Abstract
Increased intracranial pressure following traumatic brain injury is correlated with poor outcome and death in several studies. Decompressive craniectomy is a neurosurgical emergency procedure in which a large bone flap is removed and the underlying dura mater is left open in order to decrease refractory elevated intracranial pressure that is resistant to the standard measures. Currently, the results from clinical randomised trials show that decompressive craniectomy effectively lowers intracranial pressure and reduces mortality rate, but that these benefits are translated almost directly into survival with severe disability. There are, however, aspects to consider when interpreting these results and delivering the treatment to individual patients, especially in case of younger individuals. In this chapter, decompressive craniectomy and its operative technique and acute-phase complications are reviewed and discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Tips, Tricks and Pitfalls-
Decompressive craniectomy effectively lowers intracranial pressure and reduces the mortality rate following severe diffuse TBI and intractable intracranial pressure.
-
Major randomised trials indicate that the benefits are translated almost directly into survival with severe disability.
-
The temporal bone should be removed to the floor of the middle fossa to prevent upper brainstem compression.
-
The dura should always be opened and closed without sutures.
Level I
Decompressive craniectomy does not improve the rate of patients surviving with favourable functional outcome. Decompressive craniectomy effectively lowers intracranial pressure (ICP) and reduces mortality rate following severe diffuse traumatic brain injury and diffuse traumatic brain injury with surgical intracranial lesions.
Level II
There are insufficient data to support a Level II recommendation for this topic.
Level III
Decompressive craniectomy is a surgical option to control ICP in patients with refractory ICP not responding to first tier of therapy.
1 Background
1.1 Overview
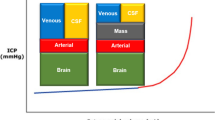
According to the Monroe-Kelly doctrine, the intracranial volume is constant and dictated by the confines of the skull, brain tissue, cerebrospinal fluid (CSF) and intracranial blood (Wilson 2016):
where V IC is intracranial volume, V BR is brain tissue volume, V BL is blood volume and V CSF is CSF volume.
The volume of these compartments is rigorously regulated, and cerebral perfusion pressure (CPP) is maintained by cerebral autoregulation. When the physiological equilibrium is disturbed by expansion in some of these volumes, compensatory mechanisms are activated in order to keep ICP constant (Stocchetti and Maas 2014).
Traumatic brain injury (TBI) is a complex continuum, which causes both cerebral and systemic events. These events may exacerbate an already sustained brain injury, often referred to as secondary brain injury. Brain injury might disturb the Monroe-Kellie equation by increased volumes on the right-hand side of the equation due to (1) focal pathologies such as contusions, intracerebral haematomas and hydrocephalus or (2) diffuse brain swelling from oedema (Unterberg et al. 2004), hyperaemia and microvascular injury (congestive brain swelling) (Kelly et al. 1996; Logsdon et al. 2015). Systemic responses include, e.g. pro-inflammatory responses (Bulstrode et al. 2014), coagulopathy (Maegele et al. 2017) and fever. In severe TBI, these phenomena appear in innumerable combinations.
Due to the rigidness of the skull after closing of the sutures, an intracranial volume increase causes increased pressure when the compensatory mechanisms of reduced CSF and intracranial blood have been exhausted. When the standard measures for decreasing ICP have failed, an alternative option is to increase the intracranial volume (V IC) utilising decompressive craniectomy (DC). DC is a neurosurgical emergency procedure in which a large bone flap is removed and the underlying dura mater is left open in order to allow brain tissue expansion and thus to lower ICP (Timofeev et al. 2012).
Primary DC refers to the decompression procedure combined with the evacuation of a space-occupying intracranial lesion or, in case of an initial diffuse hemispheric swelling, the decompression alone. A secondary DC refers to an intervention that is a part of tiered therapeutic protocol in the intensive care setting in order to reduce intractable ICP and ensure adequate CPP (Kolias et al. 2016). Along with medically refractory elevated ICP due to severe TBI, other indications for DC are intractable brain swelling due to, e.g. stroke, subarachnoid haemorrhage and intracerebral haemorrhage.
1.2 Major Randomised Controlled Trials
Increased ICP following TBI is correlated with poor outcome and death in several studies (Sahuquillo and Arikan 2006). Although not supported by Level I evidence (Carney et al. 2017), ICP monitoring and administration of ICP lowering measures are widely utilised in the intensive care unit setting. In some patients, brain swelling may result in refractory intracranial hypertension despite tiered medical treatment (Grindlinger et al. 2016).
The current evidence leaves little uncertainty about the lifesaving effect of DC in patients with severe TBI and refractory ICP. However, there is a risk that survival from injury following DC may come at the expense of severe functional disability and dependency on others. So far, two major clinical randomised controlled trials (RCTs) have investigated survival and neurological outcome of patients with TBI and refractory ICP following DC.
The international multicentre DECRA study investigated the role of early bi-frontotemporoparietal DC in patients with intractable ICP following TBI (Cooper et al. 2011). Out of 3478 evaluated patients, 155 patients with TBI with either Glasgow Coma Score (GCS) <8 or CT demonstrating moderate diffuse brain injury were considered eligible and enrolled. Patients with refractory ICP, defined as having ICP > 20 mmHg for 15 min within a 1-h period, were randomly assigned to the following groups: 73 underwent early bi-frontotemporoparietal DC and 82 received standard medical care. DC decreased ICP in patients belonging to the surgical group, but the intervention did not result in improved neurological outcome: 70% of patients in the DC group had an unfavourable outcome vs. 51% of patients in the standard care group at 6-month follow-up.
The ensuing international multicentre RESCUEicp study involved 409 eligible patients from 2008 assessed patients (Hutchinson et al. 2016). The inclusion criteria for the RESCUEicp study differed from the DECRA study in two important aspects: the ICP threshold was higher (25 mmHg for 1–12 h despite maximal medical treatment excluding barbiturates) and included patients who had previously undergone evacuation of an earlier intracranial space-occupying lesion without DC. Two hundred and two eligible patients were randomly assigned to undergo DC with medical therapy, and 196 patients were assigned to receive continued medical therapy with an option for barbiturates. The surgical technique was either a large unilateral frontotemporoparietal DC or a bifrontal craniectomy depending on the imaging characteristics and the discretion of the surgeon. DC resulted in reduced ICP and mortality rate, higher incidence of vegetative state, lower severe disability and upper severe disability (independent at home) when compared with medical therapy at 6 months. Because upper severe disability (Glasgow Outcome Scale Extended 4) (Wilson et al. 1998) was included in favourable outcome, 43% of the patients in the DC group had favourable outcome vs. 35% of the patients in the medical care group.
There are issues that should be considered when translating the findings of these two studies into clinical practice. First, the aims of the studies were different. The DECRA study examined the role of early DC in moderate ICP elevation, which can be considered under a prophylactic neuroprotective perspective, whereas the RESCUEicp study applied DC as the last tier of intervention, which can be considered rescue or salvage therapy. In the DECRA study, the trial enrolment ICP threshold (>20 mmHg for 15 min as recruitment criterion) is criticised to be unjustified as any potential improvement obtained by DC was offset by surgical morbidity. It should be noted that the DECRA study enrolled more patients with fixed pupils in the DC group (27%) than in the standard care group (12%), but after adjustment, there were no differences in the rate of unfavourable outcome in these groups. Third, in both studies, there was substantial crossover of patients from the medical care group to the DC group: 23% and 37% in the DECRA and RESCUEicp studies, respectively. Fourth, the surgical procedures in the studies were different. The RESCUEicp trial allowed both bi-frontotemporoparietal (bifrontal) and frontotemporoparietal (hemicraniectomy) craniectomies, in contrast to DECRA that only allowed bifrontal craniectomies. Patients with surgical lesions were not included in the DECRA study, but in the RESCUEicp trial, patients with intracranial haematoma accounted for nearly 20% of cases.
1.3 Other Randomised Controlled Trials
Along with the major randomised trials (DECRA and RESCUEicp), another RCT has examined the effect of DC on ICP following TBI. Qiu et al. conducted a RCT including 74 adult patients divided into unilateral DC following medical therapy and medical therapy alone (Qiu et al. 2009): mean ICP was lower at all assessed time points in the patients in the DC group.
Similar to DECRA and RESCUEicp, the smaller RCT indicated that DC by and large halved the risk for death (Cooper et al. 2011; Hutchinson et al. 2016; Qiu et al. 2009). In that particular study, favourable outcome was reported in 32% of the patients in the medical therapy group vs. 57% in the DC group (p = 0.035) (Qiu et al. 2009).
1.4 Conclusion
RCTs in patients with severe TBI face a number of problems such as lack of clinical equipoise, lack of patient consent, strong clinician preference, imbalance in surgical expertise, crossover of patients, difficulty with blinding and problems in translating the findings into clinical practice due to the uncontrollable heterogeneity in practice of study centres (Ergina et al. 2009; Kolias et al. 2016).
Results from these four clinical randomised trials imply that DC effectively lowers ICP and reduces the mortality rate but that these benefits are translated almost directly into survival with severe disability (DECRA and RESCUEicp). There are some aspects to consider when interpreting these results. In most clinical situations, DC is usually carried out once medical therapy has failed, especially in young patients with TBI. The validated prognostic models (MRC CRASH Trial Collaborators et al. 2008; Steyerberg et al. 2008) demonstrated that young age is an important prognostic factor for favourable outcome after TBI. Hence, in young patients suffering from intractable ICP after failed medical therapy, neurosurgeons need to assess and balance the chance of survival with an acceptable level of disability with the possibility of permanent severe neurological disability.
2 Operative Technique
The goal of surgery is to provide the brain with room for expansion. Therefore, a large craniectomy is preferred (De Bonis et al. 2013; Güresir et al. 2009) (Fig. 26.1). Usually, DCs are divided into hemicraniectomies (frontotemporoparietal) or bifrontal (bi-frontotemporoparietal) craniectomies. The indications are unilateral or bifrontal expansion, respectively. The authors advocate only the use of hemicraniectomy, and hence, only its operative technique is described.
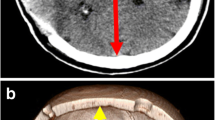
(a) Severe diffuse right-sided TBI with complex frontal bone fracture in a 37-year-old male patient, fall from height accident, (b) blossoming of the haemorrhages causing diffuse swelling seen on postoperative head CT after DC. The patient survived and became independent with upper moderate disability (Glasgow Outcome Scale Extended 6/8)
2.1 Incision
The shape and placement for the skin incision depends on the localisation of DC. For hemicraniectomies, generally three variants are utilised. We prefer using the expanded trauma flap (Fig. 26.2a) or the reversed question mark (Fig. 26.2b), because the incisions can be placed outside the craniectomy margins in order to minimise wound problems related to cranioplasty. Another option is the T-incision (Fig. 26.2c). All the aforementioned incisions provide room for temporal decompression in order to prevent upper brainstem compression (Fig. 26.2d). There are pros and cons for all the incisions in terms of preservation of the vasculature. The superficial temporal artery, including both frontal and parietal branches, is preserved using the expanded trauma flap and the reversed question mark incisions, while the posterior auricular artery and the occipital artery with its branches may be sacrificed depending on the inferior and posterior extension of the incisions. The T-incision is a modification of the reversed question mark incision. It usually preserves the posterior auricular artery and the occipital artery, while it sacrifices the parietal branch of the superficial temporal artery (Kurzbuch 2015). In both extended trauma flap and T-incision, the incision should be placed close to the ear in order to provide protection of temporal and zygomatic branches of the facial nerve (Fig. 26.2a and c). Dissecting the scalp and temporal muscle en bloc helps to avoid injuries to vascular and neural structures.
2.2 Craniectomy
The size of the craniotomy is of paramount importance. If the bone flap is too small, the swelling brain can herniate during the procedure causing severe strangulation. A bone flap with a diameter of 12–15 cm is considered adequate (De Bonis et al. 2013; Güresir et al. 2009). It is imperative to decompress the middle fossa; hence, the hemicraniectomy should be extended caudally so that the floor of the middle fossa is exposed (Fig. 26.2d). The cranio-caudal and anteroposterior dimensions determine the size of the DC. The anatomical landmarks for the DC are anteriorly the superior area above the orbital rim and the sinus of the frontal bone, posteriorly the lambdoid suture, cranially the superior sagittal sinus and inferiorly the zygomatic arch. We recommend placing the first burr hole behind the zygomatic process of the frontal bone (Fig. 26.2d, burr hole (1), the second as close to the zygomatic arch as possible (Fig. 26.2d, burr hole (2), the third on the lambdoid suture approximately 3–4 cm from the midline (Fig. 26.2d, burr hole (3) and an optional fourth behind the coronal suture not closer than 2.5–3 cm to the midline in order to avoid lesions of superior sagittal sinus and large bridging veins (Fig. 26.2d, burr hole (4). Additionally, the cranial border of the craniectomy should not be closer than 2.5 cm to the midline in order to decrease a possible risk of postoperative subdural hygroma and hydrocephalus (Fig. 26.2d, the cyan arrow). The temporal decompression is important, and the bony decompression must be flushed with the floor of the middle fossa. Thus, any remaining temporal bone should be removed with a small rongeur or a rose burr bit. We advocate the use of bone wax to seal the exposed cancellous bone in the temporal area in order to prevent CSF leak.
The middle meningeal artery may bleed following fracture of the temporal bone due to trauma or surgical decompression. The bleeding can be controlled by using bipolar, bone wax or placing tenting sutures between the dura and skull edges in order to eliminate the dead space.
2.3 Durotomy and Duraplasty
Durotomy is a key element in DC as it decreases ICP substantially (Yoo et al. 1999). There are a multitude of techniques for opening the dura when performing hemicraniectomy. In the absence of hard evidence on the efficacy of the different techniques, we consider that an inverse T-shaped dural incision centred on the temporal lobe is preferable to other incisions centred more superiorly due to the increased decompression of the temporal region. Haematomas that can be easily accessed should be evacuated.
Regarding the duraplasty, the gold standard is a synthetic sutureless, non-adhesive, on-lay dura patch of variable materials. It is claimed that the on-lay collagen dural substitutes produce less tissue reaction and easier dissection of the dura-galea interface when the time has come to perform cranioplasty (Biroli et al. 2008; Horaczek et al. 2008). The use of double dural patch has been advocated by some neurosurgeons. In this technique, a second dural sheet is positioned to separate the inner dural patch from the temporal muscle in order to facilitate safe surgical dissection of the temporal muscle in the subsequent cranioplasty (Missori et al. 2008).
2.4 Closure
Due to the large surface area of the skin flap, an epidural drain is recommended in selected cases if there is concern about haemostasis (coagulopathy is common in trauma patients). The drain should be removed within 24 h. Suturing the temporal fascia should not be done due to the restrictive effect this can have on brain expansion. Instead, the skin is closed by subcutaneous sutures followed by cutaneous staples or sutures.
2.5 Bone Storage
Synthetic implants should be preferred in cranioplasty (Malcolm et al. 2018; van de Vijfeijken et al. 2018). Autologous bone should not be used in young patients and smokers or in case of a fracture in the bone flap (Dünisch et al. 2013; Korhonen et al. 2018). However, if cranioplasty using autologous bone is planned, the bone flap can be frozen, or it can be stored autologously. In the case of freezing, the bone flap is immediately dried off, marked and deep-frozen (at least −70 °C). We recommend obtaining swab sample before storage and discarding flaps with positive culture. Otherwise, the bone can be implanted in the subcutaneous tissue of the abdomen.
3 Acute-Phase Complications
An early postoperative CT should be performed to assess blossoming of contusions (Fig. 26.1b) and contralateral injuries that often increase (Fig. 26.3a) postoperatively due to the tamponading effect of preoperative intracranial hypertension. In case of a contralateral mass lesion following DC, a subsequent lesion evacuation is recommended if the prognosis of the patient is not futile. Furthermore, the CSF circulation is often influenced resulting in convexity hygromas (Fig. 26.3b) and ventriculomegaly. Usually these CSF abnormalities should be left untreated if possible, since cranioplasty usually rectifies the problem (Stiver, 2009). However, in some patients, CSF diversion is recommended with an adjustable valve to prevent excessive sinking flap syndrome (Timofeev et al. 2012).
Cerebral autoregulation may be compromised after DC resulting in hyperaemia, possibly contributing to brain swelling and worsening of the secondary injury.
4 Decompressive Craniectomy in Paediatric Patients
The current Guidelines for the Management of Pediatric Severe Traumatic Brain Injury reports Level III evidence for DC for controlling ICP in paediatric patients with severe TBI. More specifically, the evidence suggests DC in treating neurologic deterioration, brain herniation or intracranial hypertension refractory to conservative management (Kochanek et al. 2019).
In a systematic review, Ardissino et al. reported a possible benefit in the use of DC in paediatric patients with TBI for reducing high ICP (>25 mmHg) that is refractory to medical treatment. The authors reported that the quality of evidence remains extremely low, and the findings from RCTs exhibit substantial uncertainty in translating the benefits of DC into long-term neurological outcome (Ardissino et al. 2019). The only RCT included in the systematic review by Ardissino et al. was an older study by Taylor et al. who studied the role of very early DC in paediatric patients with elevated ICP following TBI (Taylor et al. 2001). Altogether 27 patients were divided into (unconventional) bi-temporal DC following medical therapy and medical therapy alone groups. The results demonstrated reduced ICP and fewer episodes of intracranial hypertension in the DC group than in the conventional medical treatment group.
References
Ardissino M, Tang A, Muttoni E, Tsang K. Decompressive craniectomy in paediatric traumatic brain injury: a systematic review of current evidence. Childs Nerv Syst. 2019;35(2):209–16. https://doi.org/10.1007/s00381-018-3977-5.
Biroli F, Fusco M, Bani GG, Signorelli A, Esposito F, De Divitiis O, et al. Novel equine collagen-only dural substitute. Neurosurgery. 2008;62(3 Suppl 1):273–4. https://doi.org/10.1227/01.neu.0000317404.31336.69.
Bulstrode H, Nicoll JAR, Hudson G, Chinnery PF, Di Pietro V, Belli A. Mitochondrial DNA and traumatic brain injury. Ann Neurol. 2014;75(2):186–95.
Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GWJ, Bell MJ, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017;80(1):6–15. https://doi.org/10.1227/NEU.0000000000001432.
Collaborators MRCCT, Perel P, Arango M, Clayton T, Edwards P, Komolafe E, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ (Clinical Research Ed). 2008;336:425–9.
Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364(16):1493–502. https://doi.org/10.1056/NEJMoa1102077.
De Bonis P, Sturiale CL, Anile C, Gaudino S, Mangiola A, Martucci M,et al. Decompressive craniectomy, interhemispheric hygroma and hydrocephalus: A timeline of events? Clin Neurol Neurosurg. 2013.
Dünisch P, Walter J, Sakr Y, Kalff R, Waschke A, Ewald C. Risk factors of aseptic bone resorption: a study after autologous bone flap reinsertion due to decompressive craniotomy. J Neurosurg. 2013;118(5):1141–7. https://doi.org/10.3171/2013.1.JNS12860.
Ergina PL, Cook JA, Blazeby JM, Boutron I, Clavien PA, Reeves BC, Seiler CM. Challenges in evaluating surgical innovation. Lancet. 2009;374(9695):1097–104. https://doi.org/10.1016/S0140-6736(09)61086-2.
Grindlinger G, Skavdahl D, Ecker R, Sanborn M. Decompressive craniectomy for severe traumatic brain injury: clinical study, literature review and meta-analysis. SpringerPlus. 2016;5:1605.
Güresir E, Schuss P, Vatter H, Raabe A, Seifert V, Beck J. Decompressive craniectomy in subarachnoid hemorrhage. Neurosurg Focus. 2009;26(6):E4. https://doi.org/10.3171/2009.3.FOCUS0954.
Horaczek JA, Zierski J, Graewe A. Collagen matrix in decompressive hemicraniectomy. Neurosurgery. 2008;63(1 Suppl):ONS176–81. https://doi.org/10.1227/01.NEU.0000312707.25073.CB.
Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 2016;375:1119–30. https://doi.org/10.1056/NEJMoa1605215.
Kelly DF, Kordestani RK, Martin NA, Nguyen T, Hovda DA, Bergsneider M, et al. Hyperemia following traumatic brain injury: relationship to intracranial hypertension and outcome. J Neurosurg. 1996;85:762–71. https://doi.org/10.3171/jns.1996.85.5.0762.
Kochanek P, Tasker R, Carney N, Totten A, Adelson P, Selden N, et al. Guidelines for the management of pediatric severe traumatic brain injury, Third Edition: update of the Brain Trauma Foundation guidelines. Pediatr Crit Care Med. 2019;20:S1–S82.
Kolias AG, Adams H, Timofeev I, Czosnyka M, Corteen EA, Pickard JD, et al. Decompressive craniectomy following traumatic brain injury: developing the evidence base. Br J Neurosurg. 2016;30(2):246–50. https://doi.org/10.3109/02688697.2016.1159655.
Korhonen TK, Tetri S, Huttunen J, Lindgren A, Piitulainen JM, Serlo W, et al. Predictors of primary autograft cranioplasty survival and resorption after craniectomy on behalf of the Finnish National Cranial Implant Registry (FiNCIR) study group. J Neurosurg. 2018;130:1409–788. https://doi.org/10.3171/2017.12.jns172013.
Kurzbuch AR. Does size matter? Decompressive surgery under review. Neurosurg Rev. 2015;38(4):629–40. https://doi.org/10.1007/s10143-015-0626-2.
Logsdon AF, Lucke-Wold BP, Turner RC, Huber JD, Rosen CL, Simpkins JW. Role of microvascular disruption in brain damage from traumatic brain injury. Compr Physiol. 2015;5(3):1147–60. https://doi.org/10.1002/cphy.c140057.
Maegele M, Schöchl H, Menovsky T, Maréchal H, Marklund N, Buki A, Stanworth S. Coagulopathy and haemorrhagic progression in traumatic brain injury: advances in mechanisms, diagnosis, and management. Lancet Neurol. 2017;16(8):630–47. https://doi.org/10.1016/S1474-4422(17)30197-7.
Malcolm JG, Mahmooth Z, Rindler RS, Allen JW, Grossberg JA, Pradilla G, Ahmad FU. Autologous cranioplasty is associated with increased reoperation rate: a systematic review and meta-analysis. World Neurosurg. 2018;116:60–8. https://doi.org/10.1016/j.wneu.2018.05.009.
Missori P, Polli FM, Peschillo S, D’Avella E, Paolini S, Miscusi M. Double dural patch in decompressive craniectomy to preserve the temporal muscle: technical note. Surg Neurol. 2008;70(4):437–9. https://doi.org/10.1016/j.surneu.2007.03.029.
Qiu W, Guo C, Shen H, Chen K, Wen L, Huang H, et al. Effects of unilateral decompressive craniectomy on patients with unilateral acute post-traumatic brain swelling after severe traumatic brain injury. Crit Care (London, England). 2009;13(6):R185. https://doi.org/10.1186/cc8178.
Sahuquillo J, Arikan F. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database Syst Rev. 2006;12:CD003983. https://doi.org/10.1002/14651858.CD003983.pub2.
Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, McHugh GS, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5:e165; discussion e165.
Stocchetti N, Maas A. Traumatic intracranial hypertension. N Engl J Med. 2014;371(10):972. https://doi.org/10.1056/NEJMra1208708.
Taylor A, Butt W, Rosenfeld J, Shann F, Ditchfield M, Lewis E, et al. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Childs Nerv Syst. 2001;17(3):154–62. https://doi.org/10.1007/s003810000410.
Timofeev I, Santarius T, Kolias AG, Hutchinson PJA. Decompressive craniectomy - operative technique and perioperative care. Adv Tech Stand Neurosurg. 2012;38:115–36.
Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004;129(4):1019–27. https://doi.org/10.1016/j.neuroscience.2004.06.046.
van de Vijfeijken SECM, Münker TJAG, Spijker R, Karssemakers LHE, Vandertop WP, Becking AG, et al. Autologous bone is inferior to alloplastic cranioplasties: safety of autograft and allograft materials for cranioplasties, a systematic review. World Neurosurg. 2018;117:443–452.e8. https://doi.org/10.1016/j.wneu.2018.05.193.
Wilson MH. Monro-Kellie 2.0: the dynamic vascular and venous pathophysiological components of intracranial pressure. J Cereb Blood Flow Metab. 2016;36(8):1338–50. https://doi.org/10.1177/0271678X16648711.
Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15:573–85.
Yoo DS, Kim DS, Cho KS, Huh PW, Park CK, Kang JK. Ventricular pressure monitoring during bilateral decompression with dural expansion. J Neurosurg. 1999;91(6):953–9. https://doi.org/10.3171/jns.1999.91.6.0953.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Posti, J.P., Rønning, P.A. (2020). Decompressive Craniectomy. In: Sundstrøm, T., Grände, PO., Luoto, T., Rosenlund, C., Undén, J., Wester, K. (eds) Management of Severe Traumatic Brain Injury. Springer, Cham. https://doi.org/10.1007/978-3-030-39383-0_26
Download citation
DOI: https://doi.org/10.1007/978-3-030-39383-0_26
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-39382-3
Online ISBN: 978-3-030-39383-0
eBook Packages: MedicineMedicine (R0)