Abstract
Cellular senescence is a stress response characterized by a permanent loss of proliferative ability. Different types of senescence reflect different forms of stress. Although cellular senescence plays a key role in maintaining tissue homeostasis and in suppressing tumorigenesis, it is widely appreciated that accumulation of senescent cells in tissue contributes to development of age-related dysfunctions and limits the efficacy of chemo- and radiotherapy. Consequently, novel therapeutic strategies targeting senescent cells, or their pro-inflammatory secretome, have been recently developed. The application of these novel therapeutic approaches requires biomarkers for in vivo detection of senescent cells for ageing and age-associated diseases, as well as for therapy-induced senescence in cancer patients. Aptamers are short oligonucleotide or peptide sequences able to bind with high affinity and specificity a wide range of cellular targets, including cell surface epitopes, thanks to their unique three-dimensional folding. Oligonucleotide aptamers have been widely applied for the discovery of biomarkers, since the early 1990s, and Affimers have recently emerged as valuable tools for biomarker discovery. In this chapter we briefly introduce applications of aptamer technologies for recognition of cell surface antigens and suggest that aptamers provide the perfect technology to identify novel senescence-specific biomarkers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keyword
1 Cellular Senescence and the Growing Field of Senotherapy
Cellular senescence is a stress response characterized by a permanent loss of proliferative ability that can be activated by different damaging stimuli. As such, cellular senescence represents an evolutionarily conserved mechanism that prevents the proliferation of damaged and potentially tumorigenic cells. Senescence also participates in mammalian embryonic development and critically contributes to embryonic remodeling and patterning (Muñoz-Espín et al. 2013; Storer et al. 2013). The activation of p16INK4a-pRb and p53-p21CIP1 signaling pathways sustain the senescence cell cycle arrest. Cellular senescence is frequently associated with irreparable DNA damage and a persistent DNA damage response (DDR) (Hewitt et al. 2012; Fumagalli et al. 2012; Sedelnikova et al. 2004), which is triggered as a result of telomere shortening during replicative senescence (d’Adda di Fagagna et al. 2003), oncogene activation in oncogene-induced senescence (OIS) (Bartkova et al. 2006; Di Micco et al. 2006; Hills and Diffley 2014), and radiotherapy or chemotherapy in therapy-induced senescence (TIS) (te Poele et al. 2002; Mirzayans et al. 2005). However, senescence may also be induced in the absence of DNA damage. For instance, PTEN inactivation induces senescence in the absence of DDR activation: PTEN-loss activates mTOR which enhances p53 translation and p21 expression, thus leading to permanent cell cycle arrest (Alimonti et al. 2010). Induction of cellular senescence with drugs that modify chromatin structure, such as histone deacetylase inhibitors, occurs in the absence of DNA damage (Pazolli et al. 2012). Sodium butyrate induces p21 expression and causes cellular senescence. Interestingly, sodium butyrate induced-senescent cells display activation of DDR proteins, such as gamma-H2AX foci, without detectable DNA damage (Pospelova et al. 2009). Curcumin treatment induces senescence in vascular smooth muscle cells in a DNA damage-independent manner, although activation of ATM is observed (Grabowska et al. 2015). Finally, developmental senescence is not associated with DNA damage (Muñoz-Espín et al. 2013; Storer et al. 2013).
Cellular organelles undergo senescence-specific alterations and critically contribute to the development of the senescent phenotype. Enlargement of the lysosomal compartment likely explain the increased activity of a well-established marker of senescence, the so-called Senescence-Associated beta-galactosidase (SA-β-gal) (Kurz et al. 2000). The DDR promotes PGC-1b-dependent mitochondrial biogenesis, thereby increasing mitochondrial content in senescent cells. Mitochondria critically contribute to cellular senescent-associated changes (Correia-Melo et al. 2016).
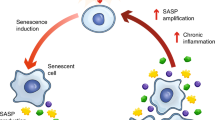
Senescent cells are transcriptionally and metabolically active, and develop a distinctive phenotype characterized by a flat and enlarged morphology. In addition, senescent cells acquire an enhanced secretory ability, called senescence-associated secretory phenotype (SASP) or senescence messaging secretome (SMS) (Coppé et al. 2008; Kuilman and Peeper 2009). The release of proinflammatory SASP factors is critical for reinforcing the growth arrest, for triggering immune-mediated removal of senescent cells and for activating wound healing (Salama et al. 2014; Serrano 2014). Main transcriptional regulators of the SASP include NF-κB and C/EBPβ that cooperatively induce SASP expression (Acosta et al. 2008; Kuilman et al. 2008; Chien et al. 2011; Crescenzi et al. 2011). For instance, Acosta and colleagues demonstrated that NF-κB and C/EBPβ cooperatively induce the expression of CXCR2 ligands IL-8 and GROα in fibroblasts undergoing OIS (Acosta et al. 2008). Similarly, IL6 expression is induced by the transcription factor C/EBPβ upon exposure of cells to oncogenic stress (Kuilman et al. 2008). Both IL-8 and IL-6 play an important role in OIS reinforcing the senescence growth arrest. A critical role of RelA/p65 as a major transcription factor that accumulates on chromatin of senescent cells was demonstrated by Chien et al. (2011). Interestingly, suppression of RelA/p65 function not only abrogates the induction of proinflammatory cytokines IL-6, IL-8, CXCL1, but also reduces expression of intercellular adhesion molecules, such as ICAM-1, and several matrix metalloproteinases (Chien et al. 2011). RelA/p65 induces the expression of TNF-α and IFN-γ in drug-induced tumor senescent cells thereby sensitizing cells to Fas-mediated apoptosis (Crescenzi et al. 2011).
Activation of NF-κB during senescence depends on the cGAS-STING cytosolic DNA sensor pathway, in which cGAS detects endogenous senescence-specific cytoplasmic chromatin fragments (reviewed in Glück and Ablasser 2019). More recent data demonstrate that during OIS cGAS-STING controls the induction of TLR2 which acts as critical upstream mediator for the initiation of the SASP. TLR2 is activated by senescence-associated Damage-Associated Molecular Patterns (identified as acute-phase serum amyloids A1 and A2) and signals, downstream of STING, through NF-κB and p38MAPK to regulate the SASP and to reinforce the senescence growth arrest (Hari et al. 2019). Finally, a role for the JAK/STAT pathway in modulation of proinflammatory SASP components has been demonstrated (Novakova et al. 2010; Xu et al. 2015).
The SASP develops over several days in culture. Hence, expression of several SASP factors, notably the proinflammatory cytokines IL-6 and IL-8, requires up to 20d after DDR initiation before reaching maximal level (Coppé et al. 2008; Crescenzi et al. 2011). Furthermore, secretome composition is temporally modulated, changing with time from a TGF-β-driven to a C/EBPβ-driven profile (Hoare et al. 2016).
Interestingly, emerging evidences show that senescent cells manage to communicate with their neighbors not only through the SASP, but also through the release of small extracellular vesicles (EVs), namely exosomes and microvesicles (Takasugi 2018). Accordingly, various stresses known to induce cellular senescence increase the secretion of EVs (Takasugi 2018). EVs produced by senescent cells play a double role by removing harmful senescence-related cytoplasmic DNA fragments (Takahashi et al. 2017) and by delivering cargos of cell type-specific bioactive molecules (Takasugi et al. 2017; Sagini et al. 2018). Both normal and neoplastic senescent cells have been shown to modulate local tissue microenvironment through EVs. For instance, EVs secreted from senescent chondrocytes, isolated from osteoarthritis patients, induce paracrine senescence in nearby cells, thereby inhibiting cartilage extracellular matrix deposition. These effects are mediated by osteoarthritis-specific microRNA (miRNA) packaged in the EVs (Jeon et al. 2019). Similarly, primary human foreskin fibroblasts undergoing Ras-mediated senescence secrete exosomes which mediate paracrine senescence. The interferon-induced transmembrane protein 3 (IFITM3), specifically accumulated within EVs, is responsible for transmitting senescence to normal neighboring cells (Borghesan et al. 2019). Interestingly, while EVs secreted from senescent cells elicit paracrine senescence, EVs isolated from human induced pluripotent stem cells reduce reactive oxygen species levels in senescent mesenchymal stem cells in culture, thereby reversing the senescent phenotype. Stem cells derived EVs also alleviate senescence in a progerin-induced premature aging cell model. High levels of EVs antioxidant enzymes are responsible for the anti-aging effects (Liu et al. 2019). In line with this, are the results of Zhang and colleagues that demonstrate that EVs isolated from hypothalamic neural stem cells ameliorate age-related cognitive declines in mice. Exosomal miRNA mediate these anti-aging effects (Zhang et al. 2017). Notably, senescent EVs can also promote protumorigenic effects: EVs enriched in EphA2 receptor bind to ephrin-A1 ligand expressed on cancer cells and promote their proliferation through reverse signaling (Takasugi et al. 2017). Notably, increased expression of EphA2 was detected in the stroma of human breast and ovarian cancer (Takasugi et al. 2017). Finally, there is also evidence that EVs deliver membrane-derived bioactive lipids that impact on immune system functions (Sagini et al. 2018).
An additional form of cell communication described in senescent cells is a direct intercellular protein transfer through cytoplasmic bridges. Although the functional consequences of direct intercellular transfer are not completely understood, is interesting to note that this kind of communication facilitates the clearance of senescent cells by NK cells (Biran et al. 2015).
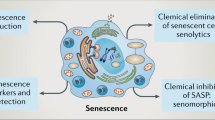
It is now widely accepted that a prolonged permanence of senescent cells in tissue and their sustained secretory activity, result in chronic inflammation and critically contributes to both age-related dysfunctions and to a protumoral microenvironment (Sun et al. 2018; Wei and Ji 2018; Jackson et al. 2012). In the context of TIS, concerns have been raised regarding the ability of cancer cells to escape the senescence arrest, to resume proliferation and to drive tumor recurrence after radio- and chemotherapy (Roberson et al. 2005; Karimi-Busheri et al. 2010). Cancer cells that evade senescent growth arrest are endowed with stem cell properties, are more malignant, and resistant to drug therapies (Yang et al. 2017), representing a likely risk for tumor relapse. Notably, the SASP promotes cell plasticity and stemness also through paracrine actions (Mosteiro et al. 2016; Ritschka et al. 2017). In line with these observations, in vivo studies support the idea that accumulation of senescent cells results in detrimental tissue remodelling, both in normal and in neoplastic tissues. For instance, selective elimination of senescent cells by genetic approaches delays the onset and attenuates progression of age-related disorders, and extends lifespan (Jeon et al. 2017; Bussian et al. 2018; Baker et al. 2016; Baker et al. 2011). Similarly, specific ablation of TIS cells in the tumor microenvironment reduces both tumor relapse and the adverse effects of chemotherapy (Demaria et al. 2017). Notably, TIS induction was shown to be associated with adverse treatment outcome and decreased overall survival in cancer patients (Wu et al. 2012; Supiot et al. 2008; Sidi et al. 2011; Kim et al. 2016). Hence, available data suggest that senescent cells and the SASP contribute to organismal ageing and limit the efficacy of chemo- and radiotherapy (Muñoz-Espín and Serrano 2014). Consequently, removal of senescent cells or the neutralization of their secretome, have become attractive therapeutic strategies to prevent senescence-associated dysfunctions, thus opening the new field of senotherapy research. Drugs that suppress or modulate the SASP without interfering with senescent growth arrest and senolytic agents that selectively induce apoptosis in senescent cells have been studied and are under development.
The possibility to pharmacologically reprogram the SASP towards an anti-tumorigenic rather than pro-tumorigenic profile was first highlighted by Toso and colleagues in the context of docetaxel-induced senescence in PTEN-null tumors, where pharmacological inhibition of the Jak2/Stat3 pathway resulted in activation of an antitumor immune response and enhanced chemotherapy efficacy (Toso et al. 2014). The neutralization of detrimental SASP components in the tumor microenvironment (TME), where senescent stromal cells promote proliferation and survival of nearby neoplastic cells, has also been studied focusing on senescent fibroblasts. In this setting, inhibition of p38MAPK was found to inhibit SASP-mediated tumor growth driven by senescent fibroblasts (Alspach et al. 2014). Pharmacological inhibition of the chromatin regulator MLL1 in fibroblasts also abrogated the SASP preventing its pro-carcinogenic effects. Furthermore, similar results were obtained in etoposide-induced senescent MCF7 breast cancer cells, mimicking the clinical scenario of TIS in tumor cells (Capell et al. 2016). Simvastatin, an HMG-coA reductase inhibitor, decreased the SASP of senescent human fibroblasts, thereby reducing endocrine resistance in breast cancer (Liu et al. 2015). Similarly, the flavonoid apigenin suppressed the SASP and reduced detrimental paracrine effects of fibroblasts on breast cancer cells (Perrott et al. 2017). Rapamycin selectively suppressed the mTOR and NF-κB-dependent secretion of proinflammatory cytokines in senescent fibroblasts, thereby suppressing prostate tumor growth in mice (Laberge et al. 2015). Trabectedin, a DNA minor-groove binding anticancer drug, which causes a gene- and promoter-dependent modulation of transcription (Larsen et al. 2016), markedly decreased the expression of proinflammatory SASP cytokines in tumor cells and limited their pro-tumoral activities in vitro (Camorani et al. 2018). Importantly, Georgilis and colleagues identified 50 druggable, potential targets for therapeutic intervention, whose knockdown suppressed pro-tumorigenic effects of the senescent fibroblasts secretome on tumor growth (Georgilis et al. 2018).
However, expression profiles of different senescent cells are not uniform and the SASP appears to be stimulus-, time- and cell type-dependent (Hernandez-Segura et al. 2017; Wiley et al. 2017; Maciel-Barón et al. 2016) which makes the modulation of the SASP a complex task. Furthermore, in the context of TIS, the SASP has both tumor-suppressive and tumor-promoting effects: SASP cytokines are known to induce immune-mediated clearance of senescent tumor cells (Iannello et al. 2013; Tasdemir et al. 2016; Eggert et al. 2016) and interfering with these immunostimulatory signals might result in detrimental effects. For instance, in a murine model of lymphoma, the reduction of SASP proteins through NF-κB inhibition hampered immunosurveillance following TIS and led to drug resistance, early relapse and reduced survival (Chien et al. 2011). Together, these observations suggest that activation of cell-, time- and stimulus-specific senescence signaling pathways in tumor cells results in different and even functionally-opposite SASPs (Burgess 2011; Llanos and Serrano 2016). Hence, therapeutic interference with key SASP regulators might require individual molecular profiles and monitoring of SASP signatures in cancer patients post-treatment.
As an alternative therapeutic strategy to prevent senescence-associated dysfunctions, senolysis, i.e. the selective removal of senescent cells, has been approached. Senolytic agents have been tested in pre-clinical models and have proved to be effective in alleviating age-related diseases and symptoms of progeria (Xu et al. 2018; Yousefzadeh et al. 2018; Farr et al. 2017; Roos et al. 2016). These studies support the clinical potential of senolysis for promoting healthy ageing. Comprehensive reviews have recently been published on this subject (Kirkland and Tchkonia 2017; Myrianthopoulos et al. 2019). In contrast, the effects of senolytic agents in TIS appear to be more multifaceted. For instance, the senolytic agent panobinostat effectively removed chemotherapy-induced senescent non-small cell lung cancer and head and neck squamous cell carcinoma cells, and proved more efficacious than multiple cycles of chemotherapy (Samaraweera et al. 2017). In contrast, the senolytic cocktail dasatinib plus quercetin, which effectively eliminated senescent fibroblasts (Schafer et al. 2017), not only showed no efficacy on TIS liver cancer cells but also displayed pro-tumorigenic effects in the absence of chemotherapy (Kovacovicova et al. 2018). These data again underline the complexity of senescent cancer cells and emphasize the need for further in vivo investigation of their features and their role(s) in the tumor microenvironment.
Future steps in senotherapy research would include the study of combination therapy with radiotherapy-chemotherapy plus senotherapy for cancer treatment, and advancing senotherapeutics from preclinical studies into the clinic for age-related diseases. Several clinical trials are ongoing for age-related diseases, aimed at evaluate the feasibility of senotherapy. For instance, a two-center, open-label study (ClinicalTrials.gov Identifier: NCT02874989) of senolytic treatment with Dasatinib and Quercetin was conducted in patients with idiopathic pulmonary fibrosis, supporting safety of the treatment and providing initial evidence of senolytic efficacy (Justice et al. 2019). In an open label Phase 1 pilot study (ClinicalTrials.gov Identifier: NCT02848131), the combination of Dasatinib plus Quercetin, administered to subjects with diabetic kidney disease, significantly decreased senescent cell burden (Hickson et al. 2019). A phase 1 clinical trial testing the safety and tolerability of a senolytic small molecule (UBX0101) for osteoarthritis treatment is ongoing (ClinicalTrials.gov Identifier: NCT03513016).
Application of senotherapies to cancer and age-related diseases will require specific and sensitive biomarkers for the detection of normal and neoplastic senescent cells in patients. In particular, assessment of senescence biomarkers in biological samples of cancer patients following neoadjuvant chemo-radiotherapy could help predict treatment responses and contribute to designing personalized therapies.
2 Markers for Senescence
The lack of specific markers for the in vivo detection of senescent cells hampers both the molecular characterization of cellular senescence in ageing, in ageing-related diseases and in cancer patients, and the development of senescence-specific molecular tools and probes for diagnostic, prognostic and therapeutic purposes. Assessment of SA-β-gal activity (Kurz et al. 2000) still represents the most commonly used method for identification of senescent cells, although this marker lacks appropriate specificity, being constantly expressed by senescent cells but also in several other conditions such as in confluent cells in culture (Severino et al. 2000; Yang and Hu 2005). Also, the use of fresh tissue, required for the detection of enzymatic activity of SA-β-gal, is incompatible with routine formalin-fixation and paraffin-embedding of clinical specimens. However, the high lysosomal β-galactosidase activity of senescent cells was successfully exploited for the delivery of galacto-oligosaccharide-encapsulated cytotoxic drugs to live senescent cells in vitro and in vivo, given that release of encapsulated material depended on digestion of the sugar-coat by the senescent-associated lysosomal β-galactosidase (Muñoz-Espín et al. 2018).
Combinations of markers indicative of cell cycle arrest, active DDR and distinctive morphological changes have been sometimes employed to detect senescent cells. For instance, co-staining of either SA-β-gal activity or histone H3K9 tri-methylation (H3K9me3, a repressive heterochromatin mark denoting cell cycle arrest) together with low Ki67 expression has allowed for detection of senescence in therapy-exposed cancer cells (Reimann et al. 2010). An irregular morphology and enlargement of nuclei is frequently observed in senescent cells, which has been related to alterations in components of the nuclear envelope and in specific downregulation of lamin B1 (Freund et al. 2012). Accordingly, the combination of SA-β-gal positivity and double Ki67-Lamin B1 negativity was used to detect senescent cells within untreated human invasive breast carcinomas (Cotarelo et al. 2016). However, although panels of biomarkers might improve our ability to identify senescent cells, there are technical challenges for the practical use of such panels in the clinic and in research.
While senescent cells differ from proliferating and quiescent cells in several aspects (Rodier and Campisi 2011), alterations in cell surface proteins are of greatest interest since surface proteins can be used both as senescence-specific biomarkers and as potential druggable targets. In addition, cell surface markers are easily detected using conventional methods on fresh samples and on fixed specimens. A screening for plasma membrane-associated proteins preferentially expressed in senescent cells led to the identification of DEP1, STX4 and B2MG proteins, whose co-expression marked senescent cells in culture and in tissue samples (Althubiti et al. 2014), although it was noted that STX4 was a better marker of p21CIP1-induced senescence, whereas DEP1 was more specific for p16INK4a-induced senescence. The combination of DEP1 and B2MG also allowed for flow cytometric identification of senescent cells. Mass spectrometry analysis of membrane- and cell surface-associated proteins from replicative senescent human diploid fibroblasts identified DPP4 (CD26) as a senescence surface marker. Interestingly, anti-DPP4 antibodies induced efficient antibody-dependent cellular cytotoxicity against senescent fibroblasts, thus enabling their preferential elimination by natural killer cells (Kim et al. 2017). More recently, the plasma membrane-localized fatty acid translocase CD36 was found upregulated in various cell types during replicative senescence, OIS, and TIS (Chong et al. 2018).
Proteomic approaches unveiled not only specific quantitative changes but also qualitative modification of surface plasma membrane-associated proteins in senescent cells, such as protein glycation (or glycoxidation), carbonylation, and carbamylation (Itakura et al. 2016; Vanhooren et al. 2015). Indeed, specific non-enzymatic post-translational modifications of proteins and lipids have been proposed as hallmarks of ageing (Friguet 2002). For instance, the products of non-enzymatic glycation and oxidation (glycoxidation) of proteins and lipids, called advanced glycation end-products (AGEs), are known to accumulate in organisms in an age-dependent manner. It is also well known that reactive oxygen species induce progressive accumulation of oxidized and carbonylated proteins during aging (Baraibar et al. 2013). This age-related accumulation of oxidized proteins likely depends on a decreased efficacy of repair systems and/or failure of removal mechanisms (Chondrogianni et al. 2014). Among oxidized products, a non-degradable material referred to as lipofuscin, accumulates in the lysosomal compartment in aged tissues, and has been used as a senescence marker (von Zglinicki et al. 1995; Georgakopoulou et al. 2013). Recently, a novel sensitive technique for the detection of lipofuscin has been developed and applied for the detection of senescent cells in fresh, frozen and formalin-fixed biological specimens (Evangelou et al. 2017). Carbamylation, the spontaneous binding of isocyanic acid to proteins, also occurs continuously throughout life (Gorisse et al. 2016), and is easily detected on long-lifespan proteins such as extracellular matrix structural proteins. In order to characterize novel aging biomarkers, the key next step is to identify specific cell surface proteins targeted by these modifications in senescent cells. In line with this are the results of Frescas and colleagues that recently described the identification of a senescence-specific oxidative modification of vimentin (malondialdehyde (MDA)-modified vimentin), which is exposed on the surface of senescent fibroblasts and is secreted into the blood of senescence-prone SAMP8 mice (Frescas et al. 2017).
It is important to note that the acquisition of a senescent phenotype is associated to cell type- and stimulus-dependent variations in gene expression. For instance, by using differential display and DNA arrays, Toussaint and coworkers (Pascal et al. 2005) showed that, despite displaying a wide variety of common biomarkers of senescence at the morphological level, WI-38 foetal lung human diploid fibroblasts acquired different expression profiles when induced to undergo senescence by replicative exhaustion or by treatment with tert-butylhydroperoxide or ethanol. Similarly, using RNA-seq, Purcell compared expression profiles of replicative senescence with that of various types of stress-induced senescence in fibroblasts, and highlighted both similarities and differences in expression between different pro-senescence stimuli (Purcell et al. 2014). Interestingly, comparative bioinformatic analyses of high-throughput microarray data from nerve and muscle biopsies from young and aged healthy donors showed a limited number of common differentially expressed genes among the skeletal muscle and nervous tissues, thus confirming the great heterogeneity that characterizes cellular senescence in vitro and in vivo (Voutetakis et al. 2015). Using numerous whole-transcriptome profiles of different fibroblast strains, Hernandez-Segura not only confirmed the stress- and cell-specific transcriptional response to senescence-inducing stimuli, but also showed that the gene expression profiles are temporally modulated (Hernandez-Segura et al. 2017). Recently, transcriptional heterogeneity of replicative senescent or stress-induced premature senescent fibroblasts was confirmed at single cell level using droplet-based single-cell mRNA sequencing (Tang et al. 2019). In this context, it is worth noting that gene expression analyses showed dramatically different profiles between senescent fibroblasts and senescent epithelial cells (Zhang et al. 2003). This observation, together with the stimulus-specific regulation of cellular senescence, suggests that the search for biomarkers of senescence for ageing and age-associated diseases, as well as for TIS in cancer patients, might require a large sampling effort, or that different biomarkers would eventually be used to identify different senescent phenotypes.
3 Potential Applications of Aptamers for Senescent Cells Biomarker Discovery and Targeting
The term aptamer (derived from the Latin aptus = to fit) was coined by Ellington and Szostak (http://ellingtonlab.org/blog/2014/12/1/on-aptamers) to describe molecules (oligonucleotides and, later, peptides) that bind to specific targets with high affinity and specificity, through structural recognition, thanks to their particular three-dimensional conformation, in the same way an antibody binds to an antigen (Ellington and Szostak 1990; Tuerk and Gold 1990; Ellington and Szostak 1992). Non-covalent interactions such as hydrophobic interactions, electrostatic interactions and van der Waals bonds mediate the binding of aptamers to their target molecules. Nucleic acid aptamers, usually ranging from 20 to 100 nt, are isolated from pools of random oligonucleotide sequences through an iterative process of selection and amplification, called SELEX (systemic evolution of ligands by exponential enrichment) (Tuerk and Gold 1990). Both RNA and single-strand DNA aptamers have been successfully selected using SELEX that can bind similar targets with similar affinity (McKeague and Derosa 2012; Lin and Patel 1997). However DNA aptamers are more stable than RNA aptamers (White et al. 2000), and the latter are usually chemically modified in order to enhance their stability (Kratschmer and Levy 2017; Germer et al. 2013).
Compared to antibodies, nucleic acid aptamers have a number of advantages. First, aptamers are chemically synthesized in the lab so their production does not require animals, is relatively easy and cheap, with low variability between batches. Aptamers can also be raised against non-immunogenic targets, such as small inorganic and organic molecules (https://www.aptagen.com/aptamer-index). Due to their small size, aptamers are minimally immunogenic (for instance, Zheng et al. 2017). Their small size also results in enhanced tissue penetration and reduced circulation time (Kaur et al. 2018; Keefe et al. 2010). Aptamers are amenable to chemical modification in the sugar, base or phosphate backbone, which can improve both their resistance to nuclease degradation and their affinity for targets (Ni et al. 2017). Finally, aptamers can be easily coupled to different probes, nanoparticles or other biomolecules.
Focusing on the potential application of nucleic acid aptamer technology for the discovery of senescent cells biomarkers, it is important to note that SELEX can be carried out using as targets both purified proteins or complex targets, such as whole cells (cell-SELEX) and plasma (Zhuo et al. 2017; Shamah et al. 2008; Fitter and James 2005). Hence, SELEX could be employed to generate aptamers that specifically recognize (and possibly modulate the function(s)) of already known senescence biomarkers, such as DEP1 (Althubiti et al. 2014), DPP4 (Kim et al. 2017) and CD36 (Chong et al. 2018). On the other hand, cell-SELEX is a powerful strategy for discovery of unknown biomarkers and the cell-SELEX method could be used to identify aptamers that show high affinity for still unidentified membrane molecules on the surface of senescent cells (Berezovski et al. 2008; Sefah et al. 2010). The inclusion of a counter-selection step, with proliferating and/or quiescent cells as negative controls, would allow for enrichment of senescence-specific aptamers. It is important to note that aptamers generated through cell-SELEX recognize various kinds of biological molecules, for instance proteins, lipids and carbohydrates, in their native conformation (Li 2007). Aptamers are able to distinguish between closely related molecules. For instance, Chen and colleagues isolated an RNA aptamer able to distinguish the single amino acid mutant protein p53R175H from the wild type p53 protein (Chen et al. 2015). Furthermore, aptamers are able to distinguish post-translational modifications (PTM) of target proteins (Díaz-Fernández et al. 2018; Gilbert et al. 1997). For instance, aptamers were produced that could recognize the hydrophobic farnesyl moiety in K-Ras (Gilbert et al. 1997). Highly specific DNA aptamers that specifically bound to histone H4 acetylated at lysine 16 (H4-K16Ac) were identified by Williams et al. (2009). DNA aptamers able to distinguish between peptides differing by a single glycosylation were developed, using N-glycosylated vascular endothelial growth factor (VEGF) (Rose et al. 2010). Notably, these aptamers were not specific for the sugar moiety itself, but rather the combination of the peptide sequence and the glycosylation. Since many types of PTM present in mammalian proteins are absent or different in bacteria (for instance ubiquitination and acetylation), it is important that recombinant proteins used as targets for SELEX are purified from mammalian cells if the aptamers are supposed to be used in mammalian cell systems, as demonstrated with the anti-cyclophilin B aptamer M9-5 (Ray et al. 2013). By binding to their target proteins, aptamers can modulate their functions, and aptamers working as both antagonists or agonists have been isolated. For example, an antagonistic DNA aptamer targeting PD-L1 efficiently blocked the binding between human PD-1 and PD-L1, thus restoring T cell functions and enhancing anti-tumor immune responses (Lai et al. 2016). An agonistic DNA aptamer, which bound to and stimulated the immune inhibitory receptor CD200R1, was shown to suppress inflammatory responses in vivo (Prodeus et al. 2018). Interestingly, this aptamer was capable of agonizing both murine and human CD200R1 (Prodeus et al. 2018).
Since their development in early 1990s nucleic acid aptamers have become important tools in a wide range of both clinical and research applications, such as ELISA and pull-down assays, flow cytometry, immunohistochemistry, drug delivery, functionalization of nanoparticles, and bioimaging (Zhu et al. 2015; Vandghanooni et al. 2018; He et al. 2018; Zhou and Rossi 2017). These observations strongly support the use of aptamers in the growing field of senotherapy. Although only one aptamer, Pegaptanib sodium, (Macugen; Eyetech Pharmaceuticals/Pfizer), an RNA aptamer directed against vascular endothelial growth factor (VEGF)-165, has been approved for therapeutic use in humans (Ng et al. 2006), other nucleic acid aptamers are at advanced stages of clinical trials (Kaur et al. 2018; Maier and Levy 2016).
Peptide aptamers were first described by Brent and coworkers (Colas et al. 1996), who based their approach on the concept that short peptides anchored at both amino and carboxy termini (i.e. conformationally constrained) are capable of high-affinity and specific interactions with other molecules. By using Escherichia coli Thioredoxin A protein as a rigid scaffold to display 20-residue random peptides, Brent and coworkers were able to select peptide aptamers that specifically bound human Cdk2, and, notably, inhibited its kinase activity. Since then, many other non-antibody protein scaffolds have been designed, which constrain variable peptide sequences for display, reviewed in Yu et al. 2017 and Simeon and Chen 2018. Here, we will focus on a recently developed, promising peptide aptamer technology known as Adhiron or Affimer system (Tiede et al. 2014, Tiede et al. 2017), developed by the BioScreening Technology Group (BSTG), from University of Leeds (UK) and their industrial partner Avacta (https://www.avacta.com/). Two different scaffolds were developed in this system: the first derived from human protease inhibitor Stefin A, the other derived from Cystatin A, a plant cysteine protease inhibitor. Each scaffold is capable of displaying one or two peptide variable regions of varying lengths (Tiede et al. 2014). Peptide aptamers derived from either of these two scaffolds are collectively called Affimers. Compared to antibodies, Affimers have a number of advantages. First, Affimers are easily expressed in Escherichia coli as well as in mammalian cells, and their production requires no animals (Dos Remedios and Peckham 2017). Both scaffolds are monomeric, highly stable (to both pH and temperature, with melting temperature between 70 and 100 °C) and small (~100 amino acids, 11 kDa, 3 nm in diameter). Their small size allows for better penetration into cellular and subcellular structures and tissue. Finally, the Affimer scaffolds show low immunogenicity (https://www.avacta.com/blogs/no-immunogenicity-issues-Affimer-technology), and can be subjected to chemical modification and conjugation.
Affimers recognizing specific targets are selected using a phage display strategy: a library of phage display Affimer reagents is screened against either purified target molecules or complex targets, such as whole cells, and a step of counter-screening may be used to increase specificity of isolated Affimers (Tang et al. 2017). Affimer libraries have been successfully screened against more than 500 target proteins and used as reagents in diagnostics, imaging and biomedical applications (recently reviewed in Kyle 2018).
Similar to nucleic acid aptamers, Affimers specifically recognize proteins with different types of post-translation modifications and are able to inhibit protein function and/or protein-protein interactions by binding to their specific targets. For instance, Affimers directed against VEGFR2 inhibited VEGF-stimulated receptor phosphorylation and downstream signaling in HUVEC cells, eventually reducing VEGF-induced HUVEC tubulogenesis (Tiede et al. 2017). Affimers that specifically bound SUMO-1 or SUMO-2, or both, inhibited SUMO-mediated protein-protein interactions (Hughes et al. 2017). Inhibition of protein-protein interaction by Affimers was also described by Robinson and colleagues, who generated Affimers against the immunoglobulin receptor FcγRIIIa. These Affimers were able to selectively block IgG immune complex binding to FcγRIIIa (but not to FcγRIIa or FcγRIIIb), and abrogated FcγRIIIa-dependent effector functions in macrophage (Robinson et al. 2018). Affimers were also developed for in vivo imaging of tumor markers. For instance, high affinity Affimers recognizing Tenascin C, an extracellular matrix glycoprotein that is highly expressed in the tumor stroma of most epithelial malignancies (Yoshida et al. 2015), were isolated by Tiede et al. (2017). This Affimer, conjugated with biotin, allowed for the detection of Tenascin C in human colorectal cancer and glioblastoma xenograft tissue sections, with a specificity similar to antibodies. In addition, Rhodamine Red-labelled Affimer accumulated in Tenascin C expressing tumour in vivo. Interestingly, Affimers were quickly cleared from the circulatory system, compared to antibodies, allowing for rapid imaging of tumor (Tiede et al. 2017).
On the whole, these studies highlight the potentiality of Affimers as tools for the identification of novel senescence-specific cell surface biomarkers.
4 Conclusions
In this chapter we have highlighted the need for novel senescence biomarkers and the development of diagnostic, prognostic and therapeutic interventions for aging and cancer-related TIS. A wealth of data from high-throughput transcriptomics and proteomics studies indicate that the acquisition of a senescent phenotype, in normal as well as neoplastic cells, is associated with alterations of cell surface proteins and/or their modifications. These studies support the possibility of utilizing these quantitative and qualitative differences for developing senescence-specific markers.
Since the first reports in the 1990s, nucleic acid aptamers and the SELEX technology have been widely applied for the discovery of biomarkers, useful in research and in the clinic. More recently, the peptide aptamer (Affimers) technology has emerged as valuable tools for biomarker discovery. We propose that aptamers represent a promising strategy to succeed in identifying new senescence-specific markers.
References
Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S et al (2008) Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133(6):1006–1018
Alimonti A, Nardella C, Chen Z, Clohessy JG, Carracedo A, Trotman LC et al (2010) A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J Clin Invest. 120(3):681–693
Alspach E, Flanagan KC, Luo X, Ruhland MK, Huang H, Pazolli E et al (2014) p38MAPK plays a crucial role in stromal-mediated tumorigenesis. Cancer Discov 4(6):716–729
Althubiti M, Lezina L, Carrera S, Jukes-Jones R, Giblett SM, Antonov A et al (2014) Characterization of novel markers of senescence and their prognostic potential in cancer. Cell Death Dis 5:e1528
Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B et al (2011) Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479(7372):232–236
Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J et al (2016) Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530(7589):184–189
Baraibar MA, Ladouce R, Friguet B (2013) Proteomic quantification and identification of carbonylated proteins upon oxidative stress and during cellular aging. J Proteomics 92:63–70
Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N et al (2006) Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444(7119):633–637
Berezovski MV, Lechmann M, Musheev MU, Mak TW, Krylov SN (2008) Aptamer-facilitated biomarker discovery (AptaBiD). J Am Chem Soc 130(28):9137–9143
Biran A, Perelmutter M, Gal H, Burton DG, Ovadya Y, Vadai E et al (2015) Senescent cells communicate via intercellular protein transfer. Genes Dev 29(8):791–802
Borghesan M, Fafián-Labora J, Eleftheriadou O, Carpintero-Fernández P, Paez-Ribes M, Vizcay-Barrena G et al (2019) Small extracellular vesicles are key regulators of non-cell autonomous intercellular communication in senescence via the interferon protein IFITM3. Cell Rep. 27(13):3956–3971.e6
Burgess DJ (2011) Senescence. NF-κB shows its beneficial side. Nat Rev Cancer 11(12):832–833
Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ (2018) Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562(7728):578–582
Camorani S, Cerchia L, Fedele M, Erba E, D’Incalci M, Crescenzi E (2018) Trabectedin modulates the senescence-associated secretory phenotype and promotes cell death in senescent tumor cells by targeting NF-κB. Oncotarget. 9(28):19929–19944
Capell BC, Drake AM, Zhu J, Shah PP, Dou Z, Dorsey J et al (2016) MLL1 is essential for the senescence-associated secretory phenotype. Genes Dev 30(3):321–336
Chen L, Rashid F, Shah A, Awan HM, Wu M, Liu A et al (2015) The isolation of an RNA aptamer targeting to p53 protein with single amino acid mutation. Proc Natl Acad Sci USA 112(32):10002–10007
Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE et al (2011) Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev 25(20):2125–2136
Chondrogianni N, Petropoulos I, Grimm S, Georgila K, Catalgol B, Friguet B et al (2014) Protein damage, repair and proteolysis. Mol Aspect Med 35:1–71
Chong M, Yin T, Chen R, Xiang H, Yuan L, Ding Y et al (2018) CD36 initiates the secretory phenotype during the establishment of cellular senescence. EMBO Rep 19(6)
Colas P, Cohen B, Jessen T, Grishina I, McCoy J, Brent R (1996) Genetic selection of peptide aptamers that recognize and inhibit cyclin-dependent kinase 2. Nature 380(6574):548–550
Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J et al (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6(12):2853–2868
Correia-Melo C, Marques FD, Anderson R, Hewitt G, Hewitt R, Cole J et al (2016) Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J 35(7):724–742
Cotarelo CL, Schad A, Kirkpatrick CJ, Sleeman JP, Springer E, Schmidt M et al (2016) Detection of cellular senescence within human invasive breast carcinomas distinguishes different breast tumor subtypes. Oncotarget 7(46):74846–74859
Crescenzi E, Pacifico F, Lavorgna A, De Palma R, D’Aiuto E, Palumbo G et al (2011) NF-κB-dependent cytokine secretion controls Fas expression on chemotherapy-induced premature senescent tumor cells. Oncogene 30(24):2707–2717
d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T et al (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426(6963):194–198
Demaria M, O’Leary MN, Chang J, Shao L, Liu S, Alimirah F et al (2017) Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov 7(2):165–176
Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C et al (2006) Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444(7119):638–642
Díaz-Fernández A, Miranda-Castro R, de-Los-Santos-Álvarez N, Lobo-Castañón MJ (2018) Post-translational modifications in tumor biomarkers: the next challenge for aptamers? Anal Bioanal Chem 410(8):2059–2065
Dos Remedios C, Peckham M (2017) 3Rs and biophysics. Biophys Rev 9(4):277–278
Eggert T, Wolter K, Ji J, Ma C, Yevsa T, Klotz S et al (2016) Distinct functions of senescence-associated immune responses in liver tumor surveillance and tumor progression. Cancer Cell 30(4):533–547
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346(6287):818–822
Ellington AD, Szostak JW (1992) Selection in vitro of single-stranded DNA molecules that fold into specific ligand-binding structures. Nature 355(6363):850–852
Evangelou K, Lougiakis N, Rizou SV, Kotsinas A, Kletsas D, Muñoz-Espín D et al (2017) Robust, universal biomarker assay to detect senescent cells in biological specimens. Aging Cell 16(1):192–197
Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL et al (2017) Targeting cellular senescence prevents age-related bone loss in mice. Nat Med 23(9):1072–1079
Fitter S, James R (2005) Deconvolution of a complex target using DNA aptamers. J Biol Chem 280(40):34193–34201
Frescas D, Roux CM, Aygun-Sunar S, Gleiberman AS, Krasnov P, Kurnasov OV et al (2017) Senescent cells expose and secrete an oxidized form of membrane-bound vimentin as revealed by a natural polyreactive antibody. Proc Natl Acad Sci USA 114(9):E1668–E1677
Freund A, Laberge RM, Demaria M, Campisi J (2012) Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell 23(11):2066–2075
Friguet B (2002) Aging of proteins and the proteasome. Prog Mol Subcell Biol 29:17–33
Fumagalli M, Rossiello F, Clerici M, Barozzi S, Cittaro D, Kaplunov JM et al (2012) Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol 14(4):355–365
Georgakopoulou EA, Tsimaratou K, Evangelou K, Fernandez Marcos PJ, Zoumpourlis V, Trougakos IP et al (2013) Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging (Albany NY) 5(1):37–50
Georgilis A, Klotz S, Hanley CJ, Herranz N, Weirich B, Morancho B et al (2018) PTBP1-mediated alternative splicing regulates the inflammatory secretome and the pro-tumorigenic effects of senescent cells. Cancer Cell 34(1):85–102.e9
Germer K, Leonard M, Zhang X (2013) RNA aptamers and their therapeutic and diagnostic applications. Int J Biochem Mol Biol 4(1):27–40
Gilbert BA, Sha M, Wathen ST, Rando RR (1997) RNA aptamers that specifically bind to a K Ras-derived farnesylated peptide. Bioorg Med Chem 5(6):1115–1122
Glück S, Ablasser A (2019) Innate immunosensing of DNA in cellular senescence. Curr Opin Immunol 56:31–36
Gorisse L, Pietrement C, Vuiblet V, Schmelzer CE, Köhler M, Duca L et al (2016) Protein carbamylation is a hallmark of aging. Proc Natl Acad Sci USA 113(5):1191–1196
Grabowska W, Kucharewicz K, Wnuk M, Lewinsk A, Suszek M, Przybylska D et al (2015) Curcumin induces senescence of primary human cells building the vasculature in a DNA damage and ATM-independent manner. Age (Dordr) 37(1):9744
Hari P, Millar FR, Tarrats N, Birch J, Quintanilla A, Rink CJ et al (2019) The innate immune sensor Toll-like receptor 2 controls the senescence-associated secretory phenotype. Sci Adv 5(6):eaaw0254
He F, Wen N, Xiao D, Yan J, Xiong H, Cai S et al (2018) Aptamer based targeted drug delivery systems: current potential and challenges. Curr Med Chem [Epub ahead of print]
Hernandez-Segura A, de Jong TV, Melov S, Guryev V, Campisi J, Demaria M (2017) Unmasking Transcriptional Heterogeneity in Senescent Cells. Curr Biol 27(17):2652–2660.e4
Hewitt G, Jurk D, Marques FD, Correia-Melo C, Hardy T, Gackowska A et al (2012) Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun 3:708
Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK et al (2019) Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 47:446–456
Hills SA, Diffley JF (2014) DNA replication and oncogene-induced replicative stress. Curr Biol 24(10):R435–R444
Hoare M, Ito Y, Kang TW, Weekes MP, Matheson NJ, Patten DA et al (2016) NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat Cell Biol 18(9):979–992
Hughes DJ, Tiede C, Penswick N, Tang AA, Trinh CH, Mandal U et al (2017) Generation of specific inhibitors of SUMO-1- and SUMO-2/3-mediated protein-protein interactions using Affimer (Adhiron) technology. Sci Signal 10(505):pii:eaaj2005
Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH (2013) p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med 210(10):2057–2069
Itakura Y, Sasaki N, Kami D, Gojo S, Umezawa A, Toyoda M (2016) N- and O-glycan cell surface protein modifications associated with cellular senescence and human aging. Cell Biosci 6:14
Jackson JG, Pant V, Li Q, Chang LL, Quintás-Cardama A, Garza D et al (2012) p53-mediated senescence impairs the apoptotic response to chemotherapy and clinical outcome in breast cancer. Cancer Cell 21(6):793–806
Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP et al (2017) Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med 23(6):775–778
Jeon OH, Wilson DR, Clement CC, Rathod S, Cherry C, Powell B et al (2019) Senescence cell-associated extracellular vesicles serve as osteoarthritis disease and therapeutic markers. JCI Insight 4(7):pii:125019
Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK et al (2019) Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 40:554–563
Karimi-Busheri F, Rasouli-Nia A, Mackey JR, Weinfeld M (2010) Senescence evasion by MCF-7 human breast tumor-initiating cells. Breast Cancer Res 12(3):R31
Kaur H, Bruno JG, Kumar A, Sharma TK (2018) Aptamers in the therapeutics and diagnostics pipelines. Theranostics 8(15):4016–4032
Keefe AD, Pai S, Ellington A (2010) Aptamers as therapeutics. Nat Rev Drug Discov 9(7):537–550
Kim SB, Bozeman RG, Kaisani A, Kim W, Zhang L, Richardson JA et al (2016) Radiation promotes colorectal cancer initiation and progression by inducing senescence-associated inflammatory responses. Oncogene 35(26):3365–3375
Kim KM, Noh JH, Bodogai M, Martindale JL, Yang X, Indig FE et al (2017) Identification of senescent cell surface targetable protein DPP4. Genes Dev 31(15):1529–1534
Kirkland JL, Tchkonia T (2017) Cellular senescence: a translational perspective. EBioMedicine 21:21–28
Kovacovicova K, Skolnaja M, Heinmaa M, Mistrik M, Pata P, Pata I et al (2018) Senolytic cocktail Dasatinib+Quercetin (D+Q) does not enhance the efficacy of senescence-inducing chemotherapy in liver cancer. Front Oncol 8:459
Kratschmer C, Levy M (2017) Effect of chemical modifications on aptamer stability in serum. Nucleic Acid Ther 27(6):335–344
Kuilman T, Peeper DS (2009) Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer 9(2):81–94
Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ et al (2008) Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133(6):1019–1031
Kurz DJ, Decary S, Hong Y, Erusalimsky JD (2000) Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci 113(Pt 20):3613–3622
Kyle S (2018) Affimer proteins: theranostics of the future? Trends Biochem Sci 43(4):230–232
Laberge RM, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L et al (2015) MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 17(8):1049–1061
Lai WY, Huang BT, Wang JW, Lin PY, Yang PC (2016) A novel PD-L1-targeting antagonistic DNA aptamer with antitumor effects. Mol Ther Nucleic Acids 5(12):e3977
Larsen AK, Galmarini CM, D’Incalci M (2016) Unique features of trabectedin mechanism of action. Cancer Chemother Pharmacol 77(4):663–671
Li L (2007) Detection of protein biomarkers using RNA aptamer microarrays and enzymatically amplified surface plasmon resonance imaging. Anal Chem 79(3):1082–1088
Lin CH, Patel DJ (1997) Structural basis of DNA folding and recognition in an AMP-DNA aptamer complex: distinct architectures but common recognition motifs for DNA and RNA aptamers complexed to AMP. Chem Biol 4(11):817–832
Liu S, Uppal H, Demaria M, Desprez PY, Campisi J, Kapahi P (2015) Simvastatin suppresses breast cancer cell proliferation induced by senescent cells. Sci Rep 5:17895
Liu S, Mahairaki V, Bai H, Ding Z, Li J, Witwer KW et al (2019) Highly purified human extracellular vesicles produced by stem cells alleviate aging cellular phenotypes of senescent human cells. Stem Cells 37(6):779–790
Llanos S, Serrano M (2016) Senescence and cancer: in the name of immunosuppression. Cancer Cell 30(4):507–508
Maciel-Barón LA, Morales-Rosales SL, Aquino-Cruz AA, Triana-Martínez F, Galván-Arzate S, Luna-López A et al (2016) Senescence associated secretory phenotype profile from primary lung mice fibroblasts depends on the senescence induction stimuli. Age (Dordr) 38(1):26
Maier KE, Levy M (2016) From selection hits to clinical leads: progress in aptamer discovery. Mol Ther Methods Clin Dev 5:16014
McKeague M, Derosa MC (2012) Challenges and opportunities for small molecule aptamer development. J Nucleic Acids 2012:748913
Mirzayans R, Scott A, Cameron M, Murray D (2005) Induction of accelerated senescence by gamma radiation in human solid tumor-derived cell lines expressing wild-type TP53. Radiat Res 163(1):53–62
Mosteiro L, Pantoja C, Alcazar N, Marión RM, Chondronasiou D, Rovira M et al (2016) Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science 354(6315):pii:aaf4445
Muñoz-Espín D, Serrano M (2014) Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 15(7):482–496
Muñoz-Espín D, Cañamero M, Maraver A, Gómez-López G, Contreras J, Murillo-Cuesta S et al (2013) Programmed cell senescence during mammalian embryonic development. Cell 155(5):1104–1118
Muñoz-Espín D, Rovira M, Galiana I, Giménez C, Lozano-Torres B, Paez-Ribes M et al (2018) A versatile drug delivery system targeting senescent cells. EMBO Mol Med 10(9):pii:e9355
Myrianthopoulos V, Evangelou K, Vasileiou PVS, Cooks T, Vassilakopoulos TP, Pangalis GA et al (2019) Senescence and senotherapeutics: a new field in cancer therapy. Pharmacol Ther 193:31–49
Ng EW, Shima DT, Calias P, Cunningham ET Jr, Guyer DR, Adamis AP (2006) Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov 5(2):123–132
Ni S, Yao H, Wang L, Lu J, Jiang F, Lu A et al (2017) Chemical modifications of nucleic acid aptamers for therapeutic purposes. Int J Mol Sci 18(8):pii:E1683
Novakova Z, Hubackova S, Kosar M, Janderova-Rossmeislova L, Dobrovolna J, Vasicova P et al (2010) Cytokine expression and signaling in drug-induced cellular senescence. Oncogene 29(2):273–284
Pascal T, Debacq-Chainiaux F, Chrétien A, Bastin C, Dabée AF, Bertholet V et al (2005) Comparison of replicative senescence and stress-induced premature senescence combining differential display and low-density DNA arrays. FEBS Lett 579(17):3651–3659
Pazolli E, Alspach E, Milczarek A, Prior J, Piwnica-Worms D, Stewart SA (2012) Chromatin remodeling underlies the senescence-associated secretory phenotype of tumor stromal fibroblasts that supports cancer progression. Cancer Res 72(9):2251–2261
Perrott KM, Wiley CD, Desprez PY, Campisi J (2017) Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells. Geroscience 39(2):161–173
Pospelova TV, Demidenko ZN, Bukreeva EI, Pospelov VA, Gudkov AV, Blagosklonny MV (2009) Pseudo-DNA damage response in senescent cells. Cell Cycle 8(24):4112–4118
Prodeus A, Sparkes A, Fischer NW, Cydzik M, Huang E, Khatri I et al (2018) A synthetic cross-species CD200R1 agonist suppresses inflammatory immune responses in vivo. Mol Ther Nucleic Acids 12:350–358
Purcell M, Kruger A, Tainsky MA (2014) Gene expression profiling of replicative and induced senescence. Cell Cycle 13(24):3927–3937
Ray P, Sullenger BA, White RR (2013) Further characterization of the target of a potential aptamer biomarker for pancreatic cancer: cyclophilin B and its posttranslational modifications. Nucleic Acid Ther 23(6):435–442
Reimann M, Lee S, Loddenkemper C, Dörr JR, Tabor V, Aichele P et al (2010) Tumor stroma-derived TGF-beta limits myc-driven lymphomagenesis via Suv39h1-dependent senescence. Cancer Cell 17(3):262–272
Ritschka B, Storer M, Mas A, Heinzmann F, Ortells MC, Morton JP et al (2017) The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev 31(2):172–183
Roberson RS, Kussick SJ, Vallieres E, Chen SY, Wu DY (2005) Escape from therapy-induced accelerated cellular senescence in p53-null lung cancer cells and in human lung cancers. Cancer Res 65(7):2795–2803
Robinson JI, Baxter EW, Owen RL, Thomsen M, Tomlinson DC, Waterhouse MP et al (2018) Affimer proteins inhibit immune complex binding to FcγRIIIa with high specificity through competitive and allosteric modes of action. Proc Natl Acad Sci USA 115(1):E72–E81
Rodier F, Campisi J (2011) Four faces of cellular senescence. J Cell Biol 192(4):547–556
Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM et al (2016) Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15(5):973–977
Rose CM, Hayes MJ, Stettler GR, Hickey SF, Axelrod TM, Giustini NP et al (2010) Capillary electrophoretic development of aptamers for a glycosylated VEGF peptide fragment. Analyst 35(11):2945–2951
Sagini K, Costanzi E, Emiliani C, Buratta S, Urbanelli L (2018) Extracellular vesicles as conveyors of membrane-derived bioactive lipids in immune system. Int J Mol Sci 19(4).pii:E1227
Salama R, Sadaie M, Hoare M, Narita M (2014) Cellular senescence and its effector programs. Genes Dev 28(2):99–114
Samaraweera L, Adomako A, Rodriguez-Gabin A, McDaid HM (2017) A novel indication for panobinostat as a senolytic drug in NSCLC and HNSCC. Sci Rep 7(1):1900
Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ et al (2017) Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 8:14532
Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC (2004) Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol 6(2):168–170
Sefah K, Shangguan D, Xiong X, O’Donoghue MB, Tan W (2010) Development of DNA aptamers using Cell-SELEX. Nat Protoc 5(6):1169–1185
Serrano M (2014) Senescence helps regeneration. Dev Cell 31(6):671–672
Severino J, Allen RG, Balin S, Balin A, Cristofalo VJ (2000) Is beta-galactosidase staining a marker of senescence in vitro and in vivo? Exp Cell Res 257(1):162–171
Shamah SM, Healy JM, Cload ST (2008) Complex target SELEX. Acc Chem Res 41(1):130–138
Sidi R, Pasello G, Opitz I, Soltermann A, Tutic M, Rehrauer H et al (2011) Induction of senescence markers after neo-adjuvant chemotherapy of malignant pleural mesothelioma and association with clinical outcome: an exploratory analysis. Eur J Cancer 47(2):326–332
Simeon R, Chen Z (2018) In vitro-engineered non-antibody protein therapeutics. Protein Cell 9(1):3–14
Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V et al (2013) Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155(5):1119–1130
Sun Y, Coppé JP, Lam EW (2018) Cellular senescence: the sought or the unwanted? Trends Mol Med 24(10):871–885
Supiot S, Shubbar S, Fleshner N, Warde P, Hersey K, Wallace K et al (2008) A phase I trial of pre-operative radiotherapy for prostate cancer: clinical and translational studies. Radiother Oncol 88(1):53–60
Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S et al (2017) Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun 8:15287
Takasugi M (2018) Emerging roles of extracellular vesicles in cellular senescence and aging. Aging Cell 17(2)
Takasugi M, Okada R, Takahashi A, Virya Chen D, Watanabe S, Hara E (2017) Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat Commun 8:15729
Tang AA, Tiede C, Hughes DJ, McPherson MJ, Tomlinson DC (2017) Isolation of isoform-specific binding proteins (Affimers) by phage display using negative selection. Sci Signal 10(505):pii:eaan0868
Tang H, Geng A, Zhang T, Wang C, Jiang Y, Mao Z (2019) Single senescent cell sequencing reveals heterogeneity in senescent cells induced by telomere erosion. Protein Cell 10(5):370–375
Tasdemir N, Banito A, Roe JS, Alonso-Curbelo D, Camiolo M, Tschaharganeh DF et al (2016) BRD4 connects enhancer remodeling to senescence immune surveillance. Cancer Discov 6(6):612–629
te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP (2002) DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res 62(6):1876–1883
Tiede C, Tang AA, Deacon SE, Mandal U, Nettleship JE, Owen RL et al (2014) Adhiron: a stable and versatile peptide display scaffold for molecular recognition applications. Protein Eng Des Sel 27(5):145–155
Tiede C, Bedford R, Heseltine SJ, Smith G, Wijetunga I, Ross R et al (2017) Affimer proteins are versatile and renewable affinity reagents. Elife 6:pii:e24903
Toso A, Revandkar A, Di Mitri D, Guccini I, Proietti M, Sarti M et al (2014) Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep 9(1):75–89
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249(4968):505–510
Vandghanooni S, Eskandani M, Barar J, Omidi Y (2018) Recent advances in aptamer-armed multimodal theranostic nanosystems for imaging and targeted therapy of cancer. Eur J Pharm Sci 117:301–312
Vanhooren V, Navarrete Santos A, Voutetakis K, Petropoulos I, Libert C, Simm A et al (2015) Protein modification and maintenance systems as biomarkers of ageing. Mech Ageing Dev 51:71–84
von Zglinicki T, Nilsson E, Döcke WD, Brunk UT (1995) Lipofuscin accumulation and ageing of fibroblasts. Gerontology 41(Suppl 2):95–108
Voutetakis K, Chatziioannou A, Gonos ES, Trougakos IP (2015) Comparative meta-analysis of transcriptomics data during cellular senescence and in vivo tissue ageing. Oxid Med Cell Longev 2015:732914
Wei W, Ji S (2018) Cellular senescence: molecular mechanisms and pathogenicity. J Cell Physiol 233(12):9121–9135
White RR, Sullenger BA, Rusconi CP (2000) Developing aptamers into therapeutics. J Clin Invest 106(8):929–934
Wiley CD, Flynn JM, Morrissey C, Lebofsky R, Shuga J, Dong X et al (2017) Analysis of individual cells identifies cell-to-cell variability following induction of cellular senescence. Aging Cell 16(5):1043–1105
Williams BA, Lin L, Lindsay SM, Chaput JC (2009) Evolution of a histone H4-K16 acetyl-specific DNA aptamer. J Am Chem Soc 131(18):6330–6331
Wu PC, Wang Q, Grobman L, Chu E, Wu DY (2012) Accelerated cellular senescence in solid tumor therapy. Exp Oncol 34(3):298–305
Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T et al (2015) JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci USA 112(46):E6301–E6310
Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM et al (2018) Senolytics improve physical function and increase lifespan in old age. Nat Med 24(8):1246–1256
Yang NC, Hu ML (2005) The limitations and validities of senescence associated-beta-galactosidase activity as an aging marker for human foreskin fibroblast Hs68 cells. Exp Gerontol 40(10):813–819
Yang L, Fang J, Chen J (2017) Tumor cell senescence response produces aggressive variants. Cell Death Discov 3:17049
Yoshida T, Akatsuka T, Imanaka-Yoshida K (2015) Tenascin-C and integrins in cancer. Cell Adh Migr 9(1–2):96–104
Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann-Stroissnigg H, Xu M et al (2018) Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36:18–28
Yu X, Yang YP, Dikici E, Deo SK, Daunert S (2017) Beyond antibodies as binding partners: the role of antibody mimetics in bioanalysis. Annu Rev Anal Chem (Palo Alto Calif) 10(1):293–320
Zhang H, Pan KH, Cohen SN (2003) Senescence-specific gene expression fingerprints reveal cell-type-dependent physical clustering of up-regulated chromosomal loci. Proc Natl Acad Sci USA 100(6):3251–3256
Zhang Y, Kim MS, Jia B, Ya J, Zuniga-Hertz J P, Han C et al (2017). Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature 548(7665):52–57
Zheng J, Zhao S, Yu X, Huang S, Liu HY (2017) Simultaneous targeting of CD44 and EpCAM with a bispecific aptamer effectively inhibits intraperitoneal ovarian cancer growth. Theranostics 7(5):1373–1388
Zhou J, Rossi J (2017) Aptamers as targeted therapeutics: current potential and challenges. Nat Rev Drug Discov. 16(3):181–202
Zhu Q, Liu G, Kai M (2015) DNA aptamers in the diagnosis and treatment of human diseases. Molecules 20(12):20979–20997
Zhuo Z, Yu Y, Wang M, Li J, Zhang Z, Liu J et al (2017) Recent Advances in SELEX Technology and Aptamer Applications in Biomedicine. Int J Mol Sci 18(10):pii:E2142
Acknowledgements
We wish to thank Dr Darren Tomlinson and Dr Thomas A. Hughes (University of Leeds, UK) for introducing us to Affimers technology.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Leonardi, A., Pacifico, F., Crescenzi, E. (2020). Potential Applications of Aptamers for Targeting Senescent Cells. In: Muñoz-Espin, D., Demaria, M. (eds) Senolytics in Disease, Ageing and Longevity. Healthy Ageing and Longevity, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-030-44903-2_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-44903-2_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-44902-5
Online ISBN: 978-3-030-44903-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)