Abstract
This chapter reviews the potential of the predatory bacteria Bdellovibrio bacteriovorus, an obligate predator of other gram-negative bacteria, as a biotechnological tool. Due to the unique lifestyle and the different applications, predatory bacteria have awakened interest to be developed as a lytic tool. The lack of physiological and metabolic information makes difficult this development. However, in the last years, different approaches have been described in order to understand the physiology, morphology, and metabolism of the predators, as well as the population dynamics of the prey-predator interactions. Besides its potential of “living antibiotic”, predatory bacteria have been proposed as a biocontrol agent in the food industry or aquaculture. A recent work using B. bacteriovorus as a biological lytic tool for the recovery of intracellular bioproducts highlighted the potential use of predators in industrial bioprocesses. The bottlenecks of using other Bdellovibrio and like organisms (BALOs) have been also considered and discussed during this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
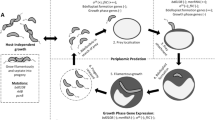
Industrial microbiology and metabolic engineering are becoming key strategies for the biotechnological industries due to the increasing interest in circular economy strategies (Ortiz-Marquez et al. 2013). Environmental protection and sustainability are the central promises. However, successful microbial processes have to be economically efficient in order to compete with traditional manufacturing routes. The economic success of a microbial strategy in a bioprocess is led by three main points: the renewable carbon source, the specific process (i.e. bioconversion), and the downstream process (i.e. purification of the product) (Du et al. 2011). The “microbial factory” has to be adapted to the specific process, which can be achieved using three different approaches (Fig. 1): (i) classical strain improvement, that involves the screening of the desired phenotype, random mutagenesis, and re-screening, (ii) development of cell factories using metabolic engineering by a cyclic process of analysis and engineering of the desired strains, iii) rational strain engineering, where the strategy was initially based on the comprehension of the biochemical stoichiometry and the expected metabolic pathways (Goel et al. 2012).
In the last few decades, Bdellovibrio and like organisms (BALOs) have attracted the attention of the scientific community due to their particular lifestyle, their physiological and metabolic versatility to colonize different niches and their ability to diminish bacterial populations (Sockett 2009). The extraordinary repertoire of species susceptible to predation by BALOs (see below) enables a wide range of potential applications based on their predatory capabilities, such as biocontrol agents in medicine, in agriculture, aquaculture and water treatment (Fig. 2) (Atterbury et al. 2011; Lin et al. 2007; Loozen et al. 2015; Scherff 1973). Apart from their well-documented application as clinical biocontrol agents, predatory bacteria have been proposed as an excellent source of valuable biotechnological enzymes (Bratanis et al. 2017; Lambert and Sockett 2013; Martinez et al. 2012; Rendulic et al. 2004) and as a biological lytic tool for intracellular product release, due to their hydrolytic arsenal (Martinez et al. 2013, 2016). In view of their unique lifestyle, they represent a sound model for evolution studies. Penetration into other cells, as observed with periplasmic BALOs, constitutes a new adaptation that could be subject to studies focusing on the origin of the eukaryotic cells (Davidov and Jurkevitch 2009; Margulis 1996).
BALOs are the group of predatory bacteria best characterized. This group is composed by small vibrioid to rod-shaped gram-negative aerobic and mesophilic bacteria (0.2–0.5 μm wide, 0.5–2.5 μm long) propelled by a single sheathed flagellum, that confers them high motility, reaching velocities of 160 μm s−1 (Thomashow and Rittenberg 1978). Although they were first isolated in soil, they are ubiquitous in nature and can be found in aquatic and terrestrial environments, including hypersaline systems (Piñeiro et al. 2008), biofilms (Kadouri and O’Toole 2005), mammalian guts (Hobley et al. 2012; Schwudke et al. 2001) and cystic fibrosis lung microbiota (de Dios Caballero et al. 2017).
Although predatory bacteria have been proposed as promising microorganisms to be applied in different fields, there is still poor knowledge available to control and use them efficiently. Thus, a deeper understanding of their lifestyle, genetics, and metabolism becomes necessary for BALOs to be developed as microbial cell factories.
In this chapter, we will address the state of the art of the potential use of Bdellovibrio strains in industrial applications. We will expose the applications that have been proposed so far, as well as discuss the drawbacks of the use of BALOs considering the cultivability, the prey range and the possible genetic manipulations to improve the predatory bacteria to be used as a biotechnological tool.
2 BALOs from an Industrial Perspective
Different applications in agriculture, food industry or aquaculture have been recently reported in which B. bacteriovorus is used (Fig. 2). Most of these applications are focussed on the direct application of the wild type predator cells. Until the work by Martínez et al. (2016), it had not been proposed the engineering and optimization of the predator as a biotechnological catalyst.
The first attempt to use predatory bacteria as biocontrol agents was in 1973 when Sherff described the effectiveness of B. bacteriovorus preying on Pseudomonas syringae to avoid the development of bacterial blight of soybean (Scherff 1973). In 2011 this predatory bacterium was used in vivo, highlighting its successful use as living antibiotic in chicken guts with Salmonella infection (Atterbury et al. 2011). B. bacteriovorus was later applied to treat and prevent the spoilage in post-harvest steps for mushrooms (Agaricus bisporus) infected with Pseudomonas tolaasi, which causes blotches on their surface decreasing the quality of the product resulting in economic losses (Saxon et al. 2014).
Bdellovibrio spp. have been also found in several bacterial communities in bioreactors for wastewater treatment. During this process, the contaminants or pathogenic microorganisms potentially present in the industrial or domestic wastewater are removed. In the biological-based steps of the processes, the predator cells could be involved in the process of auto-purification of water by shaping the microbial community and favouring the proliferation of some beneficial bacteria (anaerobic in most of the cases) that remove the more persistent contaminants during the treatment (Guelin et al. 1967; Paoletti et al. 1967). Moreover, BALOs can even be employed to kill pathogenic bacteria from water avoiding the use of hazardous chemicals (Chen et al. 2014).
Apart from the use of the predators directly to decontaminate equipments or the soil from pathogenic bacteria, the interest on biological remediation of land contaminated with hazardous chemicals, such as aromatics compounds, is increasing in the last decades due to the adverse effects on human health and the environment. To this aim, several microorganisms are being used due to their naturally or synthetically ability to degrade those compounds. However, the effectiveness of the treatment is determined by the dispersion of the degrader microorganism (Banitz et al. 2012; Furuno et al. 2010). A very peculiar application of B. bacteriovorus based on its ability to reduce prey strains from the predatory zone has recently been described. In this study of the potential of B. bacteriovorus as an adjuvant for the bioremediation of phenanthrene, it was found that under certain conditions, the predator increased phenanthrene degradation by promoting prey dispersion (Otto et al. 2017).
The susceptibility of biofilms to the attack of B. bacteriovorus has been described (Kadouri and O’Toole 2005). The hydrolytic arsenal encoded in its genome allows the dispersion on the surface of the biofilm releasing the potential prey bacteria to the medium. Also, biofilms degradation products can be used by B. bacteriovorus for protein synthesis and as a source of energy generating ATP (Im et al. 2018). Although there are no examples reported in the literature yet, this capability could be important for use in different bioprocess, where the formation of these scaffolds supposes a bottleneck in the process, because, besides the contamination issue, it could affect the functionality of the equipments (Chmielewski and Frank 2015; Kumar and Anand 1998).
Aquaculture, beyond doubt, is the fastest growing food-producing sector in the world. Its important role is to provide aquatic animal protein to balance out the deficit in the wild fisheries. Likewise, its socio-economic role in providing livelihood opportunities and economic security, particularly for the less-developed regions in the world, is being recognized (Naylor et al. 2000). The threat of diseases has now become a primary constraint and risk to the growth of this sector. The importance of prevention and control of disease risks as a measure to reduce production losses in commercial and small-scale aquaculture systems has thus received increased attention. In particular, outbreaks caused by fish pathogens such as Aeromonas hydrophyla or Yersinia ruckeri among others are considered to be a major problem to fish farming and quality, leading to severe losses on the production (Cao et al. 2012). These infections are now partially controlled by fish farmers with direct application of antibiotics such as terramycin and florfenicol. However, antibiotic treatment is cost-prohibitive to farmers in many undeveloped and developing countries, and antibiotic use may be detrimental to the environment and human health (Harikrishnan et al. 2010). The use of predatory bacteria constitutes an attractive alternative and several reports using them have been published (Cao et al. 2012; Lu and Cai 2010).
The most considered application of BALOs has been as potential antimicrobial agent against animal and human pathogens. Over last decades there has been a decrease in the discovery/development of new antibiotics alongside with an increment in resistance to current antibiotics. Therefore, the need to develop new therapies to treat bacterial infections points at predatory bacteria as a good alternative and they have been proposed as “living antibiotic”. In this sense, there has been increasing research assessing predatory bacteria both in vitro and in vivo for being able to eradicate the population of a wide range of gram-negative bacteria from diverse genera, including multi-drug resistant clinical isolates (Dashiff et al. 2011a; Im et al. 2017).
Taking into account the interesting lifecycle of BALOs (for details, see Chapter “The Ecology of Bdellovibrio and Like Organisms in Wastewater Treatment Plants”, by Jurkevitch) and the crucial role played by their hydrolytic arsenal, it is unsurprising that they are considered to constitute a rich source of hydrolytic enzymes of great interest for industry. Lipases, nucleases, glucanases or hydrolases are some of the potential candidates contained within their genomes (Rendulic et al. 2004). The use of enzymes in industry provides high and superior performances of catalytic processes and can be used on different fields: pharmaceutical and analytical industry, food and feed industry, paper and pulp industry, leather and textile industry and polymer industry among others (Singh et al. 2016). Interestingly, B. bacteriovorus possesses two depolymerases of polyhydroxyalkanoate (PHA) as part of its hydrolytic repertoire. These enzymes are able to specifically degrade short- or medium-chain-length PHA, respectively, in an efficient manner (Martinez et al. 2012). PHA are biodegradable polyesters composed by R-3-hydroxyalkanoate monomers. They are produced by a wide variety of bacteria and have similar physicochemical properties than the conventional polymers, being attractive alternatives to petroleum-based plastics (Prieto et al. 2016). Apart from its use as promising biomaterial, several biotechnological applications have been described for the PHAs involving their synthesis and degradation mechanisms. For instance, as all the 3-hydroxyalkanoates (HAs) incorporated to the pathway are pure enantiomers (R form), they are an important source of quiral syntons in medicine (Philip et al. 2007). Hence, the development of sustainable bioprocesses for producing these quiral intermediates are interesting in industry (Sudesh et al. 2000). One of the more commonly used methods for obtaining HAs is the in vivo and in vitro depolymerization of the PHA, which is based on PHA depolymerase enzymes (de Eugenio et al. 2007). In relation with PHA and taking into account the lytic ability of B. bacteriovorus, this predator has been used as a biological lytic tool for extracting PHA as a value-added intracellular bio-product. This would entail employing a PHA-producing bacterium, such as Pseudomonas putida, as prey (Martinez et al. 2016). This application is explained in detail in the next sections.
3 B. bacteriovorus as an Industrial Lytic System
B. bacteriovorus is the model microorganism among BALOs. It exhibits a biphasic growth cycle, including a free-swimming attack phase (AP) in which B. bacteriovorus search for its prey, and an intraperiplasmic growth phase (GP) inside the prey’s periplasm, forming the so-called bdelloplast structure, where it will digest the prey cellular components to synthesize its own. It is worthwhile to note that, within its large genome (~3.8 Mb), this predator contains a wide-ranging hydrolytic arsenal (150 genes coding for proteases, 10 glycanases, 20 DNases, 9 RNases and 15 lipases) which is crucial during the penetration to the prey cell and also for the lysis of the ghost prey cells, when the progeny is released (Rendulic et al. 2004). From an industrial perspective, B. bacteriovorus is attractive not only for its predation ability but also for its enormous hydrolytic arsenal.
To implement B. bacteriovorus as a biotechnological cell catalyst it should be possible to be controlled rationally (Fig. 3a). This requires a deep knowledge of its physiology and metabolism that allows the construction of metabolic models. Specifically, for predatory bacteria, the understanding of the growth cycle is crucial as well as the prey range in which the predator is efficient. All these along with a set of genetic tools would allow for predator domestication. However, the particular requirements of B. bacteriovorus, such as the prey and high concentrations of oxygen (please see Chapter “Environmental and Biotic Factors Impacting the Activities of Bdellovibrio bacteriovorus”, by Im et al. for more information), will be crucial for the bioprocess design. An optimal inoculum of predator needs to be determined according to the prey concentration reached during the fermentation as well as the moment in which predation will be maximal. Taking everything into account specific parameters for scaling-up processes needs to be calculated. The requirements for B. bacteriovorus to be used as a cell catalyst will be explained in detail in this section.
B. bacteriovorus in industrial bioprocesses as a biological catalyst. (a) Requirements for B. bacteriovorus to be used as a biotechnological tool. In an industrial bioprocess the microorganism employed needs to be domesticated. That means to have a rational control over it with a battery of genetic tools. In the case of B. bacteriovorus, the bioprocess needs to be adapted to the prey range, i.e. it has to be susceptible to be preyed by B. bacteriovorus. The last step in the design of a bioprocess involving B. bacteriovorus is the scale-up: culture parameters, such as agitation rate, flow gas rate or inoculum size, must be calculated to achieve the highest yields. (b) Schematic representation of a bioprocess. In this integrated bioprocess, P. putida KT2440 produces PHA granules intracellularly from a pool of feedstock. P. putida cells are subjected to a biological disruption using B. bacteriovorus, which will facilitate downstream processing to recover the final product (purified PHA). Figure partially made with biorender (https://www.biorender.com)
3.1 Domestication of B. bacteriovorus
One of the principal requirements of B. bacteriovorus to be used as a biotechnological tool is for it to be domesticated, i.e. to have a repertoire of genetic tools that allows its manipulation at a genomic level. Most genetic tools that have been developed to date are addressed for model organisms, which divide by binary fission or gemmation. In contrast, B. bacteriovorus elongates to form an intracellular filament inside the bdelloplast and septates into daughter cells afterwards, promoting an unequal partition of plasmids and making it difficult to develop fully controlled expression systems.
B. bacteriovorus was genetically modified in 1992 for the first time. B. bacteriovorus 109J and its host-independent (HI) derivative B. bacteriovorus BB5, which is able to grow in a rich medium in the absence of prey, were transformed to elucidate the mechanism which drives the axenic growth of HI strains. In that report, two plasmid incompatibility groups were tested, IncQ and IncP, to confer antibiotic resistance to B. bacteriovorus. Constructed plasmids were transferred by conjugation to B. bacteriovorus strains from E. coli SM10 derivatives, which has RK2 transfer functions integrated into its genome (Simon et al. 1983). The RSF1010 (IncQ) derivative plasmids (pSUP204, pSUP304.1 and pMMB33) yielded antibiotic resistance to B. bacteriovorus whereas the RK2 (IncP) derivative plasmids (pRK290, pVK100 and pTC3) did not. Nevertheless, when the latter included a B. bacteriovorus chromosomal region, they conferred antibiotic resistance. Therefore, they concluded that it was possible to perform conjugal transformation of B. bacteriovorus employing RK2 machinery resulting in either autonomous replication with RSF1010 derivative plasmids or chromosomal homologous recombination if the plasmid replicon is an RK2 derivative (Cotter and Thomashow 1992a). In later experiments, Cotter and Thomashow, demonstrated that the cosmid pVK100 including chromosomal sequences of B. bacteriovorus led to merodiploid formation via homologous recombination. They used pVK100 derivative cosmids to identify the hit locus and to restore plaque-forming ability of HI Bdellovibrio isolates (Cotter and Thomashow 1992b). Overall, the works of Cotter and Thomashow described for the first time the possibility to genetically modify B. bacteriovorus as well as described some of the genetic features of the HI phenotype.
The capability of B. bacteriovorus to incorporate exogenous DNA to its chromosome via homologous recombination was exploited to carry out directed mutagenesis experiments. In 2003, a methyl-accepting chemotaxis protein (MCP), mcp2, and a homologous gene (mviN) were disrupted with a kanamycin cassette. Suicide plasmids derived from the pSET151 plasmid (IncP) with disrupted versions of those genes were transferred by conjugation to B. bacteriovorus 109J, resulting in merodiploid strains (Lambert et al. 2003). Following this strategy, several genes of B. bacteriovorus HD100 have been disrupted to better understand predation mechanism: flagellar genes (Lambert et al. 2006), type IV pili (Evans et al. 2007), cytoskeletal elements (Fenton et al. 2010a), shape related proteins (Fenton et al. 2010b), flagellar genes (Morehouse et al. 2011), transporters (Chang et al. 2011) and sigma factors genes (Lambert et al. 2012). To identify more predation related genes, random mutagenesis using a Tn5 transposon was exploited (Medina et al. 2008; Roschanski et al. 2011; Tudor et al. 2008).
The next step forward in the genetic modification of B. bacteriovorus was the development of a system to generate markerless mutants. This system included an stringent suicide vector (pSSK10) with an R6K origin of replication, that only replicates in pir+ strains (Rakowski and Filutowicz 2013). To counterselect recombinant strains, the pSSK10 vector included the sacB gene, a toxic gene when 5% sucrose is present in the culture media. Employing this system, they eliminated the gene that confers streptomycin resistance, strB, from B. bacteriovorus HD100. Mutant strains were complemented with the expression of this gene in a pMMB206 derivative plasmid, demonstrating that this plasmid can be autonomously replicative in B. bacteriovorus HD100 (Steyert and Pineiro 2007). They used the same technique to delete a dGTPase from B. bacteriovorus HD100 (Steyert et al. 2008).
The widely used pK18mobsacB vector (Schafer et al. 1994), with the same counter-selection gene as pSSK10, was used for the first time in B. bacteriovorus HD100 to fluorescently tag proteins fusing the gene of interest to a green fluorescent protein (GFP) and conjugating the plasmid to obtain recombinant strains (Fenton et al. 2010b). This vector can be also used to generate markerless deletion mutants.
As it is shown in Table 1, all replicative plasmids that have been used in B. bacteriovorus are RSF1010 derivatives. Although these plasmids were employed to complement mutant strains in general, few experiments to express heterologous proteins have been also carried out. Plasmids carrying green or red fluorescent proteins were also transferred by conjugation into B. bacteriovorus resulting in fluorescent strains (Flannagan et al. 2004; Mukherjee et al. 2016; Roschanski and Strauch 2011). These experiments demonstrated the viability to use Bdellovibrio strains as a cell catalyst suitable for producing heterologous proteins. However, there is still a remarkable lack of genetic tools to domesticate B. bacteriovorus. For instance, there is not any inducible nor repressible promoter reported so far. To overcome this problem, a recent work has been lately published where synthetic theophylline–responsive riboswitches are employed to control GFP expression (Dwidar and Yokobayashi 2017). This system was used also to control predation by regulating the flagellar sigma factor FliA which may control up to 66% of attack phase genes. In terms of biotechnological tools, it would be interesting to develop suitable genetic tools allowing not only multiple genes deletions or under-expression, but also the expression of heterologous genes in order to recreate metabolic routes or to produce heterologous proteins.
3.2 Prey Range
Predatory bacteria attack and digest other bacteria and may therefore play a role in shaping microbial populations. This ability might be very useful and challenging in biotechnological processes driven by microbial communities. The prey range will determine the efficiency or feasibility to use predators in specific processes, such as the recovery of interesting intracellular bioproducts. To develop predatory bacteria as a biotechnological tool, it is important to characterize the variation in predation characteristics, such as prey range, and to examine the evolution of predatory bacteria lineages at different scales.
The manner in which BALOs shape microbial communities depends in part on which bacterial species are susceptible to predation and how efficient it is. Traditionally, the most common prey used to isolate and characterize BALOs were almost exclusively from the phylum Proteobacteria: Escherichia coli, Pseudomonas spp. and Erwinia spp. for terrestrial habitats and Vibrio parahaemolyticus for marine ecosystems (Jurkevitch and Davidov 2006).
Despite the wide range of susceptible prey for BALOs, predatory efficiency is strain-dependent. Indeed, Bdellovibrio spp. has been reported to be able to distinguish between different prey species in heterogenic co-cultures (Rogosky et al. 2006). Moreover, several reported cases describe B. bacteriovorus as unable to prey upon specific gram-negative bacteria. One example involves the presence of an extracellular proteinaceous layer (S-layer) that can block attachment between predator cells and the lipopolysaccharide (LPS) layer in Caulobacter sp. (Koval and Hynes 1991). Another example refers to predation by B. bacteriovorus on α-proteobacteria, such as Rhodobacter, which possess a lipopolysaccharide in its envelope that differs significantly from that of other gram-negative bacteria (Strittmatter et al. 1983), and predation on these strains is therefore generally slower.
Table 2 compiles the list of susceptible preys of the BALOs commonly studied and relevant in industry due to the production of some high-value products. It is important to highlight the value that E. coli and P. putida entail for the biotechnology industry, since they are involved in a multitude of bioprocesses. Hence B. bacteriovorus emerge as an important downstream tool for intracellular bioproducts such as the above-mentioned biopolymer PHA (Martinez et al. 2016) or as lytic agent of gram negative cell catalysts whenever required for the bioprocess.
3.3 Cultivation: The Major Drawback
Designing microbes as successful biotechnological catalysts requires some considerations, such as the complexity of the particular industrial process, the nature or toxicity of the products or by-products in the process, and the physiological and metabolic requirements of the selected bacteria. Then, during the selection and evaluation of a cell catalyst for a specific process, the potential bottlenecks must be identified. In the case of predatory bacteria, which have never been applied in industrial processes, several obstacles derived from their own physiology emerge, for example the co-culture requirement and predation inhibition.
Routinely, Bdellovibrio strains are propagated by growing them in a co-culture on gram-negative prey cells such as E. coli or Pseudomonas strains by the double-layer technique or in liquid co-cultures (Herencias et al. 2017; Lambert and Sockett 2008). This particularity makes the bioprocess especially challenging. Remarkably, it is well reported that part of the population cells of Bdellovibrio culture mutates to being able to grow axenically in the absence of prey in rich medium. These cells are the so-called host-independent (HI) derivatives (Seidler and Starr 1969). Since the isolation of B. bacteriovorus in 1962, it has been noted that it can also form saprophytic colonies on hard agar plates in the presence of heat-treated prey bacteria. The successful isolation of HI variants requires a much higher number of predatory cells compared to that needed for plating on prey lawns (Stolp and Starr 1963). This is due to the low frequency of development of these saprophytic predators (one in 106–107 cells) in rich medium (Dwidar et al. 2017). This rate is similar to the mutational rate of bacteria (Schaaper 1993). It was not until the 1990s that the HI phenotype was attributed to mutations in the predator’s genome. The region containing these mutations is called the “hit” locus (host-interaction locus) and no metabolic function is assigned so far. This region has heretofore been associated to the Type IVa pili (Capeness et al. 2013). In addition, some HI isolates lack mutations at the hit locus, and other genes may therefore be involved in the switching pathway from host dependent to HI phenotype (Capeness et al. 2013; Wurtzel et al. 2010). The genomic alteration of the hit locus was analyzed by means of next-generation sequencing (NGS) and the gene bd0108 was identified as being related to the HI phenotype. This gene encodes a 101 amino acid protein and has no homologs outside the Bdellovibrionaceae family. The gene bd0108, those in the surroundings (bd0109-bd0113, bd0118, bd0119) and other ones associated with the HI phenotype (bd3461, bd3464 or bd3852) are related to the formation of the Type IV pili, which is involved in the prey invasion process (Chanyi and Koval 2014). Mutant strains in some of these genes are unable to recognize and to attach to the prey cell in liquid co-cultures. In the context of industrial bioprocesses, the rational development of axenic predator cultures for generating predator cells suitable of preying under controlled conditions remains as a challenge. Meanwhile, to produce B. bacteriovorus at a large scale, or to use it as a lytic tool, it is necessary to establish a liquid predator-prey co-culture. The axenic growth of Bdellovibrio HI strains would be applicable as well in processes focused to purifying hydrolytic enzymes with industrial interest from the Bdellovibrio’s arsenal.
Bdellovibrio strains, high oxygen-demanding microorganisms, are unable to grow under anoxic conditions but capable of surviving for a limited period of time (Schoeffield et al. 1996). Under microaerobic conditions, the predator cells are able to prey, albeit more slowly than in the optimal oxygen conditions (Kadouri and Tran 2013). Hence, oxygen concentration is a crucial variable that needs to be considered in industrial bioprocesses involving B. bacteriovorus. Fermenter agitation, gas flow rate, and oxygen uptake are parameters to be controlled for ensuring an adequate oxygen concentration during the predation events in the bioreactor (Garcia-Ochoa and Gomez 2009). This is particularly relevant in high cell density cultivations.
Finally, predation and survival of B. bacteriovorus could be affected by the presence of certain compounds. Although, this is discussed more thoroughly in the Chapter “Environmental and Biotic Factors Impacting the Activities of Bdellovibrio bacteriovorus” by Im et al., it is possible to take advantage of them to control predation adapting it to the requirements of an industrial bioprocess. Several compounds have been reported to enhance or inhibit predation. For example, carbohydrates play an important role in predation inhibition provoking a medium acidification (pH ~4.0) due to the release of by-products (Dashiff et al. 2011b). This pH predation dependence might be exploited to precisely control the predation along the process. On the opposite side, certain ions enhance predation such as copper sulphate, a widely used algicidal in aquaculture. In concentrations ranging from 0.1 to 1.0 mg · L−1 it stimulates Bdellovibrio sp. strain BDF-H16 predation as calcium chloride or magnesium sulphate do (Huang and Starr 1973), suggesting that copper ions may act synergistically with other cations improving the bacteriolytic activity of the predator (Cao et al. 2018).
In conclusion, B. bacteriovorus has a tremendous potential as a biotechnological tool, but there are many issues that need to be addressed before it can be considered as a scalable industrial microorganism.
4 The Case of Polyhydroxyalkanoates
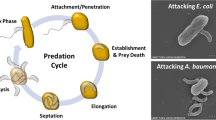
Given its ability to lyse other bacteria, B. bacteriovorus has been proposed as a novel downstream living lytic agent for the production of valuable intracellular bio-products (Figs. 3b and 4). One of the most challenging downstream processes is the isolation of bacterial polyesters or polyhydroxyalkanoates (PHAs) at industrial scale. The PHA is accumulated as intracellular granules in the bacterial cytoplasm and can reach up to 90% of cell dry weight.
(a) The predatory cycle of B. bacteriovorus preying on PHA accumulating P. putida KT2440. (1) Attack phase: Bdellovibrio cells move towards prey-rich regions. (2) Attachment: predator anchors to the host cell, which leads the infection. 3) Penetration: it enters the periplasm of the prey cell. (4 and 5) Growth in bdelloplast: the prey turns rounded due to cell wall modification and the predator grows in the periplasm and replicates its DNA. (6 and 7) Septation and development: B. bacteriovorus uses the prey as a source of nutrients. When resources become limited the predator septates and matures into individual attack phase cells. (8) Lysis: mature attack-phase cells lyse the cell-wall of the bdelloplast, beginning the search of fresh prey. PHA granules are therefore released to medium. The complete cycle takes about 4 h. Figure partially made with biorender (https://www.biorender.com) (b) Microphotographies show the different steps of P. putida KT2440/B. bacteriovorus predation event when the prey is producing PHA. (1) Transmission electronic microphotography of P. putida KT2440 cell of containing PHA granules inside the cytoplasm. (2) Scanning electronic microphotography of the attachment of the predator to the surface membrane of the prey cell. (3) Transmission electronic microphotography of the bdelloplast structure containing the predator and the PHA granules inside. (4) Phase-contrast images of PHA granules released by B. bacteriovorus after 24 h of predation upon P. putida KT2440
Depending on the length of the lateral chain, these polymers have different mechanical and physicochemical properties. Several short-chain-length-PHAs (scl-PHA) such as poly-3-hydroxybutyrate (PHB), are currently produced at large scale by several companies (Chanprateep 2010) and have extensive applications in packaging, moulding, fibre production and other commodities. Medium-chain-length-PHAs (mcl-PHA, with carbon numbers ranging from 6 to 14) are also promising candidates as bioplastics given their longer-side-chain-derived properties of reduced crystallinity, elasticity, hydrophobicity, low oxygen permeability and biodegradability. Moreover, mcl-PHA are being used as resorbable materials for medical applications, and as food coatings, pressure-sensitive adhesives, paint binders and biodegradable rubbers (Sudesh et al. 2011). However, their condition as intracellular bio-products makes their recovery difficult and costly (Jacquel et al. 2008; Madkour et al. 2013).
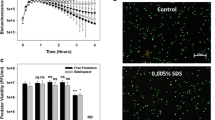
In the last years, a great effort has been made for isolating these biopolymers, which is one of the key step for process profitability in the fermentation system (Fig. 3b) (Madkour et al. 2013). Different methods such as mechanical cell disruption, separation processes (filtration, froth flotation, continuous centrifugation), enzymatic digestion or use of detergents and solvents have been investigated (Jacquel et al. 2008). However, the high costs of the traditional downstream processing or the reduced quality of the recovered polymer suppose a handicap for high-scale biopolymers production. It has been shown that B. bacteriovorus can prey upon PHA-producers such as P. putida KT2440 while the latter accumulates large amounts of mcl-PHA within its cells (Martinez et al. 2013). After lysing the prey, the predator hydrolyzes and consumes part of the PHA released into the extracellular environment; indeed, significant quantities of PHA granules and free hydroxyalkanoic acid (HAs) oligomers (54% and 25%, respectively, of PHA accumulated by the prey bacteria) can be recovered. This is due to the activity of an extracellular-like mcl-PHA depolymerase (PhaZBd, encoded by the gene bd3709), which forms part of the hydrolytic arsenal of B. bacteriovorus (Martinez et al. 2012, 2013; Rendulic et al. 2004). In order to optimize polymer recovery, B. bacteriovorus was engineered to avoid the degradation of prey-produced PHA by mutating bd2637 and bd3709 genes (which encoded for two different PHA depolymerases). The use of these mutant strains in the PHA depolymerase enzymes led to the recovery of larger amounts of the polymer (more than 80% of the PHA accumulated in the prey cells). Moreover, the use of these predator mutant strains provided a high-quality polymer, due to the lack of hydrolyzation by the PHA depolymerases. Besides, it was shown that B. bacteriovorus has the ability to attack high cell density prey cultures, allowing the release of the polymer (Martinez et al. 2016). Thus, although the system needs to be tested at larger scales in an industrially relevant environment, the results suggest that the industrial-scale upgrade is possible. To further demonstrate the feasibility of the system, engineered B. bacteriovorus strains were tested against different gram-negative bacteria that accumulate PHA (including scl-PHA).
Regarding the metabolism of the predator and the impact that the PHA has into its physiology, mcl-PHA degradation provided ecological advantages in terms of motility and predation efficiency, associated to an increment of the ATP intracellular levels. In contrast, preying on scl-PHA rewards the predator fitness in terms of the number of progenies. Overall, the results obtained in that report provide a proof-of-principle that this system could be used for intracellular bio-products recovery.
Taking into account the successful development of the lytic system by using predatory bacteria, other compounds with industrial interest could be considered for extraction: polyphosphates, hormones or pigments (Table 2).
5 Future Perspectives
With the renewed excitement and the successive promising findings opening for BALOs application, the possibility to use predators designed “à la carte” to treat bacterial infections and to exploit their possibilities seems endless. However, the future use of BALOs needs a deeper understanding of the predatory lifestyle and metabolism in order to control them rationally and to develop predators as cell factories. For that, some points should be addressed: (i) control the growth conditions taking into account that the group of BALOs have a biphasic growth cycle, (ii) control the predatory ability in terms of killing efficiency, (iii) control the metabolic state and be able to switch between the different growth phases by identifying the responsible factor/s and (iv) predator storage in suitable formulations preserving their viability over the time.
There is a need to develop genetics tools that allow the use of predatory bacteria as a lytic tool. To this aim, computational modelling and simulation are becoming crucial strategies for metabolic engineering of microorganisms. Computational models are focused on characterizing and engineering the cell at the systems level. Genome-scale metabolic modelling aims to predict gene targets to be engineered taking into account the different components of the biological system and their connections at the same time.
Currently, the availability of high-throughput experimental tools and quantitative analytical techniques allows for the design of more robust metabolic engineering strategies aimed at providing a better understanding of the behaviour of predatory bacteria. Furthermore, integration of the information and omics data at a system level constitutes a useful platform in order for BALOs to be developed as a biotechnological chassis for different purposes.
The abundance and the ubiquitous presence of BALOs in the environment highlights their potential use for control pathogenic bacteria in human, animal, plants and food as well as to be use as co-adjuvant in different processes such as wastewater treatment.
References
Abdelhafez AA, et al. Optimization of β-carotene production from agro-industrial by-products by Serratia marcescens ATCC 27117 using Plackett–Burman design and central composite design. Annals of Agricultural Sciences, Faculty of Agriculture, Ain Shams University. 2016;61(1):87–96. https://doi.org/10.1016/j.aoas.2016.01.005.
Allouche N, et al. Use of whole cells of. 2004;70(4):2105–9. https://doi.org/10.1128/AEM.70.4.2105.
Atterbury RJ, Hobley L, Till R, Lambert C, Capeness MJ, Lerner TR, et al. Effects of orally administered Bdellovibrio bacteriovorus on the well-being and Salmonella colonization of young chicks. Appl Environ Microbiol. 2011;77:5794–803.
Avidan O, et al. Identification and Characterization of Differentially-Regulated Type IVb Pilin Genes Necessary for Predation in Obligate Bacterial Predators. Scientific reports Nature Publishing Group. 2017;7(1):1013. https://doi.org/10.1038/s41598-017-00951-w.
Bagheri Lotfabad T, et al. Two schemes for production of biosurfactant from Pseudomonas aeruginosa MR01: Applying residues from soybean oil industry and silica sol–gel immobilized cells. Colloids and Surfaces B: Biointerfaces Elsevier BV. 2017;152:159–68. https://doi.org/10.1016/j.colsurfb.2017.01.024.
Banitz T, Johst K, Wick LY, Fetzer I, Harms H, Frank K. The relevance of conditional dispersal for bacterial colony growth and biodegradation. Microb Ecol. 2012;63:339–47.
Bratanis E, Molina H, Naegeli A, Collin M, Lood R. BspK, a serine protease from the predatory bacterium Bdellovibrio bacteriovorus with utility for analysis of therapeutic antibodies. Appl Environ Microbiol. 2017;83:e03037–16. https://doi.org/10.1128/AEM.03037-03016.
Cao H, He S, Wang H, Hou S, Lu L, Yang X. Bdellovibrios, potential biocontrol bacteria against pathogenic Aeromonas hydrophila. Vet Microbiol. 2012;154:413–8.
Cao HP, Yang YB, Lu LQ, Yang XL, Ai XH. Effect of copper sulfate on Bdellovibrio growth and bacteriolytic activity towards gibel carp-pathogenic Aeromonas hydrophila. Can J Microbiol. 2018;64:1054–8.
Capeness MJ, Lambert C, Lovering AL, Till R, Uchida K, Chaudhuri R, et al. Activity of Bdellovibrio hit locus proteins, Bd0108 and Bd0109, links type IVa pilus extrusion/retraction status to prey-independent growth signalling. Plos One. 2013;8:e79759. https://doi.org/10.71371/journal.pone.0079759.
Chang CY, et al. The Bdellovibrio bacteriovorus twin-arginine transport system has roles in predatory and prey-independent growth. Microbiology. 2011;157(11):3079–93. https://doi.org/10.1099/mic.0.052449-0.
Chanprateep S. Current trends in biodegradable polyhydroxyalkanoates. J Biosci Bioeng. 2010;110:621–32.
Chanyi RM, Koval SF. Role of type IV Pili in predation by Bdellovibrio bacteriovorus. PLoS One. 2014;9(11) https://doi.org/10.1371/journal.pone.0113404.
Chen CY, Yen SH, Chung YC. Combination of photoreactor and packed bed bioreactor for the removal of ethyl violet from wastewater. Chemosphere. 2014;117:494–501.
Chen Z, et al. Metabolic engineering of klebsiella pneumoniae for the production of 2-butanone from glucose. PLoS One. 2015;10(10):1–10. https://doi.org/10.1371/journal.pone.0140508.
Chmielewski RAN, Frank JF. Biofilm formation and control in food processing facilities. Compr Rev Food Sci Food Saf. 2015;2:22–32.
Cotter TW, Thomashow MF. A conjugation procedure for Bdellovibrio bacteriovorus and its use to identify DNA sequences that enhance the plaque-forming ability of a spontaneous host-independent mutant. J Bacteriol. 1992a;174(19):6011–7.
Cotter TW, Thomashow MF. Identification of a Bdellovibrio bacteriovorus genetic locus, hit, associated with the host-independent phenotype. J Bacteriol. 1992b;174(19):6018–24.
Dashiff A, Junka RA, Libera M, Kadouri DE. Predation of human pathogens by the predatory bacteria Micavibrio aeruginosavorus and Bdellovibrio bacteriovorus. J Appl Microbiol. 2011a;110:431–44.
Dashiff A, Keeling TG, Kadouri DE. Inhibition of predation by Bdellovibrio bacteriovorus and Micavibrio aeruginosavorus via host cell metabolic activity in the presence of carbohydrates. Appl Environ Microb. 2011b;77:2224–31.
Davidov Y, Jurkevitch E. Predation between prokaryotes and the origin of eukaryotes. BioEssays. 2009;31:748–57.
de Dios Caballero J, Vida R, Cobo M, Maiz L, Suarez L, Galeano J, et al. Individual patterns of complexity in cystic fibrosis lung microbiota, including predator Bacteria, over a 1-year period. MBio. 2017;8(5):e00959–17. https://doi.org/10.01128/mBio.00959-00917.
de Eugenio LI, Garcia P, Luengo JM, Sanz JM, San Roman J, Garcia JL, et al. Biochemical evidence that phaZ gene encodes a specific intracellular medium chain length polyhydroxyalkanoate depolymerase in Pseudomonas putida KT2442 – characterization of a paradigmatic enzyme. J Biol Chem. 2007;282:4951–62.
Di Gioia D, et al. Metabolic engineering of Pseudomonas fluorescens for the production of vanillin from ferulic acid. Journal of Biotechnology Elsevier BV. 2011;156(4):309–16. https://doi.org/10.1016/j.jbiotec.2011.08.014.
Dori-Bachash M, et al. Bacterial intein-like domains of predatory bacteria: a new domain type characterized in Bdellovibrio bacteriovorus. Funct Integr Genomics. 2009;9(2):153–66. https://doi.org/10.1007/s10142-008-0106-7.
Du J, Shao ZY, Zhao HM. Engineering microbial factories for synthesis of value-added products. J Ind Microbiol Biot. 2011;38:873–90.
Dwidar M, Yokobayashi Y. Controlling Bdellovibrio bacteriovorus gene expression and predation using synthetic riboswitches. ACS Synth Biol. 2017;6:2035–41.
Dwidar M, Im H, Seo JK, Mitchell RJ. Attack-phase Bdellovibrio bacteriovorus responses to extracellular nutrients are analogous to those seen during late Intraperiplasmic growth. Microbial Ecol. 2017;74:937–46.
Elkenawy NM, et al. Optimization of prodigiosin production by Serratia marcescens using crude glycerol and enhancing production using gamma radiation. Biotechnology Reports Elsevier BV. 2017;14:47–53. https://doi.org/10.1016/j.btre.2017.04.001.
Evans KJ, Lambert C, Sockett RE. Predation by Bdellovibrio bacteriovorus HD100 requires type IV pili. J Bacteriol. 2007;189(13):4850–9. https://doi.org/10.1128/JB.01942-06.
Fenton AK, Hobley L, Butan C, Subramaniam S, Sockett RE. A coiled-coil-repeat protein ‘Ccrp’ in Bdellovibrio bacteriovorus prevents cellular indentation, but is not essential for vibroid cell morphology. FEMS Microbiol Lett. 2010a;313:89–95.
Fenton AK, et al. Manipulating each MreB of Bdellovibrio bacteriovorus gives diverse morphological and predatory phenotypes. J Bacteriol. 2010;192(5):1299–311. https://doi.org/10.1128/JB.01157-09.
Flannagan RS, Valvano MA, Koval SF. Downregulation of the motA gene delays the escape of the obligate predator Bdellovibrio bacteriovirus 109J from bdelloplasts of bacterial prey cells. Microbiology. 2004;150(3):649–56. https://doi.org/10.1099/mic.0.26761-0.
Furuno S, Pazolt K, Rabe C, Neu TR, Harms H, Wick LY. Fungal mycelia allow chemotactic dispersal of polycyclic aromatic hydrocarbon-degrading bacteria in water-unsaturated systems. Environ Microbiol. 2010;12:1391–8.
Garcia-Ochoa F, Gomez E. Bioreactor scale-up and oxygen transfer rate in microbial processes: an overview. Biotechnol Adv. 2009;27:153–76.
Goel A, Wortel MT, Molenaar D, Teusink B. Metabolic shifts: a fitness perspective for microbial cell factories. Biotechnol Lett. 2012;34:2147–60.
Guelin A, Lepine P, Lamblin D. Water bactericidal activity and the part played by Bdellovibrio bacteriovorus. Ann Inst Pasteur (Paris). 1967;113:660–5.
Gutnick DL, Allon R, Levy C, Petter R, M. W. Applications of acinetobacter as an industrial microorganism. The Biology of Acinetobacter. 1991:411–41.
Harada T, et al. Production of a new acidic polysaccharide, succinoglucan by alcaligenes faecalis var. myxogenes. Agric Biol Chem. 1965;29(8):757–62. https://doi.org/10.1080/00021369.1965.10858462.
Harikrishnan R, Balasundaram C, Heo MS. Effect of probiotics enriched diet on Paralichthys olivaceus infected with lymphocystis disease virus (LCDV). Fish Shellfish Immunol. 2010;29:868–74.
Herencias C, Prieto MA, Martínez V, Smith KS. Determination of the predatory capability of Bdellovibrio bacteriovorus HD100. Bio Protocol. 2017;7:e2177.
Hobley L, et al. Genome analysis of a simultaneously predatory and prey-independent, novel Bdellovibrio bacteriovorus from the River Tiber, supports in silico predictions of both ancient and recent lateral gene transfer from diverse bacteria. BMC Genomics. 2012;13:670. https://doi.org/10.1186/1471-2164-13-670.
Huang JCC, Starr MP. Effects of calcium and magnesium ions and host viability on growth of Bdellovibrios. Antonie Van Leeuwenhoek. 1973;39:151–67.
Im H, Choi SY, Son S, Mitchell RJ. Combined application of bacterial predation and Violacein to kill polymicrobial pathogenic communities. Sci Rep. 2017;7:14415. https://doi.org/10.11038/s41598-14017-14567-14417.
Im H, Dwidar M, Mitchell RJ. Bdellovibrio bacteriovorus HD100, a predator of gram-negative bacteria, benefits energetically from Staphylococcus aureus biofilms without predation. ISME J. 2018;12:2090–5.
Jacquel N, Lo CW, Wei YH, Wu HS, Wang SS. Isolation and purification of bacterial poly (3-hydroxyalkanoates). Biochem Eng J. 2008;39:15–27.
Jurkevitch E, Davidov Y. Phylogenetic diversity and evolution of predatory prokaryotes. ACS Division of Fuel Chemistry, Preprints. 2006.
Kadouri D, O’Toole GA. Susceptibility of biofilms to Bdellovibrio bacteriovorus attack. Appl Environ Microbiol. 2005;71:4044–51.
Kadouri DE, Tran A. Measurement of predation and biofilm formation under different ambient oxygen conditions using a simple gasbag-based system. Appl Environ Microbiol. 2013;79:5264–71.
Kim SH, et al. Histamine production by Morganella morganii in mackerel, albacore, mahi-mahi, and salmon at various storage temperatures. J Food Sci. 2002;67(4):1522–8. https://doi.org/10.1111/j.1365-2621.2002.tb10316.x.
Koval SF, Hynes SH. Effect of paracrystalline protein surface layers on predation by Bdellovibrio bacteriovorus. J Bacteriol. 1991;173:2244–9.
Kumar CG, Anand SK. Significance of microbial biofilms in food industry: a review. Int J Food Microbiol. 1998;42:9–27.
Lambert C, Sockett RE. Laboratory maintenance of Bdellovibrio. Curr Protoc Microbiol. 2008; Chapter 7: Unit 7B 2.
Lambert C, Sockett RE. Nucleases in Bdellovibrio bacteriovorus contribute towards efficient self-biofilm formation and eradication of preformed prey biofilms. FEMS Microbiol Lett. 2013;340(2):109–16. https://doi.org/10.1111/1574-6968.12075.
Lambert C, Smith MCM, Sockett RE. A novel assay to monitor predator-prey interactions for Bdellovibrio bacteriovorus 109 J reveals a role for methyl-accepting chemotaxis proteins in predation. Environ Microbiol. 2003;5(2):127–32. https://doi.org/10.1046/j.1462-2920.2003.00385.x.
Lambert C, et al. Characterizing the flagellar filament and the role of motility in bacterial prey-penetration by Bdellovibrio bacteriovorus. Mol Microbiol. 2006;60(2):274–86. https://doi.org/10.1111/j.1365-2958.2006.05081.x.
Lambert C, et al. Mutagenesis of RpoE-like sigma factor genes in Bdellovibrio reveals differential control of groEL and two groES genes. BMC Microbiol. 2012;12(1):99. https://doi.org/10.1186/1471-2180-12-99.
Lin B, Chen SW, Cao Z, Lin YQ, Mo DZ, Zhang HB, et al. Acute phase response in zebrafish upon Aeromonas salmonicida and Staphylococcus aureus infection: striking similarities and obvious differences with mammals. Mol Immunol. 2007;44:295–301.
Loozen G, Boon N, Pauwels M, Slomka V, Herrero ER, Quirynen M, et al. Effect of Bdellovibrio bacteriovorus HD100 on multispecies oral communities. Anaerobe. 2015;35:45–53.
Lu F, Cai J. The protective effect of Bdellovibrio-and-like organisms (BALO) on tilapia fish fillets against Salmonella enterica ssp enterica serovar Typhimurium. Lett Appl Microbiol. 2010;51:625–31.
Madkour MH, Heinrich D, Alghamdi MA, Shabbaj II, Steinbuchel A. PHA recovery from biomass. Biomacromolecules. 2013;14:2963–72.
Maier RM, Soberón-Chávez G. Pseudomonas aeruginosa rhamnolipids: Biosynthesis and potential applications. Appl Microbiol Biotechnol. 2000;54(5):625–33. https://doi.org/10.1007/s002530000443.
Margulis L. Archaeal-eubacterial mergers in the origin of Eukarya: phylogenetic classification of life. Proc Natl Acad Sci USA. 1996;93:1071–6.
Martinez V, de la Pena F, Garcia-Hidalgo J, de la Mata I, Garcia JL, Prieto MA. Identification and biochemical evidence of a medium-chain-length Polyhydroxyalkanoate Depolymerase in the Bdellovibrio bacteriovorus predatory hydrolytic arsenal. Appl Environ Microbiol. 2012;78:6017–26.
Martinez V, Jurkevitch E, Garcia JL, Prieto MA. Reward for Bdellovibrio bacteriovorus for preying on a polyhydroxyalkanoate producer. Environ Microbiol. 2013;15:1204–15.
Martinez V, Herencias C, Jurkevitch E, Prieto MA. Engineering a predatory bacterium as a proficient killer agent for intracellular bio-products recovery: the case of the polyhydroxyalkanoates. Sci Rep. 2016;6:24381. https://doi.org/10.21038/srep24381.
Martínez V, et al. Engineering a predatory bacterium as a proficient killer agent for intracellular bio- products recovery: The case of the polyhydroxyalkanoates. Nat Publ Group. 2016; https://doi.org/10.1038/srep24381.
Medina, A. a, Shanks, R. M. and Kadouri, D. E. Development of a novel system for isolating genes involved in predator-prey interactions using host independent derivatives of Bdellovibrio bacteriovorus 109J. BMC Microbiol. 2008;8:33. https://doi.org/10.1186/1471-2180-8-33.
Milner DS, et al. Ras GTPase-like protein MglA, a controller of bacterial social-motility in Myxobacteria, has evolved to control bacterial predation by Bdellovibrio. PLoS Genet. 2014;10(4):e1004253. https://doi.org/10.1371/journal.pgen.1004253.
Morehouse KA, et al. Three motAB stator gene products in Bdellovibrio bacteriovorus contribute to motility of a single flagellum during predatory and prey-independent growth. J Bacteriol. 2011;193(4):932–43. https://doi.org/10.1128/JB.00941-10.
Mukherjee S, et al. Visualizing Bdellovibrio bacteriovorus by using the tdTomato fluorescent protein. Appl Environ Microbiol. 2016;82(6):1653–61. https://doi.org/10.1128/AEM.03611-15.
Naylor RL, Goldburg RJ, Primavera JH, Kautsky N, Beveridge MCM, Clay J, et al. Effect of aquaculture on world fish supplies. Nature. 2000;405:1017–24.
Nikel PI, de Lorenzo V. Pseudomonas putida as a functional chassis for industrial biocatalysis: From native biochemistry to trans-metabolism. Metabolic Engineering Elsevier Inc. 2018;50:142–55. https://doi.org/10.1016/j.ymben.2018.05.005.
Nikel PI, et al. From dirt to industrial applications: Pseudomonas putida as a Synthetic Biology chassis for hosting harsh biochemical reactions. Curr Opin Chem Biol. 2016;34:20–9. https://doi.org/10.1016/j.cbpa.2016.05.011.
Ortiz-Marquez JCF, Do Nascimento M, Zehr JP, Curatti L. Genetic engineering of multispecies microbial cell factories as an alternative for bioenergy production. Trends Biotechnol. 2013;31:521–9.
Otto S, Harms H, Wick LY. Effects of predation and dispersal on bacterial abundance and contaminant biodegradation. Fems Microbiol Ecol. 2017;93:fiw241. https://doi.org/10.1093/femsec/fiw1241.
Paoletti A, De Simone E, Ferro V, Orsi C, Campanile E. A new factor in autodepuration of water: Bdellovibrio batteriovorus. Riv Ital Ig. 1967;27:466–80.
Perego P, et al. 2,3-Butanediol production by Enterobacter aerogenes: Selection of the optimal conditions and application to food industry residues. Bioprocess Eng. 2000;23(6):613–20. https://doi.org/10.1007/s004490000210.
Philip S, Keshavarz T, Roy I. Polyhydroxyalkanoates: biodegradable polymers with a range of applications. J Chem Technol Biotechnol. 2007;82:233–47.
Piñeiro SA, Williams HN, Stine OC. Phylogenetic relationships amongst the saltwater members of the genus Bacteriovorax using rpoB sequences and reclassification of Bacteriovorax stolpii as Bacteriolyticum stolpii gen. nov., comb. nov. Int J Syst Evol Micrbiol. 2008;58:1203–9.
Prieto A, Escapa IF, Martinez V, Dinjaski N, Herencias C, de la Pena F, et al. A holistic view of polyhydroxyalkanoate metabolism in Pseudomonas putida. Environ Microbiol. 2016;18:341–57.
Rakowski SA, Filutowicz M. Plasmid R6K replication control. Plasmid. 2013;69:231–42.
Rendulic S, Jagtap P, Rosinus A, Eppinger M, Baar C, Lanz C, et al. A predator unmasked: life cycle of Bdellovibrio bacteriovorus from a genomic perspective. Science. 2004;303:689–92.
Rogosky AM, Moak PL, Emmert EAB. Differential predation by Bdellovibrio bacteriovorus 109J. Curr Microbiol. 2006;52:81–5.
Roschanski N, Strauch E. Assessment of the Mobilizable Vector Plasmids pSUP202 and pSUP404.2 as Genetic Tools for the Predatory Bacterium Bdellovibrio bacteriovorus. 2010; https://doi.org/10.1007/s00284-010-9748-5.
Roschanski N, Strauch E. Assessment of the Mobilizable vector plasmids pSUP202 and pSUP404.2 as genetic tools for the predatory bacterium Bdellovibrio bacteriovorus. Curr Microbiol. 2011;62:589–96.
Roschanski N, et al. Identification of genes essential for prey-independent growth of Bdellovibrio bacteriovorus HD100. J Bacteriol. 2011;193(7):1745–56. https://doi.org/10.1128/JB.01343-10.
Rotem O, et al. Cell-cycle progress in obligate predatory bacteria is dependent upon sequential sensing of prey recognition and prey quality cues. Proc Natl Acad Sci U S A. 2015;112(44):E6028–37. https://doi.org/10.1073/pnas.1515749112.
Saxon EB, Jackson RW, Bhumbra S, Smith T, Sockett RE. Bdellovibrio bacteriovorus HD100 guards against Pseudomonas tolaasii brown-blotch lesions on the surface of post-harvest Agaricus bisporus supermarket mushrooms. BMC Microbiol. 2014;14:163. https://doi.org/10.1186/1471-2180-1114-1163.
Schaaper RM. Base selection, proofreading, and mismatch repair during DNA-replication in Escherichia-Coli. J Biol Chem. 1993;268:23762–5.
Schäfer A, et al. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994;145(1):69–73. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8045426
Scherff RH. Control of bacterial blight of soybean by Bdellovibrio-Bacteriovorus. Phytopathology. 1973;63:400–2.
Schoeffield AJ, Williams HN, Turng BF, Falkler WA. A comparison of the survival of intraperiplasmic and attack phase bdellovibrios with reduced oxygen. Microbial Ecol. 1996;32:35–46.
Schwudke D, Strauch E, Krueger M, Appel B. Taxonomic studies of predatory bdellovibrios based on 16S rRNA analysis, ribotyping and the hit locus and characterization of isolates from the gut of animals. Syst Appl Microbiol. 2001;24:385–94.
Seidler RJ, Starr MP. Isolation and characterization of host-independent Bdellovibrios. J Bacteriol. 1969;100:769–85.
Simon R, Priefer U, Puhler A. A broad host range mobilization system for Invivo genetic-engineering – transposon mutagenesis in gram-negative Bacteria. Bio-Technology. 1983;1:784–91.
Singh R, Kumar M, Mittal A, Mehta PK. Microbial enzymes: industrial progress in 21st century. 3Biotech. 2016;6:174. https://doi.org/10.1007/s13205-13016-10485-13208.
Sinumvayo JP. Agriculture and Food Applications of Rhamnolipids and its Production by Pseudomonas Aeruginosa. Journal of Chemical Engineering & Process Technology. 2015;06(02):2–9. https://doi.org/10.4172/2157-7048.1000223.
Sockett RE. Predatory lifestyle of Bdellovibrio bacteriovorus. Annu Rev Microbiol. 2009;63:523–39.
Steyert SR, Pineiro SA. Development of a novel genetic system to create markerless deletion mutants of Bdellovibrio bacteriovorus. Appl Environ Microbiol. 2007;73(15):4717–24. https://doi.org/10.1128/AEM.00640-07.
Steyert SR, Messing SAJ, Amzel LM, Gabelli SB, Pineiro SA. Identification of Bdellovibrio bacteriovorus HD100 Bd0714 as a Nudix dGTPase. J Bacteriol. 2008;190:8215–9.
Stolp H, Starr MP. Bdellovibrio Bacteriovorus gen. Et Sp. N., a predatory, Ectoparasitic, and Bacteriolytic microorganism. Antonie Van Leeuwenhoek. 1963;29:217.
Strittmatter W, Weckesser J, Salimath PV, Galanos C. Nontoxic lipopolysaccharide from Rhodopseudomonas-Sphaeroides Atcc-17023. J Bacteriol. 1983;155:153–8.
Sudesh K, Abe H, Doi Y. Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog Polym Sci. 2000;25:1503–55.
Sudesh K, Bhubalan K, Chuah JA, Kek YK, Kamilah H, Sridewi N, et al. Synthesis of polyhydroxyalkanoate from palm oil and some new applications. Appl Microbiol Biotechnol. 2011;89:1373–86.
Theisen M, Liao JC. Industrial Biotechnology: Escherichia coli as a Host. Ind Biotechnol. 2016:149–81. https://doi.org/10.1002/9783527807796.ch5.
Tomás-Cortázar J, et al. Identification of a highly active tannase enzyme from the oral pathogen Fusobacterium nucleatum subsp. polymorphum. Microbial Cell Factories BioMed Central. 2018;17(1):1–10. https://doi.org/10.1186/s12934-018-0880-4.
Thomashow MF, Rittenberg SC. Penicillin-induced formation of osmotically stable Spheroplasts in nongrowing Bdellovibrio-Bacteriovorus. J Bacteriol. 1978;133:1484–91.
Tudor JJ, et al. Isolation of predation-deficient mutants of Bdellovibrio bacteriovorus by using transposon mutagenesis. Appl Environ Microbiol. 2008;74(17):5436–43. https://doi.org/10.1128/AEM.00256-08.
Wurtzel O, Dori-Bachash M, Pietrokovski S, Jurkevitch E, Sorek R. Mutation detection with next-generation resequencing through a mediator genome. Plos One. 2010;5:e15628. https://doi.org/10.11371/journal.pone.0015628.
Acknowledgments
This work was supported by the European Union’s Horizon 2020 Research and Innovation Programme, grant agreement no. 760994-2 (ENGICOIN), the Spanish Ministry of Science, Innovation and Universities (BIO2017-83448-R) and the Community of Madrid (P2018/NMT4389). Sergio Salgado is a recipient of a predoctoral FPU grant (FPU17/03978) from the Spanish Ministry of Universities.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Herencias, C., Salgado-Briegas, S., Prieto, M.A. (2020). Emerging Horizons for Industrial Applications of Predatory Bacteria. In: Jurkevitch, E., Mitchell, R. (eds) The Ecology of Predation at the Microscale. Springer, Cham. https://doi.org/10.1007/978-3-030-45599-6_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-45599-6_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-45598-9
Online ISBN: 978-3-030-45599-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)