Abstract
This chapter will introduce students to different types of bioreactors that help to culture 3D tissue-engineered constructs, providing cell growth in biocompatible matrices. The desirable properties of these devices include ease of assembly, a possibility for multiple sterilizations, absence of toxic materials, and endurance for cell culture conditions. Depending on the tissue of choice, various forms of bioreactors can be used with the common goal of enabling the flow of nutrients and oxygen through the scaffold and providing an optimal cell growth environment, including pH, temperature, and oxygen tension. For many tissues, external stimuli such as electrical stimulation, mechanical stretch, or compression must be also included in the bioreactor design. Finally, we briefly discuss the importance of monitoring cells’ viability and activity within cultured 3D scaffolds using a variety of live and fixed tissue markers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Students will be given basic information about different types of bioreactors that allow to culture 3D tissue-engineered constructs and the ways to monitor cells within them. During the practical session, students will be putting together a simple bioreactor of their choice.
11.1 Basic Types of Bioreactors
A bioreactor is a manufactured or engineered device that supports a biologically active environment. In the context of tissue engineering, bioreactors are used to support cell growth in the decellularized tissue, artificially created recellularized scaffold, or any other type of biocompatible matrix. Several operational conditions within the reactor can be modified and controlled. They include pH, temperature, oxygen tension, and the rate of media perfusion as well as the ability to apply external stimuli such as mechanical forces or electrical stimulation. The key principles to be employed in any bioreactor are as follows:
-
Simplicity and quickness of assembly
-
Efficiency of tissue formation in a short span of time
-
Sterility
-
Non-toxicity of materials of assembly
-
Ease of sterilization for repeated use
The specific design of bioreactors varies depending on the targeted tissue. For engineered cardiac tissue, for example, a bioreactor can have electrodes in order to pace the tissue. For bone or cartilage, it has to provide a specified degree of compression, preferably in a time-cyclic fashion. Below we briefly discuss several types of bioreactors based on a comprehensive review by Martin et al. [1].
-
I.
Spinner flask bioreactor. A spinner flask bioreactor is the most basic and simple form of bioreactor used in tissue engineering. It assumes the fixed position of the scaffold in the flask, while the magnetic stirrer constantly mixes the media around (◘ Fig. 11.1). This enables the constant flow of nutrients and oxygen through the scaffold. Typically, spinner flasks are around 120 mL in volume and run at 50–80 rpm, and 50% of the medium used in them is changed every 2–3 days. These types of bioreactors are usually used for the recellularization of tissues with a high value of surface-to-volume ratio, that is, for relatively small or “flat” scaffolds. For example, spinner-flask bioreactors have been successfully used in articular cartilage production in vitro.
-
II.
Rotating wall bioreactor. Rotating wall bioreactor consists of inner and outer cylinders whose walls are rotating at a constant speed (◘ Fig. 11.2). It is commonly used when there is a need to reduce shear stress since cells here grow in a microgravity environment. The scaffold in this bioreactor is not fixed; it moves freely in the media. Media can be exchanged by stopping the rotation temporarily or by adding a fluid using a pump, whereby media are constantly pumped through the vessel. Gas exchange occurs through a gas exchange membrane, and the bioreactor is typically rotated at speeds of 15–30 rpm [2].
-
III.
Compression bioreactor. As the name implies, compression bioreactors are used for the cultivation of tissues that need pressure in order to develop, for example, cartilage or bone. The compression bioreactor consists of a chamber where the scaffold is placed, a motor, a system providing linear motion, and one or several pistons applying static or dynamic compression loads upon the tissue [3].
-
IV.
Strain bioreactor. Strain bioreactors are very similar to the compression systems in their structure and mechanism. However, instead of flat plates as in a compression bioreactor, a way of clamping the scaffold into the device is needed so that a tensile force can be applied [4]. These systems have been used to engineer diverse tissues including tendon, bone, cartilage, ligament, cardiac, and vascular tissues.
-
V.
Hydrostatic pressure bioreactors. Hydrostatic pressure system normally consists of a chamber where the scaffold is placed, a pressure-applying means (e.g., a piston controlled by an actuator), a water-filled pressure chamber controlled using a variable backpressure valve, and an actuator. The pressure is created via an impermeable membrane to assure sterility. HP bioreactors are used, for example, to mimic the conditions of intervertebral discs [5].
-
VI.
Flow perfusion bioreactor. Perfusion systems typically consist of a chamber where the scaffold is placed, a liquid reservoir chamber, a pump, and connecting tubes (◘ Fig. 11.3). The cell-containing suspension is continuously pumped through the scaffold providing optimal cell attachment and growth. Flow perfusion bioreactors, in contrast to the spinner flask or rotating wall bioreactors, solve the problems caused by the static composition of media. The constant flow in perfusion systems not only combats the problem of the cell concentration on the exterior of a scaffold caused by static media but also provides a constant waste elimination and satisfies nutrient inflow necessity. Furthermore, the media can easily be replaced in the media reservoir. The perfusion systems have been shown to result in more homogeneous cell distribution compared to the static media systems [6]. One of the disadvantages of flow perfusion bioreactor is the alignment of the cells parallel to the scaffold lining, while some systems (e.g., articular cartilage) would require perpendicular alignment.
-
VII.
Microfluidics-based bioreactors. To monitor and record fluorescent signals from live cells within tissue constructs new types of bioreactors are being developed. They enable perfusion of small pieces of tissue placed inside chambers with #1 coverslip glass bottoms. One such example is a miniaturized optically accessible bioreactor (MOAB), which has several optically clear chambers with magnetically attachable lids connected to a microfluidic device that controls the flow. The device allows keeping cell constructs perfused with cell culture media while doing real-time microscopy. The chambers can also be connected to each other to mimic paracrine signaling (paracrine signaling involves substances released from neighboring cells or tissues).
-
VIII.
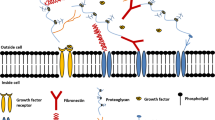
In vivo bioreactors. One of the biggest challenges of bioreactors is the long-term maintenance of cells within scaffold, in terms of nutrition and oxygen delivery. Within the body, most cells are found no more than 100–200 μm from the nearest capillary, which provides them with necessary substances and eliminates the waste. In vitro, the effective delivery mechanism remains a limiting factor. Therefore, in most cases of tissue-engineered organs, oxygen delivery relies merely on surface diffusion, resulting in viable tissues only a few hundred microns in thickness. Several ways to address this problem are being developed. They are shown in a cartoon form in ◘ Fig. 11.4. based on the article by Lovett et al. [7]. Current approaches include incorporation of angiogenic factors into scaffold material, addition of endothelial cells to generate capillary-like sprouts that can then connect to the vessels of the host, use of perfusion bioreactors that increase passive flow through the tissue, integration of microfluidic channels made from synthetic or natural polymers, insertion of pre-formed channels from endothelial cells into hydrogel scaffolds, in vivo vascularization of multilayered cell sheets followed by addition of more layers, and finally printing of vessels within the rest of 3D printed tissue. By combining these approaches, recently it became possible to create dense tissues up to 1 cm thick [8]. Newer materials and approaches to aid in vascularization of engineered tissues are continuously being developed, and students are encouraged to read recent articles and reviews on this subject [9,10,11].
Schematic diagrams of different scaffold vascularization approaches. a Incorporation of angiogenic factors, b addition of endothelial cells to generate capillary-like sprouts, c use of perfusion bioreactors, d integration of microfluidic channels made from synthetic or natural polymers, e insertion of preformed channels from endothelial cells into hydrogel scaffolds, f in vivo vascularization of multilayered cell sheets followed by addition of more layers
As for now at least, the most efficient way to solve the vascularization problem is to implant tissue constructs into a living organism so it can be fed by the host blood vessels. In time, smaller vessels sprout into the construct forming a new vasculature. This approach essentially uses “in vivo bioreactor” with the host body taking over the vascularization process. When vascularized constructs reach maturity, they can be re-implanted to their final implantation site or even a new host.
11.2 How to Monitor Cells Within a Scaffold
Tissue assays described in ► Chap. 5 can also be used to evaluate either viability or specific activity of the cells within scaffold material. In addition, as briefly described in ► Chap. 7, confocal microscopy enables visualization of the cells labeled with fluorescent markers through the thickness of the sample (to about 100–200 micron depth). Examining different layers of the tissue allows for the confirmation of the presence of viable cells within the whole volume of the recellularized tissue or scaffold and provides 3D information for data representation.
Another way to image live constructs without fixing them is to use bioluminescence. Bioluminescence detects photons emitted by an enzymatic reaction in which a luciferase oxidizes a substrate. Bioluminescence imaging has been used extensively for tracking live cells within a living organism, giving real-time information on their survival, growth, and proliferation. It requires additional steps such as the introduction of the luciferase gene into the target cell genome and injection of luciferin. Depending on the intensity of the luminescence signal obtained, the time needed to acquire an image may be as little as 1 s or as long as 10–20 min.
11.3 Histology of 3D Engineered Tissue
Histological techniques are the traditional methods employed for the assessment of tissue structure. They can be used to confirm the presence of cells in 3D scaffolds by analysis of two-dimensional slices prepared from tissue samples. Using the proper staining, it’s possible to identify the specific types of cells in the sample. By analyzing multiple histological sections from the same sample, the results are often extrapolated to the three-dimensional structure. Measuring histomorphometric parameters such as percent of the viable cells yields quantitative results. Histology, in contrast to the confocal imaging or other types of live imaging, is a destructive technique that does not allow further analysis of the samples as cells are fixed.
Session I
Demonstration
The instructor builds a simple bioreactor using available tools. It is important to demonstrate the simplicity and minimalism in construction. Yet, being simply built, the bioreactor still needs to support all the features listed in the section above: non-toxicity of material, ease of sterilization, capacity to exchange media for nutrient exchange as well as being compatible with cell incubator conditions including high humidity and 37 °C (for example one needs to consider that metal parts can rust).
Homework
Teams are tasked with searching the literature to find design of a feasible bioreactor suitable for long-term culturing of their organ of choice.
Session II
Team Exercises
Each team builds a different type of bioreactor using available tools. The type of bioreactor can match the tissue that the team is planning to cultivate. The practice will help students to build a more elaborated device for the actual recellularization.
Homework
Team members meet to decide how they will proceed with engineering the organ of their choice within the next 2–3 weeks. Methods, hypotheses to be tested, animal species from which to obtain organs and cells, required reagents, and assignment of individual tasks—all these items must be debated and presented to the instructor for further discussion. Each team then meets with the instructor individually to discuss the design of their planned experiments.
Sample Protocols
Static Media Bioreactor 1
-
1.
Pick a suitable flask/bottle with a cap to keep the scaffold in.
-
2.
Place the scaffold in.
-
3.
Fill the flask with media.
-
4.
Place the flask into the thermoshaker and adjust the temperature and shaking motion.
-
5.
Stop the shaker and change the media when required.
Static Media Bioreactor 2
-
1.
Pick a suitable flask/bottle with a cap to keep the scaffold in.
-
2.
Put in a magnet.
-
3.
Affix a porous membrane (any round-shaped material stiff enough to hold a scaffold and permeable for media) inside the flask (4–5 cm above the floor) so that the flask is divided into lower and upper parts.
-
4.
Place the scaffold on the membrane.
-
5.
Fill the flask with media.
-
6.
Place the flask on the magnetic stirrer and turn it on (adjust the motion).
-
7.
Put the whole construction in the cell incubator with adjusted temperature.
-
8.
Stop the device, take it out, and change the media when required.
Flow Perfusion Bioreactor
-
1.
Pick a suitable flask/plate/bottle with a cap and place the scaffold in side.
-
2.
Pick a suitable container and fill it with media.
-
3.
Fix one lab hose going from the media container into the scaffold flask and another one from the flask to the container.
-
4.
If the scaffold has vasculature, adjust the corresponding edges of hoses in a way that they carry media into the incoming vessel and take the media out from the outgoing vessel (e.g., fix the pipette tips on the hoses’ edges and insert them into the corresponding vessel entrances).
-
5.
Connect pumps to the hoses so that they push the liquid in corresponding directions.
-
6.
Put the whole construction into the cell incubator with adjusted temperature.
-
7.
Change the media regularly.
Take-Home Message/Lessons Learned
After reading this chapter and performing the requested assignments and exercises, students should:
-
Understand the basic principles that any type of bioreactor must comply with
-
Be familiar with the main categories of bioreactors and types of engineered tissue they are designed for
-
Be aware of different ways to vascularize engineered tissue
-
Be capable of building a simple bioreactor using commonly available plasticware and tubing.
References and Further Reading
I. Martin, D. Wendt, M. Heberer, R. Langer, E. Al, The role of bioreactors in tissue engineering. Trends Biotechnol. 22(2), 80–86 (2004)
L.E. Freed, G. Vunjak-Novakovic, Microgravity tissue engineering. Vitr. Cell Dev. Biol. Anim. 33(5), 381–385 (1997)
M. Sladkova, G. de Peppo, Bioreactor systems for human bone tissue engineering. Processes 2(2), 494–525 (2014)
N. Plunkett, F.J. O’Brien, IV.3. Bioreactors in tissue engineering. Stud. Health Technol. Inform. 152, 214–230 (2010)
J. Zvicer, B. Obradovic, Bioreactors with hydrostatic pressures imitating physiological environments in intervertebral discs. J. Tissue Eng. Regen. Med. 12(2), 529–545 (2018)
J. Glowacki, S. Mizuno, J.S. Greenberger, Perfusion enhances functions of bone marrow stromal cells in three-dimensional culture. Cell Transplant. 7(3), 319–326 (1998)
M. Lovett, K. Lee, A. Edwards, D.L. Kaplan, Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 15(3), 353–370 (2009)
D.B. Kolesky, K.A. Homan, M.A. Skylar-Scott, J.A. Lewis, Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. 113(12), 3179–3184 (2016)
M.D. Sarker, S. Naghieh, N.K. Sharma, X. Chen, 3D biofabrication of vascular networks for tissue regeneration: A report on recent advances. J. Pharm. Anal. 8(5), 277–296 (2018)
D. Richards, J. Jia, M. Yost, R. Markwald, Y. Mei, 3D bioprinting for vascularized tissue fabrication. Ann. Biomed. Eng. 45, 132–147 (2017)
L.A. Herron, C.S. Hansen, H.E. Abaci, Engineering tissue-specific blood vessels. Bioeng. Transl. Med. 4(3) (2019)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Self-Check Questions
Self-Check Questions
-
Q.11.1.
Select the best type of reactor for a tissue-engineered vessel.
-
A.
Rotating wall bioreactor
-
B.
Flow perfusion bioreactor
-
C.
Compression bioreactor
-
D.
Spinner flask
-
A.
-
Q.11.2.
To recreate in vivo environment while growing engineered heart constructs, a bioreactor’s design should include
-
A.
Electrical stimulation and cyclical stretch
-
B.
Daily media change
-
C.
Highly periodic changes in media calcium concentrations
-
D.
Mechanical tissue compression
-
A.
-
Q.11.3.
To recreate in vivo environment while growing engineered bone constructs, a bioreactor’s design should include
-
A.
Electrical stimulation and cyclical stretch
-
B.
Daily media change
-
C.
Highly periodic changes in media calcium concentrations
-
D.
Mechanical tissue compression
-
A.
-
Q.11.4.
Tissue vascularization strategies include
-
A.
In vivo vascularization of sequential layers of engineered tissue
-
B.
Creation of small artificial channels using microfluidics
-
C.
Incorporation of angiogenic factors stimulating endothelial cell proliferation
-
D.
All of the above
-
A.
-
Q.11.5.
General bioreactor design must enable a user to do all of the following, EXCEPT the ability to ___________ which is optional.
-
A.
Regulate temperature and oxygen delivery
-
B.
Control rate of media perfusion
-
C.
Observe cells while they are forming tissue
-
D.
Sterilize bioreactor for repeated use
-
A.
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Simonyan, A., Sarvazyan, N. (2020). Bioreactors. In: Sarvazyan, N. (eds) Tissue Engineering. Learning Materials in Biosciences. Springer, Cham. https://doi.org/10.1007/978-3-030-39698-5_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-39698-5_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-39697-8
Online ISBN: 978-3-030-39698-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)