Abstract
Cellular senescence is a hallmark of aging because senescent cells (SnCs) accumulate with age and play a causative role in many age-related diseases. Selectively eliminating SnCs has been emerging as a new strategy for treating age-related diseases and extending healthspan. Small molecules that targeting different SnC anti-apoptotic pathways (SCAPs) to selectively kill SnCs are termed senolytics. Up to date, several classes of senolytic agents, including naturally occurring compounds and their derivatives, and targeted therapeutics, have been identified. Here we discuss the biological significances of cellular senescence in aging, and summarize some of the known naturally occurring and targeted senolytic agents and their targets. As most of the known naturally occurring compounds or targeted senolytics have limitations to be developed as therapeutics for human applications, development of more specific and potent senolytic agents that can reduce the on-target and/or off-target toxicity of senolytics, is urgently needed to improve healthy aging in humans.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Cellular Senescence
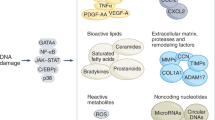
Cells become permanently growth arrested after extensive replication or as a result of exposure to stress, which prevents propagation of genetically unstable and damaged cells, and promotes their removal by the immune system (Childs et al. 2015). Therefore, cellular senescence normally functions as a vital tumor suppressive mechanism and also plays an important role in tissue damage repair. However, if the increase in senescent cell (SnC) production persists beyond the immune clearance capacity or the immune system is compromised and cannot efficiently remove SnCs, SnCs can accumulate. Under such circumstances, SnCs can play a causative role in aging and age-related diseases by inducing chronic oxidative stress and inflammation via increasing the production of reactive oxygen species (ROS) and secretion of a plethora of inflammatory mediators (e.g., cytokines and chemokines), growth factors, and extracellular proteases—termed the senescence-associated secretory phenotype (SASP) (Campisi 2013). Therefore, it has been suggested that inhibiting the induction of senescence might be detrimental, but promoting SnC clearance is beneficial. This suggestion is supported by the findings: (1) SnCs accumulate with aging, particularly at the sites of age-related pathologies (Childs et al. 2015); (2) SnCs can contribute to ‘inflammaging’ and other age-related pathologies in part via expression of SASP (Tchkonia et al. 2013); and (3) clearance of SnCs using a transgenic approach delays the onset of several age-related diseases and disorders including cancer in naturally aged mice and prolongs their lifespan (Baker et al. 2016). These findings demonstrate that SnCs are novel therapeutic targets of aging and age-related diseases.
2 SnCs are Emerging Therapeutic Targets
Although SnCs have been associated with various biological and pathological processes with aging, the causal relationship between SnCs and age-related diseases and disorders remained unclear until the year of 2011 (Baker et al. 2011). Baker et al. designed a transgenic strategy for the clearance of SnCs in progeroid mice and demonstrated that SnC removal can significantly delay the onset of several age-related pathologies and prolong the healthspan. The study provides solid evidence supporting that SnCs are causally implicated in generating age-related phenotypes (Baker et al. 2011). Using the same genetic approach, they revealed that clearance of SnCs not only delayed the onset of tumorigenesis and attenuated age-related deterioration of several organs, but also significantly extended the lifespan of normal mice (Baker et al. 2016). Since then, an increasing body of evidence has accumulated to demonstrate that SnCs play a causative role in a variety of diseases using mouse models, including atherosclerosis (Childs et al. 2016), osteoarthritis (Jeon et al. 2017), Parkinson’s disease (Chinta et al. 2018), Alzheimer’s disease (Zhang et al. 2019), diabetes (Palmer et al. 2015; Aguayo-Mazzucato et al. 2019), cancer (Takasugi et al. 2017; Demaria et al. 2017), pulmonary fibrosis (Schafer et al. 2017; He et al. 2019) and many other diseases (Childs et al. 2015; Tchkonia et al. 2013; Kirkland and Tchkonia 2017; Childs et al. 2017; Niedernhofer and Robbins 2018). Therefore, SnCs have been emerged as therapeutic targets for many of these age-related diseases.
3 Senolytics
SnCs can endure sustained DNA damage, oxidative stress, proteotoxicity and other stressors because they are protected from induction of apoptosis by various SnC anti-apoptotic pathways (SCAPs) (Kirkland and Tchkonia 2017; Childs et al. 2017; Niedernhofer and Robbins 2018). However, these SCAPs can also function as the Achilles’ heel of SnCs. Molecularly targeted inhibition of SCAPs with a small molecule can selectively kill SnCs. These small molecules are termed senolytics, whereas those that can suppress SASP, named senomorphics. Both senolytics and senomorphics have the potential to prevent and treat age-related diseases and to extend healthspan (Kirkland and Tchkonia 2017; Childs et al. 2017; Niedernhofer and Robbins 2018). However, compared to senomorphics, senolytics may provide greater promise and better benefits as anti-aging therapeutics because permanent elimination of SnCs by senolytics requires less drug exposure, produces less drug toxicity, and leads to a more durable effect than suppression of SASP by senomorphics. Therefore, development of senolytics has become a more attractive strategy to combat aging and age-related diseases. This hypothesis has yet to be tested in future studies.
To date, several classes of senolytic agents have been identified (Fig. 1.1), including (1) naturally occurring compounds and their derivatives such as quercetin (Xu et al. 2018; Zhu et al. 2015; Hickson et al. 2019), fisetin (Yousefzadeh et al. 2018; Zhu et al. 2017), piperlongumine and analogs (Wang et al. 2016; Zhang et al. 2018; Liu et al. 2018), curcumin analogs (Li et al. 2019) and cardiac glycosides (Guerrero et al. 2019; Triana-Martínez et al. 2019); and (2) targeted therapeutics such as dasatinib (Xu et al. 2018; Zhu et al. 2015; Hickson et al. 2019), a non-specific tyrosine kinase inhibitor; inhibitors of the anti-apoptotic Bcl-2 family proteins (Zhu et al. 2015; Zhu et al. 2017; Chang et al. 2016; Yosef et al. 2016); HSP90 inhibitor and histone deacetylase (Fuhrmann-Stroissnigg et al. 2017); UBX0101 (Jeon et al. 2017), an inhibitor of the MDM2/p53 protein interaction; and a modified FOXO4-p53 interfering peptide (IP) (Baar et al. 2017). The following is a brief description of each of these known senolytics.
3.1 Senolytic Natural Compounds
Many natural products have anti-aging effects and are used as traditional medicines and nutritional supplements to prevent or treat various age-related diseases, such as resveratrol (Knutson and Leeuwenburgh 2008), berberine (Xu et al. 2017), rutin (Li et al. 2016; Yang et al. 2012), catechin (Assuncao and Andrade 2015; Bernatoniene and Kopustinskiene 2018), proanthocyanidin (Liu et al. 2018), ginkgo biloba extract (EGb 761) (Sastre et al. 1998), ursolic acid (He et al. 2014; He et al. 2013) and other phytomolecules (Mukherjee et al. 2011). Most of them are antioxidants and exert their anti-aging functions mainly by reducing oxidative damage. However, only a few of them have been identified as senolytics, including quercetin (Xu et al. 2018; Zhu et al. 2015; Hickson et al. 2019), fisetin (Yousefzadeh et al. 2018; Zhu et al. 2017), piperlongumine and analogs (Wang et al. 2016; Zhang et al. 2018; Liu et al. 2018), the curcumin analog EF24 (Li et al. 2019) and goldenrod extracts (Lämmermann et al. 2018), some of which have been validated by a recent study done by Yousefzadeh et al. (2018). They tested a panel of flavonoids, including resveratrol, curcumin, luteolin, fisetin, rutin, epigallocatechin gallate, apigenin, pirfenidone, myricetin, catechin and quercetin. Of which, fisetin showed the best senolytic activity, luteolin and curcumin showed weak activity, while the others had almost no senolytic activity (Yousefzadeh et al. 2018). In addition, two recent studies demonstrate that cardiac glycosides (CGs) such as ouabain and digoxin function as broad-spectrum senolytics (Guerrero et al. 2019; Triana-Martínez et al. 2019).
3.1.1 Quercetin
Quercetin is a dietary flavonoid that can be found in a variety of vegetables and fruits as well as in tea and red wine (Formica and Regelson 1995). Quercetin shows broad biological activities, such as anti-obesity, antioxidant, anti-viral, anti-carcinogenic, anti-bacterial and anti-inflammatory (Anand David et al. 2016). Indeed, quercetin is widely used as a nutritional supplement and as a phytochemical remedy for various diseases, such as cardiovascular dysfunction, diabetes/obesity, inflammation and mood disorders. The strong antioxidant activity of quercetin enables it to quench free radicals from forming resonance-stabilized phenoxyl radicals. Nevertheless, the low bioavailability, chemical instability and poor water solubility greatly hinder its applications (Wang et al. 2016). Thus, various strategies have been developed to improve its stability, efficacy and bioavailability.
Levels of oxidative stress increase with age. Considering that quercetin is a potent antioxidant, it has been hypothesized that quercetin may delay aging via reducing oxidative damage. Administration of quercetin can reverse cognitive deficits in aged mice (Singh et al. 2003) and promote longevity in Saccharomyces cerevisiae (Belinha et al. 2007), which was attributed to its antioxidant activity. It was not identified as a senolytic until 2015 when Dr. Kirkland’s group first discovered that quercetin is a senolytic agent (Zhu et al. 2015). However, its senolytic activity was moderate and cell type specific as it can only kill senescent human endothelial cells but not senescent preadipocytes. Interestingly, when it was combined with dasatinib, they became more effective than either agent alone in killing not only senescent human endothelial cells but also preadipocytes and SnCs from many other tissue origins (Zhu et al. 2015). For example, it was shown that naturally aged, radiation-exposed, and progeroid Ercc1−/Δ mice exhibited a significant reduction in SnC burden after the treatment with the combination of quercetin and dasatinib. More importantly, this combination treatment improved the functions of multiple organs and delayed many age-related pathologies in these mice, particularly extending the healthspan in Ercc1−/Δ mice. Since then, the combination of quercetin and dasatinib have been widely used to treat a variety of age-related diseases in mouse models (Zhang et al. 2019; Schafer et al. 2017; Roos et al. 2016; Ogrodnik et al. 2017; Nath et al. 2018; Musi et al. 2018; Ogrodnik et al. 2019), including atherosclerosis (Roos et al. 2016), pulmonary fibrosis (Schafer et al. 2017), hepatic steatosis (Ogrodnik et al. 2017), chronic kidney disease (Nath et al. 2018), Alzheimer’s disease (Zhang et al. 2019; Musi et al. 2018), and obesity (Ogrodnik et al. 2019). Moreover, two clinical studies have been conducted to evaluate the safety of the combination of quercetin and dasatinib in patients with idiopathic pulmonary fibrosis (Justice et al. 2019) and diabetic kidney disease (Hickson et al. 2019). The results from these clinical studies show that quercetin and dasatinib treatment was well tolerated and could reduce SnC burden in these patients. However, quercetin is a polypharmacologic agent and its mechanisms of action have not been well defined nor have their molecular targets been identified and characterized. It remains unclear whether its therapeutic effects are mediated by its senolytic activity, particularly considering that it is not a potent senolytic agent and can only kill SnCs derived from a limited number of tissue origins alone or in combination with dasatinib in vitro (Yousefzadeh et al. 2018; Hwang et al. 2018; Grezella et al. 2018).
3.1.2 Fisetin
Fisetin is widely studied flavonoid extracted from various fruits and vegetables such as apples, persimmons, grapes, cucumbers, strawberries and onions (Arai et al. 2000). It is commonly used as a nutritional supplement and has a highly favorable safety profile. In Japan, the average dietary intake of naturally occurring fisetin is approximately 0.4 mg/day (Arai et al. 2000; Kimira et al. 1998), apparently without any adverse effects. Fisetin has numerous beneficial biological effects, including anti-oxidant, anti-tumor, anti-angiogenic, anti-inflammatory, anti-hyperlipidemic and neuroprotective effects (Pal et al. 2016; Khan et al. 2013; Sundarraj et al. 2018). Like many other flavonoids, fisetin acts as an antioxidant that can scavenge free radicals to confer marked antioxidant activity and significant biological effects. Its anti-oxidative activity has been confirmed by both cyclic voltammetry assays and quantum-chemical-based calculations (Marković et al. 2009). Accumulating data suggest fisetin as a potent anti-tumor agent that can inhibit cancer cell proliferation and induce cancer cell apoptosis in a variety of cancer cell lines (Lall et al. 2016). Interestingly, the effects are limited to cancer cells, as normal cells are less sensitive to fisetin treatment (Lall et al. 2016), showing good selectivity against normal and cancer cells.
In 2017, fisetin was first found to selectively cause cell death in SnCs but not in proliferating human umbilical vein endothelial cells (HUVECs) (Zhu et al. 2017). However, it had no senolytic activity on senescent IMR-90 cells or primary human preadipocytes (Zhu et al. 2017), indicating that its senolytic activity is cell-specific. The senolytic activity of fisetin was validated by another study in which a series of flavonoid polyphenols were tested for senolytic activity using SnCs (Yousefzadeh et al. 2018). Among the flavonoids tested, fisetin was the most potent one to induce SnC death. More importantly, treatment of progeroid Ercc1−/Δ and naturally aged mice with fisetin reduced SnC burden in multiple tissues, which resulted in a significant improvement in tissue homeostasis, reduced age-related pathology and moderately extended median and maximum lifespan of naturally aged mice (Yousefzadeh et al. 2018). Again, it has yet to be determined whether the therapeutic effects are mediated by its senolytic activity as fisetin is also a polypharmacologic agent that has been shown to extend the replicative lifespan of S. cerevisiae (Howitz et al. 2003) and the lifespan of D. melanogaster (Wood et al. 2004) in part via activation of sirtuins.
3.1.3 Piperlongumine
Piperlongumine is a biologically active extract from Piper species. It is the major alkaloid from long pepper and other important medicinal plants (Bezerra et al. 2013). Piperlongumine has wide pharmacological activities, such as anti-tumor, anti-angiogenic, anti-platelet aggregation, anti-metastatic, anti-nociceptive, anti-depressant, anti-atherosclerotic, anti-diabetic, and anti-bacterial (Bezerra et al. 2013). The anti-cancer activities of piperlongumine have been widely studied. It can kill various cancer types, including leukemia and solid tumors, such as skin, colon, breast, lung, central nervous system (CNS), nasopharyngeal, pancreatic, osseous, renal, bladder and prostate cancers (Bezerra et al. 2013; Piska et al. 2018). Interestingly, piperlongumine shows selective cytotoxicity over cancer cells and only displays weak cytotoxicity to normal cells (Bezerra et al. 2013). For example, it can suppress leukemia cell growth and reduce tumor cell viability by inducing apoptosis, but only has weak cytotoxicity to normal lymphocytes (Bezerra et al. 2007). Mechanistic studies reveal that piperlongumine functions as an antitumor agent via regulating multiple signal transduction pathways, including the mitochondrial apoptosis pathway, receptor tyrosine kinase (Raf-1) and extracellular signal-regulated kinases (ERK1/2) (Bezerra et al. 2013). Additionally, piperlongumine can suppress tumor progression and migration in vivo. The anticancer effect of piperlongumine has been proposed through its inhibition of oxidative stress response enzymes such as GSTp1 and CRB1, resulting in selective induction of ROS production in cancer cells but not in normal cells (Bezerra et al. 2013).
By screening a library of small molecules that target pathways predicted to be important for SnCs survival, Wang et al. identified piperlongumine as a novel lead for the development of senolytic agents (Wang et al. 2016). Piperlongumine selectively kills senescent human WI-38 fibroblasts induced by ionizing radiation, replicative exhaustion, or ectopic expression of the oncogene Ras. It induces SnCs apoptosis via activing the caspase cascades as pretreatment with the pan-caspase inhibitor Q-VD-OPh (QVD) can significantly block the apoptosis. Piperlongumine was reported to kill cancer cells by inducing the production of ROS. However, it cannot induce ROS production in SnCs (Wang et al. 2016). Interestingly, piperlongumine synergistically killed SnCs in combination with ABT263, a Bcl-2/Bcl-xL inhibitor. Initial structural modifications to piperlongumine identified a series of analogs with improved potency and/or selectivity in inducing SnC death (Liu et al. 2018). However, the mechanisms by which piperlongumine kills SnCs are largely unknown. Dr. Zhou’s lab identified a series of potential molecular targets of piperlongumine using a piperlongumine-based chemical probe to pull-down piperlongumine-binding proteins from live cells. One of them is oxidation resistance 1 (OXR1), an important antioxidant protein that regulates the expression of a variety of antioxidant enzymes. They found that OXR1 was upregulated in senescent WI-38 fibroblasts. Piperlongumine can bound to OXR1 directly and induce its degradation through the ubiquitin-proteasome system in an SnC-specific manner (Zhang et al. 2018). These findings provide new insights into the mechanism by which SnCs are highly resistant to oxidative stress and suggest that OXR1 is a novel senolytic target of piperlongumine that can be further exploited for the development of new senolytic agents. However, whether piperlongumine and its analogs can function as a senolytic agent in vivo has yet to be determined.
3.1.4 EF24
Curcumin is a hydrophobic polyphenol derived from the rhizome of the herb Curcuma longa, and is a well-defined natural compound with a variety biological activities, such as anti-cancer, anti-oxidation, anti-inflammation, and anti-microbial (Aggarwal and Harikumar 2009; Anand et al. 2008; Gupta et al. 2013; Hatcher et al. 2008; Maheshwari et al. 2006). Curcumin was found to have therapeutic potential and benefits in delaying aging and has been used to prevent and treat certain age-associated diseases (Grill et al. 2018; Takano et al. 2018; Yang et al. 2017). It has been shown to extend lifespan and healthspan in Drosophila melanogaster (fruit fly) (Chandrashekara et al. 2014) and Caenorhabditis elegans (Liao et al. 2011). However, its low potency and poor bioavailability limit its clinical applications (Shoba et al. 1998). A series of curcumin analogs have thus been developed in order to improve its bioavailability and therapeutic efficacy, such as EF24 (Adams et al. 2005; He et al. 2018), HO-3867 (Selvendiran et al. 2009), 2-HBA (Dinkova-Kostova et al. 2007) and dimethoxycurcumin (DIMC) (Tamvakopoulos et al. 2007), which were demonstrated to be more active than curcumin in reducing age-dependent deterioration, such as cancer and inflammation.
Curcumin was reported to have weak senolytic activity in a recent study (Yousefzadeh et al. 2018). However, Li et al. identified EF24 as a more potent senolytic agent than other curcumin analogs tested including HO-3867, 2-HBA and DIMC (Li et al. 2019). They revealed that EF24 reduced cell viability not only in ionizing radiation induced SnCs, but also in SnCs induced by extensive replication or ectopic transfection of the Ras oncogene. Moreover, EF24 displayed broad-spectrum senolytic activity against different types of SnCs, including human IMR-90 fibroblasts, HUVECs and human renal epithelial cells (Li et al. 2019). EF24 was reported to induce apoptosis in various tumor cells in part by inducing ROS production and endoplasmic reticulum stress. However, EF24 did not induce ROS production in SnCs, indicating that its senolytic activity is ROS-independent. Instead, they found that EF24 could reduce the expression of Bcl-xL and Mcl-1 in SnCs but not in normal cells, probably via proteasomal degradation. The findings provide new insights into the mechanisms by which curcumin analogs function as anti-aging agents, and suggest the potential of EF24 to be a novel senolytic agent for the treatment of age-related diseases.
3.1.5 Cardiac Glycosides (CGs)
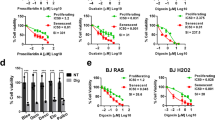
Two groups recently discovered that CGs including ouabain and digoxin are potent and broad-spectrum senolytics (Guerrero et al. 2019; Triana-Martínez et al. 2019). These CGs could kill a variety of SnCs from different species and tissues origins and induced by different stressors in vitro. They selectively killed SnCs primarily by inhibiting the Na+/K+ ATPase on the plasma membrane, which caused the disturbance of intracellular concentrations of Na+, K+ and H+ more profoundly in SnCs than non-SnCs, and subsequently led to the loss of membrane potential and acidification of the cells. In addition, SnCs are more susceptible to this disturbance than non-SnCs because SnCs exhibit partial depolarization of their plasma membrane and have a higher concentration of H+ than non-SnCs under basal conditions.
CGs also exhibit strong senolytic activity in vivo. For example, it was shown that administration of ouabain or digoxin to mice effectively eliminated oncogene-induced pre-neoplastic SnCs in the liver and pituitary, SnCs induced by radiation in the lungs, and SnCs accumulated in different tissues with aging. The elimination of SnCs in these mice led to decrease in the expression of SASP, suppression of tissue fibrosis and improvement of various physiological functions. Furthermore, CGs were highly cytotoxic to senescent cancer cells induced by various chemotherapeutic agents, resulting in a significant improvement in tumor response to chemotherapy in a lung cancer xenograft mouse model and a patient-derived breast cancer xenograft mouse model. These findings suggest that CGs have the potential to be used as effective treatments for a variety of age-related diseases including cancer.
3.2 Targeted Senolytics
Almost all targeted senolytics identified to date are repurposed anticancer agents that target SCAPs. These senolytics are in general more potent than naturally occurring senolytic compounds with the exception of CGs. However, these repurposed senolytics usually possess various on-target and/or off-target toxicities, which may preclude their clinical use as anti-aging agents as older people are more susceptible to adverse drug effects than younger individuals and less tolerant of cancer drug toxicity. Therefore, strategies to reduce on-target and/or off-target toxicity of known targeted senolytics are needed to generate safer targeted senolytics for clinical translation.
3.2.1 Dasatinib—A Pan Tyrosine Kinase Inhibitor
Dasatinib was one of the first senolytics discovered by Zhu et al. (2015). Dasatinib is a pan tyrosine kinase inhibitor that is known to promote tumor cell apoptosis via inhibiting a variety of cell survival pathways, including the down-stream pathway of ephrins or ephrin-B (EFNB) family members that are upregulated in SnCs. Dasatinib preferentially reduced the viability in senescent human preadipocytes, but was much less cytotoxic to senescent HUVECs. However, when it was combined with quercetin, they were more potent than either agent alone in killing different types of SnCs. Therefore, the combination of dasatinib and quercetin have been widely used to clear SnCs in various mouse models to treat different age-related diseases and tested in two clinical trials as we discussed earlier in Sect.1.3.1.1. However, the mechanism of action of dasatinib and the specific tyrosine kinase targeted by dasatinib to mediate its senolytic activity have yet to be determined. Identification of the specific senolytic target of dasatinib can lead to the development of more specific and potent senolytic agents that can reduce the off-target toxicity of dasatinib.
3.2.2 Inhibitors of the Bcl-2 Family Antiapoptotic Proteins
Resistance to apoptosis is a hallmark of SnCs (Wang 1995; Childs et al. 2014; Sasaki et al. 2001; Hampel et al. 2005; Soto-Gamez et al. 2019). Various SnCs may use different SCAPs to resist apoptosis. The Bcl-2 family proteins, consisting of both antiapoptotic and proapoptotic proteins, play important roles in the regulation of apoptosis (Youle and Strasser 2008). These proteins share sequence homology within conserved regions known as Bcl-2 homology (BH) domains. The Bcl-2 antiapoptotic proteins are multi-BH-domain proteins including Bcl-2, Bcl-xL, Mcl-1, Bcl-w and Bfl1. They can inhibit apoptosis by binding to the multi-BH-domain and BH3-only proapoptotic proteins. Among the Bcl-2 antiapoptotic proteins, Bcl-xL has been primarily implicated in SnC resistance to apoptosis because inhibition of Bcl-xL with a Bcl-xL specific inhibitor (such as A-1331852) or a Bcl-2 and Bcl-xL dual inhibitor (such as ABT263 or ABT737) can potently and selectively induce apoptosis in a variety of SnCs (Zhu et al. 2017; Chang et al. 2016; Yosef et al. 2016; Zhu et al. 2016), whereas inhibition of Bcl-2 and Mcl-1 alone with their specific inhibitors has no or weak effect on SnC survival (Chang et al. 2016; Yosef et al. 2016). However, inhibition of Bcl-2 and Bcl-w may contribute to the cytotoxic effect of the Bcl-xL inhibitors ABT263 and ABT737 on SnCs.
The mechanism by which Bcl-xL inhibition selectively induces apoptosis in SnCs may be attributable to the persistent stress endured by SnCs, which can upregulate the expression of some of the proapoptotic proteins such as Bcl-2 antagonist/killer (BAK) (Chang et al. 2016). To counteract the effect of these proapoptotic proteins for survival, SnCs also express a higher level of antiapoptotic proteins such as Bcl-xL (Chang et al. 2016; Yosef et al. 2016). Therefore, inhibition of Bcl-xL with an inhibitor can release Bcl-2-interacting mediator of cell death (BIM) and other BH3 proteins, which in turn activates BAK and/or Bcl-2-associated X protein (BAX). The activation of BAX and/or BAK at the mitochondrial membrane induces their oligomerization and formation of the macropores that causes mitochondrial outer membrane permeabilization (MOMP). MOMP results in the release of cytochrome C from mitochondria to the cytoplasm, which binds to the apoptotic protease-activating factor 1 (APAF1) to form the apoptosome. The apoptosome then induces a cascade activation of the initiator caspase (caspase 9) and executioner caspases (caspases 3, 6 and 7) to dismantle the cells (Czabotar et al. 2014).
Because ABT263 is one of the most advanced Bcl-2 and Bcl-xL dual inhibitor drug candidate, it has been extensively evaluated as a senolytic agent. Dr. Zhou’s and other labs have found that ABT263 can potently kill a variety of SnCs in cell culture with a few exceptions (such as senescent chondrocytes and synovial fibroblasts in the osteoarthritic joint), whereas it has minimal effect on their non-senescent counterparts (Zhu et al. 2015; Chang et al. 2016; Yosef et al. 2016). These findings suggest that ABT263 is a potent and broad-spectrum senolytic agent. This suggestion is supported by the finding that treatment of mice with ABT263 can effectively clear SnCs in various murine tissues. More importantly, clearance of SnCs with ABT263 can rejuvenate aged hematopoietic stem cells (HSCs) and the senescent hematopoietic system in aged mice (Chang et al. 2016) and ameliorate several pathological conditions associated with aging such as atherosclerosis, dementia and pulmonary fibrosis (Childs et al. 2016; Pan et al. 2017; Bussian et al. 2018). However, the on-target toxicity of thrombocytopenia induced by Bcl-xL inhibition prevents the use of ABT263 and other Bcl-xL specific inhibitors in clinic even for cancer patients, because platelets also depend on Bcl-xL for survival (Ashkenazi et al. 2017; Gandhi et al. 2011; Leverson et al. 2015; Souers et al. 2013). Therefore, strategies that can be used to overcome this on-target toxicity will be needed in order to generate a safer and more effective Bcl-xL targeting senolytic agent for clinical translation. Alternatively, a combination therapy with lower doses of different senolytic agents may provide a synergy to more effectively clear SnCs while reducing their on-target and off-target toxicity as seen with the combination of quercetin and dasatinib (Zhu et al. 2015) and ABT263 plus piperlongumine (Wang et al. 2016).
3.2.3 HSP90 Inhibitors
HSP90 is a molecular chaperone ubiquitously expressed in cells and tissues. It plays an important role in the regulation of protein stability. It is upregulated in many different types of cancers and required for the stability and function of numerous oncogenic signaling proteins as well as certain anti-apoptotic factors (Solárová et al. 2015). Therefore, several HSP90 inhibitors have been developed as potential anticancer agents. It has been well established that SnCs are under proteotoxic stress (Pluquet et al. 2015) and thus potentially are more dependent on HSP90 for survival than non-SnCs. Indeed, Fuhrmann-Stroissnigg et al. recently reported that HSP90 inhibitors such as 17-DMAG are senolytics (Fuhrmann-Stroissnigg et al. 2017). These HSP90 inhibitors can selectively kill a variety of SnCs from mouse and human. Mechanic study reveals that inhibition of HSP90 with an inhibitor disrupts the interaction of HSP90 with the phosphorylated AKT, leading to the destabilization of the active form of AKT that is important for the induction of cellular senescence and SnC survival. More importantly, it was shown that periodic treatment of Ercc1−/Δ progeroid mice with 17-DMAG reduced the tissue burden of SnCs and delayed the onset of several age-related phenotypes and pathologies. However, to translate 17-DMAG into clinic for the treatment of age-related diseases, we need to generate analogs of 17-DMAG to improve its pharmacokinetic and pharmacodynamic properties and reduce its side effects (Mellatyar et al. 2018).
3.2.4 FOXO4-p53 Interfering Peptide (IP) and MDM2 Inhibitors
p53 is a well-known tumor suppressor that acts as a double-edged sword in regulation of cellular senescence and aging (Wu and Prives 2018; Johmura and Nakanishi 2016). Increases in the levels and activity of p53 occur when cells enter a pre-senescent stage upon activation of the DNA damage response (DDR) pathway, which plays an important role in the initiation of cellular senescence. In addition, direct activation of p53 by MDM2 inhibition with nutlin-3a can also induce senescence in mouse fibroblasts without induction of DNA damage and activation of the DDR pathway (Efeyan et al. 2007). However, in many types of cells, p53 levels reduce to a level that is below the basal levels of p53 in non-SnCs when they become senescent (Kim et al. 2015). The reduction of p53 in SnCs may protect them from apoptosis because p53 is one of the most important apoptosis determinants and can induce apoptosis through both transcription-dependent and -independent mechanisms (Fridman and Lowe 2003). In addition, the reduction of p53 activity was found in various tissues in aged mice (Feng et al. 2007), which may contribute to the accumulation of SnCs and higher prevalence of cancer due to reduced apoptosis during aging. Therefore, restoration of p53 activity has the potential to eliminate SnCs via induction of SnC apoptosis. This hypothesis was supported by the finding that increases in p53 transcriptional activity via disruption of the interaction between FOXO4 and p53 using a FOXO4 peptide selectively induced apoptosis in SnCs in cell culture and effectively cleared SnCs in mice (Baar et al. 2017). In their study, they found that FOXO4 was elevated to maintain cell viability in SnCs. Subsequently, they designed a peptide called FOXO4-DRI which can disrupt PML/DNA-SCARS, release active p53 in SnCs, and selectively and potently target SnCs for p53-dependent apoptosis. In vivo, FOXO4-DRI counteracted chemotherapy-induced senescence and loss of liver function, as well as loss of renal function in fast-aging mice.
However, there are still some challenges to use peptides as a therapeutics in clinic. Alternatively, p53 can be activated by inhibition of the interaction between MDM2 and p53 to selectively kill SnCs. This is because the levels and activities of p53 are primarily regulated at the level of post-transcription via the MDM2-mediated ubiquitination and proteasome degradation (Kruse and Gu 2009), and inhibition of the interaction between MDM2 and p53 with an inhibitor can increase p53 stability and activity (Moll and Petrenko 2003). Indeed, it was reported recently that UBX0101, an inhibitor of MDM2, could selectively kill SnCs in vitro and effectively clear them in mice with post-traumatic osteoarthritis after local therapy. However, systemic treatment with MDM2 inhibitors can be risky because it causes substantial hematopoietic suppression and gastrointestinal toxicity (Tisato et al. 2017). It has yet to be determined whether these adverse effects are on-target toxicities or off-target side effects. Therefore, MDM2 inhibitors may be only suitable for clearing SnCs to treat age-related diseases such as osteoarthritis via local administration. It will be important to find an alternative strategy to activate p53 without causing significant normal tissue toxicity. This would be more desirable for the development of a better senolytic agent that can be safely used in elderly individuals who are more susceptible to drug adverse effects than young people.
4 Conclusions
While natural senolytics may have the advantages of low toxicity, they are usually less potent than targeted senolytics and thus have to be combined with other senolytic agents to be effective in clearing SnCs (Zhu et al. 2015), except CGs (Guerrero et al. 2019; Triana-Martínez et al. 2019). The mechanisms of action of most natural senolytics have not been well defined nor have their molecular targets been identified and characterized, making it very difficult to rationally modify the compounds to improve their senolytic activity. In contrast, almost all the targeted senolytics discovered are repurposed anticancer agents except the FOXO4-p53-IP (Kirkland and Tchkonia 2017; Childs et al. 2017; Niedernhofer and Robbins 2018). These repurposed senolytics usually possess various on-target and/or off-target toxicities, which could preclude their clinical use as anti-aging agents because older people are more susceptible to adverse drug effects than younger individuals and less tolerant to cancer drug toxicity. Therefore, strategies to reduce on-target and/or off-target toxicity of known targeted senolytics are urgently needed.
References
Adams BK, Cai J, Armstrong J, Herold M, Lu YJ, Sun A et al (2005) EF24, a novel synthetic curcumin analog, induces apoptosis in cancer cells via a redox-dependent mechanism. Anticancer Drugs 16(3):263–275
Aggarwal BB, Harikumar KB (2009) Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol 41(1):40–59
Aguayo-Mazzucato C, Andle J, Lee TB, Midha A, Talemal L, Chipashvili V et al (2019) Acceleration of β cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab 30(1):129–142.e4
Anand David AV, Arulmoli R, Parasuraman S (2016) Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn Rev 10(20):84–89
Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B et al (2008) Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol 76(11):1590–1611
Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N (2000) Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr 130(9):2243–2250
Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ (2017) From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov 16(4):273–284
Assuncao M, Andrade JP (2015) Protective action of green tea catechins in neuronal mitochondria during aging. Front Biosci (Landmark Ed). 20:247–262
Baar MP, Brandt RMC, Putavet DA, Klein JDD, Derks KWJ, Bourgeois BRM et al (2017) Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 169(1):132–147.e16
Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B et al (2011) Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479(7372):232–236
Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J et al (2016) Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530(7589):184–189
Belinha I, Amorim MA, Rodrigues P, de Freitas V, Moradas-Ferreira P, Mateus N et al (2007) Quercetin increases oxidative stress resistance and longevity in Saccharomyces cerevisiae. J Agric Food Chem 55(6):2446–2451
Bernatoniene J, Kopustinskiene DM (2018) The role of catechins in cellular responses to oxidative stress. Molecules 23(4)
Bezerra DP, Militão GCG, de Castro FO, Pessoa C, de Moraes MO, Silveira ER et al (2007) Piplartine induces inhibition of leukemia cell proliferation triggering both apoptosis and necrosis pathways. Toxicol in Vitro 21(1):1–8
Bezerra DP, Pessoa C, de Moraes MO, Saker-Neto N, Silveira ER, Costa-Lotufo LV (2013) Overview of the therapeutic potential of piplartine (piperlongumine). Eur J Pharm Sci 48(3):453–463
Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ (2018) Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562(7728):578–582
Campisi J (2013) Aging, cellular senescence, and cancer. Annu Rev Physiol 75:685–705
Chandrashekara KT, Popli S, Shakarad MN (2014) Curcumin enhances parental reproductive lifespan and progeny viability in Drosophila melanogaster. Age (Dordr) 36(5):9702
Chang J, Wang Y, Shao L, Laberge R-M, Demaria M, Campisi J et al (2016) Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 22(1):78–83
Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM (2014) Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep 15(11):1139–1153
Childs BG, Durik M, Baker DJ, van Deursen JM (2015) Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 21(12):1424–1435
Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM (2016) Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354(6311):472–477
Childs BG, Gluscevic M, Baker DJ, Laberge R-M, Marquess D, Dananberg J et al (2017) Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov 16(10):718–735
Chinta SJ, Woods G, Demaria M, Rane A, Zou Y, McQuade A, et al (2018) Cellular senescence is induced by the environmental neurotoxin paraquat and contributes to neuropathology linked to Parkinson’s disease. Cell Rep 22(4):930–940
Czabotar PE, Lessene G, Strasser A, Adams JM (2014) Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 15(1):49–63
Demaria M, O’Leary MN, Chang J, Shao L, Liu S, Alimirah F et al (2017) Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov 7(2):165–176
Dinkova-Kostova AT, Cory AH, Bozak RE, Hicks RJ, Cory JG (2007) Bis(2-hydroxybenzylidene)acetone, a potent inducer of the phase 2 response, causes apoptosis in mouse leukemia cells through a p53-independent, caspase-mediated pathway. Cancer Lett 245(1–2):341–349
Efeyan A, Ortega-Molina A, Velasco-Miguel S, Herranz D, Vassilev LT, Serrano M (2007) Induction of p53-dependent senescence by the MDM2 antagonist nutlin-3a in mouse cells of fibroblast origin. Cancer Res 67(15):7350–7357
Feng Z, Hu W, Teresky AK, Hernando E, Cordon-Cardo C, Levine AJ (2007) Declining p53 function in the aging process: a possible mechanism for the increased tumor incidence in older populations. Proc Natl Acad Sci USA 104(42):16633–16638
Formica JV, Regelson W (1995) Review of the biology of Quercetin and related bioflavonoids. Food Chem Toxicol 33(12):1061–1080
Fridman JS, Lowe SW (2003) Control of apoptosis by p53. Oncogene 22(56):9030–9040
Fuhrmann-Stroissnigg H, Ling YY, Zhao J, McGowan SJ, Zhu Y, Brooks RW, et al (2017) Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun 8(1):422
Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, et al (2011) Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol 29(7):909–916
Grezella C, Fernandez-Rebollo E, Franzen J, Ventura Ferreira MS, Beier F, Wagner W (2018) Effects of senolytic drugs on human mesenchymal stromal cells. Stem Cell Res Ther 9(1):108
Grill AE, Shahani K, Koniar B, Panyam J (2018) Chemopreventive efficacy of curcumin-loaded PLGA microparticles in a transgenic mouse model of HER-2-positive breast cancer. Drug Deliv Transl Res 8(2):329–341
Guerrero A, Herranz N, Sun B, Wagner V, Gallage S, Guiho R et al (2019) Cardiac glycosides are broad-spectrum senolytics. Nat Metab 1(11):1074–1088
Gupta SC, Patchva S, Aggarwal BB (2013) Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J 15(1):195–218
Hampel B, Wagner M, Teis D, Zwerschke W, Huber LA, Jansen-Dürr P (2005) Apoptosis resistance of senescent human fibroblasts is correlated with the absence of nuclear IGFBP-3. Aging Cell 4(6):325–330
Hatcher H, Planalp R, Cho J, Torti FM, Torti SV (2008) Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci 65(11):1631–1652
He Y, Li Y, Zhao T, Wang Y, Sun C (2013) Ursolic acid inhibits adipogenesis in 3T3-L1 adipocytes through LKB1/AMPK pathway. PLoS ONE 8(7):e70135
He Y, Li W, Li Y, Zhang S, Wang Y, Sun C (2014) Ursolic acid increases glucose uptake through the PI3K signaling pathway in adipocytes. PLoS ONE 9(10):e110711
He Y, Li W, Hu G, Sun H, Kong Q (2018) Bioactivities of EF24, a novel curcumin analog: a review. Front Oncol 8:614
He Y, Thummuri D, Zheng G, Okunieff P, Citrin DE, Vujaskovic Z et al (2019) Cellular senescence and radiation-induced pulmonary fibrosis. Transl Res 209:14–21
Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK et al (2019) Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 47:446–456
Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG et al (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425(6954):191–196
Hwang HV, Tran DT, Rebuffatti MN, Li C-S, Knowlton AA (2018) Investigation of quercetin and hyperoside as senolytics in adult human endothelial cells. PLoS ONE 13(1):e0190374
Jeon OH, Kim C, Laberge R-M, Demaria M, Rathod S, Vasserot AP et al (2017) Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med 23(6):775–781
Johmura Y, Nakanishi M (2016) Multiple facets of p53 in senescence induction and maintenance. Cancer Sci 107(11):1550–1555
Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK et al (2019) Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 40:554–563
Khan N, Syed DN, Ahmad N, Mukhtar H (2013) Fisetin: a dietary antioxidant for health promotion. Antioxid Redox Signal 19(2):151–162
Kim RH, Kang MK, Kim T, Yang P, Bae S, Williams DW et al (2015) Regulation of p53 during senescence in normal human keratinocytes. Aging Cell 14(5):838–846
Kimira M, Arai Y, Shimoi K, Watanabe S (1998) Japanese intake of flavonoids and isoflavonoids from foods. J Epidemiol 8(3):168–175
Kirkland JL, Tchkonia T (2017) Cellular senescence: a translational perspective. EBioMedicine 21:21–28
Knutson MD, Leeuwenburgh C (2008) Resveratrol and novel potent activators of SIRT1: effects on aging and age-related diseases. Nutr Rev 66(10):591–596
Kruse J-P, Gu W (2009) Modes of p53 regulation. Cell 137(4):609–622
Lall RK, Adhami VM, Mukhtar H (2016) Dietary flavonoid fisetin for cancer prevention and treatment. Mol Nutr Food Res 60(6):1396–1405
Lämmermann I, Terlecki-Zaniewicz L, Weinmüllner R, Schosserer M, Dellago H, de Matos Branco AD et al (2018) Blocking negative effects of senescence in human skin fibroblasts with a plant extract. NPJ Aging Mech Dis 4:4
Leverson JD, Phillips DC, Mitten MJ, Boghaert ER, Diaz D, Tahir SK, et al (2015) Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med 7(279):279ra40
Li T, Chen S, Feng T, Dong J, Li Y, Li H (2016) Rutin protects against aging-related metabolic dysfunction. Food Funct. 7(2):1147–1154
Li W, He Y, Zhang R, Zheng G, Zhou D (2019) The curcumin analog EF24 is a novel senolytic agent. Aging (Albany NY) 11(2):771–782
Liao VH-C, Yu C-W, Chu Y-J, Li W-H, Hsieh Y-C, Wang T-T (2011) Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech Ageing Dev 132(10):480–487
Liu X, Wang Y, Zhang X, Gao Z, Zhang S, Shi P, et al (2018) Senolytic activity of piperlongumine analogues: Synthesis and biological evaluation. Bioorg Med Chem 26(14):3925–3938
Liu X, Lin X, Mi Y, Li J, Zhang C (2018b) Grape Seed Proanthocyanidin Extract Prevents Ovarian Aging by Inhibiting Oxidative Stress in the Hens. Oxid Med Cell Longev. 2018:9390810
Maheshwari RK, Singh AK, Gaddipati J, Srimal RC (2006) Multiple biological activities of curcumin: a short review. Life Sci 78(18):2081–2087
Marković ZS, Mentus SV, Dimitrić Marković JM (2009) Electrochemical and density functional theory study on the reactivity of fisetin and its radicals: implications on in vitro antioxidant activity. J Phys Chem A 113(51):14170–14179
Mellatyar H, Talaei S, Pilehvar-Soltanahmadi Y, Barzegar A, Akbarzadeh A, Shahabi A et al (2018) Targeted cancer therapy through 17-DMAG as an Hsp90 inhibitor: overview and current state of the art. Biomed Pharmacother 102:608–617
Moll UM, Petrenko O (2003) The MDM2-p53 interaction. Mol Cancer Res 1(14):1001–1008
Mukherjee PK, Maity N, Nema NK, Sarkar BK (2011) Bioactive compounds from natural resources against skin aging. Phytomedicine 19(1):64–73
Musi N, Valentine JM, Sickora KR, Baeuerle E, Thompson CS, Shen Q et al (2018) Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell 17(6):e12840
Nath KA, O’Brien DR, Croatt AJ, Grande JP, Ackerman AW, Nath MC, et al (2018) The murine dialysis fistula model exhibits a senescence phenotype: pathobiological mechanisms and therapeutic potential. Am J Physiol Renal Physiol 315(5):F1493–1499
Niedernhofer LJ, Robbins PD (2018) Senotherapeutics for healthy ageing. Nat Rev Drug Discov 17(5):377
Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A et al (2017) Cellular senescence drives age-dependent hepatic steatosis. Nat Commun 13(8):15691
Ogrodnik M, Zhu Y, Langhi LGP, Tchkonia T, Krüger P, Fielder E et al (2019) Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab 29(5):1061–1077.e8
Pal HC, Pearlman RL, Afaq F (2016) Fisetin and its role in chronic diseases. Adv Exp Med Biol 928:213–244
Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL (2015) Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes 64(7):2289–2298
Pan J, Li D, Xu Y, Zhang J, Wang Y, Chen M, et al (2017) Inhibition of Bcl-2/xl with ABT-263 selectively kills senescent type II pneumocytes and reverses persistent pulmonary fibrosis induced by ionizing radiation in mice. Int J Radiat Oncol Biol Phys 99(2):353–361
Piska K, Gunia-Krzyżak A, Koczurkiewicz P, Wójcik-Pszczoła K, Pękala E (2018) Piperlongumine (piplartine) as a lead compound for anticancer agents-Synthesis and properties of analogues: a mini-review. Eur J Med Chem 156:13–20
Pluquet O, Pourtier A, Abbadie C (2015) The unfolded protein response and cellular senescence. A review in the theme: cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. Am J Physiol Cell Physiol 308(6):C415–425
Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM et al (2016) Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15(5):973–977
Sasaki M, Kumazaki T, Takano H, Nishiyama M, Mitsui Y (2001) Senescent cells are resistant to death despite low Bcl-2 level. Mech Ageing Dev 122(15):1695–1706
Sastre J, Millán A, García de la Asunción J, Plá R, Juan G, Pallardó null, et al (1998) A Ginkgo biloba extract (EGb 761) prevents mitochondrial aging by protecting against oxidative stress. Free Radic Biol Med 24(2):298–304
Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ et al (2017) Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 23(8):14532
Selvendiran K, Kuppusamy ML, Bratasz A, Tong L, Rivera BK, Rink C et al (2009) Inhibition of vascular smooth-muscle cell proliferation and arterial restenosis by HO-3867, a novel synthetic curcuminoid, through up-regulation of PTEN expression. J Pharmacol Exp Ther 329(3):959–966
Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS (1998) Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 64(4):353–356
Singh A, Naidu PS, Kulkarni SK (2003) Reversal of aging and chronic ethanol-induced cognitive dysfunction by quercetin a bioflavonoid. Free Radic Res 37(11):1245–1252
Solárová Z, Mojžiš J, Solár P (2015) Hsp90 inhibitor as a sensitizer of cancer cells to different therapies (review). Int J Oncol 46(3):907–926
Soto-Gamez A, Quax WJ, Demaria M (2019) Regulation of Survival Networks in Senescent Cells: From Mechanisms to Interventions. J Mol Biol 431(15):2629–2643
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J et al (2013) ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 19(2):202–208
Sundarraj K, Raghunath A, Perumal E (2018) A review on the chemotherapeutic potential of fisetin: in vitro evidences. Biomed Pharmacother 97:928–940
Takano K, Tatebe J, Washizawa N, Morita T (2018) Curcumin inhibits age-related vascular changes in aged mice fed a high-fat diet. Nutrients 10(10)
Takasugi M, Okada R, Takahashi A, Virya Chen D, Watanabe S, Hara E (2017) Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat Commun 8:15729
Tamvakopoulos C, Dimas K, Sofianos ZD, Hatziantoniou S, Han Z, Liu Z-L et al (2007) Metabolism and anticancer activity of the curcumin analogue, dimethoxycurcumin. Clin Cancer Res 13(4):1269–1277
Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL (2013) Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 123(3):966–972
Tisato V, Voltan R, Gonelli A, Secchiero P, Zauli G (2017) MDM2/X inhibitors under clinical evaluation: perspectives for the management of hematological malignancies and pediatric cancer. J Hematol Oncol 10(1):133
Triana-Martínez F, Picallos-Rabina P, Da Silva-Álvarez S, Pietrocola F, Llanos S, Rodilla V et al (2019) Identification and characterization of Cardiac Glycosides as senolytic compounds. Nat Commun 10(1):4731
Wang E (1995) Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res 55(11):2284–2292
Wang Y, Chang J, Liu X, Zhang X, Zhang S, Zhang X, et al (2016) Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging (Albany NY) 8(11):2915–2926
Wang W, Sun C, Mao L, Ma P, Liu F, Yang J et al (2016b) The biological activities, chemical stability, metabolism and delivery systems of quercetin: a review. Trends Food Sci Technol 56:21–38
Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M et al (2004) Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430(7000):686–689
Wu D, Prives C (2018) Relevance of the p53-MDM2 axis to aging. Cell Death Differ 25(1):169–179
Xu Z, Feng W, Shen Q, Yu N, Yu K, Wang S et al (2017) Rhizoma Coptidis and berberine as a natural drug to combat aging and aging-related diseases via anti-oxidation and AMPK activation. Aging Dis 6:760–777
Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM et al (2018) Senolytics improve physical function and increase lifespan in old age. Nat Med 24(8):1246–1256
Yang Y-C, Lin H-Y, Su K-Y, Chen C-H, Yu Y-L, Lin C-C et al (2012) Rutin, a flavonoid that is a main component of saussurea involucrata, attenuates the senescence effect in D-galactose aging mouse model. Evid Based Complement Alternat Med. 2012:980276
Yang L, Zheng Z, Qian C, Wu J, Liu Y, Guo S et al (2017) Curcumin-functionalized silk biomaterials for anti-aging utility. J Colloid Interface Sci 15(496):66–77
Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S et al (2016) Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun 7:11190
Youle RJ, Strasser A (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9(1):47–59
Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann-Stroissnigg H, Xu M et al (2018) Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36:18–28
Zhang X, Zhang S, Liu X, Wang Y, Chang J, Zhang X et al (2018) Oxidation resistance 1 is a novel senolytic target. Aging Cell 17(4):e12780
Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S et al (2019) Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat Neurosci 22(5):719–728
Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N et al (2015) The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14(4):644–658
Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB et al (2016) Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15(3):428–435
Zhu Y, Doornebal EJ, Pirtskhalava T, Giorgadze N, Wentworth M, Fuhrmann-Stroissnigg H, et al (2017) New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging (Albany NY) 9(3):955–963
Acknowledgements
This work was supported in part by US National Institutes of Health (NIH) grants R01CA211963 (D.Z.), R01CA219836 (D.Z.), R01AG063801 (D.Z.), and R56AG056372 (G.Z.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
G.Z. and D.Z. are inventors of a pending patent application for the development of Bcl-xL targeted senolytic agents. D.Z. is a co–founder and an advisor of Unity that develops senolytic agents to treat various age-related diseases in humans.
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
He, Y., Zheng, G., Zhou, D. (2020). Senolytic Drug Development. In: Muñoz-Espin, D., Demaria, M. (eds) Senolytics in Disease, Ageing and Longevity. Healthy Ageing and Longevity, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-030-44903-2_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-44903-2_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-44902-5
Online ISBN: 978-3-030-44903-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)