Abstract
This chapter provides a summary of key landmark clinical trials in ophthalmology and guidelines from the Royal College of Ophthalmologists (RCOphth). Additionally, information on Creutzfeldt-Jakob disease (CJD) and laser safety in hospitals is provided. The principles of screening with reference to the NHS diabetic eye screening programme is also provided.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Steroids
-
Mechanisms of action
-
Reduction of inflammation by corticosteroids is via inhibition of phospholipase A2, thereby blocking the production of prostaglandins and leukotrienes
-
Corticosteroids have an immunosuppressive role via inhibition of NF-kB transcription factor signaling, thereby blocking the production of IL-2 and other pro-inflammatory cytokines
-
-
Clinical applications of intravitreal corticosteroids
-
Ozurdex (Dexamethasone 700 μg intravitreal implant):
-
NICE Guidance [TA349]: option for treatment of DMO if eye is pseudophakic and CSMO does not respond to non-corticosteroid treatment or such treatment is unsuitable
-
NICE Guidance [TA229]: recommend as an option for treatment of macular oedema due to a CRVO or a BRVO when treatment with laser photocoagulation has not been beneficial or treatment with laser photocoagulation is not considered suitable because of the extent of retinal haemorrhages
-
NICE Guidance [TA460]: recommended as an option for treating non-infectious uveitis in the posterior segment of the eye in adults only if there is active disease (current inflammation in the eye) and worsening vision with a risk of blindness
-
Clinical trials:
-
MEAD study (Boyer et al. 2014): diabetic macular oedema
-
GENEVA study (Haller et al. 2010): retinal vein occlusion (BRVO/CRVO)
-
HURON study (Lowder et al. 2011): non-infectious posterior uveitis
-
-
-
Iluvien (Flucinolone Acetonide 170 μg):
-
NICE Guidance [TA301]: option for the treatment of chronic diabetic macular oedema that is insufficiently responsive to available therapies if an eye is pseudophakic
-
NICE Guidance [TA590]: option for preventing relapse in recurrent non-infectious uveitis affecting the posterior segment of the eye
-
Clinical trials:
-
FAME study (Cunha-Vaz et al. 2014): diabetic macular oedema
-
PSV-FAI-001 Study (NICE Guidance [TA590]): non-infectious posterior uveitis
-
-
-
-
Monitoring
-
Pre-treatment:
-
BP, glucose, weight
-
CXR if there is any possibility of TB
-
-
During treatment:
-
BP, glucose, weight every 3 months
-
Lipids every year
-
Bone density (DXA scan) if steroid course ≥3 months
-
-
-
Side-effects of corticosteroids
-
Ocular:
-
Glaucoma
-
Cataracts — posterior subcapsular cataracts
-
Microbial keratitis
-
-
Systemic:
-
Endocrine:
-
Cushing’ syndrome
-
Adrenal suppression — risk of Addisonian crisis with withdrawal
-
Weight gain
-
-
GI:
-
Peptic ulcer
-
Pancreatitis
-
-
Musculoskeletal:
-
Osteoporosis
-
Osteopenia
-
-
Skin:
-
Hirsutism
-
-
Haematological:
-
Immunosuppression
-
-
Psychiatric:
-
Insomnia
-
Psychosis
-
-
Neurological:
-
Raised ICP ± papilloedema
-
-
-
-
Prophylaxis of corticosteroid-induced osteoporosis:
-
Risk assessment (NICE Guidance [CG146]):
-
Consider assessment of fracture risk:
-
In all women aged ≥65 years and all men ≥75 years
-
In women aged under 65 years and men aged under 75 years in the presence of risk factors, e.g. current use or frequent recent use of oral or systemic glucocorticoids, previous fragility fracture, smoking, history of falls, family history of hip fracture, BMI <18.5 kg/m2
-
-
Tools for risk assessment (see Table 5.1):
-
Use either FRAX (without a BMD value if DXA scan has not been previously undertaken) or QFracture to estimate 10-year predicted absolute fracture risk of fracture. Above the upper age limits defined by the tools, consider people to be at high risk. Which computes the 10-year probability of hip fracture or a major osteoporotic fracture (spine, hip, forearm, or humerus fracture)
-
Following risk assessment with FRAX (without a BMD value) or QFracture, consider measuring BMD with DXA in people whose fracture risk is in the region of an intervention threshold for a proposed treatment, and recalculate absolute risk using FRAX with the BMD value
-
Do not routinely measure BMD with DXA to assess fracture risk without prior assessment using FRAX (without a BMD value) or QFracture
-
Measure bone mineral density (BMD) with DXA to assess fracture risk in people aged under 40 years who have a major risk factor, e.g. history of multiple fragility fractures, major osteoporotic fracture, or current or recent use of high-dose oral or high-dose systemic glucocorticoids (more than 7.5 mg prednisolone or equivalent per day for 3 months of longer)
-
-
-
Treatment (Compston et al. 2017):
-
Women and men age ≥70 years with a previous fragility fracture, or taking high doses of glucocorticoids (≥7.5 mg/day prednisolone) should be considered for bone protective therapy
-
In other individuals fracture probability should be estimated using FRAX with adjustment for glucocorticoid dose. FRAX assumes an average dose of prednisolone (2.5–7.5 mg/day or its equivalent) and may overestimate fracture risk in those taking lower doses and underestimate fracture risk in patients taking higher risks
-
Bone-protective treatment should be started at the onset of glucocorticoid therapy in individuals at high risk of fracture.
-
Adequate calcium intake should be achieved through dietary intake if possible, with the use of supplementation if required. An adequate vitamin D status should be maintained, using supplements if required.
-
Alendronate (see Table 5.2) and risedronate are first line treatment options. Where these are not tolerated, zoledronic acid, teriparatide or denosumab are alternative options
-
If glucocorticoid therapy is stopped, withdrawal of bone protective therapy may be considered, but if glucocorticoids are continued long term, bone protection should be maintained in the majority of cases
-
Bone protective therapy may be appropriate in some premenopausal women and younger man, particularly in individuals with a previous history of fracture or receiving high doses of glucocorticoids
-
-
-
Prophylaxis of GI side effects:
-
Higher doses of corticosteroids
-
History of GI disease
-
Co-administration of NSAIDs (avoid if possible)
-
-
Withdrawal of corticosteroids:
-
Tapering of corticosteroids is required if there is a risk of adrenal suppression:
-
Daily dose has been >40 mg/day prednisolone (or equivalent)
-
Duration has been >3 weeks
-
Frequency has been >1×/day
-
There have been other courses recently, or long-term steroid administration within the last year
-
-
-
5 mg prednisolone is equivalent to: Dexamethasone 750 μg, Betamethasone 750 μg, Methylprednisolone 4 mg, Triamcinolone 4 mg, Hydrocortisone 20 mg
2 Trials in Glaucoma Involving Trabeculectomy
2.1 Collaborative Initial Glaucoma Treatment Study (Lichter et al. 2001)
-
Primary outcome: A RCT to determine whether patients with newly diagnosed OAG are best treated by initial treatment with topical medications or by immediate trabeculectomy
-
Methods:
-
Inclusion criteria: newly diagnosed open angle glaucoma (POAG, PXF glaucoma, pigmentary glaucoma); one of three combinations of qualifying IOP (IOP ≥20 mmHg), VF changes, and optic disc findings; BCVA of 20/40 or better in both eyes; age 25–75 years; no prior ocular surgery (laser, refractive, conjunctival, intraocular); little (≤14 cumulative days of topical therapy) or no prior treatment of glaucoma
-
Exclusion criteria: use of glaucoma medication >14 cumulative days; CIGST VF score >16 in either eye; ocular disease that might affect measurement of IOP, VA, or VF; undergone ophthalmic laser, refractive, conjunctival, or intraocular surgery in either eye; PDR, DMO, or NPDR with >10 MA’s by clinical count; current or expected chronic use of corticosteroids; likely require cataract surgery within 1 year of randomisation
-
Groups: topical medication group — escalating drops, if further treatment was required start with ALT, then trabeculectomy ± 5-FU, drops, then trabeculectomy + anti-fibrotic agent, then medication/trabeculectomy group — trabeculectomy ± 5-FU, if further treatment was required start with ALT, then escalating drops, then repeat trabeculectomy + anti-fibrotic agent, then medication
-
Primary endpoint: increasing CIGST VF score (0–20) reflecting increased VF loss
-
Secondary endpoints: change in VA, change in IOP, occurrence of cataract extraction, QOL (questionnaire)
-
Follow up: 5 years (initial report)
-
-
Results: 607 patients
-
Primary endpoint: no significant difference in VF scores at 5 years in both groups
-
Secondary endpoints: initial decrease in VA in the trabeculectomy group that was not observed in the topical medication group and resulted in lower mean VA in the trabeculectomy group, that persisted through 3.5 years after surgery. After that time, mean VA levels were comparable in the two treatment groups up to 5 years of follow up (VA less in trabeculectomy group compared to topical medications group); there were no significant differences in the QOL between the two groups; both groups had significantly decreased mean IOP after treatment initiation (3 mmHg better reduction with trabeculectomy) although the amount of decrease was greater in the trabeculectomy group (48% in trabeculectomy group vs 35% in the topical medication group), and the difference was maintained over 5 years of observation; the trabeculectomy group had a higher cataract extraction probability over time compared to the topical medications group
-
Risk factors for VF progression: older age, non-white race, DM, development of cataract, maximal IOP, IOP fluctuation between visits
-
-
Conclusion of study: CIGTS clinical outcomes do not suggest a change in the way ophthalmologists currently manage their patients with newly diagnosed OAG
2.2 Advanced Glaucoma Intervention Study (The AGIS Investigators 1998, 2001)
-
Primary outcome: A RCT that assessed the effects of two surgical intervention sequences in patients with advanced POAG after the failure of medical therapy.
-
Methods:
-
Inclusion criteria: eyes with either advanced (defined as glaucoma that can no longer be controlled adequately despite maximum tolerated medical therapy in the presence of some glaucomatous VF defect) POAG without previous surgery or advanced POAG in a phakic eye 4 weeks or more after PI, phakic VA better than 20/80 [6/24]), age 35–80 years old, reproducible glaucomatous VF defects in at least one eye, a table of specific combinations of elevated IOP and VF defect (range of very mild to severe) was used to define uncontrolled glaucoma and was used to determine if a second or third operation was required
-
Exclusion criteria: secondary glaucoma or congenital angle anomalies, other active eye diseases particularly those that cause field of loss or previous surgery (except PI or localised retinopexy)
-
Groups: A-T-T group: ALT followed if necessary by trabeculectomy, followed if necessary by repeat trabeculectomy / T-A-T group: trabeculectomy followed if necessary by ALT, followed if necessary by repeat trabeculectomy
-
Primary endpoints: VA and/or VF (score 0 normal to-20 blind)
-
Follow up: 7 years (initial report)
-
-
Results: 332 black patients, 249 white patients, 10 patients of other races
-
Low post intervention IOP is associated with reduced progression of VF defect
-
Predictive analysis (IOP averaged over the first three 6-month visits — designed to assess whether IOP during early follow up is predictive of subsequent change from baseline in VF defect score): Initial mean IOP <14 mmHg over the first 18 months after surgery had a mean VF score deterioration of less than 1 point from baseline and those with an initial IOP ≥18 mmHg had a mean score deterioration of three points over 7 years
-
Associative analysis (% of visits over the first 6 years of follow up for which an eye presented with IOP <18 mmHg): IOP <18 mmHg on 100% of follow up visits over 6 years resulted in a mean score deterioration of close to zero, but those achieving IOP <18 mmHg on <100% of visits had a mean deterioration of two to three points
-
-
After 7 years of follow-up, overall (in both black and white patients) the mean decrease in IOP from baseline is greater in eyes assigned to T-A-T than in those assigned to A-T-T
-
In white patients, VF was better preserved by T-A-T only after the first year of follow-up and thereafter favour the A-T-T sequence, and acuity was better preserved by A-T-T throughout follow up.
-
For black patients, the VF and acuity loss was less for eyes in the A-T-T sequence
-
Complications of trabeculectomy: relative risk of cataract in the 5 years after trabeculectomy was 1.78 compared to those participants who avoided trabeculectomy. Youth and high IOP were key risk factors for failure of either ALT or trabeculectomy. DM or persistent postop inflammation were also significant risk factors for trabeculectomy failure
-
-
Conclusion of study: Low IOP reduces risk of VF progression. Data supports the use of the A-T-T sequence for all black patients. For white patients the data supports the use of the T-A-T sequence
2.3 Tube Versus Trabeculectomy (TVT) Study (Gedde et al. 2012)
-
Primary outcome: A RCT designed to prospectively compare the safety and efficacy of tube shunt surgery and trabeculectomy with mitomycin C (MMC 0.4 mg/ml) in eyes with prior ocular surgery (cataract extraction with IOL implantation or failed trabeculectomy) with uncontrolled glaucoma
-
Methods:
-
Inclusion criteria: age 18–85 years; previous trabeculectomy and/or cataract extraction with IOL implantation; IOP ≥18 mmHg and ≤40 mmHg on maximum tolerated medical therapy
-
Exclusion criteria: NPL vision; pregnant or nursing women; active NVI or proliferative retinopathy; ICE syndrome; aphakia; epithelial or fibrous downgrowth; vitreous in the AC for which a vitrectomy was anticipated; chronic or recurrent uveitis; severe posterior blepharitis; previous cyclodestructive procedure; prior scleral buckling procedure; presence of silicone oil; conjunctival scarring precluding a superior trabeculectomy; unwillingness to discontinue contact lens use after surgery
-
Groups: 350 mm2 Baerveldt glaucoma implant group/trabeculectomy + MMC group
-
Endpoints: IOP, VA, use of supplemental medical therapy, surgical complications, visual fields, failure (IOP >21 mmHg or less than 20% reduction below baseline on two consecutive follow up visits after 3 months, IOP ≤5 mmHg on two consecutive follow up visits after 3 months, reoperation for glaucoma — additional glaucoma surgery requiring a return to the OR, loss of light perception vision)
-
Follow up: 5 years
-
-
Results: 212 eyes of 212 patients
-
IOP reduction: mean IOP was similar between the two treatment groups at 5 years (14.3 mmHg in the tube group vs 13.6 mmHg in the trabeculectomy group)
-
Use of supplemental medical therapy: no significant difference in the mean number of supplemental medications between treatment groups at 5 years
-
Failure rate: a significantly higher failure rate was seen in the trabeculectomy group than the tube group at 5 years (33% in the tube group vs 50% in the trabeculectomy group)
-
Reoperation for glaucoma: a significantly higher rate of reoperation for glaucoma was observed in the trabeculectomy group compared with the tube group at 5 years (9% in the tube group vs 29% in the trabeculectomy group)
-
-
Conclusion of study: Tube shunt surgery had a higher success rate compared to trabeculectomy with MMC at 5 years. Both procedures were associated with similar IOP reductions and use of supplemental medical therapy at 5 years. Additional glaucoma surgery was needed more frequently after trabeculectomy with MMC than tube shunt surgery
2.4 Primary Tube Versus Trabeculectomy (PTVT) Study (Gedde et al. 2018)
-
Primary outcome: A RCT designed to prospectively compare the safety and efficacy of tube shunt surgery and trabeculectomy with mitomycin C (MMC 0.4 mg/ml) in eyes with no prior incisional ocular surgery with uncontrolled glaucoma
-
Methods:
-
Inclusion criteria: age 18–85 years; previous trabeculectomy and/or cataract extraction with IOL implantation; IOP ≥18 mmHg and ≤40 mmHg on maximum tolerated medical therapy
-
Exclusion criteria: NPL vision; pregnant or nursing women; active NVI or proliferative retinopathy; ICE syndrome; aphakia; epithelial or fibrous downgrowth; vitreous in the AC for which a vitrectomy was anticipated; chronic or recurrent uveitis; severe posterior blepharitis; previous cyclodestructive procedure; prior scleral buckling procedure; presence of silicone oil; conjunctival scarring precluding a superior trabeculectomy; unwillingness to discontinue contact lens use after surgery
-
Groups: 350 mm2 Baerveldt glaucoma implant group/trabeculectomy + MMC group
-
Endpoints: IOP, VA, use of supplemental medical therapy, surgical complications, visual fields, failure (IOP >21 mmHg or less than 20% reduction below baseline on two consecutive follow up visits after 3 months, IOP ≤5 mmHg on two consecutive follow up visits after 3 months, reoperation for glaucoma — additional glaucoma surgery requiring a return to the OR, loss of light perception vision)
-
Follow up: 1 year
-
-
Results: 242 eyes of 242 patients
-
IOP reduction: mean IOP was significantly lower in the trabeculectomy group at 1 year (13.8 mmHg in the tube group vs 12.4 mmHg in the trabeculectomy group)
-
Use of supplemental medical therapy: a significantly lower mean number of supplemental glaucoma medications was used in the trabeculectomy group at 1 year (2.1 in the tube group vs 0.9 in the trabeculectomy group)
-
Failure rate: a significantly higher failure rate was seen in the tube group than the trabeculectomy group at 1 year (17.3% in the tube group vs 7.9% in the trabeculectomy group)
-
Reoperation for glaucoma: a significantly higher rate of reoperation for glaucoma was observed in the trabeculectomy group compared with the tube group at 1 year (1% in the tube group vs 7% in the trabeculectomy group)
-
-
Conclusion of study: Trabeculectomy + MMC had a higher surgical success rate than tube shunt surgery at 1 year. Lower IOP with use of fewer glaucoma medications was achieved after trabeculectomy + MMC compared with tube shunt surgery at 1 year. Additional glaucoma surgery was needed more frequently after trabeculectomy with MMC than tube shunt surgery
3 The RCOphth Guideline on Standards for the Retrieval of Human Ocular Tissue Used in Transplantation, Research and Training 2008
-
Eyebanks
-
Four in the UK: Moorfields, East Grinstead, Manchester, Bristol
-
Two Corneal Transplant Service (CST): Bristol, Manchester
-
-
Consent
-
If a person has expressed a wish to be an eye donor, for example through the National Organ Donor Register or in a will, that consent is paramount and cannot be overridden by relatives
-
In the absence of prior consent given by a potential donor, consent may be given by a nominated representative of the donor or by a person in a qualifying relationship/nearest relative
-
Inform relatives that not every cornea will be suitable for transplantation, but that suitability cannot be determined before the eyes have been collected
-
Consent should also be obtained for a sample of the donor’s blood to be taken for the testing of viral and other microbiological markers of transmissible disease
-
Relatives should also be asked for their permission to seek further information about a donor’s medical history and behavioural background from the donor’s medical records, GP and other relevant healthcare professionals
-
Research consent using a separate consent/authorisation form
-
Strongly recommended good practice that consent is recorded by a specially trained healthcare professional, such as a transplant or tissue coordinator, using the NHSBT Consent/Authorisation forms and Management Process Document or their equivalent
-
-
Donor age
-
Upper age:
-
Currently no need to set an upper age limit for eye donation
-
Corneal endothelium is to be carefully examined by microscopy before transplantation to exclude those corneas with low endothelial cell densities endothelial damage, or other abnormalities
-
-
Lower age:
-
There will be very little demand for corneas from donors under 3 years old
-
-
-
Post-mortem time
-
Enucleation can be performed up to 24 h post-mortem time after a donors death
-
Blood sample must be taken within 24 h of a donors death
-
-
Medical and behavioural history
-
Sources of information about donors:
-
Hospital medical records
-
Consultant/Senior Nursing Staff with clinical responsibility for the deceased
-
Family/most relevant life partner
-
GP
-
Post-mortem examination request form
-
-
NHSBT assessment form used to record the family/partner interview
-
NHSBT GP form used to obtain information from the donors GP
-
Check for medical contraindications for donation and transplantation of ocular tissue
-
-
Eye retrieval
-
Eye retrievers:
-
Must be carried out by a person who is competent in enucleation
-
Check:
-
Consent/authorization has been obtained
-
All relevant sources of medical information has been checked
-
-
-
NHS Blood Transport (NHSBT) Human Tissue Transport box:
-
Contains:
-
A set of sterile, single-use instruments with a paper wrapper for use as a drape
-
Blood sample tube
-
Alcohol swabs for cleaning the skin around the eyes and the eyelids
-
Sterile saline for irrigating eyes
-
Sterile pots, 25G needles, eye stands, cotton balls and saline for creating moist chambers
-
Eye caps and cotton balls for restoring the donor’s appearance
-
Enucleation protocol, list of medical contraindications, NHSBT Ocular Tissue Donor Information and Retrieval Site Risk Assessment forms
-
-
Additional required items not included in the transport box:
-
At least 1 kg of ice is needed to keep the contents of the transport box below 5 °C for up to 24 h during transportation to the eye bank
-
10 ml syringe and 19G needle for taking the blood sample
-
Sterile gloves and appropriate protective clothing
-
-
-
Retrieval site risk assessment:
-
A requirement that a risk assessment is carried out to ensure that the retrieval site is suitable and appropriate for the removal of tissue from a deceased donor
-
-
Donor identification:
-
In hospitals and hospices, the donor should be identified by the wrist or ankle tag using name, DOB, hospital number and any other available identifiers
-
Strongly recommended good practice for identification of the donor to be confirmed by the eye retriever and another person
-
-
Physical examination of the donor:
-
Examine those parts of a donor’s body that are readily accessible, noting the areas examined and findings such as tattoos, piercings and scars on the body map provided on the NHSBT Ocular Tissue Donor information form
-
-
Blood sample:
-
If the mandatory blood tests for transmissible disease are not carried out locally, a sample of the donors blood must be sent to the eye bank with the donor’s eyes
-
If an ante-mortem blood sample taken not more than 7 days before death is not available, a blood sample should be taken from the deceased as soon after death as possible and not more than 24 h after death
-
-
Enucleation:
-
A standard enucleation protocol, such as that provided in the NHSBT Human Tissue Transport Box should be followed
-
Carefully transfer the eye to a plastic eye stand, passing the stump of the optic nerve through the hole in the base of the stand. Secure the eye on the stand by placing a sterile 25G hypodermic needle through the side of the optic nerve. Place the eye stand and eye (cornea uppermost) on top of a cotton wool ball moistened with saline in a sterile pot (moist chamber). The eye must not be immersed in any liquid in the moist chamber
-
-
Restoring the donor’s appearance:
-
Orbits should be packed with cotton wool and the lids closed over plastic eye caps to restore the original profile of the lids
-
-
Packaging, labeling and transport to a Corneal Transplant Service (CTS) eye bank:
-
Labelling:
-
Essential that the moist chambers and the blood sample tube are clearly and correctly labelled with the date, donor’s name, DOB and at least one other identifier (e.g. hospital name)
-
-
Packaging:
-
Eyes must be packed in an NHSBT Human Tissue Transport Box with the blood sample, NHSBT Retrieval Site Risk Assessment form, an NHSBT Ocular Tissue Donor form completed to the best of the eye retrievers knowledge, and any other information that may be available at the time such as a consent form, a medical history check list, or an NHSBT GP form
-
Box must be packed according to the instructions provided, including at least 1 kg of ice to ensure correct maintenance of temperature during transport
-
-
Transport:
-
Box should be closed using the supplied tamper-evident security tag
-
Eye retriever should contact UK Transplant (UKT) when the eyes are ready for collection, providing specific details of the location and reporting the security tag number
-
UKT will specify the eye bank address, which should then be clearly written on the label provided and attached to the side of the box
-
The eyes must be kept at a secure location until they are collected
-
-
-
-
Contraindications to ocular tissue transplantation
-
Infections:
-
HIV/AIDS
-
Viral hepatitis (A-C)
-
TB
-
HTLV
-
Syphilis
-
Septicaemia
-
Congenital rubella
-
Rabies
-
Behaviour leading to risk of contracting HIV, hepatitis or HTLV
-
Tattoos and body piercing within the 6 months before death
-
Acupuncture within the 6 months before death
-
Imprisonment within the 12 months before death
-
-
Previous surgery/medical treatment:
-
Immunosuppression
-
Receipt of an organ transplant
-
Receipt of dura mater or brain/spinal surgery before August 1992
-
Receipt of human pituitary hormones
-
Receipt of a cornea, sclera or other human tissue allograft
-
-
Unknown aetiology and CNS disorders:
-
Death from unknown cause
-
CJD, Alzheimer’s disease, Parkinson’s disease, MS, motor neurone disease
-
-
Malignancies:
-
Leukaemia
-
Lymphoma
-
Myeloma
-
Polycythaemia Ruba Vera
-
Myelodysplastic syndrome
-
-
Intrinsic eye disease:
-
Active ocular inflammation/uveitis
-
Any congenital or acquired disorders of the eye, or previous ocular surgery (including corneal laser surgery), that would preclude successful graft outcome
-
Retinoblastoma
-
Malignant tumours of the anterior segment
-
-
4 Recent Pivotal Age-Related Macular Degeneration Clinical Trials
4.1 ANCHOR Study (Brown et al. 2006)
-
Primary outcome: To compare ranibizumab with photodynamic therapy with verteporfin (vPDT) in the treatment of predominantly classic neovascular AMD
-
Methods:
-
Groups — 0.3 mg ranibizumab + sham vPDT group, 0.5 mg ranibizumab + sham vPDT group, sham injections + active vPDT group. Injections were administered monthly and vPDT (sham or active) was administered at day 0 and then if needed on the basis of investigator’s evaluation of angiography at 3, 6, 9 and 12 months
-
Primary endpoint — proportion of patients losing fewer than 15 letters from baseline VA at 12 months
-
Secondary endpoints — structural outcomes on fluorescein angiography
-
Follow up — 12 months
-
-
Results — 423 patients
-
Primary endpoint
-
94.3% of patients in the 0.3 mg ranibizumab group and 96.4% in the 0.5 mg ranibizumab group lost fewer than 15 letters from baseline VA, as compared with 64.3% in the vPDT group
-
The proportion of patients whose VA improved from baseline by 15 or more letters was significantly greater among those receiving ranibizumab treatment (35.7% in the 0.3 mg ranibizumab group and 40.3% in the 0.5 mg ranibizumab group, as compared with 5.6% in the vPDT group)
-
Significantly greater proportions of ranibizumab-treated patients than patients in the vPDT group had VA of 20/40 or better and smaller proportions had VA of 20/200 or worse
-
A severe loss of vision (defined as decrease of 30 letters or more) did not occur in any patient in the ranibizumab groups but occurred in 13.3% of patients in the vPDT group
-
At 12 months, 7.1% of patients in the 0.3 mg ranibizumab group and 6.4% of patients in the 0.5 mg ranibizumab group had VA of 20/20 or better, as compared with 0.7% of patients in the vPDT group
-
-
Secondary endpoints
-
At 12 months, the area occupied by classic CNV decreased by a mean of 0.52 optic disc area in the 0.3 mg ranibizumab group and 0.67 optic disc area in the 0.5 mg ranibizumab group, as compared with a mean increase of 0.54 optic disc area in the vPDT group
-
The area of leakage from CNV plus intense, progressive staining of the RPE at 12 months decreased by a mean of 2.05 optic disc area in the 0.5 mg ranibizumab group and 1.80 optic disc area in the 0.3 mg ranibizumab group, as compared with a mean increase of 0.32 optic disc area in the vPDT group
-
-
-
Conclusion of study: Ranibizumab was superior to vPDT as treatment of predominantly classic neovascular AMD
4.2 MARINA Study (Rosenfeld et al. 2006)
-
Primary outcome: To evaluate ranibizumab for the treatment of minimally classic or occult with no classic CNV associated with AMD
-
Methods:
-
Groups — 0.3 mg ranibizumab group, 0.5 mg ranibizumab group, sham injection. Injections were administered monthly for 2 years
-
Primary endpoint — proportion of patients who had lost fewer than 15 letters from baseline VA
-
Secondary endpoint — structural outcomes on fluorescein angiography
-
Follow up — 2 years
-
-
Results — 716 patients
-
Primary endpoints
-
At 12 months, 94.5% of the patients receiving 0.3 mg ranibizumab and 94.6% of the patients receiving 0.5 mg ranibizumab had lost fewer than 15 letters from baseline VA, as compared with 62.2% in the sham-injection group
-
At 24 months, 92% of the patients receiving 0.3 mg ranibizumab and 90% of the patients receiving 0.5 mg ranibizumab had lost fewer than 15 letters from baseline VA, as compared with 52.9% in the sham-injection group
-
At 12 and 24 months, approximately 25% of patients treated with 0.3 mg ranibizumab and 33% of patients treated with 0.5 mg ranibizumab had gained 15 or more letters in VA, as compared with 5% or less of those in the sham-injection group
-
At 12 months, mean increases in VA were 6.5 letters in the 0.3 mg ranibizumab group and 7.2 letters in the 0.5 mg ranibizumab group, as compared with a decrease of 10.4 letters in the sham-injection group. The benefit in VA was maintained at 24 months
-
At 12 months, approximately 40% of patients receiving ranibizumab had 20/40 vision or better, as compared with 11.3% in the sham-injection group. At 24 months, of the patients receiving ranibizumab, 34.5% of those in the 0.3 mg ranibizumab group and 42.1% in the 0.5 mg ranibizumab group had at least 20/40 vision, whereas the proportion in the sham injection group had dropped to 5.9%
-
Among patients receiving ranibizumab, 3.8% in the 0.3 mg ranibizumab group and 7.9% in the 0.5 mg ranibizumab group had 20/20 vision or better at 24 months. In the sham injection group, 0.8% of patients had 20/20 vision or better at 12 months and 0.4% of patients had 20/20 vision or better at 24 months
-
-
Secondary endpoints
-
Ranibizumab treatment was associated with arrested growth of and leakage from CNV
-
-
-
Conclusion of study: Intravitreal administration of ranibizumab for 2 years prevented vision loss and improved mean VA in patients with minimally classic or occult with no classic CNV secondary to AMD
4.3 PrONTO Study (Fung et al. 2007)
-
Primary outcome: To evaluate an OCT-guided, variable-dosing regimen with intravitreal ranibizumab for the treatment of patients with neovascular AMD (eligibility — BCVA 20/40 to 20/400 in the study eye and OCT central retinal thickness ≥300 μm)
-
Methods:
-
Groups — all patients received intravitreal injections of ranibizumab at baseline, month 1, and month 2. Additional reinjections were given if: (1) VA loss of at least 5 letters with OCT evidence of fluid in the macula, (2) an increase in OCT central retinal thickness ≥100 μm, (3) new macular haemorrhage, (4) new area of classic CNV, or (5) evidence of persistent fluid on OCT at least 1 month after the previous injection
-
Primary endpoints — change in VA and OCT measurements from baseline
-
Secondary endpoints — number of consecutive monthly injections required from baseline to achieve a fluid-free macula as determined by OCT
-
Follow up — 12 months
-
-
Results — 40 patients
-
Primary endpoints:
-
At 12 months, the mean and median VA scores improved compared with baseline by 9.3 letters and 11 letters, respectively
-
At 12 months, the mean and median central retinal thickness measurements decreased by 177.8 and 185.5 μm, respectively
-
-
Secondary endpoints
-
The mean number of injections for the first year were 5.6 (SD 2.3) and 5.0 (range, 3–13), respectively, of a possible 13 injections from day 0 through month 12
-
A total of 39 eyes eventually became fluid-free; 37 of these eyes eventually developed some recurrent fluid during the first year. Of the 37 eyes that developed some recurrent fluid, 32 received a retreatment during the first 12 months
-
After the first 3 injections, 7 patients never needed another injection. One eye never became fluid-free and received a total of 13 injections
-
Of the 39 eyes that eventually achieved a fluid-free macula, the mean and median number of monthly consecutive injections from baseline that were required to achieve a fluid-free macula were 1.5 (SD 1.1) and 1.0 (range, 1–6), respectively
-
-
-
Conclusion of study: OCT-guided, variable-dosing regimen with ranibizumab resulted in VA outcomes similar to the phase III clinical trials MARINA and ANCHOR. OCT appears useful for determining when retreatment with ranibizumab is necessary
4.4 PIER Study (Regillo et al. 2008)
-
Primary outcome: To evaluate the efficacy and safety of ranibizumab administered monthly for 3 months and then quarterly in patients with subfoveal CNV secondary to AMD
-
Methods:
-
Groups — 0.3 mg ranibizumab group, 0.5 mg ranibizumab group, sham treatment group. Injections were administered monthly, for the first three doses, followed by three-monthly intervals. Verteporfin photodynamic therapy (vPDT) was permitted at the investigator’s discretion
-
Primary endpoint — mean change from baseline to 12 months in VA score
-
Secondary endpoint — proportion of subjects losing 15 letters or less from baseline; proportion gaining ≥15 letters from baseline; proportion with a Snellen equivalent of 20/200 or worse; mean change from baseline in the near activities, distance activities, and vision-specific dependency NEI VFQ-25 subscales; and mean change from baseline in total area of CNV and total area of leakage from CNV
-
Follow up — 12 months
-
-
Results — 184 patients
-
Primary endpoints
-
At 12 months, sham-treated eyes had lost a mean of 16.3 letters, whereas ranibizumab-treated subjects had lost a mean of 1.6 letters (0.3 mg ranibizumab group) or 0.2 letters (0.5 mg ranibizumab group)
-
On average, there was a 4.5 letter decline in VA between month 3 and month 12 for both ranibizumab dose groups, reflecting the effect of quarterly dosing; these declines were statistically significant
-
-
Secondary endpoints
-
Significantly greater proportions of the ranibizumab groups than the sham group had lost fewer than 15 letters from baseline VA: 83.3% and 90.2% of the 0.3 and 0.5 mg ranibizumab groups, respectively, compared with 49.2% of the sham group
-
The three treatment groups did not differ significantly in the proportions gaining at least 15 letters: 9.5% in the sham group, 11.7% in the 0.3 mg ranibizumab group, and 13.1% in the 0.5 mg ranibizumab group
-
Significant smaller proportions of the ranibizumab groups than the sham group had VA of 20/200 of worse snellen equivalent at month 12: 23.3% and 24.6% of the 0.3 and 0.5 mg ranibizumab groups, respectively, compared with 52.4% of the sham group
-
There was no statistically significant difference between either ranibizumab dose group and the sham control for any of the 3 NEI VFQ-25 subscales that were prespecified as secondary endpoints
-
Ranibizumab reduced the total area of leakage of CNV plus intense progressive RPE staining on average, whereas the sham group exhibited an increase trend
-
-
-
Conclusion of study: Ranibizumab administered monthly for 3 months and then quarterly provided significant VA benefits to patients with AMD-related subfoveal CNV
4.5 The Comparison of Age-Related Macular Degeneration Treatment Trial (The CATT Research Group 2011)
-
Primary outcome — A RCT to assess the relative efficacy and safety of ranibizumab (0.5 mg) and bevacizumab (1.25 mg) and to determine whether an as-needed regimen would compromise long term VA, as compared to a monthly regimen
-
Methods:
-
Groups — ranibizumab monthly group, bevacizumab monthly group, ranibizumab as needed group, bevacizumab as needed group
-
Primary endpoint — mean change in VA between baseline and 1 year
-
Secondary endpoints — proportion of patients with a change in VA of 15 letters or more, the number of injections, the change in fluid and foveal thickness on OCT, change in lesion size on FA, the incidence of ocular and systemic adverse effects
-
Follow up — 1 year
-
-
Results — 1208 patients
-
Primary endpoint
-
Bevacizumab monthly (+8.0 letter) was equivalent to ranibizumab monthly (+8.5 letters)
-
Bevacizumab as needed (+5.9 letters) was equivalent to ranibizumab monthly (+6.8 letters)
-
Ranibizumab as needed was equivalent to monthly ranibizumab
-
Comparison of bevacizumab as needed and bevacizumab monthly was inconclusive
-
-
Secondary endpoints
-
The proportion of patients who did not have a decrease in VA of 15 letters or more from baseline was 94.4% in the ranibizumab monthly group, 94.0% in the bevacizumab monthly group, 95.4% in the ranibizumab as needed group, and 91.5% in the bevacizumab as needed group
-
The proportion of patients who gained at least 15 letters did not differ significantly among the groups, ranging from 24.9% in the group that received ranibizumab as needed to 34.2% in the group that received ranibizumab as needed
-
The proportion of patients with arteriothrombotic events (CVA, MI, death from vascular causes) were similar among the groups
-
The proportion of patients with serious systemic adverse events (hospitalisation from infections, e.g. pneumonia, UTI, GI disorders, e.g. haemorrhage, nausea and vomiting) was higher with bevacizumab (24.1%) than with ranibizumab (19.0%)
-
-
-
Conclusion of study
-
At 1 year, effect on visual acuity of bevacizumab were non-inferior to that ranibizumab when administered according to the same schedule. Ranibizumab given as needed with monthly evaluation had effects on vision that were similar to those of ranibizumab administered monthly.
-
At 2 years, bevacizumab and ranibizumab had similar effects on visual acuity. Treatment as needed resulted in less gain in VA, whether instituted at enrolment or after 1 year of monthly treatment
-
Non-inferiority was not shown between as required bevacizumab and monthly ranibizumab or monthly bevacizumab
-
As required ranibizumab was non-inferior to monthly ranibizumab
-
In order to achieve similar effects, prn bevacizumab needs to be administered more often than prn ranibizumab
-
There was a higher incidence of adverse events associated with bevacizumab compared to ranibizumab
-
4.6 VIEW 1 and VIEW 2 Studies (Heier et al. 2012)
-
Primary outcome: To compare intravitreal aflibercept, monthly or every 2 months, with monthly ranibizumab in treatment of nAMD
-
Methods
-
Groups — 0.5 mg aflibercept every 4 weeks group, 2 mg aflibercept every 4 weeks group, 2 mg aflibercept every 8 weeks after 3 injections at week 0, 4 and 8 group, 0.5 mg ranibizumab every 4 weeks group
-
Primary endpoint — noninferiority (margin of 10%) of the intravitreal aflibercept regimens to ranibizumab in the proportions of patients maintaining vision at week 52 (losing less than 15 ETDRS letters)
-
Secondary endpoint — compare baseline and 52-week data regarding mean change in BCVA; gaining 15 or more letters; change in total National Eye Institute 25-Item Visual Function Questionnaire (NEI VFG-25) score; change in CNV area on fluorescein angiography
-
Follow up — 12 months
-
-
Results — 2419 patients
-
Primary endpoints
-
All aflibercept groups achieved statistical noninferiority compared with monthly ranibizumab in the treatment of CNV secondary to AMD
-
-
Secondary endpoints
-
Similar VA scores across the entire 52-week study for all treatment groups
-
On the basis of the hierarchical testing sequence, only the 2 mg aflibercept every 4 weeks group was statistically superior to ranibizumab, and only in VIEW 1, with a gain of +10.9 versus +8.1 letters
-
In both studies, the proportion of patients gaining 15 or more ETDRS letters from baseline to week 52 was similar in all treatment groups
-
Vision-related quality of life, assessed by the change of total score of the NEI VFQ-25, improved in all groups in both studies
-
All groups demonstrated a comparable decrease in area of active CNV
-
All aflibercept groups in both studies had reductions in central retinal thickness similar to those for monthly ranibizumab as assessed by OCT, with a large and rapid reduction evident by week 4 that was maintained to week 52
-
-
-
Conclusion of study: Intravitreal aflibercept dosed monthly or every 2 months after 3 initial monthly doses produced similar efficacy and safety outcomes as monthly ranibizumab in the treatment of nAMD
4.7 Inhibition of VEGF in Age-Related Choroidal Neovascularisation (IVAN) Trial (Chakravarthy et al. 2013)
-
Primary outcome — A RCT to compare the efficacy and safety of ranibizumab (0.5 mg) and bevacizumab (1.25 mg) to treat neovascular age-related macular degeneration
-
Methods
-
Groups — ranibizumab continuous group, bevacizumab continuous group, ranibizumab discontinuous group, bevacizumab discontinuous group
-
Primary endpoint — BCVA at 2 years
-
Secondary endpoints — near VA, reading index, contrast sensitivity, lesion morphology and metrics from FA and OCTs, adverse events
-
Follow up — 2 years
-
-
Results — 525 patients reached the visit at 2 years
-
Primary endpoint
-
BCVA was similar between ranibizumab and bevacizumab groups and continuous and discontinuous treatment groups.
-
Bevacizumab was neither inferior or non-inferior to ranibizumab
-
Discontinuous regimen was neither inferior or non-inferior to the continuous regimen
-
-
Secondary endpoints
-
Near VA, reading index, and contrast sensitivity did not differ significantly between drug groups
-
Near VA and contrast sensitivity were significantly worse with the discontinuous regimen
-
Mortality was higher at 2 years with discontinuous treatment than continuous treatment
-
-
-
Conclusion of study: ranibizumab and bevacizumab have similar efficacy. Reduction in the frequency of retreatment resulted in a small loss of efficacy irrespective of drug. Safety was worse when treatment was administered discontinuously.
4.8 HAWK and HARRIER (Dugel et al. 2020)
-
Primary outcome – To demonstrate that brolucizumab is noninferior to fixed-dose aflibercept with respect to the change in best corrected visual acuity (BCVA) from baseline to week 48 in patients with neovascular AMD
-
Methods:
-
Groups — HAWK: brolucizumab 3 mg group, brolucizumab 6 mg group, or aflibercept 2 mg group; HARRIER: brolucizumab 6 mg group or aflibercept 2 mg group. For both trials, all treatment arms had three loading injections at weeks 0, 4, and 8 followed by 8 weeks before the next possible treatment. Brolucizumab was injected every 12 weeks (q12w) unless disease activity was identified, resulting in permanent adjustment to 8 weekly injections (q8w). Aflibercept was injected every 8 weeks (q8w)
-
Primary endpoint — mean BCVA change from baseline to week 48
-
Secondary endpoints — BCVA change from baseline averaged over the period of week 36 through week 48 (to account for differences in timing of treatment), q12w treatment status at week 48 (brolucizumab groups only), q12w treatment status at week 48 among eyes with no q8w need during the first q12w cycle (to evaluate the predictive value of the first q12w cycle; brolucizumab groups only), anatomic retinal fluid outcomes
-
Follow up — 48 weeks
-
-
Results — total of 1817 patients (HAWK + HARRIER)
-
Primary endpoint
-
In both trials, each brolucizumab arm demonstrated noninferiority versus aflibercept in least squares (LS) mean BCVA change from baseline to week 48
-
In HAWK, brolucizumab 3 mg — and brolucizumab 6 mg — treated eyes gained +6.1 and +6.6 letters, respectively, versus +6.8 letters among aflibercept-treated eyes
-
In HARRIER, brolucizumab 6 mg-treated eyes gained +6.9 letters versus +7.6 letters among aflibercept-treated eyes
-
-
Secondary endpoints
-
For brolucizumab-treated eyes, the probabilities for exclusively maintaining q12w dosing after loading through week 48 were 49.4% (brolucizumab 3 mg group) and 55.6% (brolucizumab 6 mg group) in HAWK and 51.0% (brolucizumab 6 mg group) in HARRIER
-
Under the condition that a brolucizumab treated eye did not show disease activity during the first q12w interval, the probabilities for remaining on q12w dosing up to week 48 increased to 80.9% (brolucizumab 3 mg group) and 85.4% (brolucizumab 6 mg group) in HAWK and 81.7% (brolucizumab 6 mg group) in HARRIER
-
Anatomic retinal fluid outcomes favoured brolucizumab over aflibercept
-
-
-
Conclusions of study: Brolucizumab was noninferior to aflibercept in visual function at week 48, and >50% of brolucizumab 6 mg treated eyes were maintained on q12w dosing interval through week 48. Anatomic outcomes favoured brolucizumab over aflibercept. Overall safety with brolucizumab was similar to aflibercept
4.9 The Age-Related Eye Disease Study (Age-Related Eye Disease Study Group 2001)
-
Primary outcome: To evaluate the effect of high-dose vitamins C and E, beta carotene, and zinc supplements on AMD progression and visual acuity
-
Methods:
-
Groups — antioxidants alone (500 mg of vitamin C, 400 IU of vitamin E, and 15 mg of beta carotene) group, zinc alone group (80 mg of zinc as zinc oxide and 2 mg of copper as cupric oxide to prevent potential anaemia), combination of anti-oxidants and zinc group, placebo group
-
Primary endpoints — (1) progression to advanced AMD and (2) at least a 15-letter decrease in VA score from baseline
-
Secondary endpoints — development of neovascular AMD, incidence of GA, progression to advanced AMD with an associated VA decrease of at least 15 letters, and worsening of AMD classification in Category 2 (multiple small drusen, single or non-extensive intermediate drusen, pigment abnormalities, or any combination of these, and VA of 20/32 or better in both eyes) participants to Category 3 (absence of advanced AMD in both eyes and at least 1 eye with VA of 20/32 or better with at least 1 large druse, extensive intermediate drusen, or geographic atrophy that did not involve the center of the macula) or 4 (VA of 20/32 or better and no advanced AMD in the study eye, and the fellow eye had either lesions of advanced AMD or VA less than 20/32 and AMD abnormalities sufficient to explain reduced VA) during follow up
-
Follow up — 5 years
-
-
Results — 4757 participants
-
Primary endpoints:
-
Category 2 participants had only a 1.3% probability of progression to advanced AMD by year 5. Category 3 participants had a 18% probability of progression to advanced AMD by year 5. Category 4 participants had a 43% probability of progression to advanced AMD in the fellow study eye at 5 years
-
The estimated probability of progression to advanced AMD was 28% for those assigned to placebo, 23% and 22% for those assigned to antioxidants only and zinc only, respectively, and 20% for those assigned to antioxidants plus zinc
-
Estimates of relative risks derived from odds ratios suggest risk reductions for those taking antioxidants alone or zinc alone of 17% and 21%, respectively. The risk reduction for those taking antioxidants plus zinc was 25%.
-
At 5 years, the estimated probability of at least a 15-letter decrease in VA score from baseline was 29% for those assigned to placebo, 26% for those assigned to antioxidants alone, 25% for those assigned to zinc alone, and 23% for those assigned to antioxidant plus zinc
-
-
Secondary endpoints:
-
A statistically significant benefit of treatment with antioxidants plus zinc compared with placebo was observed for neovascular AMD outcomes in participants in Categories 3 and 4
-
There is no evidence of treatment benefit in delaying the progression of AMD in participants who began the study in Category 2
-
-
-
Conclusions of study: Those with extensive intermediate size drusen, at least 1 large druse, noncentral geographic in 1 or both eyes, or advanced AMD or vision loss due to AMD in 1 eye, and without contraindications such as smoking, should consider taking a supplement of antioxidants plus zinc such as that used in AREDS
4.10 The Age-Related Eye Disease Study 2 (The Age-Related Eye Disease Study 2 (AREDS2) Research Group 2013)
-
Primary outcome: To determine whether adding lutein + zeaxanthin, DHA + EPA, or both to the AREDS formulation decreases the risk of developing advanced AMD and to evaluate the effect of eliminating beta carotene, lowering zinc doses, or both in the AREDS formulation
-
Methods:
-
Groups — lutein + zeaxanthin group, DHA + EPA group, lutein + zeaxanthin and DHA + EPA group, or placebo. All participants were also asked to take the original AREDS formulation or accept a secondary randomisation to four variations of the AREDS formulation, including elimination of beta carotene, lowering of zinc doses, or both
-
Primary endpoint — development of advanced AMD
-
Secondary endpoints — progression to moderate vision loss (three lines or more) from baseline or treatment for choroidal neovascularisation
-
Follow up — 5 years
-
-
Results — 4203 participants
-
Primary endpoint:
-
Kaplan-Meier probabilities of progression to advanced AMD by 5 years were 31% for placebo, 29% for lutein + zeaxanthin, 31% for DHA + EPA, and 30% for lutein + zeaxanthin and DHA + EPA
-
Daily supplementation with lutein + zeaxanthin, DHA + EPA, or lutein + zeaxanthin and DHA + EPA in addition to the original AREDS formulation showed no statistically significant overall effect on progression to advanced AMD or changes in VA
-
There was no apparent effect of beta carotene elimination or lower dose zinc on progression to advanced AMD
-
-
Secondary endpoints:
-
None of the nutrients affected development of moderate or worse vision loss. No apparent effect on vision of eliminating beta carotene and reducing zinc dose was observed
-
-
-
Conclusion of study: Addition of lutein + zeaxanthin, DHA + EPA, or both to the AREDS formulation in primary analyses did not further reduce risk of progression to advanced AMD. However, because of potential increased incidence of lung cancer in former smokers, lutein + zeaxanthin could be an appropriate carotenoid substitute in the AREDS formulation
5 The Optic Neuritis Treatment Trial (ONTT)
-
The ONTT was a randomised trial that evaluated the use of corticosteroids in the treatment of acute optic neuritis
-
Methods — three treatment regimens
-
250 mg of IV methylprednisolone every 6 h for 3 days followed by 1 mg of oral prednisolone per kg of body weight per day for 11 days (IV-methylprednisolone group)
-
1 mg of oral prednisolone per kg per day for 14 days (oral prednisolone group)
-
Oral placebo for 14 days (placebo group)
-
-
Results
-
A 3-day course of pulsed IV treatment with methylprednisolone (250 mg QDS) followed by an 11-day course of oral prednisolone (1 mg/kg) accelerated visual recovery but did not significantly improve visual outcome at 6 months (Beck et al. 1992). However, this regimen temporarily reduced the rate of new demyelinating events over a 6–24 month follow up period (Beck et al. 1993)
-
A regimen of oral prednisolone alone (1 mg/kg for 14 days) did not improve visual outcome at 6 months and was associated with an increased rate of new attacks of optic neuritis (Beck et al. 1992)
-
At 10 years, the probability of developing MS was 22% for patients with no white matter lesions on MRI and 56% for patients with one or more white matter lesions on MRI (Optic Neuritis Study Group 2003)
-
The aggregate cumulative probability of developing MS by the 15-year examination was 50% and was strongly related to the presence of lesions on the baseline MRI (The Optic Neuritis Study Group 2008)
-
At 15 years, the probability of developing MS was 25% for patients with no white matter lesions on MRI and 72% for patients with one or more white matter lesions on MRI (The Optic Neuritis Study Group 2008)
-
Among patients without MS at the 10-year examination, the probability of developing MS by the 15-year examination was 32% when 1 or more baseline lesions were present vs 2% when there were no baseline lesions (The Optic Neuritis Study Group 2008)
-
6 Principles of Screening Applied to the NHS Diabetic Eye Screening Programme
-
Screening is the presumptive identification of unrecognised disease or defect by the application of tests, examinations, or other procedures which can be applied rapidly (Wilson et al. 1968)
-
World Health Organisation (WHO) Criteria for screening (Wilson et al. 1968)
-
Condition:
-
The condition should be an important health problem:
-
Sight threatening DR is an important public health problem
-
In 1990–1991 DR was the leading cause of people registered blind among people of working age in England and Wales (Facey et al. 2002)
-
-
There should be a recognisable latent or early symptomatic stage:
-
Sight threatening DR has a recognisable latent or early symptomatic stage in both type 1 and 2 diabetes (Early Treatment Diabetic Retinopathy Study 1991)
-
-
The natural history of the condition, including development from latent to declared disease, should be adequately understood
-
The natural history of sight-threatening diabetic retinopathy is well understood (Kohner 1991)
-
-
-
Test:
-
There should be a suitable test or examination:
-
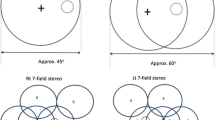
Two field mydriatic digital photography with a sensitivity of 87.8%, specificity of 86.1%, and poor image quality rate of 3.7% (Scanlon et al. 2003a, b)
-
-
The test should be acceptable to the population
-
Two field mydriatic digital photography (Scanlon et al. 2003b)
-
-
Facilities for diagnosis and treatment should be available
-
Hospital Eye Service (HES)
-
-
-
Treatment:
-
There should be an acceptable treatment for patients with recognised disease:
-
PRP is of benefit in preventing severe visual loss in eyes with PDR (The Diabetic Retinopathy Study Research Group 1981)
-
Focal photocoagulation of CSMO is of benefit in reducing moderate visual loss (Early Treatment Diabetic Retinopathy Study 1991)
-
-
There should be an agreed policy on whom to treat as patients
-
All patients with PDR and CSMO require treatment (The Diabetic Retinopathy Study Research Group 1981; Early Treatment Diabetic Retinopathy Study 1991)
-
-
-
Cost:
-
The cost of case finding (including diagnosis and treatment of patients diagnosed) should be economically balanced in relation to possible expenditure on medical care as a whole:
-
The costs of screening and effective treatment of sight threatening DR are balanced economically in relation to total expenditure on health care including the consequences of leave the disease untreated (Scanlon 2008)
-
-
-
Process:
-
Case finding should be a continuing process and not a “once and for all” project
-
Annual eye examination for DR is offered to all people with diabetes over the age of 12
-
-
-
7 Public Health England NHS Diabetic Eye Screening Programme (DESP) (Table 5.3)
7.1 NHS DESP Grading Definitions for Diabetic Retinopathy
-
R0: no retinopathy
-
R1 (background DR/mild NPDR):
-
Venous loop
-
Microaneurysms
-
Retinal haemorrhage
-
Any exudate or cotton wool spot in presence of other non-referable DR features
-
-
R2 (pre-proliferative DR/moderate NPDR):
-
Venous beading
-
Venous reduplication
-
Multiple blot haemorrhages (if uncertain, refer only in the presence of IRMA that are definitely seen)
-
Blot haemorrhages (located in OPL and INL) are larger than the width of the smallest of the four branches of the central retinal vein as it crosses the edge of the disc
-
-
IRMA (check that they can still be seen on the colour image as well as the red-free image that has not been enlarged)
-
-
R3 (proliferative DR):
-
R3A (active PDR):
-
NVD
-
NVE
-
Pre-retinal or vitreous haemorrhage
-
Pre-retinal fibrosis ± tractional RD
-
-
R3S (stable treated PDR):
-
Evidence of peripheral laser retinal treatment AND
-
Stable retina with respect to reference images taken at or shortly after discharge from the hospital eye service (HES)
-
-
7.2 NHS DESP Grading Definitions for Diabetic Maculopathy
-
Macula is defined as that part of the retina which lies within a circle centered on the centre of the fovea whose radius is the distance between the centre of the fovea and the temporal margin of the disc
-
M0
-
No maculopathy
-
-
M1:
-
Exudate within 1 DD of the centre of the fovea
-
Circinate or group of exudates within the macula:
-
A group of exudates is an area of exudates that is greater than or equal to half the disc area and this area is all within the macular area
-
To work out the area, the outer points of the exudates are joined and compared to half the area of the optic disc
-
-
Any microaneurysm or haemorrhage within 1 DD of the centre of the fovea only if associated with a best VA of 6/12 or worse
-
7.3 NHS DESP Referral Criteria to Hospital Eye Service (HES)
-
Retinopathy
-
R0: screen annually
-
R1: screen annually
-
R2: Referral to HES — seen ≤13 weeks
-
R3A: Urgent referral to HES — seen ≤2 weeks
-
R3S: screen annually
-
-
Maculopathy
-
M0: screen annually
-
M1: Referral to HES — seen ≤13 weeks
-
7.4 Treatment Time at HES
-
PRP for PDR within 2 weeks
-
Focal/grid laser for maculopathy within 10 weeks
8 Diabetic Retinopathy Clinical Research Network (DRCR.net) Protocols
-
PDR
-
Protocol S (Diabetic Retinopathy Clinical Research Network 2015):
-
Purpose:
-
Prompt PRP vs 0.5 mg ranibizumab ± deferred PRP
-
-
Results:
-
Treatment with ranibizumab resulted in VA that was non-inferior to PRP treatment at 2 years
-
-
-
-
DMO
-
Protocol B (Diabetic Retinopathy Clinical Research Network 2008):
-
Purpose:
-
Intravitreal triamcinolone vs focal/grid laser for DMO
-
-
Results:
-
At 2 years, focal/grid laser is more effective and has fewer side effects than intravitreal triamcinolone
-
For center-involving DMO, focal/grid laser produces gradual VA improvement of ≥2 lines in approximately one-third of eyes with VA of ≤20/40 at 2 years
-
For center-involving DMO, approximately 20% of laser treated eyes worsen by ≥2 lines at 2 years
-
-
-
Protocol I (Elman et al. 2010):
-
Purpose:
-
Sham injection + prompt focal/grid laser (within 3–10 days after injection) vs Intravitreal 0.5 mg ranibizumab + prompt (within 3–10 days after injection) focal/grid laser vs Intravitreal 4 mg triamcinolone + prompt (within 3–10 days after injection) focal/grid laser vs Intravitreal ranibizumab + deferred (≥24 weeks) focal/grid laser
-
-
Results:
-
Intravitreal ranibizumab with prompt or deferred focal/grid laser resulted in superior VA and OCT outcomes compared with prompt focal/grid laser alone for the treatment of DMO involving the central macula at 1 year
-
In pseudophakic eyes, intravitreal triamcinolone with prompt focal/grid laser resulted in superior VA and OCT outcomes than focal/grid laser alone and the VA and OCT outcomes were comparable to intravitreal ranibizumab with prompt or deferred focal/grid laser for the treatment of DMO involving the central macula at 1 year
-
-
-
Protocol T (The Diabetic Retinopathy Clinical Research Network 2015; Wells et al. 2016):
-
Purpose:
-
Aflibercept (2 mg), bevacizumab (1.25 mg) and ranibizumab (0.3 mg) comparison for center-involved DMO
-
-
Results:
-
If initial VA letter score was 78 to 69 (20/32 to 20/40), there was no significant difference in mean improvement in VA letter score from baseline at 1 year between the three anti-VEGF drugs
-
If initial VA score was <69 (<20/40), the mean improvement in VA letter score from baseline at 1 year was significantly greater with aflibercept than with bevacizumab or ranibizumab
-
If initial VA letter score was 78–69 (20/32–20/40), there was no significant difference in mean improvement in VA letter score from baseline at 2 years between the three anti-VEGF drugs
-
If initial VA score was (<20/40), the mean improvement in VA letter score from baseline at 2 years was significantly greater with aflibercept than bevacizumab but not with ranibizumab
-
-
-
9 Hydroxychloroquine and Chloroquine Retinopathy
9.1 Pathogenesis
-
Hydroxychloroquine and chloroquine can cause toxic retinopathy due to their binding of melanin in the RPE as well as direct toxicity to retinal ganglion cells
9.2 History
-
Symptoms: asymptomatic, reduced vision, reduced colour vision, paracentral scotomas, reduced night vision, photopsias, glare, metamorphopsia
-
Ask about risk factors
-
Concomitant tamoxifen use
-
Impaired renal function with eGFR less than 60
-
>5-year duration of hydroxychloroquine use and >5 mg/kg/day dose of hydroxychloroquine
-
9.3 Examination
-
Mottling of the RPE (early sign)
-
Blunted foveal reflex (early sign)
-
Bull’s eye maculopathy (late sign): ring of depigmentation surrounding the fovea
-
Geographic atrophy
-
Optic atrophy
9.4 Investigation
-
OCT: loss of IS/OS junction in early toxicity, parafoveal thinning of the ONL in moderate toxicity, widespread RPE atrophy and retinal thinning in severe cases, “flying saucer” sign — ovoid appearance of central fovea created by preservation of central foveal outer retinal structures surrounded by perifoveal loss of IS/OS junction and perifoveal outer retinal thinning
-
Fundus autofluorescence (FAF): ring of increased autofluorescence initially with parafoveal hypofluorescence in severe cases
-
HVF: Paracentral scotomas
9.5 The RCOphth Clinical Guidelines on Hydroxychloroquine and Chloroquine Retinopathy: Recommendations on Screening 2018
-
Screening criteria
-
Annual screening for patients who have taken hydroxychloroquine for more than 5 years
-
Annual screening for patients who have taken chloroquine for more than 1 year
-
Annual screening for patients taking hydroxychloroquine less than 5 years who have additional risk factors for retinal toxicity (concomitant tamoxifen use, impaired renal function with eGFR <60 ml/min/1.75 m2, dose of hydroxychloroquine >5 mg/kg/day)
-
It is the responsibility of the prescribing physician to refer patients eligible for screening to the local HES
-
-
Baseline screening examination
-
All patients planning to be on long term therapy (>5 years for hydroxychloroquine and >1 year for chloroquine)
-
Ideally performed within 6 months of starting hydroxychloroquine or chloroquine but definitely within 12 months
-
Fundus photograph + SD-OCT
-
HVF 10-2 if macular pathology present
-
-
Screening tests
-
All patients should have the following:
-
10-2 HVF
-
SD-OCT
-
FAF
-
-
Multifocal ERG:
-
Performed only if persistent and significant VF defects that are consistent with hydroxychloroquine retinopathy are present but without evidence of structural defects on SD-OCT or FAF
-
-
-
Interpretation of screening results
-
No toxicity: no abnormalities suggestive of toxicity detected on any test
-
Possible toxicity: one test result typical of hydroxychloroquine retinopathy, but typical abnormalities not present in other tests
-
Definite toxicity: two test results (one subjective and one objective) with abnormalities typical of hydroxychloroquine retinopathy
-
-
Management of patients with possible retinopathy
-
Continue drug treatment
-
Patients with one abnormal test result on retinal imaging (SD-OCT or FAF) but normal VF including 30-2 should return for an annual review
-
Patients with persistent VF abnormalities in the context of normal structural imaging (SD-OCT or FAF) may be referred for multifocal ERG. Treatment should continue until the outcome of mfERG is known.
-
-
Management of patients with definite toxicity
-
Recommendation to stop hydroxychloroquine should be made to the prescribing physician
-
Inappropriate for ophthalmologists to stop hydroxychloroquine treatment
-
Patients should be referred for appropriate support at the point of detection of hydroxychloroquine retinopathy, e.g. low vision or eye clinic liaison officer (ECLO) services, certification of visual impairment, and referral to local and/or national charities
-
Patient should inform the DVLA and be advised not to drive until an Estermann VF test confirms it is legal to do so.
-
-
Termination of screening
-
Screening discontinued if patients stop taking hydroxychloroquine
-
10 The RCOphth Review of the Ocular Side Effects of Topiramate 2010
10.1 Indications
-
Monotherapy or adjunct in the control of partial and primary generalised epilepsy in adults and children above the age of 2
-
Migraine prophylaxis
-
Trigeminal neuralgia
-
Bipolar disorder, depression, eating disorders
-
IIH
10.2 Ocular Side-Effects
-
Secondary acute angle closure glaucoma
-
Occurs within 2 weeks of initiation of treatment
-
Suprachoroidal or cilio-choroidal detachments and ciliary body oedema: causes a forward rotation of the ciliary body which displaces the iris-lens plane anteriorly to close the AC angle
-
Treatment:
-
Withdrawal of topiramate
-
Topical atropine
-
Topical ocular hypotensive agents
-
Cautious use of oral acetazolamide — may worsen ciliary body oedema
-
-
-
Acute myopia on its own or with angle closure
-
Suprachoroidal or cilio-choroidal detachments and ciliary body oedema — causes a forward rotation of the ciliary body which displaces the iris-lens plane anteriorly
-
Myopia on its own resolves following discontinuation of the drug
-
-
Diplopia and nystagmus
-
Posterior scleritis
10.3 Recommendations
-
Screening patient on topiramate for asymptomatic disease is not useful
-
In case of visual blurring or ocular pain, initial advice from their local optometrist should be encouraged
-
Patients referred to Ophthalmologists with acute myopia should consider drug replacement following advice from a neurologist
-
Acute angle closure should be managed with:
-
Withdrawal or replacement of topiramate with an alternative drug
-
Topical atropine drops + topical ocular hypotensive agents
-
11 The Ocular Side-Effects of Vigabatrin (Sabril) Information and Guidance for Screening (The RCOphth 2008a)
11.1 Indications for Vigabatrin
-
Anti-epileptic drug (selective irreversible inhibitor of GABA-transaminase) licensed for first line treatment of infantile spasms and for the treatment of partial epilepsy ± secondary generalization which is not satisfactorily controlled by other drugs
-
Vigabatrin is not recommended for patients with pre-existing visual field defects
-
No relationship between the daily or cumulative dose of vigabatrin and the risk of visual field constriction
11.2 History
-
Symptoms: normal VA, asymptomatic absolute field loss
-
Risk factors: male (two-fold higher chance of developing VF constriction compared with females — effect independent of any differences in dose duration or cumulative dose of vigabatrin)
11.3 Examination
-
Absolute field loss can occur in the absence of any demonstratable fundal pathology observed clinically
-
Optic nerve pallor
-
RNFL atrophy
11.4 Investigations
-
ERG: reduced or absent oscillatory potentials
-
HVF: bilateral concentric predominantly peripheral and nasal constriction of the VF with temporal and macular sparing (spares the central field)
11.5 Prognosis
-
VF constriction does not reverse on cessation of the drug
-
Progression of VF constriction after stopping vigabatrin has not been reported to date
11.6 Screening
-
Baseline VF (Humphrey 120/Octopus 07 or Goldmann kinetic perimetry — less sensitive compared to Humphrey) should be obtained before starting treatment (age ≥9 years). Threshold testing is not recommended
-
VF testing after baseline should be repeated every 6 months for 5 years. Test interval can then be extended to annually in patients who have no defect detected.
-
If VF constriction is detected it is advisable, if possible, to conduct a confirmatory field test within 1 month before considering cessation of vigabatrin
-
If the drug is discontinued VF should be repeated at a future date to monitor the field loss
12 Uveitis
12.1 Classification of Uveitis
-
International Uveitis Study Group Anatomical Classification (Bloch-Michel and Nussenblatt 1987)
-
Anterior uveitis: anterior chamber
-
Intermediate uveitis: vitreous
-
Posterior uveitis: retina or choroid
-
Panuveitis: anterior chamber, vitreous, and retina or choroid
-
-
The Standardisation of Uveitis Nomenclature (SUN) Working Group 2005 Descriptors of Uveitis
-
Onset:
-
Sudden
-
Insidious
-
-
Course:
-
Acute: episode with sudden onset and limited duration
-
Chronic: persistent uveitis with relapse in <3 months after discontinuing treatment
-
Recurrent: repeated episodes separated by periods of inactivity without treatment >3 months duration
-
-
Duration:
-
Limited <3 months
-
Persistent >3 months
-
-
12.2 The SUN Working Group Grading Scheme of Anterior Chamber Cells
-
Field size of 1 × 1 mm slit beam:
-
0: no cells
-
0.5+: 1–5 cells
-
1+: 6–15 cells
-
2+: 16–25 cells
-
3+ 26–50 cells
-
4+: >50 cells
-
12.3 The SUN Working Group Grading Scheme for Anterior Chamber Flare
-
0: none
-
1+: faint
-
2+: moderate — iris and lens details clear
-
3+: severe — iris and lens details hazy
-
4+: intense — fibrin or plastic aqueous
12.4 Nussenblatt Scale for Vitreous Haze 1985
-
Performed with an indirect ophthalmoscope and 20 D lens by visually comparing the degree of haze on examination to a colour fundus photograph printout of the six-step ordinal scale
-
0: no evident vitreal haze at all
-
Trace (0.5+): slight blurring of optic disc margin, no visualisation of the normal striations and reflex of the nerve fiber layer
-
1+: permits better definition of both the optic nerve head and the retinal vessels
-
2+: permits better visualisation of the retinal vessels
-
3+: optic nerve head is visible to the observer but its borders are blurry
-
4+: optic nerve head is obscured
-
12.5 The SUN Working Group Activity of Uveitis Terminology 2005
-
Inactive: grade 0 cells
-
Worsening activity: two-step increase in level of inflammation (AC cells or vitreous haze) or increase from grade 3+ to 4+
-
Improved activity: two-step decrease in level of inflammation (AC cells or vitreous haze) or decrease to grade 0
-
Remission: inactive disease for >3 months after discontinuing all treatments for eye disease
12.6 NICE Guidance [TA460] on Adalimumab
-
Based on evidence from the VISUAL I (Jaffe et al. 2016) and VISUAL II (Nguyen et al. 2016) trials
-
Recommended as an option for treating of non-infectious uveitis in the posterior segment of the eye in adults with inadequate response to corticosteroids, only if there is:
-
Active disease (current inflammation in the eye) AND
-
Inadequate response or intolerance to immunosuppresants AND
-
Systemic disease or both eyes are affected (or one eye is affected if the second eye has poor visual acuity) AND
-
Worsening vision with a high risk of blindness
-
-
Stop adalimumab for non-infectious uveitis in the posterior segment of the eye in adults with inadequate response to corticosteroids if there is one of the following:
-
New active inflammatory chorioretinal or inflammatory retinal vascular lesions, or both or
-
A two step increase in vitreous haze or AC cell grade or
-
Worsening of BCVA by three or more lines or 15 letters
-
12.7 NICE Guidance [TA460] on Ozurdex
-
Based on evidence from the HURON study (Lowder et al. 2011)
-
Dexamethasone intravitreal implant is recommended as an option for treating non-infectious uveitis in the posterior segment of the eye in adults, only if there is:
-
Active disease (that is, current inflammation in the eye) AND
-
Worsening vision with a risk of blindness
-
13 Creutzfeldt-Jakob Disease (CJD)
-
CJD is a progressive, fatal neurological disease that belongs to a wider group of neurodegenerative disorders known as transmissible spongiform encephalopathies (TSE) or prion diseases
-
A novel form of human prion disease, variant CJD (vCJD) is believed to result from consumption of food derived from cattle infected with bovine spongiform encephalopathy (BSE) — ingestion of contaminated beef
-
The prion protein is a normal cellular protein that is widely expressed in almost all human tissues, with the highest levels seen in nerve cells. Prions are infectious particles composed of abnormally folded forms of the prion protein.
-
Transmission (NICE Guidance [IPG196])
-
Iatrogenic:
-
Recipient of human cadaveric derived pituitary hormones
-
Antecedent neurosurgery with dura mater transplantation
-
Blood transfusion
-
Contaminated surgical instruments:
-
High risk procedures for transmission of CJD:
-
Posterior eye procedures that involve the retina or optic nerve:
-
Excision of eye, e.g. evisceration + orbital implant
-
Operations on the optic nerve, e.g. optic nerve decompression
-
Operations of vitreous body ONLY if they come into contact with the posterior hyaloid face, e.g. PPV + membrane peel
-
Scleral buckling with drainage of SRF
-
Photocoagulation of retina for detachment (only when the retina is handled directly)
-
Destruction of lesion of retina
-
Operations on the retina and RPE
-
-
-
Medium risk procedures for transmission of CJD:
-
Anterior eye procedures
-
-
-
-
Familial
-
Variant: contaminated foods of bovine origin
-
Sporadic: de novo spontaneous generation of self-replicating protein
-
-
Decontamination practices to reduce transmission (NICE Guidance [IGG196])
-
Keep instruments wet immediately after use until they are cleaned
-
Eliminate instrument migration between sets (keep instrument sets together)
-
Single use instruments for patients who have previously undergone high-risk procedures with incineration of instrument post usage
-
Decontamination of re-useable instruments: immerse in sodium hypochlorite for 1 h, rinse in water and subject to routine sterilisation
-
Decontamination of work surfaces: disinfection by flooding, for 1 h, with sodium hypochlorite, followed by water rinses (Table 5.4)
-
14 The RCOphth Ophthalmic Services Guidance on Ophthalmic Instrument Decontamination 2016
-
Decontamination is the term used to describe a combination of processes (cleaning, disinfection and/or sterilisation) used to make re-usable items safe for further use
-
Cleaning
-
Most important stage in the decontamination process
-
Removes dust, dirt, excretions, secretions, organic matter and all contamination including harmful and undesirable substances as well as a large proportion of micro-organisms which may be present
-
Mechanical/automated cleaning is the most effective and reproducible method
-
-
Disinfection
-
Process that removes or destroys potentially harmful micro-organisms (apart from spores) to a level non-harmful to health
-
Achieved by use of liquid chemicals or by moist heat
-
Moist heat is the first-choice method except for devices unable to withstand high temperatures
-
-
Sterilisation
-
Process that removes or destroys all micro-organisms and spores
-
Preferred method for instruments is autoclaving which achieves sterilisation by applying steam under pressure at the highest temperature compatible with the instruments being processed
-
-
All patients undergoing elective or emergency surgery must be asked, “Have you ever been notified that you are at increased risk of CJD or vCJD for public health purposes?” — consult local infection control team if there is a positive response
-
For high risk procedures, a further set of questions should be asked about FHx of CJD, growth hormone treatment, brain or spinal cord surgery, blood transfusions
-
Guidance for use of instruments in the ophthalmic clinic
-
For patients with known or potential CJD (e.g. dementia of unknown cause or unexplained neurodegenerative condition):
-
Single use devices or non-contact devices where possible
-
For reusable contact devices, either the device must be under strict quarantine and only reused on that patient or be disposed of
-
-
For the majority of patients:
-
Use of non-contact or disposable devices where possible
-
For reusable contact devices use disposable covers if possible and after usage:
-
Do not allow the device to dry
-
Rinse immediately for at least 30 s in water for irrigation
-
Clean with liquid soap or detergent for at least 20 s
-
Rinse again with water for 30 s
-
Immerse in freshly prepared hypochlorite 10,000 ppm of chlorine (1%) for at least 10 min
-
Rinse in three changes of sterile water or saline for at least 10 min
-
Shake, dry with tissue and store dry
-
-
-
-
Guidance for use of surgical instruments
-
For patients with no extra risk of carrying CJD prion protein:
-
Low risk surgery: no extra precautions
-
High risk surgery:
-
Ensure reusable instruments do not migrate between sets and that they should be fully trackable
-
Single use supplementary instruments
-
-
-
For patients with extra risk of carrying CJD or who have CJD:
-
Low risk surgery:
-
Minimise instrument migration and instruments should be trackable
-
-
High risk surgery:
-
Consider whether procedure is required at all or whether deferral might allow the diagnosis of CJD to be excluded if not certain
-
Perform procedure with minimal number of staff in theatre and at the end of the list
-
Use single instruments and incinerate at the end of the procedure
-
Reusable instruments should be kept quarantined and for reuse solely on that individual patient
-
-
-
15 The RCOphth Ophthalmic Services Guidance on the Prevention of Transmission of Blood-Borne Viruses in Ophthalmic Surgery
-
Standard precautions
-
General measures:
-
Hand-washing
-
Barrier protection: wearing of intact gloves, surgical face masks, protective clothing
-
-
Avoiding sharps usage wherever possible, and exercising care in their handling and disposal:
-
Sharps should not be recapped
-
Sharps should not be handed from one person to another
-
Avoid manipulation of sharps by hands or fingers
-
Surgeons should announce the movement of sharps
-
Magnetic pads should be used for keeping discarded needles
-
-
Exclude theatre personnel with open skin wounds on their hands or arms
-
-
Non-exposure prone procedures
-
Hands and fingers of the surgeon are completely visible at all times during the procedure
-
Pose no risk for transmission of a blood borne virus from an infected healthcare worker to a patient
-
-
Exposure prone procedures (EPPs)
-
Hands or fingers of the surgeon are inside a wound or body cavity and not completely visible at all times during the procedure
-
Orbital surgery and some operations in oculoplastic and lacrimal surgery
-
-
Surgeons who are HIV, hepatitis B (HBV) or Hepatitis C (HCV) positive should inform their occupational health department. An infected practitioner does not need to disclose to prospective patients of his/her infective status
-
Preventing doctor to patient transmission of HIV
-
After a single needle stick injury the risk of seroconversion is approximately 0.3%
-
No risk of transmission when blood is in contact with intact skin
-
There is a less than 1 in 1000 risk for exposure to intact mucous membranes
-
EPPs should not be performed by HIV positive surgeons
-
There are no restrictions on performing non-exposure prone procedures
-
Not all patients who have undergone an EPP by an HIV positive surgeon need to be notified and tested
-
-
Preventing doctor to patient transmission of HBV
-
After a single needle stick injury from an e-antigen (high infectivity) positive source to a non-immunised recipient the risk of seroconversion is up to 30% (the presence of hepatitis B surface antigen — HbsAg — indicates infection)
-
If the injured party is fully up to date with their immunisation and has shown a good antibody response to the vaccine (immunity is recognised by anti-hepatitis B surface antibodies: anti-Hbs), they are protected against infection with HBV
-
Hepatitis B surface antigen positive and e-antigen positive surgeons should not perform EPP’s
-
-
Preventing doctor to patient transmission of HCV
-
Risk of seroconversion in a healthcare worker following a single percutaneous injury from a HCV positive source is probably between 1.2% and 3%
-
Surgeons who are hepatitis C RNA positive should not perform EPPs
-
Hepatitis C infected surgeons who have responded successfully to treatment with antiviral therapy may resume EPPs.
-
-
General principles of management of needlestick injury
-
Gently squeezing the wound to encourage bleeding
-
Washing the wound with plain soap and copious water and then covering it with a waterproof dressing
-
Injury reported to a line manager
-
Healthcare practitioner designated to manage exposure should conduct a risk assessment
-
Blood from the source, taken with their consent, should be tested for blood borne virus status — when the source lacks capacity for consent, their tissue can only be tested if held to be in their best interests in accordance with the Mental Capacity Act 2005
-
-
HIV post-exposure prophylaxis (PEP)
-
Estimated risk of HIV transmission from a known HIV positive source after a needlestick injury is estimated at 0.3%
-
Testing of the source is performed (with consent and after pre-test counselling, and not by the direct health professional involved) ideally within 8 h and not more than 24 h after source blood is taken in order to minimise unnecessary use of PEP
-
PEP is typically given as a three-drug combination for the duration of 4 weeks. It should be commenced within 72 h after exposure
-
Post exposure HIV antibody testing should be performed at least 12 weeks after exposure or completion of PEP
-
16 Audit
-
Systematic examination of current practice to assess how well an institution or a practitioner is performing against set standards. Essentially it is a method for systematically reflection on, reviewing and improving practice.
-
Audit cycle
-
Identify a problem or issue
-
Identify standards
-
Collect data on current practice
-
Data analysis with comparison to the standard
-
Identify changes required and implement change
-
Re-audit to monitor effect of change
-
17 Laser Safety in Hospitals
-
Designated laser protection advisor (medical engineer who is responsible for ensuring that the safety and use of lasers is in accordance with the health and safety law and regulations) is present in every hospital in the UK
-
There are two designated laser safety officers (responsible for ensuring that all staff are trained to safely use lasers and that all laser procedures and clinics are in keeping with the health and safety requirements) at the local departmental level
-
Safety measures for lasering patients:
-
Laser personnel:
-
Ensure that any member of staff who is to perform a laser treatment on a patient has attended an up to date training session on the fundamentals of laser safety
-
-
Prior to laser treatment of a patient:
-
Ensure laser machines are working correctly
-
Ensure any windows or reflective surfaces are covered
-
Ensure you have the correct patient
-
Lock the laser room door before you commence the laser treatment
-
Switch the “laser in use” sign on
-
-
During laser treatment of a patient:
-
Ensure the correct protective eyewear for the correct laser machine is worn by any relatives or members of staff who will be in the laser room during the laser treatment
-
-
Suggested Reading
Age-Related Eye Disease Study Research Group. A randomised, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–36.
Beck RW, Cleary PA, Anderson MM, Keltner JL, Shults WT, Kaufman DI, Buckley EG, Corbett JJ, Kupersmith MJ, Miller NR, Savino PJ, Guy JR, Trobe JD, McCrary JA, Smith CH, Chrousos GA, Thomson S, Katz BJ, Brodsky MC, Goodwin JA, Atwell CW. A randomised, controlled trial of corticosteroids in the treatment of acute optic neuritis. N Engl J Med. 1992;326:581–8.
Beck RW, Cleary PA, Trobe JD, Kaufman DI, Kuppersmith MJ, Paty DW, Brown CH. The effect of corticosteroids for acute optic neuritis on the subsequent development of multiple sclerosis. N Engl J Med. 1993;329:1764–9.
Bloch-Michel E, Nussenblatt RB. International Uveitis Study Group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol. 1987;103:234–5.
Boyer DS, Yoon YH, Belfort R, Bandello F, Maturi RK, Augustin AJ, Li XY, Cui H, Hashad Y, Whitcup SM. Three-year, randomised, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904–14.
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44.
Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, Reeves BC. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382:1258–67.
Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, Hope S, Kanis JA, McCloskey EV, Poole KES, Reid DM, Selby P, Thompson F, Thurston A, Vine N. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12:43.
Cunha-Vaz J, Ashton P, Iezzi R, Campochiaro P, Dugel PU, Holz FG, Weber M, Danis RP, Kuppermann BD, Bailey C, Billman K, Kapik B, Kane F, Green K. Sustained delivery fluocinolone acetonide vitreous implants. Long term benefits in patients with chronic diabetic macular oedema. Ophthalmology. 2014;121:1892–903.
Diabetic Retinopathy Clinical Research Network. A randomised trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447–59.
Diabetic Retinopathy Clinical Research Network. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy. A randomised clinical trial. JAMA. 2015;314:2137–46.
Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, Gomes AV, Warburton J, Weichselberger A, Holz FG. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127:72–84.
Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology. 1991;98:823–33.
Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL III, Friedman SM, Glassman AR, Miller KM, Scott IU, Stockdale CR, Sun JK. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–77.
Facey K, Cummins E, Macpherson K, Morris A, Reay L, Slattery J. Organisation of services for diabetic retinopathy screening. Glasgow: Health Technology Board for Scotland; 2002. p. 1–224.
Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ, Puliafito CA, Davis JL, Flynn HW, Esquiabro M. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–83.
Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153:789–803.
Gedde SJ, Feuer WJ, Shi W, Lim KS, Barton K, Goyal S, Ahmed IIK, Brandt J. Treatment outcomes in the Primary Tube Versus Trabeculectomy Study after 1 year of follow up. Ophthalmology. 2018;125:650–63.
Haller JA, Bandello F, Belfort R, Blumenkranz MS, Gillie M, Heier J, Loewenstein A, Yoon YH, Jacques ML, Jiao J, Li XY, Whitcup SM. Randomised, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134–46.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U. Intraviteal aflibercept (VEGF Trap-Eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48.
Jaffe GJ, Dick AD, Brezin AP, Nguyen QD, Thorne JE, Kestelyn P, Barisani-Asenbauer T, Franco P, Heiligenhaus A, Scales D, Chu DS, Camez A, Kwatra NV, Song AP, Kron M, Tari S, Suhler EB. Adalimumab in patients with active non-infectious uveitis. N Engl J Med. 2016;375:932–43.
Kohner EM. The natural history of proliferative diabetic retinopathy. Eye. 1991;5:222–5.
Lichter PR, Musch DC, Gillespie BW, Guire KE, Janz NK, Wren PA, Mills RP. Interim clinical outcomes in the collaborative initial glaucoma treatment study comparing initial treatment randomised to medications or surgery. Ophthalmology. 2001;108:1943–53.
Lowder C, Belfort R Jr, Lightman S, Foster CS, Robinson MR, Schiffman RM, Li XY, Cui H, Whitcup SM. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129:545–53.
Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration. Two-year results. Ophthalmology. 2012;119:1388–98.
National Institute for Health and Care Excellence. Patient safety and reduction of risk of transmission of Creutzfeldt-Jakob disease (CJD) via interventional procedures [IPG196]. [Online]. London: NICE; 2006. https://www.nice.org.uk/guidance/ipg196. Accessed 13 Dec 2019.
National Institute for Health and Care Excellence. Dexamethasone intravitreal implant for the treatment of macular oedema secondary to retinal vein occlusion [TA229]. [Online]. London: NICE; 2011. https://www.nice.org.uk/guidance/ta229. Accessed 11 Dec 2019.
National Institute for Health and Care Excellence. Fluocinolone acetonide intravitreal implant for treating chronic diabetic macular oedema after an inadequate response to prior therapy [TA301]. [Online]. London: NICE; 2013. https://www.nice.org.uk/guidance/ta301. Accessed 11 Dec 2019.
National Institute for Health and Care Excellence. Dexamethasone intravitreal implant for treating diabetic macular oedema [TA349]. [Online]. London: NICE; 2015. https://www.nice.org.uk/guidance/ta349. Accessed 11 Dec 2019.
National Institute for Health and Care Excellence. Adalimumab and dexamethasone for treating non-infectious uveitis [TA460]. [Online]. London: NICE; 2017a. https://www.nice.org.uk/guidance/ta460. Accessed 13 Dec 2019.
National Institute for Health and Care Excellence. Osteoporosis: assessing the risk of fragility fracture [CG146]. [Online]. London: NICE; 2017b. https://www.nice.org.uk/guidance/cg146. Accessed 13 Dec 2019.
National Institute for Health and Care Excellence. Fluocinolone acetonide intravitreal implant for treating recurrent non-infectious uveitis [TA590]. [Online]. London: NICE; 2019. https://www.nice.org.uk/guidance/ta590. Accessed 13 Dec 2019.
Nguyen QD, Merrill PT, Jaffe GJ, Dick AD, Kurup SK, Sheppard J, Schlaen A, Pavesio C, Cimino L, Van Calster J, Camez AA, Kwatra NV, Song AP, Kron M, Tari S, Brezin AP. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicenter, double-masked, randomised, placebo-controlled phase 3 trial. Lancet. 2016;388:1183–92.
Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92:467–71.
Optic Neuritis Study Group. High- and low-risk profiles for the development of multiple sclerosis within 10 years after optic neuritis. Experience of the optic neuritis treatment trial. Arch Ophthalmol. 2003;121:944–9.
Public Health England. NHS Diabetic Eye Screening Programme. Grading definitions for referable disease. Public Health England leads the NHS Screening Programmes. [Online]. London: Public Health England; 2017. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/582710/Grading_definitions_for_referrable_disease_2017_new_110117.pdf. Accessed 11 Dec 2019.
Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S, Shams N. Randomised, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 1. Am J Ophthalmol. 2008;145:239–48.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Scanlon PH. The English national screening programme for sight-threatening diabetic retinopathy. J Med Screen. 2008;15:1–4.
Scanlon PH, Malhotra R, Thomas G, Foy C, Kirkpatrick JN, Lewis-Barned N, Harney B, Aldington SJ. The effectiveness of screening for diabetic retinopathy by digital imaging photography and technician ophthalmoscopy. Diabet Med. 2003a;20:467–74.
Scanlon PH, Malhotra R, Greenwood RH, Aldington SJ, Foy C, Flatman M, Downes S. Comparison of two reference standards in validating two field mydriatic digital photography as a method of screening for diabetic retinopathy. Br J Ophthalmol. 2003b;87:1258–63.
The Age-Related Eye Disease Study 2 (AREDS2) Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–15.
The AGIS Investigators. The advanced glaucoma intervention study (AGIS): 4. Comparison of treatment outcomes within race. Ophthalmology. 1998;105:1146–64.
The AGIS Investigators. The advanced glaucoma intervention study (AGIS): 9. Comparison of glaucoma outcomes in black and white patients within treatment groups. Am J Ophthalmol. 2001;132:311–20.
The CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908.
The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–203.
The Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of diabetic retinopathy study (DRS) findings, DRS report number 8. Ophthalmology. 1981;88:583–600.
The Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis. Final optic neuritis treatment trial follow-up. Arch Neurol. 2008;65:727–32.
The Royal College of Ophthalmologists. The ocular side-effects of vigabatrin (sabril) information and guidance for screening. [Online]. London: The Royal College of Ophthalmologists; 2008a. https://www.rcophth.ac.uk/wp-content/uploads/2015/01/2008-SCI-020-The-Ocular-Side-Effects-of-Vigabatrin-Sabril.pdf. Accessed 12 Dec 2019.
The Royal College of Ophthalmologists. Standards for the retrieval of human ocular tissue used in transplantation, research and training. [Online]. London: The Royal College of Ophthalmologists; 2008b. http://bmec.swbh.nhs.uk/wp-content/uploads/2013/03/RETRIEVAL-OF-HUMAN-OCULAR-TISSUE.pdf. Accessed 12 Dec 2019.
The Royal College of Ophthalmologists. Ophthalmic Services Guidance. Prevention of transmission of blood-borne viruses in ophthalmic surgery. [Online]. London: The Royal College of Ophthalmologists; 2008c. https://www.rcophth.ac.uk/wpcontent/uploads/2014/12/2010_PROF_053_Blood_Borne_Viruses.pdf. Accessed 12 Dec 2019.
The Royal College of Ophthalmologists. A review of the ocular side effects of topiramate. [Online]. London: The Royal College of Ophthalmologists; 2010. https://www.rcophth.ac.uk/wp-content/uploads/2014/12/2010_PROF_122_Review_of_Ocular_side_effects_of_Topiramate-2.pdf. Accessed 12 Dec 2019.
The Royal College of Ophthalmologists. Ophthalmic Services Guidance. Ophthalmic instrument decontamination. [Online]. London: The Royal College of Ophthalmologists; 2016. https://www.rcophth.ac.uk/wp-content/uploads/2014/12/Ophthalmic-Instrument-Decontamination.pdf. Accessed 12 Dec 2019.
The Royal College of Ophthalmologists. Hydroxychloroquine and chloroquine retinopathy: recommendations on screening. [Online]. London: The Royal College of Ophthalmologists; 2018. https://www.rcophth.ac.uk/wp-content/uploads/2018/07/Hydroxychloroquine-and-Chloroquine-Retinopathy-Screening-Guideline-Recommendations.pdf. Accessed 12 Dec 2019.
The Standardisation of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140:509–16.
Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, Brucker AJ, Ferris FL, Hampton GR, Jhaveri C, Melia M, Beck RW. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. Two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123:1351–9.
Wilson JMG, Jungner G, World Health Organisation. Principles and practice of screening for disease. [Online]. Geneva: World Health Organization; 1968. https://apps.who.int/iris/bitstream/handle/10665/37650/WHO_PHP_34.pdf?sequence=17.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Fung, T.H.M., Amoaku, W.M.K. (2020). Health Promotion, Audit, Research and Evidence-Based Medicine. In: Fung, T., Amoaku, W. (eds) Viva and OSCE Exams in Ophthalmology. Springer, Cham. https://doi.org/10.1007/978-3-030-43063-4_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-43063-4_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-43062-7
Online ISBN: 978-3-030-43063-4
eBook Packages: MedicineMedicine (R0)