Abstract
Despite a large number of clinical trials, no study using potentially neuroprotective pharmacological treatments has demonstrated robust clinical improvement. There is robust experimental and clinical evidence showing that the tissue injury processes continue over a prolonged period of time post-injury, implying that future neuroprotective therapies may be initiated beyond the acute post-injury phase. It is possible that drugs targeting more prolonged injury mechanisms, such as mitochondrial dysfunction, axonal injury, and neuroinflammation, have a better chance of succeeding. In the present chapter, the current evidence for neuroprotection in severe clinical TBI is presented.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Neuroprotection in TBI
Recommendations
Level I
There is no Level 1 evidence suggesting that any pharmacological treatment option can improve the outcome of TBI patients. Corticosteroids, magnesium sulfate, erythropoietin, cannabinoids, and progesterone are without demonstrated efficacy for TBI patients and should not be routinely used. Hypothermia decreases ICP although an improved outcome of patients with severe TBI has not been demonstrated.
Level II
There are studies at a Level II evidence with suggested benefits. None of these pharmacological treatments have as of yet been supported at a Level I and cannot be recommended.
Level III
Numerous studies at a Level III evidence exist. These studies are currently not sufficient to recommend or suggest a pharmacological compound to be administered to TBI patients. Thus a Level III recommendation for this topic cannot be provided.
2 Overview
A major problem in the management of severe traumatic brain injury (TBI) is that clinical outcome has not been markedly improved over the last decades (Rosenfeld et al. 2012). To date, >1000 studies with a huge variety of exploratory targets for the treatment of TBI are registered at www.clinicaltrials.gov, and important pharmacological treatment targets are also continuously being explored in the experimental TBI setting. A key concept in the management of TBI is that not all cell death occurs at the time of primary injury; instead a cascade of molecular and neurochemical secondary events occur during the initial hours and days with a complex temporal profile. Ultimately, this secondary injury cascade markedly exacerbates the primary injury as outlined in Chap. 6 of this book. As shown in numerous experimental TBI studies, there should be a possibility to attenuate the secondary injury cascades by pharmacological means. This possibility for improving TBI outcome has been explored over several decades, and many drugs with promising preclinical documentation have reached the clinical trial stage (see Maas et al. (2010)), most of which are regarded as neuroprotective.
Neuroprotection in TBI can be defined as “interventions aimed at improving the patient’s outcome, and preserving and restoring the integrity, function, and connectivity of the brain cells not irremediably damaged by the initial injury” (Zoerle et al. 2017). With few exceptions, most TBI trials to date have been rather small, only rarely enrolling more than 1000 patients. In their comprehensive overview published in 2016, Bragge and co-workers evaluated multicenter randomized control trials (RCTs) and found 47 completed pharmacological RCTs and 8 ongoing. None of these pharmacological treatments showed a robust clinical benefit, and the vast majority targeted early neuroprotective mechanisms (Bragge et al. 2016). The use of fluid management, hypertonic saline, mannitol, and various sedative compounds is covered in other chapters of this book, and in the following paragraphs, outlining key pharmacological compounds as well as hypothermia, evaluated in several clinical trials for severe TBI.
2.1 Early Mechanisms
Rapid intracellular influx of calcium is an immediate event in severe TBI (see Chap. 6). Based on the notion that excess calcium influx is detrimental, the calcium antagonist nimodipine was evaluated in the Head Injury Trials (HITs) (see Vergouwen et al. (2006)). A potential benefit in patients with traumatic subarachnoid hemorrhage was suggested and was explored in the HIT3 and HIT4 studies. In the larger HIT4 trial, a significantly impaired outcome in nimodipine-treated patients was observed, and nimodipine cannot be recommended for any subtype of severe TBI. Other immediate events of key pathophysiological importance are glutamate release leading to excitotoxicity and increased formation of reactive oxygen species (ROS), factors consistently identified as important targets in animal models of TBI (Marklund 2016; Marklund and Hillered 2011). Based on the observations that extracellular concentrations of glutamate and aspartate increase early after TBI, their N-methyl-d-Aspartate (NMDA) receptors were targeted in several large placebo-controlled trials evaluating compounds such as aptiganel, SNX-111, D-CPP-ene, selfotel, and eliprodil. Invariably, they all failed or even impaired outcome despite successful evaluation in preclinical models. To date, NMDA receptor antagonists should not be used for neuroprotection in severe TBI. A positive role for NMDA receptor agonists was suggested in animal models of TBI, finding that they could influence outcome by modifying post-injury plasticity (Shohami and Biegon 2014).
Another early event in severe TBI is calcium- and glutamate-induced mitochondrial dysfunction that may be prolonged. An important event is the opening of the mitochondrial permeability transition pore (mPTP), resulting in a reduced capacity for ATP production as well as the release of apoptosis-inducing factors and generation of ROS (Karlsson et al. 2019). Mitochondrial dysfunction may lead to ongoing tissue atrophy (Xu et al. 2010) and is suggested by microdialysis monitoring (Stovell et al. 2018; Lakshmanan et al. 2010). Ciclosporin (CsA) is a commonly used drug for immunosuppression and was also found neuroprotective due to, e.g., inhibition of the calcium-mediated mPTP activation and attenuation of ROS formation. Across numerous TBI models and time windows, CsA treatment has consistently resulted in neuroprotection with improved histological and/or behavioral outcome. In the clinical trial stage, it was found safe with promising, although not significant treatment results (Mazzeo et al. 2009; Brophy et al. 2013). Microdialysis has also been used in combination with whole blood and CSF sampling to determine the optimal dosing of CsA (Brophy et al. 2013). To date, the available human data is insufficient to recommend its use in severe TBI. The open-label phase II Copenhagen Head Injury Ciclosporin study, evaluating two dosing regimens, is currently ongoing for severe TBI. In a first feasibility study, ciclosporin was found safe, it passed the blood-brain barrier and showed signs of favorable changes in the levels of the biomarkers GFAP, NF-L, Tau, and UCH-L1 (Karlsson et al. 2019).
Thus, mitochondrial dysfunction and glutamate excitotoxicity pave the way for increased oxidative stress post-TBI (O’Connell and Littleton-Kearney 2013). Following the formation of highly reactive, oxygen-based radicals, ROS-induced lipid peroxidation that includes oxidative damage to cellular and organelle membranes ensues. Although the extremely short-acting and reactive radical hydroxyl ion (OH-) is highly toxic to virtually every structure in the cell, others such as the superoxide anion (O2−) and the nitrogen-based radicals (reactive nitrogen species (RNS)), nitric oxide (NO), and peroxynitrite (ONOO−) have been evaluated as pharmacological targets in TBI (Frati et al. 2017). However, both the superoxide radical scavenger polyethylene glycol-conjugated superoxide dismutase (PEG-SOD) and the 21-aminosteroid lipid peroxidation inhibitor tirilazad failed to improve survival or functional outcome in large phase III trials for TBI. Since increased formation of O2−, NO, and ONOO− influences cerebral blood flow, they remain interesting targets for TBI, although at this point there is no evidence for, or sufficiently tested, a ROS scavening drug being ready for clinical use.
Finally, both the endocannabinoid dexanabinol and magnesium sulfate had unusually solid preclinical documentation showing efficacy in numerous animal models using prolonged time windows. Disappointingly, both compounds failed to demonstrate clinical efficacy, and there were clear suggestions, at least in the high-dose group, that magnesium sulfate impaired the outcome of severe TBI patients (Temkin et al. 2007; Maas et al. 2006). Some of the pharmacological targets are outlined in Fig. 56.1.

Fig. 56.1 Key events following severe TBI. The secondary injury process provides numerous pharmacological treatment targets that have been successfully evaluated in the experimental TBI setting, while all being unsuccesful in the clinical trial setting. rCBF regional cerebral blood flow; EAA excitatory amino acids; BBB blood-brain barrier
2.2 Steroids
Clinical trials initiated in the late 1970s and early 1980s began evaluating the corticosteroid dexamethasone, and it was found to be ineffective in improving outcome in cohorts of severely brain-injured patients. The multicenter randomized MRC CRASH trial, enrolling more than 10,000 patients, was by far the largest TBI trial (until the recently published CRASH-3 trial), and it evaluated methylprednisolone for patients with severe TBI. The results showed a significant increase in death and severe disability (Roberts et al. 2004). It should be remembered that in this trial excessive doses of corticosteroids were administered. In an early study evaluating 396 severe TBI patients, the synthetic corticosteroid triamcinolone was administered within 4 h after trauma and the outcome was improved by treatment in patients with a focal lesion and a GCS score of <8 (Grumme et al. 1995). Currently, there is some renewed interest in a lower-dose corticosteroid approach for selected TBI patients such as those with cortical contusions. There are significant adverse effects, however, emphasizing that corticosteroids should not routinely be administered to TBI patients.
2.3 Hormone Treatment
The most widely studied sex hormone progesterone repeatedly showed neuroprotective effects in several animal TBI models, attenuating cerebral edema and neuronal death and improving behavioral outcome. Many, but not all preclinical studies were performed on the bifrontal contusion model. An early clinical study found an improved 3- and 6-month outcome when progesterone was administered to patients within 8 h following severe TBI (Xiao et al. 2008; Skolnick et al. 2014). Thus, two phase III randomized trials were initiated (the ProTECT and SYNAPSE trials), of which the ProTECT trial used a very early <4-hour administration time window. Both the ProTECT and SYNAPSE trials were negative on the primary outcome measures, and even though a recent meta-analysis, evaluating eight RCTs, found an improved outcome at 3 although not at 6 months post-injury (Pan et al. 2019), to date, progesterone cannot be recommended as a routine treatment for severe TBI.
Pituitary deficiency is a common finding in survivors of severe TBI (Tritos et al. 2015; Marina et al. 2015) and may influence clinical outcome. Thus, this is an important factor in the clinical management of severe TBI. This does not imply, however, that early routine supplementation of hormones early post-injury for neuroprotection is warranted.
2.4 Hypothermia
Increased body and brain temperature is a well-known secondary injury insult in TBI since it increases brain metabolism and exacerbates neuronal injury. Hypothermia may effectively attenuate the inflammatory response, glutamate release, and ROS production and positively influence neuronal metabolism. In animal models of TBI, it consistently improved histological outcome (Dietrich and Bramlett 2017). This archetypical neuroprotectant has been applied in several clinical trials aiming at a temperature of 32–36.5 °C using various methods of cooling (cooling blankets, gastric lavage, selective head cooling, intravascular methods, etc.). A recent Cochrane assessment (Lewis et al. 2017), updated from an earlier 2009 review, evaluated 37 eligible trials including a total of 3110 randomized participants in both the adult and pediatric age groups. It was concluded that there are no high-quality evidence showing that hypothermia is beneficial in the treatment of severe TBI. The recent POLAR study (Cooper et al. 2018) evaluated early hypothermia (33–35 °C) for a minimum of 72 h up to 7 days post-injury compared to normothermia. A total of 500 patients were included. Even though hypothermia was initiated rapidly (at a median of 1.8 h post-injury) and rewarming was slow, neurological outcomes were not improved by 6 months. However, there were no changes in the rates of pneumonia and intracerebral hemorrhages. The Eurotherm study (Andrews et al. 2018) enrolled 387 patients from 47 centers in 18 countries. This trial used hypothermia titrated to ICP control, where core temperature was initially reduced to 35 °C followed by incremental decreases to a lower limit of 32 °C if needed to maintain ICP at <20 mmHg. Here, the titrated hypothermia approach successfully reduces ICP although mortality was higher and functional outcome worse than in patients treated with normothermia. This study builds on many other findings that hypothermia reduced ICP and it is currently used in many stepwise ICP management protocols.
The reasons for the limited success by hypothermia treatment are likely multifactorial. However, the treatment is risky and should be used with caution and only by experienced physicians since adverse effects include arrhythmias, coagulopathies, sepsis, and, in particular, pneumonia. To avoid the risks associated with systemic hypothermia, selective brain cooling was attempted in a small single-center trial in China, showing reduced ICP and beneficial outcomes at 1 and 2 years post-injury (Qiu et al. 2006), results that await additional, multicenter studies.
In the pediatric population, similar findings of unaltered or even impaired outcome have been found (Bragge et al. 2016; Hutchison et al. 2008). Together, these reports suggest that hypothermia cannot currently be recommended for routine use for TBI, although coming studies may help defining if subsets of TBI patients may benefit from hypothermia, and the most effective hypothermia protocol.
2.5 Neuroinflammation
There is robust clinical and experimental evidence showing a rapid and complex inflammatory response post-TBI. A very rapid upregulation at 1 h post-injury of cytokine and chemokine mRNA, followed by their corresponding proteins, has been found in the experimental TBI setting. Immune cells are also found invading the injured brain tissue, initially neutrophils at ca 24 h post-injury and later T cells and macrophages at 3–5 days post-injury (Clausen et al. 2019). Locally, there is also a very early activation of resident microglial cells that may then persist for years post-injury. Cellular membrane disruption caused by the mechanical impact as well as secondary injury factors may result in the release of damage-associated molecular patterns (DAMPs) that can trigger and amplify neuroinflammation (Simon et al. 2017).
Neuroinflammation should be recognized as an interaction between central and peripheral components that are influenced by age, gender, type of TBI and its severity, and other factors. Inflammation is often referred to as a double-edged sword possessing both beneficial and detrimental functions. The removal of injury debris may be one such positive action of the inflammatory mediators. However, chronic neuroinflammation is associated with an ongoing white matter atrophy (Johnson et al. 2013; Ramlackhansingh et al. 2011) and may be associated with impaired regeneration, posing a logical pharmacological treatment target. Although there are many potential treatment targets in this complex system, ever-increasing interest is generated for the action of interleukin (IL)-1β and its receptor. IL-1β is a key pro-inflammatory mediator, and when neutralized in experimental TBI, improved histological and behavioral outcome is consistently found.
Using a novel microdialysis approach in a single-center, phase II randomized control study of recombinant human IL1ra (rhIL1ra, anakinra) in severe TBI, the drug was found safe, to penetrate the extracellular fluid of the brain and to modify the inflammatory response as evident by comparison of the concentrations of 41 cytokines and chemokines. This proof-of-concept study provided evidence that an anti-inflammatory drug may alter the local inflammatory response and suggest an important role for microdialysis in pharmacological studies on TBI (Helmy et al. 2014). In a follow-up study, the IL1ra compound shifted the chemokine profile from an M2 microglia phenotype to an M1, highlighting that the microglial response may be modified by a study drug (Helmy et al. 2016).
Glucocorticoids may be considered anti-inflammatory compounds, and did not improve outcome in severe TBI, although the excessive doses used do not provide arguments either for or against inflammation as a treatment target in TBI. The optimal neuroinflammatory targets have not yet been defined, nor in what TBI subtype neuroinflammation may be most efficacious. Furthermore, the complex temporal response of neuroinflammation makes timing of treatment a challenge. However, neuroinflammation remains a promising target for pharmacological treatment and neuroprotection in severe TBI. To date, there are no specific drugs that can be recommended for administration to severe TBI patients.
2.6 Others
2.6.1 Erythropoietin (EPO)
EPO is a glycoprotein first used to treat patients with anemia. It has numerous other functions and is considered a neuroprotective drug with roles in apoptosis, radical oxygen species defense, inflammation, and angiogenesis. In experimental TBI, it is neuroprotective and improves functional outcome in animal models (Peng et al. 2014). The large interest in EPO for the treatment of clinical TBI was mainly based on robust preclinical evidence (Liu et al. 2017). In general trauma patients, thromboembolic events were increased by the treatment although early mortality was reduced. More recently, the Erythropoietin in Traumatic Brain Injury (EPO-TBI) was published (Nichol et al. 2015). It was a double-blind, placebo-controlled trial in >600 patients with moderate or severe TBI. EPO did neither improve neurological outcome nor result in an increased number of patients with deep venous thrombosis. In a follow-up post hoc analysis, it was suggested that a reduction in mortality did occur with EPO although only in those patients receiving one to two doses of the compound, not three (Gantner et al. 2018). At present, EPO cannot be recommended to severe TBI patients outside of clinical trials.
2.6.2 Beta-Blockers
Severe TBI elicits a severe stress reduction with increased pulse rate and, often, increased blood pressure. Stress reduction using, e.g., clonidine and beta-blockers, is part of the Lund concept for the treatment of severe TBI covered elsewhere in this book. Whether beta-blockers such as propranolol and others have neuroprotective mechanisms of action remains a topic of debate. Emerging observational studies find improved outcome and reduced mortality in those patients provided beta-blockers during neurocritical care. For instance, in an observational study of >2200 patients in 15 North American trauma centers, 50% of TBI patients received beta-blockers, most on day 1 post-injury. Administration of beta-blockers resulted in lower mortality, and propranolol was superior to the other compounds (Ley et al. 2018). In a smaller observational study, the use of beta-blockers shortened hospital stay and markedly improved clinical outcome in severe TBI patients (Ahl et al. 2017). Although these trials showed much promise in the treatment of severe TBI, beta-blockers have not been carefully evaluated in a randomized, systematic fashion, and their use for the purpose of neuroprotection cannot be recommended. Furthermore, there are studies finding increased infection rates and prolonged stay in intensive care unit with their use (Chen et al. 2017), suggesting caution and that better evidence is needed prior to beta-blockers being generally recommended to severe TBI patients.
2.6.3 Tranexamic Acid
Coagulation disorders are covered elsewhere in this book. However, rapid correction of coagulation abnormalities may be the best option to achieve neuroprotection. In this chapter, only tranexamic acid (TXA) is discussed. In >20,000 adult trauma patients enrolled in the CRASH-2 study, early <3 h post-injury administration of THX reduced the mortality due to bleeding (Roberts et al. 2013). Early intracranial bleeding is common in severe TBI and associated with increased risk of death and disability, and increased fibrinolysis may worsen the injury progression. The rationale for using TXA is that it inhibits the enzymatic breakdown of fibrinogen and fibrin and may thus prevent hemorrhage, albeit at the price of an increased risk of thromboembolic complications. In the CRASH-3 study, 12,737 TBI patients (9,202 patients treated within 3 hours post-injury) were administered THX or placebo where the primary outcome was death due to TBI within 28 days of injury for patients treated within 3 h of injury. Secondary outcome measures included, e.g., vascular occlusive events, neurosurgical blood loss, days in intensive care, and adverse events (Roberts et al. 2018). The results of the CRASH-3 study showed that there was a small but significant reduction in head-injury related deaths (mortality was 12.5% in the TXA group versus 14.0% in the placebo group), implying and important role for fibrinolysis inhibitors in severe TBI (CRASH-3 trial collaborators 2019).
Tips, Tricks, and Pitfalls
-
If you see edema surrounding a traumatic hematoma and consider using corticosteroids, evaluate the available evidence—which will make you refrain from using it. To date, these drugs have not been shown to benefit TBI patients; instead high-dose treatment was shown to impair the outcome of TBI patients in large clinical trials. Still, the interest for using a lower and more refined corticosteroid dose remains in selected centers, particularly in patients with intracerebral contusions.
-
Hypothermia reduces ICP when applied to patients with severe TBI and is used as a late step in several ICP-lowering protocols worldwide. The value of hypothermia for neuroprotection in severe TBI is at best limited, and the reader should be aware of the studies finding impaired clinical outcome. Thus, routine use of systematic hypothermia for severe TBI cannot be recommended which is supported by the two recent Eurotherm and POLAR RCTs. In pediatric cases, hypothermia has not resulted in improved outcome, and several studies show impaired outcome by the treatment suggesting caution to induce hypothermia in children. The optimal cooling and rewarming strategies, as well as the time window of efficacy, have not been defined.
-
Recent RCTs evaluating erythropoietin or progesterone did not show a clinical benefit over control treatment.
-
Attenuation of coagulation disorders, although not strictly neuroprotection, may be a pharmacological way of attenuating lesion progression in TBI.
-
Large observational studies find that administration of beta-blockers for blood pressure reduction and/or stress relief is associated with reduced mortality and improved outcome. However, they have not as of yet been administered in a randomized fashion, and their role remains debatable.
-
Mitochondrial dysfunction may be a key to the energy metabolic disturbance observed post-TBI and be related to the slow recovery experienced by many patients.
-
Neuroinflammation remains a promising target for neuroprotection in severe TBI, although the precise treatment targets, timing, and compounds have not been established.
-
Is pharmacological neuroprotection dead for severe TBI? Well, not really but clearly suffering. The limitation of providing a drug in sufficient concentrations to the injured brain early enough post-injury will remain a challenge. Likely, to target delayed and/or persistent mechanisms such as mitochondrial dysfunction, axonal injury and neuroinflammation, may have a higher chance for success. Furthermore, the attempt to improve outcome by using a pharmacological compound targeting a single disease mechanism—the “silver bullet”—is likely futile.
-
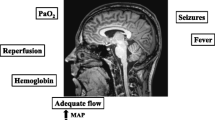
The strict avoidance, detection, and treatment of avoidable factors (seizures, fever, hypotension, hypoxemia, hyper- and hypoglycemia, low CPP, high ICP, etc.) probably remain the best available therapy at the moment to provide neuroprotection for patients suffering from severe TBI.
3 Background
In severe TBI, it is well-established that neuronal and glial cells as well as blood vessels may be disrupted at time of impact and that the ensuing hemorrhage and tissue laceration result in exacerbated injury to the brain tissue. One key component of the injury cascade is a massive disturbance of the cellular ion homeostasis initiated by the marked release of the excitatory amino acid neurotransmitters glutamate and aspartate, in turn resulting in an activation of glutamate receptors leading to excitotoxicity (for overview, see Marklund and Hillered (2011) and Chap. 6 in this volume). As a consequence of the glutamate release, cellular influx of Na+ and Ca2+ and efflux of K+ ensue and lead to traumatic depolarization. The rapid influx of calcium leads to mitochondrial damage, axonal injury, an increase in free radical production, and activation of calcium-dependent destructive proteases such as caspases and calpains resulting in cytoskeletal damage. The mitochondrial dysfunction post-TBI (Hiebert et al. 2015) occurs at the time of increased energy demand due to activation of energy-consuming ion transport systems and cell repair enzymes. A high demand for glucose also occurs at time of reduced regional cerebral blood flow, and this uncoupling of blood flow and cerebral metabolism negatively influences the injured brain (Marklund et al. 2002; Chen et al. 2004). Injured mitochondria are also a potential source for increased production of reactive oxygen/nitrogen species (ROS/RNS), and in combination with a decreased anti-oxidant defense that also occurs following TBI, induces damage to cellular membranes and organelles by lipid peroxidation, protein oxidation, and nucleotide breakdown (Hall 2015).
For obvious reasons, neuronal cell death has attracted the majority of attention in TBI research, although the presence of delayed traumatic axonal injury is increasingly recognized following TBI (Tsitsopoulos et al. 2017).
Inflammation may be a double-edged sword following TBI. Although some inflammatory pathways may be important for regenerative responses and repair, numerous experimental studies suggest that other parts of the immune response is exacerbating the primary injury. The acute inflammatory response following TBI includes breakdown of the blood-brain barrier (BBB) with edema formation, infiltration of peripheral immune cells with production of ROS, activation of resident microglia and astrocytes, and intrathecal release of cytokines (Corps et al. 2015).
In this chapter, I reviewed the key clinical evidence for neuroprotection in patients with severe TBI. Promising preclinical trials have failed when attempting to translate findings from animal models into the clinical setting. Importantly, injury mechanisms, genetic background, gender, age, type and severity of injury, metabolic state of the brain, and other conditions (e.g., other diseases, medication, and coagulation abnormalities) associated with TBI may clearly influence the brain injury and are insufficiently evaluated in preclinical models. Also, much detailed pathophysiological knowledge from experimental TBI has not been confirmed in the injured human brain, and the brain penetration of putative neuroprotective compounds is frequently unknown. Finally, careful selection of patients in terms of injury type and severity based on detailed neuroradiology, age, and additional injuries combined with improved secondary outcome measures is likely crucial in the future development of neuroprotective compounds for human TBI.
The pharmacological and hypothermia TBI trials presented here have all frequently been criticized in terms of study design, route of administration, time window, and patient selection (e.g., see Bragge et al. (2016); Marklund and Hillered (2011)). In particular, it should be emphasized that TBI is not one disease; instead the different subtypes of TBI may require markedly different treatments. It is inevitable that the vast clinical heterogeneity creates difficulties when aiming to design a clinical trial (Saatman et al. 2008). Presumably, many previous trials included patients in too good or too severe condition to enable detection of a treatment effect. Also, preclinical studies use rodent TBI models reaching at most a moderate level of injury, and time windows for drug administration beyond the first post-injury hours are rather scarce in the experimental setting. Lack of early mechanistic endpoints and the insensitivity of the more global outcome measures are specific problems in clinical TBI research. Important lessons for future trials include improved patient classifications, knowledge of brain penetration of the study drug and mechanism of its actions, and more carefully defined and detailed clinical outcome measures. The difficulty of achieving high enough concentrations of the drug early enough post-injury is another important obstacle in the pharmacology of TBI. Perhaps may other administration protocols, using for instance intranasal administration (Guennoun et al. 2019), be evaluated in future RCTs. More sophisticated RCT design, large multicenter RCTs in priority areas, increased focus on preclinical research, and alternatives to RCTs, such as comparative effectiveness research and precision medicine, are needed to fully implement acute TBI research for the benefit of severe TBI patients.
4 Summary
Currently, despite a relatively large number of phase III randomized clinical trials, there is no neuroprotective compound with proven clinical benefit available for TBI patients. Whether or not these failures are caused by inadequate trial design, poor brain penetration and/or efficacy of the compound, patient heterogeneity, insufficient preclinical documentation, or insensitive outcome measures, among many other plausible reasons, remains a matter of debate. Furthermore, there is seldom consensus among centers on general neurointensive care management protocols, which further increase patient heterogeneity. It is obvious that numerous mistakes have been made in the past when attempting to translate preclinical information into the complex human situation. The search for the “silver bullet” (Fig. 56.2)—a compound targeting a single neuroprotective mechanism showing efficacy in all subtypes of TBI and in all TBI severities—is not likely to be succesful. The future pharmacological management of TBI patients will probably include both neuroprotective drugs and compounds enhancing regeneration. Until such pharmacological treatments are developed, clinicians should aim for further improvement in the monitoring and neurointensive care management to improve the outcome of severe TBI patients.
The future pharmacological treatment of TBI may need to include a combination of drugs instead of attempting to use the “silver bullet.” Modified from Pitkänen et al. 2005
References
Ahl R, Thelin EP, Sjolin G, Bellander BM, Riddez L, Talving P, et al. Beta-Blocker after severe traumatic brain injury is associated with better long-term functional outcome: a matched case control study. Eur J Trauma Emerg Surg. 2017;43(6):783–9.
Andrews PJ, Sinclair HL, Rodriguez A, Harris B, Rhodes J, Watson H, et al. Therapeutic hypothermia to reduce intracranial pressure after traumatic brain injury: the Eurotherm3235 RCT. Health Technol Assess. 2018;22(45):1–134.
Bragge P, Synnot A, Maas AI, Menon DK, Cooper DJ, Rosenfeld JV, et al. A state-of-the-science overview of randomized controlled trials evaluating acute management of moderate-to-severe traumatic brain injury. J Neurotrauma. 2016;33(16):1461–78.
Brophy GM, Mazzeo AT, Brar S, Alves OL, Bunnell K, Gilman C, et al. Exposure of cyclosporin A in whole blood, cerebral spinal fluid, and brain extracellular fluid dialysate in adults with traumatic brain injury. J Neurotrauma. 2013;30(17):1484–9.
Chen SF, Richards HK, Smielewski P, Johnstrom P, Salvador R, Pickard JD, et al. Relationship between flow-metabolism uncoupling and evolving axonal injury after experimental traumatic brain injury. J Cereb Blood Flow Metab. 2004;24(9):1025–36.
Chen Z, Tang L, Xu X, Wei X, Wen L, Xie Q. Therapeutic effect of beta-blocker in patients with traumatic brain injury: a systematic review and meta-analysis. J Crit Care. 2017;41:240–6.
Clausen F, Marklund N, Hillered L. Acute inflammatory biomarker responses to diffuse traumatic brain injury in the rat monitored by a novel microdialysis technique. J Neurotrauma. 2019;36(2):201–11.
Cooper DJ, Nichol AD, Bailey M, Bernard S, Cameron PA, Pili-Floury S, et al. Effect of early sustained prophylactic hypothermia on neurologic outcomes among patients with severe traumatic brain injury: the POLAR randomized clinical trial. JAMA. 2018;320(21):2211–20.
Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015;72(3):355–62.
CRASH-3 trial collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet. 2019;394(10210):1713–23. https://doi.org/10.1016/S0140-6736(19)32233-0. Epub 2019 Oct 14.
Dietrich WD, Bramlett HM. Therapeutic hypothermia and targeted temperature management for traumatic brain injury: experimental and clinical experience. Brain Circ. 2017;3(4):186–98.
Frati A, Cerretani D, Fiaschi AI, Frati P, Gatto V, La Russa R, et al. Diffuse axonal injury and oxidative stress: a comprehensive review. Int J Mol Sci. 2017;18(12):E2600.
Gantner DC, Bailey M, Presneill J, French CJ, Nichol A, Little L, et al. Erythropoietin to reduce mortality in traumatic brain injury: a post-hoc dose-effect analysis. Ann Surg. 2018;267(3):585–9.
Grumme T, Baethmann A, Kolodziejczyk D, Krimmer J, Fischer M, von Eisenhart Rothe B, et al. Treatment of patients with severe head injury by triamcinolone: a prospective, controlled multicenter clinical trial of 396 cases. Res Exp Med (Berl). 1995;195(4):217–29.
Guennoun R, Frechou M, Gaignard P, Liere P, Slama A, Schumacher M, et al. Intranasal administration of progesterone: a potential efficient route of delivery for cerebroprotection after acute brain injuries. Neuropharmacology. 2019;145(Pt B):283–91.
Hall ED. The contributing role of lipid peroxidation and protein oxidation in the course of CNS injury neurodegeneration and neuroprotection: an overview. In: Kobeissy FH, editor. Brain neurotrauma: molecular, neuropsychological, and rehabilitation aspects. Frontiers in neuroengineering. Boca Raton: CRC Press/Taylor & Francis; 2015.
Helmy A, Guilfoyle MR, Carpenter KL, Pickard JD, Menon DK, Hutchinson PJ. Recombinant human interleukin-1 receptor antagonist in severe traumatic brain injury: a phase II randomized control trial. J Cereb Blood Flow Metab. 2014;34(5):845–51.
Helmy A, Guilfoyle MR, Carpenter KLH, Pickard JD, Menon DK, Hutchinson PJ. Recombinant human interleukin-1 receptor antagonist promotes M1 microglia biased cytokines and chemokines following human traumatic brain injury. J Cereb Blood Flow Metab. 2016;36(8):1434–48.
Hiebert JB, Shen Q, Thimmesch AR, Pierce JD. Traumatic brain injury and mitochondrial dysfunction. Am J Med Sci. 2015;350(2):132–8.
Hutchison JS, Ward RE, Lacroix J, Hebert PC, Barnes MA, Bohn DJ, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358(23):2447–56.
Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136(Pt 1):28–42.
Karlsson M, Pukenas B, Chawla S, Ehinger JK, Plyler R, Stolow M, et al. Neuroprotective effects of cyclosporine in a porcine pre-clinical trial of focal traumatic brain injury. J Neurotrauma. 2019;36(1):14–24.
Lakshmanan R, Loo JA, Drake T, Leblanc J, Ytterberg AJ, McArthur DL, et al. Metabolic crisis after traumatic brain injury is associated with a novel microdialysis proteome. Neurocrit Care. 2010;12(3):324–36.
Lewis SR, Evans DJ, Butler AR, Schofield-Robinson OJ, Alderson P. Hypothermia for traumatic brain injury. Cochrane Database Syst Rev. 2017;9:CD001048.
Ley EJ, Leonard SD, Barmparas G, Dhillon NK, Inaba K, Salim A, et al. Beta blockers in critically ill patients with traumatic brain injury: results from a multicenter, prospective, observational American Association for the Surgery of Trauma study. J Trauma Acute Care Surg. 2018;84(2):234–44.
Liu WC, Wen L, Xie T, Wang H, Gong JB, Yang XF. Therapeutic effect of erythropoietin in patients with traumatic brain injury: a meta-analysis of randomized controlled trials. J Neurosurg. 2017;127(1):8–15.
Maas AI, Murray G, Henney H 3rd, Kassem N, Legrand V, Mangelus M, et al. Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol. 2006;5(1):38–45.
Maas AI, Roozenbeek B, Manley GT. Clinical trials in traumatic brain injury: past experience and current developments. Neurotherapeutics. 2010;7(1):115–26.
Marina D, Klose M, Nordenbo A, Liebach A, Feldt-Rasmussen U. Early endocrine alterations reflect prolonged stress and relate to 1-year functional outcome in patients with severe brain injury. Eur J Endocrinol. 2015;172(6):813–22.
Marklund N. Rodent models of traumatic brain injury: methods and challenges. Methods Mol Biol. 2016;1462:29–46.
Marklund N, Hillered L. Animal modelling of traumatic brain injury in preclinical drug development: where do we go from here? Br J Pharmacol. 2011;164(4):1207–29.
Marklund N, Sihver S, Langstrom B, Bergstrom M, Hillered L. Effect of traumatic brain injury and nitrone radical scavengers on relative changes in regional cerebral blood flow and glucose uptake in rats. J Neurotrauma. 2002;19(10):1139–53.
Mazzeo AT, Brophy GM, Gilman CB, Alves OL, Robles JR, Hayes RL, et al. Safety and tolerability of cyclosporin a in severe traumatic brain injury patients: results from a prospective randomized trial. J Neurotrauma. 2009;26(12):2195–206.
Nichol A, French C, Little L, Haddad S, Presneill J, Arabi Y, et al. Erythropoietin in traumatic brain injury (EPO-TBI): a double-blind randomised controlled trial. Lancet. 2015;386(10012):2499–506.
O’Connell KM, Littleton-Kearney MT. The role of free radicals in traumatic brain injury. Biol Res Nurs. 2013;15(3):253–63.
Pan ZY, Zhao YH, Huang WH, Xiao ZZ, Li ZQ. Effect of progesterone administration on the prognosis of patients with severe traumatic brain injury: a meta-analysis of randomized clinical trials. Drug Des Devel Ther. 2019;13:265–73.
Peng W, Xing Z, Yang J, Wang Y, Wang W, Huang W. The efficacy of erythropoietin in treating experimental traumatic brain injury: a systematic review of controlled trials in animal models. J Neurosurg. 2014;121(3):653–64.
Pitkänen A, Marklund N, Morales, McIntosh TK. Mechanisms of neuronal death and neuroprotective strategies after traumatic brain injury. Drug Discov Today Dis Mech. 2005;2(4):408–18.
Qiu W, Shen H, Zhang Y, Wang W, Liu W, Jiang Q, et al. Noninvasive selective brain cooling by head and neck cooling is protective in severe traumatic brain injury. J Clin Neurosci. 2006;13(10):995–1000.
Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, et al. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol. 2011;70(3):374–83.
Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G, et al. Effect of intravenous corticosteroids on death within 14 days in 10,008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet. 2004;364(9442):1321–8.
Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17(10):1–79.
Roberts I, Belli A, Brenner A, Chaudhri R, Fawole B, Harris T, et al. Tranexamic acid for significant traumatic brain injury (The CRASH-3 trial): statistical analysis plan for an international, randomised, double-blind, placebo-controlled trial. Wellcome Open Res. 2018;3:86.
Rosenfeld JV, Maas AI, Bragge P, Morganti-Kossmann MC, Manley GT, Gruen RL. Early management of severe traumatic brain injury. Lancet. 2012;380(9847):1088–98.
Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT, et al. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25(7):719–38.
Shohami E, Biegon A. Novel approach to the role of NMDA receptors in traumatic brain injury. CNS Neurol Disord Drug Targets. 2014;13(4):567–73.
Simon DW, McGeachy MJ, Bayir H, Clark RS, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13(3):171–91.
Skolnick BE, Maas AI, Narayan RK, van der Hoop RG, MacAllister T, Ward JD, et al. A clinical trial of progesterone for severe traumatic brain injury. N Engl J Med. 2014;371(26):2467–76.
Stovell MG, Mada MO, Helmy A, Carpenter TA, Thelin EP, Yan JL, et al. The effect of succinate on brain NADH/NAD(+) redox state and high energy phosphate metabolism in acute traumatic brain injury. Sci Rep. 2018;8(1):11140.
Temkin NR, Anderson GD, Winn HR, Ellenbogen RG, Britz GW, Schuster J, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: a randomised controlled trial. Lancet Neurol. 2007;6(1):29–38.
Tritos NA, Yuen KC, Kelly DF, Neuroendocrine A, Pituitary Scientific C. American Association of Clinical Endocrinologists and American College of Endocrinology Disease State Clinical Review: a neuroendocrine approach to patients with traumatic brain injury. Endocr Pract. 2015;21(7):823–31.
Tsitsopoulos PP, Abu Hamdeh S, Marklund N. Current opportunities for clinical monitoring of axonal pathology in traumatic brain injury. Front Neurol. 2017;8:599.
Vergouwen MD, Vermeulen M, Roos YB. Effect of nimodipine on outcome in patients with traumatic subarachnoid haemorrhage: a systematic review. Lancet Neurol. 2006;5(12):1029–32.
Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit Care. 2008;12(2):R61.
Xu Y, McArthur DL, Alger JR, Etchepare M, Hovda DA, Glenn TC, et al. Early nonischemic oxidative metabolic dysfunction leads to chronic brain atrophy in traumatic brain injury. J Cereb Blood Flow Metab. 2010;30(4):883–94.
Zoerle T, Carbonara M, Zanier ER, Ortolano F, Bertani G, Magnoni S, et al. Rethinking neuroprotection in severe traumatic brain injury: toward bedside neuroprotection. Front Neurol. 2017;8:354.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Marklund, N. (2020). Pharmacological Neuroprotection. In: Sundstrøm, T., Grände, PO., Luoto, T., Rosenlund, C., Undén, J., Wester, K. (eds) Management of Severe Traumatic Brain Injury. Springer, Cham. https://doi.org/10.1007/978-3-030-39383-0_56
Download citation
DOI: https://doi.org/10.1007/978-3-030-39383-0_56
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-39382-3
Online ISBN: 978-3-030-39383-0
eBook Packages: MedicineMedicine (R0)