Abstract
Approximately 800 people are diagnosed with osteosarcoma (OSA) per year in the USA. Although 70% of patients with localized OSA are cured with multiagent chemotherapy and surgical resection, the prognosis for patients with metastatic or relapsed disease is guarded. The small number of patients diagnosed annually contributes to an incomplete understanding of disease pathogenesis, and challenges in performing appropriately powered clinical trials and detecting correlative biomarkers of response. While mouse models of OSA are becoming increasingly sophisticated, they generally fail to accurately recapitulate tumor heterogeneity, tumor microenvironment (TME), systemic immune dysfunction, and the clinical features of tumor recurrence, metastases, and chemoresistance, which influence outcome. Pet dogs spontaneously develop OSA with an incidence that is 30–50 times higher than humans. Canine OSA parallels the human disease in its clinical presentation, biological behavior, genetic complexity, and therapeutic management. However, despite therapy, most dogs die from metastatic disease within 1 year of diagnosis. Since OSA occurs in immune-competent dogs, immune factors that sculpt tumor immunogenicity and influence responses to immune modulation are in effect. In both species, immune modulation has shown beneficial effects on patient outcome and work is now underway to identify the most effective immunotherapies, combination of immunotherapies, and correlative biomarkers that will further improve clinical response. In this chapter, the immune landscape of canine OSA and the immunotherapeutic strategies used to modulate antitumor immunity in dogs with the disease will be reviewed. From this immunological viewpoint, the value of employing dogs with spontaneous OSA to accelerate and inform the translation of immunotherapies into the human clinic will be underscored.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Canine osteosarcoma
- Immunotherapy
- Tumor microenvironment
- Animal model
- Immune modulation
- Comparative oncology
Introduction
OSA affects approximately 800 people per year in the USA, and, as such, it is subject to the challenges that orphan diseases present for therapeutic advances. The relatively small number of patients contributes to an incomplete understanding of the disease pathogenesis, challenges to performing appropriately powered, randomized, controlled clinical trials, and identifying correlative biomarkers of response that might direct patient stratification and improve outcomes. Furthermore, in comparison to more common cancers, funding opportunities and dollars for basic and clinical research are limited. These factors have contributed to the lack of significant advances in the treatment of OSA for more than 30 years [1]. While mouse models of OSA are becoming increasingly sophisticated, they often fail to recapitulate tumor heterogeneity and the immunosuppressive microenvironment and systemic immune dysfunction that are frequently encountered in cancer patients [2,3,4]. These shortcomings are emphasized when using murine models to evaluate the safety and efficacy of immunotherapeutic agents, that act on the immune system to augment antitumor immunity and prevent metastatic disease. Metastasis to the lung, bone, and soft tissues is the principal cause of death in OSA patients, and this natural progression of the disease is also poorly modeled in murine systems.
Pet dogs spontaneously develop OSA with an incidence that is 30–50 times higher than humans (~45,000 cases/year in the USA) and has a lifetime risk of up to 10% in predisposed breeds [5]. OSA arising spontaneously in large breed dogs parallels the human disease in its clinical presentation, biological behavior, genetic complexity, and therapeutic management [6, 7]. However, despite therapy , most dogs will develop metastatic disease, which is ultimately responsible for the death of the canine patient within 1 year of diagnosis [6]. As such, dogs with spontaneous OSA have been recognized as a valuable comparative model of pediatric OSA in which to investigate disease pathogenesis and evaluate therapies to prevent and treat metastatic disease [8]. Over 75% of canine OSA occurs in the long bones with the metaphyseal region of the distal radius, proximal humerus and distal femur being the most common sites affected [9]. Commonly affected sites in humans are the distal femur and proximal tibia, with the proximal humerus and distal radius, both non-weight-bearing bones in humans compared to dogs, being less frequently affected [10, 11]. In contrast to OSA in humans, middle- to older-aged adult, skeletally mature dogs are most commonly affected although there is a second, smaller peak incidence at 12–24 months of age [12]. Similar to pediatric OSA, the majority of canine OSAs are high grade, and elevated serum levels of alkaline phosphatase and metastatic disease are poor prognostic indicators in both species [13, 14]. In both species, metastatic disease occurs in the lungs, bone, and soft tissue. Unlike pediatric patients, standard of care in dogs consists of surgical resection followed by four to six cycles of adjuvant carboplatin and/or doxorubicin chemotherapy [15, 16]. However, although improved survival is seen with the addition of chemotherapy after primary tumor removal [15, 17], up to 90% of dogs develop metastatic disease despite standard of care with overall survival times ranging from 8–12 months [18]. Indeed, as many as 25% of dogs receiving chemotherapy will develop gross metastatic disease within 14 weeks of amputation, underscoring the aggressive nature of the disease in dogs and suggesting that neoadjuvant therapies might be worthy of investigation [19]. Multiple chemotherapeutic strategies have been investigated in dogs with OSA over the last 30 years; however, none have significantly improved disease-free intervals or median survival times [16, 19,20,21,22].
Given the similarities identified between OSA in dogs and humans, researchers from diverse scientific disciplines have taken advantage of the canine translational “model” to inform diagnostics, prognostics, and therapeutics for both species. For example, taking advantage of the reduced genetic heterogeneity that occurs within specific dog breeds, molecular biologists have utilized genome-wide gene-expression profiling, exomic profiling, comparative genomic hybridization, and comparative transcriptomics to identify molecular subtypes, recurrent driver/suppressor gene mutations, and signatures that are predictive of outcome in dogs with OSA [23,24,25,26,27]. These signatures have been successfully applied to tumor samples from a more genetically diverse human patient population where they are also predictive of outcome [23, 24]. Surgeons and bioengineers have taken advantage of the canine OSA patient to develop limb-sparing techniques that have been applied to human patients [28]. The similar body size, genetic make-up, metabolism, and drug distribution kinetics between species have resulted in the canine OSA patient being a valuable asset for medical oncologists in evaluating safety and determining optimal dosing schedules of novel cytotoxic agents, small molecule inhibitors, and immune modulating agents to prevent metastatic disease. Finally, radiation oncologists have explored different radiation types and dosing schedules to optimize management of nonresectable lesions in the dog and to induce immunogenic cell death (ICD), which may augment immunotherapeutic strategies aimed at preventing metastatic disease [29].
While the canine OSA patient has already contributed much to our understanding of disease biology and the development of surgical and chemotherapeutic strategies to manage human OSA patients, perhaps, its greatest contribution will be realized in the development of safe and effective immunotherapies or combination immunotherapies to prevent metastatic disease. OSA is an immune-responsive tumor, and William Coley’s observations in the late 1800s that concurrent bacterial infections increased patient survival provided some of the first evidence of this concept [30, 31]. Similar observations have been made more recently in canine OSA patients that experience surgical site infections following limb-sparing surgeries [32,33,34]. Although rare, spontaneous regression of primary OSA has also been reported in both species and is considered to be immunologically mediated [35,36,37]. Conversely, tumor-mediated suppression of innate and adaptive immunity occurs with many different types of neoplasia including OSA and this contributes to disease progression, metastases, and therapeutic resistance [38, 39]. Given the recent unprecedented success of immunotherapies including chimeric antigen receptor (CAR), T cells, and checkpoint inhibitors for the treatment of hematological and solid tumors with high mutational load, and the known immune responsiveness of OSA, there is increasing interest in evaluating immunotherapeutic strategies to treat OSA [40, 41]. Unlike immune-compromised rodent models of OSA that employ subcutaneous or orthotopic implantation of human tumor tissue or cell lines for research purposes, dogs that spontaneously develop OSA are immune competent, making them much better suited to evaluate therapies that act on the immune system to promote antitumor immunity. Furthermore, the spontaneity of tumor development means that tumor heterogeneity is preserved in dogs [42, 43] and the complex interplay that exists between the developing tumor and the immune system that sculpts tumor immunogenicity and directs the development of an immunosuppressive microenvironment is expected to be intact. Similar to pediatric OSA patients, dogs with OSA also exhibit systemic immune dysfunction that may serve as a significant barrier that needs to be overcome to improve response toimmunotherapies [38]. Since standard of care for dogs with OSA is surgical resection followed by cytotoxic chemotherapy, most canine OSA tissues are from chemotherapy-naïve primary samples , which may provide a more accurate assessment of tumor, tumor microenvironment (TME), and immune infiltrates than pediatric OSA samples taken at resection after multiagent chemotherapy. Finally, since owners of canine cancer patients may choose not to pursue standard of care, due to cost, patient size, or concerns surrounding quality-of-life issues, novel immunotherapies can be used at an earlier stage of disease compared to pediatric patients, increasing the likelihood of a favorable response that is not adversely affected by prolonged chemotherapy or advanced disease status.
Many questions now face immunotherapists aiming to improve the outcome of patients with OSA [44]. These include what is the immune status of the patient and of the tumor microenvironment (TME); how will these factors influence the clinical and immunological responses to immunotherapy; which tumor targets are relevant and safe; how can immunotherapies be rationally combined to broaden and augment antitumor immunity and provide a permissive TME to optimize antitumor effect; what are accurate biomarkers of response; and can they be employed to improve outcome via patient stratification? These clinically relevant questions can only be answered in patients with spontaneous tumors that exhibit tumor heterogeneity, recapitulate the tumor microenvironment, have intact and functional innate and adaptive immune responses, and either are known to develop metastatic disease with high frequency or already have metastatic disease.
Canine OSA patients present a spontaneous, immune competent “model” system that may be used to address a number of these questions and accelerate our understanding of OSA pathogenesis. Furthermore, they provide a valuable parallel patient population in which to evaluate the safety and efficacy of combination immunotherapeutic strategies and identify correlative biomarkers of clinical responses [45]. In this chapter, the immune landscape of canine OSA will be reviewed and compared with human OSA. Furthermore, the immunotherapeutic strategies that have been employed to modulate antitumor immunity in dogs with OSA will be presented. The review will examine the evidence that supports the use of canine patients to evaluate immunotherapeutic strategies, accelerate their translation into the human clinic, and identify correlative biomarkers that will assist in patient stratification for human clinical trials.
The Immunology of Canine OSA
Mutational Burden
The genetics of canine OSA have been well studied and are reviewed in detail elsewhere in this book. However, given that a tumor’s somatic mutational load is the best predictor of neoepitope burden and, in turn, neoepitope burden is the most predictive measure of immune therapeutic response, it is worth mentioning here what is known about the mutational status of canine OSA [46]. Similar to humans, canine OSA exhibits considerable genomic instability from a karyotypic standpoint and shares microaberrations in commonly mutated genes such as p53 and Rb [47, 48]. However, OSA arising in humans and dogs generally exhibits a low point-mutation burden (median 1.98 mutations per Mb canine DNA) with a trend toward higher mutational loads in metastatic lesions [49,50,51]. Although the mutational burden of OSA is comparably low across the spectrum of evaluated human cancers, it is high in relation to other pediatric cancers, and nonsynonymous mutations may serve as potential neoantigens for tumor-specific T cells that may be augmented by immunotherapeutic strategies in both species [51, 52].
Immune Landscape
Understanding the factors that influence the immune responsiveness of OSA and identifying correlative biomarkers that predict this response are key to improving the outcome of human and canine patients with this disease. Here we provide a comparative overview of what is known about the immunological landscape of canine OSA, identifying key players that may be manipulated by immunotherapeutic strategies to enhance patient response.
One of the most comprehensive studies that investigated the comparative immunological landscape of OSA utilized RNAseq to evaluate transcriptional profiles from primary appendicular OSA of humans, dogs, and genetically engineered mouse models to identify shared transcriptional profiles that influenced tumor development and progression [24]. Three highly conserved gene clusters were identified across species that were enriched in cell cycle transcripts, immune transcripts associated with monocytes, and transcripts associated with T cells. In humans and dogs, increased expression of transcripts associated with cell cycle correlated with poor patient outcome. In humans, patients whose tumors showed loss of immune cell transcripts had the shortest survival time, suggesting this may serve as a prognostic biomarker for metastatic disease. However, the lack of immune transcripts was not significantly correlated with survival times in the dog. The authors postulate that this may be due to the aggressive nature of canine OSA, with dogs not surviving long enough for the role of immune activation to be recognized. Taking advantage of the reduced genetic heterogeneity seen within dog breeds, the same investigators performed genome-wide gene-expression profiling, which separated OSA tumor samples into two different molecular subgroups distinguished by expression of G2/M transition, DNA damage checkpoints, and microenvironment-interaction signatures. These different subtypes had different metastatic potentials that correlated with the presence or absence of immune cell infiltrates within the stroma [23].
Monocytes/Macrophages
During tumor development , circulating monocytes/macrophages traffic into tumors where they are co-opted by the tumor microenvironment, shifting from a classical proinflammatory type 1 (M1) phenotype to an anti-inflammatory, protumorigenic type 2 (M2) phenotype [53]. Accumulations of M2 macrophages in tumors such as breast and cervical cancer have been associated with a poor clinical outcome [53, 54]. Buddingh et al. used gene-expression analysis and IHC to show that high-grade human OSA samples contained both type 1 (CD14/HLA-DRα+) and type 2 (CD14/CD163+) TAMs and that the presence of TAMs was associated with reduced metastases and improved survival [55, 56]. Similar findings were reported by Gomez-Bruchet who analyzed pretreatment biopsy samples from patients enrolled on the French phase 3 trial (OS 2006) and demonstrated that patients with core biopsies showing >50% of cells as CD163+ TAM experienced improved overall survival [56]. Finally, recent evidence in a murine xenograft model of metastatic OSA showed that the beneficial effects of PD-1 antagonism on pulmonary metastases were associated with increased infiltration by M1 macrophages and a reduction in M2 macrophages and depletion of macrophages in this model system negated the therapeutic effect of the checkpoint inhibitor [57]. Indeed, it is thought that the balance between M1 and M2 macrophages, which is controlled by the tumor cells themselves, plays a key role in determining the outcome of T cell responses within the tumor, with recent evidence suggesting that this outcome is dictated by PD-1/PD-L1 interactions. These findings underscore the complexity of immune interactions with the tumor and suggest that therapeutic strategies that influence the M1/M2 balance and promote a predominantly proinflammatory milieu may enhance antitumor T cell responses to control metastases and promote a more favorable outcome.
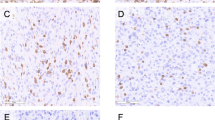
Using quantitative IHC to determine the presence of CD204+ macrophages, CD3+ T cells, and FOXP3+ (forkhead box P3) cells in primary tumors of 24 dogs with appendicular OSA, Withers et al. reported that the only prognostic subset was CD204+ cells, with dogs with high levels of CD204+ infiltrate experiencing prolonged disease-free intervals (DFI) [58]. Dogs with proximal humeral OSA, a location that is generally associated with a poor prognosis, tended to have lower CD204+ infiltrates compared to all other tumor locations and experienced shorter median survival times (MST) [58, 59]. In the same study, the authors demonstrated that tumors that contained high numbers of CD204+ TAMs also had greater lymphocytic infiltrates and patients with lymphocytic infiltrates above the top quartile showed a statistically significant prolongation of survival [58]. It is worth noting that CD204 expression is commonly associated with an M2 phenotype; however, the presence of lymphocytic infiltrates that correlate with improved survival might suggest that functionally, these TAMs are more consistent with a proinflammatory subset. As the canine reagent toolbox expands, further investigation into the phenotype and functional properties of these TAMs will ensue. These studies suggest that at a basic level, canine and pediatric OSAs share comparable immune infiltrate features and suggest dogs with OSA are relevant to further investigations into agents that manipulate the TME to promote effective antitumor immunity.
Several studies have demonstrated that high-circulating monocyte counts (>400 cells/μl in dogs, but still within the normal range) are associated with shorter DFI in dogs and in pediatric patients with appendicular and axial OSA [60,61,62,63]. Recently, the mechanistic basis for this has been investigated in dogs. Researchers found that circulating monocytes from dogs with OSA had reduced expression of cell adhesion molecules and chemokine receptors including CD62L, CCR7, CCR2, and CXCR2 [64]. They also exhibited decreased chemotactic function. These findings are consistent with the idea of tumor-mediated monocyte dysfunction in which monocytes from OSA patients have a reduced ability to traffic into tumor sites and initiate an antitumor immune response. This idea is further supported by the finding that canine OSA patients that express higher levels of CCR2 on circulating monocytes, enabling them to migrate into areas of inflammation in response to chemoattractant proteins, have improved survival [64]. Interestingly, when monocyte counts were high in these dogs, the cells tended to express higher levels of CD14 and lower levels of CD16 compared with patients with lower monocyte counts. In humans, this macrophage subset (CD14hi, CD16int) denotes a proinflammatory macrophage phenotype, that is MHCII high , produces TNF-α, IL-1β, and IL-6, and is a potent T cell stimulator [65]; however, the functional attributes of a CD14hi, CD16int/lo subset have not been explored in canines. Consistent with systemic monocyte dysfunction, stimulation of circulating monocytes from canine OSA patients with LPS led to the production of significantly more TNF-α and PGE2 than monocytes from healthy dogs. TNF-α is classically proinflammatory; however, it also promotes PGE2 production and can exhibit protumorigenic effects in part via IL-34 production in the TME [66, 67]. PGE2 plays an important direct role in immune dysfunction through multiple mechanisms in patients with cancer [68]. It inhibits the function of neutrophils, monocytes, and macrophages; disrupts cross-talk between DCs and T cells; skews T cells to a type 2 protumorigenic phenotype; and promotes the accumulation of regulatory T cells (Tregs) and myeloid-derived suppressor cell (MDSCs) [68]. Canine OSA cell lines and tumor tissues have also been shown to produce PGE2 [69, 70]. Millanta et al. confirmed these findings by IHC showing that 93% of canine OSA tissues expressed COX-2, 85% expressed microsomal PGE2 synthase-1, and 89% of tumors expressed the PGE2 receptor [71]. In similar studies, Wasserman et al. showed that myeloid cells exposed to tumor-derived soluble factors from OSA cell lines had reduced phagocytic activity, downregulated MHCII and CD80 expression reducing their capacity to activate antigen-specific CD4+ T cells, and suppressed responding effector cell proliferation [72]. Although not confirmed, it is possible that tumor-derived exosomes exert this immunosuppressive influence and contribute to the broad, tumor-mediated immune dysfunction seen in OSA patients. Similar immunosuppressive leukocyte profiles have been identified in pediatric sarcoma patients [61]. Together these data suggest that as in human patients, canine OSA avoids the immune response by adversely affecting the function and chemotactic capabilities of monocytes/macrophages [73]. Further investigations into the phenotype and function of different macrophage subsets are required in healthy and tumor-bearing dogs, but the current data suggests that canines with OSA can serve as a clinically relevant, patient population in which to investigate the biological and therapeutic effects of agents that modulate monocyte/macrophage subsets in the oncology clinic such as L-MTP-PE [74] and All-trans retinoic acid (ATRA) [75].
Myeloid-Derived Suppressor Cells
Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells that are produced in the bone marrow and traffic to tumor microenvironments under the influence of certain chemokines [76]. They are potent suppressors of T cell responses through a variety of different mechanisms and have the capacity to differentiate into TAMs within the tumor microenvironment [76, 77]. MDSCs play an important role in tumor progression and metastases, and their presence has been shown to predict response to immunotherapy and correlate with poor clinical outcome in a number of different solid tumor types [76]. Recently, MDSCs that resemble fibroblasts and have T cell suppressor capabilities have been described in pediatric sarcoma patients, although no OSA patients were included in the dataset [78]. Canine MDSCs have recently been characterized into monocytic and granulocytic subsets both phenotypically and functionally, and both subsets were shown to be increased in the peripheral blood of dogs with hematological and solid tumors compared to healthy controls [79]. Several earlier studies evaluated the presence and function of circulating MDSC in dogs with cancer [80, 81]. Sherger et al. identified a functionally immunosuppressive subset of MDSCs (CD11blo CADO48lo) that were increased in the peripheral blood of dogs with different cancer types including OSA [81]. Similarly, Goulart et al. found a significantly higher percentage of CD11b+CD14−MHCII− granulocytic MDSCs in the peripheral blood of dogs with advanced or metastatic cancers, including OSA. This group further showed that these cells expressed hallmark features of human MDSC including ARG1, iNOS2, TGF-β, and IL-10, which mediate suppressor activity against T cells [80]. Although our understanding of the role that MDSCs play either directly or indirectly in OSA remains rudimentary, these results suggest that they contribute to the systemic and local immune dysfunctions in both species that must be overcome to improve the clinical response to immunotherapies .
Regulatory T Cells
Regulatory T cells are thought to play a central role in suppressing antitumor immunity and contributing to poor outcomes in human cancer patients. As such, their presence has been evaluated in dogs with various cancers including OSA [82, 83]. Utilizing a combination of an anti-canine CD4 antibody and a cross-reactive antimouse FOXP3 antibody [82], Biller et al. showed that prior to amputation, dogs with OSA had significantly higher numbers of circulating Tregs and reduced numbers of CD8+ T cells compared to their healthy counterparts, resulting in a low CD8:Treg ratio that was predictive of shorter overall survival [38]. These aberrations in cell numbers and CD8:Treg ratio remained unchanged for at least 24 hours after amputation. Percentages of Tregs in the draining and distant lymph nodes of dogs with OSA and healthy controls were comparable [38]. Recently, proteins such as TGF-β [84], alpha fetoprotein, and heat shock proteins (HSP) within exosomes released from cultured canine OSA cell lines have been shown to suppress T cell proliferation, decrease CD25 expression on T cells, and direct a regulatory T cell phenotype, providing a potential mechanism for tumor-associated immune suppression in canine OSA patients [39]. However, in a follow-up study, Rissetto et al. used CD4, FOXP3, and CD25 to identify canine Tregs and found no difference in the percentage of Tregs in the peripheral blood or the draining lymph node of dogs with appendicular OSA when compared to healthy control dogs [83, 85]. Both studies evaluated samples from a small number of canine OSA patients, and evaluation of a larger cohort of dogs will be required to confirm the presence and predictive value of circulating Tregs in canine OSA.
T Cells
Tumor-infiltrating lymphocytes are identified in the majority of OSA biopsy samples, and multiple studies suggest that the presence of cytotoxic T cells controls the development of metastatic disease. Recently, Scott et al. reported that the presence of T cell infiltrates in human primary appendicular OSA predicted increased survival [24]. This supported previous findings from a multi-institutional European study that revealed a high ratio of intratumoral CD8+:FOXP3+ cells (>3.08) was predictive of improved survival [86]. Furthermore, Lussier et al. demonstrated that tumor-infiltrating cytotoxic T cells express PD-1 and that PD-1/PD-L1 blockade increases CTL activity, leading to a decrease in tumor burden and improved survival in a mouse model of OSA [87]. In dogs with OSA, RNAseq [24], IHC [58], flow cytometry [38], and histomorphometry [88] have been used to evaluate the presence of tumor-infiltrating lymphocytes. Histomorphometric and IHC studies on treatment of naïve, primary appendicular canine tumors showed that seven out of ten dogs had mild inflammation with a median of 8% of nucleated cells in the tumor being CD3+ T cells [88]. These cells were found in areas of necrosis and fibrosis as well as in viable tumor. Withers et al. used IHC and showed accumulations of B and T lymphocytes that resembled tertiary lymphoid structures (TLSs) in some canine patients [59]. Interestingly, neither RNAseq nor IHC data showed a correlation between T cells in canine primary appendicular tumors and overall survival [24, 58]. This is in contrast to the data obtained from human primary appendicular OSA tumors [24]. This discrepancy between species may arise due to the more rapid progression of OSA in the dog and the lack of time available to mount an immune response and/or the fact that overall survival time in dogs is highly influenced by the owners’ perception of quality of life and their capability of paying for treatment, leading to earlier euthanasia of the canine patient and highly variable overall survival times. In dogs, the comparative lack of reagents that enable T cell subset identification by IHC and flow cytometry makes it challenging to define the T cell subsets within TILs that might influence outcome. Furthermore, no studies have yet addressed whether TILs present in canine OSA are tumor specific .
Checkpoint Molecule Expression
The expression of immune checkpoint molecules such as PD-L1 on OSA cell lines and tumor tissues has been investigated as another mechanism by which OSA can inhibit immune function within the TME. Shen et al. reported that using IHC, 23.7% and 50% of human OSA tissue samples expressed high and intermediate levels of PD-L1, respectively, and that PD-L1 expression levels correlated with metastatic disease and poor overall prognosis [89, 90]. These findings suggest that OSA cells actively participate in antitumor immunity and that checkpoint blockade in the form of anti-PD-L1 and anti-PD-1 therapies may have therapeutic benefit in pediatric OSA. However, clinical trial results with PD-1/PD-L1 and CTLA4 inhibitors used as monotherapies have been disappointing in pediatric OSA, and combination therapies aimed at inducing antitumor immunity together with checkpoint blockade may represent important areas of research moving forward [91,92,93]. With the advent of canine-specific or cross-reactive antibodies that recognize key checkpoint molecules, the role that they play in restricting antitumor immune responses and the benefit of checkpoint inhibition in dogs with different cancers including OSA is beginning to be explored. A recent study using a murine anti-canine PD-L1 antibody demonstrated expression of PD-L1 on the surface of three different canine OSA cell lines, and expression was increased following treatment with recombinant canine (rc) IFN-γ or supernatants from mitogen-stimulated T cells [94]. Using IHC, Maekawa et al. demonstrated that PD-L1 was also expressed in primary canine OSA, suggesting that as in pediatric OSA, strategies to inhibit PD-1:PD-L1 interaction might have a beneficial effect [95]. However, unlike pediatric OSA, no studies have yet been performed in canine primary or metastatic OSA lesions to determine whether PD-L1 is positively correlated with the amount of TILs or whether it serves as a prognostic indicator [87, 96, 97]. Circulating canine monocytes did not express PD-L1 but did upregulate its expression following treatment with rcIFN-γ [98]. Similar results were obtained using canine monocyte-derived macrophages [94], suggesting that these mononuclear cells may contribute to systemic and tumor-associated T cell suppression. Functional studies using checkpoint inhibitors have shown that blockade of the PD-1/PD-L1 axis promotes CTL responses and enhances IFN-γ production in vitro and in vivo, leading to reduced metastatic tumor burden in murine models [87]. These findings support the notion that this key checkpoint axis is intact and open to manipulation to enhance antitumor immunity in dogs with OSA and other tumors [95, 99]. With the development of canine anti-PD-1 and PD-L1-blocking antibodies, it is likely that pet dogs with spontaneous OSA will serve as valuable subjects in which to evaluate the effectiveness of combination vaccine or cellular therapies with checkpoint inhibition and to identifycorrelative biomarkers that predict response .
Metastatic Lesions
While several studies have been performed that compare the genetic makeup of paired primary appendicular OSA with metastatic lesions, studies evaluating the immunological landscape of metastatic lesions are rare [49, 100]. In human patients, immune infiltrates have been identified in primary and metastatic OSA lesions although lymphocytic infiltrate in metastatic lesions was shown to be higher than in the paired primary samples [101]. Withers et al. used IHC to evaluate CD3+ T cells, FOXP3+ cells, B cells, and CD204+ macrophages in 21 paired primary and metastatic canine OSA samples [59]. They showed positive correlations of CD3+ T cells and FOXP3+ cells between primary and metastatic samples and that metastatic lesions had significantly more CD3+, PAX-5+, and CD204+ cells compared with the primary tumor. In human patients, CD3+ T cells were also higher in metastatic lesions compared with the primary tumor, but T cell subsets in the primary and metastatic lesions were the same [101]. Although B cells were the least prevalent immune cells in OSA lesions, they were observed to form clusters at the edge of half of the primary and 1/3 of the metastatic lesions, a feature that is reminiscent of tertiary lymphoid structures (TLSs) and has rarely been reported in human OSA.Unfortunately, the lack of canine-specific reagents to identify FDCs, follicular helper T cells, and chemokines makes further interrogation of these structures challenging. Indeed, additional geospatial molecular studies will be required to further define the immune cell types and their function within primary and metastatic lesions, to determine whether they have tumor-promoting or antitumor activity and perhaps to provide additional biomarkers of response to immunotherapies .
In summary, to the extent to which the immune status of canine patients and the immune landscape of their primary and metastatic OSA lesions have been explored, remarkable similarities have been identified with the human disease. These findings suggest that dogs with OSA will be valuable in investigating the benefit of microenvironment modulators such as macrophage activators, inhibitors of Tregs and MDSCs and their suppressive factors, and checkpoint inhibitors. More work is required to better understand the TME in both human and canine OSA patients particularly to identify biomarkers that may predict the patient’s ability to favorably respond to immunomodulatory agents and immunotherapies. As the diagnostic reagent toolbox continues to develop for canine tissues and new technologies including geospatial gene-expression analysis are adopted, it is anticipated that our understanding of the immune microenvironment will improve, and this will guide the rational selection of immune therapies and combination immune therapies that aim to improve outcome for human and canine patients with OSA .
Immunotherapy of Canine OSA
The evidence outlined above indicates that the innate and adaptive arms of the immune system play a role in controlling OSA progression and that tumor-associated local and systemic immune dysfunction enables tumor progression. Therefore, therapeutic strategies aimed at augmenting antitumor activity and reversing immune dysfunction have the potential to improve patient outcome. The studies that have been performed to evaluate the safety and efficacy of immunotherapies aimed at improving the prognosis of humans and pet dogs with OSA are outlined below.
Immune Modulatory Agents
Coley’s Toxins
In the late 1800s, William Coley made the seminal observation that patients with bone sarcoma suffering from concurrent streptococcal infections had prolonged durable remission times, suggesting that nonspecific immune activation was able to delay metastatic disease. Coley’s efforts to recapitulate the favorable effects of natural streptococcal infection on patient outcome resulted in the development of Coley’s toxins. This Streptococcus/Serratia concoction of either live or heat-killed bacteria was administered repeatedly to patients with sarcoma or following surgical resection of their sarcoma with favorable outcomes documented in a number of cases [30, 31]. Although the mechanism of improved overall response was unknown at the time, the adjuvant effects of bacterial components particularly on macrophages, promoting a permissive milieu that supports effective antitumor immunity , appear central to the effect [102].
BCG
Similar to Coley’s toxins, Bacillus Calmette-Guerin (BCG) , a live, attenuated strain of Mycobacterium bovis , promotes antitumor immunity and is FDA approved for first-line use in patients with high-risk nonmuscle invasive bladder cancer. Its therapeutic effect is thought to be mediated by T cells, NK cells, granulocytes, macrophages, and dendritic cells, plus a potential direct effect on the bladder cancer cells themselves [103]. Bech-Nielsen and colleagues treated dogs with spontaneous OSA after amputation with q2 weekly flank injections of BCG and noted a significant increase in survival extending from 13 weeks (control group n = 5) to 40 weeks in the vaccinated group (n = 6) [104]. The Kaplan-Meier survival curve reflected those of many medical immunotherapy trials performed today, with an elevation of the tail of the curve representing a greater proportion of vaccinated patients experiencing prolonged, durable remissions. A similar study by Owen and Bostock reported prolonged survival in dogs (n = 6) with appendicular OSA who underwent amputation followed by intravenous injections of 107–108 BCG organisms 1, 2, 4, and 8 weeks postoperatively [105]. Observations of a transient, low-grade fever within hours of administration suggested innate immune activation. Follow-up studies in healthy dogs showed that intravenous BCG mediated an increase in NK cell cytotoxicity and enhanced pulmonary macrophage activation, which likely played a role in controlling micrometastatic disease [106, 107]. These studies were performed in the absence of adjuvant chemotherapy. Almost 100 years after Coley made his seminal observation that bacterial infections improve patient outcome, similar observations were reported for dogs that had undergone limb salvage surgery for the treatment of appendicular OSA [32,33,34, 108]. Bacterial infections of Staphylococcus spp. , Pseudomonas spp. , and/or Streptococcus spp. were reported [33]. Similar to the studies using BCG, the mechanisms resulting in decreased pulmonary metastases and prolonged survival associated with bacterial infections are thought to be mediated by macrophages and NK cells, a concept supported in part by the finding that the survival benefit associated with osteomyelitis in murine OSA models is lost if monocytes/macrophages are depleted [102, 109].
Muramyl Tripeptides
The role of monocyte and macrophage activation in delaying or preventing metastatic disease in humans and dogs with OSA has been further underscored by favorable clinical responses in both species to liposome-encapsulated muramyl tripeptide-phosphatidylethanolamine (L-MTP-PE) – a mycobacterial wall extract. In a randomized, double-blinded, clinical trial, L-MTP-PE was administered intravenously twice a week for 8 weeks to 14 dogs with appendicular OSA after amputation [110]. Thirteen amputated dogs received empty liposomes as placebo controls. L-MTP-PE produced a transient, low-grade fever but was otherwise well tolerated. Median metastasis-free interval and median survival time for dogs receiving L-MTP-PE was 168 and 222 days, respectively, and 58 and 77 days for the placebo group. Follow-up studies demonstrated a similar beneficial effect of L-MTP-PE when administered after adjuvant cisplatin chemotherapy [111]. Here the MST of placebo dogs (n = 14) was 9.8 months compared with 14.4 months for dogs receiving L-MTP-PE. Interestingly, the survival benefit of L-MTP-PE was lost when treatment was administered concurrently with cisplatin [111], suggesting that concurrent cisplatin may either obviate the effects of L-MTP-PE or pre-treatment with cisplatin is required for the effects of L-MTP-PE to be realized. In vitro studies have revealed that L-MTP-PE is a potent activator of canine monocytes and macrophages, increasing their production of TNF-α and IL-6 and enhancing their cytostatic capabilities against tumor cells [112, 113]. Furthermore, pulmonary alveolar macrophages taken from canine patients treated with L-MTP-PE plus doxorubicin showed greater cytotoxic activity against OSA cells when compared to dogs treated with either agent alone [112]. In contrast to the in vivo results suggesting that cisplatin suppresses the beneficial effects of L-MTP-PE, monocyte cytotoxicity and TNF-α production were increased in dogs with splenic hemangiosarcoma treated with doxorubicin (a known inducer of ICD) plus L-MTP-PE [114]. Thus, it appears that different chemotherapies differentially influence the immunomodulatory and antitumor activities of L-MTP-PE. Additional work is required to identify the optimal combination and order of chemotherapy and immunomodulatory agents to achieve the most beneficial outcome. Understanding this order, which may depend upon the specific agents involved, remains an important challenge in the field of cancer immunotherapy today .
Cytokines
Given the pivotal role that innate and adaptive immune responses play in controlling tumor progression, several groups have investigated whether administration of IL-2, a potent T and NK cell growth factor, can augment antitumor immunity and delay progression or induce regression of pulmonary metastases [115]. Since high-dose systemic administration of IL-2 has a narrow therapeutic index, Khanna et al. explored the effects of aerosolized IL-2 liposomes on local and systemic immune effectors of normal healthy dogs [116]. The study showed that inhalation of human IL-2 liposomes significantly increased the number and activation status of leucocytes in bronchoalveolar lavage (BAL) fluid and skewed their composition toward lymphocytes and eosinophils rather than monocytes and macrophages, demonstrating biological activity of the administeredIL-2 [116]. Significant activation of systemic immune effectors was not observed and the aerosolized IL-2 was well tolerated, providing much needed safety data. To determine the clinical effects of aerosolized IL-2 in canine patients with metastatic OSA, four dogs with metastatic disease received aerosolized IL-2 liposomes two and three times a day for 30 days [117]. Two out of four dogs had complete and durable regression of metastases. Similar effects were observed on the composition of BAL cells with a statistically significant increase in lymphocytes, eosinophils, and macrophages after treatment. Cytolytic activity of BAL cells was also increased after 2 weeks of treatment , an effect that was attributed to NK cell activity and also possibly eosinophilic cytotoxicity [118]. However, cytotoxic activity declined thereafter, which may have been caused in part by the recorded development of antibodies against human IL-2. To circumvent formation of antidrug antibodies, cationic liposome–DNA complexes encoding canine IL-2 were delivered via intravenous infusion to 20 dogs with metastatic pulmonary OSA [119]. IL-2 expression was identified in the lung tissue and systemic immune activation in the form of transient fever, lymphopenia and thrombocytopenia, upregulation of costimulatory molecules and MHCII on monocytes, and increased NK cell cytotoxicity occurred. Overall survival of treated dogs was significantly increased compared with historical controls matched for disease stage. Furthermore, three dogs showed partial or complete regression of pulmonary metastases. These effects are most likely to be associated with a combination of local IL-2 production and innate immune responses induced by liposomes. In this study, the effects of IL-2 production on the local environment including leukocyte composition within BAL fluid were not evaluated.
Losartan
Losartan is a type I angiotensin II receptor antagonist. It has immunomodulatory effects on monocytes and macrophages and reduces pulmonary metastatic tumor burden in several mouse models of metastatic cancer (CT26 and 4T1) in part through inhibition of monocyte recruitment to the TME and a reduction in granulocytic MDSCs [120]. Losartan acts similarly to block CCL2-mediated migration in canine monocytes [121]. A clinical trial in dogs with metastatic OSA showed that a combination of high-dose losartan and the tyrosine kinase inhibitor toceranib was well tolerated in dogs and showed reduced monocyte trafficking to the metastatic lesions and exhibited antitumor activity (Steve Dow, personal communication). The results of these canine studies have supported the initiation of a pediatric trial (NCT03900793) for patients with relapsed/refractory OSA investigating the value of losartan in combination with sunitinib . The results of this trial are eagerly awaited.
Bisphosphonates
Bisphosphonates have been employed in the palliative setting to alleviate pain and reduce bone resorption by inhibiting osteoclast function. In addition to their effects on osteoclast apoptosis and inhibition of osteoclastogenesis, recent studies have indicated that bisphosphonates such as zoledronate and pamidronate have immunomodulatory functions through effects on innate and adaptive immune responses [122, 123]. In vitro, zoledronate inhibits regulatory T cell expansion, migration, and immunosuppressive activity [122]. In an HER2/neu (ErbB-2) transgenic mouse model, zoledronate switched tumor-associated macrophages from an M2 to M1 phenotype, reduced infiltration of macrophages into mammary tumors, and reduced VEGF concentrations and vascularization of the tumor [124]. However, in a murine model of OSA, where canine OSA cells were implanted orthotopically, zoledronate administered alone or following amputation did not reduce the incidence of pulmonary metastases [125]. The immunomodulatory effects of bisphosphonates have not yet been evaluated in vivo in the dog; however, given the common clinical use of these agents in canines with OSA, their effects on enabling antitumor immunity that is induced or augmented by other immunomodulatory agents, vaccines, or adoptive cellular therapies could be readily evaluated .
Active Vaccination
As nonspecific immune activation has shown moderate clinical benefit in delaying or preventing metastatic disease, attempts have been made to further improve outcomes by combining innate immune activation with tumor-specific adaptive immune responses using bacterial or viral vectors that supply TAA in the context of immune activation or provide additional cytokine support for T cell responses. Antigens identified and specifically targeted for therapeutic gain in human OSA patients include the epidermal growth cell factor receptor HER2/neu [126], GD2 and GD3 antigens [127, 128], TP-3-PAP [129, 130], and IGF-1R [84].
Bacterial Vaccines
Listeria monocytogenes
Listeria monocytogenes is a facultative, aerobic, intracellular bacteria that is a potent stimulator of innate and adaptive immunity. Through its ability to secrete the pore-forming lysin listeriolysin O (LLO), the bacteria can escape the phagosome and access the class I processing machinery of antigen-presenting cells [131]. As such, attenuated strains of recombinant Listeria, modified to express TAA fused to a truncated LLO, have been used in mouse models and in human patients to deliver antigens to APCs and generate tumor-specific CD4+ and CD8+ T cell responses [132]. The potent tumor-specific T cell responses generated break peripheral tolerance and lead to tumor regression [132, 133]. The epidermal growth factor receptor HER2/neu is expressed by 40–60% of primary OSA samples and serves as a relevant target for T cell therapies in humans and dogs with OSA [126, 134,135,136]. As such, active vaccination strategies to prime and expand HER2/neu specific T cells may be employed effectively to prevent or treat metastatic OSA. The ability of a recombinant chimeric huHER2/neu-expressing Listeria to prevent metastatic disease when administered in the setting of minimal residual disease after amputation and chemotherapy was evaluated in an 18-dog prospective clinical trial [137]. Vaccinated dogs experienced a median DFI of 650 days and OS of 956 days compared with an OS of 423 days for a historical, HER2/neu positive control group that received the same standard of care treatment without vaccination. This promising result has led to a larger prospective, controlled, national clinical trial conducted through the Comparative Oncology Trials Consortium. Tumor tissue and serial plasma, serum, and PBMC samples are being prospectively collected during this trial to evaluate immune responses and identify correlative predictive biomarkers.
Salmonella Typhimurium
Anaerobic bacteria that preferentially home to and accumulate in the hypoxic microenvironment of tumors have been used to promote antitumor immunity [138]. A highly attenuated Salmonella typhimurium (VNP20009) that only induces low levels of TNF-α and is dependent upon external purines for growth was administered intravenously every week or every 2 weeks to dogs with different tumor types in a phase I basket clinical trial to evaluate toxicity and early antitumor efficacy [139]. The majority of patients had soft tissue sarcomas or carcinomas, while 4 out of 41 treated dogs had OSA. CRs were seen in 10% of patients and 10% of patients experienced stable disease. One dog with metastatic OSA showed a partial response for 68 days. Fever, nausea, vomiting, and diarrhea were common side effects. Although immunological endpoints were not addressed in this study, the antitumor responses may have been associated with the organism’s direct tumoricidal activity, innate and adaptive immune activation, depletion of nutrients, and/or alteration of the TME. A second phase I study evaluated the safety of orally administered, attenuated Salmonella typhimurium as a vector to deliver IL-2 to 19 dogs with appendicular OSA. Dogs received oral dosing once 10 days prior to amputation and then after surgery concurrently with each of five doses of adjuvant doxorubicin for a total of six doses [140]. Salmonella was safe and well tolerated, and treated dogs experienced longer DFI but not OS when compared to two comparable historical control groups. An inflammatory leucogram (lymphocytosis and monocytosis) was seen in 18 out of 19 dogs 10 days after the first Salmonella administration, suggesting it was biologically active. Salmonella was not detected in any tumor tissue cultured after amputation, suggesting that any observed beneficial effects were more likely associated with antitumor immunity rather than a direct tumoricidal activity of the vector. Randomized, placebo-controlled, prospective trials are warranted to determine the true value of this approach .
DNA Vaccines
Alternative approaches to induce HER2/neu-specific T cell responses have been explored in dogs using DNA encoding the extracellular and transmembrane domains of human HER2/neu and electroporation as a priming strategy followed by a boost with an adenovirus 6 vector expressing the same HER2/neu construct [141]. This regime induced HER2/neu-specific IFN-γ responses and HER2/neu-specific IgG responses, although the adenoviral vector was found to be highly immunogenic, limiting the efficacy of any subsequent attempts to boost immunity using this serovar. Although no studies have yet been published using this approach in dogs with OSA to assess therapeutic effectiveness, the approach has been shown to be safe and induces durable HER2/neu-specific T cell responses in healthy dogs. Overcoming vector immunogenicity to enable effective booster treatments will be important to take this approach further clinically .
Oncolytic Viruses
Defects in antiviral defense mechanisms in tumor cells provide an ideal opportunity for oncolytic viruses (OVs) to be used therapeutically to selectively infect and destroy tumor cells. Tumor lysis and ICD results in induction of systemic polyclonal T cell responses that aim to control both primary and metastatic diseases. Many OVs also exert immunomodulatory effects on the microenvironment through their ability to induce the release of pathogen-associated molecular patterns (PAMPs) from tumor cells and promote the production of type I interferons [142]. Le Boeuf and colleagues demonstrated the ability of the oncolytic rhabdovirus, Maraba (MG1), to infect and kill both canine sarcoma cell lines and human sarcoma explants and confirmed these cytotoxic effects in a murine sarcoma model [143]. Similarly, Naik et al. evaluated VSV expressing IFNβ in dogs with different tumor types, including one dog with axial OSA and metastatic disease. All dogs tolerated intravenous oncolytic viral therapy well and the one dog with OSA showed stabilization of primary and metastatic disease for 6 months [144]. Laborda et al. utilized a locally delivered, hyaluronidase-armed, oncolytic adenovirus in a total of six dogs, including two dogs with OSA [145]. No adverse side effects occurred that could be directly attributed to the adenoviral therapy and partial responses were seen in two dogs, although neither had OSA. Although experience with oncolytic viral therapy in dogs with OSA is limited, these studies lay the groundwork for further evaluation of this approach either as a monotherapy or in combination with immunomodulatory agents or immune checkpoint inhibition to augment clinical effect .
FasL-Mediated Inflammation
The death receptor Fas (CD95) is expressed by many different tumor types and its engagement by FasL (CD95L) triggers apoptosis, leading to the hypothesis that FasL may represent a promising cancer therapeutic [146]. Both innate and adaptive immune responses are induced by intratumoral delivery of FasL, effects that are mediated via apoptosis of Fas+ macrophages and the resulting influx of neutrophils that are ultimately responsible for tumor cell death. Subsequent recruitment and activation of APCs promotes a systemic antitumor immune response that aims to control metastatic spread. However, controversy surrounds its use in part, because Fas-signaling has also been shown to be required for tumor cell survival [147]. Furthermore, systemic administration of FasL results in lethal hepatotoxicity in mouse models [148]. To mitigate these risks while evaluating the effects of neoadjuvant FasL in dogs with OSA, Modiano et al. delivered a single intratumoral dose of adenovirus expressing canine FasL (Ad-FasL) to 56 dogs [88]. Ten days later, dogs underwent standard amputation and adjuvant carboplatin chemotherapy. Ad-FasL was generally well tolerated, with adverse effects associated with transient increases in transaminases and creatine phosphokinase. Adenoviral delivery of FasL induced a potent inflammatory response with increased lymphocytic infiltration within the tumor compared to dogs who did not receive Ad-FasL. Furthermore, dogs with high inflammation scores within the treated tumor experienced improved overall survival [88]. Dogs with reduced Fas expression on their tumors had greater inflammation scores supporting the notion that the improved survival effects of FasL are primarily associated with its induction of inflammation rather than direct Fas-mediated tumor apoptosis .
Cell-Based Therapies
Tall 104 Cells
The earliest recorded use of adoptive T cell therapy for dogs with OSA was in 1999, when Daniela Santoli’s group at the Wistar Institute evaluated the safety and efficacy of the human cytotoxic T cell line, TALL104 cells, to prevent metastatic disease. Dogs that had undergone amputation and adjuvant cisplatin chemotherapy received 1 × 108 γ-irradiated cells/kg systemically daily for 5 days and then every month for up to 9 months . Only mild and transient grade 1 and 2 related GI toxicities were reported. Although the overall median survival was 11.5 months and the median DFI was 9.8 months, the Kaplan-Meier survival curve demonstrated uncharacteristic long-term survival of some patients. These effects were speculated to be in part mediated through enhanced endogenous NK cell activity that occurred as a direct result of TALL104 administration. Perhaps unsurprisingly, the unmodified, xenogenic, adoptively transferred cells did not persist and antibody responses against them were detected in all treated dogs and cellular immune responses against them were detected in 80% of treated dogs. Although performed 20 years ago, these studies have set the stage for evaluating genetic modifications of human T cells that will enable them to cross xenogenic barriers and persist to mediate antitumor immunity in canine patients. Employment of a comparative approach in these endeavors aims to provide greater clarity surrounding the modifications that will be required for successful allogeneic adoptive T cell therapy in human patients (N. Mason, personal communication).
Polyclonal Activated T Cells
Isolation , ex vivo expansion, and reinfusion of autologous tumor-infiltrating lymphocytes have proven effective in the treatment of immunogenic tumors such as melanoma; however, this strategy is underexplored in OSA patients [149]. Instead, veterinary researchers are currently evaluating active vaccination of canine patients with appendicular OSA using an autologous tumor lysate vaccine to prime circulating T cells. Primed, tumor-specific T cells are harvested by apheresis and polyclonally expanded ex vivo using a proprietary cocktail, before being adoptively transferred back into the patient after amputation. Adjuvant cytotoxic chemotherapy is not employed in the protocol (J. Bryan, personal communication). Early results suggest the procedure is safe, but outcome data is yet to be published.
NK Cell Therapies
NK cells play a fundamental role in tumor surveillance and elimination, and, as such, efforts have been made to employ autologous or allogeneic NK cells in adoptive transfer strategies to treat or prevent metastatic disease in human cancer patients. Activation of NK cells is MHC independent and mediated via receptors that recognize cell surface proteins that are upregulated on stressed target cells or are non-self proteins [150]. In addition to direct killing, NK cell activity is augmented in response to antibodies, cytokines, and immunomodulatory agents including chemotherapy and radiation therapy. As such, strategic combinations of adoptive NK cell transfer with immunomodulatory agents, sensitizing chemotherapy, or radiation therapy are being put forward for clinical evaluation. Advances in the use of adoptive immunotherapy (AI) with NK cells in dogs have previously been hampered by the lack of specific, validated antibodies to identify canine NK cells. A CD5 low, CD8+, CD3+/− subset has been described that cytologically displays features consistent with NK cell morphology, expresses high levels of message for NK cell receptors , and displays cytotoxic activity against MHC null, thyroid adenocarcinoma cells [151]. More recently, an anticanine NKp46 mAb was generated, and NKp46+CD3− canine cells showed cytolytic activity against canine OSA cell lines. Furthermore, these cells were effectively expanded ~20,000-fold over 3 weeks in coculture with irradiated K562 feeder cells that express hu4-1BBL and membrane-bound huIL-21 and huIL-2 [152]. Cytolytic activity of expanded CD5dimCD3−NKp46+ cells was significantly increased in vitro against allogeneic OSA cell lines after their treatment with γ-radiation [153]. Furthermore, radiation of canine OSA xenografts in NSG mice significantly increased homing of ex vivo expanded adoptively transferred canine NK cells and tumor killing. In contrast to human OSA treatments, radiation therapy is commonly employed in canine patients that do not undergo amputation to alleviate pain [154,155,156]. Canter et al. combined palliative radiation with intratumoral delivery of ex vivo expanded autologous NK cells once a week for 2 weeks after palliative radiation [153]. Limited toxicity was observed with this approach and posttreatment biopsies demonstrated persistence of labeled NK cells within the tumor for at least 1 week. Five out of ten dogs remained metastases-free at 6 months, and one dog showed resolution of a suspicious pulmonary nodule following treatment. Overall survival times were favorable compared with historical controls. Follow-up studies are now planned to evaluate the effects of the NK cell activating cytokine IL-15 as monotherapy and then in combination with autologous NK cell transfer in patients with metastatic OSA (R. Rebhun, personal communication). Taken together, this work described the successful isolation , activation, expansion, and transfer of canine NK cells and illustrates the enhancing effects of RT on NK cell cytotoxicity. Furthermore, it sets the stage for future studies evaluating AI with NK cells alone or in combination with the sensitizing effects of RT and supportive cytokines (IL-2/IL-15).
CAR T Cell Technology
Several groups have described protocols for generating canine CAR T cells either via RNA transfection with a first-generation CD20-targeting CAR or a second-generation IL-13Rα2-targeting CAR construct [157, 158] or transduction with an RD114 pseudotyped retroviral vector containing a second-generation HER2-targeting CAR construct [136]. Second-generation canine CAR T cells expressing the humanized, cross-reactive anti-IL-13Rα2 scFv (Hu08) produced IFN-γ when cocultured with three different canine OSA cell lines expressing IL-13Rα2 [158]. Furthermore, lentiviral transduced human CAR T cells expressing the same scFv effectively inhibited tumor growth when administered intravenously to NOD/SCID mice bearing established canine MC-KOSA xenografts [158]. Similarly, Mata et al. demonstrated that second-generation canine CAR T cells expressing the cross-reactive antiHER2/neu (FRP5) scFv and canine intracellular signaling domains secrete IFN-γ and effectively kill HER2-positive canine OSA cell lines in an antigen-specific manner. Furthermore, adoptive transfer of HER2-redirected T cells into SCID mice with established intraperitoneal OSA xenografts leads to tumor regression [159]. Similar tumor regression occurred following adoptive transfer of HER2-specific CART cells into mice with established OSA pulmonary metastases [159]. The same group of investigators showed low levels of HER2 expression on the surface of CD133+ OSA tumor-initiating cells (TICs) and that HER2-specific CAR T cells specifically killed TICs in established orthotopic OSA tumors [160]. These data suggest that HER2-targeted CAR T cells may be valuable in targeting micrometastases to prevent metastatic disease. Given that up to 95% of canine patients will have micrometastatic disease at the time of initial presentation, they again represent a valuable patient population in which to evaluate CAR T cell strategies to prevent or treat metastatic disease.
Together this work sets the stage for evaluating both IL-13Rα2- and HER2-targeting CARs in pet dogs with OSA either alone or in combination with other immunotherapies such as checkpoint inhibitors or immunomodulatory agents for the treatment of both primary and metastatic disease. Furthermore, the identification of anticanine or cross-reactive antibodies against GD2 (e.g., 14G2a), IL-11Rα, and FAP will enable additional canine CARs to be constructed against these OSA-associated cell surface targets and then evaluated in dogs for their safety and ability to improve outcome [161].
In all cases, it remains to be seen whether adoptive cell transfer alone will be sufficient to control or prevent metastatic disease or whether combination with immunomodulatory agents that influence the TME will be required to achieve optimal clinical results. Indeed, as our understanding of the immune landscape of OSA increases and validated biomarkers emerge that predict immune responsiveness, it is anticipated that rational combinations of agents that augment tumor-specific T cells with agents that reverse systemic immune dysfunction and immune suppression within the TME will lead to improved patient outcome for both species.
Additional Strategies for Induction of ICD
It is now apparent that standard-of-care cancer therapeutic modalities such as certain chemotherapeutic agents and radiation therapy can induce ICD of the tumor, which is valuable in broadening antitumor immune responses initiated or augmented by immunotherapeutic strategies [29, 162]. In the last decade in particular, radiation has been shown to promote antitumor immune responses via increasing expression of target antigens, activating dendritic cells and inducing tumor-specific CD8+ T cell responses, promoting CD4+ and CD8+ T cell infiltration into tumors, upregulating MHCI, and downregulating immunosuppressive molecules within the TME including arginase-I, CTLA4, PD-1, PD-L1, IDO, FOXP3, TGF-β, and IL-10 [163, 164]. These local effects translate into systemic antitumor immunity and are responsible for abscopal effects that have been reported following RT therapy. Although radiation therapy is infrequently employed in the treatment of pediatric OSA, its combined use with hyperthermia therapy, checkpoint inhibitors, locally delivered cytokines, vaccination, and adoptive cellular therapies is being actively pursued in other cancer types [165,166,167,168]. Conversely, both coarse fraction external beam radiation and megadose stereotactic radiosurgery are commonly used in canine patients with OSA that do not undergo primary tumor removal [169]. Palliative radiation is employed often as monotherapy, providing pain relief in up to 74% of dogs for 2–3 months [154, 155, 170]. Thus, dogs with OSA provide a readily available model system in which to explore the immunogenic effects of RT on the primary tumor immunome and to evaluate the safety and therapeutic effectiveness of its combination with vaccines, cellular therapies, and immunomodulatory agents to control primary disease and prevent metastatic disease [29, 171].
In summary, compelling evidence exists to indicate that OSA is an immune responsive tumor and that therapies aimed at initiating, enabling, and broadening antitumor immunity hold great promise for preventing and treating metastatic disease and improving patient outcome. A number of challenges lie ahead, not least of which is the design and implementation of rational combinations of immunotherapies and immunomodulatory agents that will promote tumor-specific adaptive T cell responses and enable them to function effectively within the TME. It is likely that not all patients will require the same immune modulation regime, and identifying biomarkers that can predict each patient’s requirement enabling therapy to be tailored to their needs, may lead to the improvement in patient survival that the field has been searching for over the last Four decades. Given the remarkable similarity between canine and pediatric OSA, particularly as it relates to the local and systemic immune landscape, and the large number of pet dogs diagnosed with OSA per year, it seems that we have a remarkable opportunity to address some of these key challenges in the veterinary setting, leading to improved outcome for both patient populations .
References
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30
Guijarro MV, Ghivizzani SC, Gibbs CP (2014) Animal models in osteosarcoma. Front Oncol 4:189
Sanmamed MF, Chester C, Melero I, Kohrt H (2016) Defining the optimal murine models to investigate immune checkpoint blockers and their combination with other immunotherapies. Ann Oncol 27:1190–1198
Rangarajan A, Weinberg RA (2003) Opinion: comparative biology of mouse versus human cells: modelling human cancer in mice. Nat Rev Cancer 3:952–959
Anfinsen KP, Grotmol T, Bruland OS, Jonasdottir TJ (2011) Breed-specific incidence rates of canine primary bone tumors—a population based survey of dogs in Norway. Can J Vet Res 75:209–215
Fenger JM, London CA, Kisseberth WC (2014) Canine osteosarcoma: a naturally occurring disease to inform pediatric oncology. ILAR J 55:69–85
Fan TM, Khanna C (2015) Comparative aspects of osteosarcoma pathogenesis in humans and dogs. Vet Sci 2:210–230
Owen LN (1967) Comparative aspects of bone tumours in man and dog. Proc R Soc Med 60:1309–1310
Morello E, Martano M, Buracco P (2011) Biology, diagnosis and treatment of canine appendicular osteosarcoma: similarities and differences with human osteosarcoma. Vet J 189:268–277
Ottaviani G, Jaffe N (2009) The epidemiology of osteosarcoma. Cancer Treat Res 152:3–13
Unni K (1996) Dahlin’s bone tumors: general aspects and data on 11,087 cases, 5th edn. Lippincott-Raven, Philadelphia
Misdorp W, Hart AA (1979) Some prognostic and epidemiologic factors in canine osteosarcoma. J Natl Cancer Inst 62:537–545
Garzotto CK, Berg J, Hoffmann WE, Rand WM (2000) Prognostic significance of serum alkaline phosphatase activity in canine appendicular osteosarcoma. J Vet Intern Med 14:587–592
Hillers KR, Dernell WS, Lafferty MH, Withrow SJ, Lana SE (2005) Incidence and prognostic importance of lymph node metastases in dogs with appendicular osteosarcoma: 228 cases (1986-2003). J Am Vet Med Assoc 226:1364–1367
Phillips B, Powers BE, Dernell WS et al (2009) Use of single-agent carboplatin as adjuvant or neoadjuvant therapy in conjunction with amputation for appendicular osteosarcoma in dogs. J Am Anim Hosp Assoc 45:33–38
Kent MS, Strom A, London CA, Seguin B (2004) Alternating carboplatin and doxorubicin as adjunctive chemotherapy to amputation or limb-sparing surgery in the treatment of appendicular osteosarcoma in dogs. J Vet Intern Med 18:540–544
Chun R, Garrett LD, Henry C, Wall M, Smith A, Azene NM (2005) Toxicity and efficacy of cisplatin and doxorubicin combination chemotherapy for the treatment of canine osteosarcoma. J Am Anim Hosp Assoc 41:382–387
Withrow SJ, Powers BE, Straw RC, Wilkins RM (1991) Comparative aspects of osteosarcoma. Dog versus man. Clin Orthop Relat Res 270:159–168
London CA, Gardner HL, Mathie T et al (2015) Impact of toceranib/piroxicam/cyclophosphamide maintenance therapy on outcome of dogs with appendicular osteosarcoma following amputation and carboplatin chemotherapy: a multi-institutional study. PLoS One 10:e0124889
McMahon M, Mathie T, Stingle N, Romansik E, Vail D, London C (2011) Adjuvant carboplatin and gemcitabine combination chemotherapy postamputation in canine appendicular osteosarcoma. J Vet Intern Med 25:511–517
Selmic LE, Burton JH, Thamm DH, Withrow SJ, Lana SE (2014) Comparison of carboplatin and doxorubicin-based chemotherapy protocols in 470 dogs after amputation for treatment of appendicular osteosarcoma. J Vet Intern Med 28:554–563
Berg J, Weinstein MJ, Springfield DS, Rand WM (1995) Results of surgery and doxorubicin chemotherapy in dogs with osteosarcoma. J Am Vet Med Assoc 206:1555–1560
Scott MC, Sarver AL, Gavin KJ et al (2011) Molecular subtypes of osteosarcoma identified by reducing tumor heterogeneity through an interspecies comparative approach. Bone 49:356–367
Scott MC, Temiz NA, Sarver AE et al (2018) Comparative transcriptome analysis quantifies immune cell transcript levels, metastatic progression, and survival in osteosarcoma. Cancer Res 78:326–337
Shao YW, Wood GA, Lu J et al (2019) Cross-species genomics identifies DLG2 as a tumor suppressor in osteosarcoma. Oncogene 38:291–298
Sakthikumar S, Elvers I, Kim J et al (2018) SETD2 is recurrently mutated in whole-exome sequenced canine osteosarcoma. Cancer Res 78:3421–3431
Davis LE, Jeng S, Svalina MN et al (2017) Integration of genomic, transcriptomic and functional profiles of aggressive osteosarcomas across multiple species. Oncotarget 8:76241–76256
Straw RC, Powers BE, Withrow SJ, Cooper MF, Turner AS (1992) The effect of intramedullary polymethylmethacrylate on healing of intercalary cortical allografts in a canine model. J Orthop Res 10:434–439
Fan TM, Selting KA (2018) Exploring the potential utility of pet dogs with cancer for studying radiation-induced immunogenic cell death strategies. Front Oncol 8:680
Coley WB (1907) Sarcoma of the long bones: the diagnosis, treatment and prognosis, with a report of sixty-nine cases. Ann Surg 45:321–368
Coley WB (1910) The treatment of inoperable sarcoma by bacterial toxins (the mixed toxins of the Streptococcus erysipelas and the Bacillus prodigiosus). Proc R Soc Med 3:1–48
Culp WT, Olea-Popelka F, Sefton J et al (2014) Evaluation of outcome and prognostic factors for dogs living greater than one year after diagnosis of osteosarcoma: 90 cases (1997-2008). J Am Vet Med Assoc 245:1141–1146
Lascelles BD, Dernell WS, Correa MT et al (2005) Improved survival associated with postoperative wound infection in dogs treated with limb-salvage surgery for osteosarcoma. Ann Surg Oncol 12:1073–1083
Liptak JM, Dernell WS, Ehrhart N, Lafferty MH, Monteith GJ, Withrow SJ (2006) Cortical allograft and endoprosthesis for limb-sparing surgery in dogs with distal radial osteosarcoma: a prospective clinical comparison of two different limb-sparing techniques. Vet Surg 35:518–533
Mehl ML, Withrow SJ, Seguin B et al (2001) Spontaneous regression of osteosarcoma in four dogs. J Am Vet Med Assoc 219:614–617
Ogihara Y, Takeda K, Yanagawa T, Hirasawa Y (1994) Spontaneous regression of lung metastases from osteosarcoma. Cancer 74:2798–2803
Carlsen NL (1999) Spontaneous regression of malignant tumors. Ugeskr Laeger 161:3980
Biller BJ, Guth A, Burton JH, Dow SW (2010) Decreased ratio of CD8+ T cells to regulatory T cells associated with decreased survival in dogs with osteosarcoma. J Vet Intern Med 24:1118–1123
Troyer RM, Ruby CE, Goodall CP et al (2017) Exosomes from osteosarcoma and normal osteoblast differ in proteomic cargo and immunomodulatory effects on T cells. Exp Cell Res 358:369–376
Wedekind MF, Wagner LM, Cripe TP (2018) Immunotherapy for osteosarcoma: where do we go from here? Pediatr Blood Cancer 65:e27227
Dyson KA, Stover BD, Grippin A et al (2019) Emerging trends in immunotherapy for pediatric sarcomas. J Hematol Oncol 12:78
Fox MH, Armstrong LW, Withrow SJ et al (1990) Comparison of DNA aneuploidy of primary and metastatic spontaneous canine osteosarcomas. Cancer Res 50:6176–6178
LaRue SM, Fox MH, Withrow SJ et al (1994) Impact of heterogeneity in the predictive value of kinetic parameters in canine osteosarcoma. Cancer Res 54:3916–3921
Roberts RD, Lizardo MM, Reed DR et al (2019) Provocative questions in osteosarcoma basic and translational biology: a report from the Children’s Oncology Group. Cancer 125:3514–3525
Khanna C, Fan TM, Gorlick R et al (2014) Toward a drug development path that targets metastatic progression in osteosarcoma. Clin Cancer Res 20:4200–4209
Alexandrov LB, Nik-Zainal S, Wedge DC et al (2013) Signatures of mutational processes in human cancer. Nature 500:415–421
Angstadt AY, Thayanithy V, Subramanian S, Modiano JF, Breen M (2012) A genome-wide approach to comparative oncology: high-resolution oligonucleotide aCGH of canine and human osteosarcoma pinpoints shared microaberrations. Cancer Genet 205:572–587
Schiffman JD, Breen M (2015) Comparative oncology: what dogs and other species can teach us about humans with cancer. Philos Trans R Soc Lond Ser B Biol Sci 370:20140231
Gardner HL, Sivaprakasam K, Briones N et al (2019) Canine osteosarcoma genome sequencing identifies recurrent mutations in DMD and the histone methyltransferase gene SETD2. Commun Biol 2:266
Kovac M, Blattmann C, Ribi S et al (2015) Exome sequencing of osteosarcoma reveals mutation signatures reminiscent of BRCA deficiency. Nat Commun 6:8940
Bousquet M, Noirot C, Accadbled F et al (2016) Whole-exome sequencing in osteosarcoma reveals important heterogeneity of genetic alterations. Ann Oncol 27:738–744
Grobner SN, Worst BC, Weischenfeldt J et al (2018) The landscape of genomic alterations across childhood cancers. Nature 555:321–327
Chanmee T, Ontong P, Konno K, Itano N (2014) Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 6:1670–1690
Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S (2005) Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep 14:425–431
Buddingh EP, Kuijjer ML, Duim RA et al (2011) Tumor-infiltrating macrophages are associated with metastasis suppression in high-grade osteosarcoma: a rationale for treatment with macrophage activating agents. Clin Cancer Res 17:2110–2119
Gomez-Brouchet A, Illac C, Gilhodes J et al (2017) CD163-positive tumor-associated macrophages and CD8-positive cytotoxic lymphocytes are powerful diagnostic markers for the therapeutic stratification of osteosarcoma patients: an immunohistochemical analysis of the biopsies fromthe French OS2006 phase 3 trial. Onco Targets Ther 6:e1331193
Dhupkar P, Gordon N, Stewart J, Kleinerman ES (2018) Anti-PD-1 therapy redirects macrophages from an M2 to an M1 phenotype inducing regression of OS lung metastases. Cancer Med 7:2654–2664
Withers SS, Skorupski KA, York D et al (2019) Association of macrophage and lymphocyte infiltration with outcome in canine osteosarcoma. Vet Comp Oncol 17:49–60
Withers SS, York D, Choi JW et al (2019) Metastatic immune infiltrates correlate with those of the primary tumour in canine osteosarcoma. Vet Comp Oncol 17(3):242–252
Sottnik JL, Rao S, Lafferty MH et al (2010) Association of blood monocyte and lymphocyte count and disease-free interval in dogs with osteosarcoma. J Vet Intern Med 24:1439–1444
Hingorani P, Maas ML, Gustafson MP et al (2015) Increased CTLA-4(+) T cells and an increased ratio of monocytes with loss of class II (CD14(+) HLA-DR(lo/neg)) found in aggressive pediatric sarcoma patients. J Immunother Cancer 3:35
Liu T, Fang XC, Ding Z, Sun ZG, Sun LM, Wang YL (2015) Pre-operative lymphocyte-to-monocyte ratio as a predictor of overall survival in patients suffering from osteosarcoma. FEBS Open Bio 5:682–687
Selmic LE, Lafferty MH, Kamstock DA et al (2014) Outcome and prognostic factors for osteosarcoma of the maxilla, mandible, or calvarium in dogs: 183 cases (1986-2012). J Am Vet Med Assoc 245:930–938
Tuohy JL, Lascelles BD, Griffith EH, Fogle JE (2016) Association of canine osteosarcoma and monocyte phenotype and chemotactic function. J Vet Intern Med 30:1167–1178
Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, Wong SC (2012) The three human monocyte subsets: implications for health and disease. Immunol Res 53:41–57
Zhang W, Dziak R (1996) Tumor necrosis factor alpha stimulates arachidonic acid metabolism in human osteoblastic osteosarcomal cells. Prostaglandins Leukot Essent Fatty Acids 54:427–431
Segaliny AI, Mohamadi A, Dizier B et al (2015) Interleukin-34 promotes tumor progression and metastatic process in osteosarcoma through induction of angiogenesis and macrophage recruitment. Int J Cancer 137:73–85
Kalinski P (2012) Regulation of immune responses by prostaglandin E2. J Immunol 188:21–28
Mohammed SI, Coffman K, Glickman NW et al (2001) Prostaglandin E2 concentrations in naturally occurring canine cancer. Prostaglandins Leukot Essent Fatty Acids 64:1–4
Shor S, Fadl-Alla BA, Pondenis HC et al (2015) Expression of nociceptive ligands in canine osteosarcoma. J Vet Intern Med 29:268–275
Millanta F, Asproni P, Cancedda S, Vignoli M, Bacci B, Poli A (2012) Immunohistochemical expression of COX-2, mPGES and EP2 receptor in normal and reactive canine bone and in canine osteosarcoma. J Comp Pathol 147:153–160
Wasserman J, Diese L, VanGundy Z, London C, Carson WE, Papenfuss TL (2012) Suppression of canine myeloid cells by soluble factors from cultured canine tumor cells. Vet Immunol Immunopathol 145:420–430
Dumars C, Ngyuen JM, Gaultier A et al (2016) Dysregulation of macrophage polarization is associated with the metastatic process in osteosarcoma. Oncotarget 7:78343–78354
Pahl JH, Kwappenberg KM, Varypataki EM et al (2014) Macrophages inhibit human osteosarcoma cell growth after activation with the bacterial cell wall derivative liposomal muramyl tripeptide in combination with interferon-gamma. J Exp Clin Cancer Res 33:27
Zhang X, Zhou H, Wang J et al (2002) Arsenic trioxide, retinoic acid and Ara-c regulated the expression of annexin II on the surface of APL cells, a novel co-receptor for plasminogen/tissue plasminogen activator. Thromb Res 106:63–70
Diaz-Montero CM, Finke J, Montero AJ (2014) Myeloid-derived suppressor cells in cancer: therapeutic, predictive, and prognostic implications. Semin Oncol 41:174–184
Corzo CA, Condamine T, Lu L et al (2010) HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med 207:2439–2453
Zhang H, Maric I, DiPrima MJ et al (2013) Fibrocytes represent a novel MDSC subset circulating in patients with metastatic cancer. Blood 122:1105–1113
Goulart MR, Hlavaty SI, Chang YM et al (2019) Phenotypic and transcriptomic characterization of canine myeloid-derived suppressor cells. Sci Rep 9:3574
Goulart MR, Pluhar GE, Ohlfest JR (2012) Identification of myeloid derived suppressor cells in dogs with naturally occurring cancer. PLoS One 7:e33274
Sherger M, Kisseberth W, London C, Olivo-Marston S, Papenfuss TL (2012) Identification of myeloid derived suppressor cells in the peripheral blood of tumor bearing dogs. BMC Vet Res 8:209
Biller BJ, Elmslie RE, Burnett RC, Avery AC, Dow SW (2007) Use of FoxP3 expression to identify regulatory T cells in healthy dogs and dogs with cancer. Vet Immunol Immunopathol 116:69–78
O’Neill K, Guth A, Biller B, Elmslie R, Dow S (2009) Changes in regulatory T cells in dogs with cancer and associations with tumor type. J Vet Intern Med 23:875–881
Portela RF, Fadl-Alla BA, Pondenis HC et al (2014) Pro-tumorigenic effects of transforming growth factor beta 1 in canine osteosarcoma. J Vet Intern Med 28:894–904
Rissetto KC, Rindt H, Selting KA, Villamil JA, Henry CJ, Reinero CR (2010) Cloning and expression of canine CD25 for validation of an anti-human CD25 antibody to compare T regulatory lymphocytes in healthy dogs and dogs with osteosarcoma. Vet Immunol Immunopathol 135:137–145
Fritzsching B, Fellenberg J, Moskovszky L et al (2015) CD8(+)/FOXP3(+)-ratio in osteosarcoma microenvironment separates survivors from non-survivors: a multicenter validated retrospective study. Onco Targets Ther 4:e990800
Lussier DM, O’Neill L, Nieves LM et al (2015) Enhanced T-cell immunity to osteosarcoma through antibody blockade of PD-1/PD-L1 interactions. J Immunother 38:96–106
Modiano JF, Bellgrau D, Cutter GR et al (2012) Inflammation, apoptosis, and necrosis induced by neoadjuvant fas ligand gene therapy improves survival of dogs with spontaneous bone cancer. Mol Ther 20:2234–2243
Zhu Z, Jin Z, Zhang M et al (2017) Prognostic value of programmed death-ligand 1 in sarcoma: a meta-analysis. Oncotarget 8:59570–59580
Shen JK, Cote GM, Choy E et al (2014) Programmed cell death ligand 1 expression in osteosarcoma. Cancer Immunol Res 2:690–698
Merchant MS, Wright M, Baird K et al (2016) Phase I clinical trial of ipilimumab in pediatric patients with advanced solid tumors. Clin Cancer Res 22:1364–1370
Le Cesne A, Marec-Berard P, Blay JY et al (2019) Programmed cell death 1 (PD-1) targeting in patients with advanced osteosarcomas: results from the PEMBROSARC study. Eur J Cancer 119:151–157
Tawbi HA, Burgess M, Bolejack V et al (2017) Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol 18:1493–1501
Hartley G, Faulhaber E, Caldwell A et al (2017) Immune regulation of canine tumour and macrophage PD-L1 expression. Vet Comp Oncol 15:534–549
Maekawa N, Konnai S, Ikebuchi R et al (2014) Expression of PD-L1 on canine tumor cells and enhancement of IFN-gamma production from tumor-infiltrating cells by PD-L1 blockade. PLoS One 9:e98415
Koirala P, Roth ME, Gill J et al (2016) Immune infiltration and PD-L1 expression in the tumor microenvironment are prognostic in osteosarcoma. Sci Rep 6:30093
Palmerini E, Agostinelli C, Picci P et al (2017) Tumoral immune-infiltrate (IF), PD-L1 expression and role of CD8/TIA-1 lymphocytes in localized osteosarcoma patients treated within protocol ISG-OS1. Oncotarget 8:111836–111846
Coy J, Caldwell A, Chow L, Guth A, Dow S (2017) PD-1 expression by canine T cells and functional effects of PD-1 blockade. Vet Comp Oncol 15:1487–1502
Nemoto Y, Shosu K, Okuda M, Noguchi S, Mizuno T (2018) Development and characterization of monoclonal antibodies against canine PD-1 and PD-L1. Vet Immunol Immunopathol 198:19–25
Giraldo NA, Becht E, Remark R, Damotte D, Sautes-Fridman C, Fridman WH (2014) The immune contexture of primary and metastatic human tumours. Curr Opin Immunol 27:8–15
Sundara YT, Kostine M, Cleven AH, Bovee JV, Schilham MW, Cleton-Jansen AM (2017) Increased PD-L1 and T-cell infiltration in the presence of HLA class I expression in metastatic high-grade osteosarcoma: a rationale for T-cell-based immunotherapy. Cancer Immunol Immunother 66:119–128
Tuohy JL, Somarelli JA, Borst LB, Eward WC, Lascelles BDX, Fogle JE (2020) Immune dysregulation and osteosarcoma: Staphylococcus aureus downregulates TGF-beta and heightens the inflammatory signature in human and canine macrophages suppressed by osteosarcoma. Vet Comp Oncol 18(1):64–75
Redelman-Sidi G, Glickman MS, Bochner BH (2014) The mechanism of action of BCG therapy for bladder cancer—a current perspective. Nat Rev Urol 11:153–162
Bech-Nielsen S, Brodey RS, Fidler IJ, Abt DA, Reif JS (1977) The effect of BCG on in vitro immune reactivity and clinical course in dogs treated surgically for osteosarcoma. Eur J Cancer 13:33–41
Owen LN, Bostock DE (1974) Effects of intravenous BCG in normal dogs and in dogs with spontaneous osteosarcoma. Eur J Cancer 10:775–780
Betton GR, Gorman NT, Owen LN (1979) Cell mediated cytotoxicity in dogs following systemic or local BCG treatment alone or in combination with allogeneic tumour cell lines. Eur J Cancer 15:745–754
Gorman NT (1979) Alveolar macrophage cytotoxicity in dogs following intravenous BCG. Eur J Cancer 15:1051–1059
Thrall DE, Withrow SJ, Powers BE et al (1990) Radiotherapy prior to cortical allograft limb sparing in dogs with osteosarcoma: a dose response assay. Int J Radiat Oncol Biol Phys 18:1351–1357
Sottnik JL, U’Ren LW, Thamm DH, Withrow SJ, Dow SW (2010) Chronic bacterial osteomyelitis suppression of tumor growth requires innate immune responses. Cancer Immunol Immunother 59:367–378
MacEwen EG, Kurzman ID, Rosenthal RC et al (1989) Therapy for osteosarcoma in dogs with intravenous injection of liposome-encapsulated muramyl tripeptide. J Natl Cancer Inst 81:935–938
Kurzman ID, MacEwen EG, Rosenthal RC et al (1995) Adjuvant therapy for osteosarcoma in dogs: results of randomized clinical trials using combined liposome-encapsulated muramyl tripeptide and cisplatin. Clin Cancer Res 1:1595–1601
Kurzman ID, Shi F, Vail DM, MacEwen EG (1999) In vitro and in vivo enhancement of canine pulmonary alveolar macrophage cytotoxic activity against canine osteosarcoma cells. Cancer Biother Radiopharm 14:121–128
Smith BW, Kurzman ID, Schultz KT, Czuprynski CJ, MacEwen EG (1993) Muramyl peptides augment the in vitro and in vivo cytostatic activity of canine plastic-adherent mononuclear cells against canine osteosarcoma cells. Cancer Biother 8:137–144
Shi F, MacEwen EG, Kurzman ID (1993) In vitro and in vivo effect of doxorubicin combined with liposome-encapsulated muramyl tripeptide on canine monocyte activation. Cancer Res 53:3986–3991
Mitchell DH, Withrow SJ, Johnston MR, Kruse CA (1991) Cytotoxicity against autologous, allogeneic, and xenogeneic tumor targets by human recombinant interleukin-2-activated lymphocytes from healthy dogs and dogs with lung tumors. Am J Vet Res 52:1132–1136
Khanna C, Hasz DE, Klausner JS, Anderson PM (1996) Aerosol delivery of interleukin 2 liposomes is nontoxic and biologically effective: canine studies. Clin Cancer Res 2:721–734
Khanna C, Anderson PM, Hasz DE, Katsanis E, Neville M, Klausner JS (1997) Interleukin-2 liposome inhalation therapy is safe and effective for dogs with spontaneous pulmonary metastases. Cancer 79:1409–1421
Rivoltini L, Viggiano V, Spinazze S et al (1993) In vitro anti-tumor activity of eosinophils from cancer patients treated with subcutaneous administration of interleukin 2. Role of interleukin 5. Int J Cancer 54:8–15
Dow S, Elmslie R, Kurzman I, MacEwen G, Pericle F, Liggitt D (2005) Phase I study of liposome-DNA complexes encoding the interleukin-2 gene in dogs with osteosarcoma lung metastases. Hum Gene Ther 16:937–946
Regan DP, Coy JW, Chahal KK et al (2019) The angiotensin receptor blocker losartan suppresses growth of pulmonary metastases via AT1R-independent inhibition of CCR2 signaling and monocyte recruitment. J Immunol 202:3087–3102
Regan D, Dow S (2015) Manipulation of innate immunity for cancer therapy in dogs. Vet Sci 2:423–439
Liu H, Wang SH, Chen SC, Chen CY, Lo JL, Lin TM (2016) Immune modulation of CD4(+)CD25(+) regulatory T cells by zoledronic acid. BMC Immunol 17:45
Green J, Clezardin P (2010) The molecular basis of bisphosphonate activity: a preclinical perspective. Semin Oncol 37(Suppl 1):S3–S11
Coscia M, Quaglino E, Iezzi M et al (2010) Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J Cell Mol Med 14:2803–2815
Wolfe TD, Pillai SP, Hildreth BE 3rd et al (2011) Effect of zoledronic acid and amputation on bone invasion and lung metastasis of canine osteosarcoma in nude mice. Clin Exp Metastasis 28:377–389
Gorlick R, Huvos AG, Heller G et al (1999) Expression of HER2/erbB-2 correlates with survival in osteosarcoma. J Clin Oncol 17:2781–2788
Roth M, Linkowski M, Tarim J et al (2014) Ganglioside GD2 as a therapeutic target for antibody-mediated therapy in patients with osteosarcoma. Cancer 120:548–554
Dobrenkov K, Ostrovnaya I, Gu J, Cheung IY, Cheung NK (2016) Oncotargets GD2 and GD3 are highly expressed in sarcomas of children, adolescents, and young adults. Pediatr Blood Cancer 63:1780–1785
Maniscalco L, Iussich S, Morello E et al (2015) Increased expression of insulin-like growth factor-1 receptor is correlated with worse survival in canine appendicular osteosarcoma. Vet J 205:272–280
Anderson PM, Meyers DE, Hasz DE et al (1995) In vitro and in vivo cytotoxicity of an anti-osteosarcoma immunotoxin containing pokeweed antiviral protein. Cancer Res 55:1321–1327
Mostowy S, Cossart P (2012) Virulence factors that modulate the cell biology of Listeria infection and the host response. Adv Immunol 113:19–32
Wood LM, Paterson Y (2014) Attenuated Listeria monocytogenes: a powerful and versatile vector for the future of tumor immunotherapy. Front Cell Infect Microbiol 4:51
Shahabi V, Seavey MM, Maciag PC, Rivera S, Wallecha A (2011) Development of a live and highly attenuated Listeria monocytogenes-based vaccine for the treatment of Her2/neu-overexpressing cancers in human. Cancer Gene Ther 18:53–62
Ahmed N, Salsman VS, Yvon E et al (2009) Immunotherapy for osteosarcoma: genetic modification of T cells overcomes low levels of tumor antigen expression. Mol Ther 17:1779–1787
Flint AF, U’Ren L, Legare ME, Withrow SJ, Dernell W, Hanneman WH (2004) Overexpression of the erbB-2 proto-oncogene in canine osteosarcoma cell lines and tumors. Vet Pathol 41:291–296
Mata M, Vera JF, Gerken C et al (2014) Toward immunotherapy with redirected T cells in a large animal model: ex vivo activation, expansion, and genetic modification of canine T cells. J Immunother 37:407–415
Mason NJ, Gnanandarajah JS, Engiles JB et al (2016) Immunotherapy with a HER2-targeting Listeria induces HER2-specific immunity and demonstrates potential therapeutic effects in a phase I trial in canine osteosarcoma. Clin Cancer Res 22:4380–4390
Al-Ramadi BK, Fernandez-Cabezudo MJ, El-Hasasna H et al (2008) Attenuated bacteria as effectors in cancer immunotherapy. Ann N Y Acad Sci 1138:351–357
Thamm DH, Kurzman ID, King I et al (2005) Systemic administration of an attenuated, tumor-targeting Salmonella typhimurium to dogs with spontaneous neoplasia: phase I evaluation. Clin Cancer Res 11:4827–4834
Fritz SE, Henson MS, Greengard E et al (2016) A phase I clinical study to evaluate safety of orally administered, genetically engineered Salmonella enterica serovar Typhimurium for canine osteosarcoma. Vet Med Sci 2:179–190
Peruzzi D, Mesiti G, Ciliberto G, La Monica N, Aurisicchio L (2010) Telomerase and HER-2/neu as targets of genetic cancer vaccines in dogs. Vaccine 28:1201–1208
Marchini A, Daeffler L, Pozdeev VI, Angelova A, Rommelaere J (2019) Immune conversion of tumor microenvironment by oncolytic viruses: the protoparvovirus H-1PV case study. Front Immunol 10:1848
Le Boeuf F, Selman M, Son HH et al (2017) Oncolytic Maraba virus MG1 as a treatment for sarcoma. Int J Cancer 141:1257–1264
Naik S, Galyon GD, Jenks NJ et al (2018) Comparative oncology evaluation of intravenous recombinant oncolytic vesicular stomatitis virus therapy in spontaneous canine cancer. Mol Cancer Ther 17:316–326
Laborda E, Puig-Saus C, Rodriguez-Garcia A et al (2014) A pRb-responsive, RGD-modified, and hyaluronidase-armed canine oncolytic adenovirus for application in veterinary oncology. Mol Ther 22:986–998
Trauth BC, Klas C, Peters AM et al (1989) Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science 245:301–305
Modiano JF, Bellgrau D (2016) Fas ligand based immunotherapy: a potent and effective neoadjuvant with checkpoint inhibitor properties, or a systemically toxic promoter of tumor growth? Discov Med 21:109–116
Rensing-Ehl A, Frei K, Flury R et al (1995) Local Fas/APO-1 (CD95) ligand-mediated tumor cell killing in vivo. Eur J Immunol 25:2253–2258
Theoleyre S, Mori K, Cherrier B et al (2005) Phenotypic and functional analysis of lymphocytes infiltrating osteolytic tumors: use as a possible therapeutic approach of osteosarcoma. BMC Cancer 5:123
Gasser S, Raulet DH (2006) Activation and self-tolerance of natural killer cells. Immunol Rev 214:130–142
Huang YC, Hung SW, Jan TR et al (2008) CD5-low expression lymphocytes in canine peripheral blood show characteristics of natural killer cells. J Leukoc Biol 84:1501–1510
Foltz JA, Somanchi SS, Yang Y, Aquino-Lopez A, Bishop EE, Lee DA (2016) NCR1 expression identifies canine natural killer cell subsets with phenotypic similarity to human natural killer cells. Front Immunol 7:521
Canter RJ, Grossenbacher SK, Foltz JA et al (2017) Radiotherapy enhances natural killer cell cytotoxicity and localization in pre-clinical canine sarcomas and first-in-dog clinical trial. J Immunother Cancer 5:98
Ramirez O 3rd, Dodge RK, Page RL et al (1999) Palliative radiotherapy of appendicular osteosarcoma in 95 dogs. Vet Radiol Ultrasound 40:517–522
Green EM, Adams WM, Forrest LJ (2002) Four fraction palliative radiotherapy for osteosarcoma in 24 dogs. J Am Anim Hosp Assoc 38:445–451
Knapp-Hoch HM, Fidel JL, Sellon RK, Gavin PR (2009) An expedited palliative radiation protocol for lytic or proliferative lesions of appendicular bone in dogs. J Am Anim Hosp Assoc 45:24–32
Panjwani MK, Smith JB, Schutsky K et al (2016) Feasibility and safety of RNA-transfected CD20-specific chimeric antigen receptor T cells in dogs with spontaneous B cell lymphoma. Mol Ther 24:1602–1614
Yin Y, Boesteanu AC, Binder ZA et al (2018) Checkpoint blockade reverses anergy in IL-13Ralpha2 humanized scFv-based CAR T cells to treat murine and canine gliomas. Mol Ther Oncolytics 11:20–38
Ahmed N, Salsman VS, Yvon E et al (2009) Immunotherapy for osteosarcoma: genetic modification of T cells overcomes low levels of tumor antigen expression. Mol Ther 17:1779–1787
Rainusso N, Brawley VS, Ghazi A et al (2012) Immunotherapy targeting HER2 with genetically modified T cells eliminates tumor-initiating cells in osteosarcoma. Cancer Gene Ther 19:212–217
DeRenzo C, Gottschalk S (2014) Genetically modified T-cell therapy for osteosarcoma. Adv Exp Med Biol 804:323–340
Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G (2017) Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 17:97–111
Gupta A, Probst HC, Vuong V et al (2012) Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol 189:558–566
Sharma A, Bode B, Studer G et al (2013) Radiotherapy of human sarcoma promotes an intratumoral immune effector signature. Clin Cancer Res 19:4843–4853
Dewan MZ, Galloway AE, Kawashima N et al (2009) Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 15:5379–5388
Seetharam S, Staba MJ, Schumm LP et al (1999) Enhanced eradication of local and distant tumors by genetically produced interleukin-12 and radiation. Int J Oncol 15:769–773
Rech AJ, Dada H, Kotzin JJ et al (2018) Radiotherapy and CD40 activation separately augment immunity to checkpoint blockade in cancer. Cancer Res 78:4282–4291
Qu C, Ping NN, Wu Q et al (2018) Radiotherapy priming chimeric antigen receptor T cell therapy is a safe and promising approach in relapsed/refractory diffuse large B cell lymphoma patients with high tumor burden. In: 60th American Society of Hematology Annual Meeting and Exposition, San Diego
Coomer A, Farese J, Milner R, Liptak J, Bacon N, Lurie D (2009) Radiation therapy for canine appendicular osteosarcoma. Vet Comp Oncol 7:15–27
McEntee MC (1997) Radiation therapy in the management of bone tumors. Vet Clin North Am Small Anim Pract 27:131–138
Takahashi Y, Yasui T, Tamari K et al (2017) Radiation enhanced the local and distant anti-tumor efficacy in dual immune checkpoint blockade therapy in osteosarcoma. PLoS One 12:e0189697
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mason, N.J. (2020). Comparative Immunology and Immunotherapy of Canine Osteosarcoma. In: Kleinerman, E., Gorlick, R. (eds) Current Advances in the Science of Osteosarcoma. Advances in Experimental Medicine and Biology, vol 1258. Springer, Cham. https://doi.org/10.1007/978-3-030-43085-6_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-43085-6_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-43084-9
Online ISBN: 978-3-030-43085-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)