Abstract
The prognosis for metastatic osteosarcoma (OS) is poor and has not changed in several decades. Therapeutic paradigms that target and exploit novel molecular pathways are desperately needed. Recent preclinical data suggests that modulation of the Fas/FasL pathway may offer benefit in the treatment of refractory osteosarcoma. Fas and FasL are complimentary receptor-ligand proteins. Fas is expressed in multiple tissues, whereas FasL is restricted to privilege organs, such as the lung. Fas expression has been shown to inversely correlate with the metastatic potential of OS cells; tumor cells which express high levels of Fas have decreased metastatic potential and the ones that reach the lung undergo cell death upon interaction with constitutive FasL in the lung. Agents such as gemcitabine and the HDAC inhibitor, entinostat/Syndax 275, have been shown to upregulate Fas expression on OS cells, potentially leading to decreased OS pulmonary metastasis and improved outcome. Clinical trials are in development to evaluate this combination as a potential treatment option for patients with refractory OS.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Metastatic osteosarcoma (OS) carries a poor prognosis and options for successful treatment and eventual cure are few. Despite dramatic progress in the 1970s and 1980s in the treatment of non-metastatic OS, the outcomes have not changed in several decades. The exact molecular mechanisms underlying drug resistance and development of metastatic disease remain unknown. Furthermore, the contribution of the organ microenvironment remains unexplored. Novel therapeutic approaches for OS lung metastasis and refractory/recurrent disease are desperately needed [1,2,3,4,5,6,7,8,9].
Similar to other cancer types, targeted therapy and immunotherapy are potential treatment alternatives which have yet to be fully evaluated in OS. Immunomodulatory agents have long been considered for OS as a way to enhance the immune response [2, 3]. In fact, several studies suggest that OS may be amenable to treatment with immune-based therapies including immune checkpoint inhibitors [4,5,6,7,8,9]. Furthermore, there are several ongoing clinical trials which focus on the use of targeted therapies for recurrent and refractory OS. These include denosumab (anti-NFκB ligand), glembatumumab vedotin (anti-glycoprotein NMB), dinutuximab (anti-GD2), sirolimus (mTOR inhibitor), and VEGFR inhibitors (apatinib, lenvatinib, cabozantinib). In the present chapter, we provide the rationale for an alternative combination therapy using a specific histone deacetylase (HDAC) inhibitpr, entinostat/Syndax 275 in combination with the nucleoside analog, gemcitabine for the treatment of OS.
Fas and the Fas Signaling Pathway
Fas (CD95) is a cell surface death receptor that belongs to the tumor necrosis factor receptor (TNFR) superfamily. Interaction of Fas with its cognate ligand, FasL (CD95L), induces apoptosis in Fas-expressing cells. Fas is expressed on several different cell types including tumor cells, whereas FasL expression is restricted to immune cells (activated T and NK cells) and privilege organs, such as the lung [10]. The Fas/FasL signaling pathway is involved in immune homeostasis and immune and tumor surveillance.
As with all death receptors, Fas has a conserved death domain (DD) in its cytoplasmic tail that is crucial for the initiation of Fas-induced apoptosis. Fas and FasL ligation results in oligomerization and aggregation of the Fas receptor, which then leads to death-inducing signaling complex (DISC) assembly at the cellular membrane. DISC consists of Fas receptor, Fas associated with a death domain (FADD) adaptor molecule, procaspase-8, procaspase-10, and the cellular FLICE-like inhibitory protein (c-FLIP). DISC formation results in procaspase-8 activation, which later leads to cleavage of various intracellular proteins and ultimately apoptosis.
Fas Expression and Its Role in OS Lung Metastasis Formation
Fas-induced apoptosis is involved in tumor cell death and regulation of tumor development. Multiple studies have demonstrated that the absence of the Fas signaling pathway in primary tumors is associated with poor prognosis [11,12,13,14]. Tumor cells downregulate their Fas expression to escape from FasL-mediated apoptosis induced by activated immune cells [11, 14, 15]. Altered Fas expression can also affect a tumor’s metastatic potential [16, 17].
OS most commonly metastasizes to the lungs. Metastases to the lungs are often resistant to salvage chemotherapy [18]. Our laboratory has previously demonstrated an inverse correlation between the metastatic potential of human OS cells with Fas expression [19]. The LM7 cell, a subline of the SAOS human OS cell line obtained by recycling the cells seven times through the lungs of nude mice, expresses low levels of Fas [20], whereas the SAOS cells express high levels of Fas. SAOS cells cannot induce pulmonary metastasis when injected intravenously (i.v.), whereas LM7 cells form metastasis in the lung when injected i.v [21]. Similarly, K7 mouse OS cells, which express high levels of Fas, are not metastatic whereas K7M3 cells, derived from K7 after recycling the cells through the lungs, express low levels of Fas and form lung metastases when injected i.v. In addition, K7M3 cells form primary tumors in the bone if injected into the tibia and metastasize to the lung spontaneously. The primary bone tumor that develops in the tibia homogeneously expresses Fas, while lung metastases have low to no Fas expression [22]. Because FasL is constitutively expressed in the lung, we hypothesized that when OS cells express a functional Fas receptor, they will undergo cell death due to Fas/FasL-mediated apoptosis as they approach the lung microenvironment. On the other hand, Fas− OS cells will survive and form lung metastasis. We also showed that LM7 cells transfected with the full-length Fas gene expressed a higher level of Fas and formed significantly fewer and smaller pulmonary nodules compared to control-transfected LM7 cells [21]. Conversely, blocking the Fas signaling pathway in K7M3 and K7 mouse OS cells by transfection with Fas-associated death domain (FADD) dominant-negative (FDN) plasmid resulted in lower sensitivity to FasL-mediated apoptosis in vitro and enhanced metastatic potential to the lungs. Lung nodules from mice injected with the FADD_DN-transfected cells contained both Fas-positive and Fas-negative cells [15, 22]. These results support our hypothesis that Fas expression influences OS cells metastatic potential. A functional and intact Fas/FasL signaling pathway is key to the development of OS lung metastases. We further confirm these findings by injecting wild-type K7M3 and K7 cells into an FasL-deficient gld mice and found an increase in the number of lung tumors with both Fas-positive and Fas-negative cells [15, 22] suggesting that in the absence of FasL in the pulmonary epithelium, Fas+ tumor cells can survive and grow in the lungs. Subsequent analysis of patient samples supported our pre-clinical findings. Immunohistochemistry staining for Fas expression of 38 OS lung metastatic patient samples revealed 60% of the samples to be Fas negative, 32% to be weakly positive, and 3.2% (only one sample) to be strongly positive. Fas-positive expression was only detected in patients who had received chemotherapy prior to lung metastasis resection suggesting that treatment may contribute to Fas upregulation in OS tumors. Indeed, we further demonstrated that gemcitabine [23], interleukin -12 [24], entinostat/syndax275 [25], and 9-Nitrocamptothecin [26] upregulated Fas expression on OS cells which then resulted in the regression of established lung metastases.
Taken together, our findings address the importance of the Fas/FasL signaling pathway in the metastatic potential of OS and suggest that therapies able to upregulate Fas expression may add benefit in the treatment of OS lung metastases.
Gemcitabine and Its Effect on Osteosarcoma
Gemcitabine (2′,2′-difluorodeoxycytidine, dFdC) is a chemotherapeutic agent that has been approved for the treatment of various solid tumors including non-small-cell lung carcinoma, pancreatic, breast, and ovarian cancers. Gemcitabine is a deoxycytidine analog and its antitumor activity is the result of its ability to inhibit DNA replication and ultimately lead to cell death [27]. It has been tested in multiple pre-clinical and clinical settings [28,29,30,31,32,33], including OS [34,35,36,37,38,39,40,41,42,43,44,45]. Gemcitabine in combination with docetaxel remains a standard well-tolerated salvage chemotherapy regimen in the treatment of multiple sarcomas. However, it has only shown modest efficacy in relapsed/refractory OS [46,47,48,49,50]. Ofer Merimsky and colleagues reported gemcitabine treatment prolongs disease stabilization in 70% of patients with bone sarcomas resistant to doxorubicin [35]. A phase II clinical trial of the combination of gemcitabine and sirolimus demonstrated promising results in patients with relapsed and progressing OS [43]. Other gemcitabine combinations have not been as successful, however. Specifically, the addition of gemcitabine to carboplatin, for example, did not show benefit as compared to carboplatin alone in dogs with OS [37].
Based on our preliminary findings in the laboratory that the Fas-FasL pathway is implicated in the metastatic potential of OS, we hypothesized that agents that upregulate Fas expression could provide therapeutic benefit as the presence of FasL in the lung microenvironment will lead to cell death. Indeed, we demonstrated [23,24,25,26] in vitro that gemcitabine upregulated Fas expression in various OS cell lines and enhanced cell sensitivity to FasL in the lung. Inhibition of the Fas/FasL signaling pathway abolished the gemcitabine therapeutic effect, suggesting that an intact Fas pathway is important to the therapeutic efficacy of gemcitabine [22]. Other groups have similarly reported that gemcitabine induced growth inhibition, cell cycle arrest, and apoptosis in canine OS cell lines [36, 38]. Consistent with our findings, in vitro culture with relatively low concentrations of gemcitabine significantly increased functional Fas receptor expression in lung, colon, breast, and pancreatic tumor cell lines [51, 52].
Using two OS mouse models (K7M3 and LM7), we demonstrated aerosol gemcitabine to have therapeutic effect. Gemcitabine therapy resulted in significant increase in Fas expression, enhanced apoptosis, and subsequent regression of lung metastases. Aerosol gemcitabine further inhibited the growth of a subcutaneous OS primary tumor [22, 53]. We also confirmed in vivo the importance of the Fas/FasL pathway in the therapeutic efficacy of gemcitabine as aerosol gemcitabine therapy given to gld mice whose FasL function is impaired, resulted in increased Fas expression in OS lung metastasis but no therapeutic effect [22]. Similarly, dogs with OS lung metastasis treated with aerosol gemcitabine demonstrated increased Fas expression, apoptosis, and percentage of tumor necrosis [54]. Takashi Ando and colleagues have also demonstrated that systemic administration of gemcitabine results in a decrease in primary tumor growth, increased cell apoptosis, and decreased pulmonary metastasis in an OS mouse model [38]. Taken together, these results provide a rationale for the use of gemcitabine in combination with other agents shown to upregulate Fas expression to further enhance gemcitabine therapeutic effect against OS.
Histone Deacetylase (HDAC) Inhibitors
Epigenetic modifications, such as DNA methylation and acetylation, induce chromatin remodeling and altered gene expression. Defects in epigenetic regulation may result in loss or gain of gene function and lead to onset and progression of human diseases including cancer [55].
Histone acetyltransferases (HATs) and histone deacetylases (HDACs) are responsible for histone modifications. HAT stimulates gene transcription through the transferring of acetyl moieties to histone’s N-terminal lysine residues, which results in a less compact chromatin state. The opposing activity of the HDAC enzymes contributes to transcriptional repression by removing the acetyl moieties, creating a more compact chromatin leading to less gene expression. 18 HDACs have been identified in humans and are classified into four groups. Class I contains HDAC 1, 2, 3, and 8; Class II contains HDAC 4, 5, 6, 7, 9, and 10; Class III contains sirtuins and Class IV contains HDAC 11. Studies suggest that aberrant function of HAT and HDAC is often linked to tumorigenesis and poor prognosis in cancer [56]. Therefore, targeting these two enzymatic activities may provide therapeutic means to treat several malignancies associated with faulty epigenetic modifications [57, 58].
Several HDAC inhibitors have been shown to have anti-cancer effects. HDAC inhibitors regulate gene transcription by limiting the accessibility of transcription factors and RNA polymerase activities at the promoter level. HDAC inhibitors belong to four structural classes: (I) hydroxamic acids (hydroxamates); (II) benzamides; (III) short-chain fatty (aliphatic) acids; (IV) cyclic tetrapeptides; and (V) sirtuin inhibitors. In recent years, several HDAC inhibitors, with various target specificities and pharmacokinetics, have been under evaluation in clinical and preclinical studies. Thus far, four have received FDA approval for cancer treatment: vorinostat (SAHA), Belinostat (PXD-101), panobinostat (LBH589), and Istodax (romidepsin) [59, 60].
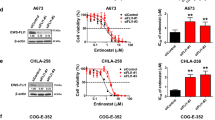
HDAC inhibitors have demonstrated a broad range of effects on tumor cells including cell death, growth arrest, and cell cycle suppression. In the clinical setting, tumor debulking and differentiation, prevention of angiogenesis, and enhancement of host immune response have been attributed to HDAC inhibitors [58]. Studies demonstrated that HDAC inhibitors are selectively more cytotoxic to cancer cells than normal cells, suggesting a potential therapeutic benefit of these drugs for the treatment of cancer [61, 62]. It has been shown that class I HDACs (1, 2, 3 and 8) play a key role in the pathogenesis of OS [63, 64]. Entinostat/syndax-275, a member of the benzamide group, is a narrow-spectrum HDAC inhibitor and affect HDAC class I with limited effect on HDAC 8 [65]. Entinostat/syndax-275 is in several phase I/II clinical trials for the treatment of solid and hematologic malignancies.
Entinostat/Syndax-275 and Its Effect on Osteosarcoma
It is well known that HDAC inhibitors can inhibit human and canine OS cell growth by promoting apoptosis, mostly through Fas-mediated or caspase-dependent pathways. For example, treatment with valproic acid prior to incubation with doxorubicin resulted in less cell growth and more apoptosis both in canine and human OS cells. In addition, valproic acid and doxorubicin combination therapy in a canine OS subcutaneous xenograft model led to significantly less tumor growth compared to either alone [66]. Further combination of two epigenetic modifying drugs, the DNA methylation inhibitor, Zebularine, and the HDAC inhibitor suberoylanilide hydroxamic acid (SAHA) showed significant human and canine OS cell growth inhibition. Inhibition was more effective in cell lines with a more aggressive gene expression profile [67]. Similarly, co-treatment with a DNA methyltransferase inhibitor, 5-Aza-dC, and HDAC inhibitor trichostatin A effectively reduced cell proliferation of the multi-drug resistance OS cell line HosDXR150, whereas single treatment had only a minor effect on cell viability [27]. Lastly, SAHA in combination with cisplatin decreased cell proliferation and enhanced OS cell apoptosis via caspase activation [68, 69].
HDAC Effect on the Fas/FasL Apoptotic Pathway
HDAC inhibitors can sensitize tumor cells to Fas-mediated apoptosis using different mechanisms. For example, apicidin and depsipeptide (FR901228) increased apoptosis in acute promyelocytic leukemia cells and uveal melanoma by inducing upregulation of Fas/FasL expression [70,71,72]. Another study demonstrated the HDAC inhibitor PCI-24781 to induce apoptosis in acute leukemia cells through activation of caspase-8 and FADD [73]. In OS cells, FR901228 inhibited cell growth both in vitro and in xenograft mouse models. FR901228 upregulated FasL mRNA and cell surface expression, activated caspase-8 and -3 and ultimately induced Fas-mediated apoptosis [74].
We also have demonstrated that therapeutically achievable doses of entinostat/syndax-275 while having limited cytotoxic effect on OS cell growth in vitro activate the Fas pathway and enhance Fas mRNA and protein expression. Combination treatment entinostat/syndax-275 and FasL significantly increased OS cells’ sensitivity to FasL as demonstrated by enhanced caspase cleavage/activity and reduced clonogenic growth. Blocking the Fas pathway reversed this effect [25, 75]. Intranasal administration of entinostat/syndax-275 at a dose of 0.13 mg/kg (which is approximately 200-fold less than the therapeutically effective oral dose described before) in mice with established OS lung metastasis resulted in reduced metastatic tumor growth [13]. In addition, oral administration of entinostat/syndax-275 in mice with OS pulmonary metastasis resulted in tumor growth inhibition and increased survival rate. Histopathological examination showed a higher level of apoptosis and lower level of cellular FLICE inhibitory protein (c-FLIP) expression in the lung tissues of treated mice. No evidence of drug toxicity was observed in the treated group of mice [75].
Despite sufficient evidence to demonstrate that entinostat/syndax-275 activates the Fas pathway in OS, studies in our lab demonstrated that this HDAC inhibitor did not increase the expression of Fas on the cell surface. Instead, entinostat/syndax-275 treatment led to redistribution of Fas to membrane lipid rafts and downregulation of cellular c-FLIPmRNA and protein expression. c-FLIP knockdown in OS cells resulted in the redistribution of Fas to lipid rafts and enhanced sensitivity to FasL-induced cell death [75, 76]. Our findings were consistent with other studies demonstrating that the HDAC inhibitor FR901228 downregulated c-FLIP in both chronic lymphocytic leukemia cells and Fas-resistant OS cells and enhanced their sensitivity to Fas-mediated apoptosis [77, 78]. Entinostat/syndax-275 has also been shown to downregulate c-FLIP in chronic lymphocytic leukemia (CLL) cells and induce caspase-dependent apoptosis [79]. Similarly, 7 days of treatment with valproic acid sensitized OS cells to Fas-mediated cell death without enhancing Fas expression on the cell surface [80].
c-FLIP is a key regulator of Fas-mediated apoptosis. c-FLIP, a catalytically inactive caspase-8/-10 homolog, interferes with activation of procaspase-8 at the death-inducing signaling complex (DISC) level and prevents Fas-induced apoptosis [81]. Many studies showed that c-FLIP was overexpressed in various cancer cells and its expression is liked with tumorigenesis and poor survival [75, 82,83,84], highlighting a potential mechanism by which cancer cells resist to death receptor-induced apoptosis. The expression of c-FLIP has also been correlated with resistance to several chemotherapy drugs [70, 85]. We also evaluated c-FLIP expression in patient primary and pulmonary OS samples using immunohistochemistry. C-FLIP expression was significantly higher in pulmonary nodules than in primary tumors. Similar results were observed in our human xenograft models [76]. Taken together, these findings suggest that the overexpression of c-FLIP as an inhibitor of the Fas-signaling pathway may contribute to the survival and growth of OS cells in a FasL+ lung microenvironment. Therefore, the downregulation of c-FLIP in entinostat/syndax-275-induced Fas signaling may be therapeutically beneficial for the treatment of OS lung metastasis.
Gemcitabine and Entinostat/Syndax-275 as Potential Salvage Regimen for Osteosarcoma Lung Metastasis
The above preclinical data suggests that the use of therapeutic agents able to upregulate Fas expression, increase Fas localization to lipid rafts, or decrease cFLIP expression may offer benefit in the treatment of OS. The combination of gemcitabine and entinostat/syndax-275 – both of which have been shown to enhance Fas expression in OS cells – has not been studied in pediatric patients with refractory or recurrent pulmonary OS. Therefore exploitation of the Fas/FasL pathway as a potential therapeutic option for patients with refractory OS seems appropriate. A phase I/II clinical trial of the combination is under development at MD Anderson Cancer Center. This clinical trial will evaluate feasibility and safety of the combination therapy entinostat/syndax-275 and gemcitabine and determine whether there is potential utility for patients with refractory/relapsed OS. To this end, the primary objective of the study is to determine the maximum tolerated dose (MTD) of entinostat/syndax-275 when it is given in combination with gemcitabine in pediatric patients with recurrent sarcoma and recommend a phase 2 dose of the combination therapy. Secondary objectives include: 1) To determine the disease control rate at 4 months for pediatric patients with recurrent unresectable pulmonary OS when treated with gemcitabine in combination with entinostat/syndax-275 and 2) To estimate the disease-free survival for the subset of pediatric patients with recurrent pulmonary OS that has been fully resected after treatment with gemcitabine in combination with entinostat/syndax-275. It is expected that this trial will serve as a potential therapeutic alternative for patients with refractory OS. Several approaches have been taken to treat OS. However, none have shown significant benefit as there has been no impact in survival. It is of paramount importance that therapies that move into clinical trials have a scientific rationale. Here we present enough preclinical evidence to support combination therapy gemcitabine and entinostat/syndax275 for refractory OS. Therefore, results from this study holds promise as an alternative to treat patients with OS.
References
Kaste SC et al (1999) Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer 86(8):1602–1608
Meyers PA et al (2005) Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol 23(9):2004–2011
Meyers PA et al (2008) Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children’s Oncology Group. J Clin Oncol 26(4):633–638
Guma SR et al (2014) Aerosol interleukin-2 induces natural killer cell proliferation in the lung and combination therapy improves the survival of mice with osteosarcoma lung metastasis. Pediatr Blood Cancer 61(8):1362–1368
Zhu Z et al (2017) Prognostic value of programmed death-ligand 1 in sarcoma: a meta-analysis. Oncotarget 8(35):59570–59580
Bielack SS et al (2015) Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon Alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: first results of the EURAMOS-1 good response randomized controlled trial. J Clin Oncol 33(20):2279–2287
Shen JK et al (2014) Programmed cell death ligand 1 expression in osteosarcoma. Cancer Immunol Res 2(7):690–698
Lussier DM et al (2015) Enhanced T-cell immunity to osteosarcoma through antibody blockade of PD-1/PD-L1 interactions. J Immunother 38(3):96–106
Longhi A et al (2006) Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev 32(6):423–436
French LE et al (1996) Fas and Fas ligand in embryos and adult mice: ligand expression in several immune-privileged tissues and coexpression in adult tissues characterized by apoptotic cell turnover. J Cell Biol 133(2):335–343
Wang WS et al (2006) Matrix metalloproteinase-7 increases resistance to Fas-mediated apoptosis and is a poor prognostic factor of patients with colorectal carcinoma. Carcinogenesis 27(5):1113–1120
Liu B et al (2019) Leucine-rich repeat neuronal protein-1 suppresses apoptosis of gastric cancer cells through regulation of Fas/FasL. Cancer Sci 110(7):2145–2155
Volm M, Koomagi R (2000) Relevance of proliferative and pro-apoptotic factors in non-small-cell lung cancer for patient survival. Br J Cancer 82(10):1747–1754
Wang WS et al (2004) Overexpression of the thymosin beta-4 gene is associated with increased invasion of SW480 colon carcinoma cells and the distant metastasis of human colorectal carcinoma. Oncogene 23(39):6666–6671
Koshkina NV et al (2007) Fas-negative osteosarcoma tumor cells are selected during metastasis to the lungs: the role of the Fas pathway in the metastatic process of osteosarcoma. Mol Cancer Res 5(10):991–999
Yang D et al (2008) Downregulation of IFN-gammaR in association with loss of Fas function is linked to tumor progression. Int J Cancer 122(2):350–362
Liu K, Abrams SI (2003) Alterations in Fas expression are characteristic of, but not solely responsible for, enhanced metastatic competence. J Immunol 170(12):5973–5980
Marina N et al (2004) Biology and therapeutic advances for pediatric osteosarcoma. Oncologist 9(4):422–441
Worth LL et al (2002) Fas expression inversely correlates with metastatic potential in osteosarcoma cells. Oncol Rep 9(4):823–827
Jia SF, Worth LL, Kleinerman ES (1999) A nude mouse model of human osteosarcoma lung metastases for evaluating new therapeutic strategies. Clin Exp Metastasis 17(6):501–506
Lafleur EA et al (2004) Increased Fas expression reduces the metastatic potential of human osteosarcoma cells. Clin Cancer Res 10(23):8114–8119
Gordon N et al (2007) Corruption of the Fas pathway delays the pulmonary clearance of murine osteosarcoma cells, enhances their metastatic potential, and reduces the effect of aerosol gemcitabine. Clin Cancer Res 13(15 Pt 1):4503–4510
Gordon N, Kleinerman ES (2010) Aerosol therapy for the treatment of osteosarcoma lung metastases: targeting the Fas/FasL pathway and rationale for the use of gemcitabine. J Aerosol Med Pulm Drug Deliv 23(4):189–196
Jia SF et al (2003) Aerosol gene therapy with PEI: IL-12 eradicates osteosarcoma lung metastases. Clin Cancer Res 9(9):3462–3468
Koshkina NV, Rao-Bindal K, Kleinerman ES (2011) Effect of the histone deacetylase inhibitor SNDX-275 on Fas signaling in osteosarcoma cells and the feasibility of its topical application for the treatment of osteosarcoma lung metastases. Cancer 117(15):3457–3467
Koshkina NV et al (2000) 9-Nitrocamptothecin liposome aerosol treatment of melanoma and osteosarcoma lung metastases in mice. Clin Cancer Res 6(7):2876–2880
Capobianco E et al (2014) Separate and combined effects of DNMT and HDAC inhibitors in treating human multi-drug resistant osteosarcoma HosDXR150 cell line. PLoS One 9(4):e95596
Marchi E et al (2005) Gemcitabine as frontline treatment for cutaneous T-cell lymphoma: phase II study of 32 patients. Cancer 104(11):2437–2441
Perez-Manga G et al (2000) Gemcitabine in combination with doxorubicin in advanced breast cancer: final results of a phase II pharmacokinetic trial. J Clin Oncol 18(13):2545–2552
Rizzieri DA et al (2003) Phase I evaluation of prolonged-infusion gemcitabine with fludarabine for relapsed or refractory acute myelogenous leukemia. Clin Cancer Res 9(2):663–668
Santoro A et al (2000) Gemcitabine in the treatment of refractory Hodgkin’s disease: results of a multicenter phase II study. J Clin Oncol 18(13):2615–2619
Turner AI et al (2006) Single agent gemcitabine chemotherapy in dogs with spontaneously occurring lymphoma. J Vet Intern Med 20(6):1384–1388
Marconato L et al (2008) Adjuvant gemcitabine after surgical removal of aggressive malignant mammary tumours in dogs. Vet Comp Oncol 6(2):90–101
Zak D et al (2005) Combination of gemcitabine and irinotecan for recurrent metastatic osteogenic sarcoma. Clin Adv Hematol Oncol 3(4):297–9; discussion 300-2
Merimsky O et al (2000) Gemcitabine in bone sarcoma resistant to Doxorubicin-based chemotherapy. Sarcoma 4(1–2):7–10
McMahon MB et al (2010) Biological activity of gemcitabine against canine osteosarcoma cell lines in vitro. Am J Vet Res 71(7):799–808
McMahon M et al (2011) Adjuvant carboplatin and gemcitabine combination chemotherapy postamputation in canine appendicular osteosarcoma. J Vet Intern Med 25(3):511–517
Ando T et al (2005) Gemcitabine inhibits viability, growth, and metastasis of osteosarcoma cell lines. J Orthop Res 23(4):964–969
Anderson PM et al (2005) Gemcitabine radiosensitization after high-dose samarium for osteoblastic osteosarcoma. Clin Cancer Res 11(19 Pt 1):6895–6900
Okuno S et al (2002) Phase II trial of gemcitabine in advanced sarcomas. Cancer 94(12):3225–3229
Okuno S et al (2003) Phase II trial of gemcitabine in patients with advanced sarcomas (E1797): a trial of the Eastern Cooperative Oncology Group. Cancer 97(8):1969–1973
Wagner-Bohn A et al (2006) Phase II study of gemcitabine in children with solid tumors of mesenchymal and embryonic origin. Anti-Cancer Drugs 17(7):859–864
Martin-Broto J et al (2017) Gemcitabine plus sirolimus for relapsed and progressing osteosarcoma patients after standard chemotherapy: a multicenter, single-arm phase II trial of Spanish Group for Research on Sarcoma (GEIS). Ann Oncol 28(12):2994–2999
Wang Y et al (2018) Licoricidin enhances gemcitabine-induced cytotoxicity in osteosarcoma cells by suppressing the Akt and NF-kappaB signal pathways. Chem Biol Interact 290:44–51
Caliskan Y et al (2019) A new therapeutic combination for osteosarcoma: gemcitabine and Clofazimine co-loaded liposomal formulation. Int J Pharm 557:97–104
Palmerini E et al (2016) Gemcitabine and docetaxel in relapsed and unresectable high-grade osteosarcoma and spindle cell sarcoma of bone. BMC Cancer 16:280
Lee JA et al (2016) Higher gemcitabine dose was associated with better outcome of osteosarcoma patients receiving gemcitabine-docetaxel chemotherapy. Pediatr Blood Cancer 63(9):1552–1556
Rapkin L et al (2012) Gemcitabine and docetaxel (GEMDOX) for the treatment of relapsed and refractory pediatric sarcomas. Pediatr Blood Cancer 59(5):854–858
Navid F et al (2008) Combination of gemcitabine and docetaxel in the treatment of children and young adults with refractory bone sarcoma. Cancer 113(2):419–425
Hara H et al (2019) Gemcitabine and docetaxel combination chemotherapy for advanced bone and soft tissue sarcomas: protocol for an open-label, non-randomised, phase 2 study. BMC Cancer 19(1):725
Gravett AM, Dalgleish AG, Copier J (2019) In vitro culture with gemcitabine augments death receptor and NKG2D ligand expression on tumour cells. Sci Rep 9(1):1544
Pei Q et al (2015) Gemcitabine sensitizes pancreatic cancer cells to the CTLs antitumor response induced by BCG-stimulated dendritic cells via a Fas-dependent pathway. Pancreatology 15(3):233–239
Koshkina NV, Kleinerman ES (2005) Aerosol gemcitabine inhibits the growth of primary osteosarcoma and osteosarcoma lung metastases. Int J Cancer 116(3):458–463
Rodriguez CO Jr et al (2010) Aerosol gemcitabine: preclinical safety and in vivo antitumor activity in osteosarcoma-bearing dogs. J Aerosol Med Pulm Drug Deliv 23(4):197–206
Berdasco M, Esteller M (2013) Genetic syndromes caused by mutations in epigenetic genes. Hum Genet 132(4):359–383
Xu WS, Parmigiani RB, Marks PA (2007) Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene 26(37):5541–5552
Barneda-Zahonero B, Parra M (2012) Histone deacetylases and cancer. Mol Oncol 6(6):579–589
Bose P, Dai Y, Grant S (2014) Histone deacetylase inhibitor (HDACI) mechanisms of action: emerging insights. Pharmacol Ther 143(3):323–336
Guha M (2015) HDAC inhibitors still need a home run, despite recent approval. Nat Rev Drug Discov 14(4):225–226
Eckschlager T et al (2017) Histone deacetylase inhibitors as anticancer drugs. Int J Mol Sci 18(7)
Qiu L et al (2000) Histone deacetylase inhibitors trigger a G2 checkpoint in normal cells that is defective in tumor cells. Mol Biol Cell 11(6):2069–2083
Marks PA, Xu WS (2009) Histone deacetylase inhibitors: potential in cancer therapy. J Cell Biochem 107(4):600–608
Chaiyawat P et al (2018) Expression patterns of class I histone deacetylases in osteosarcoma: a novel prognostic marker with potential therapeutic implications. Mod Pathol 31(2):264–274
Deng Z et al (2016) Histone deacetylase inhibitor trichostatin a promotes the apoptosis of osteosarcoma cells through p53 signaling pathway activation. Int J Biol Sci 12(11):1298–1308
Dokmanovic M, Marks PA (2005) Prospects: histone deacetylase inhibitors. J Cell Biochem 96(2):293–304
Wittenburg LA et al (2011) The histone deacetylase inhibitor valproic acid sensitizes human and canine osteosarcoma to doxorubicin. Cancer Chemother Pharmacol 67(1):83–92
Thayanithy V et al (2012) Combinatorial treatment of DNA and chromatin-modifying drugs cause cell death in human and canine osteosarcoma cell lines. PLoS One 7(9):e43720
Hou M et al (2018) Synergistic antitumor effect of suberoylanilide hydroxamic acid and cisplatin in osteosarcoma cells. Oncol Lett 16(4):4663–4670
Pettke A et al (2016) Suberanilohydroxamic acid (vorinostat) synergistically enhances the cytotoxicity of doxorubicin and cisplatin in osteosarcoma cell lines. Anti-Cancer Drugs 27(10):1001–1010
Matta H et al (2002) Role of MRIT/cFLIP in protection against chemotherapy-induced apoptosis. Cancer Biol Ther 1(6):652–660
Klisovic DD et al (2003) Depsipeptide (FR901228) inhibits proliferation and induces apoptosis in primary and metastatic human uveal melanoma cell lines. Invest Ophthalmol Vis Sci 44(6):2390–2398
Kwon SH et al (2002) Apicidin, a histone deacetylase inhibitor, induces apoptosis and Fas/Fas ligand expression in human acute promyelocytic leukemia cells. J Biol Chem 277(3):2073–2080
Rivera-Del Valle N et al (2010) PCI-24781, a novel hydroxamic acid HDAC inhibitor, exerts cytotoxicity and histone alterations via Caspase-8 and FADD in leukemia cells. Int J Cell Biol 2010:207420
Imai T et al (2003) FR901228 induces tumor regression associated with induction of Fas ligand and activation of Fas signaling in human osteosarcoma cells. Oncogene 22(58):9231–9242
Rao-Bindal K et al (2013) The histone deacetylase inhibitor, MS-275 (Entinostat), downregulates c-FLIP, sensitizes osteosarcoma cells to FasL, and induces the regression of osteosarcoma lung metastases. Curr Cancer Drug Targets 13:411
Rao-Bindal K et al (2013) Expression of c-FLIP in pulmonary metastases in osteosarcoma patients and human xenografts. Pediatr Blood Cancer 60(4):575–579
Watanabe K, Okamoto K, Yonehara S (2005) Sensitization of osteosarcoma cells to death receptor-mediated apoptosis by HDAC inhibitors through downregulation of cellular FLIP. Cell Death Differ 12(1):10–18
Aron JL et al (2003) Depsipeptide (FR901228) induces histone acetylation and inhibition of histone deacetylase in chronic lymphocytic leukemia cells concurrent with activation of caspase 8-mediated apoptosis and down-regulation of c-FLIP protein. Blood 102(2):652–658
Lucas DM et al (2004) The histone deacetylase inhibitor MS-275 induces caspase-dependent apoptosis in B-cell chronic lymphocytic leukemia cells. Leukemia 18(7):1207–1214
Yamanegi K et al (2012) Valproic acid cooperates with hydralazine to augment the susceptibility of human osteosarcoma cells to Fas- and NK cell-mediated cell death. Int J Oncol 41(1):83–91
Lavrik IN, Krammer PH (2012) Regulation of CD95/Fas signaling at the DISC. Cell Death Differ 19(1):36–41
de Hooge AS et al (2007) Expression of cellular FLICE inhibitory protein, caspase-8, and protease inhibitor-9 in Ewing sarcoma and implications for susceptibility to cytotoxic pathways. Clin Cancer Res 13(1):206–214
Korkolopoulou P et al (2004) c-FLIP expression in bladder urothelial carcinomas: its role in resistance to Fas-mediated apoptosis and clinicopathologic correlations. Urology 63(6):1198–1204
Bullani RR et al (2001) Selective expression of FLIP in malignant melanocytic skin lesions. J Invest Dermatol 117(2):360–364
Longley DB et al (2006) c-FLIP inhibits chemotherapy-induced colorectal cancer cell death. Oncogene 25(6):838–848
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kiany, S., Harrison, D., Gordon, N. (2020). The Histone Deacetylase Inhibitor Entinostat/Syndax 275 in Osteosarcoma. In: Kleinerman, E.S., Gorlick, R. (eds) Current Advances in Osteosarcoma . Advances in Experimental Medicine and Biology, vol 1257. Springer, Cham. https://doi.org/10.1007/978-3-030-43032-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-43032-0_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-43031-3
Online ISBN: 978-3-030-43032-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)