Abstract
Structural features of pure water and aqueous solutions of the electrolytes NaCl, NaCO3 and Zn[NO3]2 were comprehensively studied using the X-ray diffraction, infrared absorption spectroscopy and Raman spectroscopy methods. Spectral in situ measurements of the test samples at pressures up to 1000 bar and temperatures 25–500 °C were performed with the use of the high-temperature high-pressure cells with transparent sapphire windows. It was experimentally determined a temperature and pressure impact on the infinite clusters of the hydrogen-bound molecules that is inherent to the liquid water. Therewith, the critical isotherm of the liquid water is defined as the conditional boundary of the percolation threshold below which the clusters of finite sizes can exist only. An evaluation of the effect of the electrolytes, dissolved in water, onto the hydrogen bonds and structural features of water of the aqueous solutions as well as determination of the polyatomic anions stability under high temperatures and pressures was also performed. The vibrational spectra of NaCl are not revealed a significant difference between the properties of the aqueous solution and pure water under the test conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Supercritical water
- Fluids
- Critical isotherm
- Percolation threshold
- Hydrogen bonds
- Correlation functions
- Molecular spectroscopy
1 Introduction
Hydrothermal fluid is a necessary participant of the most important geological processes and shows highly active in the formation of the Earth’s crust, volcanic activity, transport and concentration of ore-forming components in the deposits. Due to its high mobility and solubility, it makes a decisive contribution to the processes of heat and mass transport in the earth’s crust and mantle. In geological terms, hydrothermal fluid is a hot, highly compressed water solution of many components, which exists, as a rule, at supercritical temperatures and pressures. The basis of the hydrothermal fluid is supercritical water (scH2O), which is directly involved in the most important geological processes.
The studies of water under supercritical conditions are also of interest in connection with the possibility of important practical applications in environmentally safe chemical technologies, such as oxidation in supercritical water (Bermejo and Cocero 2006). Indeed, the scH2O easily dissolves oxygen and organic compounds and facilitates their interaction (Wang et al. 2018). Mixed with other substances, the scH2O can be used not only for oxidation processes but also for hydrogenation (Fedyaeva et al. 2013), various chemical reactions (Akiya and Savage 2002) to produce nanoparticles of oxidies (Cabanos and Poliakoff 2001; Viswanathan and Gupta 2003) and many other practical applications (Purkarová et al. 2018).
Due to the enormous role of the scH2O in geological and technological processes, we will try to understand what the physical state of water is at supercritical temperatures and pressures, to summarize the available data on the intermolecular interaction and the nature of near-ordering in liquid and supercritical water, i.e. what is commonly referred to as “structure”. On the one hand, due to the specific interaction between water molecules caused by hydrogen bonds, experimental data (especially vibrational spectra) are difficult to interpret. However on the other hand, it is this interaction that can be used as a kind of probe to look inside this complex, continuously changing system. Exactly hydrogen bonds determine the high critical temperature of water.
At normal pressure, we can heat water only to 100 °C. In this range of temperatures, changes in the network of hydrogen bonding that bonds water molecules are noticeable poorly. But at high pressures, we can heat the water to very high temperatures, keeping it liquid or liquid-like. At the same time, radical changes occur in the network of hydrogen bonds, which are well noticeable in vibrational spectra and paired correlation functions. But before we begin to consider these phenomena, we must remember how the substance goes into a supercritical state and what it is.
There is no qualitative difference between gas and liquid. They differ only in a greater or lesser degree of interaction between molecules. Gas and liquid can be differed from each other only when they exist simultaneously. At temperatures and pressures above critical, there are no various phases, and we cannot characterize the supercritical state as a gas or liquid. Thus, the term “fluid” is used to describe the supercritical phase. In fact, when using this term, we mean that the supercritical phase of high density should approach the properties of a liquid. A phase with a lower density at a small supercritical pressure and at a high temperature is close in properties to a gas. Therefore, it is usually to talk about the “liquid-like” and “gas-like” states of the supercritical phase.
Numerous attempts have been made to define the approximate boundaries of the “liquid-like” and “gas-like” supercritical states in the phase diagram, in particular of water. For example, as a possible boundary between the “liquid-like” and “gas-like” states of the supercritical fluid, critical isochore and maximum lines of some thermodynamic parameters were proposed. (such as thermal expansion coefficient, maximums Cp and Cv.). However, the fact that the lines of the maxima of the thermodynamic parameters above the critical point diverge shows that there can be no continuation of the two-phase equilibrium curve to the supercritical region (Gorbaty and Bodarenko 2007). Another assumption shared by many scientists is that supercritical fluid is simply, or at least technically a gas (Poliakoff and King 2001).
Hydrogen bonds exist in supercritical water, but the infinite network of hydrogen bonds disappears. Based on the experimental data, we will try to clarify two important questions: under what conditions does the infinite network of hydrogen bonds disappear and what is the form of the final clusters formed by remaining hydrogen bonds.
2 Experimental
The specific feature of the approach to the study of water and systems modeling hydrothermal fluid was the simultaneous application of several methods of investigation of the same object, if it is possible under the same thermodynamic conditions. The X-ray scattering intensity was measured. The data obtained were supplemented by the results of vibrational spectroscopy studies (Infrared Absorption and Raman spectroscopy). For each of the experimental methods, a unique high-temperature high-pressure equipment was created and special methods of measurement and processing of the obtained data were developed.
X-ray scattering intensity measurements were performed by the energy dispersion method (Giessen and Gordon 1968). The advantage of this method is that it significantly simplifies the task of creating a high-temperature high-pressure cell. The description of the cell used in our experiments and the procedure of data processing were discussed in detail (Gorbaty and Okhulkov 1994). At a constant pressure of 1000 bar, paired correlation functions of the studied systems were obtained in the temperature range of 25–500 °C.
Infrared absorption spectra (IR-absorption) were obtained at temperatures of 20–550 °C and pressures up to 1000 bar. To obtain absorption spectra, a high-temperature high-pressure cell with a variable absorption layer thickness was produced (Gorbaty and Bondarenko 1999). The use of such a cell avoids a number of errors associated with the distortion of the optimal geometry of the incident radiation due to the small linear and angular aperture of the cell, the loss of light for reflection and absorption in the windows, the absorption of water vapor and carbon dioxide in the atmosphere, the lens effect. In addition, the measurement method automatically takes into account the own radiation of the cell heated to a high temperature.
A miniature cell with 180° geometry was used for Raman spectroscopy (Gorbaty et al. 2004; Gorbaty 2007). In some cases, it was more convenient to use another cell equipped with an internal vessel to isolate the sample from the pressure transfer medium (Gorbaty and Bondarenko 1995). Raman spectra were obtained at a constant pressure of 1000 bar up to 500 °C. The advantage of the Raman spectroscopy over IR absorption is that the materials most usually used for optical windows, such as sapphire or diamond, are transparent across the entire spectrum. On the other hand, Raman spectroscopy, unlike absorption spectroscopy, is not a quantitative method. It does not work with absolute scattering intensities, except for specially designed experiments. Only the relative intensity of the lines in the spectrum can be measured using an internal or external standard. In addition, the intensity of Raman spectra depends on many factors that are very difficult, or not impossible, to control and correct.
3 Results and Discussion
3.1 Supercritical State of Water
The specific feature of the water structure is the tetrahedral nearest-ordering, arising due to the hydrogen bonds between the water molecules. Each molecule can be join four nearest molecules by means of hydrogen bonds. The main indicator of the tetrahedral structure is the peak at ~4.5 Å in the paired correlation function (Bernal and Fowler 1933). The peak corresponds to a distance between tops of more or less ideal tetrahedron. The paired correlation functions of water g(r) obtained at temperatures up to 500 °C and a constant pressure of 1000 bar are shown in Fig. 2.1 (Gorbaty and Bondarenko 2007). Only part of the g(r) function is presented to focus on behavior of peak at 4.5 Å. With increasing temperature the peak amplitude decreases and near the critical temperature the peak almost disappears. It is clear that with increasing temperature the number of relatively strong hydrogen bonds decreases, the bonds themselves become longer and more distorted. However, at temperatures above the critical one it reappears again and tends to grow with a further increase of temperature. The same is observed for aqueous solutions (Okhulkov and Gorbaty 2001).
This peak behavior consists in the fact that molecules enveloped by hydrogen bonds tend to unite into aggregates with tetrahedral ordering. An important role in the formation of such aggregates is played by the of effect cooperativity of hydrogen bonds (Barlow et al. 2002; Yukhnevich 1997). This effect consists in increasing the average hydrogen bond energy in the H-bound aggregate with each new molecule attached to it. Therefore, striving to minimize the free energy, the molecules are combined into clusters of finite size. This is the reason for the appearance of a peak at 4.5 Å in the pair correlation function, which indicates a tetrahedral ordering above the critical isotherm.
Calculations of MD (Kalinashev and Churakov 1999) and MC (Krishtal et al. 2001) did not reveal the appearance of tetrahedral ordering at temperature above critical. One of the reason is that these methods cannot yet take into the effect of cooperativity of hydrogen bonds. The results of such calculations are close to the statistical distribution of molecules with different numbers of hydrogen bonds. This leads to the conviction that, at high supercritical temperature, molecules with four and three hydrogen bonds practically absent.
But the experimental functions of g(r) (Fig. 2.1) show that this conclusion does not reflect the real situation. It is quite clear that it is impossible to explain the behavior of experimental paired correlation functions using theoretical or computational methods. To explain the behavior of experimental paired correlation functions, an imaginary speculative two-dimensional model is proposed (Gorbaty and Bondarenko 2017).
The temperature dependence of probability of hydrogen bond formation Pb constructed from experimental datas, is shown in Fig. 2.2 (Gorbaty and Kalinuchev 1995). The value Pb decreases throughout the studied temperature range, and at critical temperature is 0.34 ± 0.03. This value is quite close to percolation threshold Pc calculated for a diamond-like crystal lattice (Stanley and Teixeira 1980).
This observation gives rise to an assumption that the line of percolation threshold for the network of hydrogen bonding in water is close to a critical isotherm. Above the percolation threshold, the substance is in a liquid state when the molecules are joined into a three-dimensional network, forming an infinite cluster. The infinity of the cluster means that one can always mentally “walk” through the connections from one wall of the vessel to another, no matter how big the vessel. But as the temperature rises, the number of hydrogen bonds decreases, until such a time as any path we choose is inevitably cut off. This means that the system has crossed the percolation threshold, below which there are clusters of only finite sizes. In the supercritical state, an infinite cluster of hydrogen bound molecules can no exist. The destruction of an infinite cluster leads to the appearance of free (inertial) rotating monomers and water molecules aggregates bound by remaining hydrogen bonds.
There is some evidence of specific water behavior near the critical isotherm. For example, the temperature derivative of the sound velocity in water has minima on the critical isotherm in a wide pressure range (Frank 1987). The isotope distribution coefficient undergoes a jump near the critical temperature (Driesner 1996).
Another example showing the special role of the critical isotherm shows in Fig. 2.3. These data were obtained with the use Raman spectroscopy directly at high temperatures and a constant pressure of 1000 bar (Bondarenko and Gorbaty 1997). A piece of sulphur was placed in an inner sapphire vessel filled with water in a high-temperature high-pressure cell for Raman spectroscopy. Temperature dependences of concentrations of the components of the solution reach their maximum values close to the critical temperature even at a pressure much higher than the critical one.
Free rotation of monomers and aggregates of water molecules, covered by hydrogen bonds, is well visible in the spectra of infrared absorption obtained at temperatures of 20–550 °C for three isobars 100, 250 and 500 bar. Figure 2.4 shows the IR spectra of (ν2 + ν3) combination mode of the D2O molecule. Unbound molecules are involved in free (inertial) rotation, when the molecule has time to make several turns between the collisions. This is manifested in the occurrence of the envelope P-, Q- and R- branches of the vibrational-rotational spectrum. The envelopes P, Q and R of the branches are particularly well visible in the spectra of gas phase at 350 and 400 °C, obtained at a pressure of 100 bar. At higher pressures, rotational branches are poorly resolved, but there is a sharp increase in the bandwidth above critical isotherm (Gorbaty and Bondarenko 1998).
Thus, the infinite cluster of hydrogen-bound molecules inherent in liquid water cannot exist in a supercritical state, but the probability of hydrogen binding is still high. Critical isotherm is a formal boundary separating qualitatively different states of the substance. Near the critical isotherm, the probability of hydrogen binding in the water is close to the percolation threshold.
4 Influence of Electrolytes on Hydrothermal Fluid Properties
Water solutions of electrolytes: NaCl, Na2SO4, Na2CO3, NaNO3, Zn[NO3]2 and orthophosphoric acid H3PO4 were studied by molecular spectroscopy at high temperatures and pressures. The most discussed question is the effect of ions on hydrogen bonded network of water molecules. The effect of some salt on Raman spectra of uncoupled OD vibration mode HDO is shown in Fig. 2.5. The important feature of spectra is a weak high frequency shoulder in the counter of band. This shoulder usually assigned OD to the non-bound or weakly bound groups. The weak shoulder is seen in spectra of HDO, NaCl, Na2CO3 and maybe Na2SO4. The shoulder turns into a peak in spectrum of NaClO4 solution. From viewpoint of structure-making/breaking it may assume that ion ClO4− is structure-weakening (chaotropic) what then the other ions are structure-strengthening (kosmotropic) (Bondarenko and Gorbaty 2011).
Stretching mode of water molecule is very sensitive to the formation of hydrogen bonds. Especially strong is the dependence of the intensity of absorption on the energy of hydrogen bonds. Due to the strong overlap of symmetrical and antisymmetrical stretching modes H2O, it is more productive to study the modes OD and OH of HDO molecules, which are approximately 1000 cm−1 apart.
The most complete results were obtained from the water-NaCl system, which was studied at the same parameters of the state of vibrational spectroscopy (IR and Raman), X-ray diffraction and computer simulation (Bondarenko et al. 2006). The concentration of the electrolyte was chosen so that 100 water molecules per two formulae units of salt (about 1.1 M). It is believed that the nearest environment of ions includes from 4 to 8 water molecules. Thus, the hydrate shell of ions should contain at least 25% of all water molecules. This seems to have a very strong affect on the extend of coverage of water molecules by hydrogen bonds and have to be well visible in the vibrational spectra. Only a few of the large number of results will be presented here.
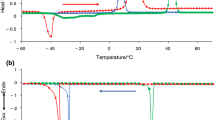
The infrared spectra of band of absorption of νOD HDO for pure solvent (H2O + HDO) and for 1.1 M solution NaCl are not very different (Fig. 2.6). So only a careful analyzed of the shape of observed bands one can help to understand whether ions of dissolved salt influences the character of hydrogen bonding in water. Indeed the integrated intensity (Fig. 2.7a) and position of the band maximum (Fig. 2.7b) for pure solvent and solution, which are very sensitive to the extent of hydrogen bonding are practically in the limit of experimental error. Nevertheless the temperature trends show that most likely hydrogen bonds are stronger in water at low temperatures while at high temperatures they are stronger in solution. The temperature dependence of the band half-width in the infrared spectra of the solution and solvent is shown in Fig. 2.8. The increase in half-width at supercritical temperatures can easily be explained. It has been shown (Gorbaty and Bondarenko 1998) that above the critical temperature free (inertial) rotation of water molecules becomes possible. This leads to an increase half-width of the stretching band with increasing temperature and, at lower density, to the appearance of unauthorized P, Q and R-rotational branches. The maximum half-width observed at 100–150 °C seems to reflect the change in the energy distribution between the different species of the structure. In fact, the maximum half-width is a purely quantitative consequence of the monotonic decreasing of the degree of hydrogen bonding with the increase in temperature.
In Raman spectra of NaCl aqueous solution at the same parameters as in the absorption spectra, the quantitative parameters of solution and water spectra practically do not differ. One can only notice that the half-width of the solution spectra in the Raman scattering spectra as well as in the absorption is somewhat smaller than in spectra of the solvent, which probably means a higher degree of orderliness.
The results of the study of vibrational spectra of aqueous NaCl solution show that a large number (not less than a quarter) of all molecules in the nearest environment of ions have practically no or very little influence on the state of hydrogen bonds. There are two possible explanations for this unexpected fact.
-
1.
Energy of interaction of a water molecule, in particular, with chlorine ion is close to energy of normal hydrogen bonding.
-
2.
Chlorine and sodium ions do not form clearly defined hydrate shells, but are simply built into the cavities between the bonded molecules, with little or no effect on the near order in such a matrix.
Water solutions of salts NaNO3, Zn[NO3]2 and orthophosphoric acid H3PO4 were investigated by the method of Raman spectroscopy. These studies were mainly aimed at determining the stability of multi-atomic anions at high temperatures and pressures. All obtained spectra were reduced to the intensity of bending mode of the water, taken as an internal standard, taking into account the change in the density of the solution.
Figure 2.9 shows the behavior of aqueous solution Zn[NO3]2 at heating up to 450 °C at constant pressure 1000 bars. Narrow intensive line at 980 cm−1 belongs to anion [NO3]−. It can be clearly seen that at a temperature of 350 °C the intensity of the line begins to decrease rapidly. At 450 °C, it disappears completely, which indicates the complete decomposition of the anion. The appearance of a weak line at 1850 cm−1 may indicate the formation of NO. The same result was obtained for NaNO3 aqueous solution
Raman spectra of H3PO4 aqueous solution with concentration of 2.91 mol% were obtained at a constant pressure of 1000 bar in the temperature range of 25–400 °C. The results are shown in Fig. 2.10. It can be assumed that the spectra change little between 25 and 250 °C, and only at 300 °C does the spectrum change dramatically and especially the scattering intensity. In fact, the composition of the solution changes continuously, starting from the lowest temperatures. It is quite clear that at 300 °C, a new compound appears in the solution, which has not yet been confidently identified, and the concentration of anion [PO4]− is greatly reduced. It is known that for ions containing phosphorus, there is a strong temperature dependence of the forms of their existence. In addition, the phosphorus-containing compounds in the aqueous solution are more prone to polymerization. All this, along with the fact that phosphates are a valuable mineral raw material and the basis for some semiconductor compounds, makes such an object extremely interesting.
Near the critical water temperature, the solution content falls into the sediment. The solution becomes opaque and it is no longer possible to obtain the spectrum at 400 °C. A microscopic study of the sediment deposited on a sapphire window shows that it is mainly composed of rounded particles and needle crystal fragments. This is likely to indicate that, at high temperatures, not one but several compounds are formed in the solution. At supercritical temperatures, the solubility of phosphorus-containing compounds in the hydrothermal fluid decreases dramatically.
References
Akiya N, Savage PE (2002) Roles of water for chemical reaction in high temperature water. Chem Rev 102(8):2725–2750
Barlow SJ, Bondarenko GV, Gorbaty YE, Yamaguchi T, Poliakoff M (2002) An IR study of hydrogen bonding in liquid and supercritical alcohols. J Phys Chem A 106(43):10452–10460
Bermejo MD, Cocero MJ (2006) Supercritical water oxidation: a technical review. AlChE J 52(11):3933–3951
Bernal J, Fowler RH (1933) A theory of water and ionic solution with particular reference to hydrogen and hydroxyl ions. Chem Phys 1:515–548
Bondarenko GV, Gorbaty YE (1997) In situ Raman spectroscopic study of sulphur-saturated water at 1000 bar between 200 and 500 °C. Geochim et Cosmochim Acta 61(7):1413–1420
Bondarenko GV, Gorbaty YE (2011) Hydrogen bonding in aqueous solution of NaClO4. Mol Phys 109(5):783–788
Bondarenko GV, Gorbaty YE, Okhulkov AV, Kalinichev AG (2006) Structure and hydrogen bonding in liquid and supercritical aqueous NaCl solutions at a pressure of 1000 bar and temperature up to 500 C: a comprehensive experimental and computational study. J Phys Chem A 110(11):4042–4052
Cabanas A, Poliakoff M (2001) The continuous hydrothermal synthesis of nano-particulate ferrites in near critical and supercritical water. J Mater Chem 11:1408–1416
Driesner T (1996) The effect of pressure on deuterium-hydrogen fractionation in high temperature. Science 277:791–794
Fedyaeva ON, Vostricov AA, Sokol MY, Fedorova NI (2013) Hydrogenezation of bitumen in supercritical water flow and the effect of zinc addition. Russ J Phys Chem B7(7):820–828
Franck EU (1987) Fuids at high pressure and temperature. Pure Appl Chem 59(1):25–29
Giessen BC, Gordon GE (1968) X-ray diffraction new high-speed technique based on X-ray spectrography. Science 159(3818):973–975
Gorbaty YE (2007) Spectroscopic techniques for studying liquids and supercritical fluids at high temperature and pressure. Sverhcriticheskie fluidy Teoriya Pract 2(1):40–53
Gorbaty YE, Bodarenko GV (1995) High-pressure high-temperature Raman cell for corrosive liquids. Rev Sci Instrum 66(8):4347–4349
Gorbaty YE, Bodarenko GV (1999) Experimental technique for quantitative IR studies of highly absorbing substances at high temperature and pressure. Appl Spectoscopy 53(8):908–913
Gorbaty YE, Bodarenko GV (2007) Water in supercritical state. Sverhcriticheskie fluidy Teoriya Pract 2(2):5–18
Gorbaty YE, Bodarenko GV (2017) Transition of liquid water to the supercritical state. J Mol Liq 239:5–9
Gorbaty YE, Bondarenko GV (1998) The physical state of supercritical fluids. J Supercrit Fluids 14:1–8
Gorbaty YE, Kalinichev AG (1995) Hydrogen bonding in supercritical water. 1. Experimental results. J Phys Chem 99(15):5336–5340
Gorbaty YE, Okhulkov AV (1994) High-pressure x-ray cell for studying the structure of fluids with the energy-dispersive technique. Rev Sci Instrum 65(7):2195–2198
Gorbaty YE, Bodarenko GV Venardow E, Barlow S, Garsia-Verdugo E, Poliakoff M (2004) Experimental spectroscopic high-temperature high-pressure techniques for studying liquids and supercritical fluids. Vibr Spectrosc 35:97–101
Kalinashev AG, Churakov SV (1999) Size and topology of molecular clusters in supercritical water: a molecular dynamic simulation. Chem Phys Lett 302(5–6):411–417
Krishtal S, Kiselev M, Puhovski Y, Kerdcharoen T, Hannongbua S, Heinzinger K (2001) Study of hydrogen bond network in sub- and supercritical water by molecular dynamics simulation. Z Naturforsch 56a:579–584
Okhulkov AV, Gorbaty YE (2001) The pair correlation functions of 1.1 M NaCl aqueous solution at constant pressure of 1000 bar in the temperature range 20–500 °C. J Mol Liq 93:39–42
Poliakoff M, King P (2001). Phenomenal fluids. Nature 412:125–125
Purcarova E, Ciahotny K, Svab M, Skoblia S, Beno Z (2018) Supercritical water gasification of wastes from the paper industry. J Supercrit Fluids 135:130–136
Stanley HE, Teixeira J (1980) Interpretation of the unusual behavior of H2O and D2O at temperatures: test of a percolation model. J Chem Phys 73(7):3404–3422
Viswanathan R, Gupta RB (2003) Formation of zinc oxide nanoparticles in supercritical water. J Supercrit Fluids 27(2):187–193
Wang J, Zhang Y, Zheng W, Chou I-M, Pan Z (2018) Using Raman spectroscopy and a fused quartz tube reactor to study the oxidation of o-dichlorobenzene in hot compressed water. J Supercrit Fluids 140:380–386
Yukhnevich GV (1997) The mechanism of occurrence of cooperative properties in conjugate hydrogen-bonds. Spectrs Lett 30(5):901–914
Acknowledgements
The study was support by the draft AAAA-A18-118020590149-0
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bondarenko, G.V., Gorbaty, Y.E. (2020). Experimental Studies of Hydrothermal Fluid. In: Litvin, Y., Safonov, O. (eds) Advances in Experimental and Genetic Mineralogy. Springer Mineralogy. Springer, Cham. https://doi.org/10.1007/978-3-030-42859-4_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-42859-4_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-42858-7
Online ISBN: 978-3-030-42859-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)