Abstract
Traditionally, it is assumed that singing of birds is a male-typical testosterone-dependent behavior. In this review I point out that singing outside the breeding season is common in males of many songbird species while their testosterone levels are low. Further, females of many tropical and temperate songbird species sing in various context in- or outside of the breeding season but testosterone levels of singing females are low in most cases. These findings question the testosterone-hypothesis of song production of songbirds. However, a key problem is the interpretation of “high” versus “low” testosterone levels, which would require a basic understanding of the quantitative interaction of testosterone and its estrogenic derivate with their specific receptors, as well as the dynamic abundance of these receptors in song controlling systems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Bird songs are thought to mainly function in the realm of female mate attraction and defense of breeding territories against other males (Searcy and Andersson 1986; Kroodsma and Byers 1991; Catchpole and Slater 1995). Individual variation in song characteristics does affect reproductive success through mate choice and male–male competition (Andersson 1994). In relation, there is evidence of sexual selection for song traits such as song rate, repertoire size, structure of song motor units (syllables), and speed of syllable repetitions (for review: Podos et al. 2004). Current theory predicts that when senders and receivers have different evolutionary interests, as in sexual selection, signals must be subject to some costly constraint to constitute stable, honest indicators of quality (Grafen 1990). Individual variation in vocalizations will therefore depend on the condition of the adult male and its developmental history (Rowe and Houle 1996), but the costs of vocalizations are not well understood (Gil and Gahr 2002).
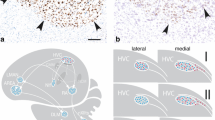
Many temperate and tropical species, that breed seasonally, show seasonal singing activities, although there are surprisingly few quantitative data of circannual singing of bird species. In male songbirds, we can distinguish three categories (Fig. 7.1a): (MI.) males’ singing activity is restricted to the breeding period; (MII.) males sing in the breeding season, and show a second period of singing activity in their post-molt nonbreeding period; (MIII.) males sing year-round. In the second and third cases the uttered song types and or the song pattern might change during the course of the year despite continuous singing activity. Although singing of females of temperate zone species is rare or not well documented, females of some temperate and of many tropical songbird species sing regularly. We can distinguish the following categories (Fig. 7.1b): (FI.) females’ singing activity is restricted to the breeding period; (FII.) Females sing in the breeding season, are quiet during the autumnal molt and show again singing activity in their post-molt nonbreeding period; (FIII.) Females sing year-round; (FIV.) Females sing only in the nonbreeding season; (FV.) Females never sing. Clearly, these classifications must be an oversimplification in light of ca 4500 songbird species, but are useful for the discussion of (neuro)endocrine control of singing of males and females.
Schematic representations of seasonal singing activity of male (a) and female (b) songbirds. In a, schematic drawings of three types of males’ seasonal singing activities are depicted. The timing is based on a hypothetical average breeding season of the Northern Hemisphere: (MI.) Males’ singing activity is restricted to the breeding period, (MII.) Males sing in the breeding season, and show a second period of singing activity in their post-molt nonbreeding period, (MIII.) Males sing year-round. In the second and third case the uttered song types and or the song pattern might change during the course of the year despite continuous singing activity. In b, schematic drawings of five types of females’ seasonal singing activities are depicted: (FI.) Females’ singing activity is restricted to the breeding period, (FII.) Females sing in the breeding season, are quite during the autumnal molt and show again singing activity in their post-molt nonbreeding period, (FIII.) Females sing year-round, (FIV.) Females sing only in the nonbreeding season, (FV.) Females never sing. % Singing activity is normalized to the month with highest singing activity of a species type. Month [1–12] represents January to December. (Modified after: Cox 1944; Immelmann 1969; Ritchison 1983; Langmore 1998; Schwabl 1992; Brunton and Li 2006; Price et al. 2008; Bezzel 2011; York et al. 2016; Gahr, unpublished observations)
Due to the functions of male song for mate attraction and territorial defense, it is generally assumed that singing depends on the gonadal hormone testosterone. This predicts that males sing primarily in the breeding season and that females are not singing. However, as stated before, males of some temperate species and of many tropical species are known to sing outside of the breeding season and females of many tropical species sing regularly. In this review, I discuss the evidence for (1) seasonal singing activity, (2) seasonality of song structure, (3) testosterone dependency of song activity and song structure, (4) neural mechanisms of seasonal singing activity, and (5) neural mechanisms of hormone-dependent seasonal song structure. Based on the present survey of seasonal singing of songbirds, in the conclusion (6) I discuss the concept of singing as a testosterone-sensitive male sexual behavior. Although seasonal changes in singing/vocal activity are common in species of most avian orders (e.g., woodpeckers: Tremain et al. 2008; Malacarne et al. 1991) and seasonal changes in song pattern, too, are likely widespread (e.g., Galliformes: Fusani et al. 1993; suboscine-Passeriformes: Robertson et al. 2009), my review is focused on seasonal singing of songbirds (oscine Passeriformes). Songbirds are particularly interesting for this review, since they represent about half of today’s bird species and since all songbirds have a homologous brain circuit that controls the song pattern, which facilitates species comparisons of underlying mechanisms.
7.1 Seasonal Singing Activity and Song Type Usage
7.1.1 Seasonal Singing Activity (Fig. 7.1)
In males of most temperate zone species singing activity varies during the breeding cycle being highest during mating and/or territory establishment and declines toward the end of the breeding season. Typically, after the breeding season individuals of temperate species molt and show no singing activity in this period. In fall, the majority of species prepare for migration, while about 40% of species of the Northern hemisphere are resident or short-distance migrants. Observations by Alexander (1935) and a survey of Cramp and Perrins (1994) indicate that ca 60% of Paleartic-South-European or Palearctic-African migrants sing in their respective wintering grounds during northern winter, but good quantitative data are lacking. Thus, since the singing behavior during migration and in wintering areas is not well studied, the classification of species as “seasonal singing” is bleared for migratory species that depart briefly after the end of the breeding season. Likewise, little data about singing outside of the breeding season are available for tropical species that migrate within the tropics. In addition, for tropical species, the main problem is determining the breeding period per se that might differ strongly between species and between populations and individuals within a species (Stouffer et al. 2013). Further, since the amplitudes of fall and winter songs are frequently lower than that of breeding period songs, some species of category MI (FI) or MII (FII) might be category II or III, respectively. However, a confounding factor that might lead to false-positive category III species is the song development of juveniles; juvenile male songbirds practice singing in their first autumn/winter which might be confounded with singing adults in un-ringed populations. Last, the singing behavior in the nonbreeding period might differ within a species, in particular between sedentary and migrant populations.
With these caveats, in the following I list examples of the before mentioned three categories of seasonal singing activity of male songbirds: (MI.) males’ singing activity is restricted to the breeding period: temperate zone examples of the first category are the chaffinch (Fingilla coelebs; Bezzel 1988), the willow tit (Parus montanus; Bezzel 2011), the wood warbler (Phylloscopus sibilatrix; Fouarge 1968; Bezzel 2011), and the black-capped chickadee (Poecile atricapillus; Philmore et al. 2006). A tropical such species is the silver-beaked tanager (Ramphocelus carbo; Quispe et al. 2015, 2017).
(MII.) Males sing in the breeding season and show a second period of singing activity in their post-molt nonbreeding period: Examples are the black redstart (Phoenicurus ochruros; Apfelbeck et al. 2013), the canary (Serinus canaria; Leitner et al. 2001a, b; Voigt and Leitner 2008), the serin (Serinus serinus; Bezzel 2011), the European robin (Erithacus rubecula; Lack 1943; Bezzel 2011), the garden warbler (Sylvia borin; Bezzel 2011), the common chiffchaff (Phylloscopus collybita; Bezzel 2011), the song trush (Turdus philomelos; Hegelbach and Spaar 2000; Alexander 1935), the great tit (Parus major; Bezzel 2011; Van Duyse et al. 2003), the stonechats (Saxicola torquata: V. Canoine personal communication), the nightingale (Luscinia megarhynchos: Kunc et al. 2006; Alexander 1935), the marsh warbler (Acrocephalus palustris: Kelsey 1988), and the great reed warbler (Acrocephalus arundinaceus; Sorensen et al. 2016). In these species, the postbreeding period of singing varies: some species start in fall and are able to sing throughout winter [e.g., the European robin (Lack 1943; Bezzel 2011) and black redstart (Apfelbeck et al. 2012; Bezzel 2011)] while others start singing only after their fall migration at the wintering grounds [e.g., the great reed warbler (Sorensen et al. 2016)]. Further, interindividual differences in singing activity seem much more pronounced in fall and winter singing periods as compared to the reproductive season, to the extent that not all males of a species are singing or at least sing infrequent outside the breeding season (e.g., Alexander 1935; Bezzel 2011; Sorensen et al. 2016).
(MIII.) Males sing year-round: Temperate such species are the winter wren (Troglodytes troglodytes: Bezzel 2011), the coal tit (Parus ater: Bezzel 2011), the Eurasian nuthatch (Sitta europaea; Bezzel 2011), and the white-crowned sparrow (Zonotrichia leucophrys: DeWolfe and Baptista 1995). Tropical examples are the white-browed sparrow weaver (York et al. 2016; Voigt and Gahr 2011), the forest weaver (Ploceus bicolor: Wickler and Seibt 1980; Schmidl and Gahr unpublished data) and the red-cheeked cordon-bleus (Uraeginthus bengalus: Gahr and Güttinger 1986). The zebra finch (Taeniopygia guttata), originating of inner Australia, sings year-round, depending on water availability (Zann 1996; Johnson and Rashotte 2002). As stated above, for most tropical species quantitative data covering the whole year are missing.
Even in species that sing only in the breeding season (Category I) large call repertoires might be uttered year-round, e.g., the black-capped chickadee sings in the breeding season (Phillmore et al. 2006) but produces learned contact calls (the chick-a-dee call and the gargle call) year-round (Ficken et al. 1978; Ficken and Weise 1984; Hughes et al. 1998). This indicates a general complication of the categorization of seasonal singing, that is, to distinguish songs from calls in the first place. As the chickadee example indicates even vocal learning does not always help to distinguish songs from calls. In most studies included in this review, authors have used complexity such as being composed of several sequentially uttered sounds, of more than one sound type, and the length of the sounds to distinguish between songs and calls. This structural definition of a song must of course be a species-specific definition and there is not always a clear dichotomy between call and song, e.g., female canaries utter a call, the female-specific trill that is composed of several syllables in a mating context (Amy et al. 2015). This call is more complex than all other calls uttered by female canaries (Mulligan and Olson 1969), more structured than plastic songs uttered by some female canaries (Pesch and Güttinger 1985; Gahr, personal observation) but less complex than male canary songs. However, this canary call is as complex as, e.g., the “chit” songs of female red-winged blackbirds that are produced in a mating context (Agelaius phoeniceus) and more complex than the “teer” songs of these females (Beletsky 1983; Yasukawa et al. 1987).
In difference to all male songbirds, females of some species do not sing at all (e.g., the zebra finch: Morris 1954) and if they sing do not always do so in the breeding season (e.g., the European robin: Lack 1943; Hoelzel 1986; Schwabl 1992). Further, in reports of female singing of Northern temperate zone species, only a fraction of all females of a particular species are observed to sing (e.g., Arcese et al. 1988; Sandell and Smith 1997; Schwabl 1992; Bensch and Hasselquist 1992; Pesch and Güttinger 1985; Langmore and Davies 1997). Thus, the seasonal context of female singing seems to be more variable within a species and between species (for reviews: Ritchison 1983; Langmore 1998; Slater and Mann 2004). Like in males, female songs might be part of duets or solo songs, a distinction that is relevant in the frame of seasonality only in case of multiple distinct song types of a species. Further, most examples of seasonal or year-round female singing lack circannual quantitative data. According to the females’ singing behavior, species can be classified as follows (Fig. 7.1):
(FI.) Females’ singing activity is restricted to the breeding period: Temperate zone examples are the alpine accentors (Prunella collaris: Langmore et al. 1996), the black-headed grosbeaks (Pheuticus melanocephalus: Ritchison 1983), the American dipper (Cinclus americanus: Bakus 1959), the Northern cardinal (Cardinalis cardinalis: Ritchison 1986; Laskey 1944). Although females of these and other species sing only in the breeding season, the context is varied, including nest-building, nest-relief, incubation, brooding, feeding chicks, pair-bond maintenance, family-group maintenance, and only rarely courtship and mate attraction (for review Ritchison 1983; Langmore 1998; Slater and Mann 2004). The example for the latter is the alpine accentor (Langmore et al. 1996).
(FII.) Females sing in the breeding season and have a second singing activity in their post-molt nonbreeding period: An example might be the streak-backed oriole (Price et al. 2008), but data covering the entire year would be required.
(FIII.) Females sing year-round: Examples are the New Zealand bellbird (Anthornis melanura; Brunton and Li 2006), the superb fairy wrens (Malurus cyaneus; Cooney and Cockburn 1995), the white-browed sparrow weaver (York et al. 2016; Voigt and Gahr 2011), and the forest weaver (Wickler and Seibt 1980; Schmidl and Gahr unpublished data), and likely females of many duetting tropical oscines, such as of the wren family (Slater and Mann 2004). As stated above, for most tropical species quantitative data in relation to phenology are missing.
(FIV.) Females sing only outside of the breeding season: Such examples are the canary (Pesch and Güttinger 1985; Ko and Gahr unpublished data), the European robin (European robin; Lack 1943; Schwabl 1992), and the starling (Pavlova et al. 2007). In the latter, presence of males and nest-boxes suppresses singing.
(FV.) Female never sing, e.g., the zebra finch and the Bengalese finch (Lonchura striata domestica) (Morris 1954; Immelmann 1969).
7.1.2 Seasonal Song Type Usage
In some species males produce several song types of which one or several are restricted to the breeding season. The species-typical songs uttered in the breeding season are frequently called full song (also called primary song or crystallized song). Here we use the term full song for songs loudly uttered in the breeding season or year-round. A well-studied such species is the colony-living white-browed sparrow weaver in which the dominant males sing two types of full song: the so-called morning song throughout the breeding season and the duet song throughout the year (Voigt et al. 2006; York et al. 2016) (Fig. 7.2a). The duet song is shared between the dominant male, all subordinate males and the females of a colony (Fig. 7.2b). Duet song, which extends into chorus song if more than two individuals join, is uttered year-round. Solo song and duet song of the dominant male white-browed sparrow weaver differ in the temporal organization and in syllables’ structures (Voigt et al. 2006).
White-browed sparrow weavers possess an extraordinary vocal communication system with two completely different types of song. Sonagrams from field recordings showing a duet song (a, similar in structure to chorus song) and a sequence from morning (solo) song (b) of white-browed sparrow weavers. While all group members engage in duet and chorus singing, morning song is only produced by the dominant male of the group. Note that time scales are different
There is no species known in which females sing multiple song types with at least one type restricted to the reproductive context. A potential such case might be the courtship songs of cordon bleus, in which males and females combine singing with other vocal and with visual displays (Gahr and Güttinger 1986; Ota et al. 2015).
However, the courtship song per se is similar to the song produced outside of the breeding season that might serve a pair-maintaining function. Song types that might be restricted to certain seasons are the so-called soft songs (also called quiet song, twitter song, whisper song) of males of certain species, which are low amplitude songs. According to calculations of Anderson et al. (2008), the full songs might be perceived by humans as about 4–8 louder than the soft songs of the same species, e.g., European robins (Lack 1943; Dabelsteen et al. 1997) and European blackbirds (Turdus merula; Dabelsteen 1984; Dabelsteen and Pedersen 1990) utter soft songs in winter. Likewise, the dunnock (Prunella modularis: Snow 1988), the alpine accentor (Prunella collaris: Langmore et al. 1996), the dark-eyed junco (Junco hyemalis: Titus 1998), the whitethroat (Sylvia communis: Balsby 2000), the swamp sparrow (Melospiza georgiana: Ballentine et al. 2008), and the song sparrow (Melospiza melodia: Anderson et al. 2008) produce soft songs that are only audible over a few meters. In case of the song sparrow, males broadcasted loudly their full song and two forms of soft songs, one similar in structure to the full song but with low amplitude and one that is unstructured, variable and sang with low amplitude (Anderson et al. 2007, 2008). The same males produce these songs types within short-time windows during the breeding season (Anderson et al. 2007, 2008). Likewise, male red-wings (Lampe 1991), European blackbirds (Dabelsteen 1984), and dark-eyed juncos (Titus 1998) produce soft songs that are described as being different in temporal organization, including more high-pitched elements and/or being more variable than the full songs during the breeding season. However, since soft songs are difficult to hear and to record over distance, it is in most species unclear if these songs represent a year-round song type that is uttered in the breeding season next to the loudly advertised full song, or a song type that is restricted to the nonbreeding season. Further, in most cases the low audibility of soft songs resulted in the lack of suitable recordings that would allow qualifying the soft songs as different or similar in structure to the full songs (Dabelsteen et al. 1998; Morton 2000; Anderson et al. 2008). It is unlikely that soft songs simply reflect production errors of the singer since the less structured soft songs of the swamp sparrows are nevertheless distinct reoccurring motor sequences (Anderson et al. 2007, 2008).
7.2 Seasonal Change of Song Structure
Autumnal/winter singing, i.e., “audible” singing outside of the breeding season as opposed to soft singing, has been reported for males of a number of nonmigratory temperate zone species such as the European robin, the winter wren, the black redstart, and the great tit (Armstrong 1955; Schwabl 1992; Apfelbeck et al. 2012; Van Duyse et al. 2003; Bezzel 2011). Likewise, males of various but not all long-distance migratory species have been reported to sing regularly on their wintering grounds (Cramp and Perrins 1994). In some of these species singing outside of the breeding period songs appear similar to the full song (e.g., the winter wren: Kreutzer 1973; the European robin: Lack 1943; Schwabl 1992; Ramenda and Gahr unpublished data). However, the males’ songs uttered in the nonbreeding period are in many species described as being less structured than the full songs and were termed subsongs. The term subsong originally described quite singing (i.e., soft songs) (Nicholson and Koch 1936) but was later used to label songs that were less structured than the typical full songs broadcasted during the breeding season (Lister 1953a). Nowadays, the term subsong is frequently used to describe the unstructured songs of juveniles uttered during song development. To avoid confusion, in this review we use the term “plastic song” for songs of adults uttered outside of the breeding season, i.e., autumnal/wintering songs that are structurally different from full songs.
Very few data are available in which the song pattern of the breeding period and the nonbreeding period within a species were compared statistically, and even fewer data are available, in which the song of the same individual singer uttered in different seasons were compared (Table 7.1). The best-studied example of seasonal song structure is the canary (Nottebohm et al. 1986; Leitner et al. 2001a, b; Voigt and Leitner 2008). Wild canaries form nonmigratory island populations at the Canary Islands, the Azores and Madeira. Both wild and domesticated canaries sing a less structured version of their full songs in autumn (Fig. 7.3). Besides a lower song amplitude and singing in groups, the males change their syllable repertoire partially, the syllable repetition rate, the stereotypy of syllables, the stereotypy of syllable sequences, and the song length (Nottebohm et al. 1986; Leitner et al. 2001a, b; Voigt and Leitner 2008). Other temperate zone examples likely singing plastic songs in autumn and winter are the song thrush, the European blackbird, the mistle trush, the marsh warbler, the willow warbler, and the chaffinch (Thorpe 1958, 1961; Thorpe and Pilcher 1958; Kelsey 1988; Sorensen 2014). In fact, Thorpe states: “we may safely guess that as further material comes to hand, subsong will be found to be a very widespread phenomenon amongst song birds” (Thorpe 1961, p. 70). Similar, Lister (1953b) reported plastic song for many species of the Indian subcontinent. However, about 60 years later, the data situation is still mainly descriptive if not anecdotic:
Seasonal changes of the song pattern of wild canary males. Full songs of the breeding season (a) differ from plastic songs of the autumn (b) but are similar to the full songs uttered in the next breeding season (c). The repertoire composition, the number of unrepeated syllables and the song length change seasonally in wild canaries (Leitner et al. 2001a, b; Voigt and Leitner 2008); in domesticated canaries the syllable repetition rate changes in addition (Nottebohm et al. 1986; Voigt and Leitner 2008). Shown are spectrograms of song segments recorded from the same male in the field in April 1996 (breeding season 1, a), in November 1996 (nonbreeding season, b), and again in April 1997 (breeding season 2, c). Each syllable is a stereotyped set of one to several continuous sounds each with a typical frequency modulation. To illustrate this, some syllables are labeled with gray, green, or red. In a sequence, a syllable is repeated one to several times. Syllables that the bird produced year-round are labeled gray, those that are produced only in the breeding seasons are labeled red, those that were found only in one period are indicated green. A sequence of syllables from the 1996 breeding season (a) was marked with letters. Syllables that were produced in the following nonbreeding (b) and breeding season (c) are marked with the letters used in (a). About 75% of syllables such as b, d, e, i, j, l had correlation coefficients between 0.85 and 0.95 and are, therefore, produced year-round. About 25% of the syllables were produced seasonally. Half of those syllables (red) reappeared in the following year, i.e., are produced annually. Correlation coefficients for such syllables (for example c, g, h, k) were between 0.86 and 0.93. Certain syllables such as “a” and “x” (green) were not found in the subsequent seasons. “f” (a) and “f∗” (c) are related syllables but with a correlation coefficients below 0.75 (after Leitner et al. 2001a, b)
Including the canary, quantitative comparisons of songs and plastic songs are available for only seven species (Fig. 7.4): In the song sparrow, song parts (trills) were composed of more syllables and syllables of the entire song were more stereotyped in the full songs as compared to plastic songs (Smith et al. 1997). In black redstarts, full songs had more elements in two subparts (A and C) than songs of the nonbreeding period while other structural song parameters did not differ significantly between (Apfelbeck et al. 2013). In mockingbirds (Mimus polyglottos) the full song phrases are composed of significantly more repetitions of a given syllable type and the same individuals use partially different syllable repertoires in the spring breeding versus the autumnal nonbreeding season (Howard 1974; Logan 1983). In the New Zealand honeyeater, the tui (Prosthemadera novaeseelandiae), in the breeding season males’ songs contained significantly greater proportions of trill components compared with songs in the nonbreeding season (Hill et al. 2015). In European starlings, full songs are characterized by longer song bouts and a larger repertoire (Van Hout et al. 2009). Further, although many species seem to sing during migration and on the wintering grounds (see above), only the great reed warbler (Sorensen et al. 2016; Wegrzyn and Leniowski 2009) was studied in detail in both periods. In difference to the song of the breeding season, songs of males wintering in Zambia were longer and less repetitive (Sorensen et al. 2016). In the willow warbler (Phylloscopus trochilus), songs recorded at the wintering grounds in Zambia seem to be somewhat longer with more variable syllable structure as compared to songs of breeding birds in Europe (Sorensen 2014), but this has not been analyzed statistically.
Testosterone dependency of singing activity (song rate) and song structure (song length, segment length, syllable repertoire size, stereotypy, syllable rate, frequency range) of songbirds. Classifications are based on published studies that employed castration, hormone treatment and/or inhibiting of endogenous hormone production and androgen and estrogen receptor activity. Red: testosterone sensitive. Green: Estrogen sensitive. Yellow: Testosterone and estrogen sensitive. N.A. = not analyzed in the respective publication. References: 1 = Apfelbeck et al. 2012; 2 = Arnold 1975; 3 = Boseret et al. 2006; 4 = Cynx et al. 2005; 5 = Fusani et al. 2003; 6 = Harding et al. 1983; 7 = Harding et al. 1988; 8 = Heid et al. 1985; 9 = Ketterson et al. 1992; 10 = Konishi 1965; 11 = Kunc et al. 2006; 12 = Kurvers et al. 2008; 13 = Maney et al. 2009; 14 = Meitzen et al. 2007a; 15 = Nowicki and Ball 1989; 16 = Pinxten et al. 2002; 17 = Poulsen 1951; 18 = Pröve 1974; 19 = Ritschard et al. 2011; 20 = Silverin 1980; 21 = Smith et al. 1997; 22 = Soma et al. 1999; 23 = Strand et al. 2008; 24 = Thorpe 1958; 25 = Van Hout et al. 2009; 26 = Van Hout et al. 2012; 27 = Van Roo 2004; 28 = Walters et al. 1991; 29 = Weatherhead et al. 1993; 30 = Wingfield 1994 (From Gahr 2014)
In summary, seasonality of the song structure has been studied quantitatively only in very few species (Table 7.1). Nevertheless, anecdotic reports on the seasonal occurrence of plastic songs suggest that it is a broad phenomenon occurring in various songbird families (e.g., Acrocephalidae; Fingillidae; Meliphagidae; Mimidae; Passerellidae; Phylloscopidae; Sturnidae; Turdidae). Seasonal structural changes such as song length, syllable repetition rates, syllable consistency, and repertoire composition are species specific (Table 7.1). However, individuals of some species such as the winter wren and the skylark (Alauda arvensis) change song parameters dependending on the social context (Camacho-Schlenker et al. 2011; Geberzahn and Aubin 2014), which might be confounded with seasonal changes. Further, some observations of seasonal song structures might be due to juveniles that undergo song development in their first autumn and winter.
Another indication of seasonal changes in song structure is the occurrence of adult song learning. Adult learning of new song syllables likely affects overall song structure, particular in species that sing stereotyped songs in the breeding season such as canaries or song sparrow, in which changes of the syllable repertoire might affect syllable sequencing, song stereotypy, and or song length (Nottebohm and Nottebohm 1978; Leitner et al. 2001a, b; Nordby et al. 2001). Well-documented examples of changes of adult repertoires are the song sparrow (Nordby et al. 2001), the starling (Adret-Hausberger et al. 1990; Eens et al. 1992), the brown-headed cowbird (Molothrus ater; O’Loghlen and Rothstein 2002), and the mockingbird (Derrickson 1987). In light of seasonal structural changes, it is not relevant if adult song learning is de novo learning or altered syllable usage of material learned as juveniles.
Structural seasonal changes of the song of adult female songbirds have not been reported.
7.3 Testosterone-Dependent Seasonal Singing
7.3.1 Testosterone-Dependent Singing Activity
The gonadal dependency of singing of male birds was known for centuries based on the castration of roosters. Berthold with his seminal testis transplantation experiments was the first to recognize that a gonadal hormone, now known as testosterone, is controlling vocal activity and vocal structure (Berthold 1849). Likewise, in songbirds, seasonal singing activity was found to correlate with the hormonal activity of the testicles (Bullough 1942; Davis 1958; Armstrong 1973; Wingfield and Farner 1975). Testosterone is the main androgen released by the testicles and can be converted by the enzyme 5α-reductase into the androgen 5α-dihydrotestosterone (both androgens bind to the androgen receptor) and into the estrogen 17β-estradiol (a potent ligand of both estrogen receptor α [ERα] and estrogen receptor β [ERβ]) via the enzyme aromatase, which occurs in various tissues including the brain (Schlinger and Arnold 1991; Saldanha et al. 2000; Fusani et al. 2000, 2001; Schlinger and Remage-Healey 2012).
In temperate zones, the annual increase in day length triggers seasonal gonadal growth, which results in increased levels of circulating testosterone, an important initiator of reproductive behaviors. Supporting data of testosterone-dependent vocal activity (Fig. 7.4) come from hormone treatments of species of several avian orders including songbirds (e.g., Heid et al. 1985; Dloniak and Deviche 2001; Van Hout et al. 2009), suboscine passerines (Kroodsma 1985), parrots (Brockway 1968; Nespor et al. 1996), galliformes species (Marler et al. 1962; Andrew 1963; Chiba and Hosokawa 2006; Beani et al. 2000; Fusani et al. 1994), night herons (Noble and Wurm 1940), doves (Bennett 1940), and gulls (Terkel et al. 1976; Groothuis and Meeuwissen 1992). In particular, in all testosterone treatment experiments, male birds increased their singing activities (for review Gahr 2014). There is so far no report that testosterone treatment would fail to affect singing activity of birds. Further, males of MI species have maximal testosterone levels at the beginning of the breeding/singing period (e.g., Schwabl 1992; Fusani 2008; Goymann and Landys 2011; Apfelbeck et al. 2017).
In contrast to the testosterone hypothesis of singing, male birds of category MII and MIII sing outside of the breeding season when testicles are regressed (Armstrong 1973) and the levels of circulating testosterone are in many cases very low, around or below 100 pg per mL blood plasma (canary: Leitner et al. 2001a, b; Voigt and Leitner 2008/black redstart: Apfelbeck et al. 2013/song sparrow: Smith et al. 1997; Wingfield and Hahn 1994; but Soma et al. 1999/great tit: van Duyse et al. 2003/stonechats: Gwinner et al. 1994; Goymann et al. 2006). Because these values are close to the detection limits of most testosterone assays, (Wingfield and Farner 1975; Leitner et al. 2001a, b; Gwinner et al. 1994; Goymann et al. 2006; Apfelbeck et al. 2013) the real blood testosterone concentration might even be lower. Since the detection of low amounts of testosterone is particularly sensitive to the sample volume and assay procedures, species differences of low testosterone levels might in part be procedural. In the breeding seasons, the amounts of circulating testosterone are in average 10–200 times higher: In the canary (Leitner et al. 2001a, b; Voigt and Leitner 2008), the great tit (Röhss and Silverin 1983; Van Duyse et al. 2003), the willow tit (Silverin et al. 1986), the black redstart (Apfelbeck et al. 2013), the European robin (Schwabl 1992), the mockingbird (Logan and Wingfield 1995), and the stonechats (Gwinner et al. 1994; Goymann et al. 2006) testosterone is high in the breeding singing season but low in the nonbreeding singing periods. It is unclear if these low levels of testosterone are relevant for the control of singing activity outside of the breeding season. In relation, inhibition of the androgen receptor did not affect singing outside of the breeding periods in male black redstarts and European robins (Schwabl and Kriner 1991; Apfelbeck et al. 2012, 2013).
Thus, testosterone data of seasonal (category MII) and year-round (category MIII) singing males support the notion that elevated levels are important for initiating singing in the breeding season but are not supportive for a testosterone-sensitive mechanism underlying autumnal and wintery singing. Further, although the overall relationship between testosterone and high singing activities is well established in the breeding season, there is mixed evidence of how individual variation in testosterone levels relate to individual variation in singing activity (e.g., Ketterson et al. 1992 but Saino and Moller 1995).
The effect of testosterone on song performance might involve androgenic and estrogenic action of testosterone and its metabolites. In the male zebra finch, aromatase inhibitor reduced the amount of directed (presumably courtship related) singing but not that of undirected singing (Walters et al. 1991). In great tits, estrogen might play a role for singing activity but this pathway was tested together with the androgenic control of singing (Van Duyse et al. 2005). However, singing rate was not estrogen sensitive in other species tested in this way, the canary (Fusani et al. 2003; Rybak and Gahr 2004), the black redstart (Apfelbeck et al. 2012), and the house finch (Carpodacus mexicanus: Strand et al. 2008).
As stated above, females of many species sing too. Testosterone is produced in the ovary as a precursor of estrogens (Johnson 1990). In relation, testosterone is highest in the chicken hen about 6–8 h before ovulation (Etches and Cheng 1981; Robinson et al. 1988). However, maximal concentrations of circulating testosterone (range: 50 pg–3.8 ng/mL) of female birds are four- to tenfold lower than in male conspecifics during the breeding season (Ketterson et al. 2005), and are around or below the detection limits in the nonbreeding periods (Ketterson et al. 2005). Concentrations of circulating estrogens in female songbirds are highest during the nest-building and the egg-laying period with concentration between 100 and 700 pg per mL blood plasma and are low throughout the rest of the year (Silverin et al. 1986; Wingfield and Farner 1978; Dawson 1983; Schwabl et al. 1980; Schwabl 1992; Gwinner et al. 1994).
In support of testosterone dependency of female singing, females that sing periodically or circumstantially are thought to secrete higher levels of testosterone in these periods. Such examples might be the female song sparrow (Melospizia melodia) (Arcese et al. 1988), the European robin (Schwabl 1992), the blue-capped cordon blues (Geberzahn and Gahr 2011), and female dunnocks, in which competition for male reproductive investment elevates testosterone (Langmore et al. 2002). Further, testosterone treatment induced singing in all adult female birds that differentiated the necessary song controlling brain structures during development (for reviews: Gahr 2007, 2014). However, these experimental concentrations of circulating testosterone are in average 10–500 times higher than those measured in untreated females and are higher than those found in males during the breeding season (e.g., Kriner and Schwabl 1991; Fusani et al. 2003; Hartog et al. 2009). On the other hand, female dark-eyed juncos have relatively high testosterone levels but do not sing regularly (Ketterson et al. 2005). Unfortunately, there are only little hormone data of females that spontaneously singing when circulating levels of testosterone are expected being low (e.g., female white-browed sparrow weaver: Wingfield et al. 1991 but York et al. 2016). Although estrogens have been shown to mediate some of the activational action of testosterone on vocalizations (Fusani et al. 2003; Fusani and Gahr 2006), these actions are due to estrogens derived from testosterone in the brain. Unfortunately, there are no estrogen data of females that were singing during nest-building/egg-laying period. Female European robins singing in winter had low to undetectable levels of estradiol (Schwabl 1992).
As stated above for the male birds, it is unclear if the low testosterone levels are relevant for the control of female singing behavior.
Thus, in summary, elevated levels of circulating testosterone correlate with males’ singing activity in the breeding season but not with males’ singing outside of the breeding season and not with the females’ singing activities. A major problem of behavior–hormone correlations is the temporal resolution of data sampling, which is dense (e.g., daily) concerning the singing but sparse (weakly or biweekly or even less frequent) in case of the blood hormone measurements. In addition, testosterone production might undergo circadian changes. For these reasons short periods of high or elevated testosterone concentration are easily missed. Further, birds need to be caught for blood sampling. Thus, in most longitudinal field studies that cover one or more seasons, the singing activity data and testosterone data originate frequently from different individuals. Correlations of this type are particularly worrisome if the data sets originate from entirely different populations and years (e.g., Rost 1990, 1992) and should be avoided. Last, unfortunately the current testosterone assays cannot resolve low blood hormone concentrations. Thus, it needs to be seen if low levels of circulating testosterone are meaningful for song control.
7.3.2 Testosterone-Dependent Seasonal Song Structure
Vocalizations of some species (e.g., the canary, starling) change in structure after castration of adult males (Berthold 1849; Heid et al. 1985; Van Hout et al. 2009), can be reinstated with testosterone treatment after castration in adulthood (Heid et al. 1985), and can be induced in adult females by testosterone treatment (e.g., Shoemaker 1939; Leonard 1939; Konishi 1965; Kern and King 1972; Vallet et al. 1996). However, little attention has been paid to verify if testosterone-induced vocalizations of females are indeed “male-typical,” e.g., testosterone-treated female canaries sing male-like songs (Shoemaker 1939; Leonard 1939), which in average are composed of much fewer syllables compared to male canaries (Hartley and Suthers 1990; Fusani et al. 2003). However, some testosterone-treated female canaries produce songs with sexual release quality (Vallet et al. 1996). Likewise, in autumn, testosterone-induced songs of male chaffinches were reported being less structured than the full songs (Thorpe 1958).
In difference to overall testosterone sensitivity of singing activity, song structure such as song length, song fragment length (e.g., motif, tour, phrase), song unit repertoire (element, syllable, song type), song unit stereotypy, song unit repetition rates, or the frequency range are sensitive to testosterone treatment in a species-specific manner (Fig. 7.4; Gahr 2014). Thus, there are large species differences to the extent of which the song pattern is testosterone-sensitive, from little in the zebra finch to stark in the canary. In relation, acute levels of testosterone do not correlate with song structure such as repertoire size in the red-winged blackbird (Agelaius phoeniceus) (Weatherhead et al. 1993) or the canary. This might be due to fact that song structure depends on the organizational effect of testosterone, that is, there is a delay between testosterone’s effect on neuronal and neural endophenotypes and the occurrence of the overt phenotypes such as the song. In general, the relation between individual changes in testosterone levels and changes in song structure as well as interindividual differences in testosterone levels and such differences in song structure are vague.
The only example of brain-derived estrogens that control the song structure comes from the adult canary; estrogens are required to sing songs with high syllable-repetition rates (Fusani et al. 2003; Rybak and Gahr 2004; Fusani and Gahr 2006), a feature that is important for the sexual quality of canaries’ songs (Kreutzer and Vallet 1991).
7.4 Neural Mechanisms of Seasonal Singing Activity
Singing activity seems not controlled directly by the neural vocal control system that controls the structure of the song as detailed in the next paragraph. Even male canaries with bilaterally lesioned HVC (an important song control region of the songbird brain, see Fig. 7.5), try to sing although with no or little audible outcome (Nottebohm et al. 1976). In a non-songbird, the adult male ring dove (Streptopelia risoria), estrogens derived from testosterone in the preoptic area are important to stimulate sexual vocal displays (Hutchison and Steimer 1984). Likewise, in songbirds the testosterone-inducible singing activity (see above; Gahr 2014) might be mediated by androgen or estrogen receptors that are abundant in preoptic neurons: Alward et al. (2013) showed that singing rate was increased by testosterone implants into the medial preoptic area of castrated male canaries while this treatment did not enhance the song structure such as song stereotypy. In relation, implants of estrogens plus dihydrotestosterone near HVC of white-crowned sparrows did not affect song rate (Meitzen et al. 2007a). How testosterone affects cellular mechanisms of preoptic neurons and how those would activate the vocal control areas needs to be seen.
Distribution of androgen receptors (AR) and estrogen receptors (ERα) in the vocal control system. In a, we depict the expression of AR mRNA in the HVC of a male canary and in b, the ERα mRNA in the HVC of a great tit (Parus major) of the reproductive season. The mRNA expressing cells (brown) were labeled with a nonradioactive in situ hybridization method. In c, we show the distribution of AR (blue dots) and of ERα (red triangles) in the areas of a schematic vocal control system of songbirds. Some thalamic brain areas that appear important for coordination of the left and right vocal control network are omitted (see Wild 1997). Note that ER expression in vocal areas is limited to HVC and differs strongly between species (see d and Table 7.1). In d, we represent the distribution of AR and ERα in the lateral and medial part of the HVC: in type I, ERα is expressed throughout the entire HVC, in type II ERα is expressed in the medial HVC but not or very low in the lateral part, in type III ERα expression is low even in the medial part of HVC. In all songbirds, AR is expressed throughout HVC and ERα is found ventromedial to HVC. In Area X, AR is abundant only in some individuals. Abbreviations: Area X; DLM, nucleus dorsolateralis anterior, pars medialis; DM, dorsomedial nucleus of the midbrain nucleus intercollicularis; HVC, proper name; Field L; lMAN, lateral magnocellular nucleus of the anterior nidopallium; mMAN, medial magnocellular nucleus of the anterior nidopallium; NC, caudal nidopallium; NIF, nucleus interface of the nidopallium; nXIIts, tracheosyringeal portion of the nucleus hypoglossus; RA, robust nucleus of the arcopallium; RAm, nucleus retroambigualis; rVRG, rostro-ventral respiratory group (after Frankl-Vilches and Gahr 2017)
However, such testosterone-dependent mechanisms acting in the preoptic region would not explain singing in seasons when testosterone levels are baseline. Riters and colleagues suggest that song intensity involves neuro-peptidergic mechanisms in limbic regions outside of the vocal control system such as preoptic and septal nuclei (Riters and Ball 1999; Riters 2012; Kelm-Nelson et al. 2013; Merullo et al. 2016). Others speculated that the hormone DHEA (dehydroepiandrosterone) would mediate singing in periods of low testosterone levels (e.g., Soma et al. 2002; Maddison et al. 2012), but there is conflicting evidence (e.g., Pintér et al. 2011). Further, limbic neurons are sensitive for modulatory action of monoaminergic systems, melatonin and opioids and might increase or decrease the probability to sing, although as stated before, the connections of these regions to the vocal control areas need to be identified (e.g., Wild 2017). Such neuro-modulating mechanisms might explain the impact of the sociosexual and physical environment (e.g., food and water availability in zebra finches: Rashotte et al. 2001; Johnson and Rashotte 2002) on singing activity independent of gonadal hormones (e.g., Gulledge and Deviche 1998; Bentley et al. 1999; Deviche and Gulledge 2000; Tramontin et al. 1999).
An alternative possibility of testosterone-sensitive singing activity in times of low testosterone is the increased sensitivity of vocal neurons for testosterone and its androgenic and estrogenic derivatives. Such an example is the vocal neurons of the silver-beaked tanager (Ramphocelus carbo), an endemic neotropical songbird from the Amazon region. In these males, seasonal activation of full song correlated with an increased expression of androgen receptors in the HVC, while testosterone levels remained basal for several weeks after onset of song activity (Quispe et al. 2016). Circulating levels of testosterone started to rise later in the breeding season and coincided with consummatory sexual behaviors. This indicates that equatorial silver-beaked tanagers expands the annual period of singing by transiently increasing the sensitivity of the vocal control system to testosterone, a mechanism that avoids potentially detrimental effects of prolonged periods of elevated testosterone (Quispe et al. 2016).
7.5 Neural Mechanisms of Testosterone-Dependent Seasonal Song Structure
As detailed before, in many tropical and some temperate zone birds, the same song pattern (full song) can be heard year-round (e.g., winter wren: Kreutzer 1973; the great tit): while others sing additional song types in the breeding period (e.g., morning song of white-browed sparrow weaver: Voigt et al. 2006; directed song of zebra finches: Pröve 1974; Sossinka and Böhner 1980; Kao and Brainard 2006) or sing plastic songs in the nonbreeding period (e.g., great reed warbler: Sorensen et al. 2016; canary: Nottebohm et al. 1986; Leitner et al. 2001a, b; Voigt and Leitner 2008). Species and sex differences in seasonal stability versus seasonal plasticity might dependent on the testosterone sensitivity of neural vocal control regions, on the sensitivity of the genome to changing testosterone levels, and might depend on species-specific effects of testosterone-induced gene expression on neural circuit formation. Alternatively, seasonal vocal pattern might be influenced by testosterone sensitivity of auditory processing (Henry and Lucas 2009; Velez et al. 2015) in auditory areas that give input to the vocal control system. In relation, androgen receptors (ARs) are expressed in many neurons of the auditory forebrain (Metzdorf et al. 1999). This scenario is not further discussed in the present review.
7.5.1 Seasonal Testosterone Sensitivity of Vocal Control Regions: Androgen and Estrogen Receptors in Vocal Neurons
In songbirds, neural song control is achieved by a chain of interconnected brain areas in the fore-, mid-, and hindbrain (Nottebohm et al. 1976; Wild 1997; Hahnloser et al. 2002; Amador et al. 2013) (Fig. 7.5c). In particular, forebrain vocal control areas such as the HVC are evolutionary novelties of songbirds (Gahr 2000; Petkov and Jarvis 2012). In addition to the control of the song pattern, these areas are active during call-based vocal communication (Ter Maat et al. 2014; Benichov et al. 2016). The forebrain vocal circuit of songbirds connects to general avian vocal areas in the mid- and hindbrain via a projection of archistriatal neurons (the RA, robust nucleus of the arcopallium), in particular to the syringeal motonucleus (nucleus hypoglossus pars tracheosyringealis) and to respiratory pre-motor nuclei (Wild 1997).
One mode of steroid action in the brain is the alteration of gene expression by binding to intracellular steroid receptors that transactivate transcription of target genes in a ligand-dependent manner (Carson-Jurica et al. 1990). The androgen receptor (AR) has a high affinity for the testosterone and 5α-dihydrotestosterone, but not for 5β-dihydrotestosterone (Grino et al. 1990). The two types of estrogen receptors (ERα, ERβ) bind 17β-estradiol with high affinity. The AR gene codes for the AR protein, the ESR1 gene for ERα protein, and the ESR2 gene for ERβ protein. The AR and ERα but not ERβ occur in the vocal control system (see below).
In songbirds, AR mRNA or AR protein were reported for HVC, RA, and lMAN of all species studied (Balthazart et al. 1992; Bernard et al. 1999; Gahr et al. 1998, 2008; Metzdorf et al. 1999; Fusani et al. 2000; Voigt and Gahr 2011; Fraley et al. 2010; Quispe et al. 2016). Since these include species of various songbird families, among which are the basal Maluridae, the Corvidae, the Malaconotidae, and the derived Fringillidae and Thraupidae, the AR expression in HVC, RA and lMAN seems a general characteristic of songbirds (Frankl-Vilches and Gahr 2017). Further, AR mRNA and protein are reported for mMAN (medial magnocellular nucleus of the anterior nidopallium) and NIF (nucleus interfacialis) in canaries and zebra finches (Balthazart et al. 1992; Metzdorf et al. 1999; Fusani et al. 2000), but these areas have not yet been surveyed in other species. Extrapolating from the HVC, RA and lMAN data, we assume that AR expression in mMAN and NIF is also a common feature of songbirds. In Area X of zebra finches and canaries, ARs occur in only some individuals for unknown reasons (Gahr 2004; Kim et al. 2004). In another Estrildid finch, the wild white-rumped munia (Lonchura striata) and its domesticated relative the Bengalese finch (Lonchura striata domestica), ARs are expressed in a strain-specific pattern in Area X (Wada et al. 2013). In the brainstem, ARs occur in all respiratory-vocal areas and in syringeal motoneurons (Gahr and Wild 1997; Gahr 2000).
Among forebrain vocal areas, ESR1 mRNA and ERα protein is only expressed in HVC and around the dorsal aspect of RA of canaries and zebra finches (Gahr et al. 1993; Metzdorf et al. 1999). Further comparative data are available for ERα expression and protein abundance in the HVC of various species. These data suggest three types of distribution pattern (Frankl-Vilches and Gahr 2017) (Fig. 7.5d): (I) High expression of ERα throughout the entire HVC (e.g., canary, East-African shrike); (II) High expression of ERα only in the medial part of HVC (e.g., the forest weaver, the black redstart); and (III) No expression in the lateral part and low levels of ERα in the medial part of HVC (e.g., zebra finch, Bengalese finch). In all songbird species, large populations of ERα expressing neurons are found ventromedial to HVC aligning the lateral ventricle, an area including the so-called para-HVC (Johnson and Bottjer 1995), but extending much further medial than the latter (Gahr et al. 1993, and unpublished data).
In summary, all forebrain vocal control areas express or have the potential to express AR. Females of species of Maluridae, the Corvidae, the Malaconotidae, and the derived Fringillidae and Thraupidae that develop a vocal system, AR and ERα are expressed in the same areas as in their male conspecifics (Gahr unpublished data). Thus, since the expression pattern between species is very similar, AR and ERα distribution does not explain species differences in the degree of testosterone sensitivity of song features. In difference, ERα distribution of HVC varies considerably between species and suggests species indifference in estrogen dependency of vocal structures. Further, sexual differences of testosterone-dependent singing are not explained by sex differences in hormone receptor abundance in vocal areas.
Nevertheless, species differences in seasonal dynamics of both AR and ERα expression in vocal control areas might be involved in seasonality of song pattern and neural endophenotypes in a species-specific way (Fusani et al. 2000; Fraley et al. 2010). In the canary, AR expression in HVC is similar in the breeding and the nonbreeding season and low during the molt while ERα expression is high in the nonbreeding season and at the beginning of the breeding season but lower during the later breeding season and the molt (Gahr and Metzdorf 1997; Fusani et al. 2000). In the white-crowned sparrow, AR expression in HVC is higher during the breeding season as compared to the nonbreeding season while AR expression in other vocal areas is similar in the two seasons (Fraley et al. 2010). In general, AR expression in vocal control areas is rather invariant throughout the year except during the molt (Gahr and Metzdorf 1997; Gahr unpublished data).
7.5.2 Seasonal Changes of Transcription of Vocal Control Neurons
The same set of genes was found in genomes of various songbird species (Frankl-Vilches et al. 2015; Lovell et al. 2014; Warren et al. 2010). These findings suggest that the evolution of species-specific testosterone-sensitive song structures and related endophenotypes of songbirds does not result from the gain and loss of genes, but from the species-specific hormone-sensitive gene regulation (Frankl-Vilches et al. 2015). In contrast to the global similarity of songbird genomes, on the nucleotide level there are considerable species differences as shown above for the AR promoter of chicken and zebra finch. Such species differences can impact binding motifs of the ERα, the so-called estrogen response element (ERE) and of the AR, the so-called androgen response element (ARE) as shown for genes expressed in the HVC of the canary and the zebra finch (Frankl-Vilches et al. 2015). About 35% of the ERE-bearing and about 11% of ARE-bearing genes expressed in HVC of canaries were lacking these sites in the corresponding zebra finch orthologous promoters (Frankl-Vilches et al. 2015). Thus, species-specific evolutionary loss or gain (e.g., through point mutations) of EREs and AREs might underlie a species-specific gene pool that can be regulated by the activation of AR and ERα via testosterone and its androgenic and estrogenic metabolites in HVC.
This species-specific sensitivity of genes for AR and ERα together with species-specific testosterone profiles might lead to species differences in seasonal transcriptomes. In the white-crowned sparrow, seasonal comparisons of HVC and RA showed that gene expression is area-specific and time-specific across different reproductive conditions (Thompson et al. 2012). In the European robin, examination of HVC transcriptomes and histological analyses of song control nuclei showed testosterone-induced differentiation processes related to neuron growth and spacing, angiogenesis and neuron projection morphogenesis. Similar effects were found in female canaries treated with testosterone (Dittrich et al. 2014). In contrast, the expression of genes related to synaptic transmission was not enhanced in the HVC of testosterone treated female robins but was strongly upregulated in female canaries. A comparison of the testosterone-stimulated transcriptomes indicated that brain-derived neurotrophic factor (BDNF) likely functions as a common mediator of the testosterone effects in HVC (Dittrich et al. 2014; Frankl-Vilches et al. 2015). BDNF is an important regulator of neuronal and neural plasticity (Kowiański et al. 2017). However, other modes of testosterone action, notably related to synaptic transmission, appeared to be regulated in a more species-specific manner in the HVC of robins and canaries. Divergent effects of testosterone on the HVC of different species might be related to differences between species in regulatory mechanisms of the singing behavior.
7.5.3 Comparisons of Seasonal Testosterone-Induced Differentiation of Song Control Regions and Song Structure
Comparisons of the neuroanatomy of male songbirds suggest that seasonal changes in song structure parallel seasonal changes in HVC size of several species (Nottebohm 1981; Tramontin and Brenowitz 2000). However, this pattern is not universal and a number of species do not show such a correlation (Gahr 1990, 1997; Brenowitz et al. 1991; Leitner et al. 2001a, b; Reeves et al. 2003; Phillmore et al. 2006). Next to HVC size, testosterone and its estrogenic metabolites affect various parameters of song control neurons of adult songbirds in a song-area-specific manner. These effects including synapse density and dendritic arbores in RA (DeVoogd and Nottebohm 1981), GAP-junctions in HVC (Gahr and Garcia-Segura 1996), spiking activity of RA neurons (Meitzen et al. 2007b, 2009), synapse protein expression in HVC (Voigt et al. 2004), and neurotransmitter content (Ball and Balthazart 2010). Although these neuronal features are closer to neural function than song-area size, their relations to testosterone-dependent vocal pattern remains to be seen.
Next, I discuss the consequences of local manipulation of testosterone and estrogen concentration and of testosterone- and/or estrogen-controlled proteins in particular song control regions. First, testosterone infusion into song areas suggest that local action of steroids in one song control area affects downstream areas (Brenowitz and Lent 2002; Meitzen et al. 2007a). In particular the works of Meitzen et al. (2007a) show that estrogenic and androgenic metabolites of testosterone act in HVC to increase firing rate of RA neurons, which correlates with song stereotypy but not with song performance in adult white-crowned sparrows. Such testosterone-induced firing rates of RA neurons reflect seasonality of RA neurons since spontaneous firing rates of such neurons are much higher in spring compared to autumn in white-crowned and song sparrows (Meitzen et al. 2007b, 2009). Likewise, testosterone infusion into RA of adult canaries increased the firing of RA neurons (Breutel and Gahr unpublished data). How HVC neurons affect the firing rate of RA neurons is not known.
In canaries, songs are composed of syllables that are repeated identically several times (so called tours) before switching to the next syllable. Fusani et al. (2003) induced singing in adult female canaries through testosterone treatment, but inhibited in one group of such females the aromatization of testosterone into estrogens with an aromatase inhibitor. Testosterone-induced development of male-like song in female canaries is accompanied by an increase in the expression and enzymatic activity of aromatase in the telencephalon near HVC (Fusani et al. 2001), the only vocal areas with higher levels of estrogen receptors (Fig. 7.2). After 3–4 weeks of testosterone treatment, females developed a male-like song, with exception that such females sang few different syllables. In correlation with the male-like songs (long sequences of repeated syllables), the HVC size of singing females increased and was different from untreated non-singing control females (Fusani et al. 2003). The estrogen-deprived singing females differed, however, in that they produced syllable sequences with lower repetition rates compared to the non-deprived singing females (Fusani et al. 2003). On the behavioral level, similar results were obtained for male canaries (Rybak and Gahr 2004).
The local action of testosterone on HVC and the song structure has been studied in some detail in canaries. Testosterone induces the production of vascular endothelial growth factor (VEGF) and its receptor (VEGFR2 tyrosine-kinase) in the canary HVC, which in turn leads to an upregulation of BDNF production in HVC endothelial cells (Louissaint et al. 2002). Systemic inhibition of the VEGFR2 tyrosine-kinase was sufficient to block testosterone-induced singing of female canaries, even though testosterone exerts its inductive action on HVC morphology such as increased volume and increased total number of HVC neurons (Hartog et al. 2009). Expression of exogenous BDNF in HVC, induced locally by in situ transfection, reversed this VEGFR2 inhibitor-associated blockage of song development. The VEGFR2-inhibited, BDNF-expressing females developed elaborate male-like full song features such as syllable repertoires and high repetition rates of song syllables (Hartog et al. 2009).
The work of Fusani et al. (2003) and Hartog et al. (2009) suggests that androgen-dependent overall HVC morphology (reflected in HVC volume) is necessary for the production of certain (basic canary pattern such as repetition of identical syllables) song structure of canaries while other (estrogen-dependent) song structures (high repetition rates of syllables) require neuronal properties that are independent of vocal area size. The association/dissociation of brain area size and a particular neuronal phenotype might be a species-specific phenomenon since HVC volumes appear estrogen sensitive in song sparrows (Soma et al. 2004) and white-crowned sparrows (Tramontin et al. 2003 but Baker et al. 1984) but androgen sensitive in the canary (Fusani et al. 2003; Fusani and Gahr 2006) and hormone insensitive in the Mexican house finch (Strand et al. 2008).
7.5.4 Males Versus Females
As stated above, the differences between testosterone sensitivity of singing activity and of song pattern, seasonal or not, of male and female songbirds are not explained by the expression of AR and ERα in vocal control areas. A similar conclusion comes from the comparison of the neuroanatomy of the song system of males and females of several species. In species with singing females sexual dimorphisms are found in a species-specific, area-specific way to a varying degree at all organizational levels of the song control areas, the gross-anatomical, ultrastructural, electrophysiological, biochemical, and molecular (gene-expression) level (Nottebohm and Arnold 1976; Nottebohm 1980; Gahr and Metzdorf 1997; Del Negro and Edeline 2002; Riters and Ball 2002; Nealen 2005; Voigt and Gahr 2011; Duncan et al. 2011). Most notable, even in species such as the forest weaver, the East-African shrike and the Northern cardinal, in which male and female sing identical or nearly identical songs, respectively, there are sex differences in the size and organization of forebrain song control areas (Gahr et al. 1998, 2008; Jawor and MacDougall-Shackleton 2008; Voigt and Gahr 2011). Likewise, there is no correlation between song performance and song system anatomy since song areas are smaller in female streak-backed oriols that have a larger song output than the males of this species (Hall et al. 2010). Further, even in females that produce male typical songs due to testosterone treatment, the neuroanatomy is sexually dimorphic and does not allow relating neural features and hormone-dependent song pattern of males or females (Nottebohm 1980; Fusani et al. 2003; Hartog et al. 2009; Voigt and Leitner 2013).
7.5.5 Testosterone-Independent Seasonal Control of Song Structure
Vocal neurons express receptors for glucocorticoids (Suzuki et al. 2011; Senft et al. 2016), retinoic acid (Denisenko-Nehrbass et al. 2000), and melatonin (Gahr and Kosar 1996; Jansen et al. 2005; Fusani and Gahr 2015). Further, vocal neurons are sensitive for modulatory action of monoaminergic systems (Li and Sakaguchi 1997; Ball and Balthazart 2010) and opioids (Gulledge and Deviche 1998; Dloniak and Deviche 2001). These neurochemical phenotypes suggest that song phenotypes are under the control of multiple environmental signals that directly act on song neurons, bypassing the gonadal hormones or acting in concert with the gonadal hormones. The most direct evidence suggesting non-gonadal plasticity of singing comes from works on adult male zebra finches: melatonin affects their song pattern and the electrophysiology of RA neurons transiently (Jansen et al. 2005; Deregnaucourt et al. 2012). Unfortunately, the impact of melatonin on song and vocal neurons of seasonal singers such as the canary has not been tested.
A common mechanism of song control, dependent and independent of gonadal hormone, might be BDNF-sensitive mechanisms of HVC. As explained above for the canary, BDNF activity in the HVC is important for the production of sexually salient song pattern (Hartog et al. 2009). Whatever upregulates BDNF in HVC might facilitate differentiation of song structure. This notion is supported by the findings that BDNF expression, at least in rodents can be modulated by many factors that reflect environmental conditions, next to sex hormones (Dittrich et al. 1999; Louissaint et al. 2002; Scharfman and MacLusky 2005; Berton et al. 2006). In this scenario, BDNF upregulation in the vocal control system, even in birds with low levels of gonadal hormone production should show some form of singing.
7.6 Conclusion. Seasonal Singing: Who, How, and What for?
Traditionally, it is assumed that singing of songbirds and song-like vocalizations of other bird species such as the crowing of the chicken are male-typical behaviors (Thorpe 1961, pp. 41–48). This idea was particularly sponsored by the observation of many naturalists that male birds of the Northern hemisphere sing intensely during the breeding season but not in fall or early winter (Fry 1916; Cox 1944; Saunders 1947, 1948). However, the reconsideration of old observations (Fry 1916; Alexander 1935) and new studies of species of the Northern and Southern hemisphere (e.g., Kelsey 1988; DeWolfe and Baptista 1995; van Duyse et al. 2003; Kunc et al. 2006; Bezzel 2011; Apfelbeck et al. 2013; Sorensen 2014; Sorensen et al. 2016) clearly demonstrate that singing outside the breeding season is a common behavior of male songbirds while their testosterone levels remain low (e.g., Schwabl 1992; Apfelbeck et al. 2013; York et al. 2016). Further, detailed studies of tropical and temperate species in the last three decades and reconsideration of old studies of Australo-Asian species led to the conclusion that singing of female songbirds is an ancient and widespread behavior (e.g., Gahr and Güttinger 1986; Hoelzel 1986; Ritchison 1986; Odom et al. 2014), while testosterone levels of singing females are low in most cases (for review: Ketterson et al. 2005). These findings question the testosterone hypothesis of song production. However, a key problem is the interpretation of “high” and “low” testosterone levels, which would require a basic understanding of the quantitative interaction of testosterone molecules, androgen receptor (AR) and estrogen receptor (ER) densities, and transcriptional regulation, e.g., would the transient upregulation of AR in HVC or the vocal system in general be a mechanism to maintain song structures in seasons when testosterone production is low? Or do other endocrine systems induce singing in males outside of the breeding season and in females at various annual periods? Or are males and females able to regulate AR and ER dependent mechanisms via non-endocrine pathways? These are just a few possibilities to explain the mismatch of the seasonal profiles of testosterone and of singing behavior. More detailed field studies of hormone production and singing of males and females as well as detailed molecular analysis of hormone-receptor–hormone interactions are needed to elucidate the neuroendocrine control of seasonal singing. Insights into the endocrine control of male singing outside the breeding season and of female singing are essential for understanding the proximate and ultimate mechanisms of seasonal singing.
References
Adret-Hausberger M, Güttinger HR, Merkel FW (1990) Individual life-history and song repertoire changes in a colony of starlings (Sturnus vulgaris). Ethology 84:265–280
Alexander HG (1935) A chart of bird song. Br Birds 29:190–198
Alward BA, Balthazart J, Ball GF (2013) Differential effects of global versus local testosterone on singing behavior and its underlying neural substrate. Proc Natl Acad Sci USA 110:19573–19578. https://doi.org/10.1073/pnas.1311371110
Amador A, Perl YS, Mindlin GB, Margoliash D (2013) Elemental gesture dynamics are encoded by song premotor cortical neurons. Nature 495:59–64. https://doi.org/10.1038/nature11967
Amy M, Salvin P, Naguib M, Leboucher G (2015) Female signalling to male song in the domestic canary, Serinus canaria. R Soc Open Sci 2:140196. https://doi.org/10.1098/rsos.140196
Anderson RC, Nowicki S, Searcy WA (2007) Soft song in song sparrows: response of males and females to an enigmatic signal. Behav Ecol Sociobiol 61:1267–1274. https://doi.org/10.1007/s00265-007-0357-7
Anderson RC, Searcy WA, Peters S, Nowicki S (2008) Soft song in song sparrows: acoustic structure and implications for signal function. Ethology 114:662–676. https://doi.org/10.1111/j.1439-0310.2008.01518.x
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Andrew RJ (1963) Effects of testosterone on the behavior of the domestic chick. J Comp Physiol Psychol 56:933–940. https://doi.org/10.1037/h0045105
Apfelbeck B, Kiefer S, Mortega KG, Goymann W, Kipper S (2012) Testosterone affects song modulation during simulated territorial intrusions in male black redstarts (Phoenicurus ochruros). PLoS One 7:e52009. https://doi.org/10.1371/journal.pone.0052009
Apfelbeck B, Mortega K, Kiefer S, Kipper S, Vellema M, Villavicencio CP, Gahr M, Goymann W (2013) Associated and disassociated patterns in hormones, song, behavior and brain receptor expression between life-cycle stages in male black redstarts, Phoenicurus ochruros. Gen Comp Endocrinol 184:93–102. https://doi.org/10.1016/j.ygcen.2012.11.027
Apfelbeck B, Mortega KG, Flinks H, Illera JC, Helm B (2017) Testosterone, territorial response, and song in seasonally breeding tropical and temperate stonechats. BMC Evol Biol 17:101. https://doi.org/10.1186/s12862-017-0944-9
Arcese P, Stoddard PK, Hiebert SM (1988) The form and function of song in female song sparrows. Condor 90:44–50. https://doi.org/10.2307/1368431
Armstrong EA (1955) The wren. Collins, London
Armstrong EA (1973) A study of bird song, vol 201. Dover, New York
Arnold AP (1975) The effects of castration and androgen replacement on song, courtship, and aggression in zebra finches (Poephila guttata). J Exp Zool 191:309–326. https://doi.org/10.1002/jez.1401910302
Baker MC, Bottjer SW, Arnold AP (1984) Sexual dimorphism and lack of seasonal changes in vocal control regions of the white-crowned sparrow brain. Brain Res 295:85–89
Bakus GJ (1959) Observations on the life history of the dipper in Montana. Auk 76:190–207. https://doi.org/10.2307/4081776
Ball GF, Balthazart J (2010) Seasonal and hormonal modulation of neurotransmitter systems in the song control circuit. J Chem Neuroanat 39:82–95. https://doi.org/10.1016/j.jchemneu.2009.08.005
Ballentine B, Searcy WA, Nowicki S (2008) Reliable aggressive signalling in swamp sparrows. Anim Behav 75:693–703. https://doi.org/10.1016/j.anbehav.2007.07.025
Balsby TJS (2000) The function of song in whitethroats Sylvia communis. Bioacoustics 11:17–30. https://doi.org/10.1080/09524622.2000.9753447
Balthazart J, Foidart A, Wilson EM, Ball GF (1992) Immunocytochemical localization of androgen receptors in the male songbird and quail brain. J Comp Neurol 317:407–420. https://doi.org/10.1002/cne.90317040
Beani L, Briganti F, Giuseppe C, Concetta L, Dess X, Fulgheri F (2000) Effect of androgens on structure and rate of crowing in the Japanese quail (Coturnix japonica). Behaviour 137:417–435
Beletsky LD (1983) Aggressive and pair bond maintenance songs of female red-winged blackbirds (Agelaius, Phoeniceus). Z Tierpsychol 62:47–54
Benichov JI, Benezra SE, Vallentin D, Globerson E, Long MA, Tchernichovski O (2016) The forebrain song system mediates predictive call timing in female and male zebra finches. Curr Biol 26:309–318. https://doi.org/10.1016/j.cub.2015.12.037
Bennett MA (1940) The vocal hierarchy in ring doves. II. The effects of testosterone propionate. Ecology 21:148–165
Bensch S, Hasselquist D (1992) Evidence for active female choice in a polygynous warbler. Anim Behav 44:301–311. https://doi.org/10.1016/0003-3472(92)90036-9
Bentley GE, Van’t Hof TJ, Ball GF (1999) Seasonal neuroplasticity in the songbird telencephalon: a role for melatonin. Proc Natl Acad Sci USA 96:4674–4679
Bernard DJ, Bentley GE, Balthazart J, Turek FW, Ball GF (1999) Androgen receptor, estrogen receptor alpha, and estrogen receptor beta show distinct patterns of expression in forebrain song control nuclei of European starlings. Endocrinology 140:4633–4643. https://doi.org/10.1210/en.140.10.4633
Berthold AA (1849) Transplantation der Hoden Archiv für Anatomie. Physiologie und Wissenschaftliche Medizin 16:42–46
Berton O, Berton O, McClung CA, DiLeone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ (2006) Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311:864–868. https://doi.org/10.1126/science.1120972
Bezzel E (1988) Die Gesangszeiten des Buchfinken (Fringilla coelebs). Eine Regionalstudie. J Ornithol 129:71–81. https://doi.org/10.1007/BF01641533
Bezzel E (2011) Gesangsphänologie und Methodenstandards der Brutvogelerfassung – langfristige lokale Erfahrungen. Limicola 25:1–36
Boseret G, Carere C, Ball GF, Balthazart J (2006) Social context affects testosterone-induced singing and the volume of song control nuclei in male canaries (Serinus canaria). J Neurobiol 66:1044–1060. https://doi.org/10.1002/neu.20268
Brenowitz EA, Lent K (2002) Act locally and think globally: intracerebral testosterone implants induce seasonal-like growth of adult avian song control circuits. Proc Natl Acad Sci USA 99:12421–12426. https://doi.org/10.1073/pnas.192308799
Brenowitz EA, Nalls B, Wingfield JC, Kroodsma DE (1991) Seasonal-changes in avian song nuclei without seasonal-changes in song repertoire. J Neurosci 11:1367–1374
Brockway BF (1968) Influences of sex hormones on the loud and soft warbling of male budgerigars. Anim Behav 16:5–12
Brunton DH, Li XL (2006) The song structure and seasonal patterns of vocal behavior of male and female bellbirds (Anthornis melanura). J Ethol 24:17–25. https://doi.org/10.1007/s10164-005-0155-5
Bullough WS (1942) The reproductive cycles of the British and continental races of the starling (Sturnus vulgaris L). Phil Trans R Soc B 231:165–U142
Camacho-Schlenker A, Courvoisier H, Aubin T (2011) Song sharing and singing strategies in the winter wren Troglodytes troglodytes. Behav Process 87:260–267
Carson-Jurica MA, Schrader WT, O’Malley BW (1990) Steroid-receptor family - structure and functions. Endocr Rev 11:201–220. https://doi.org/10.1210/edrv-11-2-201
Catchpole CJB, Slater P (1995) Bird song: biological themes and variations. Cambridge University Press, Cambridge, UK. https://doi.org/10.1017/CBO9780511754791
Chiba A, Hosokawa N (2006) Effects of androgens and estrogens on crowings and distress callings in male Japanese quail, Coturnix japonica. Horm Behav 49:4–14. https://doi.org/10.1016/j.yhbeh.2005.05.020
Cooney R, Cockburn A (1995) Territorial defense is the major function of female song in the superb fairy-wren, Malurus-cyaneus. Anim Behav 49:1635–1647. https://doi.org/10.1016/0003-3472(95)90086-1
Cox PR (1944) A statistical investigation into bird song. Br Birds 38:3–9
Cramp SP, Perrins CM (1994) The birds of the Western Palearctic. Oxford University Press, Oxford
Cynx J, Bean NJ, Rossman I (2005) Testosterone implants alter the frequency range of zebra finch songs. Horm Behav 47:446–451. https://doi.org/10.1016/j.yhbeh.2004.11.018
Dabelsteen T (1984) An analysis of the full song of the blackbird Turdus-Merula with respect to message coding and adaptations for acoustic communication. Ornis Scand 15:227–239. https://doi.org/10.2307/3675931
Dabelsteen T, Pedersen SB (1990) Song and information about aggressive responses of blackbirds, Turdus-Merula - evidence from interactive playback experiments with territory owners. Anim Behav 40:1158–1168. https://doi.org/10.1016/S0003-3472(05)80182-4
Dabelsteen T, McGregor PK, Holland J, Tobias JA, Pedersen SB (1997) The signal function of overlapping singing in male robins. Anim Behav 53:249–256
Dabelsteen T, McGregor PK, Lampe HM, Langmore NE, Holland JO (1998) Quiet song in song birds: an overlooked phenomenon. Bioacoustics 9:89–105. https://doi.org/10.1080/09524622.1998.9753385
Davis J (1958) Singing behaviour and the gonad cycle of the rufous-sided towhee. Condor 60:308–336
Dawson A (1983) Plasma gonadal-steroid levels in wild starlings (Sturnus-vulgaris) during the annual cycle and in relation to the stages of breeding. Gen Comp Endocrinol 49:286–294. https://doi.org/10.1016/0016-6480(83)90146-6
Del Negro C, Edeline JM (2002) Sex and season influence the proportion of thin spike cells in the canary HVC. Neuroreport 13:2005–2009. https://doi.org/10.1097/00001756-200211150-00003
Denisenko-Nehrbass NI, Jarvis E, Scharff C, Nottebohm F, Mello CV (2000) Site-specific retinoic acid production in the brain of adult songbirds. Neuron 27:359–370. https://doi.org/10.1016/S0896-6273(00)00043-X
Deregnaucourt S, Saar S, Gahr M (2012) Melatonin affects the temporal pattern of vocal signatures in birds. J Pineal Res 53:245–258. https://doi.org/10.1111/j.1600-079X.2012.00993.x
Derrickson KC (1987) Behavioral-correlates of song types of the northern mockingbird (Mimus-polyglottos). Ethology 74:21–32
Deviche P, Gulledge CC (2000) Vocal control region sizes of an adult female songbird change seasonally in the absence of detectable circulating testosterone concentrations. J Neurobiol 42:202–211. https://doi.org/10.1002/(Sici)1097-4695(20000205)42:2/202::Aid-Neu4/3.0.Co;2-G
DeVoogd T, Nottebohm F (1981) Gonadal-hormones induce dendritic growth in the adult avian brain. Science 214:202–204. https://doi.org/10.1126/science.7280692
Dewolfe BB, Baptista LF (1995) Singing behavior, song types on their wintering grounds and the question of leap-frog migration in Puget-sound white-crowned sparrows. Condor 97:376–389. https://doi.org/10.2307/1369024
Dittrich F, Feng Y, Metzdorf R, Gahr M (1999) Estrogen-inducible, sex-specific expression of brain-derived neurotrophic factor mRNA in a forebrain song control nucleus of the juvenile zebra finch. Proc Natl Acad Sci USA 96:8241–8246. https://doi.org/10.1073/pnas.96.14.8241
Dittrich F, Ramenda C, Grillitsch D, Frankl-Vilches C, Ko MC, Hertel M, Goymann W, ter Maat A, Gahr M (2014) Regulatory mechanisms of testosterone-stimulated song in the sensorimotor nucleus HVC of female songbirds. BMC Neurosci 15:125. https://doi.org/10.1186/s12868-014-0128-0
Dloniak SM, Deviche P (2001) Effects of testosterone and photoperiodic condition on song production and vocal control region volumes in adult male dark-eyed juncos (Junco hyemalis). Horm Behav 39:95–105. https://doi.org/10.1006/hbeh.2000.1621
Duncan KA, Jimenez P, Carruth LL (2011) Distribution and sexually dimorphic expression of steroid receptor coactivator-1 (SRC-1) in the zebra finch brain. Gen Comp Endocrinol 170:408–414. https://doi.org/10.1016/j.ygcen.2010.10.021
Eens M, Pinxten R, Verheyen RF (1992) No overlap in song repertoire size between yearling and older starlings Sturnus-vulgaris. Ibis 134:72–76. https://doi.org/10.1111/j.1474-919X.1992.tb07233.x
Etches RJ, Cheng KW (1981) Changes in the plasma concentrations of luteinizing hormone, progesterone, oestradiol and testosterone and in the binding of follicle-stimulating hormone to the theca of follicles during the ovulation cycle of the hen (Gallus domesticus). J Endocrinol 91:11–22
Ficken MS, Weise CM (1984) A complex call of the black-capped chickadee (Parus atricapillus) I. Microgeographic variation. Auk 101:349–360
Ficken MS, Ficken WR, Witkin S (1978) Vocal repertoire of the black-capped chickadee. Auk 95:34–48. https://doi.org/10.2307/4085493
Fouarge JG (1968) Le Pouillot Siffleur Phylloscopus sibilatrix. Le Gerfaut 58:179–368
Fraley GS, Steiner RA, Lent KL, Brenowitz EA (2010) Seasonal changes in androgen receptor mRNA in the brain of the white-crowned sparrow. Gen Comp Endocrinol 166:66–71. https://doi.org/10.1016/j.ygcen.2009.08.001
Frankl-Vilches C, Gahr M (2017) Androgen and estrogen sensitivity of bird song: a comparative view on gene regulatory levels. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 204:113–126. https://doi.org/10.1007/s00359-017-1236-y
Frankl-Vilches C et al (2015) Using the canary genome to decipher the evolution of hormone-sensitive gene regulation in seasonal singing birds. Genome Biol 16:19. https://doi.org/10.1186/s13059-014-0578-9
Fry HJ (1916) A study of the seasonal decline of bird song. Auk 33:28–40. https://doi.org/10.2307/4072114
Fusani L (2008) Testosterone control of male courtship in birds. Horm Behav 54:227–233. https://doi.org/10.1016/j.yhbeh.2008.04.004
Fusani L, Gahr M (2006) Hormonal influence on song structure and organization: the role of estrogen. Neuroscience 138:939–946. https://doi.org/10.1016/j.neuroscience.2005.08.041
Fusani L, Gahr M (2015) Differential expression of melatonin receptor subtypes MelIa, MelIb and MelIc in relation to melatonin binding in the male songbird brain. Brain Behav Evol 85:4–14. https://doi.org/10.1159/000367984
Fusani L, Beani L, Dessì-Fulgheri F (1993) Seasonal individual changes in grey partridge Perdix perdix male call. Mus reg Sci nat Torino 1:173–181
Fusani L, Beani L, Dessifulgheri F (1994) Testosterone affects the acoustic structure of the male call in the grey partridge (Perdix perdix). Behaviour 128:301–310. https://doi.org/10.1163/156853994x00307
Fusani L, Van’t Hof T, Hutchison JB, Gahr M (2000) Seasonal expression of androgen receptors, estrogen receptors, and aromatase in the canary brain in relation to circulating androgens and estrogens. J Neurobiol 43:254–268. https://doi.org/10.1002/(Sici)1097-4695(20000605)43:3/254::Aid-Neu4/3.0.Co;2-W
Fusani L, Hutchison JB, Gahr M (2001) Testosterone regulates the activity and expression of aromatase in the canary neostriatum. J Neurobiol 49:1–8
Fusani L, Metzdorf R, Hutchison JB, Gahr M (2003) Aromatase inhibition affects testosterone-induced masculinization of song and the neural song system in female canaries. J Neurobiol 54:370–379. https://doi.org/10.1002/neu.10141
Gahr M (1990) Delineation of a brain nucleus - comparisons of cytochemical, hodological, and cytoarchitectural views of the song control nucleus Hvc of the adult canary. J Comp Neurol 294:30–36. https://doi.org/10.1002/cne.902940104
Gahr M (1997) How should brain nuclei be delineated? Consequences for developmental mechanisms and for correlations of area size, neuron numbers and functions of brain nuclei. Trends Neurosci 20:58–62
Gahr M (2000) Neural song control system of hummingbirds: comparison to swifts, vocal learning (songbirds) and nonlearning (suboscines) passerines, and vocal learning (budgerigars) and nonlearning (dove, owl, gull, quail, chicken) nonpasserines. J Comp Neurol 426:182–196
Gahr M (2004) Hormone-dependent neural plasticity in the juvenile and adult song system - what makes a successful male? Ann N Y Acad Sci 1016:684–703. https://doi.org/10.1196/annals.1298.025
Gahr M (2007) Sexual differentiation of the vocal control system of birds. Adv Genet 59:67–105. https://doi.org/10.1016/S0065-2660(07)59003-6
Gahr M (2014) How hormone-sensitive are bird songs and what are the underlying mechanisms? Acta Acustica united with Acustica 100(4):705–718. https://doi.org/10.3813/AAA.918749
Gahr M, Garcia-Segura LM (1996) Testosterone-dependent increase of gap-junctions in HVC neurons of adult female canaries. Brain Res 712:69–73
Gahr M, Güttinger HR (1986) Functional-aspects of singing in male and female Uraeginthus-bengalus (Estrildidae). Ethology 72:123–131
Gahr M, Kosar E (1996) Identification, distribution, and developmental changes of a melatonin binding site in the song control system of the zebra finch. J Comp Neurol 367:308–318
Gahr M, Metzdorf R (1997) Distribution and dynamics in the expression of androgen and estrogen receptors in vocal control systems of songbirds. Brain Res Bull 44:509–517. https://doi.org/10.1016/S0361-9230(97)00233-5
Gahr M, Wild JM (1997) Localization of androgen receptor mRNA-containing cells in avian respiratory-vocal nuclei: an in situ hybridization study. J Neurobiol 33:865–876
Gahr M, Güttinger HR, Kroodsma DE (1993) Estrogen-receptors in the avian brain - survey reveals general distribution and forebrain areas unique to songbirds. J Comp Neurol 327:112–122. https://doi.org/10.1002/cne.903270109
Gahr M, Sonnenschein E, Wickler W (1998) Sex difference in the size of the neural song control regions in a dueting songbird with similar song repertoire size of males and females. J Neurosci 18:1124–1131
Gahr M, Metzdorf R, Schmidl D, Wickler W (2008) Bi-directional sexual dimorphisms of the song control nucleus HVC in a songbird with unison song. PLoS One 3:e3073. https://doi.org/10.1371/journal.pone.0003073
Geberzahn N, Aubin T (2014) How a songbird with continuous singing style modulates its song when territorially challenged. Behav Ecol Sociobiol 68:1–12
Geberzahn N, Gahr M (2011) Undirected (solitary) birdsong in female and male blue-capped cordon-bleus (Uraeginthus cyanocephalus) and its endocrine correlates. PLoS One 6:e26485. https://doi.org/10.1371/journal.pone.0026485
Gil D, Gahr M (2002) The honesty of bird song: multiple constraints for multiple traits. Trends Ecol Evol 17:133–141. https://doi.org/10.1016/S0169-5347(02)02410-2
Goymann W, Landys MM (2011) Testosterone and year-round territoriality in tropical and non-tropical songbirds. J Avian Biol 42:485–489. https://doi.org/10.1111/j.1600-048X.2011.05464.x
Goymann W, Geue D, Schwabl I, Flinks H, Schmidl D, Schwabl H, Gwinner E (2006) Testosterone and corticosterone during the breeding cycle of equatorial and European stonechats (Saxicola torquata axillaris and S. t. rubicola). Horm Behav 50:779–785. https://doi.org/10.1016/j.yhbeh.2006.07.002
Grafen A (1990) Biological signals as handicaps. J Theor Biol 144:517–546. https://doi.org/10.1016/S0022-5193(05)80088-8
Grino PB, Griffin JE, Wilson JD (1990) Testosterone at high-concentrations interacts with the human androgen receptor similarly to dihydrotestosterone. Endocrinology 126:1165–1172
Groothuis T, Meeuwissen G (1992) The influence of testosterone on the development and fixation of the form of displays in two age classes of young black-headed gulls. Anim Behav 43:189–208. https://doi.org/10.1016/S0003-3472(05)80215-5
Gulledge CC, Deviche P (1998) Photoperiod and testosterone independently affect vocal control region volumes in adolescent male songbirds. J Neurobiol 36:550–558. https://doi.org/10.1002/(Sici)1097-4695(19980915)36:4/550:Aid-Neu8/3.0.Co;2-V
Gwinner E, Rödl T, Schwabl H (1994) Pair territoriality of wintering stonechats - behavior, function and hormones. Behav Ecol Sociobiol 34:321–327. https://doi.org/10.1007/Bf00197002
Hahnloser RHR, Kozhevnikov AA, Fee MS (2002) An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature 419:65–70. https://doi.org/10.1038/nature00974
Hall ZJ, MacDougall-Shackleton SA, Osorio-Beristain M, Murphy TG (2010) Male bias in the song control system despite female bias in song rate in streak-backed orioles (Icterus pustulatus). Brain Behav Evol 76:168–175. https://doi.org/10.1159/000320971
Harding CF, Sheridan K, Walters MJ (1983) Hormonal specificity and activation of sexual-behavior in male zebra finches. Horm Behav 17:111–133. https://doi.org/10.1016/0018-506x(83)90021-1
Harding CF, Walters MJ, Collado D, Sheridan K (1988) Hormonal specificity and activation of social-behavior in male red-winged blackbirds. Horm Behav 22:402–418. https://doi.org/10.1016/0018-506x(88)90011-6
Hartley RS, Suthers RA (1990) Lateralization of syringeal function during song production in the canary. J Neurobiol 21:1236–1248. https://doi.org/10.1002/neu.480210808
Hartog TE et al (2009) Brain-derived neurotrophic factor signaling in the HVC is required for testosterone-induced song of female canaries. J Neurosci 29:15511–15519. https://doi.org/10.1523/JNEUROSCI.2564-09.2009
Hegelbach J, Spaar R (2000) Saisonaler Verlauf der Gesangsaktivität der Singdrossel (Turdus philomelos), mit Anmerkungen zum nachbrutzeitlichen Gesangsschub. J Ornithol 141:425–434. https://doi.org/10.1111/j.1439-0361.2000.00035.pp.x
Heid P, Güttinger HR, Prove E (1985) The influence of castration and testosterone replacement on the song architecture of canaries (Serinus-canaria). Z Tierpsychol 69:224–236
Henry KS, Lucas JR (2009) Vocally correlated seasonal auditory variation in the house sparrow (Passer domesticus). J Exp Biol 212(23):3817–3822. https://doi.org/10.1242/jeb.033035
Hill SD, Amiot C, Ludbrook MR, Ji WH (2015) Seasonal variation in the song structure of Tui (Prosthemadera novaeseelandiae). N Z J Ecol 39:110–115
Hoelzel AR (1986) Song characteristics and response to playback of male and female robins Erithacus-rubecula. Ibis 128:115–127. https://doi.org/10.1111/j.1474-919X.1986.tb02098.x
Howard RD (1974) The influence of sexual selection and interspecific competition on mockingbird song (Mimus polyglottos). Evolution 28:428–438. https://doi.org/10.2307/2407164
Hughes M, Nowicki S, Lohr B (1998) Call learning in black-capped chickadees (Parus atricapillus): the role of experience in the development of ‘chick-a-dee’ calls. Ethology 104:232–249
Hutchison JB, Steimer T (1984) Androgen metabolism in the brain: behavioural correlates. In: de Vries GJ, de Bruin JPC, Uylings HBM, Corner MA (eds) Sex differences in the brain. Elsevier, Amsterdam
Immelmann K (1969) Song development in the zebra finch and other estrildid finches. In: Hinde RA (ed) Bird vocalizations. Cambridge University Press, Cambridge, pp 61–77
Jansen R, Metzdorf R, van der Roest M, Fusani L, ter Maat A, Gahr M (2005) Melatonin affects the temporal organization of the song of the zebra finch. FASEB J 19:848–850. https://doi.org/10.1096/fj.04-2874fje
Jawor JM, MacDougall-Shackleton SA (2008) Seasonal and sex-related variation in song control nuclei in a species with near-monomorphic song, the northern cardinal. Neurosci Lett 443:169–173. https://doi.org/10.1016/j.neulet.2008.07.085
Johnson AL (1990) Steroidogenesis and actions of steroids in the hen ovary. Crit Rev Poult Biol 2:319–346
Johnson F, Bottjer SW (1995) Differential estrogen accumulation among populations of projection neurons in the higher vocal center of male canaries. J Neurobiol 26:87–108. https://doi.org/10.1002/neu.480260108
Johnson F, Rashotte M (2002) Food availability but not cold ambient temperature affects undirected singing in adult male zebra finches. Physiol Behav 76:9–20
Kao MH, Brainard MS (2006) Lesions of an avian basal ganglia circuit prevent context-dependent changes to song variability. J Neurophysiol 96:1441–1455
Kelm-Nelson CA, Stevenson SA, Cordes MA, Riters LV (2013) Modulation of male song by naloxone in the medial preoptic nucleus. Behav Neurosci 127:451–457. https://doi.org/10.1037/a0032329
Kelsey MG (1988) A comparison of the song and territorial behaviour of a long-distance migrant, the Marsh Warbler Acrocephalus palustris, in summer and winter. Ibis 131:403–414
Kern MD, King JR (1972) Testosterone-induced singing in female white-crowned sparrows. Condor 74:204–209. https://doi.org/10.2307/1366289
Ketterson ED, Nolan V, Wolf L, Ziegenfus C (1992) Testosterone and avian life histories - effects of experimentally elevated testosterone on behavior and correlates of fitness in the dark-eyed Junco (Junco hyemalis). Am Nat 140:980–999. https://doi.org/10.1086/285451
Ketterson ED, Nolan V Jr, Sandell M (2005) Testosterone in females: mediator of adaptive traits, constraint on sexual dimorphism, or both? Am Nat 166(Suppl 4):S85–S98. https://doi.org/10.1086/444602
Kim YH, Perlman WR, Arnold AP (2004) Expression of androgen receptor mRNA in zebra finch song system: developmental regulation by estrogen. J Comp Neurol 469:535–547. https://doi.org/10.1002/cne.11033
Konishi M (1965) The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z Tierpsychol 22:770–783
Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J (2017) BDNF: a key factor with mutipotent impact of brain signaling and synaptic plasticity. Cell Mol Neurobiol 38(3):579–593. https://doi.org/10.1007/s10571-017-0510-4
Kreutzer ML (1973) Contribution à l’étude du chant de proclamation territoriale du troglodyte (Troglodytes troglodytes): Stéréotypie, variations géographiques, dialectes, conséquences éthologiques de ces phénomènes. Université Paris V
Kreutzer ML, Vallet EM (1991) Differences in the responses of captive female canaries to variation in conspecific and heterospecific songs. Behaviour 117:106–116. https://doi.org/10.1163/156853991x00148
Kriner E, Schwabl H (1991) Control of winter song and territorial aggression of female robins (Erithacus-rubecula) by testosterone. Ethology 87:37–44
Kroodsma DE (1985) Development and use of two song forms by the eastern Phoebe. Wilson Bull 97:21–29
Kroodsma DE, Byers BE (1991) The function(s) of bird song. Am Zool 31:318–328
Kunc HP, Foerster K, Vermeirssen ELM, Kempenaers B (2006) Experimentally elevated plasma testosterone levels do not influence singing behaviour of male blue tits (Parus caeruleus) during the early breeding season. Ethology 112:984–992. https://doi.org/10.1111/j.1439-0310.2006.01254.x
Kurvers RHJM, Roberts ML, McWilliams SR, Peters A (2008) Experimental manipulation of testosterone and condition during molt affects activity and vocalizations of male blue tits. Horm Behav 54:263–269. https://doi.org/10.1016/j.yhbeh.2008.03.011
Lack DF (1943) The life of the robin. Witherby HFG, London
Lampe HM (1991) The response of male redwings Turdus-iliacus to playback of conspecific songs with or without the terminating Twitter. Ornis Scand 22:137–142. https://doi.org/10.2307/3676544
Langmore NE (1998) Functions of duet and solo songs of female birds. Trends Ecol Evol 13:136–140
Langmore NE, Davies NB (1997) Female dunnocks use vocalizations to compete for males. Anim Behav 53:881–890. https://doi.org/10.1006/anbe.1996.0306
Langmore NE, Davies NB, Hatchwell BJ, Hartley IR (1996) Female song attracts males in the alpine accentor Prunella collaris. Proc Roy Soc B 263:141–146. https://doi.org/10.1098/rspb.1996.0022
Langmore NE, Cockrem JF, Candy EJ (2002) Competition for male reproductive investment elevates testosterone levels in female dunnocks, Prunella modularis. Proc Roy Soc B 269:2473–2478. https://doi.org/10.1098/rspb.2002.2167
Laskey AR (1944) A study of the cardinal in Tennessee. Wilson Bull 56:27–44
Leitner S, Cornelia V, Gahr M (2001a) Seasonal changes in the song pattern of the non-domesticated Island Canary (Serinus canaria), a field study. Behaviour 138:885–904
Leitner S, Voigt C, Garcia-Segura LM, Van’t Hof T, Gahr M (2001b) Seasonal activation and inactivation of song motor memories in wild canaries is not reflected in neuroanatomical changes of forebrain song areas. Horm Behav 40:160–168. https://doi.org/10.1006/hbeh.2001.1700
Leonard SL (1939) Induction of singing in female canaries by injections of male hormone. Proc Soc Exp Biol Med 41:229–230
Li R, Sakaguchi H (1997) Cholinergic innervation of the song control nuclei by the ventral paleostriatum in the zebra finch: a double-labeling study with retrograde fluorescent tracers and choline acetyltransferase immunohistochemistry. Brain Res 763(2):239–246
Lister D (1953a) Secondary song: a tentative classification. Br Birds 46:139–143
Lister D (1953b) Secondary song of some Indian birds Bombay. Nat Hist Soc 51:699–706
Logan CA (1983) Biological diversity in avian vocal learning. In: Zeiler MH (ed) Advances in the analysis of behaviour, vol 3. Wiley, Sussex, pp 143–176
Logan CA, Wingfield JC (1995) Hormonal correlates of breeding status, nest construction, and parental care in multiple-brooded northern mockingbirds, Mimus polyglottos. Horm Behav 29:12–30. https://doi.org/10.1006/hbeh.1995.1002
Louissaint A Jr, Rao S, Leventhal C, Goldman SA (2002) Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron 34:945–960
Lovell PV et al (2014) Conserved syntenic clusters of protein coding genes are missing in birds. Genome Biol 15:565. https://doi.org/10.1186/s13059-014-0565-1
Maddison CJ, Anderson RC, Prior NH, Taves MD, Soma KK (2012) Soft song during aggressive interactions: seasonal changes and endocrine correlates in song sparrows. Horm Behav 62:455–463. https://doi.org/10.1016/j.yhbeh.2012.08.002
Malacarne G, Cucco M, Camanni S (1991) Coordinated visual-displays and vocal duetting in different ecological situations among Western Palearctic non-passerine birds. Ethol Ecol Evol 3:207–219. https://doi.org/10.1080/08927014.1991.9525369
Maney DL, Lange HS, Raees MQ, Reid AE, Sanford SE (2009) Behavioral phenotypes persist after gonadal steroid manipulation in white-throated sparrows. Horm Behav 55:113–120. https://doi.org/10.1016/j.yhbeh.2008.09.002
Marler P, Kreith M, Willis E (1962) An analysis of testosterone-induced crowing in young domestic cockerels. Anim Behav 10:48–54. https://doi.org/10.1016/0003-3472(62)90130-6
Meitzen J, Moore IT, Lent K, Brenowitz EA, Perkel DJ (2007a) Steroid hormones act transsynaptically within the forebrain to regulate neuronal phenotype and song stereotypy. J Neurosci 27:12045–12057. https://doi.org/10.1523/Jneurosci.3289-07.2007
Meitzen J, Perkel DJ, Brenowitz EA (2007b) Seasonal changes in intrinsic electrophysiological activity of song control neurons in wild song sparrows. J Comp Physiol A 193:677–683. https://doi.org/10.1007/s00359-007-0222-1
Meitzen J, Weaver AL, Brenowitz EA, Perkel DJ (2009) Plastic and stable electrophysiological properties of adult avian forebrain song-control neurons across changing breeding conditions. J Neurosci 29:6558–6567. https://doi.org/10.1523/jneurosci.5571-08.2009
Merullo DP, Angyal CS, Stevenson SA, Riters LV (2016) Song in an affiliative context relates to the neural expression of dopamine- and neurotensin-related genes in male European starlings. Brain Behav Evol 88:81–92. https://doi.org/10.1159/000448191
Metzdorf R, Gahr M, Fusani L (1999) Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J Comp Neurol 407:115–129
Morris D (1954) The reproductive behaviour of the zebra finch (Poephila guttata), with special reference to pseudofemale behaviour and displacement activities. Behaviour 6:271–322
Morton ES (2000) An evolutionary view of the origins and functions of avian vocal communication. Jpn J Ornitho 49:69–78. https://doi.org/10.3838/jjo.49.69
Mulligan JA, Olson KC (1969) Communication in canary courtship calls. In: Hinde RA (ed) Bird vocalisations. Cambridge University Press, London, UK, pp 165–184
Nealen PM (2005) An interspecific comparison using immunofluorescence reveals that synapse density in the avian song system is related to sex but not to male song repertoire size. Brain Res 1032:50–62. https://doi.org/10.1016/j.brainres.2004.10.059
Nespor AA, Lukazewicz MJ, Dooling RJ, Ball GF (1996) Testosterone induction of male-like vocalizations in female budgerigars (Melopsittacus undulatus). Horm Behav 30:162–169. https://doi.org/10.1006/hbeh.1996.0020
Nicholson EM, Koch L (1936) Songs of wild birds. H.F. & G. Witherby, London
Noble GK, Wurm M (1940) The effect of testosterone propionate on the black-crowned night heron. Endocrinology 26:837–850
Nordby JC, Campbell SE, Beecher MD (2001) Late song learning in song sparrows. Anim Behav 61:835–846. https://doi.org/10.1006/anbe.2000.1673
Nottebohm F (1980) Testosterone triggers growth of brain vocal control nuclei in adult female canaries. Brain Res 189:429–436. https://doi.org/10.1016/0006-8993(80)90102-X
Nottebohm F (1981) A brain for all seasons: cyclical anatomical changes in song control nuclei of the canary brain. Science 214:1368–1370
Nottebohm F, Arnold AP (1976) Sexual dimorphism in vocal control areas of the songbird brain. Science 194:211–213
Nottebohm F, Nottebohm ME (1978) Relationship between song repertoire and age in the canary, Serinus canarius. Z Tierpsychol 46:298–305. https://doi.org/10.1111/j.1439-0310.1978.tb01451.x
Nottebohm F, Stokes TM, Leonard CM (1976) Central control of song in the canary, Serinus canarius. J Comp Neurol 165:457–486. https://doi.org/10.1002/cne.901650405
Nottebohm F, Nottebohm ME, Crane L (1986) Developmental and seasonal changes in canary song and their relation to changes in the anatomy of song-control nuclei. Behav Neural Biol 46:445–471
Nowicki S, Ball GF (1989) Testosterone induction of song in photosensitive and photorefractory male sparrows. Horm Behav 23:514–525. https://doi.org/10.1016/0018-506x(89)90039-1
O’Loghlen AL, Rothstein SI (2002) Ecological effects on song learning: delayed development is widespread in wild populations of brown-headed cowbirds. Anim Behav 63:475–486. https://doi.org/10.1006/anbe.2001.1951
Odom KJ, Hall ML, Riebel K, Omland KE, Langmore NE (2014) Female song is widespread and ancestral in songbirds. Nat Commun 5:3379. https://doi.org/10.1038/ncomms4379
Ota N, Gahr M, Soma M (2015) Tap dancing birds: the multimodal mutual courtship display of males and females in a socially monogamous songbird. Sci Rep 5:16614. https://doi.org/10.1038/srep16614
Pavlova DZ, Pinxten R, Eens M (2007) Seasonal singing patterns and individual consistency in song activity in female European starlings (Sturnus vulgaris). Behaviour 144:663–680. https://doi.org/10.1163/156853907781347835
Pesch A, Güttinger H-R (1985) Der Gesang des weiblichen Kanarienvogels. J Ornithol 126:108–110. https://doi.org/10.1007/BF01640450
Petkov CI, Jarvis ED (2012) Birds, primates, and spoken language origins: behavioral phenotypes and neurobiological substrates. Front Evol Neurosci 4:12. https://doi.org/10.3389/fnevo.2012.00012
Phillmore LS, Hoshooley JS, Sherry DF, MacDougall-Shackleton SA (2006) Annual cycle of the black-capped chickadee: seasonality of singing rates and vocal-control brain regions. J Neurobiol 66:1002–1010. https://doi.org/10.1002/neu.20282
Pintér O, Péczely P, Zsebök S, Zelena D (2011) Seasonal changes in courtship behavior, plasma androgen levels and in hypothalamic aromatase immunoreactivity in male free-living European starlings (Sturnus vulgaris). Gen Comp Endocrinol 172:151–157. https://doi.org/10.1016/j.ygcen.2011.02.002
Pinxten R, De Ridder E, Balthazart J, Eens M (2002) Context-dependent effects of castration and testosterone treatment on song in male European starlings. Horm Behav 42:307–318. https://doi.org/10.1006/hbeh.2002.1824
Podos J, Huber SK, Taft B (2004) Bird song: the interface of evolution and mechanism. Annu Rev Ecol Evol Syst 35:55–87. https://doi.org/10.1146/annurev.ecolsys.35.021103.105719
Poulsen H (1951) Inheritance and learning in the song of the chaffinch (Fringilla-coelebs L). Behaviour 3:216–228. https://doi.org/10.1163/156853951x00278
Price JJ, Yunes-Jiménez L, Osorio-Beristain M, Omland KE, Murphy TG (2008) Sex-role reversal in song? Females sing more frequently than males in the streak-backed oriole. Condor 110:387–392. https://doi.org/10.1525/cond.2008.8430
Pröve E (1974) Der Einfluß von Kastration und Testosteronsubstitution auf das Sexualverhalten männlicher Zebrafinken (Taeniopygia guttata castanotis Gould). J Ornithol 115:338–347. https://doi.org/10.1007/BF01644328
Quispe Valdez R, Trappschuh M, Gahr M, Goymann W (2015) Towards more physiological manipulations of hormones in field studies: comparing the release dynamics of three kinds of testosterone implants, silastic tubing, time-release pellets and beeswax. Gen Comp Endocrinol 212:100–105. https://doi.org/10.1016/j.ygcen.2015.01.007
Quispe Valdez R, Sebe F, da Silva ML, Gahr M (2016) Dawn-song onset coincides with increased HVC androgen receptor expression but is decoupled from high circulating testosterone in an equatorial songbird. Physiol Behav 163:334–334. (vol 156, p 1, 2016)
Quispe Valdez R, Brazao Protazio JM, Gahr M (2017) Seasonal singing of a songbird living near the equator correlates with minimal changes in day length. Sci Rep 7:9140. https://doi.org/10.1038/s41598-017-08800-6
Rashotte ME, Sedunova EV, Johnson F, Pastukhov IF (2001) Influence of food and water availability on undirected singing and energetic status in adult male zebra finches (Taeniopygia guttata). Physiol Behav 74:533–541. https://doi.org/10.1016/S0031-9384(01)00600-X
Reeves BJ, Beecher MD, Brenowitz EA (2003) Seasonal changes in avian song control circuits do not cause seasonal changes in song discrimination in song sparrows. J Neurobiol 57:119–129. https://doi.org/10.1002/neu.10257
Ritchison G (1983) The function of singing in female black-headed grosbeaks (Pheucticus-melanocephalus). Family-group maintenance. Auk 100:105–116
Ritchison G (1986) The singing behavior of female northern cardinal. Condor 88:156–159
Riters LV (2012) The role of motivation and reward neural systems in vocal communication in songbirds. Front Neuroendocrinol 33:194–209. https://doi.org/10.1016/j.yfrne.2012.04.002
Riters LV, Ball GF (1999) Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris). Horm Behav 36:276–286. https://doi.org/10.1006/hbeh.1999.1549
Riters LV, Ball GF (2002) Sex differences in the densities of alpha 2-adrenergic receptors in the song control system, but not the medial preoptic nucleus in zebra finches. J Chem Neuroanat 23:269–277
Ritschard M, Laucht S, Dale J, Brumm H (2011) Enhanced testosterone levels affect singing motivation but not song structure and amplitude in Bengalese finches. Physiol Behav 102:30–35. https://doi.org/10.1016/j.physbeh.2010.10.005
Robertson BA, Fontaine JJ, Loomis E (2009) Seasonal patterns of song structure variation in a suboscine passerine. Wilson J Ornithol 121:815–818. https://doi.org/10.1676/08-155.1
Robinson FE et al (1988) Steroidogenic relationships of gonadotrophin hormones in the ovary of the hen (Gallus domesticus). Gen Comp Endocrinol 69:455–466
Röhss M, Silverin B (1983) Seasonal-variations in the ultrastructure of the Leydig-cells and plasma-levels of luteinizing-hormone and steroid-hormones in juvenile and adult male great tits Parus major. Ornis Scand 14:202–212. https://doi.org/10.2307/3676154
Rost R (1990) Hormones and behavior - a joint examination of studies on seasonal-variation in song production and plasma-levels of testosterone in the great tit Parus major. J Fur Ornithol 131:403–411. https://doi.org/10.1007/Bf01639816
Rost R (1992) Hormones and behavior - a comparison of studies on seasonal-changes in song production and testosterone plasma-levels in the willow-tit Parus montanus. Ornis Fennica 69:1–6
Rowe L, Houle D (1996) The lek paradox and the capture of genetic variance by condition dependent traits. Proc Roy Soc B 263:1415–1421. https://doi.org/10.1098/rspb.1996.0207
Rybak F, Gahr M (2004) Modulation by steroid hormones of a “sexy” acoustic signal in an Oscine species, the common canary Serinus canaria. Acad Bras Cienc 76:365–367. https://doi.org/10.1590/S0001-37652004000200026
Saino N, Moller AP (1995) Testosterone correlates of mate guarding, singing and aggressive-behavior in male barn swallows, Hirundo rustica. Anim Behav 49:465–472. https://doi.org/10.1006/anbe.1995.0060
Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA (2000) Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol 423:619–630. https://doi.org/10.1002/1096-9861(20000807)423:4/619:Aid-Cne7/3.0.Co;2-U
Sandell MI, Smith HG (1997) Female aggression in the European starling during the breeding season. Anim Behav 53:13–23. https://doi.org/10.1006/anbe.1996.0274
Saunders AA (1947) The seasons of bird song the beginning of song in spring. Auk 64:97–107. https://doi.org/10.2307/4080066
Saunders AA (1948) The seasons of bird song: the cessation of song after the nesting season. Auk 65:19–30. https://doi.org/10.2307/4080221
Scharfman HE, MacLusky NJ (2005) Similarities between actions of estrogen and BDNF in the hippocampus: coincidence or clue? Trends Neurosci 28:79–85. https://doi.org/10.1016/j.tins.2004.12.005
Schlinger BA, Arnold AP (1991) Brain is the major site of estrogen synthesis in a male songbird. Proc Natl Acad Sci USA 88:4191–4194. https://doi.org/10.1073/pnas.88.10.4191
Schlinger BA, Remage-Healey L (2012) Neurosteroidogenesis: insights from studies of songbirds. J Neuroendocrinol 24:16–21. https://doi.org/10.1111/j.1365-2826.2011.02150.x
Schwabl H (1992) Winter and breeding territorial behavior and levels of reproductive hormones of migratory European robins. Ornis Scand 23:271–276. https://doi.org/10.2307/3676649
Schwabl H, Kriner E (1991) Territorial aggression and song of male European robins (Erithacus Rubecula) in autumn and spring - effects of antiandrogen treatment. Horm Behav 25:180–194. https://doi.org/10.1016/0018-506x(91)90049-N
Schwabl H, Wingfield JC, Farner DS (1980) Seasonal variation in plasma levels of luteinizing hormone and steroid hormones in the European blackbird (Turdus merula). Vogelwarte - Zeitschrift für Vogelkunde 30:283–294
Searcy WA, Andersson M (1986) Sexual selection and the evolution of song. Annu Rev Ecol Syst 17:507–533. https://doi.org/10.1146/annurev.es.17.110186.002451
Senft RA, Meddle SL, Baugh AT (2016) Distribution and abundance of glucocorticoid and mineralocorticoid receptors throughout the brain of the great tit (Parus major). PLoS One 11:e0148516. https://doi.org/10.1371/journal.pone.0148516
Shoemaker HH (1939) Effect of testosterone propionate on behavior of the female canary. Proc Soc Exp Biol Med 41:299–302. https://doi.org/10.3181/00379727-41-10652
Silverin B (1980) Effects of long-acting testosterone treatment on freeliving pied flycatchers, Ficedula hypoleuca, during the breeding period. Anim Behav 28:906–912. https://doi.org/10.1016/S0003-3472(80)80152-7
Silverin B, Viebke PA, Westin J (1986) Seasonal-changes in plasma-levels of Lh and gonadal-steroids in free-living willow tits Parus montanus. Ornis Scand 17:230–236. https://doi.org/10.2307/3676831
Slater PJB, Mann NI (2004) Why do the females of many bird species sing in the tropics? J Avian Biol 35:289–294. https://doi.org/10.1111/j.0908-8857.2004.03392.x
Smith GT, Brenowitz EA, Beecher MD, Wingfield JC (1997) Seasonal changes in testosterone, neural attributes of song control nuclei, and song structure in wild songbirds. J Neurosci 17:6001–6010
Snow DW (1988) Prunella modularis dunnock - social pattern and behaviour; voice. In: Cramp S (ed) Handbook of the birds of Europe, the Middle East and North Africa: the birds of the Western Palearctic, vol 5. Oxford University Press, New York, pp 553–556
Soma KK, Hartman VN, Wingfield JC, Brenowitz EA (1999) Seasonal changes in androgen receptor immunoreactivity in the song nucleus HVC of a wild bird. J Comp Neurol 409:224–236
Soma KK, Wissman AM, Brenowitz EA, Wingfield JC (2002) Dehydroepiandrosterone (DHEA) increases territorial song and the size of an associated brain region in a male songbird. Horm Behav 41:203–212. https://doi.org/10.1006/hbeh.2001.1750
Soma KK, Tramontin AD, Featherstone J, Brenowitz EA (2004) Estrogen contributes to seasonal plasticity of the adult avian song control system. J Neurobiol 58:413–422. https://doi.org/10.1002/neu.10288
Sorensen MC (2014) Singing in Africa: no evidence for a long supposed function of winter song in a migratory songbird. Behav Ecol 25:909–915. https://doi.org/10.1093/beheco/aru058
Sorensen MC, Jenni-Eiermann S, Spottiswoode CN (2016) Why do migratory birds sing on their tropical wintering grounds? Am Nat 187:E65–E76. https://doi.org/10.1086/684681
Sossinka R, Böhner J (1980) Song types in the zebra finch poephila guttata castanotis. Z Tierpsychol 53:123–132
Stouffer PC, Johnson EI, Bierregaard RO (2013) Breeding seasonality in central Amazonian rainforest birds. Auk 130:529–540. https://doi.org/10.1525/auk.2013.12179
Strand CR, Ross MS, Weiss SL, Deviche P (2008) Testosterone and social context affect singing behavior but not song control region volumes in adult male songbirds in the fall. Behav Process 78:29–37. https://doi.org/10.1016/j.beproc.2007.12.002
Suzuki K, Matsunaga E, Kobayashi T, Okanoya K (2011) Expression patterns of mineralocorticoid and glucocorticoid receptors in Bengalese finch (Lonchura Striata Var. Domestica) brain suggest a relationship between stress hormones and song-system development. Neuroscience 194:72–83. https://doi.org/10.1016/j.neuroscience.2011.07.073
Ter Maat A, Trost L, Sagunsky H, Seltmann S, Gahr M (2014) Zebra finch mates use their forebrain song system in unlearned call communication. PLoS One 9:e109334. https://doi.org/10.1371/journal.pone.0109334
Terkel AS, Moore CL, Beer CG (1976) The effects of testosterone and estrogen on the rate of long-calling vocalization in juvenile laughing gulls, Larus atricilla. Horm Behav 7:49–57
Thompson CK et al (2012) Seasonal changes in patterns of gene expression in avian song control brain regions. PLoS One 7:e35119. https://doi.org/10.1371/journal.pone.0035119
Thorpe WH (1958) The learning of song patterns by birds, with especial reference to the song of the chaffinch fringilla coelebs. Ibis 100:535–570. https://doi.org/10.1111/j.1474919X.1958.tb07960.x
Thorpe WH (1961) Bird song: the biology of vocal communication and expression in birds. Cambridge University Press, London
Thorpe WH, Pilcher M (1958) The nature and characteristics of subsong. Br Birds 51:509–514
Titus R (1998) Short-range and long-range songs: use of two acoustically distinct song classes by dark-eyed juncos (Junvo hyemalis). Auk 115:386–393
Tramontin AD, Brenowitz EA (2000) Seasonal plasticity in the adult brain. Trends Neurosci 23:251–258. https://doi.org/10.1016/S0166-2236(00)01558-7
Tramontin AD, Wingfield JC, Brenowitz EA (1999) Contributions of social cues and photoperiod to seasonal plasticity in the adult avian song control system. J Neurosci 19:476–483
Tramontin AD, Wingfield JC, Brenowitz EA (2003) Androgens and estrogens induce seasonal-like growth of song nuclei in the adult songbird brain. J Neurobiol 57:130–140. https://doi.org/10.1002/neu.10263
Tremain SB, Swiston KA, Mennill DJ (2008) Seasonal variation in acoustic signals of pileated woodpeckers. Wilson J Ornithol 120:499–504
Vallet E, Kreutzer M, Gahr M (1996) Testosterone induces sexual release quality in the song of female canaries. Ethology 102:617–628
Van Duyse E, Pinxten R, Eens M (2003) Seasonal fluctuations in plasma testosterone levels and diurnal song activity in free-living male great tits. Gen Comp Endocrinol 134:1–9. https://doi.org/10.1016/S0016-6480(03)00213-2
Van Duyse E, Pinxten R, Snoeijs T, Eens M (2005) Simultaneous treatment with an aromatase inhibitor and an anti-androgen decreases the likelihood of dawn song in free-living male great tits, Parus major. Horm Behav 48:243–251. https://doi.org/10.1016/j.yhbeh.2005.02.013
Van Hout AJM, Eens M, Balthazart J, Pinxten R (2009) Complex modulation of singing behavior by testosterone in an open-ended learner, the European starling. Horm Behav 56:564–573. https://doi.org/10.1016/j.yhbeh.2009.09.010
Van Hout AJ, Pinxten R, Darras VM, Eens M (2012) Testosterone increases repertoire size in an open-ended learner: an experimental study using adult male European starlings (Sturnus vulgaris). Horm Behav 62:563–568. https://doi.org/10.1016/j.yhbeh.2012.09.008
Van Roo BL (2004) Exogenous testosterone inhibits several forms of male parental behavior and stimulates song in a monogamous songbird: the blue-headed vireo (Vireo solitarius). Horm Behav 46:678–683. https://doi.org/10.1016/j.yhbeh.2004.06.011
Velez A, Gall MD, Lucas JR (2015) Seasonal plasticity in auditory processing of the envelope and temporal fine structure of sounds in three songbirds. Anim Behav 103:53–63. https://doi.org/10.1016/j.anbehav.2015.01.036
Voigt C, Gahr M (2011) Social status affects the degree of sex difference in the songbird brain. PLoS One 6:e20723. https://doi.org/10.1371/journal.pone.0020723
Voigt C, Leitner S (2008) Seasonality in song behaviour revisited: seasonal and annual variants and invariants in the song of the domesticated canary (Serinus canaria). Horm Behav 54:373–378. https://doi.org/10.1016/j.yhbeh.2008.05.001
Voigt C, Leitner S (2013) Testosterone-dependency of male solo song in a duetting songbird - evidence from females. Horm Behav 63:122–127. https://doi.org/10.1016/j.yhbeh.2012.10.006
Voigt C, Metzdorf R, Gahr M (2004) Differential expression pattern and steroid hormone sensitivity of SNAP-25 and synaptoporin mRNA in the telencephalic song control nucleus HVC of the zebra finch. J Comp Neurol 475:83–94. https://doi.org/10.1002/cne.20151
Voigt C, Leitner S, Gahr M (2006) Repertoire and structure of duet and solo songs in cooperatively breeding white-browed sparrow weavers. Behaviour 143:159–182. https://doi.org/10.1163/156853906775900739
Wada K et al (2013) Differential androgen receptor expression and DNA methylation state in striatum song nucleus area X between wild and domesticated songbird strains. Eur J Neurosci 38:2600–2610. https://doi.org/10.1111/ejn.12258
Walters MJ, Collado D, Harding CF (1991) Oestrogenic modulation of singing in male zebra finches: differential effects on directed and undirected songs. Anim Behav 42:445–452. https://doi.org/10.1016/S0003-3472(05)80043-0
Warren WC et al (2010) The genome of a songbird. Nature 464:757–762. https://doi.org/10.1038/nature08819
Weatherhead PJ, Metz KJ, Bennett GF, Irwin RE (1993) Parasite faunas, testosterone and secondary sexual traits in male red-winged blackbirds. Behav Ecol Sociobiol 33:13–23. https://doi.org/10.1007/Bf00164342
Wegrzyn E, Leniowski K (2009) Syllable sharing and changes in syllable repertoire size and composition within and between years in the great reed warbler, Acrocephalus arundinaceus. J Ornithol 151:255–267
Wickler W, Seibt U (1980) Vocal dueting and the pair bond. Z Tierpsychol 52:217–226. https://doi.org/10.1111/j.1439-0310.1980.tb00713.x
Wild JM (1997) Neural pathways for the control of birdsong production. J Neurobiol 33:653–670. https://doi.org/10.1002/(Sici)1097-4695(19971105)33:5/653:Aid-Neu11/3.0.Co;2-A
Wild JM (2017) The ventromedial hypothalamic nucleus in the zebra finch (Taeniopygia guttata): afferent and efferent projections in relation to the control of reproductive behavior. J Comp Neurol 525:2657–2676. https://doi.org/10.1002/cne.24225
Wingfield JC (1994) Regulation of territorial behavior in the sedentary song sparrow, Melospiza melodia morphna. Horm Behav 28:1–15. https://doi.org/10.1006/hbeh.1994.1001
Wingfield JC, Farner DS (1975) The determination of five steroids in avian plasma by radioimmunoassay and competitive protein-binding. Steroids 26:311–321
Wingfield JC, Farner DS (1978) The annual cycle in plasma irLH and steroid hormones in feral populations of the white-crowned sparrow, Zonotrichia leucophrys gambelii. Biol Reprod 19:1046–1056
Wingfield JC, Hahn TP (1994) Testosterone and territorial behaviour in sedentary and migratory sparrows. Anim Behav 47:77–89. https://doi.org/10.1006/anbe.1994.1009
Wingfield JC, Hegner RE, Lewis DM (1991) Circulating levels of luteinizing-hormone and steroid-hormones in relation to social-status in the cooperatively breeding white-browed sparrow Weaver, Plocepasser Mahali. J Zool 225:43–58
Yasukawa K, Boley RA, Simon SE (1987) Seasonal change in the vocal behavior of female red-winged blackbirds, Agelaius phoeniceus. Anim Behav 35:1416–1423. https://doi.org/10.1016/S0003-3472(87)80014-3
York JE, Radford AN, de Vries B, Groothuis TG, Young AJ (2016) Dominance-related seasonal song production is unrelated to circulating testosterone in a subtropical songbird. Gen Comp Endocrinol 233:43–52. https://doi.org/10.1016/j.ygcen.2016.05.011
Zann RA (1996) The zebra finch. Oxford University Press, Oxford
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gahr, M. (2020). Seasonal Hormone Fluctuations and Song Structure of Birds. In: Aubin, T., Mathevon, N. (eds) Coding Strategies in Vertebrate Acoustic Communication. Animal Signals and Communication, vol 7. Springer, Cham. https://doi.org/10.1007/978-3-030-39200-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-39200-0_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-39199-7
Online ISBN: 978-3-030-39200-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)