Abstract
During the last few years, a great interested has been given in for nanotechnology applications in pharmaceutical technology. At the same time, there is a growing research effort in the search for healthier and safer products. In this context, the use of naturally originate raw materials, essential oils for instance, rises as an interesting approach for replacing several synthetic active pharmaceutical ingredients. However, these materials often feature low bioavailability, uncontrolled volatility or low long-term stability, which require novel encapsulation techniques. Therefore, research efforts have been focusing on nanoemulsion technology that is particularly suited to produce novel products. Nanoemulsions constitute one interesting vehicle for enhancing solubility, stability and delivering natural oils, by encapsulating them into nanosized micelles with sizes ranging from 20–200 nm. They gather some unique characteristics as small size, increased surface area and stability which can increase efficiency and biological effects of pharmaceutical dosage forms.
The wide application of nanoemulsions to the encapsulation of bioactive molecules still require much development in order to achieve the optimization of the obtention methods for large-scale production. This chapter includes an overview about nanoemulsion stability characteristics and the different approaches for obtaining nanoemulsions, including high energy methods like high-pressure homogenization, microfluidizers and ultrasonic homogenization, which are the most used approaches, and low energy methods such as phase inversion composition and phase inversion temperature, simple but still less reproduceable methods. In addition, we present the main aspects of nanoemulsion formulations, type of surfactants and oil phase, and the techniques for characterizing nanoemulsions, for example, dynamic light scattering, zeta potential, microscopy and X-ray diffraction. Moreover, applications of nanoemulsions to pharmaceutical technological approaches for essential oils encapsulation, such as the improvement of bioactive oils bioavailability and solubilization, masking unpleasant aspects of oils and enhancement of essential oils pharmacological activity are also discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Recently, the use of naturally originate bioactive molecules, especially natural antioxidants and anti-microbial agents, has been rising. The search for natural products has been triggering more and more research in the development of suitable and stable formulations. Essential oils are bioactive molecules, being considered safe and biocompatible, offering a great range of benefits due to a complex composition of fatty acids, terpenes, triterpenes and many other lipophilic components, that can offer protection against dehydration, solar radiation, inflammation, insect attack, microorganisms, and viruses (Donsì and Ferrari 2016; Andreu et al. 2015; Bonferoni et al. 2017).
Many essential oils present proved antioxidant potential, preventing oxidation reaction and reducing the formation of free radicals, which are risk factors for cellular damage and for chronicle diseases as cancer. However, one of the main challenges to formulate essential oils into a pharmaceutical product lies on low water solubility of most of oils, which impairs bioavailability of lipophilic components and stability in hydrophilic formulations. Moreover, many essential oils are highly volatile and labile, being unstable under many environmental conditions, as light exposure and oxidation. Those restrictions must be overcome during the development of pharmaceutical formulations (Badgujar et al. 2014; Herman and Herman 2015; Moghimi et al. 2016).
Nanoemulsions are nanosized colloidal dispersions with droplets within the nanometric scale, more often between 20 and 200 nm. Nanoemulsions are thermodynamically stable isotropic systems in which two immiscible liquids are mixed to form a single phase by means of an emulsifying agent, i.e., a surfactant (Helgeson 2016; Solans et al. 2005). Nanoemulsions have been considered of a great potential of industrial application in pharmaceutical, food, agrochemical, cosmetics and personal care products (McClements and Jafari 2018). Nanoemulsions are widely applied to encapsulate, protect and promote controlled release of lipophilic bioactive molecules. Besides, nanoemulsions can also act as templates to produce other types of nanoparticles, such as solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), multiple emulsions and hydrogel-thickened nanoemulsions (Sutradhar and Amin 2013; McClements and Jafari 2018; Barradas et al. 2017).
Because of the reduced droplet size, nanoemulsions promote interesting characteristics in comparison to conventional emulsions, i.e. macroemulsions. For instance, nanoemulsions present much larger surface area of dispersed phase in relation to the total volume of the dispersion than that observed in macroemulsions. Thus, droplet deformation-derived phenomena are typically higher for nanoemulsions than for conventional emulsions (Helgeson 2016). Besides, macroemulsions exhibit multiple light scattering, which provide them a white opaque appearance. In contrast, in nanoemulsions, droplets are much smaller than the wavelength of visible light. Hence, most of the nanoemulsions are optically transparent systems when droplet size is small enough, usually below 100 nm (McClements and Rao 2011).
The development of nanoemulsions as nanostructured carriers for pharmaceutical application requires an accurate selection of the components such as, surfactants, and oils as well as the preparation method selected for nanoemulsion production. Special attention must be given to oily phase used as inner phase in oil-in-water nanoemulsions. In order to obtain nanoemulsions, a minimum energy input is necessary. Energy input can be achieved by applying work to the system, usually called “high energy” methods, of by modulating composition or temperature of the system, referred as “low energy” methods. Both methods require the same amount of energy for providing nanosized droplets, regardless if the energy is provided mechanically or thermodynamically (Helgeson 2016; McClements and Jafari 2018).
Nanoemulsions and polymeric micelles can be considered promising systems for encapsulation of essential oils since because of various advantages such as: sustained and controlled release of bioactive molecules; solubilization of lipophilic substances as oils; they are suitable for administration under different routes; protection from chemical, environmental and enzymatic degradation of labile molecules; controlled volatilization of essential oils, reduction of side effects and dose.
In this chapter, we provide an overview focused on formulation of oil-in-water nanoemulsions, as they can provide encapsulation of lipophilic components, and diverse approaches for nanoemulsions production. Additionally, we summarize the instability phenomena related to nanoemulsions and the recent applications of nanoemulsions for the encapsulation of natural oils.
4.2 Nanoemulsions: General Aspects
Emulsions are heterogeneous colloidal systems, consisting of an immiscible liquid dispersed as droplets, i.e. disperse or inner phase in another immiscible liquid, i.e., continuous or outer phase, stabilized by a surfactant. When the continuous phase is an aqueous solution and the dispersed phase is an oil, the emulsion is called oil-in-water emulsion (Fig. 4.1a). On the other hand, when the dispersed phase is an aqueous solution and the continuous phase is constituted by an oil, the emulsion is called water-in-oil emulsion (Fig. 4.1b). In all cases, an emulsifier agent or surfactant is needed to reduce interfacial tension between dispersed and continuous phases and provide stable systems. The nature of the surfactant and the stabilizing film provided by it, greatly influence general aspects of nanoemulsions, as long-term stability, droplet size and charge, for example. In general, emulsified systems can be classified according to some physicochemical properties such as droplet size and preparation methods into macroemulsions, microemulsions and nanoemulsions.
Oil-in-water nanoemulsion (a) and water-in-oil nanoemulsion (b), composed by oil and aqueous phases, stabilized by surfactants. In oil-in-water nanoemulsion aqueous phase constitutes the outer phase and oil phase is the inner phase, and the surfactant molecule self-assemble into a micelle structure, with hydrophilic portion oriented towards the aqueous phase. In water-in-oil nanoemulsions oil phase and water phase constitute the outer phase and inner phase, respectively, and surfactants hydrophobic portion is in contact with the outer phase
The most important difference between nanoemulsions and macroemulsions lies in the droplet size, since both are thermodynamically unstable systems. Nanoemulsions usually show mean droplet size smaller than 200 nm, although there is no agreement regarding the size range that comprise nanoemulsion and distinguish them from micro-sized and conventional-sized macroemulsions. According to the literature, droplet size can vary from 20 nm to either 500, 300, 200, or yet 100 nm, being until now a controversial issue regarding nanoemulsions classification (Solans and Solé 2012; McClements and Rao 2011).
In general, macroemulsions are optically opaque, due to the large droplet size, which produces multiple light scattering, while nanoemulsions are often transparent or translucent, since they present small droplet size scatter light weakly (McClements and Jafari 2018; McClements 2011). Nanoemulsions are kinetically stable systems and do not require the addition of a cosurfactant, unlike microemulsions. Kinetic stability is attributed to the Brownian motion of the droplets which overcomes the gravity force and prevents droplets sedimentation and coalescence. In addition, the use of nonionic or polymeric surfactants may cause steric repulsion between the droplets and contribute to the stability of the system (Tadros et al. 2004; Solans et al. 2005).
Besides storage stability, there are other interesting physical properties that differentiate nanoemulsions from macroemulsions. For example, nanoemulsions have dispersed phase surface area relative to the total volume of the dispersion much larger than that observed in macroemulsions. Due to the high relative surface area, all phenomena related to droplet deformation are typically more relevant to nanoemulsions than to macroemulsions (Mason et al. 2006).
It is noteworthy the common misconception between nano and microemulsions. There has been much effort to clarify the difference between these two colloidal systems in this scientific field (Sonneville-Aubrun et al. 2004; Anton and Vandamme 2011). Both microemulsions and nanoemulsions contain droplets of diameters ranging from 20 to 200 nm, differing in certain aspects, especially regarding the preparation method (Abismail et al. 1999; McClements 2011). Although both systems can feature similar characteristics, as nanosized droplets as inner phase dispersed in a continuous phase, they differ in therms of stability and in physicochemical concepts (Anton and Vandamme 2011; McClements 2012). Microemulsions is a term usually used to refer to thermodynamically stable isotropic oil/surfactant/water systems, while nanoemulsions are kinetically stable conventional emulsions constituted by nanosized micelles (McClements 2012). Microemulsions can feature a wide range of structures with one, two, three ore more phases in equilibrium, depending on the concentrations between all the components and upon certain temperatures. These different structures can be water-continuous, oil-continuous or bicontinuous and nanometric swollen micelles, which gives them a bluish and translucent nanoemulsion-like aspect. However, micelles can form different geometries such as, worm-like, bicontinuous sponge-like, liquid crystalline, hexagonal, lamellar, and spherical swollen micelles, which is the most often confused with nanoemulsions (Anton and Vandamme 2011; McClements 2012).
Moreover, nanosized micelles from microemulsion are deeply afected in morphology and size upon temperature and dilution, while nano-micelles from nanoemulsions remain unaltered when submited to the same conditions, which is an important aspect to be considered when microemulsions are intended to be applied as parenteral prooducts. Parenteral route can pose some challenges as infinite dillution conditions and temperature, pH and osmolarity variations. Under biological conditions, only nanoemulsions remain stable (Lefebvre et al. 2017; Hörmann and Zimmer 2016; Anton and Vandamme 2011).
These misinterpretations are often found in literature, where one can find inadequate methods for obtaining and characterizing micro and nanoemulsions. For example, ternary phase diagrams to produce nanoemulsions constitutes a very frequent but inapropriate methodology that can be more suitable to produce microemulsions. The simplicity of low-energy emulsifying methods is the main aspect that can induce the confusion between nano and microemulsions. Besides, in order produce nanoemulsions by low-energy methods, surfactant must be mixed in oil phase before adding water, not being possible inverting the order of components incorporation. Microemulsions can be formed no matter the order in which components are added (Anton and Vandamme 2011). This difference between both systems is an easy and simple preliminary test for classification of a system in whether nano or microemulsions.
The large surface area provided by the nanostructured droplets increases drug absorption in biological membranes such as the intestinal epithelium, the cornea and skin for example (Singh et al. 2017). Moreover, because they are transparent formulations, nanoemulsions present an interesting aesthetic characteristic, even containing significant quantities of oil in the composition (Sonneville-Aubrun et al. 2004; Tadros et al. 2004).
4.3 Nanoemulsions: Composition
In general, a classical nanoemulsion formulation comprises an aqueous and an oil phase stabilized by a surfactant. The physical-chemical properties of the components will greatly impact the formation and stability of the nanoemulsions obtained from them. Typically, nanoemulsions require relatively high amounts of surfactants, usually from 10 to 15wt.%, in order to stabilize the high surface area of nanosized micelles (Azeem et al. 2009; Mason et al. 2006). The composition of nanoemulsions can be tuned by careful selection of the ingredients used to obtain them.
4.3.1 Surfactants
Surfactants are molecules whose structures comprise two parts of opposing affinities, one having a hydrophilic character and the other with a hydrophobic character. Both hydrophilic and lipophilic moieties should be in equilibrium in the surfactant molecule, so that it constitutes a good emulsifier. This hydrophilic-lipophilic equilibrium of a both moieties in the surfactant molecular structure is called hydrophilic-lipophilic balance (Abismail et al. 1999; Porras et al. 2004). Hydrophilic-lipophilic balance is a classifying system for all surfactants and provides a good indication for the right the choice of emulsifiers suitable for obtaining stable emulsions. During the production of nanoemulsions, surfactants molecules should be able to quickly adsorb on newly formed droplet surface, while maintaining droplet integrity once two droplets collide, avoiding droplet deformation or coalescence (Tadros 1994).
The formation of a stable nanoemulsion involves selecting an appropriate composition, controlling the order of addition of the components and applying a minimum energy input capable of promoting the deformation of droplets. This minimum energy input is related to the Young-Laplace Theory, which shows that the difference between the external and internal pressures of a drop is a direct function of the droplet radius (Eq. 4.1). Therefore, to break a drop into droplets of smaller size, the difference between the internal and external pressure to the drop should be considerable (Tadros et al. 2004).
where,
-
Pi = Internal pressure
-
Pe = External pressure
-
γ = Interfacial tension
-
r = Radius of droplet curvature
According to Eq. 4.1, large amounts of surfactants are required to reduce surface tension and stabilize the great interfacial surface produced. The production of nanoemulsions, therefore, is a thermodynamic unfavorable and non-spontaneous process. Besides, to obtain droplets of nanometric size it is necessary to supply energy to the system, usually provided by mechanical devices or even by chemical potential of the constituents. Nanoemulsions often present spherical droplets because both the high interfacial tension and small droplet size combined provide high Laplace pressures, which triggers the minimization of the oil–water interfacial area into a spherical shape (McClements 2011).
The success of developing a stable nanoemulsion depends on the right selection of the surfactant used, which shall act on the interface between dispersed and continuous phases. The choice of a surfactant for pharmaceutical purposes is one of the most critical steps and involves consideration of the toxicity of substances that can be applied in large quantities. (Tadros 2009; Wiedmann 2003). The selection of a surfactant can be considered the most important step to produce nanoemulsions, since emulsifiers can influence both fabrication method and performance of nanoemulsions.
Due to the amphiphilic molecular constitution, surfactants tend to migrate to the interfaces between two immiscible fluids, in a way that polar groups are oriented to the aqueous phase and the non-polar, to the organic phase. The polar group is of great importance because it defines some properties, such as water solubility and the classification of the surfactants which can be divided into ionic, which can be anionic and cationic, nonionic and amphoteric, or zwitterionic.
At low concentrations, the surfactant molecules are solubilized within the solution as free soluble surfactant molecules (Fig. 4.2a). As the surfactant concentration increases, a decrease in surface tension of the solution occurs, representing the surfactant adsorption at air-water surface (Fig. 4.2b). When reaching a certain concentration, it is observed that the variation of the surface tension is minimal in relation to the increase of the concentration, which means that, the saturation of the water-air interface is reached. At this stage, the adsorption of the surfactant on the surface is no longer observed. The surfactant concentration that causes this phenomenon is called the critical micelle concentration, where it can be observed the formation of molecular aggregates, known as micelles (Fig. 4.2c).
Micellization steps: surfactants as free soluble molecules, below critical micelle concentration (a). As surfactant concentration raises, surfactants start to self-assemble as monolayer in water-air interface (b). Above critical micelle concentration, water-air interface is saturated with surfactant molecules and interfacial tension no longer reduces. Then surfactants start to self-assemble as micelles, in a thermodynamically favorable phenomenon (c)
The moieties that are not soluble in the surrounding solvent attract strongly and produce a stable compact vesicular morphology, as to expel the solvent from this environment. On the other hand, groups that are very soluble, tend to be externally exposed producing a soluble particle, i.e., a micelle (Myers 2005b). The size of the micelles and the number of surfactant molecules per micelle depends on the type of surfactant and the physicochemical environment. When the surfactant is adsorbed at the oil-water interface, interfacial tension decreases causing a steric hindrance effect or electrostatic repulsion, both able to prevent coalescence or aggregation phenomena. These barriers not only prevent emulsion droplets from coming into direct contact, but also serve to stabilize the liquid film between two adjacent droplets (Myers 2005a).
The interfacial tension is related to the amount of surfactant adsorbed at the interface and the nature of the interfacial layer. The interfacial tension decreases with increasing surface charge, which is directly related to the concentration and size of the surfactant. However, depending on the type of surfactant, many other effects are important (Tadros et al. 2004; Myers 2005a). It can be said, then, that surfactants play a very important role in the stabilization or destabilization of nanoemulsions, increasing or reducing the electrostatic or steric repulsions of the interface, which are dependent on structural aspects, such as double electric layer, branching, aromaticity. Other factors like the presence and type of electrolytes, pH, temperature and presence of additives (Myers 2005a). The most studied for the formation of nanoemulsions are non-ionic surfactants.
Both liquids and solids feature surface tension due to the cohesive energy between their molecules. Surface tension is resultant from unbalanced forces of attraction or non-equilibrated in interfacial regions, in which there is a sudden variation of the density. Thus, the resultant force on a molecule close to the surface liquid/vapor is different from that on a molecule that is in a completely homogeneous region, in which the resulting force is null. The surface tension can also be defined as the excess of surface energy (Tsujii 1998; Shaw 1992).
Liquids tend to self-assemble into forms that minimize surface area, that is, a greater number of molecules are inside the liquid and, therefore, remain surrounded by other liquid molecules. Liquid droplets therefore tend to be spherical because a sphere is the shape with the smallest surface/volume ratio. Surface effects can be expressed according to the Helmholtz and Gibbs energies (Eq. 4.2), in which the bond between these amounts of energy and the surface area is the work (force) needed to increase the surface area (σ) of a liquid in a given quantity (Hunter 2001)
where, W means work or applied force; γ (N.m−1) means superficial tension and θ superficial tension.
The effect of surface tension is to minimize surface area, resulting in a curved surface, as in a vesicle. The intensity of this tension depends on the liquid, solvent purity and temperature in which the surfactant lies (Hunter 2001). The same considerations apply to the interface between two immiscible liquids. Again, intermolecular forces in non-equilibrium are presented, however with decreased intensity. The interfacial tensions usually lie between the individual surface tension of the two liquids above mentioned (Shaw 1992).
4.3.2 Types of Surfactants
There are several surfactants that have been used in the production of nanoemulsions, since small-molecule synthetic surfactants, which may be non-ionic and ionic, and natural surfactants such as phospholipids, proteins and polysaccharides. In general, the surfactants may be: (i) non-ionic, (ii) amphoteric (iii) cationic, or (iv) anionic.
The class of the non-ionic surfactants comprise small molecules such as sorbitan esters, like Span® and Tween® series and polymeric surfactants, such as poly(oxyethylene) ethers, for example, Brij® or block copolymers as poly(ethylene oxide)-poly(propylene oxide). Small molecules can form small-sized droplets because of the facility on easily adsorb on the surface of droplets that they feature (Jafari et al. 2017).
Natural surfactants exhibit excellent surface activity, although they present molecular structure being very voluminous compared to synthetic surfactants. Since natural surfactants are naturally produced substances, they show advantages like biodegradability, are easily produced from renewable resources, present high specificity and tend to less toxic (Salem and Ezzat 2018). Table 4.1 summarizes the most common types of natural and synthetic surfactants.
The use of natural surfactants, due to the innocuous character, provides some advantages over synthetic surfactants, including biocompatibility and biodegradability, multifunctional characteristics, stable activity under different environmental conditions, such as high or low temperatures, pH, high pressure and osmolarity. Thus, natural surfactants may be more effective in stabilizing nanoemulsions for the most diverse purposes (Dickinson 2003; Qian and McClements 2011). In general, most of natural surfactants are large molecules, which can delay the ability to adsorb on the droplets surface and provide larger droplets. However, they can provide good stability by both steric and electrostatic stabilization (Qian and McClements 2011).
One of the most common natural surfactants used in the development of nanoemulsions are phospholipids. Phospholipids can be classified as natural amphoteric surfactants and have excellent biocompatibility because they are similar in composition to cell membranes. The term lecithin is commonly used to designate a mixture of phospholipids obtained from both animal and vegetable sources, as eggs and soybean, respectively (Shchipunov 2015). The composition of lecithin may vary upon several environmental and processing conditions, however, the phospholipids most commonly found in lecithin are: phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, sphingomyelin, glycerol phospholipids of complex fatty acid composition (Klang and Valenta 2011; Shchipunov 2015).
The polar portion of lecithin is comprised by positively charged phosphate groups, which are bounded to lipophilic chains by ester groups. Moreover, nitrogen-containing moieties bounded to phosphate groups can assume negative charges, providing phospholipids such as lecithin an amphoteric character (Shchipunov 2015).
Biopolymers such as polysaccharides have been attracting attention as natural surfactants for producing stable nanoemulsions (Dickinson 2003; Bai et al. 2016). Polysaccharides feature a complex structure, water-soluble, with good emulsifying properties and thickening properties, are widely used in food, pharmaceutical and paper industries. Polysaccharides and gums such as gum arabic are often used as thickening or gelling agents in pharmaceutical industry (Prajapati et al. 2013). The polysaccharides most commonly found are hydrocolloids such as xanthan, modified starch, galactomannans and pectin (Dickinson 2003; Prabaharan 2011; Sweedman et al. 2013; Prajapati et al. 2013). Recently, they have been considered promising components to be used as natural surfactants as there is a growing demand for natural and sustainable ingredients. The emulsification properties of polysaccharides are related to the presence of non-polar groups and hydrophilic groups, providing the interaction with both oil and water. The stabilization method related to polysaccharides can occur by both electrostatic repulsion and steric effect, preventing droplet flocculation and coalescence (Jafari et al. 2017).
Gum arabic is one of the most used polysaccharides for producing stable emulsions, due to the formation of a stable layer around the droplets, providing steric repulsion among droplets by forming steric layer around them (Yadav et al. 2007; McNamee et al. 1998). Other naturally occurring polysaccharides used in producing nanoemulsion comprise pectin, a heterogenous anionic polysaccharide already used as gelling, thickening and stabilizing properties (Bai et al. 2016) and maltodextrin (Sonu et al. 2018).
Some proteins, especially those obtained from bovine milk, as casein and whey proteins, can be used as emulsifying and surfactants agents, since they feature both hydrophilic and lipophilic residues and surface activity, providing cohesive and strong films around droplets (Yerramilli and Ghosh 2017; Mayer et al. 2013; Kuhn and Cunha 2012; Adjonu et al. 2014). Proteins can stabilize droplets mainly by electrostatical repulsion because of the presence of negatively charged groups, although steric effect can also be attributed to them (Adjonu et al. 2014; Dickinson et al. 1998). As proteins are very large molecules, the adsorption on droplet surface takes longer. On the other hand, proteins feature the ability of forming a viscoelastic coating that prevent droplet deformation and, thus aggregation, providing higher stability under several conditions (McClements 2011). One of the most studied proteins used as natural surfactants is casein.
Casein comprises almost 80% of milk protein and it is a heterogeneous protein composed by four main proteins fractions: αs1 (~44 wt.%), αs2 (~11 wt.%), β (~32 wt.%) and k (~11 wt.%) with a strong aggregation behavior, which is fundamental to stabilize casein-based micelles (Wang and Zhang 2017; Sharma et al. 2017). Casein provides both electrostatic and steric stabilization against coalescence under neutral pH values (Dickinson et al. 1998). At acidic pH values, the negative charge on caseinate structure is reduced and aggregation between casein-based micelles occurs (Sharma et al. 2017).
Several works have reported the combination of proteins and polysaccharides-based emulsifiers based on the ability to form polyelectrolyte complexes between these molecules and the possibility of Maillard reaction, which contribute to increase stability of the layer around nanosized micelles end prevent coalescence (Sonu et al. 2018; Farshi et al. 2019).
There is a growing demand on replacing synthetic surfactants for natural molecules. However, the application of natural surfactants still is limited by several issues. For example, Phospholipids and protein-based surfactants, are unstable under environmental conditions such as pH, osmotic pressure and heating. Polysaccharides, on the other hand, provide stability under environmental stressful conditions (Zhang et al. 2015). Besides, polysaccharides can stabilize nanoemulsion by increasing the viscosity oh aqueous phase by stablishing hydrogen bonds water molecules (Dickinson 2009). Moreover, according to Qian and McClements 2011, small-molecule surfactants can provide smaller droplets than proteins, which can be related to the ability to rapidly adsorb to the droplet surfaces during homogenization and providing a more stable interface.
The solubility of synthetic or ionic surfactants is related to the interactions between the ionic group, which is the polar part of the surfactant, and water. The ionic surfactants most used in the production of nanoemulsion are summarized in Table 4.1. In general, ionic surfactants are resistant to changes in temperature and quite soluble in water. The applications of ionic surfactants are originated from these properties. Also, pH has great influence on the solubility of the ionic surfactants due to the neutralization reactions that occur with this type of molecules.
In the case of anionic products, a reduction in pH causes a decrease in the degree of dissociation and in the solubility of the surfactant due to the formation of the corresponding undissociated acid, which has lower solubility. The electrical charge on droplet surface plays an important role in stabilizing nanosized droplets as electrostatic repulsion, aggregation stability etc. Moreover, surface charge can be explored to provide nanoemulsions novel application by triggering the interaction of charged surfactants with other components in biological media (Tian et al. 2016). Quaternary ammonium salts belong to the class of cationic surfactants, the best-known being hexadecyl trimethyl ammonium bromide and dodecyl ammonium bromide (Bouchemal et al. 2004; Lawrence and Rees 2000).
In the case of ionic surfactants, the stability of nanoemulsions is mainly governed by electrostatic interaction between the droplets. Surface charge and, consequently, zeta potential can be modulated by mixing non-ionic and ionic surfactants and varying the proportion of both of them, providing extremely long-term stable nanoemulsion (Tian et al. 2016; Babchin and Schramm 2012). Sodium lauryl sulfate (SLS) or sodium dodecyl sulfate is the most common anionic surfactant used to produce emulsions. However, they can disorganize several cell membrane organization and affect both protein and lipid structures, being highly irritant for skin application and toxic for systemic administration (Elmahjoubi et al. 2009; Bondi et al. 2015).
Non-ionic surfactants are widely used for pharmaceutical purposes because they feature low toxicity and greater stability against changes in ionic strength and pH of the common biological media. There are different types of non-ionic surfactants, the most used to provide pharmaceutical nanoemulsions are polyglycerol alkyl ethers, glucosyl dialkyl ethers, crownethers, ester-linked surfactants, polyoxyethylene alkyl ethers, ethoxylated hydrogenated castor oil, Brij, Spans, or sorbitan esters and Tweens, or Polysorbates.
Synthetic non-ionic surfactants show no charges on the polar part of the molecular structure, which is often composed of ethylene oxide groups. For this reason, solubility of synthetic non-ionic surfactants in aqueous solution differs from most solutes: with increasing temperature, these surfactants show phase separation, and this temperature is known as cloud point temperature. This behavior occurs because of the increasing the size of the molecular aggregates, known as micelles and the inter-micellar attraction. This can be explained by the dehydration of the outer layer of the micelle of the nonionic surfactant with the increase in temperature, which is promoted by the breaking of the hydrogen bonds between the ethylene oxide group and the water (Prud’homme et al. 1996; Alexandridis et al. 1994).
Compared with low molecular weight surfactants, non-ionic polymeric surfactants produce more stable micelles, have lower critical micelle concentration values, with a slower dissociation rate, which allows controlling the release of the molecules contained in the nucleus of nano-sized droplets and also achieve greater accumulation of the drug at a specific site. In addition, these copolymers are capable of forming micelles in a wide variety of solvents (Xing and Mattice 1997; Kataoka et al. 2001).
Like Mao et al. studied the effect of different surfactants on interfacial tension, droplet size, zeta potential and morphology of β-carotene-loaded nanoemulsions produced by high pressure homogenization. High molecular weight surfactants, such as Tween 20®, decaglycerol monolaurate, modified starch, whey protein isolate were evaluated. The results showed that Tween 20® and decaglycerol monolaurate provided smaller droplet size, although with poorer stability compared with modified starch and whey protein isolate, presenting droplets aggregates. On the other hand, modified starch and whey protein isolate provided larger droplets with higher stability against droplet aggregation due to the formation of stronger interfacial layers. Moreover, the combination of Tween 20 and whey protein isolate provided nanoemulsions with higher stability regarding β-carotene content under storage conditions of 55 °C for prolonged time (Like Mao et al. 2009).
4.3.3 Oil Phase
Oil phase composes the core of oil-in-water nanoemulsions and it can influence greatly both formation and stability of nanoemulsions. Several aspects as polarity, water miscibility, interfacial tension and viscosity can change nanoemulsions characteristics such as droplet size and stability (McClements 2011). Different types of oil phases can be used for the formation of nanoemulsions: synthetic, mineral, vegetable or essential oils. In this chapter the origin and the chemical and physicochemical properties of essential oils will be the focus. Triacylglycerols, free fatty acids, fixed/vegetable, essential and mineral oils, waxes alone or in associations can be used as oil phase upon nanoemulsion development (Jafari 2017). The most common the essential oils often found are clove, peppermint, sweet fennel and bergamot (Al-Subaie et al. 2015; Saberi et al. 2014; Pengon et al. 2018; Strickley 2004; Wan et al. 2019; Barradas et al. 2015). They can act as drug carriers or solvents or be used as the only constitute of oil phase due to pharmacological properties commonly attributed to essential oils.
Very often, oil phase of nanoemulsions are liquid, as natural oils. However, they can also be solid as natural waxes and solid lipids (in the case of SLN and NLC) (Sutradhar and Amin 2013; McClements and Jafari 2018). Physical state of oil droplets can influence creaming stability, optical properties and release pattern of the encapsulates bioactive molecules. The right selection of liquid and solid lipophilic components can provide the control over physical state of oil droplets (McClements 2015).
In both oil-in-water and water-in-oil, oil phase is one of the most important issues, since it can influence several physicochemical aspects, for example: viscosity, refractive index, transparency, interfacial tension stability, sensorial aspects and droplet size. As a consequence, a range of functional properties that are consequence of physicochemical parameters are affected, as bioavailability, miscibility and digestibility (McClements and Jafari 2018). Moreover, molecular weight of the components, interfacial tension, density and viscosity of the oil phase also influence the properties of nanoemulsions and can lead the choice of a certain type of emulsification method (McClements and Rao 2011).
Droplet stability is dependent on oil solubility, which is influenced by the amount of water soluble and lipophilic oil components. Great amounts of water soluble components in oil phase can lead to Ostwald ripening, the major source of instability in nanoemulsions (Chebil et al. 2013). There are some strategies to retard droplet growth due to Ostwald ripening. Ripening inhibitors can be added to oil phase of oil-in-water nanoemulsions prepared with highly soluble oils as essential oils (Rao and McClements 2012; McClements and Jafari 2018). Ripening inhibitors are usually highly hydrophobic molecules almost water insoluble able to increase hydrophobicity inside the droplets even upon diffusion of water-soluble molecules. As a consequence, there is an increase in the concentration of the ripening inhibitor inside droplets, promoting a concentration gradient in the system that triggers the diffusion of water-soluble components from large back to small droplets, as the opposite of what occurs in Ostwald ripening (McClements and Jafari 2018).
Oils and lipophilic components can be encapsulated inside nanomicelles with the aim of protecting labile and degradable bioactive molecules from environmental conditions, controlling release, increasing bioavailability and providing an easier manipulation or incorporation of oils into aqueous formulations. Moreover, encapsulation techniques can be applied to the modification of physicochemical properties of oils, which makes it possible to convert solutions into fine powders of to mask undesirable tastes when administrated by oral route.
4.3.4 Essential Oils
Essential oils are aromatic substances extracted from aromatic plants or plants parts, including leaves, fruits, barks and seeds. They considered raw materials of great importance for the cosmetic, pharmaceutical, fragrance and food industries. These pure and extremely potent organic substances are considered the main biochemical components of the therapeutic action of medicinal and aromatic plants (Pathania et al. 2018; Pérez-Recalde et al. 2018; Donsì and Ferrari 2016).
These are volatile substances extracted from aromatic plants, being extremely potent organic substances and considered the main biochemical components of the therapeutic action of medicinal and aromatic plants. The methods of extraction vary according to the location of the volatile oil in the plant and the proposed use of it. The most common methods are: enfleurage, steam distillation, or azeotropic distillation; extraction with organic solvent in a continuous and discontinuous way; pressing or supercritical CO2 extraction (Asbahani et al. 2015).
The designation of oil is given thanks to some physical-chemical characteristics, for example, that they are often oily-appearing liquids at room temperature. The main characteristic of essential oils is the volatility, which differs them from the fixed/vegetable oils, which are mixtures of lipid substances normally obtained from seeds. Another major characteristic is given thanks to the pleasant and intense aroma of most of the volatile oils, being therefore also called essences. They are still soluble in apolar organic solvents, as ether, thus receiving the name of ethereal oils or, in Latin, aetheroleum. Although they have a limited solubility in water, they have been used to increase water solubility of poorly soluble drug by constituting the inner phase of oil-in-water nanoemulsions (Barradas et al. 2017).
Essential oils constituents range from terpene hydrocarbons, simple and terpene alcohols, aldehydes, ketones, phenols, esters, ethers, oxides, peroxides, furans, organic acids, lactones, coumarins, to sulfur compounds. In the mixture, these compounds are present in different concentrations, and usually one of them is the majority compound, others are in lower levels and some in very low quantities (Donsì and Ferrari 2016)
Essential oils are well-known as flavors, antioxidant and antimicrobial products and have been widely used as functional ingredients in food, pharmaceutical, and cosmetic formulations (Jin et al. 2016). In recent years, the application of essential oils in food products has generated great interest because essential oils are generally recognized as safe regulatory status, multiple functionalities, and wide acceptance by consumers. However, the poor solubility in aqueous solutions and high volatility during processing are two major obstacles of utilizing EOs in the industrial processes.
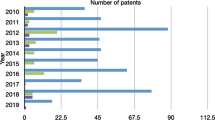
A superficial search in the main patent databases and indexed journal articles reveals the growing interest in the use of nanoemulsions in the last decade as well as the use of these systems in the pharmaceutical industry (Figs. 4.3 and 4.4). It is possible to notice a considerable increase in the number of publications describing essential oil-loaded nanoemulsions. The poor solubility in aqueous solutions and high volatility during processing are two major obstacles of utilizing essential oils as sanitizing agents or as preservatives in food matrices. Oil-in-water nanoemulsions carefully prepared using appropriate surfactants and emulsification processes are common choices to deliver essential oils in aqueous systems, for several administration routes and distinct applications. Nevertheless, there are still few studies that deal with the particularities in the development, characterization and evaluation of the pharmaceutical properties of essential oils-loaded nanoemulsions, especially regarding the evaluation of the industrial scale production.
Patents related to nanoemulsions formulations from 2010 to 2019 using Boolean operators AND (Espacenet search∗ 6/10/2019). Blue: nanoemulsions; Green: nanoemulsions AND drugs; Purple: Nanoemulsions AND essential oil. ∗https://worldwide.espacenet.com
Publications related to nanoemulsions formulations from 2010 to 2019 using Boolean operators AND (Web of Science search∗∗ 6/10/2019). Blue: nanoemulsions; Green: nanoemulsions AND drugs; Purple: nanoemulsions AND essential oil. ∗∗https://webofknowlegde.com
Essential oils are generally obtained from the peeling of fruits, flowers or leaves. Several essential oils are being used in the development of nanoemulsions, in Table 4.2 are listed some nanosystems with proven activity. For example, clove oils are the essential oils obtained by distillation of the flower buds, stems, and leaves of the clove tree (Syzygium aromaticum) (Goñi et al. 2016). Among clove oils, clove bud oil (CBO) is widely used and well-known as a potent antioxidant and for antibacterial, antifungal, and antiviral activities (Anwer et al. 2014; Chaieb et al. 2007). Eugenol, 4-allyl-2-methoxyphenol, is the primary constituent, responsible for more than 80% of CBO and is the major contributor of the above biological functions of CBO (Jirovetz et al. 2006; Chaieb et al. 2007).
Another example, Rosmarinus officinalis L. essential oil is usually isolated by hydrodistillation, steam distillation, or extraction with organic solvents. The Rosemary oil is mainly located in leaves and the flowers; it is known for antioxidant, antimicrobial, anti-inflammatory properties and studies suggest that the one chemical compounds more frequently reported molecules was 1,8-cineole, α-pinene, and camphor (Angioni et al. 2004; Hernández et al. 2016).
Basil and thyme are aromatic herbs that are also used extensively to add a distinctive aroma and flavor. The essential oils are extracted from fresh leaves and flowers are very recognized can be used as aroma additives in food, pharmaceuticals, and cosmetics (Q. X. Li and Chang 2016; Mandal and DebManda 2016). Major compounds found in volatile extracts of basil and thyme exhibited varying amounts of anti-oxidative activity in particular, eugenol, thymol, carvacrol and 4-allylphenol (Marotti et al. 1996; Hudaib et al. 2002; Politeo et al. 2007; Hussain et al. 2008).
Recognized in traditional medicine, lemongrass essential oil is a potent antimicrobial and antioxidant natural bioproduct. Lemongrass essential oil composition consists mainly of 70–85% geranial (citral), neral, geraniol, nerol, citronellol, 1,8-cineole (eucalyptol), α-terpineol, linalool, geranyl acetate. Lemongrass oil is collected by steam distillation of the herbage. Lemongrass essential oil is a viscous liquid, yellow to dark yellow or dark amber in color turning red on prolonged storage (Sharma et al. 2018).
Also, among the essential oils, there are citrus fruits, which also have countless therapeutic applications. Citrus fruits are well known and appreciated for centuries as they feature pleasant aroma and appetizing flavor. The essential oils from these fruits are generally obtained from the juice of the peels of fruits, but it can also be obtained from flowers or leaves (Asbahani et al. 2015). Among the best-known essential oils are those of orange, lemon, mint, eucalyptus, mint, citronella, clove, among others, as can be seen in Table 4.2.
The methods of extraction vary according to the location of the volatile oil in the plant and the proposed use of it. The most common methods are: enfleurage, steam distillation; extraction with organic solvent in a continuous and discontinuous way; pressing or supercritical CO2 extraction (Asbahani et al. 2015).
4.4 Preparation of Nanoemulsions
Typically, the emulsification process is based on the dispersion of an immiscible liquid into another immiscible liquid as small droplets which are surrounded by a thin interfacial layer of a surfactant that acts as an emulsifier, nanoemulsification methods are no different. In general, the methods to produce nanoemulsions can be classified into high or low-energy methods. High-energy methods have been widely used to produce nanoemulsions in large industrial scale (McClements and Jafari 2018). These methods use mechanical devices to produce nano-sized droplets. Low-energy methods depend on internal energy of the components to spontaneous produce nanoemulsions upon changes in the compositions or environmental conditions. In both type of methods, surfactants play an important role in reducing the interfacial tension of the system and thus they contribute to the reduction of the shear energy required to reduce the radius of curvature of the formed droplets (T. Tadros et al. 2004). The choice of the method to obtain nanoemulsion should be made based on the properties of the oil phase and the surfactant, physicochemical properties and functional aspects requires for the final application (McClements and Jafari 2018).
A minimum energy input required is the same no matter the nanoemulsification method. However, the preparation method may influence the properties of the produced nanoemulsions, such as: droplet size and stability. Without affecting the final nature of the dispersion phases, though (Gutiérrez et al. 2008). On the other hand, the choice of the technique adopted to produce nanoemulsions greatly affect droplet size and stability (Salem and Ezzat 2018). In this section, we have a brief overview on the most commonly used high-energy and low-energy approaches for nanoemulsion formation.
4.4.1 High-Energy Methods
High-energy methods are also known as work-based methods, since they are dependent of high amounts of intense energy, which are supplied by mechanical devices, like high pressure homogenizers, microfluidizers and ultrasonicators, which generate shear forces able to disrupt oil and water interface, providing nanosized droplets (Solans et al. 2005; Leong et al. 2009). The size and stability of nanoemulsions produced can be influenced by the type and processing conditions of the high-energy method, oil properties such as lipophilicity, viscosity and interfacial tension, surfactant type and concentration.
High energy methods are carried out by the input of mechanical energy using mechanical or ultrasonic equipment that generate high shear stress or pressure difference, disrupting and breaking the droplets into smaller sizes (Abismail et al. 1999; Sonneville-Aubrun et al. 2004; Tadros et al. 2004). In general, the preparation of nanoemulsions through high-energy methods can be divided into two distinct phases. In a first step the oil and aqueous phases are emulsified with an homogenizer as Turrax® or Politron® and the coarse emulsion obtained presents a submicron droplet size, between 500 and 1000 nm, depending on the equipment used and the operating conditions (McClements and Rao 2011; McClements 2011). Then, the droplet diameter is progressively reduced to its minimal value, ranging from 20 to 200 nm by means of high pressure homogenizers or microfluidizers, depending on the energy intensity of the homogenizer used, processing time and sample composition (T. Tadros et al. 2004; Salem and Ezzat 2018). It is worth noting that the minimum droplet size achievable may not be stabilized if the surfactant is insufficient to cover the newly created interface, as observed by Barradas et al. (2015). Final droplet size results from a balance between two phenomena that happen at the same time during homogenization: Breaking of drops into fine droplets and droplet coalescence after processing (Jafari et al. 2008). Higher surfactant concentration, increasing shear intensity or duration can contribute to reducing droplet size (Gupta et al. 2016b).
The obtention of nanoemulsions through high pressure homogenizers, ultrasonic cavitation and microfluidizers has been well described in the literature (Tadros et al. 2004; Helgeson 2016; Dias et al. 2014), and is considered as a safe method for keeping nanoemulsions from instability phenomena without the addition of stabilizers, thickeners, cosolvents or cosurfactants (Anton et al. 2008). Figure 4.5 summarizes the high-energy methods reported in this chapter.
High-energy methods based on mechanical devices, such as ultrasonicators (a), composed by a probe able to produce a cavitation effect and nanosized droplets are obtained after enough time of processing to ensure homogeneous size distribution and polydispersity index: High pressure homogenizators (b) consist basically of a pump, which injects the liquid to be homogenized under very high pressure in a restrictive homogenizing valve, which causes the disruption of the dispersed droplets. Microfluidizers (c) are composed of an interaction chamber where the fluid is injected and homogenized by cutting, impact and cavitation. Emulsification can occur inside the channels, since streams are conducted to an interaction chamber under high pressures, where they are submitted to disruptive conditions, which provide the formation of fine droplets. (Modified after Jafari 2017; Rao and McClements 2011)
Ultrasonication techniques for the preparation of nanoemulsions has also been described by several authors (Shahavi et al. 2015; Gupta et al. 2016a; Leong et al. 2009). The device comprises a sonication probe constituted by piezoelectric crystals able to expand and contract as a consequence to an altering electrical voltage (Fig. 4.5a). In consequence to sonicator probe vibration, ultrasonic high-frequency waves, higher than 20 KHz, are produced, which are able to wield a cavitation effect and causing mechanical vibration and the formation of micro-sized bubbles that collapse, causing the disruption of oil-water interface (McClements 2011). As a result, fine nanosized droplets are obtained after enough time of processing to ensure homogeneous size distribution and polydispersity index (Schwarz et al. 2012; Shahavi et al. 2015; Abismail et al. 1999; Sivakumar et al. 2014). The main parameters involved in this emulsification procedure are the interfacial properties of the emulsion, which can be controlled by the nature and concentration of surfactants and the oil properties, such as surface tension and viscosity (Gupta et al. 2016a). Moreover, droplet size is dependent on processing time, sonication intensity, type and concentration of surfactant (Dias et al. 2014).
High pressure homogenization features the advantage of being applied in the production of industrial scale nanoemulsions and greater control in droplet size reduction (Abismail et al. 1999). However, it requires large amounts of energy input, being more expensive to perform. The high-pressure valve homogenizer consists basically of a pump, which injects the liquid to be homogenized under very high pressure in a restrictive homogenizing valve. Many aspects may influence the physicochemical characteristics of the obtained nanoemulsions as temperature, viscosity and oil phase concentration of the emulsions, which affects the choice of the operational parameters that must be adjusted for each formulation (Lee and Norton 2013). In high pressure homogenizer, the sample is forced through small channels under a pressure ranging from 500 to 15,000 psi (Gupta et al. 2016a) (Fig. 4.5b).
The sample flows under high pressure through microchannels resulting in a very fine emulsion, which causes the disruption of the dispersed droplets. Therefore, the radius of the generated droplets decreases gradually according to the increase of the shear rate. However, due to the lack of homogeneity of the flow, it is often necessary to process this fluid through the device through various cycles, until adequate droplets of size and polydispersity index are obtained. The pressure and number of processing cycles can be adjusted to produce nanoemulsions with tunable droplet size (Ouzineb et al. 2006; Constantinides et al. 2008).
The use of high-pressure homogenizers and ultrasonicators can lead to nanoemulsions of equivalent physical-chemical properties upon the optimization of the operational conditions and the qualitative and quantitative composition of the formulations. However, some authors have reported some disadvantages regarding the use of ultrasonication, such as excessive heating of the sample, larger droplet distribution and low reproducibility in relation to the droplet diameter and polydispersity index, in addition to problems related to the difficulties of scale up (Tadros et al. 2004; Gutiérrez et al. 2008). Moreover, as they require high energy input, they are often considered cost-inefficient (Solans and Solé 2012).
Microfluidizers are composed of an interaction chamber where the fluid is injected and homogenized by cutting, impact and cavitation, in a design that resembles the high pressure homogenizers (Fig. 4.5c) (Lee and Norton 2013; Tadros et al. 2004; Salem and Ezzat 2018). In this case, emulsification can occur inside the channels, since both dispersed and continuous phases flow inside fine channels individually. Streams are conducted to an interaction chamber under high pressures, where they are submitted to disruptive conditions, which provide the formation of fine droplets (Lee and Norton 2013; McClements 2011).
In this method, several parameters can influence the obtention of small droplets: the viscosity of both dispersed and continuous phases and the type of the surfactant used (McClements 2011; Salem and Ezzat 2018). Droplet is shown to decrease as homogenization pressure, surfactant concentration and number of processing passes increase and viscosity decreases (McClements 2011).
4.4.2 Low-Energy Methods
All low-energy emulsification methods are based on physicochemical properties and uses the internal chemical energy of the components. In phase inversion methods nanoemulsification achieve by spontaneous inversion of the surfactant’s curvature providing small size globules (Jin et al. 2016; Solans and Solé 2012). Low-energy emulsifying methods are advantageous because they are effective in providing small-sized droplets and allow nanoemulsification by simple stirring (Solans and Solé 2012). Figure 4.6 summarizes the most common low-energy methods, which are reviewed in this chapter.
Schematic diagram of low energy methods: The spontaneous emulsification (a), is based on the diffusion of a water-miscible component from the organic phase into aqueous phase when both phases are put into contact, and one of the phases contains a component miscible in both phases. In phase inversion temperature (b), there is an increase of the volumetric fraction of the dispersed phase that is added to a microemulsion and then, the curvature of the surfactant is altered. Emulsion inversion phase (c) is known to cause a catastrophic phase inversion, which occurs through the inversion in oil-to-water ratio. (Modified after Jin et al. 2016; Anton and Vandamme 2011; Rao and McClements 2011)
Nanoemulsification occur by phase inversion in an coarse emulsion as a result of dramatic changes in the environmental conditions, in which parameters affecting the hydrophile-lipophilic balance (HLB) of the surfactant, as temperature and / or concentration are modified (Tadros et al. 2004).
The spontaneous formation of nanoemulsions is achievable by various methods based on diffusion of solutes between two phases, interfacial turbulence, surface tension gradient and dispersion or condensation mechanisms (Salem and Ezzat 2018). The spontaneous emulsification (Fig. 4.6a), also referred as self-emulsification method is based on the diffusion of a water-miscible component from the organic phase into aqueous phase when both phases are put into contact, and one of the phases contains a component miscible in both phases. Surfactant, cosurfactant or a polar organic solvent such as ethanol or acetone can be examples of dual-soluble components (McClements 2011). As a consequence, some of the components partially miscible in both phases diffuse from the original phase towards the other one in a rapid diffusion movement, without no phase transition or change in the surfactant spontaneous curvature (Solans and Solé 2012). The sudden diffusion of the components provides an increased oil-water interfacial area, which trigger other phenomena such as interfacial turbulence and thus, the spontaneous droplets assemble (Anton et al. 2008; Salem and Ezzat 2018).
Spontaneous nanoemulsification and droplet size can be highly influenced by the composition of the formulation, some physical chemical properties and the mixing conditions (Bouchemal et al. 2004; Rao and McClements 2012). The obtention of nanosized micelles can be performed with the addition of high concentrations of water-miscible component into the oil phase and very high solvent/oil ratio (McClements 2011; Solans and Solé 2012).
Spontaneous emulsification is often related to a special phenomenon called The Ouzo effect with beverages based on sweet fennel oil, rich in trans-anethole, that allow surfactant-free self-emulsification (Carteau et al. 2008). When water is added to the alcoholic oil solution, some of the ethanol molecules diffuse from the organic phase into aqueous phase, which reduce sweet fennel oil solubility and small oil droplets spontaneously assemble. Spontaneous emulsification has been recently explored to provide sweet fennel oil-based nanoemulsions with very reduced amounts of surfactants (Barradas et al. 2017).
Moreover, nanoemulsions can also be obtained from dilution of surfactants aggregates, as liquid crystalline particles and bicontinuous microemulsions. Solè et al. produced 20 nm oil-in-water nanoemulsions upon dilution of oil-in-water and water-in-oil microemulsions by self-emulsification method. The effect of dilution procedure, i.e., stepwise or at once, and cosurfactant nature on nanoemulsion formation was studied. Oil-in-water microemulsion provided small-sized 20 nm nanoemulsion regardless the dilution method and microemulsion composition. On the other hand, water-in-oil microemulsion resulted in nanoemulsion when water dilution was performed stepwise. Regarding cosurfactant nature, droplet size decreased as cosurfactant alkyl chain size increased, which enhanced nanoemulsion stability (Solè et al. 2012).
Self-emulsification is being applied by pharmaceutical industry to obtain oil-in-water nanoemulsions for being a low-cost technique. Moreover, spontaneous emulsification is being highly explored to produce self-emulsifying drug delivery systems (SEDDS) and self-nanoemulsifying drug delivery systems (SNEDDS). The main application of self-emulsifying drug delivery systems comprises the very self-emulsification obtention method increase in drug bioavailability and stability of the micelles (Wei et al. 2012; Shahba et al. 2012; Anton and Vandamme 2011). However, one of the major limitation of this method is the very high concentrations of surfactants needed to trigger self-emulsification, which can cause toxicity (McClements 2011; Azeem et al. 2009).
Phase inversion-based methods are low-energy techniques that involve the inversion of the surfactant curvature, passing through a transition phase in which surfactant curvature achieves zero curvature, such as bicontinuous microemulsions or lamellar liquid crystalline phases (Porras et al. 2008; Tadros et al. 2004; Fernandez et al. 2004; Sutradhar and Amin 2013; Mayer et al. 2013; Solans and Solé 2012). They are based on the chemical energy released from phase transitions phenomena during emulsification process. Phase inversion methods occur when some dramatic change in the environmental conditions take place, i.e., temperature or composition. These methods require an extensive control in terms of selecting the right surfactant, knowledge of surfactants phase behavior and the most adequate thermodynamic method. Droplet size provided depends on the selected surfactant, the properties of the intermediary phases formed and the interfacial properties between both fluids (Helgeson 2016; Maestro et al. 2008).
In phase inversion composition method (Fig. 4.6b), phase inversion occurs by a major change in composition of the system. There is an increase of the volumetric fraction of the dispersed phase that is added to a microemulsion and then, the curvature of the surfactant is altered. Maestro et al. showed that changes in salt concentration changed the spontaneous curvature of ionic surfactants and prepared water-in-oil nanoemulsions from oil-in-water emulsions by means of phase inversion composition. Phase inversion was achieved by the ability of salt ions to screen the charges on surfactant groups (Maestro et al. 2008).
Changing pH is also a useful approach to produce nanoemulsions by phase inversion composition. It can provide changes in electrical charge a stability of surfactants. Solè et al. reported the production of nanoemulsions by phase inversion composition method using ionic surfactant, potassium oleate, for example. At low pH values, the carboxyl groups from fatty acids are non-ionized and, thus lipid soluble. Under this condition, these molecules can stabilize water-in-oil emulsion. However, this situation is inverted if pH is raised. At higher pH values, carboxyl groups become ionized which increases water-solubility and provides the stabilization of oil-in-water emulsion (Solè et al. 2006).
Phase inversion composition method is considered an interesting approach for large-scale production of nanoemulsions, since it relies only on the addition of one component to a mixture of components without requiring high temperatures of high-energy input, being also beneficial when temperature-sensitive components are used. Moreover, phase inversion composition method is not limited a specific type of surfactant (Solans and Solé 2012).
For temperature-sensitive surfactants, a phase inversion can be achieved by changing the temperature of the system, forcing the transition of an oil-in-water emulsion, prepared at low temperatures, for a water-in-oil emulsion, formed at higher temperatures, due to changes in the physicochemical properties of the surfactant. This is a typical phase inversion composition example of a transient phase inversion, known as phase inversion temperature, in which nanoemulsions are formed under a fixed composition by changing temperature, by a drastic change in surfactants water solubility, through an intermediate liquid crystalline or bicontinuous microemulsion phase (Fernandez et al. 2004; McClements and Rao 2011).
This process is triggered by the changes in physicochemical properties of surfactant as temperature changes. Polyethoxylated nonionic surfactants are highly temperature-dependent. They tend to become lipophilic with increasing temperature due to dehydration of the chain of the polar part of the surfactant, i.e., ethylene oxide groups. During heating, the ethylene oxide groups responsible for the hydrophilic characteristic of the surfactant are “hidden” and, consequently, there is modification of the affinity for the phases. As the system undergoes cooling, the surfactant passes through a point of zero curvature and with minimum surface tension, which provides conditions to the formation of nanoemulsions (Fernandez et al. 2004; Tadros et al. 2004).
The inversion point take place at a specific temperature, i.e., phase inversion temperature, when the solubility of the surfactant in water and in oil reach equivalent values. As temperature is continuously raised, the surfactant becomes more soluble in the oil phase than in the aqueous phase (McClements 2011). Nanoemulsions are obtained by rapidly breaking up microemulsions formed at phase inversion point by sudden cooling below phase inversion temperature (McClements 2011). Through the phase inversion temperature method very small droplet sizes and stable nanoemulsions are obtained. On the other hand, the systems are highly prone to coalescence, being unstable, which makes cooling step a critical aspect to obtain stable nanoemulsions (Tadros et al. 2004; Solans and Solé 2012).
In emulsion inversion point method (Fig. 4.6c), one solvent, i.e. water or oil, is continuously added under stirring to the dispersed phase until this solvent concentration becomes predominant, at constant temperature. Once a critical amount of solvent is achieved, droplets are so highly packed together that phase inversion occurs, and the emulsion reaches a phase inversion point from water-in-oil to oil-in-water or vice-versa. This causes the spontaneous surfactant curvature to change, i.e., micelles are assembled in reverse curvature, which disrupts them into smaller structures thereby obtaining emulsions with nanometric droplets (Anton et al. 2008).
Emulsion inversion point is considered to cause a catastrophic phase inversion, which occurs through the inversion in oil-to-water ratio. The surfactants used to produce the catastrophic phase inversion comprise small molecule synthetic emulsifiers able to stabilize both oil-in-water and water-in-oil emulsions. In this method, droplet size depends on the stirring speed and the solvent addition speed (McClements and Rao 2011).
Although the phase inversion temperature method facilitates the obtention of nanoemulsions with nanometric droplet sizes by the fact that it is possible to achieve very low values of interfacial tension, the dynamics of droplets coalescence can be extremely fast. In order to obtain stable nanoemulsions, the water-oil dispersion formed from phase inversion temperature method is readily cooled at a temperature just below it. Thus, the lamellar or the bicontinuous system collapse forming small droplets kinetically stable, with very small droplet size and narrow size droplet size. If the cooling process is not fast enough, the coalescence predominates and a polydisperse mixture is obtained (Fernandez et al. 2004).
According to the literature, not only the minimum interfacial tension produced during inversion of the curvature of the surfactant, but also the formation of the bicontinuous phase or liquid-crystalline phase prior to the inversion locus is closely related to the formation of nanoemulsions, in both emulsion inversion point and phase inversion temperature methods (Fernandez et al. 2004; Tadros et al. 2004). Moreover, complete solubilization of the oil in the bicontinuous phase or in the crystalline phase is an extremely important factor for the formation of fine nanoemulsions.
4.4.3 Comparison Between Obtention Methods
High energy emulsification processes are different regarding energy input and emulsification time: the high-pressure homogenizer, for example, is a high energy and low process time method; ultrasonication is a high energy and long process method; and rotor stator is a mixer that uses less energy, requiring longer process times. On the other hand, low-energy processes can provide smaller droplet sizes compared to high-energy methods. However, there are limited options for surfactants to be used in low-energy approaches, which exclude several natural surfactants as proteins or polysaccharides (Rao and McClements 2012). Besides, low-energy methods, as they cannot count on high shear energy devices, require large amounts of surfactants, which can limit the pharmaceutical application of the obtained nanoemulsions.
Jafari, He, and Bhandari compared different methods of high energy emulsification, it was observed that the average mechanical mixer produced emulsions with large droplet size, in a range above 10,000 nm, because, in this case, the main deformations forces are shear stresses in laminar flow that are not able to efficiently break the droplets. The stator rotor mixer proved to be more efficient than the average mechanical mixer in the formation of smaller droplets, of about 1000 nm, and with narrow size distribution, due to the incorporation of more forces on the shear that can form smaller droplet sizes, for example, inertial forces (Jafari et al. 2007).
Considering the other two emulsification systems, the high-pressure homogenization, and microfluidization, provided droplet sizes and size distribution smaller than the ultrasonication due to the greater efficiency in the rupture of the drops, or with cavitation along with shear and inertial forces, in addition to the higher energy input. From these results it can be shown that the difference in droplets size obtained is directly related to the energy input (Jafari et al. 2007).
Comparing both high and low energy emulsification methods, it was verified the variation of the droplet size as a function of time for nanoemulsions prepared using the phase inversion temperature method and high-pressure homogenization. In both cases, an increase in droplet hydrodynamic size after a certain time the preparation of nanoemulsions. However, when high pressure homogenization is used, the droplet size can be maintained at lower values for longer periods of time. On the other hand, phase inversion temperature method provides a faster destabilization of the system (Tadros et al. 2004).
Phase inversion composition method and high-pressure homogenization were compared in the production of oil-in-water efavirenz-loaded nanoemulsions. Phase inversion composition method was performed by dilution in water. Nanoemulsions were evaluated regarding the physical stability when submitted to heating-cooling cycles, centrifugation and freeze-thaw cycles, water-dispersibility, droplet size, rheology and dilution impact on droplet size. Both nanoemulsions showed good physical stability and water dispersibility. Regarding viscosity, there was no significant difference between nanoemulsions prepared by each method. Nanoemulsions prepared through phase inversion composition method showed to be more transparent than those prepared by high-pressure homogenization, this is due to the concentration of surfactant and oil/surfactant ratio used to prepare nanoemulsions by each method. Droplet size showed no significant difference between both methods. However, polydispersity Index was found to be significantly different between the methods. Phase inversion temperature method provided polydispersity index from 0.150 to 0.404, while high-pressure homogenization varied from 0.637 to 0.812. Both nanoemulsions proved to be stable under infinite dilution. Droplet size and polydispersity index remained unaltered after sample dilution. Drug release also showed differences between nanoemulsions made by high-energy and low-energy emulsification methods. Drug release from nanoemulsion prepared by high-pressure homogenization was smaller than that prepared by phase inversion composition method, which can be a consequence of polydispersity index (Kotta et al. 2015).
Yang et al. compared microfluidization with spontaneous emulsification in the production of food-grade nanoemulsions with 20% of oil phase and same type and concentration of surfactants. The influence of the surfactant used end surfactant-oil ratio, stability and droplet size were also evaluated. Microfluidization produced nanosized droplets with less than 100 nm, using lower surfactant-to-oil ratio than spontaneous emulsification. Besides, microfluidization showed a linear decrease in droplet radius as surfactant concentrations increased until it reached a value where no difference in droplet size is observed, suggesting that minimum droplet size was achieved. Spontaneous emulsification also produces droplets smaller 100 nm, even though to do so, it required considerably higher, i.e., ten-fold higher of surfactant concentration. Regarding the kinetic stability, microfluidization provided stable droplets against aggregation and gravitational separation phenomena for at least 30 days. Spontaneous emulsification, on the other hand, provided droplets sizes that increased after one-month storage. Moreover, nanoemulsions showed creaming instability signs (Yang et al. 2012).
4.5 Stability of Nanoemulsions
As previously mentioned, nanoemulsions are characterized for being kinetically stable. Nanoemulsions shelf life can be further improved with some stabilization strategies that aim at maintaining bulk and droplet properties stable for longer periods of time. In order to achieve stable nanoemulsions several stabilization methods can be used, such as electrostatic, steric and mechanical stabilization (Cardoso-Ugarte et al. 2016).
Repulsive electrostatic forces between droplets can ensure proper droplet separation and prevent coalescence and/or flocculation. This is related to surfactant superficial charge and is responsible for the high stability of ionically-charged nanoemulsions. Electrostatic stabilization is more important with smaller droplets, since they have an increased superficial area (Helgeson 2016).
Superficial charge is often provided by the surfactants, especially natural surfactants, as biopolymers with surface activity like proteins, polysaccharides, which can stablish electrostatical intermolecular interactions depending on concentration, pH and isoelectric point, and ionic strength of the solution (Cardoso-Ugarte et al. 2016). When pH of the surrounding solution is far from protein isoelectric point, proteins residues are charged and there is an electrostatic repulsion that hinders droplets to aggregate (Dickinson et al. 1991).
Non-ionic surfactants also can provide stabilization, even though they are not charged. Steric stabilization is preponderant in the case of non-ionic surfactants, particularly with amphiphilic non-ionic polymers, which can form voluminous interfacial films around droplets able to prevent coalescence. Amphiphilic block polymers are advantageous since less amounts of surfactants are required to stabilize droplets. On the other hand, in some cases, macromolecular surfactants can have difficult diffusion towards droplets interface due to their size and molecular mobility (Bouyer et al. 2012; T. Tadros 2009; Qian and McClements 2011).
Steric stabilization comprises three main mechanisms: (i) non-adsorbent macromolecules which provide an elastic film around droplets that avoid droplet collision and deformation, (ii) branched macromolecules that form a voluminous surface that prevent droplets from approaching, (iii) stabilization due to hydrophobic interactions between adsorbed macromolecular surfactants (Cardoso-Ugarte et al. 2016).
The addition of viscosity agents and gelling polymers in the outer phase of nanoemulsions is often called as mechanical stabilization, since they can reduce droplets mobility by providing a mechanical network that serves as a barrier to aggregation. Viscosity agents are often called as stabilizers and produce semi-solid or gel-like systems. However, stabilizers should be used with caution, since important properties as optical appearance, droplet size and encapsulation efficiency can be modified (Behrend et al. 2000; Dickinson 2009).
4.5.1 Instability Phenomena
As nanoemulsions are formed from non-spontaneous process they tend to undergo instability phenomena such as flocculation, coalescence and Ostwald ripening and gravitational phase separation as conventional emulsions. However, the small size of the droplets in a nanoemulsion confers enhanced kinetic stability, compared to ordinary emulsions (McClements 2011).
Thus, nanoemulsions can be destabilized by several different phenomena: (i) irreversible phenomena, related to the permanent modification of the droplet size and may lead to complete phase separation; (ii) reversible flocculation of droplets, which may be followed by creaming or sedimentation, according to the respective densities of the dispersed and continuous phases (Tadros et al. 2004; Sing et al. 1999; Abismail et al. 1999). In general, nanoemulsions tend to be more stable to gravitational separation, flocculation and coalescence and more susceptible to Ostwald ripening (McClements 2011).
Reversible phenomena involve aggregation and migration of droplets, as flocculation and creaming. Irreversible phenomena are related to the modification of droplet size, for examples, coalescence. Droplets can co-exist in nanoemulsions as individually separated entities or as flocs, i.e., droplets aggregates formed as consequence of attractive interactions among them, characterizing a flocculated system (McClements 2015; McClements and Jafari 2018).
Flocculation is a reversible phenomenon in which the droplets dispersed in an emulsion aggregate and migrate, aiming to reach the thermodynamic equilibrium by decreasing the chemical potential differences that exist throughout the system. During this process, the droplets collide randomly and can remain in contact after these shocks, producing aggregates or flocs (Katsumoto et al. 2001; Starov and Zhdanov 2003). In flocculation, droplets aggregate without the rupture of the interfacial surfactant film, being a reversible phenomenon. The flocculation rate depends on attractive forces between droplets, the frequency of collisions between droplets and how long they remain in contact (Wang et al. 2009). In a dilute system, flocculation may accelerate gravitational phase separation as a consequence of the increase in droplet size. On the contrary, in concentrated systems, flocculation can prevent gravitational phase separation, since the aggregates may produce a tree-dimensional network which may be a barrier to phase segregation (McClements and Jafari 2018).
According to the difference of density between both inner and outer phases, the aggregates formed in the flocculation phenomenon may show gravitational separation such as sedimentation or creaming. When less dense aggregates are formed, they can rise to the surface, characterizing the phenomenon of creaming. Contrarywise, when inner phase shows higher density, denser aggregates are formed and deposited at the bottom of the system, constituting the sedimentation process (Fig. 4.7). Both sedimentation and creaming phenomena are related to gravitational forces that can influence whether the aggregates will move upwards or downwards, depending on the difference of density between inner or outer phases. Hence, both phenomena are most prone to occur with droplets with increased droplet size, as larger objects are more susceptible to gravitational forces. Thus, gravitational separation can be reduced with smaller droplet sizes, increasing the viscosity of continuous phase or by reducing the density difference between dispersed and continuous phases (McClements 2011; McClements and Jafari 2018).
Instability phenomena in emulsions and nanoemulsions. Reversible phenomena comprise aggregation and density-driven migration of droplets, as flocculation and creaming. Irreversible phenomena are related to a definitive increase of droplet size, for example: coalescence, Ostwald ripening and, the most drastic, phase inversion. (Modified after McClements and Jafari 2018; Taylor 1998)
Some surfactants as proteins and polysaccharides can change droplet density by forming a shell layer on droplet surface, which can prevent gravitational separation phenomena such creaming, since it reduces density differences between inner and outer phases. Moreover, it can be possible to produce tunable-density droplets by controlling droplet size and thickness of surfactant layer (McClements 2011).
Irreversible phenomena include the Ostwald ripening, phase separation and coalescence, which may lead to the eventual complete phase separation (Sing et al. 1999). Coalescence can occur when droplets collide and merge, producing one larger droplet. Coalescence can lead to complete phase separation but can be avoided when repulsive interactions are provided by either electrostatic or steric effects, which is achievable by the right choice of surfactant (McClements and Jafari 2018).
Ostwald ripening is often considered as the main cause of instability of a nanoemulsion (Liu et al. 2006). This process is dependent on the polydispersity of the system, the solubility of dispersed phase in the continuous phase and the difference in solubility between droplets of different sizes. Ostwald ripening is the process by which the larger particles grow from the smaller droplets due to the greater solubility of the smaller droplets and by the molecular diffusion of the continuous phase.
In polydisperse nanoemulsions, there is a difference between the capillary pressure from different-sized droplets which makes the small droplets of the dispersed phase to diffuse into the large droplets (Chebil et al. 2013; Taylor 1998). As a consequence, the larger droplets grow from the smaller ones that have greater chemical potential. Thus, the droplets content diffuses through the dispersing phase due to the greater solubility of the smaller droplets, which does not require contact between the droplets. This phenomenon aims to decrease the total energy of the system by reducing the total interfacial area, which can be avoided with the use of insoluble oils and with the choice of suitable polymeric surfactants (T. Tadros et al. 2004; C. Solans et al. 2005; Constantinides et al. 2008).
The rate of droplet growth depends on the product of the solubility of the dispersed oil in the aqueous continuous phase and oil diffusion coefficient and is explained by the Lifshitz-Slezov-Wagner theory (Taylor 1998). It predicts that the Ostwald ripening rate presents a linear relationship between the dispersed phase droplet radius and time. Lifshitz-Slezov-Wagner theory assumes that the droplets of the dispersed phase are spherical and furthermore the distance between them is greater than the diameters of these droplets and the kinetics is controlled by molecular diffusion of the dispersed phase in the continuous phase. Also according to this theory, the Ostwald ripening rate in oil-in-water nanoemulsions, although is predicted to be lower than in conventional emulsions, is directly proportional to the solubility of the oil in the aqueous phase (Helgeson 2016).
However, the decrease in droplet size causes the increase of oil solubility in water, which is the driving force of Ostwald ripening. As the main factor for Ostwald ripening is the solubility of oil phase in water, it does not configure a real issue when it comes to poorly water-soluble oils. On the other hand, for nanoemulsions formulated with oils with some water-solubility, Ostwald ripening might represent the main instability event (McClements 2011).
Tadros et al. presented two methods that tended to delay the increase in droplet size. In the first, a further dispersed phase that is insoluble in the continuous phase is added to the nanoemulsion (Tadros et al. 2004). The fact that it is insoluble causes the droplets to remain almost unchanged in size, generating a balance in the chemical potential due to the partitioning of the dispersed phases. This method has limited application because of the difficulty of finding a phase that fully meets this process. From these results, reduction of the Ostwald ripening process can be observed by the addition to the system of a small amount of a second oil with low solubility in the aqueous phase.
The second method consists in the modification of the interfacial film between the oil phase and water, i.e. outer phase, by adding another surfactant, preferably a block copolymer A-B-A type. The effect would be the adsorption of this copolymer at the oil-in-water interface reducing interfacial tension and balancing curvature effects, which would reduce the diffusion rate of the dispersed phase, minimizing droplet ripening (T. Tadros et al. 2004). In addition, for a system containing a nonionic surfactant based on poly (ethylene oxide), the Ostwald ripening rate can also be decreased by adding a second surfactant having the same hydrophobic group and with a higher hydrophilic content, i.e., ethylene oxide chains, than the primary surfactant (C. Solans et al. 2005).
Several studies were performed to describe and to modulate the Ostwald ripening mechanism. They suggested that the Ostwald ripening can also be delayed or disrupted by size, interfacial viscosity or elasticity of the droplets. This occurs when the interfacial tension between the dispersed phase and the continuous phase equals zero. For a number of drops of the emulsion it was also shown that the Ostwald ripening could be disrupted by interfacial elasticity, even at finite interfacial tensions (Meinders and van Vliet 2004; Liu et al. 2006).
Repeated collisions might trigger coalescence, which is the mechanical fusion of two droplets (Helgeson 2016). Coalescence occurs after prolonged contact between the particles, when the adhesion energy between two droplets is greater than the turbulent energy that causes the dispersion. The mechanism is based on the rupture of the thin film between adjacent droplets, which leads to the joining of two droplets, forming one of larger size. The origin of the rupture of this film can occur due to a mechanical instability in the emulsion. When a large number of particles coalesce, the result is complete separation of the phases. The greater the extent of this phenomenon, the greater the tendency to complete phase separation (Sing et al. 1999).
Irreversible changes caused by Ostwald ripening lead to the formation of larger droplets, i.e., the formation of less stable emulsions, which may be responsible for phase separation. Phase inversion may occur due to temperature variation and/or change in composition of the emulsion.
The composition of nanoemulsions can be tuned towards to produce kinetically stable systems. The adequate selection of oil and aqueous phase and, most importantly, surfactants are essential to an ideal nanoemulsion design.
4.6 Characterization of Nanoemulsions
A wide range of analytical methodologies are required in order to fully characterize nanoemulsions droplet size, aggregation, charge and physical state and bulk properties as rheology and stability. The most used are described in more details here.
4.6.1 Dynamic Light Scattering
Droplet size distribution are some of the most important physical characteristics of nanoemulsions and can influence a great number of characteristics, since droplet size can influence bulk properties, such as optical properties, rheology and stability (McClements 2011) and also other functional properties as solubility, release profile and bioavailability (Bourbon et al. 2018). In general, when nanoemulsions are produced, a range of different droplet sizes are distributed all over the system. Hence, droplet size is often reported as mean droplet size and polydispersity index (PdI). PdI values below 0.3 is indicative of a narrow size distribution whereas values close to 1.0 are indicative of very high size heterogeneity (Klang and Valenta 2011).
Dynamic light scattering (DLS) (λ = 500 nm) is based on measurements of photon correlation spectroscopy caused by the Brownian motion of droplets. The diffusion of small isometric particles is rapid, causing faster fluctuations in the intensity of scattering light compared to large particles that diffuse more slowly. The particle/droplet size analyzer is based on the typical principle of DLS. This principle comprises four main components. The first of all is the laser beam, which is used to provide the light source to illuminate the particles inside the vial. Most of the laser beam passes rectilinearly through the sample, but another part of it is scattered by particles or droplets dispersed in the medium (Dorfmüller 1987).
A detector is used to measure the intensity of scattered light. As one single particle scatters light in all directions, it is possible to place a detector at any position and still detect the scattering. In DLS, the position of 90°, from the detector to the incident light beam, is a classical detection arrangement. However, it provides a narrower detectable size range and thus, can only be used for low sample concentrations (Dorfmüller 1987).
Another common device used for particle size analysis feature the position of the detector in 173° from the transmitted light beam. The scattered light intensity must be within a specific range for the detector to measure it successfully. When the amount of light detectable is out of this range attenuators should be applied to reduce the light intensity of the laser and thus reduce the scattered intensity (Dorfmüller 1987).
The intensity of the scattering signal is transmitted to the detector by a digital signal processor called correlator. The correlator compares the scattering intensity at successive time intervals to derive the rate at which the intensity varies. The detection optics of this equipment measure the information of the scattering near 180°, being known as backscatter detection, patented technology known as non-invasive backscatter (Porras et al. 2004).
Analysis of apparent hydrodynamic radius (rh) are often performed under the dilute regimen, which makes it possible to obtain the diffusion coefficient D as the Eq. 4.3, where η is the viscosity of the medium (Wang et al. 2008).
With very concentrated samples the apparent droplet size can be overestimated because of multiple scattering due to long-length colloidal interactions among close droplets. In this context DLS measurements can be often limited by the necessity of diluting the samples.
The mean droplet size obtained from DLS can be determined by different manner, for instance, number, intensity (Z-average) and volume values. Hence, it is fundamental to be clear about the type of determination applied to droplet size characterization, since these values can be very discrepant, depending on sample concentration, polydispersity and refraction index.
In order to characterize droplet size, techniques such as electron microscopy, X-Ray and Neutron Scattering of liquid emulsions, spectro-turbidity and dynamic light scattering can be used, the latter being the most used in the characterization of nanoemulsions (McClements 2015).
Droplet size can also be characterized by transmission electron microscopy, which can visually provide droplet size and distribution in nanoemulsions of nanometric droplet sizes. From this technique it is also possible to observe morphological spherical shape of droplets and evaluate the destabilization of the nanoemulsions, in which droplet coalescence can observed. However, transmission electron microscopy has also limitations, which are often related to sample preparation that can induce to coalescence upon solvent evaporation during drying step. Besides, contrast with heavy metals as drying can also alter droplets environment and induce to some instability phenomena. High-energy electron beams can ruin nanostructure of droplets, which can be overcome by Cryo- transmission electron microscopy techniques.
4.6.2 Zeta Potential
Nanoemulsions formulated with ionic surfactants feature superficial charge. The type and magnitude of the superficial charge of droplets are responsible for many characteristics of nanoemulsions, such as stability against aggregation phenomena and functional properties like mucoadhesivity and absorption (McClements 2015).
The electrical characteristics of nanoemulsion can range from strongly positive, to neutral and strongly negative depending on the surfactant charge and the surrounding conditions. Droplet superficial charge can be characterized by zeta potential. Zeta potential describes the electro-kinetic potential in a system. Charged nanodroplets dispersed in a liquid system attract opposite-charged ions close to surface, providing a double layer with a certain thickness that can vary depending on the type and concentration of counter-ions (Jin et al. 2016). With the distribution of counter-ions in the surrounding droplet layer, any movement between rigid and mobile phases provides an electrokinetic potential (Bourbon et al. 2018). The stern layer plays an important role in the stabilization of nanoemulsions, since stern layer is responsible for the repulsive forces that prevent droplets from aggregate.
Zeta potential measurements results are presented in millivolts (mV) and is carried in an electrophoretic cell, where two electrodes create an electrical field and migration of colloidal particles or droplets is measured. Zeta potential can be strongly affected by pH and ionic strength of the system (Bhattacharjee 2016). Other factors can greatly influence zeta potential as the state oh hydration and droplet morphology. According to literature, zeta potential values above 60 mV suggest excellent electrostatic stability, while results from 60 to 30 mV indicate good stability and from 5 to 15 mV suggest a region of limited flocculation and from 3 to 5 mV correspond to maximum flocculation (Bourbon et al. 2018). When zeta potential values are high in module, droplets would repulse each other and the system is less prone to coalescence (de Oliveira et al. 2014; Junyaprasert et al. 2009; Svetlichny et al. 2017).
Nanoemulsion stabilized by anionic surfactants show negative superficial charge and those stabilized by cationic surfactants present positive superficial charge. By controlling the amount and the type of surfactant used when formulating nanoemulsions, it is possible to tune the superficial charge according to the characteristics needed.
4.6.3 Rheological Behavior
Bulk physicochemical properties, as rheological behavior can deeply impact nanoemulsions applicability, particularly for some applications routes as topical or dermal application (Barradas et al. 2018). Rheology is an important tool to characterize nanoemulsion stability. In general, flocculated systems are more viscous than a non-flocculated nanoemulsion with the same droplet size and concentration (McClements and Rao 2011). Moreover, rheological viscoelastic properties reflect flow behavior and the deformation during nanoemulsions industrial production in processing steps as flow through pipes, mixing and packaging (Chung and McClements 2018).
Flow behavior of nanoemulsions are measured apparent viscosity values as a function of shear rate or shear stress. Viscoelastic properties are determined under dynamic oscillation modes and are presented as viscous (G′) and elastic (G″) modulus as a function of deformation of frequency. Typically, dilute small-sized nanoemulsions can show low viscosity and a newtonian behavior as previously published by Barradas et al. (2017). The elastic modulus provides information about the elastic properties and the stored energy, being considered a solid-like property. On the other hand, the viscous modulus indicates the viscous properties and the energy dissipated as heat being a liquid-like property.
Nanoemulsions viscosity increases as droplet increases and when dispersed phase is more concentrated. As droplet concentration increases, there is a tendency to aggregation among them, which can increase both viscosity and viscoelastic behavior (Tadros 2004). Droplet aggregation can change flow behavior from newtonian to shear thinning and increase elastic modulus of concentrated nanoemulsions. Shift on rheological behavior occur once critical droplet concentration is achieved. Besides, rheological behavior of nanoemulsions can be altered by strong electrostatic attractive or repulsive interactions among the droplets, which can provide a three-dimensional network and increase viscoelastic behavior.
The viscosity of oil phase can also greatly influence rheology and viscosity of nanoemulsions. Longer fatty acids chains lengths are able to increase viscosity of vegetable oils. As oil degradation occur, the size of fatty acids chains can be reduced by hydrolysis, which often causes the reduction in nanoemulsions viscosity as reported by Barradas et al. 2017. Thus, rheology can be an important tool to access nanoemulsion stability.
Rheology can also be a useful approach to characterize nanoemulsions microstructure and classify them as viscous, viscoelastic or semi-solid materials. Transient non-destructive tests are often applied to study structural recovery time of nanoemulsions after a constant stress is applied. This measurement can provide important information regarding the strength of intermolecular bonds and microstructure of the sample. For example, for injectable nanoemulsions it is fundamental to predict formulation viscosity, rheological behavior and structural integrity after being submitted to high deformation upon passing through a syringe.
Dynamic oscillatory tests can determine viscoelastic properties of the samples, allowing to classify them into fluid-like, solid-like, gel-like and semi-solid materials (Helgeson 2016; Chung and McClements 2018). Both frequency and strain stress can be tuned to obtain information about the macrostructure of materials. Non-destructive experiments are often performed under small strains and low frequencies in order to maintain internal structure unaltered, within linear viscoelastic region. Largely destructive measurements are conducted to reproduce stresses to which nanoemulsions can be submitted during production or practical application. High stresses are applied to cause flow or large deformations. In these cases, for liquid-like fluid nanoemulsions, viscosity is reported as apparent viscosity at a particular shear stress (Chung and McClements 2018).
4.6.4 Conductivity
Calderò et al. produced nanoemulsions oil-in-water nanoemulsions from water-in-oil emulsions by phase inversion composition method as a template for polymeric nanoparticles by solvent evaporation. Conductivity determinations were used to confirm phase inversion from water-in-oil to oil-in-water (Calderó et al. 2011). As water concentration was increased, conductivity was significantly raised until a maximum, when it dropped. It was justified by the fact that at lower water concentrations, conductivity increases as the electrolytes in the system become gain mobility. However, with higher water concentrations, a dilution effect in the charged components takes place, which causes the conductivity (Calderó et al. 2011).
4.6.5 Phase Behavior
Small-angle neutron scattering (SANS) has been quite explored for structural characterization for phase behavior and surfactant curvature during low-energy emulsification methods (Solans and Solé 2012). Moreover, it is considered a valuable tool for characterizing bulk properties of nanoemulsions, since neutron wavelengths can probe nanosized materials (Graves et al. 2005). SANS provide the identification of ordered regions within the sample, which can be observed as spots in the scattering pattern. In contrast, amorphous and disordered regions show a diffuse pattern distributed all over the scattering profile. Moreover, a color-detector provide different intensity levels, which can emphasize scattering from ordered liquid crystalline regions in the presence of disordered phases (Sonneville-Aubrun et al. 2009).
Compared with DLS, SANS provides information of droplet size in a wide range of sizes and regarding the internal structure of nanosized droplets, by performing selective deuteration of the components. Besides droplet size information, SANS provide information regarding droplet structure, such as core-shell structure, globular drops or bicontinuous microemulsion (Wang et al. 2008).
Sonneville-Aubrun et al. produced 100 nm oil-in-water nanoemulsions by performing the addition water to a water-in-oil reverse microemulsions by phase transition methods and used SANS as a tool to study phase inversion stages. Measurements were performed immediately after the addition of water to the microemulsion. The SANS profiles showed that in the early stages of water addition, the initial formation of mesophase occurred, suggesting that a liquid crystalline region was formed as a transition phase. Moreover, SANS showed a strong change in surfactant curvature progressively or abruptly as the hydration of the surfactant polar group occurs, depending on the speed of water addition (Sonneville-Aubrun et al. 2009).
SANS has also been used to study silicone anionic nanoemulsions produced with different oil volume fractions, from 0.008 to 0.6. At dilute oil volume fractions, droplets are spherical. As silicon volume fractions increased above a critical jamming point, the primary peak increased, suggesting more frequent interactions and deformations among droplets due to electrostatic repulsion of neighboring droplets (Graves et al. 2005).
4.7 Application of Nanoemulsions
Nanoemulsions feature the most diverse applications in the cosmetics and pharmaceutical industry (Singh et al. 2017; Sutradhar and Amin 2013; Jaiswal et al. 2015). The small droplet size, increased surface area, high kinetic stability and optical transparency of nanoemulsions, compared to conventional emulsions, offer them advantages in many technological applications, some of them are explored below. The ability to encapsulate lipophilic bioactive molecules and improve solubility and stability of lipophilic components, makes oil-in-water nanoemulsions a useful tool to enhance the delivery of natural oils. Qian et al. produced β-lactoglobulin-stabilized nanoemulsion for encapsulation of carotenoids, natural antioxidant easily chemically degradable. The influence of ionic strength, temperature and pH on chemical stability of β-carotene and physical stability of nanoemulsions was studied. Color fading due to chemical degradation of β-carotene was significantly smaller to β-lactoglobulin-stabilized nanoemulsions, which showed to be an effective approach to increase chemical stability of bioactive molecules, such as β-carotene (Qian et al. 2012).
The encapsulation of lipophilic components, as vegetable oils provides easier handling or administration and incorporation into a pharmaceutical secondary formulation, for example, gels, lotions or creams. Besides, nanoencapsulation of vegetable oils can also increase bioavailability by increasing water solubility and due to small droplet size, promote rate and site-controlled delivery and protect from environmental degradation and prevent early evaporation (Dias et al. 2014; Sutradhar and Amin 2013).
The encapsulation of bioactive molecules and vegetable oils can be useful in developing novel pharmaceutical formulations for masking unpleasant taste or smell of some drugs, which is especially useful for pediatrics formulations (Amin et al. 2018). Moreover, nanoemulsions are useful tools to improve bioavailability of both synthetic drugs and biologically active lipids or probiotics, as polyphenols and oil-soluble vitamins, for example, which can improve the pharmacological effect (Chen et al. 2011; Salem and Ezzat 2018). Yen et al. developed nanoemulsions for improving bioavailability of andrographolide, a poorly water-soluble anti-inflammatory. The results indicated a significantly enhanced bioavailability of sixfold from nanoemulsions in comparison with conventional drug suspension. The ability of preventing gastrointestinal inflammatory disorders was much higher for nanoemulsions then for drug suspension (Yen et al. 2018). Besides, there is a general understanding that both solubility and bioavailability of poorly soluble drugs increase with reduced droplet size. The most plausible explanation is the enhancement of surface contact area, which can increase contact with solvents or cells.
Lane et al. studied the bioavailability of omega-3-rich algal oil encapsulated by nanoemulsions as a prophylactic strategy to prevent coronary heart disease and cerebrovascular disease in 11 subjects, which were fed with the formulations. This study investigated whether the ingestion of omega-3-loaded nanoemulsions increased bioavailability in comparison with free oil. The results showed that bioavailability of omega-3 and polyunsaturated oils was drastically enhanced in patients fed with nanoemulsions (Lane et al. 2014).
The solubility of oil encapsulated in nanoemulsions increases with the reduction of droplet size. According to McClements studies (McClements 2011), the solubility of a typical oil was increased by 2.24, 1.08, 1.01 and 1.00 when droplet sizes of 10, 100, 1000 and 10,000 nm, respectively, were obtained. It is due to modification in oil-water partition coefficient of the encapsulated substance (McClements 2011).
A great number of studies have reported that oil-in-water nanoemulsions can increase antimicrobial activity of essential oils against several microorganisms as bacteria and fungus (Salvia-Trujillo et al. 2015; Sonu et al. 2018; Chuesiang et al. 2019). The encapsulation of essential oils into nanosized droplets can lead to a great disruptive activity of essential oils on cell membranes of microorganisms (McClements and Jafari 2018). Chuesiang et al. produced cinnamon oil-loaded oil-in-water nanoemulsions by phase inversion temperature method and investigated water-miscibility and antimicrobial activity against Escherichia coli, Salmonella enterica serova Triphimurim, Staphylococcus aureus and Vibrio parahaemolyticus. Cinnamon oil features biological activities, which is related to cinnamaldehyde, cinnamon oil main constituent, that is able to interact with bacterial cell membrane (Chuesiang et al. 2019). Nanoemulsions increased antimicrobial activity probably due to the encapsulation of cinnamaldehyde, which is prone to chemical degradation in its free form. Besides, water dispersibility of cinnamon oil was enhanced by nanoencapsulation, which can allow the use as a natural preservative to be incorporated in food or beverages. Moreover, smaller droplets seemed to be more efficiently transported through bacterial membranes, and thus provided high antimicrobial activity in comparison with larger droplets, even though the latter contained higher amounts of cinnamon oil (Chuesiang et al. 2019).
A wide range of bioactive natural oils feature important antioxidant properties, however, as they are highly lipophilic molecules, the incorporation of these components into many aqueous pharmaceutical formulations can be limited. In this context nanoemulsions rise as an encapsulation approach to provide protection to the droplet content, while preserving bioactive molecules functional properties. Rinaldi et al. produced neem oil-loaded nanoemulsions by ultrasound sonication Tween 20® as surfactant. The antioxidant activity of neem oil alone and encapsulated into nanoemulsions were quite similar, suggesting that nanoemulsions are efficient in encapsulating bioactive molecules, while maintaining functional activity of neem oil. Moreover, cytotoxicity was significantly reduced when Neem oil was incorporated in droplets in comparison with free Neem oil (Rinaldi et al. 2017).
Nanoemulsions can be applied in the controlled release of bioactive molecules in the pharmaceutical and cosmetic area. This is due to the large surface area and low interfacial tension of droplets, which allows the effective penetration of active pharmaceutical ingredient. Because of the small size of nanosized oil-loaded droplets, they can penetrate the stratum corneum and can they also be applied in alcohol-free perfume formulations (Rai et al. 2018). The encapsulation of vegetable oils can be particularly beneficial for volatile components, such as essential oils and aromas. The encapsulation approach can control the release and evaporation rate of bioactive molecules and volatile components, which can bring important for aroma perception and duration (McClements 2015). Time-controlled oil release can be tuned by modulating the lipophilicity of the inner phase. High lipophilicity can lead to a more sustained oil release. Droplet size in the case of nanoemulsions is not a limiting parameter for release profile (McClements 2011).
Because the nanoemulsions are often transparent, they are related to freshness, purity and simplicity even when carrying great amounts of oil. This characteristic has been very much valued in both pharmaceutical and cosmetic industries. It is only achieved when droplet size is too small (<70 nm) to avoid strong light scattering and ensure optical transparency, which is also dependent on polydispersity. In that context, optically transparent products can be produced when small droplet size and narrow size distribution are obtained and maintained for a considerable period of time (Wooster et al. 2008). Transparent nanoemulsions can be prepared by both high-energy and low-energy methods by adjusting and optimizing oil and aqueous phase composition, surfactants and processing parameters to achieve small droplet sizes and prevent Ostwald ripening and aggregation phenomena (McClements 2011).
Nanoemulsions with small droplet size can be sterilized through filtration and lead to a wide variety of water-based pharmaceutical products. A wide variety of products are obtained with the use of nanoemulsions, for example: lotions, moisturizers and transparent gels, with different rheological behavior (Helgeson 2016). Parenteral, or injectable, administration of nanoemulsions is employed for a variety of purposes, i.e., nutrition, for example in the administration of vegetable oils, vitamins, among others, and topical or systemic drug release. Nanoemulsions are advantageous for intravenous administration because of the rigid requirements of this route of administration, particularly the need for a droplet size in the formulation below 1 μm (Hörmann and Zimmer 2016). The benefit of nanoemulsions in oral drug administration has also been reported in the absorption of the emulsion in the gastrointestinal tract which has been correlated with droplet size (Bali et al. 2011).
4.8 Conclusion
Nanoemulsions are unique nanocarriers for the delivery lipophilic components as they provide a more stable, bioavailable, readily manufacturable, and acceptable formulation. They also impart good protection to the entrapped bioactive molecules against the effects of external conditions, as they encapsulate bioactive molecules in the core of nanosized micelles. In addition, nanoemulsions exhibit high surface area, stability and tunable rheology, which can improve drug bioavailability, making many treatments less toxic and invasive. Recently, a growing interest in the use of natural oils has been taking place, since they are proving to feature antimicrobial, antioxidants and anti-inflammatory properties, among others. In this chapter the main aspects on nanoemulsion formulation, obtention methods, characterization techniques and applications were presented.
Both high-energy and low-energy emulsification methods provide nanoemulsions with small droplet size and stability. However, much research is still needed to the achieving scaling up of these processes and to understand the impact on size and stability aspects for nanoemulsions. The difficult to scale up nanoemulsions production is responsible for so few nanoemulsion-based products are commercially available in contrast with so much research being published in this field.
Before nanoemulsions become widespread in pharmaceutical field, other challenges must be overcome. First, pharmaceutical-grade excipients should be ideally chosen, such as synthetic polymers and surfactants. Next, there are some safety concerns involving nanotechnological products as nanoemulsion and nano-based products toxicological profile is different from conventional emulsion. In this context further research is needed to promote wide nanoemulsions production and utilization.
References
Abismail B, Canselier JP, Wilhelm AM, Delmas H, Gourdon C (1999) Emulsification by ultrasound- drop size distribution and stability.Pdf. Ultrason Sonochem 6:75–83
Adjonu R, Doran G, Torley P, Agboola S (2014) Formation of whey protein isolate hydrolysate stabilised nanoemulsion. Food Hydrocoll 41:169–177. https://doi.org/10.1016/j.foodhyd.2014.04.007
Alam P, Shakeel F, Anwer MK, Foudah AI, Alqarni MH (2018) Wound healing study of eucalyptus essential oil containing nanoemulsion in rat model. J Oleo Sci 67(8):957–968. https://doi.org/10.5650/jos.ess18005
Alexandridis P, Holzwarth JF, Hatton TA (1994) Micellization of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers in aqueous solutions: thermodynamics of copolymer association. Macromolecules 27(9):2414–2425. https://doi.org/10.1021/ma00087a009
Al-Subaie MM, Hosny KM, El-Say KM, Ahmed TA, Aljaeid BM (2015) Utilization of nanotechnology to enhance percutaneous absorption of acyclovir in the treatment of herpes simplex viral infections. Int J Nanomedicine. https://doi.org/10.2147/IJN.S83962
Amin F, Khan S, Shah SMH, Rahim H, Hussain Z, Sohail M, Ullah R, Alsaid MS, Shahat AA (2018) A new strategy for taste masking of azithromycin antibiotic: development, characterization, and evaluation of azithromycin titanium nanohybrid for masking of bitter taste using physisorption and panel testing studies. Drug Des Dev Ther 12:3855–3866. https://doi.org/10.2147/DDDT.S183534
Andreu V, Mendoza G, Arruebo M, Irusta S (2015) Smart dressings based on nanostructured fibers containing natural origin antimicrobial, anti-inflammatory, and regenerative compounds. Materials 8(8):5154–5193. https://doi.org/10.3390/ma8085154
Angioni A, Barra A, Cereti E, Barile D, Coïsson JD, Arlorio M, Dessi S, Coroneo V, Cabras P (2004) Chemical composition, plant genetic differences, antimicrobial and antifungal activity investigation of the essential oil of Rosmarinus Officinalis L. J Agric Food Chem 52(11):3530–3535. https://doi.org/10.1021/jf049913t
Anton N, Vandamme TF (2011) Nano-emulsions and micro-emulsions: clarifications of the critical differences. Pharm Res 28(5):978–985. https://doi.org/10.1007/s11095-010-0309-1
Anton N, Benoit J-P, Saulnier P (2008) Design and production of nanoparticles formulated from nano-emulsion templates – a review. J Control Release 128(3):185–199. https://doi.org/10.1016/j.jconrel.2008.02.007
Anwer MK, Jamil S, Ibnouf EO, Shakeel F (2014) Enhanced antibacterial effects of clove essential oil by nanoemulsion. J Oleo Sci. https://doi.org/10.5650/jos.ess13213
Asbahani A, El K, Miladi W, Badri M, Sala EH, Aït Addi H, Casabianca AEM et al (2015) Essential oils: from extraction to encapsulation. Int J Pharm 483(1–2):220–243. https://doi.org/10.1016/j.ijpharm.2014.12.069
Azeem A, Rizwan M, Ahmad FJ, Iqbal Z, Khar RK, Aqil M, Talegaonkar S (2009) Nanoemulsion components screening and selection: a technical note. AAPS PharmSciTech 10. https://doi.org/10.1208/s12249-008-9178-x
Babchin AJ, Schramm LL (2012) Osmotic repulsion force due to adsorbed surfactants. Colloids Surf B: Biointerfaces. https://doi.org/10.1016/j.colsurfb.2011.10.050
Badgujar SB, Patel VV, Bandivdekar AH (2014) Foeniculum Vulgare mill: a review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. Biomed Res Int. https://doi.org/10.1155/2014/842674
Bai L, Huan S, Jiyou G, McClements DJ (2016) Fabrication of oil-in-water nanoemulsions by dual-channel microfluidization using natural emulsifiers: saponins, phospholipids, proteins, and polysaccharides. Food Hydrocoll 61:703–711. https://doi.org/10.1016/j.foodhyd.2016.06.035
Bali V, Ali M, Ali J (2011) Nanocarrier for the enhanced bioavailability of a cardiovascular agent: in vitro, pharmacodynamic, pharmacokinetic and stability assessment. Int J Pharm. https://doi.org/10.1016/j.ijpharm.2010.10.018
Barradas TN, de Campos VEB, Senna JP, Coutinho CSC, Tebaldi BS, Silva KGH, Mansur CRE (2015) Development and characterization of promising o/w nanoemulsions containing sweet fennel essential oil and non-ionic sufactants. Colloids Surf A Physicochem Eng Asp 480. https://doi.org/10.1016/j.colsurfa.2014.12.001
Barradas TNTN, Juliana Perdiz Senna JP, Stephani Araujo Cardoso SA, Nicoli S, Padula C, Santi P, Rossi F, Gyselle de Holanda e Silva KG, Mansur CRE (2017) Hydrogel-thickened nanoemulsions based on essential oils for topical delivery of psoralen: permeation and stability studies. Eur J Pharm Biopharm 116:38–50. https://doi.org/10.1016/j.ejpb.2016.11.018
Barradas TN, Senna JP, Cardoso SA, de Holanda e Silva KG, Elias Mansur CR (2018) Formulation characterization and in vitro drug release of hydrogel-thickened nanoemulsions for topical delivery of 8-methoxypsoralen. Mater Sci Eng C 92. https://doi.org/10.1016/j.msec.2018.06.049
Behrend O, Ax K, Schubert H (2000) Influence of continuous phase viscosity on emulsification by ultrasound. Ultrason Sonochem. https://doi.org/10.1016/S1350-4177(99)00029-2
Bhattacharjee S (2016) DLS and zeta potential – what they are and what they are not? J Control Release. https://doi.org/10.1016/j.jconrel.2016.06.017
Bondi CA, Marks JL, Wroblewski LB, Raatikainen HS, Lenox SR, Gebhardt KE (2015) Human and environmental toxicity of sodium lauryl sulfate (SLS): evidence for safe use in household cleaning products. Environ Health Insights 9:27–32. https://doi.org/10.4137/EHI.S31765
Bonferoni MC, Rossi S, Cornaglia AI, Mannucci B, Grisoli P, Vigani B, Saporito F et al (2017) Essential oil-loaded lipid nanoparticles for wound healing. Int J Nanomedicine 13:175–186. https://doi.org/10.2147/IJN.S152529
Borges RS, Lima ES, Keita H, Ferreira IM, Fernandes CP, Cruz RAS, Duarte JL et al (2018) Anti-inflammatory and antialgic actions of a nanoemulsion of Rosmarinus officinalis L. essential oil and a molecular docking study of its major chemical constituents. Inflammopharmacology 26(1):183–195. https://doi.org/10.1007/s10787-017-0374-8
Botas G, Cruz R, de Almeida F, Duarte J, Araújo R, Souto R, Ferreira R et al (2017) Baccharis reticularia DC. and limonene nanoemulsions: promising larvicidal agents for Aedes aegypti (Diptera: Culicidae) control. Molecules 22(11):1990. https://doi.org/10.3390/molecules22111990
Bouchemal K, Briançon S, Perrier E, Fessi H (2004) Nano-emulsion formulation using spontaneous emulsification: solvent, oil and surfactant optimisation. Int J Pharm 280(1–2):241–251. https://doi.org/10.1016/j.ijpharm.2004.05.016
Bourbon AI, Gonçalves RFS, Vicente AA, Pinheiro AC (2018) Characterization of particle properties in nanoemulsions. Nanoemulsions (Elsevier):519–546. https://doi.org/10.1016/B978-0-12-811838-2.00016-3
Bouyer E, Mekhloufi G, Rosilio V, Grossiord JL, Agnely F (2012) Proteins, polysaccharides, and their complexes used as stabilizers for emulsions: alternatives to synthetic surfactants in the pharmaceutical field? Int J Pharm. https://doi.org/10.1016/j.ijpharm.2012.06.052
Calderó G, García-Celma MJ, Solans C (2011) Formation of polymeric nano-emulsions by a low-energy method and their use for nanoparticle preparation. J Colloid Interface Sci 353(2):406–411. https://doi.org/10.1016/j.jcis.2010.09.073
Cardoso-Ugarte GA, López-Malo A, Jiménez-Munguía MT (2016) Application of nanoemulsion technology for encapsulation and release of lipophilic bioactive compounds in food. Emulsions (Elsevier):227–255. https://doi.org/10.1016/B978-0-12-804306-6.00007-6
Carteau D, Bassani D, Pianet I (2008) The ‘Ouzo Effect’: following the spontaneous emulsification of trans-anethole in water by NMR. C R Chim. https://doi.org/10.1016/j.crci.2007.11.003
Cerqueira-Coutinho C, Santos-Oliveira R, dos Santos E, Mansur CR (2015) Development of a photoprotective and antioxidant nanoemulsion containing chitosan as an agent for improving skin retention. Eng Life Sci. https://doi.org/10.1002/elsc.201400154
Chaieb K, Hajlaoui H, Zmantar T, Kahla-Nakbi AB, Rouabhia M, Mahdouani K, Bakhrouf A (2007) The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium Aromaticum L. Myrtaceae): a short review. Phytother Res. https://doi.org/10.1002/ptr.2124
Chebil A, Desbrières J, Nouvel C, Six J-L, Durand A (2013) Ostwald ripening of nanoemulsions stopped by combined interfacial adsorptions of molecular and macromolecular nonionic stabilizers. Colloids Surf A Physicochem Eng Asp 425:24–30. https://doi.org/10.1016/j.colsurfa.2013.02.028
Chen H, Khemtong C, Yang X, Chang X, Gao J (2011) Nanonization strategies for poorly water-soluble drugs. Drug Discov Today. https://doi.org/10.1016/j.drudis.2010.02.009
Chuesiang P, Siripatrawan U, Sanguandeekul R, Yang JS, McClements DJ, McLandsborough L (2019) Antimicrobial activity and chemical stability of cinnamon oil in oil-in-water nanoemulsions fabricated using the phase inversion temperature method. LWT 110:190–196. https://doi.org/10.1016/j.lwt.2019.03.012
Chung C, McClements DJ (2018) Characterization of physicochemical properties of nanoemulsions: appearance, stability, and rheology. Nanoemulsions (Elsevier):547–576. https://doi.org/10.1016/B978-0-12-811838-2.00017-5
Constantinides PP, Chaubal MV, Shorr R (2008) Advances in lipid nanodispersions for parenteral drug delivery and targeting. Adv Drug Deliv Rev 60(6):757–767. https://doi.org/10.1016/j.addr.2007.10.013
de Oliveira EF, Paula HCB, de Paula RCM (2014) Alginate/cashew gum nanoparticles for essential oil encapsulation. Colloids Surf B: Biointerfaces 113:146–151. https://doi.org/10.1016/j.colsurfb.2013.08.038
Dias D d O, Colombo M, Kelmann RG, Kaiser S, Lucca LG, Teixeira HF, Limberger RP, Veiga VF, Koester LS (2014) Optimization of copaiba oil-based nanoemulsions obtained by different preparation methods. Ind Crop Prod 59:154–162. https://doi.org/10.1016/j.indcrop.2014.05.007
Dickinson E (2003) Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. https://doi.org/10.1016/S0268-005X(01)00120-5
Dickinson E (2009) Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll 23(6):1473–1482. https://doi.org/10.1016/j.foodhyd.2008.08.005
Dickinson E, Galazka VB, Anderson DMW (1991) Emulsifying behaviour of gum arabic. Part 1: effect of the nature of the oil phase on the emulsion droplet-size distribution. Carbohydr Polym 14(4):373–383. https://doi.org/10.1016/0144-8617(91)90003-U
Dickinson E, Semenova MG, Antipova AS (1998) Salt stability of casein emulsions. Food Hydrocoll 12(2):227–235. https://doi.org/10.1016/S0268-005X(98)00035-6
Donsì F, Ferrari G (2016) Essential oil nanoemulsions as antimicrobial agents in food. J Biotechnol Elsevier BV. https://doi.org/10.1016/j.jbiotec.2016.07.005
Dorfmüller T (1987) R. Pecora (Ed.): dynamic light scattering – applications of photon correlation spectroscopy, Plenum Press, New York and London 1985. 420 Seiten, Preis: $ 59.90. Ber Bunsenges Phys Chem 91(4):498–499. https://doi.org/10.1002/bbpc.19870910455
Duarte JL, Amado JRR, Oliveira AEMFM, Cruz RAS, Ferreira AM, Souto RNP, Falcão DQ, Carvalho JCT, Fernandes CP (2015) Evaluation of larvicidal activity of a nanoemulsion of Rosmarinus officinalis essential oil. Rev Bras 25(2):189–192. https://doi.org/10.1016/j.bjp.2015.02.010
Elmahjoubi E, Frum Y, Eccleston GM, Wilkinson SC, Meidan VM (2009) Transepidermal water loss for probing full-thickness skin barrier function: correlation with tritiated water flux, sensitivity to punctures and diverse surfactant exposures. Toxicol in Vitro. https://doi.org/10.1016/j.tiv.2009.06.030
Farshi P, Tabibiazar M, Ghorbani M, Mohammadifar M, Amirkhiz MB, Hamishehkar H (2019) Whey protein isolate-guar gum stabilized cumin seed oil nanoemulsion. Food Biosci 28:49–56. https://doi.org/10.1016/j.fbio.2019.01.011
Fernandez P, André V, Rieger J, Kühnle A (2004) Nano-emulsion formation by emulsion phase inversion. Colloids Surf A Physicochem Eng Asp. https://doi.org/10.1016/j.colsurfa.2004.09.029
Goñi MG, Roura SI, Ponce AG, Moreira MR (2016) Clove (Syzygium aromaticum) oils. In: Preedy VR (ed) Essential oils in food preservation, flavor and safety. Academic, Amsterdam
Graves S, Meleson K, Wilking J, Lin MY, Mason TG (2005) Structure of concentrated nanoemulsions. J Chem Phys 122(13):134703. https://doi.org/10.1063/1.1874952
Gupta A, Eral HB, Hatton TA, Doyle PS (2016a) Controlling and predicting droplet size of nanoemulsions: scaling relations with experimental validation. Soft Matter. https://doi.org/10.1039/C5SM02051D
Gupta A, Eral HB, Hatton TA, Doyle PS (2016b) Nanoemulsions: formation, properties and applications. Soft Matter 12(11). https://doi.org/10.1039/C5SM02958A
Gutiérrez JM, González C, Maestro A, Solè I, Pey CM, Nolla J (2008) Nano-emulsions: new applications and optimization of their preparation. Curr Opin Colloid Interface Sci. https://doi.org/10.1016/j.cocis.2008.01.005
Hashem AS, Awadalla SS, Zayed GM, Maggi F, Benelli G (2018) Pimpinella Anisum essential oil nanoemulsions against Tribolium Castaneum—insecticidal activity and mode of action. Environ Sci Pollut Res 25(19):18802–18812. https://doi.org/10.1007/s11356-018-2068-1
Hashemi Gahruie H, Ziaee E, Eskandari MH, Hosseini SMH (2017) Characterization of basil seed gum-based edible films incorporated with Zataria Multiflora essential oil nanoemulsion. Carbohydr Polym 166:93–103. https://doi.org/10.1016/j.carbpol.2017.02.103
Hasssanzadeh H, Alizadeh M, Rezazad Bari M (2018) Formulation of garlic oil-in-water nanoemulsion: antimicrobial and physicochemical aspects. IET Nanobiotechnol 12(5):647–652. https://doi.org/10.1049/iet-nbt.2017.0104
Helgeson ME (2016) Colloidal behavior of nanoemulsions: interactions, structure, and rheology. Curr Opin Colloid Interface Sci. https://doi.org/10.1016/j.cocis.2016.06.006
Herman AAP, Herman AAP (2015) Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: a review. J Pharm Pharmacol. https://doi.org/10.1111/jphp.12334
Hernández MD, Sotomayor JA, Hernández Á, Jordán MJ (2016) Rosemary (Rosmarinus officinalis L.) oils. In: Preedy VR (ed) Essential oils in food preservation, flavor and safety. Elsevier, San Diego, pp 677–688
Hoeller S, Sperger A, Valenta C (2009) Lecithin based nanoemulsions: a comparative study of the influence of non-ionic surfactants and the cationic phytosphingosine on physicochemical behaviour and skin permeation. Int J Pharm 370(1–2):181–186. https://doi.org/10.1016/j.ijpharm.2008.11.014
Hörmann K, Zimmer A (2016) Drug delivery and drug targeting with parenteral lipid nanoemulsions – a review. J Control Release 223:85–98. https://doi.org/10.1016/j.jconrel.2015.12.016
Hudaib M, Speroni E, Di Pietra AM, Cavrini V (2002) GC/MS evaluation of thyme (Thymus vulgaris L.) oil composition and variations during the vegetative cycle. J Pharm Biomed Anal 29(4):691–700. http://www.ncbi.nlm.nih.gov/pubmed/12093498
Hunter RJ (2001) Foundations of colloid science, 2nd edn. Oxford University Press, New York
Hussain AI, Anwar F, Hussain Sherazi ST, Przybylski R (2008) Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem 108(3):986–995. https://doi.org/10.1016/j.foodchem.2007.12.010
Jafari SM (2017) Nanoencapsulation technologies for the food and nutraceutical industries. Academic Print, London. https://doi.org/10.1016/B978-0-12-809436-5/00014-8
Jafari SM, He Y, Bhandari B (2007) Production of sub-micron emulsions by ultrasound and microfluidization techniques. J Food Eng 82(4):478–488. https://doi.org/10.1016/j.jfoodeng.2007.03.007
Jafari SM, Assadpoor E, He Y, Bhandari B (2008) Re-coalescence of emulsion droplets during high-energy emulsification. Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2007.09.006
Jafari SM, Paximada P, Mandala I, Assadpour E, Mehrnia MA (2017) Encapsulation by nanoemulsions. Nanoencapsulation Technol Food Nutraceut Ind 2017:36–73
Jaiswal M, Dudhe R, Sharma PK (2015) Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech Springer Verlag. https://doi.org/10.1007/s13205-014-0214-0
Jin W, Xu W, Lian H, Li Y, Liu S, Li B (2016) Nanoemulsions for food: properties, production, characterization and applications. In: Grumezescu A (ed) Emulsions. Elsevier Inc, London, pp 1–29. https://doi.org/10.1016/B978-0-12-804306-6.00001-5
Junyaprasert VB, Teeranachaideekul V, Souto EB, Boonme P, Müller RH (2009) Q10-loaded NLC versus nanoemulsions: stability, rheology and in vitro skin permeation. Int J Pharm. https://doi.org/10.1016/j.ijpharm.2009.05.020
Jirovetz L, Buchbauer G, Stoilova I, Stoyanova A, Krastanov A, Schmidt E (2006) Chemical composition and antioxidant properties of clove leaf essential oil. J Agric Food Chem 54:6303–6307
Kataoka K, Harada A, Nagasaki Y (2001) Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev 47(1):113–131
Katsumoto Y, Ushiki H, Lachaise J, Graciaa A (2001) Time evolution of the size distribution of nano-sphere droplets in the hexadecane-in-water miniemulsion stabilized by nonionic surfactants. Colloid Polym Sci 279(2):122–130. https://doi.org/10.1007/s003960000395
Khan MS, Krishnaraj K (2014) Phospholipids: a novel adjuvant in herbal drug delivery systems. Crit Rev Ther Drug Carrier Syst 31(5):407–428. https://doi.org/10.1615/CritRevTherDrugCarrierSyst.2014010634
Kiaei N, Hajimohammadi R, Hosseini M (2018) Investigation of the anti-inflammatory properties of calendula nanoemulsion on skin cells. Bioinspired, Biomimetic Nanobiomater 7(4):228–237. https://doi.org/10.1680/jbibn.17.00033
Klang V, Valenta C (2011) Lecithin-based nanoemulsions. J Drug Delivery Sci Technol 21(1):55–76. https://doi.org/10.1016/S1773-2247(11)50006-1
Kotta S, Khan AW, Ansari SH, Sharma RK, Ali J (2015) Formulation of nanoemulsion: a comparison between phase inversion composition method and high-pressure homogenization method. Drug Deliv 22(4):455–466. https://doi.org/10.3109/10717544.2013.866992
Kuhn KR, Cunha RL (2012) Flaxseed oil – whey protein isolate emulsions: effect of high pressure homogenization. J Food Eng 111(2):449–457. https://doi.org/10.1016/j.jfoodeng.2012.01.016
Lane KE, Li W, Smith C, Derbyshire E (2014) The bioavailability of an omega-3-rich algal oil is improved by nanoemulsion technology using yogurt as a food vehicle. Int J Food Sci Technol 49(5):1264–1271. https://doi.org/10.1111/ijfs.12455
Lawrence MJ, Rees GD (2000) Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev 45(1):89–121. https://doi.org/10.1016/S0169-409X(00)00103-4
Lee L, Norton IT (2013) Comparing droplet breakup for a high-pressure valve homogeniser and a microfluidizer for the potential production of food-grade nanoemulsions. J Food Eng 114(2):158–163. https://doi.org/10.1016/j.jfoodeng.2012.08.009
Lefebvre G, Riou J, Bastiat G, Roger E, Frombach K, Gimel J-C, Saulnier P, Calvignac B (2017) Spontaneous nano-emulsification: process optimization and modeling for the prediction of the nanoemulsion’s size and polydispersity. Int J Pharm 534(1–2):220–228. https://doi.org/10.1016/j.ijpharm.2017.10.017
Lémery E, Briançon S, Chevalier Y, Bordes C, Oddos T, Gohier A, Bolzinger MA (2015) Skin toxicity of surfactants: structure/toxicity relationships. Colloids Surf A Physicochem Eng Asp. https://doi.org/10.1016/j.colsurfa.2015.01.019
Leong TSH, Wooster TJ, Kentish SE, Ashokkumar M (2009) Minimising oil droplet size using ultrasonic emulsification. Ultrason Sonochem. https://doi.org/10.1016/j.ultsonch.2009.02.008
Li QX, Chang CL (2016) Basil (Ocimum basilicum L.) oils. In: Preedy VR (ed) Essential oils in food preservation, flavor and safety. Academic, San Diego, pp 231–238
Li Z-h, Cai M, Liu Y-s, Sun P-l (2018) Development of finger citron (Citrus medica L. Var. Sarcodactylis) essential oil loaded nanoemulsion and its antimicrobial activity. Food Control 94:317–323. https://doi.org/10.1016/j.foodcont.2018.07.009
Liu W, Sun D, Li C, Liu Q, Xu J (2006) Formation and stability of paraffin oil-in-water nano-emulsions prepared by the emulsion inversion point method. J Colloid Interface Sci 303(2):557–563. https://doi.org/10.1016/j.jcis.2006.07.055
Lou Z, Chen J, Yu F, Wang H, Kou X, Ma C, Zhu S (2017) The antioxidant, antibacterial, antibiofilm activity of essential oil from Citrus medica L. Var. Sarcodactylis and its nanoemulsion. LWT 80:371–377. https://doi.org/10.1016/j.lwt.2017.02.037
Maestro A, Solè I, González C, Solans C, Gutiérrez JM (2008) Influence of the phase behavior on the properties of ionic nanoemulsions prepared by the phase inversion composition method. J Colloid Interface Sci 327(2):433–439. https://doi.org/10.1016/j.jcis.2008.07.059
Mandal S, DebManda M (2016) Thyme (Thymus vulgaris L.) oils. In: Preedy VR (ed) Essential oils in food preservation, flavor and safety. Academic, San Diego, pp 25–834
Mao L, Xu D, Yang J, Yuan F, Gao Y, Zhao J (2009) Effects of small and large molecule emulsifiers on the characteristics of B-carotene nanoemulsions prepared by high pressure homogenization. Food Technol Biotechnol 47(3):336–342
Marotti M, Piccaglia R, Giovanelli E (1996) Differences in essential oil composition of basil (Ocimum basilicum L.) Italian cultivars related to morphological characteristics. J Agric Food Chem 44(12):3926–3929. https://doi.org/10.1021/jf9601067
Mason TG, Wilking JN, Meleson K, Chang CB, Graves SM (2006) Nanoemulsions: formation, structure, and physical properties. J Phys Condens Matter 18(18):635–666. https://doi.org/10.1088/0953-8984/18/41/R01
Mayer S, Weiss J, McClements DJ (2013) Vitamin E-enriched nanoemulsions formed by emulsion phase inversion: factors influencing droplet size and stability. J Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2013.04.016
Mazarei Z, Rafati H (2019) Nanoemulsification of Satureja khuzestanica essential oil and pure carvacrol; comparison of physicochemical properties and antimicrobial activity against food pathogens. LWT 100:328–334. https://doi.org/10.1016/j.lwt.2018.10.094
McClements DJ (2011) Edible nanoemulsions: fabrication, properties, and functional performance. Soft Matter 7(6):2297–2316. https://doi.org/10.1039/C0SM00549E
McClements DJ (2012) Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft Matter 8(6):1719–1729. https://doi.org/10.1039/C2SM06903B
McClements DJ (2015) Food emulsions: principles, practices, and techniques, 3rd edn. CRC Press, Boca Raton/London/New York
McClements DJ, Jafari SM (2018) General aspects of nanoemulsions and their formulation. Nanoemulsions (Elsevier):3–20. https://doi.org/10.1016/B978-0-12-811838-2.00001-1
McClements DJ, Rao J (2011) Food-grade nanoemulsions: formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit Rev Food Sci Nutr 51(4):285–330. https://doi.org/10.1080/10408398.2011.559558
McNamee BF, O’Riorda ED, O’Sullivan M (1998) Emulsification and microencapsulation properties of gum arabic. J Agric Food Chem. https://doi.org/10.1021/jf9803740
Meinders MBJ, van Vliet T (2004) The role of interfacial rheological properties on ostwald ripening in emulsions. Adv Colloid Interf Sci 108–109:119–126. https://doi.org/10.1016/j.cis.2003.10.005
Moghimi R, Ghaderi L, Rafati H, Aliahmadi A, McClements DJ (2016) Superior antibacterial activity of nanoemulsion of thymus daenensis essential oil against E. Coli. Food Chem 194:410–415. https://doi.org/10.1016/j.foodchem.2015.07.139
Myers D (2005a) Surfactants in solution. In: Surfactant science and technology, 3rd edn. Wiley, Hoboken, pp 107–142
Myers D (2005b) The organic chemistry of surfactants. In: Surfactant science and technology. Wiley, Hoboken, pp 1–448
Nirmal NP, Mereddy R, Li L, Sultanbawa Y (2018) Formulation, characterisation and antibacterial activity of lemon myrtle and anise myrtle essential oil in water nanoemulsion. Food Chem 254:1–7. https://doi.org/10.1016/j.foodchem.2018.01.173
Oliveira AEMFM, Bezerra DC, Duarte JL, Cruz RAS, Souto RNP, Ferreira RMA, Nogueira J et al (2017) Essential oil from Pterodon emarginatus as a promising natural raw material for larvicidal nanoemulsions against a tropical disease vector. Sustain Chem Pharm 6:1–9. https://doi.org/10.1016/j.scp.2017.06.001
Osanloo M, Sedaghat MM, Esmaeili F, Amani A (2018a) Larvicidal activity of essential oil of Syzygium aromaticum (clove) in comparison with its major constituent, eugenol, against Anopheles stephensi. J Arthropod Borne Dis 12(4):361–369. http://www.ncbi.nlm.nih.gov/pubmed/30918905
Osanloo M, Sereshti H, Sedaghat MM, Amani A (2018b) Nanoemulsion of dill essential oil as a green and potent larvicide against Anopheles stephensi. Environ Sci Pollut Res 25(7):6466–6473. https://doi.org/10.1007/s11356-017-0822-4
Ouzineb K, Lord C, Lesauze N, Graillat C, Tanguy PA, McKenna T (2006) Homogenisation devices for the production of miniemulsions. Chem Eng Sci 61(9):2994–3000. https://doi.org/10.1016/j.ces.2005.10.065
Pathania R, Khan H, Kaushik R, Khan MA (2018) Essential oil nanoemulsions and their antimicrobial and food applications. Curr Res Nutr Food Sci J. https://doi.org/10.12944/crnfsj.6.3.05
Pengon S, Chinatangkul N, Limmatvapirat C, Limmatvapirat S (2018) The effect of surfactant on the physical properties of coconut oil nanoemulsions. Asian J Pharm Sci 13(5):409–414. https://doi.org/10.1016/j.ajps.2018.02.005
Pérez-Recalde M, Ruiz Arias IE, Hermida ÉB (2018) Could essential oils enhance biopolymers performance for wound healing? a systematic review. Phytomedicine 38:57–65. https://doi.org/10.1016/j.phymed.2017.09.024
Politeo O, Jukic M, Milos M (2007) Chemical composition and antioxidant capacity of free volatile aglycones from basil (Ocimum basilicum L.) compared with its essential oil. Food Chem 101(1):379–385. https://doi.org/10.1016/j.foodchem.2006.01.045
Porras M, Solans C, González C, Martínez A, Guinart A, Gutiérrez JM (2004) Studies of formation of W/O nano-emulsions. Colloids Surf A Physicochem Eng Asp. https://doi.org/10.1016/j.colsurfa.2004.08.060
Porras M, Solans C, González C, Gutiérrez JM (2008) Properties of water-in-oil (W/O) nano-emulsions prepared by a low-energy emulsification method. Colloids Surf A Physicochem Eng Asp. https://doi.org/10.1016/j.colsurfa.2008.04.012
Prabaharan M (2011) Prospective of guar gum and its derivatives as controlled drug delivery systems. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2011.04.022
Prajapati VD, Jani GK, Moradiya NG, Randeria NP (2013) Pharmaceutical applications of various natural gums, mucilages and their modified forms. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2012.11.021
Prud’homme RK, Guangwei W, Schneider DK (1996) Structure and rheology studies of poly(oxyethylene−oxypropylene−oxyethylene) aqueous solution. Langmuir 12(20):4651–4659. https://doi.org/10.1021/la951506b
Qian C, McClements DJ (2011) Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: factors affecting particle size. Food Hydrocoll 25(5):1000–1008. https://www.sciencedirect.com/science/article/pii/S0268005X10002328
Qian C, Decker EA, Xiao H, McClements DJ (2012) Physical and chemical stability of β-carotene-enriched nanoemulsions: influence of PH, ionic strength, temperature, and emulsifier type. Food Chem 132(3):1221–1229. https://doi.org/10.1016/j.foodchem.2011.11.091
Rai VK, Mishra N, Yadav KS, Yadav NP (2018) Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: formulation development, stability issues, basic considerations and applications. J Control Release. https://doi.org/10.1016/j.jconrel.2017.11.049
Rao J, McClements DJ (2011) Food-grade microemulsions, nanoemulsions and emulsions: fabrication from sucrose monopalmitate & lemon oil. Food Hydrocoll 25(6):1413–1423. https://doi.org/10.1016/j.foodhyd.2011.02.004
Rao J, McClements DJ (2012) Food-grade microemulsions and nanoemulsions: role of oil phase composition on formation and stability. Food Hydrocoll 29(2):326–334. https://doi.org/10.1016/j.foodhyd.2012.04.008
Rinaldi F, Hanieh PN, Longhi C, Carradori S, Secci D, Zengin G, Ammendolia MG et al (2017) Neem oil nanoemulsions: characterisation and antioxidant activity. J Enzyme Inhib Med Chem 32(1):1265–1273. https://doi.org/10.1080/14756366.2017.1378190
Rosety M, Ordóñez FJ, Rosety-Rodríguez M, Rosety JM, Rosety I, Carrasco C, Ribelles A (2001) Acute toxicity of anionic surfactants sodium dodecyl sulphate (SDS) and linear alkylbenzene sulphonate (LAS) on the fertilizing capability of gilthead (Sparus aurata L.) sperm. Histol Histopathol 16(3):839–843. https://doi.org/10.14670/HH-16.839
Saberi AH, Fang Y, McClements DJ (2014) Stabilization of vitamin E-enriched mini-emulsions: influence of organic and aqueous phase compositions. Colloids Surf A Physicochem Eng Asp. https://doi.org/10.1016/j.colsurfa.2014.02.042
Salem MA, Ezzat SM (2018) Nanoemulsions in food industry. In: Some new aspects of colloidal systems in foods. IntechOpen, London. https://doi.org/10.5772/intechopen.79447
Salvia-Trujillo L, Rojas-Graü A, Soliva-Fortuny R, Martín-Belloso O (2015) Physicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2014.07.012
Schwarz JC, Weixelbaum A, Pagitsch E, Löw M, Resch GP, Valenta C (2012) Nanocarriers for dermal drug delivery: influence of preparation method, carrier type and rheological properties. Int J Pharm. https://doi.org/10.1016/j.ijpharm.2012.08.003
Seibert JB, Rodrigues IV, Carneiro SP, Amparo TR, Lanza JS, Frézard FJG, de Souza GHB, dos Santos ODH (2019) Seasonality study of essential oil from leaves of Cymbopogon densiflorus and nanoemulsion development with antioxidant activity. Flavour Fragrance J 34(1):5–14. https://doi.org/10.1002/ffj.3472
Shahavi MH, Hosseini M, Jahanshahi M, Meyer RL, Darzi GN (2015) Evaluation of critical parameters for preparation of stable clove oil nanoemulsion. Arab J Chem. https://doi.org/10.1016/j.arabjc.2015.08.024
Shahavi MH, Hosseini M, Jahanshahi M, Meyer RL, Darzi GN (2016) Clove oil nanoemulsion as an effective antibacterial agent: Taguchi optimization method. Desalin Water Treat 57(39):18379–18390. https://doi.org/10.1080/19443994.2015.1092893
Shahba AA-W, Mohsin K, Alanazi FK (2012) Novel self-nanoemulsifying drug delivery systems (SNEDDS) for oral delivery of cinnarizine: design, optimization, and in-vitro assessment. AAPS PharmSciTech 13(3):967–977. https://doi.org/10.1208/s12249-012-9821-4
Sharma M, Mann B, Sharma R, Bajaj R, Athira S, Sarkar P, Pothuraju R (2017) Sodium caseinate stabilized clove oil nanoemulsion: physicochemical properties. J Food Eng 212:38–46. https://doi.org/10.1016/j.jfoodeng.2017.05.006
Sharma A, Sharma NK, Srivastava A, Kataria A, Dubey S, Sharma S, Kundu B (2018) Clove and lemongrass oil based non-ionic nanoemulsion for suppressing the growth of plant pathogenic Fusarium Oxysporum f.Sp. Lycopersici. Ind Crop Prod 123:353–362. https://doi.org/10.1016/j.indcrop.2018.06.077
Shaw DJ (1992) The colloidal state. In: Introduction to colloid and surface chemistry, 4th edn. Elsevier, Amsterdam, p 320. https://doi.org/10.1016/C2009-0-24070-0
Shchipunov YA (2015) Lecithin. In: Somasundaran P (ed) Encyclopedia of surface and colloid science. CRC Press, Boca Raton, pp 3674–3693. https://doi.org/10.1081/E-ESCS3
Sing AJF, Graciaa A, Lachaise J, Brochette P, Salager JL (1999) Interactions and coalescence of nanodroplets in translucent O/W emulsions. Colloids Surf A Physicochem Eng Asp 152(1–2):31–39. https://doi.org/10.1016/S0927-7757(98)00622-0
Singh Y, Meher JG, Raval K, Khan FA, Chaurasia M, Jain NK, Chourasia MK (2017) Nanoemulsion: concepts, development and applications in drug delivery. J Control Release. https://doi.org/10.1016/j.jconrel.2017.03.008
Sivakumar M, Tang SY, Tan KW (2014) Cavitation technology – a greener processing technique for the generation of pharmaceutical nanoemulsions. Ultrason Sonochem. https://doi.org/10.1016/j.ultsonch.2014.03.025
Solans C, Solé I (2012) Nano-emulsions: formation by low-energy methods. Curr Opin Colloid Interface Sci 17(5):246–254. https://doi.org/10.1016/j.cocis.2012.07.003
Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ (2005) Nano-emulsions. Curr Opin Colloid Interface Sci. https://doi.org/10.1016/j.cocis.2005.06.004
Solè I, Maestro A, Pey CM, González C, Solans C, Gutiérrez JM (2006) Nano-emulsions preparation by low energy methods in an ionic surfactant system. Colloids Surf A Physicochem Eng Asp 288(1–3):138–143. https://doi.org/10.1016/j.colsurfa.2006.02.013
Solè I, Solans C, Maestro A, González C, Gutiérrez JM (2012) Study of nano-emulsion formation by dilution of microemulsions. J Colloid Interface Sci 376(1):133–139. https://doi.org/10.1016/j.jcis.2012.02.063
Sonneville-Aubrun O, Simonnet JT, L’Alloret F (2004) Nanoemulsions: a new vehicle for skincare products. Adv Colloid Interf Sci. https://doi.org/10.1016/j.cis.2003.10.026
Sonneville-Aubrun O, Babayan D, Bordeaux D, Lindner P, Rata G, Cabane B (2009) phase transition pathways for the production of 100 Nm oil-in-water emulsions. Phys Chem Chem Phys 11(1):101–110. https://doi.org/10.1039/B813502A
Sonu KS, Mann B, Sharma R, Kumar R, Singh R (2018) Physico-chemical and antimicrobial properties of d-limonene oil nanoemulsion stabilized by whey protein–maltodextrin conjugates. J Food Sci Technol 55(7):2749–2757. https://doi.org/10.1007/s13197-018-3198-7
Speranza A, Corradini MG, Hartman TG, Ribnicky D, Oren A, Rogers MA (2013) Influence of emulsifier structure on lipid bioaccessibility in oil–water nanoemulsions. J Agric Food Chem 61(26):6505–6515. https://doi.org/10.1021/jf401548r
Starov VM, Zhdanov VG (2003) Viscosity of emulsions: influence of flocculation. J Colloid Interface Sci 258(2):404–414. https://doi.org/10.1016/S0021-9797(02)00149-2
Strickley RG (2004) Solubilizing excipients in oral and injectable formulations. Pharm Res. https://doi.org/10.1023/B:PHAM.0000016235.32639.23
Sundararajan B, Moola AK, Vivek K, Kumari BDR (2018) Formulation of nanoemulsion from leaves essential oil of Ocimum basilicum L. and its antibacterial, antioxidant and larvicidal activities (Culex quinquefasciatus). Microb Pathog 125:475–485. https://doi.org/10.1016/j.micpath.2018.10.017
Sutradhar KB, Amin L (2013) Nanoemulsions: increasing possibilities in drug delivery. Eur J Nanomed. https://doi.org/10.1515/ejnm-2013-0001
Svetlichny G, Külkamp-Guerreiro IC, Dalla Lana DF, Bianchin MD, Pohlmann AR, Fuentefria AM, Guterres SS (2017) Assessing the performance of copaiba oil and allantoin nanoparticles on multidrug-resistant Candida parapsilosis. J Drug Delivery Sci Technol 40:59–65. https://doi.org/10.1016/j.jddst.2017.05.020
Sweedman MC, Tizzotti MJ, Schäfer C, Gilbert RG (2013) Structure and physicochemical properties of octenyl succinic anhydride modified starches: a review. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2012.09.040
Tadros TF (1994) Fundamental principles of emulsion applications. Colloids Surf A Physicochem Eng Asp 91:39–55
Tadros T (2004) Application of rheology for assessment and prediction of the long-term physical stability of emulsions. Adv Colloid Interf Sci. https://doi.org/10.1016/j.cis.2003.10.025
Tadros T (2009) Polymeric surfactants in disperse systems. Adv Colloid Interf Sci 147–148:281–299. https://doi.org/10.1016/j.cis.2008.10.005
Tadros T, Izquierdo P, Esquena J, Solans C (2004) Formation and stability of nano-emulsions. Adv Colloid Interf Sci. https://doi.org/10.1016/j.cis.2003.10.023
Taleb M, Abdeltawab N, Shamma R, Abdelgayed S, Mohamed S, Farag M, Ramadan M (2018) Origanum vulgare L. essential oil as a potential anti-acne topical nanoemulsion—in vitro and in vivo study. Molecules 23(9):2164. https://doi.org/10.3390/molecules23092164
Taylor P (1998) Ostwald ripening in emulsions. Adv Colloid Interf Sci 75(2):107–163. https://doi.org/10.1016/S0001-8686(98)00035-9
Tian Y, Chen L, Zhang W (2016) Influence of ionic surfactants on the properties of nanoemulsions emulsified by nonionic surfactants span 80/tween 80. J Dispers Sci Technol 37(10):1511–1517. https://doi.org/10.1080/01932691.2015.1048806
Tsujii K (1998) In: Tanaka T (ed) surface activity – principles, phenomena, and applications, 1st edn. Academic, San Diego
Wan J, Zhong S, Schwarz P, Chen B, Rao J (2019) Physical properties, antifungal and mycotoxin inhibitory activities of five essential oil nanoemulsions: impact of oil compositions and processing parameters. Food Chem 291:199–206. https://doi.org/10.1016/j.foodchem.2019.04.032
Wang L, Zhang Y (2017) Eugenol nanoemulsion stabilized with zein and sodium caseinate by self-assembly. J Agric Food Chem 65:14. https://doi.org/10.1021/acs.jafc.7b00194
Wang L, Mutch KJ, Eastoe J, Heenan RK, Dong J (2008) Nanoemulsions prepared by a two-step low-energy process. Langmuir 24(12):6092–6099. https://doi.org/10.1021/la800624z
Wang L, Tabor R, Eastoe J, Li X, Heenan RK, Dong J (2009) Formation and stability of nanoemulsions with mixed ionic–nonionic surfactants. Phys Chem Chem Phys 11(42):9772. https://doi.org/10.1039/b912460h
Wei Y, Ye X, Shang X, Peng X, Bao Q, Liu M, Guo M, Li F (2012) Enhanced oral bioavailability of silybin by a supersaturatable self-emulsifying drug delivery system (S-SEDDS). Colloids Surf A Physicochem Eng Asp. https://doi.org/10.1016/j.colsurfa.2011.12.025
Wiedmann T (2003) Surfactants and polymers in drug delivery. J Control Release 93:415. https://doi.org/10.1016/j.jconrel.2002.11.001
Wooster TJ, Golding M, Sanguansri P (2008) Impact of oil type on nanoemulsion formation and ostwald ripening stability. Langmuir 24(22):12758–12765. https://doi.org/10.1021/la801685v
Xing L, Mattice WL (1997) Strong solubilization of small molecules by triblock-copolymer micelles in selective solvents. Macromolecules 30(6):1711–1717. https://doi.org/10.1021/ma961175p
Yadav MP, Manuel Igartuburu J, Yan Y, Nothnagel EA (2007) Chemical investigation of the structural basis of the emulsifying activity of gum arabic. Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2006.05.001
Yang Y, Marshall-Breton C, Leser ME, Sher AA, McClements DJ (2012) Fabrication of ultrafine edible emulsions: comparison of high-energy and low-energy homogenization methods. Food Hydrocoll 29(2):398–406. https://doi.org/10.1016/j.foodhyd.2012.04.009
Yen C-C, Chen Y-C, Wu M-T, Wang C-C, Wu Y-T (2018) Nanoemulsion as a strategy for improving the oral bioavailability and anti-inflammatory activity of andrographolide. Int J Nanomedicine 13:669–680. https://doi.org/10.2147/IJN.S154824
Yerramilli M, Ghosh S (2017) Long-term stability of sodium caseinate-stabilized nanoemulsions. J Food Sci Technol 54(1):82–92. https://doi.org/10.1007/s13197-016-2438-y
Zhang J, Peppard TL, Reineccius GA (2015) Preparation and characterization of nanoemulsions stabilized by food biopolymers using microfluidization. Flavour Fragrance J 30(4):288–294. https://doi.org/10.1002/ffj.3244
Zhang S, Zhang M, Fang Z, Liu Y (2017) Preparation and characterization of blended cloves/cinnamon essential oil nanoemulsions. LWT 75:316–322. https://doi.org/10.1016/j.lwt.2016.08.046
Acknowledgements
This material is based upon the work supported by the Coordination for the Improvement of Higher Education Personnel (CAPES) and National Council for Scientific and Technological Development (CNPq).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Barradas, T.N., de Holanda e Silva, K.G. (2020). Nanoemulsions as Optimized Vehicles for Essential Oils. In: Saneja, A., Panda, A., Lichtfouse, E. (eds) Sustainable Agriculture Reviews 44. Sustainable Agriculture Reviews, vol 44. Springer, Cham. https://doi.org/10.1007/978-3-030-41842-7_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-41842-7_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-41841-0
Online ISBN: 978-3-030-41842-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)