 978-3-030-46725-8
eBook Packages: MedicineMedicine (R0)