Abstract
Hutchinson-Gilford progeria syndrome (HGPS) and other progeroid (premature aging) syndromes are attributable to lamin A processing defects; these result in aberrant nuclear architectures that impact normal transcription and translation, resulting in a premature aging phenotype at the cellular, tissue, and organismal levels. Patients with HGPS are characterized by severe accelerated cardiovascular disease, including valvular calcification and vessel wall stiffening and calcification as well as atherosclerosis, typically culminating in premature death due to myocardial infarction or stroke. Understanding the mechanisms of the early and aggressive vascular and valvular calcification (and vascular atherosclerosis) in the progeroid syndromes may cast important light on the pathways (and therapies) relevant for physiologic aging and senescence.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Progeria
- Mechanisms of calcification
- Aging
- Degeneration
- Lamin A mutations
- Progerin
- Accelerated atherosclerosis
Hutchinson-Gilford Progeria Syndrome (HGPS)
The gross manifestations of Hutchinson-Gilford progeria syndrome (HGPS) were recognized as early as 1754 in an Englishman man who died at age 17. The entity was initially described clinically in 1886 by Jonathan Hutchinson [1], with a subsequent case reported in 1897 by Hastings Gilford [2]; in 1904, Gilford christened the disease “progeria” (literally “before old age”) [3]. HGPS is categorized as a premature aging (progeroid) syndrome; it occurs in roughly one in 20 million live births; there are approximately 400–500 cases described in the worldwide literature. Affected individuals are initially healthy and asymptomatic, but at 18–24 months diverge from normal growth curves (“failure to thrive”), eventually exhibiting a characteristic phenotype comprising scleroderma-like skin, alopecia, osteopenia, skeletal dysplasias (including micrognathia and a “bird-beak” nose), joint contractures, and decreased subcutaneous tissue (lipodystrophy) [4]. In addition to cardiac conduction defects, individuals with HGPS most importantly develop markedly accelerated cardiovascular disease with vascular stiffening, valvular and vascular calcification, and severe atherosclerosis; patients characteristically die of myocardial infarction or stroke at 14–15 years of age. Because many of the features of HGPS phenocopy the changes associated with normal human senescence, it has been proposed as a model for physiologic aging (see final section). The implicit assumption is that therapy which can ameliorate the premature aging of progeria can potentially mitigate more typical physiologic senescence.
Mechanisms Underlying Non-HGPS Progeroid Syndromes
HGPS is actually just one of the more well-known of several progeroid (“resembling normal aging”) syndromes, each with somewhat distinct mechanisms and different manifestations [5].
Virtually all of the premature aging diseases are monogenic—attributable to mutations in a single gene. Several are associated with primary defects in DNA repair, often (although not uniformly) accompanied by heightened mutation rates and cancer incidence. Progeroid DNA repair disorders include (1) mutations in various RecQ helicases responsible for unwinding DNA during replication and repair (e.g., Werner syndrome, Bloom syndrome, and Rothmund-Thompson syndrome) and (2) mutations in a number of the proteins involved in nucleotide excision DNA repair (e.g., Cockayne syndrome and xeroderma pigmentosum). Mutations in the gene for the extracellular matrix protein fibrillin have also been linked to the Marfan-progeroid-lipodystrophy syndrome with individuals exhibiting a progeroid appearance at birth [6]. Interestingly, although all of these entities are associated with variably shortened lifespans, many patients with DNA repair defects can live into adulthood, and morbidity and mortality are not always attributable to cardiovascular causes.
Another pathway implicated in accelerated aging involves klotho, a transmembrane β-glucuronidase that acts as a co-receptor for the binding of various fibroblast growth factors; it is also an endogenous regulator of mitochondrial function and antioxidant production (reviewed in [7]). Klotho levels decrease with aging; in murine models, klotho deficiency leads to premature senescence while promoting vascular calcification. Of note, no genetic klotho deficiency disorders have been described in humans, although klotho polymorphisms have been linked to heterogeneities in aging phenotype onset and severity [8]. In cell cultures and in mouse models, reduced levels of Klotho upregulate Runx2, a central transcription factor control node for osteogenic differentiation; concurrently, klotho deficiency leads to increased levels of tissue non-specific alkaline phosphatase (TNAP) which catabolizes inorganic pyrophosphate, an endogenous inhibitor of calcification. In general, oxidative stress and reactive oxygen species (ROS) feature broadly in any disorder that involves vascular and valvular calcification. Thus, the expression levels of several bone morphogenic proteins, as well as Runx2, are all upregulated by various ROS (reviewed in [7]).
Mechanisms Underlying HGPS Syndrome
This section will focus specifically on HGPS, one of the dozen so-called laminopathies caused by abnormal abundance or aberrant post-translational processing of lamin A [9].
Lamins are intermediate filaments associated with the inner nuclear membrane of the nuclear envelope; they provide nuclear structural integrity with additional roles in regulating nuclear shape, chromatin architecture, signal transduction, and gene transcription [10]. Divided into homologous A- and B-type proteins, both lamins are expressed in most differentiated mammalian cells; they are subject to alternative splicing and also undergo significant post-translational modification [11].
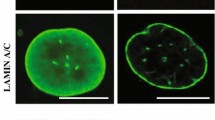
In particular, lamin A and lamin C are alternatively spliced A-type lamins encoded by the LMNA gene; HGPS is specifically a disorder of abnormal lamin A processing. Mature lamin A is generated by progressive modification from a prelamin A precursor (Fig. 11.1) [12]. After farnesylation, the C-terminal serine-isoleucine-methionine tripeptide is removed and replaced with a carboxymethyl residue . The farnesylation and carboxymethyl adduct leads to a more hydrophobic protein that intercalates into the lipid of the inner nuclear membrane. The carboxymethyl and farnesyl modifications form the substrate for zinc metalloproteinase STE24 (ZMPSTE24, also known as farnesylated protein converting enzyme-1, or FACE-1) to cleave the terminal 15 amino acids and release the mature lamin A molecule; this protein can then interact with a variety of nuclear membrane proteins to organize nuclear architecture and regulate gene activity. Importantly, if the farnesyl group is not ultimately removed, persistent membrane integration will lead to a distorted and dysfunctional nuclear architecture (see below).
Normal (left side) and classical mutant HGPS (right side) prelamin A processing and effects on cellular physiology. Wild type prelamin A is initially farnesylated at a cysteine residue near the C-terminus, followed by removal of the terminal serine-isoleucine-methionine tripeptide and then carboxymethylation; the fanesylation and carboxymethylation allow the precursor molecule to integrate into the inner nuclear membrane. This protein is then cleaved by zinc metalloproteinase STE24 (ZMPSTE24) to form the mature lamin A protein, which is no longer intercalated into the nuclear membrane, but does interact with a variety of nuclear envelope proteins to maintain normal nuclear shape and chromatin architecture. In the case of the HGPS prelamin A, a de novo synonymous mutation replaces the cytosine with a thymidine at position 1824 in the 11th exon; although this theoretically results in a conserved codon for glycine at position 608, the C>T transition generates an alternative splice site that removes 150 nucleotides from exon 11, resulting in the deletion of 50 amino acids including the cleavage site for ZMPSTE24. Subsequent farnesylation and carboxymethylation produces the progerin protein that remains associated with the inner nuclear membrane and leads to nuclear distortion with alterations in chromatin architecture, intracellular signaling, and mechanotransduction, ultimately leading to genomic instability and premature senescence. Note that low levels of progerin are synthesized ‘normally’ in aged cells and can be induced by oxidative stress and telomere shortening
HGPS typically occurs as a consequence of a de novo synonymous c.1824C>T mutation in the 11th exon of LMNA (Fig. 11.1). Although the C>T transition (GGC>GGT) codes for glycine in both cases (G608G), it also generates a new splice site in exon 11, ultimately leading to the deletion of 150 nucleotides that code for 50 amino acids, including the ZMPSTE24 cleavage site. Thus, the mutant farnesylated protein—called progerin—cannot be catabolized by FACE-1 and remains permanently attached to the nuclear membrane. As would be expected, mutations that affect the activity of ZMPSTE24 can also lead to persistent prelamin A association with the nuclear membrane and also cause a syndrome analogous to HGPS [13].
The persistent nuclear membrane association of progerin leads to a host of intracellular defects, histologically reflected by distorted and irregular nuclear profiles (Fig. 11.1; reviewed in [14, 15]). Nuclear rigidity and distortion presumably lead to grossly abnormal chromatin architecture, with subsequent defective chromatin remodeling and ineffective access of transcriptional machinery to persistently condensed genomic DNA. DNA damage will result from mechanical strand breakage, and telomere shortening and genomic instability are predictable consequences. The distorted chromatin organization will, in turn, be reflected in aberrant gene transcription and intracellular signaling. It is likely that the nuclear membrane distortion will also lead to defective mechanosensing through altered nuclear-cytoskeletal interactions, which are expected to be especially important for normal vascular function [16, 17]. The dysregulated chromatin architecture will likely also lead to dysfunctional mitoses and possible replication-associated catastrophe. Finally, the requirement for augmented DNA repair will engender excess mitochondrial demands with an additional component of oxidative stress.
Interestingly, although a number of murine models of progeria have been developed, they do not entirely recapitulate the disease phenotype in humans with complete fidelity (reviewed in [18]; (Table 11.1). Some of this is undoubtedly due to differences in cross-species protein interactions (human progerin may associate differently with nuclear membrane proteins than the mouse homologue), as well as the relative role of lamins A and C in murine nuclear architecture. Nevertheless, there are enough overlaps in the clinical course to assert that the basic theory regarding aberrant nuclear structure-function is likely correct. Moreover, the mouse models can be exploited to study disease pathogenesis, as well as the efficacy of various therapeutic interventions.
Given the specific molecular processes underlying the biochemical changes in HGPS, a number of therapeutic interventions have been investigated. In particular, farnesyl transferase inhibitors (e.g., lonafarnib) have proved effective in single-arm clinical trials [19]. There is also augmented benefit to HGPS patients from the addition of statins (blocking upstream HMG-CoA reductase) and bisphosphonates (blocking farnesyl diphosphate synthase) introducing two additional inhibitors to the prelamin farnesylation pathway [20].
Mechanisms for Vascular Pathology in HGPS
Both endothelium and smooth muscle cells are impacted by progerin effects on nuclear transcription.
Endothelial pathology in HGPS
Although less well investigated, endothelial cells (EC) with lamin A mutations exhibited accelerated senescence in vitro and a proinflammatory/atherogenic program of EC activation with sustained expression of leukocyte adhesion molecules (vascular cell adhesion molecule-1 and E-selectin), proinflammatory cytokines (interleukin-8 and monocyte chemoattractant protein-1), and prothrombotic genes (plasminogen activator inhibitor-1), with reduced expression of endothelial nitric oxide synthase (eNOS) and downregulation of Krüppel-like factor 2 (KLF2; a zinc finger transcription factor important in maintaining normal endothelial function at sites of laminar flow) [21,22,23]. Moreover, conditioned media from HGPS EC induced similar dysfunction in normal endothelium [23] suggesting that soluble mediators and/or extracellular vesicles may be involved [24, 25].
Vascular smooth muscle cell pathology in HGPS
Vascular smooth muscle cell (VSMC) attrition, with increased proteoglycan matrix expression, occurs in murine progeria models as well as in HGPS patients [26, 27]. This VSMC loss may be attributable to error-prone DNA repair mechanisms including non-homologous end joining of double-stranded DNA breaks, leading to increased cell death through caspase-independent mechanisms (e.g., mitotic catastrophe) [28]. Loss of VSMC and increased matrix synthesis will ultimately engender stiffer, less compliant vessels; loss of normal endothelial function (e.g., with diminished eNOS production) will also lead to increased vascular tone; systemically, this can promote the hypertensive changes seen in progeria.
Cardiovascular calcification in HGPS
An increased propensity to calcification underlies many of the vascular and valvular pathologies associated with HGPS (reviewed in [12]); these likely involve an increased production by VSMC of factors that drive osteogenic differentiation, as well as the loss of extracellular inorganic pyrophosphate (PPi), a major inhibitor of calcification [29] (Fig. 11.2).
Mechanisms of calcification in wild type and HGPS vascular smooth muscle cells. Nuclear distortion and instability lead to oxidative stress and mitochondrial dysfunction, which will result in reduced ATP production and ultimately diminished inorganic pyrophosphate (PPi) synthesis. Since extracellular PPi is a major inhibitor of calcification, mechanisms that reduce its synthesis or increase its degradation lead to greater matrix calcification. Thus, increased production by HGPS cells of tissue non-specific alkaline phosphatase (TNAP) and ectonucleoside triphosphate diphosphohydrolase-1(eNTPD1) reduces the functional levels of PPi. At the same time, HGPS vascular smooth muscle cells are also synthesizing osteogenic transcription factors, such as runx2, as well as a host of osteogenic regulatory proteins that are associated with a senescent secretory VSMC phenotype. The net result is markedly increased propensity for matrix calcification. eNPP1: ectonucleotide pyrophosphatase/phosphodiesterase 1, an enzyme that breaks down extracellular ATP to generate PPi
PPi synthesis is reduced when mitochondrial dysfunction results in diminished ATP synthesis (the main source of PPi); this occurs in progeroid syndromes presumably through increased oxidative stress and defective mitochondrial turnover. In addition, knock-in mice carrying the mutant Lmna G609G/+ construct exhibited concurrent upregulation of enzymes that catabolize ATP (ectonucleoside triphosphate diphosphohydrolase-1; eNTPD-1) or PPi, including tissue non-specific alkaline phosphatase (TNAP) [30]; exogenous PPi reduced vascular calcification in this model.
On the flip side of the equation (reviewed in [12]), DNA damage (associated with cellular senescence) and oxidative stress conspire to reduce ZMPSTE24 expression in VSMC, resulting in increased prelamin A levels. In turn, accumulating prelamin A drives endogenous damage repair mechanisms and a senescent VSMC secretory phenotype [31]. As a result, VSMC produce increasing levels of osteogenic regulators such as osteocalcin and osteopontin, and the transcription factor Runx2, in addition to mediators that promote calcification, including interleukin 6, bone morphogenic protein 2, and osteoprotogerin (Fig. 11.2).
Although valvular calcification (most commonly in the aortic valve) has been reported in HGPS patients [32], nothing is known about the underlying mechanisms and whether the pathways involved might actually mirror typical valvular senescent calcification. Presumably, the same pathways described for VSMC also impact the propensity for calcification in valvular interstitial cells, but this has not been formally evaluated. Although there is an understandable paucity of human HGPS valvular specimens to analyze, the various murine models provide excellent opportunities to explore altered calcification in other sites. Indeed, to date, valvular degenerative pathologies have not even been reported in any of the mouse progeria systems.
Atherosclerosis in HGPS
Severe atherosclerotic disease is a common feature in HGPS; indeed, as noted previously, the most common cause of death in affected patients is myocardial infarction or cerebrovascular accident. This predilection for early and severe atherosclerosis is not attributable to changes in circulating cholesterol levels or general inflammatory state of affected individuals [33], although EC dysfunction will likely lead to greater inflammatory cell recruitment at sites of vascular injury [22, 23]. Given that atherosclerosis also characteristically occurs at sites of low or turbulent shear stress (i.e., at branch points or sites of vascular curvature), and that EC KLF2 is down regulated in progeria, it also seems likely that defective mechanosensing in the vasculature will be a contributory factor [34]. The atherosclerotic plaques in HGPS closely resemble more typical age-related atherosclerosis although there increased adventitial fibrosis has been described; it is noteworthy that progerin is expressed in a significant fraction of all the cells (EC, intima, media, and adventitia) in these plaques [26].
Interestingly, most progeroid murine models exhibit vascular stiffening—likely as a consequence of VSMC loss and increased extracellular matrix synthesis—but atherosclerosis is not a feature in animals with progerin-only mutations (Table 11.1). This is likely due to the fact that mice are relatively resistant to atherosclerosis development, attributable to species differences in cholesterol and lipid metabolism. Nevertheless, expression of murine progerin on a background of congenital apoprotein E deficiency (ApoE−/−) led to the development of accelerated atherosclerosis, as well as atherosclerosis-related mortality [35]. VSMC-specific progerin expression alone yielded a severe atherosclerotic phenotype (and a shortened lifespan) on the ApoE-deficient background, whereas progerin expression in the macrophage cell lineage alone did not.
Relation of HGPS to “Normal” Aging
Physiologic aging is fundamentally the consequence of genomic instability, telomere loss, epigenetic changes, and abnormal protein turnover [36]. The tissues in HGPS patients exhibit many of these molecular, biochemical, and cellular signatures. Consequently, the causal intracellular accumulation of progerin in HGPS patients is promoted as an important window on the mechanisms that underlie physiologic senescence (reviewed in [18, 37]). Furthering the case that aging and progerin are mechanistically linked, oxidative stress and telomere shortening—associated with “normal” aging—also result in prelamin A synthesis [38] and progerin [39] expression in normal “aged” cells . Thus, there is at least a theoretical connection linking the aberrant nuclear membrane architecture associated with progerin to age-related pathologies such as cardiac fibrosis and diastolic dysfunction, vascular stiffening and hypertension, and atherosclerosis, stroke, and myocardial infarction. Presumably, the accelerated nature of the “physiologic” aging in HGPS results from the higher constitutive levels of progerin in virtually all cells leading to severe ongoing dysfunction, versus the relatively sparse progerin-positive cells in normal age-related lesions. In this regard, it is noteworthy that progerin levels do increase with age, but only marginally so; progerin is detectable in <0.01% of fibroblasts in primary cultures from young donor skin biopsies, but only in the range of 0.3–0.8% of fibroblasts from aged donors [40]. Nevertheless, by the time cells reach replicative senescence, the fraction of progerin-positive cells reached almost 90%. In the final analysis, HGPS probably does represent a true model of accelerated aging [18, 37]; it remains to be seen whether the interventions developed to slow the progress of progeria will also prove effective in the more physiologic setting of “normal” human senescence.
Conclusion
The fundamental pathogenesis underlying HGPS is well understood; moreover, the resulting biochemical abnormalities (e.g., persistent lamin farnesylation) are at least partially amenable to pharmacologic intervention. Thus, to the extent that the secondary manifestations of HGPS (lipodystrophy, alopecia, osteopenia, skeletal dysplasias, joint contractures, etc.) truly reflect accelerated “aging,” this incredibly rare entity has the potential to shine a very bright light on pathways that propel physiologic senescence. In particular, the endothelial and smooth muscle cell dysfunction in HGPS are almost certainly the proximate causes of the premature vascular (and valvular) calcification that occurs, as well as the accelerated atherosclerosis that represents the major cause of mortality in affected patients. Consequently, if accumulating progerin is indeed the driver for cardiovascular degenerative pathology (which is still an uncertain proposition), then the pharmacologic interventions developed to treat HGPS patients stand to provide major benefits for the entire (aging) population.
Abbreviations
- EC:
-
Endothelial cells
- eNOS:
-
Endothelial nitric oxide synthase
- eNTPD1:
-
Ectonucleoside triphosphate diphosphohydrolase-1
- FACE-1:
-
Farnesylated protein converting enzyme-1
- HGPS:
-
Hutchinson-Gilford progeria syndrome
- KLF2:
-
Krüppel-like factor 2
- PPi:
-
Inorganic pyrophosphate
- ROS:
-
Reactive oxygen species
- TNAP:
-
Tissue non-specific alkaline phosphatase
- VSMC:
-
Vascular smooth muscle cells
- ZMPSTE24:
-
Zinc metalloproteinase STE24
References
Hutchinson J. A case of congenital absence of hair with atrophic condition of the skin and its appendages. Lancet. 1886;1:923.
Gilford H. On a condition of mixed premature and immature development. Med Chir Trans. 1897;80:17–45.
Gilford H. Progeria: a form of senilism. Practitioner. 1904;73:188–217.
Meredith MA, Gordon LB, Clauss S, Sachdev V, Smith AC, Perry MB, et al. Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med. 2008;358:592–604.
Navarro CL, Cau P, Lévy N. Molecular bases of progeroid syndromes. Hum Mol Genet. 2006;15 Spec No 2:R151–61.
Takenouchi T, Hida M, Sakamoto Y, Torii C, Kosaki R, Takahashi T, et al. Severe congenital lipodystrophy and a progeroid appearance: mutation in the penultimate exon of FBN1 causing a recognizable phenotype. Am J Med Genet A. 2013;161A:3057–62.
Pescatore LA, Gamarra LF, Liberman M. Multifaceted mechanisms of vascular calcification in aging. Arterioscler Thromb Vasc Biol. 2019;39:1307–16.
Arking DE, Krebsova A, Macek M Sr, Macek M Jr, Arking A, Mian IS, et al. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci U S A. 2002;99:856–61.
Worman HJ. Nuclear lamins and laminopathies. J Pathol. 2012;226:316–25.
Gruenbaum Y, Foisner R. Lamins: nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annu Rev Biochem. 2015;84:131–64.
Dittmer TA, Misteli T. The lamin protein family. Genome Biol. 2011;12:222–36.
Dorado B, Andrés V. A-type lamins and cardiovascular disease in premature aging syndromes. Curr Opin Cell Biol. 2017;46:17–25.
Barrowman J, Wiley PA, Hudon-Miller SE, Hrycyna CA, Michaelis S. Human ZMPSTE24 disease mutations: residual proteolytic activity correlates with disease severity. Hum Mol Genet. 2012;21:4084–93.
Vidak S, Foisner R. Molecular insights into the premature aging disease progeria. Histochem Cell Biol. 2016;145:401–17.
Gonzalo S, Kreienkamp R, Askjaer P. Hutchinson-Gilford progeria syndrome: a premature aging disease caused by LMNA gene mutations. Ageing Res Rev. 2017;33:18–29.
Ji JY. Endothelial nuclear lamina in mechanotransduction under shear stress. Adv Exp Med Biol. 2018;1097:83–104.
Qi YX, Han Y, Jiang Z. Mechanobiology and vascular remodeling: from membrane to nucleus. Adv Exp Med Biol. 2018;1097:69–82.
Hamczyk MR, del Campo L, Andrés V. Aging in the cardiovascular system: lessons from Hutchinson-Gilford progeria syndrome. Annu Rev Physiol. 2018;80:27–48.
Gordon LB, Massaro J, D’Agostino RB Sr, Campbell SE, Brazier J, Brown WT, et al. Impact of farnesylation inhibitors on survival in Hutchinson-Gilford progeria syndrome. Circulation. 2014;130:27–34.
Gordon LB, Kleinman ME, Massaro J, D’Agostino RB Sr, Shappell H, Gerhard-Herman M, et al. Clinical trial of the protein farnesylation inhibitors lonafarnib, pravastatin, and zoledronic acid in children with Hutchinson-Gilford progeria syndrome. Circulation. 2016;134:114–25.
Pantsulaia I, Ciszewski WM, Niewiarowska J. Senescent endothelial cells: potential modulators of immunosenescence and ageing. Ageing Res Rev. 2016;29:13–25.
Bonello-Palot N, Simoncini S, Robert S, Bourgeois P, Sabatier F, Levy N, et al. Prelamin A accumulation in endothelial cells induces premature senescence and functional impairment. Atherosclerosis. 2014;237:45–52.
Yap B, Garcia-Cardeña G, Gimbrone MA Jr. Endothelial dysfunction and the pathobiology of accelerated atherosclerosis in Hutchinson-Gilford progeria syndrome. FASEB 22 (meeting abstract supplement). 2008; 471.11.
Hutcheson JD, Goettsch C, Bertazzo S, Maldonado N, Ruiz JL, Goh W, et al. Genesis and growth of extracellular vesicle-derived microcalcification in atherosclerotic plaques. Nat Mater. 2016;15:335–43.
Hutcheson JD, Aikawa E. Extracellular vesicles in cardiovascular homeostasis and disease. Curr Opin Cardiol. 2018;33:290–7.
Olive M, Harten I, Mitchell R, Beers JK, Djabali K, Cao K, et al. Cardiovascular pathology in Hutchinson-Gilford progeria: correlation with the vascular pathology of aging. Arterioscler Thromb Vasc Biol. 2010;30:2301–9.
Varga R, Eriksson M, Erdos MR, Olive M, Harten I, Kolodgie F, et al. Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2006;103:3250–5.
Zhang H, Xiong ZM, Cao K. Mechanisms controlling the smooth muscle cell death in progeria via down-regulation of poly (ADP-ribose) polymerase 1. Proc Natl Acad Sci U S A. 2014;111:E2261–70.
Villa-Bellosta R, Wang X, Millán JL, Dubyak GR, O’Neill WC. Extracellular pyrophosphate metabolism and calcification in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2011;301:H61–8.
Villa-Bellosta R, Rivera-Torres J, Osorio FG, Acín-Pérez R, Enriquez JA, López-Otín C, et al. Defective extracellular pyrophosphate metabolism promotes vascular calcification in a mouse model of Hutchinson-Gilford progeria syndrome that is ameliorated on pyrophosphate treatment. Circulation. 2014;127:2442–51.
Liu Y, Drozdov I, Shroff R, Beltran LE, Shanahan CM. Prelamin A accelerates vascular calcification via activation of the DNA damage response and senescence-associated secretory phenotype in vascular smooth muscle cells. Circ Res. 2013;112:e99–109.
Nair K, Ramachandran P, Krishnamoorthy KM, Dora S, Achuthan TJ. Hutchinson-Gilford progeria syndrome with severe calcific aortic valve stenosis and calcific mitral valve. J Heart Valve Dis. 2004;13:866–9.
Gordon LB, Harten IA, Patti ME, Lichtenstein AH. Reduced adiponectin and HDL cholesterol without elevated C-reactive protein: clues to the biology of premature atherosclerosis in Hutchinson-Gilford progeria syndrome. J Pediatr. 2005;146:336–41.
Song M, San H, Anderson SA, Cannon RO 3rd, Orlic D. Shear stress-induced mechanotransduction protein deregulation and vasculopathy in a mouse model of progeria. Stem Cell Res Ther. 2014;5:41–52.
Hamczyk MR, Villa-Bellosta R, Gonzalo P, Andrés-Manzano MJ, Nogales P, Bentzon JF, et al. Vascular smooth muscle-specific progerin expression accelerates atherosclerosis and death in a mouse model of Hutchinson-Gilford progeria syndrome. Circulation. 2018;138:266–82.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging. Cell. 2013;153:1194–217.
Ashapkin VV, Kutueva LI, Kurchashova SY, Kireev II. Are there common mechanisms between Hutchinson-Gilford progeria syndrome and natural aging? Front Genet. 2019;10:455.
Ragnauth CD, Warren DT, Liu Y, McNair R, Tajsic T, Figg N, et al. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation. 2010;121:2200–10.
Cao K, Blair CD, Faddah DA, Kieckhaefer JE, Olive M, Erdos MR, et al. Progerin and telomere dysfunction collaborate to trigger senescence in normal human fibroblasts. J Clin Invest. 2011;121:2833–44.
McClintock D, Ratner D, Lokuge M, Owens DM, Gordon LB, Collins FS, et al. The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PLoS One. 2007;2:e1269.
Acknowledgments
The author wishes to recognize with appreciation the outstanding and innovative research and the comprehensive HGPS reviews developed by Dr. Vicente Andrés and colleagues at Centro Nacional de Investigaciones Cardiovasculares Carlos III in Madrid, Spain. In addition, Dr. Leslie B. Gordon (Hasbro Children’s Hospital and the Alpert Medical School of Brown University in Providence, RI, and Medical Director of the Progeria Research Foundation) has been a tireless and eloquent international leader and advocate in the pursuit of the understanding and treatment of HGPS; without Dr. Gordon and her colleagues at the PRF, we would know only a fraction of what is understood about the disease.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mitchell, R.N. (2020). Cardiovascular Calcification in Hutchinson-Gilford Progeria and Correlation with Age-Related Degenerative Calcification. In: Aikawa, E., Hutcheson, J. (eds) Cardiovascular Calcification and Bone Mineralization. Contemporary Cardiology. Humana, Cham. https://doi.org/10.1007/978-3-030-46725-8_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-46725-8_11
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-46724-1
Online ISBN: 978-3-030-46725-8
eBook Packages: MedicineMedicine (R0)