Abstract
In this study, hydrogen’s role during the transition to 100% renewable energy systems is discussed thoroughly, and the importance of sustainable hydrogen production is highlighted. For a successful transition to hydrogen-based renewable energy systems, hydrogen has to be produced in a clean, reliable, affordable, efficient, and safe manner. Therefore, in the second part of this study, a comprehensive life cycle assessment of solar hydrogen production options is conducted. The selected clean hydrogen production options are steam methane reforming, conventional electrolysis, photoelectrochemical cells, PV electrolysis, and photocatalysis. A complete source to service approach is taken when evaluating the environmental and technical performance of the selected hydrogen production options. Greenhouse gas (GHG) emissions, resource use, fossil fuel use, water use, energy and exergy efficiencies, and cost of hydrogen are the selected sustainability performance criteria. The selected hydrogen production methods are compared based on these performance criteria. In the next part, the performance evaluation results of each option are normalized and ranked in the 0–10 range where 0 gives the least sustainable manner, and 10 is the hypothetical ideal case where there is no damage to the environment, zero resource and water use, and 100% energy and exergy efficiencies, and zero cost. The GHG emissions, resource use, fossil fuel use, and water use results indicate that photoelectrochemical cells (PEC) is the most advantageous. Steam methane reforming has the highest efficiencies and the lowest. When all of the selected performance criteria are considered together, PEC has the highest sustainability rankings (5.24/10), and steam methane reforming has the lowest (3.24/10).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Hydrogen has a key role during the transition to clean energy systems for a sustainable future. When produced from clean and renewable resources like solar energy and water, hydrogen can provide safe, reliable, affordable, efficient, clean, and efficient energy to a wide variety of end users. Hydrogen plays an especially important role during the transition to 100% renewable energy systems in terms of energy security in supply and demand. Therefore, the recent literature shows that there have been numerous studies towards more efficient, clean, and cheap solar hydrogen production.
The first step towards truly sustainable energy systems via hydrogen is the production. For that reason, solar hydrogen has to be produced in affordable, reliable, environmentally benign, and efficient manners with zero or minimal resource depletion (Acar and Dincer 2019). In the literature, different studies are focusing on different aspects of solar hydrogen production. For instance, Wang et al. (2017) have focused on photocatalysis and photoelectrochemical cells (PEC). The authors have reviewed the recent progress of black TiO2 for photocatalytic hydrogen evolution and PEC water splitting, along with a detailed introduction to its unique structural features, optical property, charge carrier transfer property, and related theoretical calculations. Lee et al. (2018) have provided a review of advanced hydrogen passivation applied on p-type, n-type, and upgraded metallurgical grade crystalline silicon solar cells, respectively. Acar et al. (2016) have examined photocatalytic hydrogen generation as a key to solve climate crisis issues by enabling the transition to 100% renewable energy. The authors have considered social, environmental, and economic characteristics of hydrogen production while evaluating different types of photocatalysts. De Crisci et al. (2018) have highlighted some of the methods of eliminating hydrogen sulfide pollution via partial oxidation, reformation, and decomposition techniques and approaches. The authors have proposed an approach to convert hydrogen sulfide to sulfur, water and, more importantly, hydrogen. With their approach, hydrogen is produced with zero GHG emissions, and the proposed method also helps to lower and eventually eliminate hydrogen sulfide.
In the literature, there have been numerous examples of innovative approaches to sustainable hydrogen production. For instance, Khetkorn et al. (2017) have reviewed the recent technological progress, enzymes involved, and genetic as well as metabolic engineering approaches towards sustainable hydrogen production from microalgae. Research and development activities in the field of energy and cost-effective solar and wind hydrogen have been summarized by Saeedmanesh et al. (2018). Im-orb et al. (2018) have developed a user-friendly solid oxide electrolysis cell (SOEC) model in a flowsheet simulator. The authors have developed a model to investigate the effects of key process parameters such as operating temperature, current density, steam concentration, sweep gas type and several cells, on the environmental, energetic, and economic performance of the SOEC for sustainable hydrogen production. Sharma (2019) have presented hydrogen production from biomass carbohydrates by using different types of catalysis such as chemical catalysis, biocatalysis, and their combinations. Zhang and Wang (2015) have investigated semiconductor photocatalysts for solar water splitting at 600 nm wavelength for sustainable hydrogen production.
Kadier et al. (2016) have provided a brief overview of recent advances in research on scalable microbial electrolysis cells (MEC) reactor designs and configurations for sustainable hydrogen gas production. Bolatkhan et al. (2019) have investigated the sustainable methods of hydrogen production with the help of two large groups of phototrophic microorganisms, which are microalgae and cyanobacteria. Santos et al. (2018) have designed a new family of highly effective catalysts for low-temperature water gas shift reaction based on gold modified copper-zinc mixed oxides. Nikolaidis and Poullikkas (2017) have provided a comparative overview of the major hydrogen production methods, including an overall comparison of conventional and alternative methods from fossil fuels and renewables. Wang and Yin (2018) have reviewed various biomass as feedstock, including waste activated sludge produced from the wastewater treatment plant, algae, agricultural residuals, and municipal wastes used for biological hydrogen production.
Dincer and Acar (2017) have introduced the critical perspectives of innovation for specifically for hydrogen production under a new concept (so-called: 18S concept), covering source, system, service, scope, staff, scale-up, safety, scheme, sector, solution, stakeholder, standardization, subsidy, stimulation, structure, strategy, support and sustainability. Jiang et al. (2018) have demonstrated an innovative approach to biological hydrogen production and introduced a tandem inorganic-biological hybrid by combining AglnS2/In2S3 and a facultative anaerobic bacterium, Escherichia coli, for sustainable biological hydrogen production. Show et al. (2018) have presented an elucidation on development in biohydrogen encompassing innovative biological pathways, bioreactor designs and operation, and techno-economic evaluation. The authors have also outlined the challenges and prospects of biohydrogen production. Esmieu et al. (2018) have investigated alternative methods from protein engineering to artificial enzymes and utilized innovative biological and biomimetic approaches towards sustainable hydrogen production. He et al. (2017) have taken an innovative approach to photocatalysis by introducing novel metal-free catalysts for highly efficient and stable photocatalytic hydrogen production from water splitting.
For sustainable hydrogen production, it is essential to investigate the system performance, including all system components thoroughly. For instance, Shinagawa and Takanabe (2017) have investigated the impact of electrolyte engineering on the performance and sustainability of hydrogen production systems. Ahmed and Dincer (2018) have reviewed the engineering design principles for different hydrogen production system configurations, including single, dual/tandem photoelectrodes, tandem PEC-PV, and multi-junction designs. All of these studies have pointed out the need for considerable efforts from both technical and managing aspects to achieve a full-scale application of sustainable hydrogen production from affordable, reliable, clean, and abundant sources.
In the literature, there is a need for studies comprehensively investigating solar hydrogen production systems from environmental, technical, and economic perspectives quantitatively based on their life cycle performances. For this reason, in this study, the impact of solar hydrogen production systems on the environment is quantitatively investigated by comparing their life cycle GHG emissions, fossil fuel use, water use, and resource use together with their energy and exergy efficiencies and cost. The life cycle term used in this study follows the source-system-service approach and includes all energy and material source harvesting and processing into account as well. Steam methane reforming (SMR), conventional electrolysis (CE), PEC, PV electrolysis (PE), and photocatalysis (PC) is the selected hydrogen production options for comparison purposes. For a better and clearer insight on the true sustainability of the selected options, the environmental, economic, and technical performance results of each option are normalized and ranked to highlight their strengths and weaknesses and provide future directions.
2 How Hydrogen Empowers the Transition to 100% Renewable Energy
The need for an energy transition towards 100% renewable sources is extensively recognized, and the demand for renewables has been increasing steadily. On the other hand, there are several implications and challenges which must be resolved before a complete switch from fossil fuels to renewables. These issues require a concerted, collaborative, and interdisciplinary effort to come up with innovative solutions. Among the possible solutions towards the 100% renewable energy systems, hydrogen has the potential to be an effective enabler of this transition, since it provides a clean, sustainable, reliable and safe alternative for resolving many problems that stand in the way of resilient sustainable and renewable energy systems.
Hydrogen is the candidate to replace the fossil-based energy systems with renewables, which is the key to a sustainable future. What is more, hydrogen has the potential to enable long-term energy independence and security for all types of end-users in the energy supply and distribution. With the advancements in technology and new developments in materials science through vigorous R&D efforts, most of the challenges associated with hydrogen energy systems have been tackled, and the costs have been decreasing steadily as the scale of the system keep increasing.
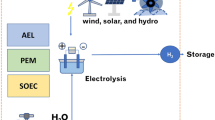
The energy transition requires an interdisciplinary approach and crosscutting technologies. And it should be noted that there is no single technology alone which can enable full decarbonization of energy systems and 100% renewable energy. Therefore, a strong collaboration of the industry, academia, and governments is needed to design, develop, build, test, and commercialize large-scale renewable energy systems. Hydrogen seems to be a promising candidate for truly sustainable energy systems: it is a multipurpose energy carrier, it can be stored in a wide variety of options, and it can be used to meet the energy demands of all kind of end-users in the market. When it is produced from 100% renewable energy, hydrogen gives the industry, buildings, transportation, and all other industries and sectors a secure and reliable access to clean energy at all times without the burden of excess renewable electricity. Hydrogen’s substantial benefits for all types of end users are summarized in Fig. 1.
Hydrogen can be used to decarbonize the global power and heat consumption with 100% renewable energy systems. Hydrogen is capable of providing high-grade heat which could be used to meet a wide range of energy requirements which is very challenging to address via electricity. As a chemical fuel, hydrogen can be transported to longer distances than electricity with considerably lower transmission losses. Besides, hydrogen can be used in mobile applications such as cars, buses, trains, trucks, planes, ships, etc. Hydrogen is also extensively used in different industrial processes including ammonia and methanol synthesis, fertilizer production, and so on. All of these advantages make hydrogen the key solution in the transformation of the global energy system towards renewables.
Currently, 95% of the global hydrogen supply is produced from fossil fuels. And the largest share of hydrogen demand comes from the industry for the synthesis of various chemicals such as ammonia. Hydrogen is also mainly used in refining for hydrocracking and desulfurization of fuels. In addition, a large portion of the global fertilizer industry is produced from ammonia synthesized in the Haber-Bosh process. Haber-Bosh process utilizes the hydrogen which is the product of natural gas steam reformation. The breakdown of global hydrogen demand by the industry is given in Table 1. Currently, hydrogen is produced mainly from fossil fuels, which is largely contributing to a wide range of issues including climate change. For that reason, it is very important for the major consumers of hydrogen to switch to renewable sources and completely eliminate the use of fossil fuels for hydrogen production. By doing so, they would not only reduce their CO2 emissions and costs in the long-term in an effective manner but also fasten the transition to 100% renewables for a truly sustainable future.
It is important to note that fossil fuels cause dependence on import and/or export of these sources since they are found only in the limited regions around the world. Importing fossil fuels needs transportation and the entire supply chain infrastructure and this process emits CO2. In addition to the social, political, and environmental impacts of the whole fossil fuel supply chain, there are noise and pollution issues related to fossil fuel consumption at the end-user site. In addition, remote locations often rely on diesel generators, which are noisy and polluting the environment with GHG emissions. With hydrogen being the storage medium for 100% renewable energy systems, these regions’ dependence on fossil fuel extraction, processing, transportation, and use, as well as import can be eliminated, which would essentially increase energy security in these regions in a clean and reliable manner.
Global coal, oil, and natural gas reserves are not distributed homogeneously around the world. For that reason, almost every country in the world relies on fossil fuel imports and/or exports, Cutting the reliance on these imports and exports enhances the resilience of these countries by making them more energy independent with energy security through 100% renewable energy systems. For this manner, microgrids can be developed with hydrogen as a storage medium for renewables. These microgrids can effectively support all types of end users have secure and uninterrupted access to reliable renewable energy. Having reliable and uninterrupted access to energy and material resources at all times is considered as the first step towards sustainable communities. Decentralization of energy systems with locally available renewables not only eliminates the transmission and distribution losses but also the negative environmental impact of the entire fossil fuel supply chain and empowers local communities.
In addition to their significant energetic, economic, and environmental benefits, 100% renewable energy based microgrids are also smarter than traditional centralized grid structures. By including hydrogen as a storage medium for renewables, new collaboration and business model opportunities are generated for cleaner communities to solve climate change issues. In these systems, hydrogen is used to provide heating, cooling, and power and as a transportation fuel. Therefore, all types of industries, from residential services to transportation, chemicals, buildings, and so on are expected to have substantial benefits from hydrogen. For that reason, hydrogen is seen as the driver for a clean, independent, and energy secure future. With renewables, hydrogen has the potential to enhance energy security and system resilience of different sectors in the following ways (Table 2):
-
In the transportation sector, decarbonizing short- and long-distance driving, trucking, shipping, and aviation requires greater energy densities than batteries currently offer. And the current grid system is not capable of delivering renewable electricity to longer distances. Besides, electrification of the entire transportation sector would generate a load that cannot be met with the current grid infrastructure. On the other hand, hydrogen has the potential to address the issues related to electrification.
-
In the steel sector, blast furnace emissions arise from the iron reduction process as well as the energy input requirements. Replacing coking coal with hydrogen as the reduction agent may be a key route to decarbonization, which has already been embraced by some major companies within the industry.
-
In other industrial processes (e.g., cement and ethylene production), the use of a decarbonized heat source such as hydrogen may prove more cost-effective than direct electrification. Because further developments are still required before electric furnaces can be commercially deployed at large-scales.
-
Last, but not least, in residential heating, it is possible to use hydrogen piped by converting the existing natural gas grid.
In this study, the importance of hydrogen and its key role towards the decarbonization of the whole global energy chain is discussed up to this point. For the hydrogen economy to successfully mature and reach larger scales in all over the world, the first challenge to tackle is hydrogen production. Sustainability requires hydrogen to be produced in a safe, affordable, reliable, clean, and effective manner. And with this motivation in mind, in this study, a comprehensive environmental, economic, and thermodynamic performance evaluation of different solar hydrogen production options is conducted.
3 Solar Hydrogen Production
PEC has become a very promising solution for the efficient, reliable, clean, and affordable hydrogen production challenge. On the other hand, PEC technologies have several challenges which can be summarized as low photon-to-hydrogen energy conversion efficiency, slow electron-hole separation rate, and rapid electron-hole recombination issue. For this reason, there have been many studies focusing on different aspects of PEC to enhance their performance by lowering costs and increasing efficiencies. When the challenges mentioned above are addressed via advancements in materials science and technologies, sustainable hydrogen production could be attainable via PEC. The chemical reactions in PEC water splitting are as follows:
Using earth-abundant catalysts for efficient and affordable hydrogen production is a common strategy used in the literature. For instance, Morales-Guio et al. (2015) have reported PEC-based hydrogen production in alkaline solutions, which are more favorable than acidic solutions for the complementary oxygen evolution half‐reaction. The authors have shown that amorphous molybdenum sulfide is a highly active hydrogen evolution catalyst in basic medium. The authors have developed catalyst coated Cu2O photoelectrodes which exhibit high PEC activity for hydrogen evolution in alkaline solutions. Ding et al. (2016) have focused on molybdenum disulfide (MoS2) and related compounds as inexpensive alternatives for hydrogen evolution reaction catalysis and PEC water splitting. In their studies, the authors have considered key approaches to improving the intrinsic catalytic activity and overall catalytic performance and the developments in combining MoS2 with semiconductors to realize solar-to-fuel conversion. The authors have also discussed different design approaches for efficient PEC water-splitting systems and some important challenges and future directions for earth abundant hydrogen evolution reaction electrocatalysis and PEC water splitting.
Kwon et al. (2016) have developed wafer-scale, transferable, and transparent thin-film catalysts based on MoS2, which consists of cheap and earth-abundant elements for highly efficient and cheaper PEC-based hydrogen production. Zhang et al. (2016) have reported the rational design of a novel 3D p-Si/NiCoSex core/shell nanopillar (NP) array photocathode via uniform photo-assisted electrodeposition of NiCoSex electrocatalyst on bamboo shoot-like Si NP array backbones. The authors have shown that the design of p-Si/NiCoSex core/shell NP arrays offers a new strategy for preparing highly efficient PEC-based solar energy conversion devices. Zhang et al. (2015) have reported amorphous MoSxCly as a high-performance electrocatalyst for both electrochemical and PEC-based hydrogen generation. The authors have shown that the MoSxCly electrocatalysts exhibit stable and high catalytic activity toward the hydrogen evolution reaction.
Fan et al. (2016) have synthesized and characterized TiO2/reduced graphene oxide/Cu2O heterostructure constituted by TiO2 nanowires, reduced graphene nanostructures, and Cu2O. The authors have shown that their complex exhibits significantly enhanced PEC performance, including high photocurrent density and hydrogen production at higher efficiencies. Zheng et al. (2016) have fabricated strongly coupled Nafion molecules and ordered porous CdS networks for efficient visible‐light PEC hydrogen evolution. The authors have shown that the Nafion layer coating shifts the band position of CdS upward and accelerates charge transfer in the photoelectrode/electrolyte interface. Tong et al. (2017) have reported an environmentally friendly, high-efficiency PEC device in which the photoanode consists of a mesoporous TiO2 film sensitized with heavy metal-free, near-infrared (NIR) colloidal CuInSexS2−x (CISeS) quantum dots. The authors have reported that their PEC device is environmentally friendly with outstanding stability, cost-effectiveness, and high efficiency.
Gross et al. (2016) have reported the improvement of a dye-sensitised p-type nickel oxide (NiO) photocathode with a hexaphosphonated Ru(2,2′-bipyridine)3 based dye (RuP3) and a tetraphosphonated molecular [Ni(P2N2)2]2+ type proton reduction catalyst (NiP) for the photoreduction of aqueous protons to H2. With this method, the authors have concluded that the PEC could achieve higher solar-to-hydrogen efficiencies in the visible light region. Tsai and Hsu (2015) have established the use of CdSe/graphene quantum dot (QD) nanoheterostructures as the photoanode for outstanding PEC-based hydrogen production. Zhang et al. (2015) have synthesized a dense array of CdS–ZnS core-shell nanorods. This photocatalyst array has been reported to have high photocorrosion resistance, charge separation and transportation efficiencies, photocurrent density, photon to electron conversion efficiency and stability, which are essential for sustainable PEC-based hydrogen production.
Hydrogen can be produced from solar electricity as well via electrolysis, which is usually referred to as PV-electrolysis. PV-electrolysis is an effective approach to utilize excess (surplus) electricity from the PV panels. In the literature, there are numerous studies focusing on the performance enhancement of solar electrolysis. These studies focus mainly on efficiency enhancement and cost reduction of hydrogen production. For instance, Tebibel et al. (2017) have investigated the energetic, economic, and environmental aspects of PV electrolysis. The authors focused on the optimal management strategy to achieve high system efficiency and safe operation. Shaner et al. (2016) have performed a techno-economic analysis of PEC and PV electrolysis hydrogen production of 10,000 kg H2/day (3.65 kilotons per year) to assess the economics of each technology and to provide a basis for comparison between these technologies as well as within the broader energy landscape. Dahbi et al. (2016) have presented a new way for hydrogen production by adapting the electrolysis to a renewable source of energy such as PV to generate the maximum quantity of hydrogen.
Bhattacharyya et al. (2017) have addressed the design of a standalone solar PV energy system that meets the energy requirements of the electrolysis process, followed by the performance analysis under different environmental conditions. The authors have also presented a step-by-step simplified approach for the preliminary PV power system design and analysis for an electrolysis-based hydrogen production unit. Kumari et al. (2016) have demonstrated a new method to produce hydrogen fuel from solar energy by splitting seawater vapor from ambient humidity at near-surface ocean conditions. The authors have used a proton exchange membrane electrolyzer with seawater-humidified air at 80% relative humidity at the anode, and dry N2 at the cathode and maintained a sufficient electrolysis current density to operate near the maximum energy-conversion point. Huang et al. (2016) have simulated hydrogen production scenarios for fuel cell electric vehicle (FCEV) hydrogen refueling stations by examining an electrolysis hydrogen production system powered by small wind turbines and a PV system.
4 Sustainability Investigation of Hydrogen Production Options
The sustainability investigation includes GHG emissions, resource use, fossil fuel use, water use, energy and exergy efficiencies, and cost. All of the sustainability performance criteria values are calculated based on the life cycle performances, from the source to the end user, by using the GaBi (Life Cycle Assessment (LCA) modelling and reporting software). It should be noted that life cycle results sum up all of the energy and emissions associated with the process inputs, including upstream. Energy and emission adjustments associated with by-products are not included. In the GHG emissions category, the following are considered:
-
CO2 emissions
-
SOx emissions
-
NOx emissions
-
CO emissions
-
CH4 emissions
-
Particulate matter (PM10 and PM2.5) emissions
-
Volatile organic compound (VOC) emissions
-
Primary organic carbon emissions
-
Black carbon emissions.
All emissions are given in units as kg emissions per kg of hydrogen produced. Resource use indicates the amount of resource energy used (MJ) per kg of hydrogen produced. This resource energy can be natural gas, grid electricity (conventional electrolysis), or solar energy (PEC, photocatalysis, or PV electrolysis). Fossil fuel use indicates the amount of fossil energy used (MJ) per kg of hydrogen produced. And water use is the total amount of water usage per kg of hydrogen production. It should be noted that the life cycle includes the preparation of resources, distribution, transportation, processing, waste handling, and the hydrogen production process itself. That is why the selected options have fossil fuel use despite the fact that the production processes alone do not involve any fossil fuel use. Cost indicates the life cycle cost of the selected options. The energy efficiency of the hydrogen production options are calculated based on the following equation:
Here, η is the energy efficiency and \({\text{HHV}}_{{{\text{H}}_{ 2} }}\) is the higher heating value (also known gross calorific value or gross energy) of hydrogen which is defined as the amount of heat released by one kg hydrogen (initially at 25 °C) once it is combusted and the products have returned to a temperature of 25 °C. \({\text{HHV}}_{{{\text{H}}_{ 2} }}\) takes into account the latent heat of vaporization of water in the combustion products. And the exergy efficiency of the selected hydrogen production options are calculated as
In this equation, ψ is the exergy efficiency and \(ex_{{{\text{H}}_{ 2} }}^{\text{ch}}\) is the chemical exergy content of one kg hydrogen. For comparison purposes, all chemical exergies are calculated at standard state, which is 25 °C and 1 atm. And the hydrogen cost is gathered from the recent literature.
In the next step, for a more comprehensive investigation, the environmental, economic, and technical performance results are normalized and ranked within the range of 0–10 where 0 indicates the least desired performance and ten is given to a hypothetical ideal case. 0 is given to the highest GHG emissions, resource use, fossil fuel use, water use, and cost and 0% energy and exergy efficiencies. On the other hand, ten is assigned to a non-existing ideal situation where the hydrogen production option has zero emissions, cost, resource use, fossil fuel use, and water use and 100% energy and exergy efficiencies. Given these conditions, the normalized rankings of the GHG emissions, resource use, fossil fuel use, water use, and cost are calculated based on:
Here, Ranki is the rank of the selected hydrogen production option (i.e., SMR, CE, PEC, PVE, or PC). And max is the maximum GHG emissions, resource use, fossil fuel use, water use, or cost among the selected options while “i” is the GHG emissions, resource use, fossil fuel use, water use, or cost of the selected hydrogen production option. And the energy and exergy efficiency rankings of the selected hydrogen production options are calculated from the following equation:
Here, since the energy and exergy efficiencies of each option are within the range of 0–1, the efficiency data are normalized by simply multiplying them with 10. The comprehensive performance investigation results are presented and discussed in detail in the next section with some possible future directions.
5 Results and Discussion
In this part of the present study, comprehensive sustainability examination outcomes of the carefully chosen hydrogen production methods are presented, and the GHG emissions, resource, fossil fuel, and water use, energy and exergy efficiencies, and the hydrogen production costs of the selected options are discussed in detail.
Achieving the objectives of the Paris Agreement, substantial greenhouse gas emission reductions are required across all sectors and all over the world. Currently, the consensus is to substantially increase the share of renewable energy in the global final energy consumption. But this study highlights the importance of 100% renewable energy systems for a carbon-free society. Despite that, one-third of global energy-related emissions come from the industry, where currently no viable economic alternative to fossil fuels exists. This study shows how hydrogen from renewable sources could be a critical element of a strategy to tackle this issue.
The second section of this study explains the advantages of hydrogen as a fuel, including providing high-grade heat; addressing a range of energy needs that direct electrification cannot meet; and replacing fossil fuel-based feedstocks, such as natural gas, in high-emission applications of the industry sector.
Hydrogen has zero GHG emissions during the storage and end-use steps. However, for a completely clean and renewable energy supply chain, hydrogen must be produced from renewables in the cleanest possible way. Therefore, it is essential to reduce, and possibly eliminate, GHG emissions of hydrogen production. Making the current hydrogen production completely renewable and emissions-free is challenging but can have a positive impact on reaching lowering global CO2 emissions target and can play an important role in realizing cost declines. For this reason, the first investigation in this study is to comparatively evaluate the lifecycle GHG emissions of the selected hydrogen production options.
In Fig. 2, GHG emissions are compared, and the results show that steam methane reforming has the highest emissions (about 9 kg GHG/kg H2 produced), followed by conventional electrolysis, which is around 7 kg GHG/kg H2 produced. The lowest GHG emissions come from PEC (less than 0.5 kg GHG/kg H2 produced). PV electrolysis has about 4 kg GHG emissions and photocatalysis has 0.6 kg GHG emissions per kg hydrogen production. During the operation part of the hydrogen generation processes, there is no GHG emissions emitted from the conventional electrolysis, PEC, PV electrolysis, and photocatalysis. The high emissions from PV electrolysis are because of the PV manufacturing process. In conventional electrolysis, grid electricity is used, and since almost 80% of the grid electricity comes from fossil fuel combustion, there are relatively high emissions associated with this method. In all options, a thorough life cycle approach is considered, and all transportation, distribution, and operational emissions are calculated from the source to the very end user. In solar hydrogen production options, construction and transportation of the PEC reactors, photocatalysts, or PV panels are taken into consideration which causes the emissions.
The ultimate objective of this study is to show the potential of hydrogen to make integration of renewables to the existing energy systems happen and eventually eliminating fossil fuel use throughout the world. In this step of the present study, the selected hydrogen production methods are compared based on their resource use (MJ) to produce one kg of hydrogen. By doing so, it is aimed to find the method that uses the least amount of resource energy to produce the maximum amount of hydrogen. It should be noted that fossil-based hydrogen production methods are given here for comparison purposes.
Figure 3 shows the resource use calculation results. In steam methane reforming, the results show the amount of natural gas energy (MJ) required to produce one kg hydrogen. Similarly, in conventional electrolysis, this option indicates the amount of energy resource, which is mainly natural gas or coal (MJ) required to produce one kg hydrogen. In PEC, photocatalysis, and PV electrolysis, the results present MJ solar energy to produce one kg hydrogen. Here, it can be seen that steam methane reforming has the highest resource use (about 251 MJ/kg H2), followed by conventional electrolysis (around 128 MJ/kg H2). PEC has the lowest resource use, which is around 100 MJ/kg H2.
In addition to the resource use, the lifecycle fossil fuel use performance of the selected options must be compared. Even though the solar hydrogen production options do not use fossil fuels at the hydrogen production site, the construction of materials, reactors, synthesis of catalysts, transportation of the components, construction of the process units, etc. might consume large amounts of fossil fuels which would cause significant GHG emissions. As the transition to fully renewabilized energy systems require the elimination of fossil fuel use, the auxiliary steps of each hydrogen production process must be carefully investigated to evaluate the actual fossil fuel consumption in the entire lifecycle. In this study, the entire lifecycles of the selected five hydrogen production methods are thoroughly investigated to accurately estimate each option’s true fossil fuel use.
The life cycle fossil fuel use performance of the carefully chosen hydrogen production options is shown in Fig. 4. Since all upstream processes and additional steps like transportation are included in this study, the highest fossil fuel use happens to be in steam methane reforming (251 MJ/kg H2). The second highest fossil fuel use belongs to conventional electrolysis (110 MJ/kg H2), which is because of the high fossil fuel contribution in the grid electricity. PEC has the lowest fossil fuel use of about 20 MJ/kg H2. PV electrolysis and photocatalysis have similar fossil fuel use of around 30 MJ/kg H2. The fossil fuel use of PEC, photocatalysis, and PV electrolysis is mostly because of the processing of the reactors, panels, photocatalysts, membranes, etc.
Even though this study’s primary aim is to highlight the possibility of the full renewabilization of energy systems through hydrogen, one cannot deny that water, as a scarce source, must also be considered while choosing the most sustainable hydrogen production option. Water is becoming more and more important every other day because of the issues related to fossil fuel use, pollution, deforestation, and so on. While evaluating the alternatives, water consumption performance has to be considered as well. The ultimate goal must be to find a method with the least water consumption performance. Ideally, the options with water recycling or wastewater usage possibilities should be selected to secure our global fresh water supplies.
In Fig. 5, the water use performances are comparatively assessed. Since clean water is an essential resource which is needed for all natural and industrial processes, it is essentially one aim of sustainable energy systems to minimize the waste of clean water. Water use includes two factors: water consumption and water degradation, such as water pollution (L/kg H2). The results show that steam methane reforming has the highest water use, which is around 140 L/kg H2, followed by conventional electrolysis, which is almost 30 L/kg H2. One reason for the considerably high water consumption of the conventional electrolysis is the grid electricity generation process. PEC has the lowest water consumption of around 15 L/kg H2, followed by PV electrolysis and photocatalysis (about 20 L/kg H2). Another advantage of PEC is the fact that it can be integrated into wastewater treatment and desalination processes. As a result, these integrated systems do not only produce hydrogen, but also provides fresh water, which is a scarce resource around the world.
While the cost and performance of hydrogen energy systems have improved in the recent years (e.g., fuel cell cost fell more than 50%), performance improvement is not capturing its full potential as industry standards have been set for specific applications but remain limited overall. Advancing the energy transition requires harmonized regional and sector-specific hydrogen standards that will allow for economies of scale in research, development, and deployment (R, D & D) and manufacturing.
In this study, hydrogen production costs are calculated by taking the life cycle approach, and the results are presented in Fig. 6. Here, it can be seen that PEC, due to its early R&D stage and smaller scales, has the highest cost, which is around 9 USD/kg H2. Following PEC, PV electrolysis and photocatalysis have the second highest production cost of about 6 USD/kg H2. The lowest production cost belongs to steam methane reforming (less than 1 USD/kg H2), followed by conventional electrolysis (almost 3 USD/kg H2). PEC, photocatalysis, and PV electrolysis have higher costs compared to steam methane reforming and conventional electrolysis because of a couple of reasons. Firstly, PEC, PV, and photocatalysis are in smaller scales than steam methane reforming, and conventional electrolysis, which increases their production costs significantly. In addition, most of the materials used in PEC, PV, and photocatalysis are still expensive, and the high initial cost of these options make hydrogen production more expensive than the more traditional options. However, it should be noted that the cost of the renewable-based hydrogen production options is expected to decrease significantly in the future. The target is to make renewable hydrogen cost-competitive with fossil fuel-based alternatives.
The last category is energy and exergy efficiencies of the selected hydrogen production options, and the results are presented in Fig. 7. In this study, in addition to energy efficiency, exergy efficiency of each hydrogen production method is calculated to provide a deeper understanding of the system performances from lifecycle perspective. Energy efficiency is the ratio of the energy content of the product hydrogen, divided by the energy content of the input. Energy efficiency is very useful to lower any waste and enhance the system performance of a process. However, it does not reflect the whole picture since it does not focus on the useful work portion of energy. This is actually mentioned in the first law of thermodynamics, stating that energy can neither be created not destroyed. On the other hand, energy conservation is not really answering the question of what happens to the quality of our energy. By using the second law of thermodynamics, exergy efficiency actually states what part of the useful exergy is conserved in a process. For instance, in a hydrogen production process, exergy efficiency is the ratio of exergetic content of the product hydrogen to the exergetic content of all the input. By doing so, exergy efficiency gives a better perspective when selecting the most sustainable hydrogen production option.
From Fig. 7, it can be seen that steam methane reforming and conventional electrolysis have significant advantages due to their larger scale operation. Steam methane reforming has about 84% energy efficiency and 52% exergy efficiency. The energy and exergy efficiencies of conventional electrolysis are 53% and 25%, respectively. Photocatalysis has the lowest efficiencies among the selected options with 3% energy efficiency and 2% exergy efficiency. With 18% energy and 12% exergy efficiency, PEC has a higher performance than PV electrolysis, which has 12% energy and 7% exergy efficiency.
After performing the environmental, energetic, exergetic, and economic performance of the selected hydrogen production methods, the results are normalized and ranked based on the procedure explained in the previous section. The results are given in detail in Table 3 and presented in Fig. 8.
The overall comparison shows that PEC has advantages in terms of its low emissions and the use of energy and material resources. Steam methane reforming has the highest results based on the energy and exergy efficiencies and cost. In contrast, steam methane reforming has the lowest performance in terms of emissions, resource use, fossil fuel use, and water use. PEC has the lowest performance in cost criteria while photocatalysis has the weakest performance in energy and exergy efficiency categories. When the averages of the normalized performance rankings are taken, it is seen that PEC has the highest normalized average ranking of 5.24 out of 10, which is immediately followed by photocatalysis with 5.02. The third highest normalized average ranking belongs to conventional electrolysis, that is 4.94. Among the selected options, steam methane reforming has the lowest average normalized ranking (3.24), and PV electrolysis has 4.45.
In this study, for the first time in the literature, a comprehensive quantitative comparison approach is taken to evaluate the environmental, economic, thermodynamic, and technical performances of the solar, conventional, and fossil fuel-based hydrogen production options. This study takes GHG emissions, resource use, fossil fuel use, water use, energy, and exergy efficiencies, and cost into account to provide a better insight into the cleaner hydrogen production methods. The results not only show a clear picture of the current status of these options, but also provides potential future directions to guide researchers, policymakers, and industries in the field of renewable energy systems for a sustainable future.
6 Conclusions
This study is conducted to explain the advantages of hydrogen as a fuel to support the transition to 100% renewable energy systems. The benefits of hydrogen include providing high-grade heat; addressing a range of energy needs that direct electrification cannot meet; and replacing fossil fuel-based feedstocks, such as natural gas, in high-emission applications of different sectors and the industry as a whole. According to the present study, renewables-based hydrogen could be critical for deeper energy transition for the following reasons:
-
Hydrogen could potentially be the missing link in the transformation of sectors, such as aviation or refining, where electrification is not suitable to replace fossil fuels.
-
Hydrogen can support higher shares of wind and solar energy in power sectors all over the world by serving as a reliable storage option for renewable electricity to balance the grid and even out variable power production.
-
Hydrogen offers possibilities to tap high-quality renewable energy resources, such as remote deserts since it is easier to transport compared to electricity and unconstrained by grid connections.
-
Hydrogen can take advantage of existing energy infrastructure, including injection into the existing natural gas grids reducing emissions of the current gas infrastructure such as gas turbines for the power sector.
-
The transportation sector powered by hydrogen can offer consumers low emissions driving performance similar to conventional vehicles using internal combustion engines.
In this study, the GHG emissions, resource use, fossil fuel use, water use, energy and exergy efficiencies, and cost of hydrogen via five different production methods are investigated in detail from a life cycle perspective. The results are then comparatively evaluated by normalizing and ranking the environmental, energetic and exergetic, and economic performances of the selected hydrogen production options. Steam methane reforming, conventional electrolysis, PEC, PV electrolysis, and photocatalysis are the selected hydrogen production options. This study is one of the first attempts to thoroughly investigate the life-cycle environmental and economic impacts of sustainable hydrogen production. And the results indicate that:
-
In terms of GHG emissions, steam methane reforming has the highest emissions (9 kg GHG/kg H2), and PEC has the lowest emissions (0.47 kg GHG/kg H2).
-
Steam methane reforming has the highest resource consumption (251 MJ natural gas energy/kg H2), and PEC has the lowest resource consumption (103 MJ solar energy/kg H2).
-
Fossil fuel consumption data shows that steam methane reforming has the highest fossil fuel consumption (251 MJ fossil fuel energy/kg H2) and PEC has the lowest resource consumption (20 MJ fossil fuel energy/kg H2).
-
Steam methane reforming has the highest water consumption (140 L water/kg H2), and PEC has the lowest water consumption (15 L water/kg H2).
-
Steam methane reforming has the highest energy and exergy efficiencies, which are 83% and 52%, respectively and photocatalysis has the lowest energy and exergy efficiencies, which are 2% and 1%.
-
Cost comparison shows that steam methane reforming has the lowest cost (0.76 USD/kg H2) while PEC has the highest cost (9.02 USD/kg H2).
-
The overall normalized performance ranking comparison shows that PEC has the highest average normalized ranking (5.24/10) and steam methane reforming has the lowest average normalized ranking (3.24/10).
References
Acar C, Dincer I (2019) Review and evaluation of hydrogen production options for better environment. J Clean Prod 218:835–849
Acar C, Dincer I, Naterer GF (2016) Review of photocatalytic water-splitting methods for sustainable hydrogen production. Int J Energy Res 40(11):1449–1473
Ahmed M, Dincer I (2018) A review on photoelectrochemical hydrogen production systems: challenges and future directions. Int J Hydrogen Energy 44(5):2474–2507
Bhattacharyya R, Misra A, Sandeep KC (2017) Photovoltaic solar energy conversion for hydrogen production by alkaline water electrolysis: conceptual design and analysis. Energy Convers Manag 133:1–13
Bolatkhan K, Kossalbayev BD, Zayadan BK, Tomo T, Veziroglu TN, Allakhverdiev SI (2019) Hydrogen production from phototrophic microorganisms: reality and perspectives. Int J Hydrogen Energy 44(12):5799–5811
Dahbi S, Aboutni R, Aziz A, Benazzi N, Elhafyani M, Kassmi K (2016) Optimised hydrogen production by a photovoltaic-electrolysis system DC/DC converter and water flow controller. Int J Hydrogen Energy 41(45):20858–20866
De Crisci AG, Moniri A, Xu Y (2018) Hydrogen from hydrogen sulfide: towards a more sustainable hydrogen economy. Int J Hydrogen Energy 44(3):1299–1327
Dincer I, Acar C (2017) Innovation in hydrogen production. Int J Hydrogen Energy 42(22):14843–14864
Ding Q, Song B, Xu P, Jin S (2016) Efficient electrocatalytic and photoelectrochemical hydrogen generation using MoS2 and related compounds. Chem 1(5):699–726
Esmieu C, Raleiras P, Berggren G (2018) From protein engineering to artificial enzymes–biological and biomimetic approaches towards sustainable hydrogen production. Sustain Energy Fuels 2(4):724–750
Fan W, Yu X, Lu HC, Bai H, Zhang C, Shi W (2016) Fabrication of TiO2/RGO/Cu2O heterostructure for photoelectrochemical hydrogen production. Appl Catal B 181:7–15
Gross MA, Creissen CE, Orchard KL, Reisner E (2016) Photoelectrochemical hydrogen production in water using a layer-by-layer assembly of a Ru dye and Ni catalyst on NiO. Chem Sci 7(8):5537–5546
He Z, Fu J, Cheng B, Yu J, Cao S (2017) Cu2(OH)2CO3 clusters: novel noble-metal-free cocatalysts for efficient photocatalytic hydrogen production from water splitting. Appl Catal B 205:104–111
Huang PH, Kuo JK, Wu ZD (2016) Applying small wind turbines and a photovoltaic system to facilitate electrolysis hydrogen production. Int J Hydrogen Energy 41(20):8514–8524
Im-orb K, Visitdumrongkul N, Saebea D, Patcharavorachot Y, Arpornwichanop A (2018) Flowsheet-based model and exergy analysis of solid oxide electrolysis cells for clean hydrogen production. J Clean Prod 170:1–13
Jiang Z, Wang B, Jimmy CY, Wang J, An T, Zhao H, Li H, Yuan S, Wong PK (2018) AglnS2/In2S3 heterostructure sensitization of Escherichia coli for sustainable hydrogen production. Nano Energy 46:234–240
Kadier A, Simayi Y, Abdeshahian P, Azman NF, Chandrasekhar K, Kalil MS (2016) A comprehensive review of microbial electrolysis cells (MEC) reactor designs and configurations for sustainable hydrogen gas production. Alex Eng J 55(1):427–443
Khetkorn W, Rastogi RP, Incharoensakdi A, Lindblad P, Madamwar D, Pandey A, Larroche C (2017) Microalgal hydrogen production—a review. Biores Technol 243:1194–1206
Kumari S, White RT, Kumar B, Spurgeon JM (2016) Solar hydrogen production from seawater vapor electrolysis. Energy Environ Sci 9(5):1725–1733
Kwon KC, Choi S, Hong K, Moon CW, Shim YS, Kim DH, Kim T, Sohn W, Jeon JM, Lee CH, Nam KT (2016) Wafer-scale transferable molybdenum disulfide thin-film catalysts for photoelectrochemical hydrogen production. Energy Environ Sci 9(7):2240–2248
Lee SH, Bhopal MF, Lee DW, Lee SH (2018) Review of advanced hydrogen passivation for high efficient crystalline silicon solar cells. Mater Sci Semicond Process 79:66–73
Morales-Guio CG, Liardet L, Mayer MT, Tilley SD, Grätzel M, Hu X (2015) Photoelectrochemical hydrogen production in alkaline solutions using Cu2O coated with earth-abundant hydrogen evolution catalysts. Angew Chem 127(2):674–677
Nikolaidis P, Poullikkas A (2017) A comparative overview of hydrogen production processes. Renew Sustain Energy Rev 67:597–611
Saeedmanesh A, Mac Kinnon MA, Brouwer J (2018) Hydrogen is essential for sustainability. Curr Opin Electrochem 12:166–181
Santos JL, Reina TR, Ivanov I, Penkova A, Ivanova S, Tabakova T, Centeno MA, Idakiev V, Odriozola JA (2018) Multicomponent Au/Cu-ZnO-Al2O3 catalysts: robust materials for clean hydrogen production. Appl Catal A 558:91–98
Shaner MR, Atwater HA, Lewis NS, McFarland EW (2016) A comparative technoeconomic analysis of renewable hydrogen production using solar energy. Energy Environ Sci 9(7):2354–2371
Sharma K (2019) Carbohydrate-to-hydrogen production technologies: a mini-review. Renew Sustain Energy Rev 105:138–143
Shinagawa T, Takanabe K (2017) Towards versatile and sustainable hydrogen production through electrocatalytic water splitting: electrolyte engineering. ChemSusChem 10(7):1318–1336
Show KY, Yan Y, Ling M, Ye G, Li T, Lee DJ (2018) Hydrogen production from algal biomass–advances, challenges and prospects. Biores Technol 257:290–300
Tebibel H, Khellaf A, Menia S, Nouicer I (2017) Design, modelling and optimal power and hydrogen management strategy of an off grid PV system for hydrogen production using methanol electrolysis. Int J Hydrogen Energy 42(22):14950–14967
Tong X, Zhou Y, Jin L, Basu K, Adhikari R, Selopal GS, Zhao H, Sun S, Vomiero A, Wang ZM, Rosei F (2017) Heavy metal-free, near-infrared colloidal quantum dots for efficient photoelectrochemical hydrogen generation. Nano Energy 31:441–449
Tsai KA, Hsu YJ (2015) Graphene quantum dots mediated charge transfer of CdSe nanocrystals for enhancing photoelectrochemical hydrogen production. Appl Catal B 164:271–278
Wang J, Yin Y (2018) Fermentative hydrogen production using various biomass-based materials as feedstock. Renew Sustain Energy Rev 92:284–306
Wang B, Shen S, Mao SS (2017) Black TiO2 for solar hydrogen conversion. J Mater 3(2):96–111
Zhang J, Wang X (2015) Solar water splitting at λ = 600 nm: a step closer to sustainable hydrogen production. Angew Chem Int Ed 54(25):7230–7232
Zhang X, Meng F, Mao S, Ding Q, Shearer MJ, Faber MS, Chen J, Hamers RJ, Jin S (2015a) Amorphous MoSxCly electrocatalyst supported by vertical graphene for efficient electrochemical and photoelectrochemical hydrogen generation. Energy Environ Sci 8(3):862–868
Zhang J, Wang L, Liu X, Li XA, Huang W (2015b) High-performance CdS–ZnS core–shell nanorod array photoelectrode for photoelectrochemical hydrogen generation. J Mater Chem A 3(2):535–541
Zhang H, Ding Q, He D, Liu H, Liu W, Li Z, Yang B, Zhang X, Lei L, Jin S (2016) A p-Si/NiCoSex core/shell nanopillar array photocathode for enhanced photoelectrochemical hydrogen production. Energy Environ Sci 9(10):3113–3119
Zheng XL, Song JP, Ling T, Hu ZP, Yin PF, Davey K, Du XW, Qiao SZ (2016) Strongly coupled nafion molecules and ordered porous CdS networks for enhanced visible-light photoelectrochemical hydrogen evolution. Adv Mater 28(24):4935–4942
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Acar, C. (2020). Solar Hydrogen’s Role for a Sustainable Future. In: Uyar, T. (eds) Accelerating the Transition to a 100% Renewable Energy Era. Lecture Notes in Energy, vol 74. Springer, Cham. https://doi.org/10.1007/978-3-030-40738-4_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-40738-4_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-40737-7
Online ISBN: 978-3-030-40738-4
eBook Packages: EnergyEnergy (R0)