Abstract
Alzheimer’s disease (AD) is one of the most devastating forms of dementia. At present, there are no treatments of value and therefore patients with AD have an uncertain future, due to the current incapability to envisage the course of the disease. This is mainly due to the lack of understanding of the underlying pathophysiology and to the huge patients’ clinical heterogeneity. In this regard, recent evidence reinforced the notion that synaptic dysfunction could be a relevant aspect of AD-related pathophysiology. In particular, it has been shown that loss of synaptic density occurs since the early phases of the disease, and that synaptic failure is an early event that precedes neuronal degeneration. Such remarkable weakening of synaptic transmission is supposed to play a key role in the pathogenesis of different forms of dementia, including AD, frontotemporal dementia, and Lewy body dementia. However, despite this emerging background, it has not been possible to quantify synaptic functioning (or dysfunction) directly in vivo in AD patients. Transcranial magnetic stimulation (TMS) has been recently introduced as a novel approach able to identify the early signatures of synaptic dysfunction characterizing the different forms of AD. In the current chapter, I review the novel emerging neurophysiological signatures of AD that have been highlighted in vivo by TMS studies. I show how TMS measurement of neurophysiological activity may provide novel biomarkers useful to increase the accuracy of differential diagnosis, to predict disease progression, and to anticipate response to therapy. Recently, different forms of noninvasive brain stimulation techniques (e.g., repetitive TMS, rTMS) have been applied to patients with AD in order to improve cognitive decline and behavioral disorders. In recent years, treatments based on multiple sessions of rTMS have represented a promising tool for influencing cognition in people with neurodegenerative diseases. In the second part of this chapter, I also consider novel therapeutic approaches based on the clinical use of rTMS.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- AD = Alzheimer’s disease
- Aβ = Amyloid-β
- DMN = Default mode network
- LTP = Long-term depression
- rTMS = Repetitive transcranial magnetic stimulation
- SAI = Short afferent inhibition
- TBS = Theta burst stimulation
- TMS = Transcranial magnetic stimulation
1 Introduction
Alzheimer’s disease (AD) is one of the most devastating forms of dementia, being considered a remarkable problem due to the ageing of the population. It is nowadays considered as one of the most serious medical, economic, and social emergencies faced by our society, and it is predicted to become even more problematic over the next decades. Unfortunately, there are no effective treatments, and patients diagnosed with AD face an uncertain future, caused by the current inability to predict the course of the disease. The only approved treatment for AD is indeed based on standard cholinergic and glutamatergic drugs, whose clinical efficacy is overall negligible and debated. Since the 1990s, symptomatic therapies have been available, which moderately improve cognition and function. The most frequently prescribed treatments for AD are Acetylcholinesterase Inhibitors (AchEIs) and memantine. These therapies may provide transient relief from some symptoms (6–12 months in most cases), but are unable to reduce the progressive decline of everyday activities, communication, and social behavior [1]. In addition, the current treatments are not effective for everyone: it is estimated that only approximately 40–70% of the patients benefit from current treatments. Based on this, and on the significant limitations of the current treatment options, more effective symptomatic therapies, particularly in the earlier stages of AD, are needed. Nonetheless, recent clinical trials based on new “putative” disease-modifying drugs have failed in reaching their principal clinical outcome.
So far, relatively well-defined criteria have been identified for the diagnosis of early AD, based on patients’ clinical presentation and biomarkers’ profile. In particular, recent consensus was found on the necessity to determine the presence of beta-amyloid- and tau-related pathology. Evidence of these abnormalities may be identified either by cerebrospinal fluid (CSF) sampling or Positron Emission Tomography (PET) imaging, using specific ligands [2]. Nonetheless, the clinical course of AD remains largely variable at single subject level. This is mainly due to the modest understanding we presently have of AD pathophysiology. Critically, the mechanisms determining the severity of AD progression, and those counteracting it, are largely unknown, thus preventing any consistent prognostic estimate at the individual patient level. Thus, there is a critical demand to explore other paths that may expand our knowledge on the pathophysiological changes occurring in AD, especially in the early phases of the disease, when the first minimal signs appear or even before.
In this perspective, I review the emerging contribution of transcranial magnetic stimulation (TMS), a noninvasive brain stimulation method that may allow to determine new key pathophysiological features characterizing the different forms of dementia. Moreover, I will consider the application of repetitive sessions of noninvasive brain stimulation such as repetitive TMS (rTMS) as a new promising therapeutic strategy to slow down the progression of cognitive decline.
2 Synaptic Dysfunction in AD
The aggregation and deposition of amyloid-β (Aβ) and tau proteins are two fundamental factors recognized in AD pathogenesis. These pathological processes are thought to start many years before the onset of cognitive impairment. However, the first signs of cognitive damage appear only when a substantial synaptic loss has occurred in vulnerable brain regions [3].
CSF concentrations of beta-amyloid 1–42, total tau (t-tau), and phosphorylated tau (p-tau) proteins have been recently put forward as a useful tool for AD diagnosis and phenotyping. Notably, AD patients with higher levels of CSF t-tau and p-tau have been reported to exhibit a more malignant disease course [4]. Recently, growing evidence has shown that the accumulation of tau pathology is highly associated with functional and structural weakening of AD brains [5]. Moreover, it has been established that the gathering of “tangles” correlates with patients’ level of cognitive deterioration, while beta-amyloid requires the presence of tau proteins to develop its toxicity. Thus, the progressive neuronal and synaptic loss mirrors the cumulative result of different pathologic substrates in AD and, therefore, may provide the best marker to follow disease progression. However, it has to be taken into account that synaptic dysfunction is an initial and noticeable pathological feature of AD preceding neuronal loss in numerous brain areas. In basic science studies, earlier investigations have mainly focused on the direct toxic effects of beta-amyloid into AD-related synaptic damages. Only recently, an emergent role of tau was established [6]. It was shown that tau overexpression is able to induce synaptic degeneration even in the absence of neurofibrillary tangles. This synaptic dysfunction has been directly associated with the onset of early memory impairments observed in patients with AD [7].
Actually, although several AD biomarkers are widely applied and considered useful for diagnosis, sufficient accuracy is still lacking in evaluating disease severity and predicting disease progression and response to therapy both considering CSF and neuroimaging parameters, such as hippocampal atrophy/whole brain volume [7]. In particular, the use of a single biomarker provides too limited information to define the complex underlying severity of disease across its entire range, from preclinical to clinical phases of AD. Moreover, AD biomarkers assessment is routinely performed by means of invasive and/or high-cost procedures, limiting their use in clinical practice. Indeed, the evidence provided by brain imaging methods is merely correlative. Thus, several efforts are underway to combine multiple biomarkers to predict the severity of AD, with the major difficulty in tracking the temporally different evolution of each biomarker throughout the disease course [7].
In recent years, growing evidence has highlighted the notion that loss of synaptic density could be an early event antecedent to neuronal degeneration, suggesting that the impairment of synaptic plasticity mechanisms should play a key role in the pathogenesis of AD [3]. Notably, in various efforts to find semiquantitative correlations between the progressive cognitive impairment and brain pathological alterations, the strongest relationship has been found between the loss of synaptic density and the degree of cognitive impairment in AD. Thus, the impairment of synaptic transmission due to toxic oligomeric species [8] could predict disease severity more precisely than neuronal loss, which is considered a more tardive event. This evidence finds support on experimental studies showing that Aβ peptides and tau proteins can interfere with physiological mechanisms of neuronal synaptic plasticity in AD animal models. In particular, it has been demonstrated that these molecules influence hippocampal long-term potentiation (LTP) [9], which is related to memory impairment occurring in AD.
These altered mechanisms have been linked to different alterations occurring at different levels of observation, including spine shrinkage, neuronal network disarrangement, and cell death [10]. Taken together, this evidence suggests that synaptic dysfunction, occurring at different levels of brain activity, could represent a key driver of AD-related cognitive decline.
Despite this promising evidence, so far it has not been possible to quantify synaptic functioning (or dysfunction) directly in vivo in AD patients. Different in vivo techniques, such as 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) [11], functional magnetic resonance imaging (fMRI) [12] and electroencephalography (EEG), have been used in order to provide useful biomarkers for synaptic dysfunction and network connectivity in AD progression [13]. However, FDG-PET and fMRI techniques provide only an indirect estimate of synaptic dysfunction, being limited by a low temporal resolution that does not allow to track synaptic activity at the physiological time scale in which neuronal interactions occur (i.e., in the range of milliseconds). Indeed, imaging methods infer alterations of synaptic activity as a consequence of slow and subtle changes in metabolic parameters, such as blood-oxygen-level-dependent contrast imaging (BOLD) used in fMRI. These signals are indeed relative, and not individually quantitative, and observe changes in blood oxygenations occurring across several seconds, being very far from real-time synaptic activity. Moreover, despite all the advances in imaging of AD in the research setting, there is a lack of translation of these methodologies into the clinical practice. Most imaging biomarkers have not been validated in unselected patient cohorts and participants in large AD studies are not representative of the general population. These techniques require special facilities and expertise to perform and interpret. The paucity of standard acquisition and analysis methods between different centers makes the widespread adoption of them even more challenging. In addition, some of the new imaging modalities are still too expensive to be considered cost effective in a community setting or in nonspecialized centers.
3 TMS to Measure Synaptic Dysfunction in AD
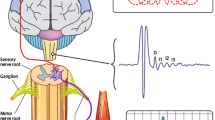
On the other hand, TMS-based methods provide the possibility to evaluate in real time the brain electrical activity in the healthy and pathological conditions. It is based on the principle that brain stimulation can be induced by generating a brief, high-intensity magnetic field by passing a brief electric current through a magnetic coil. When a substantial electrical current is induced in a stimulating coil, this is able to produce a transient time-variable magnetic field. When a magnetic field of this sort and sufficient strength is applied to the brain, it can induce an electrical current in the brain producing firing of groups of nerve cells. When stimulation of this sort is applied repeatedly, it will progressively change brain activity. The discovery and practical application of these basic techniques has led to the widespread use of TMS in neuroscientific and clinical applications [14]. Within this background, TMS-based approach may represent a valid tool to overcome the problems limiting other imaging techniques to track dysfunction of synaptic activity in incipient dementia [15,16,17].
Depending on the adopted protocol, it is possible to test key physiological aspects of synaptic activity at different levels of local and global complexity. TMS allows (1) to investigate in detail the properties of local interneural networks that are mediated by specific neurotransmitters [18], (2) to determine the capability of specific areas of the brain to form cortical plasticity [19], (3) to assess the ongoing oscillatory activity of a specific area or across broader and more distributed brain networks [20] and (4) to establish causal relationships between stimulation and subsequent changes in cerebral function and behavioral outcome, by combining measurements of network-based neural activity [21].
For instance, paired pulse TMS protocols applied over specific areas of the brain (e.g., the primary motor cortex) allow to evaluate in vivo the activity of different intracortical circuits such as short intracortical inhibition (SICI), reflecting GABAergic neurotransmission, and short afferent inhibition (SAI) probing cholinergic neurotransmission in AD patients [22, 23]. SICI is measured by paired-pulse TMS: a subthreshold conditioning stimulus and a suprathreshold test stimulus are applied at short interstimulus intervals of 1–5 ms through the same stimulating coil [24]. It has been hypothesized that SICI represents short-lasting inhibitory postsynaptic potentials (IPSPs) in corticospinal neurons through activation of a low-threshold cortical inhibitory circuit [24]. SAI refers to a MEP inhibition in a hand muscle produced by a conditioning afferent electrical stimulus applied to the median or ulnar nerve at the wrist approximately 20 ms prior to focal TMS of the hand area of the contralateral motor cortex. AchEIs increasing the availability of acetylcholine in the synaptic cleft were observed to normalize the abnormally reduced SAI in patients with Alzheimer’s disease [25], while nicotine was found to increase SAI in healthy nonsmoking subjects [26]. These data are consistent with the view that SAI represents central cholinergic activity controlled by inhibitory circuits separate from those underlying SICI.
Depending on their specific frequency and/or patterning, different rTMS protocols result in excitatory or inhibitory after-effects lasting several minutes, which have been linked to LTP or to long-term depression (LTD). Repetitive TMS over the primary motor area can be used to measure in vivo cortical plasticity mechanisms such as LTP, which is considered the main neurophysiological correlate for learning and memory [19, 27]. Theta burst stimulation (TBS) is a novel form of rTMS that was developed recently to match theta burst patterns of stimulation commonly used to induce plasticity in animal brain slices. Intermittent TBS (iTBS) enhances cortical excitability for up to 1 h inducing LTP. These after-effects are thought to reflect rTMS influences on the strength of glutamatergic synapses via NMDA receptor, AMPA receptor, and calcium channel effects [28]. Long-lasting influences on the brain depend on changing synaptic strength or causing anatomical changes such as alterations in dendritic spines or sprouting. Since the anatomical changes may well be a secondary consequence of prolonged changes of synaptic strength, the basic logic of TMS stimulation is to change synaptic strength [29].
The combination of TMS with EEG (TMS-EEG) has provided an emergent method to directly probe local and widespread cortical dynamics, through the recording of TMS-evoked potentials (TEPs) [30]. TEPs have the great advantage to be highly reproducible, demonstrating consistency over time, but also to be extremely sensitive to changes in brain state. Moreover, TMS-EEG allows to investigate brain oscillatory activity within a specific area and between anatomically distinct brain regions, which is relevant when considering AD as a disconnection syndrome. TMS-EEG can indeed verify challenging aspect of the clinical assessment of brain disorders independently from patients’ ability to interact with the external environment. Theoretical considerations suggest that efficient brain activity involves complex patterns that are, at once, distributed among interacting cortical areas (integrated) and differentiated in space and time (information-rich).
rTMS can also be applied to establish causal relationships between stimulation and subsequent changes in cerebral function and behavioral outcome, for instance by combining fMRI measurements of network-based neural activity. In this scenario, trains of rTMS can be applied over a certain brain area, presumably a key node of a certain network and the induced changes in connectivity may be analyzed by means of resting-state fMRI. These two complementary tools can be combined to optimally study brain connectivity and manipulate distributed brain networks. Important clinical applications include using resting-state fMRI to guide target selection for TMS and using TMS to modulate pathological network interactions identified with resting-state fMRI. The combination of TMS and resting-state fMRI has the potential to accelerate the translation of both techniques into the clinical realm and promises a new approach to the diagnosis and treatment of neurological and psychiatric diseases that demonstrate network pathology [21].
4 TMS-Based Biomarkers in AD
On the basis of this background, we and others recently introduced the notion that TMS can be considered a novel tool to shape early features of synaptic dysfunction at different levels of complexity in patients with dementia. We recently showed that a systematic TMS-based assessment of GABAergic and cholinergic neurotransmission reliably distinguishes AD patients from those with frontotemporal dementia (FTD) and age-matched healthy controls (HC) and, therefore, TMS could represent a sensible diagnostic tool for clinical practice. Short-latency afferent inhibition (SAI), assessing the function of cholinergic circuits indirectly, has been found to be impaired in patients with AD; conversely, short-interval intracortical inhibition (SICI) and intracortical facilitation (ICF), markers of γ-aminobutyric acid type A (GABAA)ergic and glutamatergic neurotransmission, respectively, have been found to be impaired in patients with FTD [23]. These findings stemmed from the evidence that AD is defined by both amyloid deposits and a well-established cholinergic deficit, whereas in FTD, abnormalities in glutamatergic and GABAergic neurotransmission have been reported. Thus, the assessment of TMS intracortical connectivity holds promise to be a useful tool in the differential diagnosis of neurodegenerative diseases, being free from strict exclusion criteria, not time consuming, and inexpensive. However, its clinical value needs to be further demonstrated, also taking into consideration that both conditions may show several overlapping features, such as amyloid positivity in FTD, cholinergic deficits in FTD, or glutamatergic overexpression in AD [31].
On the other hand, we were among the first to demonstrate that LTP-like cortical plasticity is consistently impaired in AD patients, as assessed with iTBS protocol applied over the primary motor cortex [27]. The motor cortex is considered a reliable model to investigate early changes in cortical plasticity and central cholinergic transmission occurring in AD patients who are affected only at later stages of the disease, when AD becomes clinically manifest. Cortical plasticity is regarded as the principal biological mechanism for learning and memory. In humans, it can be assessed by noninvasive rTMS [19], in strict analogy with the hippocampal plasticity assessable in animal models. In the case of AD, synaptic loss is the strongest pathophysiological correlate of cognitive decline, indicating that synaptic degeneration has a central role in the development of dementia [32]. Experimental animal models showed that accumulation of soluble Aβ oligomers specifically blocks mechanisms of cortical plasticity such as hippocampal LTP, which is regarded as an electrophysiological correlate of learning and memory [33]. In contrast, these oligomers have been shown to electrically facilitate evoked LTD [34]. These events can, in turn, induce changes in the conformation of tau proteins, leading to further detrimental effects on synaptic plasticity and cognition.
Similar mechanisms of cortical plasticity can be investigated in vivo and noninvasively in humans, although the plasticity-induction procedures adopted are not completely identical in humans and animals. As discussed earlier, repetitive TMS over the primary motor area can be used to measure in vivo cortical plasticity mechanisms such as LTP.
In the context of AD, TMS applied over the motor cortex is considered a reliable model to investigate early changes in cortical plasticity and central cholinergic transmission occurring early in the disease [35].
In general AD patients, as opposed to HCs, are characterized by a weakened LTP-like cortical plasticity together with an impairment of SAI, putative biomarker of central cholinergic transmission [27]. In a large cohort of newly diagnosed sporadic AD patients, it was found that overall AD patients show after iTBS an impairment of LTP-like cortical plasticity, forming a paradoxical LTD in comparison to HCs. Moreover, SAI was impaired in AD showing a strong association with the individual age of subjects rather than with disease age of onset, while there was no association between age of onset and impairment of cortical plasticity. Thus, it was argued that cortical LTP disruption is a central mechanism of AD that is independent of age of onset [17]. Moreover, LTP-like cortical plasticity impairment is selectively associated with a less efficient verbal memory, but not to other cognitive functions, independent from biomarkers and other demographic and clinical factors [36]. Remarkably, LTP-like cortical plasticity is the most powerful TMS measurement in identifying AD patients among different neurophysiological parameters [37]. Motta et al. used TMS-based parameters to evaluate LTP-like cortical plasticity and cholinergic activity as measured by short afferent inhibition (SAI) in 60 newly diagnosed patients with AD and 30 HCs. Receiver-operating characteristic (ROC) curves were used to assess TMS ability in discriminating patients with AD from HCs. It was found that the area under the ROC curve was 0.90 for LTP-like cortical plasticity, indicating an excellent accuracy of this parameter in detecting AD pathology.
Apart from determining the diagnostic accuracy of TMS, we also showed that LTP-like cortical plasticity is able to predict cognitive decline in AD patients. The probability of a faster cognitive decline increased with every point decrease of LTP-like cortical plasticity, suggesting that the level of cortical plasticity evaluated at early stages of the disease is strictly linked to the subsequent clinical worsening in these patients. This finding is supported by experimental works showing that synaptic loss is the strongest pathophysiological correlate of cognitive decline, pointing to synaptic degeneration as a central mechanism in the dementia [37]. Furthermore, more impaired LTP-like cortical plasticity was associated with higher t-tau, but not 1–42 Aβ CSF levels. Aβ peptides exist in several soluble forms (oligomers) that can be released in the extracellular space where they may induce direct detrimental effects on neuronal transmission. However, consistent with previous findings, Aβ 1–42 fragments detected in the CSF of AD patients did not correlate with measures of cortical plasticity. In particular, we found that high tau CSF levels were associated with a paradoxical response toward LTD-like cortical plasticity instead of the expected LTP-like cortical. The same patients underwent a faster clinical progression [16]. These results suggest that more aggressive tau pathology is associated with prominent LTD-like mechanisms of cortical plasticity and faster cognitive decline. In this complex picture, synaptic dysfunction is likely to be influenced also by genetic factors. For instance, there is a strong relationship between Apolipoprotein E (APOE) polymorphisms and cortical plasticity, since APOE is known to regulate both beta-amyloid clearance/aggregation and tau-related microtubule stabilization, being strictly linked with altered mechanisms of synaptic plasticity. In a recent work from our group, it was found that the presence of APOE polymorphisms implies different mechanisms of CSF tau-related dysfunction in AD patients [38]. Indeed, high CSF tau levels are associated with impaired cortical plasticity and more aggressive disease progression only in AD patients carrying APOE4, but not APOE3 genotype. In parallel, CSF tau levels influence apoptosis in normal human astrocytes when incubated with CSF collected from AD patients with APOE4, but not APOE3 genotype. Taken together, these findings reveal that CSF tau levels are linked to cortical plasticity, cognitive decline, and astrocyte survival only when associated with APOE4 genotype [38].
In the field of dementia, TMS-EEG has been scarcely used. So far, in AD patients, only a few TMS-EEG studies investigated cortical correlates of cognitive impairment. Although the findings of these studies highlighted an association of cortical activity changes with cognitive decline and showed good specificity and sensitivity in identifying healthy subjects from those with cognitive impairment, the potential of TMS-EEG in tracking longitudinally disease progression was not investigated [39]. In the context of AD, we recently showed that innovative combined TMS-EEG protocols provide the possibility to directly measure cortical functional activity in cognitive-related areas, such as the dorsolateral prefrontal cortex (DLPFC) or the posterior parietal cortex (PPC), extending the potential role of TMS-based biomarkers in assessing the effects of therapies on cortical activity outside the primary motor cortex [40].
The detection of novel TMS-EEG markers of synaptic dysfunction (in terms of cortical excitability, connectivity, and oscillation) might contribute to provide additional predictive biomarkers of response to therapies in AD.
5 TMS-Based Therapeutics in AD
To date, the mainstream treatment for AD patients is represented only by cholinergic and glutamatergic drugs. However, pharmacological treatments have limited efficacy and are accompanied by adverse side effects. For this, it is of great importance to develop alternative therapeutic approaches. Recently, different forms of noninvasive brain stimulation techniques (e.g., TMS) have been applied to patients with AD in order to improve cognitive decline and behavioral disorders. In recent years, treatments based on multiple sessions of rTMS have represented a promising tool for influencing cognition in people with neurodegenerative diseases. This procedure is noninvasive and painless, and it does not require the use of anesthesia or pharmacological substances. The key principle of rTMS is based not only on regularly “repeated” stimulation of a focal cortical area but also on “accelerated” stimulation with multiple sessions and stimuli, leading to long-lasting modulation of the brain plasticity. From a neurobiological perspective, rTMS could induce relevant clinical improvement by promoting changes in synaptic plasticity. Synaptic plasticity is the most important biological mechanism accounting for learning and memory; in particular, LTP is considered as a main neurophysiological correlate of these cognitive functions [36]. We recently demonstrated that AD patients showed a disruption in LTP-like cortical plasticity since the early stages of the disease [37]. In this context, high-frequency rTMS could enhance LTP-like cortical plasticity, thus resulting in changes both at local and network levels as revealed by TMS-EGG and fMRI studies.
Until now, several studies have exclusively explored the effects of intensive treatments, lasting 2 weeks. Recently, safety and efficacy of maintenance with rTMS treatment in early AD patients showed a long-term trend with less cognitive decline than would be expected [41]. Noninvasive brain stimulation methods have been recently proposed as a novel approach to improve cognitive functions in patients with dementia, targeting the prefrontal cortex as a key area to be stimulated [42,43,44,45]. Moreover, novel interesting approaches are considering the possibility to stimulate in the same patients more areas such as the right and left DLPFC, Broca and Wernicke areas, and the right and left parietal somatosensory association cortex in conjunction with active cognitive training targeting these same brain regions [46].
However, since the early stages of AD, prominent neuropathological abnormalities (i.e., β-amyloid plaques and neurofibrillary tangles) involve posterior cortical regions of the brain, including the precuneus (PC), the posterior cingulate, the retrosplenial, and lateral PPC. Moreover, there is an initial disruption of medial fronto-parietal functional connectivity. Specifically, AD patients show alterations of the so-called default mode network (DMN), for which the PC is a key node [47]. Interestingly, at early clinical stages of AD, disconnection of the PC precedes (and probably contributes to) the occurrence of regional brain atrophy, which becomes prominent at later disease stages [48]. This means that the PC is a vulnerable region for the transitional stage toward dementia, which may be targeted by tailored interventions. Indeed, AD patients often show a reduction of PC cortical thickness accompanied by an abnormal activation during memory tasks and decreased functional connectivity. This is especially relevant since the activity of the PC is considered necessary for episodic memory retrieval [49], whose impairment represents the clinical onset of typical AD. Thus, the PC represents an ideal target for interventions aimed at slowing down and potentially counteracting memory decline in AD patients.
This hypothesis finds support in a recent experimental work performed in healthy subjects showing that TMS [50] was effective in modulating short- and long-term memory functions when applied over the PPC and PC. Following this line of evidence, we recently showed that 20-Hz rTMS was able to increase long-term memory performance and to potentiate the cortical activity of the PC. This provides novel evidence that noninvasive treatment of network dysfunction, through stimulation of the PC, represents a potentially efficacious strategy to improve cognitive dysfunction in AD. We showed that high-frequency excitatory rTMS improved long-term memory in patients with AD, by modulating both local neural activity and the connections with parietal, frontal and temporal areas.
However, the effects were only evaluated in a short-term course temporal window of 2 weeks. Sham-controlled rTMS trials are needed to explore whether rTMS may have a clinical impact in modifying the course of AD when applied over clinically relevant periods of 6–12 months.
6 Conclusions
TMS is contributing to shape the characteristics of synaptic dysfunction in AD patients, helping to increase diagnostic accuracy, and providing relevant clinical information in terms of disease progression and response to therapy.
On the other hand, there is a great interest in developing novel rTMS protocols that may have the potential to improve cognitive functions in patients with mild dementia and eventually slow down cognitive decline, if applied during a long-term period of several months.
References
Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, Burns A, Dening T, Findlay D, Holmes C, Hughes A. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med. 2012;366(10):893–903.
Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, Bakardjian H, Benali H, Bertram L, Blennow K, Broich K. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12(3):292–323.
Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–16.
Wallin ÅK, Blennow K, Zetterberg H, Londos E, Minthon L, Hansson O. CSF biomarkers predict a more malignant outcome in Alzheimer disease. Neurology. 2010;74(19):1531–7.
Cho H, Choi JY, Hwang MS, Kim YJ, Lee HM, Lee HS, Lee JH, Ryu YH, Lee MS, Lyoo CH. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann Neurol. 2016;80(2):247–58.
Yin Y, Gao D, Wang Y, Wang ZH, Wang X, Ye J, Wu D, Fang L, Pi G, Yang Y, Wang XC. Tau accumulation induces synaptic impairment and memory deficit by calcineurin-mediated inactivation of nuclear CaMKIV/CREB signaling. Proc Natl Acad Sci. 2016;113(26):E3773–81.
Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68(18):1501–8.
Selkoe DJ. The therapeutics of Alzheimer’s disease: where we stand and where we are heading. Ann Neurol. 2013;74(3):328–36.
Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–91.
Lasagna-Reeves CA, de Haro M, Hao S, Park J, Rousseaux MW, Al-Ramahi I, Jafar-Nejad P, Vilanova-Velez L, See L, De Maio A, Nitschke L. Reduction of Nuak1 decreases tau and reverses phenotypes in a tauopathy mouse model. Neuron. 2016;92(2):407–18.
Mosconi L, Pupi A, De Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann N Y Acad Sci. 2008;1147:180.
Brickman AM, Small SA, Fleisher A. Pinpointing synaptic loss caused by Alzheimer’s disease with fMRI. Behav Neurol. 2009;21(1–2):93–100.
Cook IA, Leuchter AF. Synaptic dysfunction in Alzheimer’s disease: clinical assessment using quantitative EEG. Behav Brain Res. 1996;78(1):15–23.
Fitzgerald PB, Daskalakis ZJ. Repetitive transcranial magnetic stimulation treatment for depressive disorders: a practical guide. Berlin: Springer Science & Business Media; 2013.
Cantone M, Di Pino G, Capone F, Piombo M, Chiarello D, Cheeran B, Pennisi G, Di Lazzaro V. The contribution of transcranial magnetic stimulation in the diagnosis and in the management of dementia. Clin Neurophysiol. 2014;125(8):1509–32.
Koch G, Di Lorenzo F, Del Olmo MF, Bonni S, Ponzo V, Caltagirone C, Bozzali M, Martorana A. Reversal of LTP-like cortical plasticity in Alzheimer’s disease patients with tau-related faster clinical progression. J Alzheimers Dis. 2016;50(2):605–16.
Di Lorenzo F, Ponzo V, Bonnì S, Motta C, Negrão Serra PC, Bozzali M, Caltagirone C, Martorana A, Koch G. Long-term potentiation–like cortical plasticity is disrupted in Alzheimer’s disease patients independently from age of onset. Ann Neurol. 2016;80(2):202–10.
Ziemann U. 1. Neuromodulation of motor learning in health and after stroke. Clin Neurophysiol. 2012;3(123):e9.
Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–6.
Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M. Natural frequencies of human corticothalamic circuits. J Neurosci. 2009;29(24):7679–85.
Fox MD, Halko MA, Eldaief MC, Pascual-Leone A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). NeuroImage. 2012;62(4):2232–43.
Di Lazzaro V, Rothwell JC. Corticospinal activity evoked and modulated by non-invasive stimulation of the intact human motor cortex. J Physiol. 2014;592(19):4115–28.
Benussi A, Di Lorenzo F, Dell’Era V, Cosseddu M, Alberici A, Caratozzolo S, Cotelli MS, Micheli A, Rozzini L, Depari A, Flammini A, Ponzo V, Martorana A, Caltagirone C, Padovani A, Koch G, Borroni B. Transcranial magnetic stimulation distinguishes Alzheimer disease from frontotemporal dementia. Neurology. 2017;89(7):665–72.
Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471(1):501–19.
Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Marra C, Daniele A, Ghirlanda S, Gainotti G, Tonali PA. Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75(4):555–9.
Grundey J, Freznosa S, Klinker F, Lang N, Paulus W, Nitsche MA. Cortical excitability in smoking and not smoking individuals with and without nicotine. Psychopharmacology. 2013;229(4):653–64.
Koch G, Di Lorenzo F, Bonnì S, Ponzo V, Caltagirone C, Martorana A. Impaired LTP-but not LTD-like cortical plasticity in Alzheimer’s disease patients. J Alzheimers Dis. 2012;31(3):593–9.
Huang YZ, Chen RS, Rothwell JC, Wen HY. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118(5):1028–32.
Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55(2):187–99.
Miniussi C, Thut G. Combining TMS and EEG offers new prospects in cognitive neuroscience. Brain Topogr. 2010;22(4):249.
Benussi A, Alberici A, Ferrari C, Cantoni V, Dell’Era V, Turrone R, Cotelli MS, Binetti G, Paghera B, Koch G, Padovani A. The impact of transcranial magnetic stimulation on diagnostic confidence in patients with Alzheimer disease. Alzheimers Res Ther. 2018;10(1):94.
Klyubin I, Betts V, Welzel AT, Blennow K, Zetterberg H, Wallin A, Lemere CA, Cullen WK, Peng Y, Wisniewski T, Selkoe DJ. Amyloid β protein dimer-containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. J Neurosci. 2008;28(16):4231–7.
Palop JJ, Mucke L. Amyloid-β–induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci. 2010;13(7):812.
Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid β protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62(6):788–801.
Battaglia F, Wang HY, Ghilardi MF, Gashi E, Quartarone A, Friedman E, Nixon RA. Cortical plasticity in Alzheimer’s disease in humans and rodents. Biol Psychiatry. 2007;62(12):1405–12.
Di Lorenzo F, Motta C, Bonnì S, Mercuri NB, Caltagirone C, Martorana A, Koch G. LTP-like cortical plasticity is associated with verbal memory impairment in Alzheimer’s disease patients. Brain Stimul. 2019;12(1):148–51.
Motta C, Di Lorenzo F, Ponzo V, Pellicciari MC, Bonnì S, Picazio S, Mercuri NB, Caltagirone C, Martorana A, Koch G. Transcranial magnetic stimulation predicts cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2018;89(12):1237–42.
Koch G, Di Lorenzo F, Loizzo S, Motta C, Travaglione S, Baiula M, Rimondini R, Ponzo V, Bonnì S, Toniolo S. Sallustio F. CSF tau is associated with impaired cortical plasticity, cognitive decline and astrocyte survival only in APOE4-positive Alzheimer’s disease. Sci Rep. 2017;7(1):13728.
Ferreri F, Vecchio F, Vollero L, Guerra A, Petrichella S, Ponzo D, Määtta S, Mervaala E, Könönen M, Ursini F, Pasqualetti P. Sensorimotor cortex excitability and connectivity in Alzheimer’s disease: a TMS-EEG co-registration study. Hum Brain Mapp. 2016;37(6):2083–96.
Koch G, Bonnì S, Pellicciari MC, Casula EP, Mancini M, Esposito R, Ponzo V, Picazio S, Di Lorenzo F, Serra L, Motta C. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. NeuroImage. 2018;169:302–11.
Lefaucheur JP, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Cantello RM, Cincotta M, de Carvalho M, De Ridder D, Devanne H. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014;125(11):2150–206.
Rutherford G, Lithgow B, Moussavi Z. Short and long-term effects of rTMS treatment on Alzheimer’s disease at different stages: a pilot study. J Exp Neurosci. 2015;9:JEN-S24004.
Cotelli M, Manenti R, Cappa SF, Geroldi C, Zanetti O, Rossini PM, Miniussi C. Effect of transcranial magnetic stimulation on action naming in patients with Alzheimer disease. Arch Neurol. 2006;63(11):1602–4.
Ferrucci R, Mameli F, Guidi I, Mrakic-Sposta S, Vergari M, Marceglia SE, Cogiamanian F, Barbieri S, Scarpini E, Priori A. Transcranial direct current stimulation improves recognition memory in Alzheimer disease. Neurology. 2008;71(7):493–8.
Turriziani P. Enhancing memory performance with rTMS in healthy subjects and individuals with mild cognitive impairment: the role of the right dorsolateral prefrontal cortex. Front Hum Neurosci. 2012;6:62.
Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38.
Rabey JM, Dobronevsky E, Aichenbaum S, Gonen O, Marton RG, Khaigrekht M. Repetitive transcranial magnetic stimulation combined with cognitive training is a safe and effective modality for the treatment of Alzheimer’s disease: a randomized, double-blind study. J Neural Transm. 2013;120(5):813–9.
Gili T, Cercignani M, Serra L, Perri R, Giove F, Maraviglia B, Caltagirone C, Bozzali M. Regional brain atrophy and functional disconnection across Alzheimer’s disease evolution. J Neurol Neurosurg Psychiatry. 2011;82(1):58–66.
Lundstrom BN, Ingvar M, Petersson KM. The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. NeuroImage. 2005;27(4):824–34.
Bonnì S, Veniero D, Mastropasqua C, Ponzo V, Caltagirone C, Bozzali M, Koch G. TMS evidence for a selective role of the precuneus in source memory retrieval. Behav Brain Res. 2015;282:70–5.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Koch, G. (2020). Transcranial Magnetic Stimulation in Dementia: From Pathophysiology to Treatment. In: Dell'Osso, B., Di Lorenzo, G. (eds) Non Invasive Brain Stimulation in Psychiatry and Clinical Neurosciences. Springer, Cham. https://doi.org/10.1007/978-3-030-43356-7_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-43356-7_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-43355-0
Online ISBN: 978-3-030-43356-7
eBook Packages: MedicineMedicine (R0)