Abstract
The past two decades have marked the beginning of an unprecedented success story for cancer therapy through redirecting antitumor immunity [1]. While the mechanisms that control the initial and ongoing immune responses against tumors remain a strong research focus, the clinical development of technologies that engage the immune system to target and kill cancer cells has become a translational research priority. Early attempts documented in the late 1800s aimed at sparking immunity with cancer vaccines were difficult to interpret but demonstrated an opportunity that more than 100 years later has blossomed into the current field of cancer immunotherapy. Perhaps the most recent and greatest illustration of this is the widespread appreciation that tumors actively shut down antitumor immunity, which has led to the emergence of checkpoint pathway inhibitors that re-invigorate the body’s own immune system to target cancer [2, 3]. This class of drugs, with first FDA approvals in 2011, has demonstrated impressive durable clinical responses in several cancer types, including melanoma, lung cancer, Hodgkin’s lymphoma, and renal cell carcinoma, with the ongoing investigation in others. The biology and ultimate therapeutic successes of these drugs led to the 2018 Nobel Prize in Physiology or Medicine, awarded to Dr. James Allison and Dr. Tasuku Honjo for their contributions to cancer therapy [4]. In parallel to the emerging science that aided in unleashing the body’s own antitumor immunity with checkpoint pathway inhibitors, researchers were also identifying ways to re-engineer antitumor immunity through adoptive cellular immunotherapy approaches. Chimeric antigen receptor (CAR)-based T cell therapy has achieved an early head start in the field, with two recent FDA approvals in 2017 for the treatment of B-cell malignancies [5]. There is an explosion of preclinical and clinical efforts to expand the therapeutic indications for CAR T cell therapies, with a specific focus on improving their clinical utility, particularly for the treatment of solid tumors. In this chapter, we will highlight the recent progress, challenges, and future perspectives surrounding the development of CAR T cell therapies for solid tumors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction to Immunotherapy
The past two decades have marked the beginning of an unprecedented success story for cancer therapy through redirecting antitumor immunity [1]. While the mechanisms that control the initial and ongoing immune responses against tumors remain a strong research focus, the clinical development of technologies that engage the immune system to target and kill cancer cells has become a translational research priority. Early attempts documented in the late 1800s aimed at sparking immunity with cancer vaccines were difficult to interpret but demonstrated an opportunity that more than 100 years later has blossomed into the current field of cancer immunotherapy. Perhaps the most recent and greatest illustration of this is the widespread appreciation that tumors actively shut down antitumor immunity, which has led to the emergence of checkpoint pathway inhibitors that re-invigorate the body’s own immune system to target cancer [2, 3]. This class of drugs, with first FDA approvals in 2011, has demonstrated impressive durable clinical responses in several cancer types, including melanoma, lung cancer, Hodgkin’s lymphoma, and renal cell carcinoma, with the ongoing investigation in others. The biology and ultimate therapeutic successes of these drugs led to the 2018 Nobel Prize in Physiology or Medicine, awarded to Dr. James Allison and Dr. Tasuku Honjo for their contributions to cancer therapy [4]. In parallel to the emerging science that aided in unleashing the body’s own antitumor immunity with checkpoint pathway inhibitors, researchers were also identifying ways to re-engineer antitumor immunity through adoptive cellular immunotherapy approaches. Chimeric antigen receptor (CAR)-based T cell therapy has achieved an early head start in the field, with two recent FDA approvals in 2017 for the treatment of B-cell malignancies [5]. There is an explosion of preclinical and clinical efforts to expand the therapeutic indications for CAR T cell therapies, with a specific focus on improving their clinical utility, particularly for the treatment of solid tumors. In this chapter, we will highlight the recent progress, challenges, and future perspectives surrounding the development of CAR T cell therapies for solid tumors.
2 CAR T Cell Therapy
The development of effective CAR T cell therapies for any cancer type lies in several key variables [6]—(1) design of CAR constructs, (2) manufacturing processes that lead to the final therapeutic product, and (3) clinical study design to comprehensively assess safety and efficacy of CAR T cell therapies and combinatorial immunotherapy strategies. We will summarize key findings in these areas that have contributed to the successes in CAR T cells in treating hematological malignancies to date, as well as address many of the challenges facing CAR T cell therapy for treating solid tumors.
2.1 CAR Design
CARs are modular synthetic receptors that redirect antigen specificity of T cells to cell surface targets expressed by tumor cells, thereby eliciting a potent T cell functional output primarily through cytolytic activity and production of inflammatory cytokines. CARs consist of four major components: the antigen-binding domain, the extracellular spacer domain, the transmembrane domain, and the intracellular signaling region consisting of co-stimulatory and CD3ζ cytolytic domains (Fig. 11.1). Observations from engineering these components were recently reviewed extensively [7]. Here, we will summarize major findings that contribute to the current convention for designing new CARs.
Illustrations of a T cell receptor (TCR) and a chimeric antigen receptor (CAR). a TCR complex on the surface of a T cell, composed of six subunits including TCR alpha (α) and beta (β), a homodimer of CD3 zeta (ζ), and dimers of CD3 epsilon (ε) with either CD3 gamma (γ) or CD3 delta (δ). b CAR construct on the surface of a T cell, composed of an antigen-binding domain (e.g., a single-chain variable fragment, or scFv), an extracellular spacer domain (e.g., an IgG4 Fc molecule), a transmembrane domain, an intracellular co-stimulatory domain, and an intracellular CD3ζ cytolytic domain
The antigen-binding domain confers target specificity to the CAR. These domains are often derived from the variable regions of monoclonal antibodies termed single-chain variable fragments (scFv), although other targeting moieties have been described, including but not limited to: natural or engineered receptor ligands [8, 9], receptor extracellular domains [10, 11], and engineered non-immunoglobulin binding proteins [12, 13]. The majority of solid tumor targets evaluated to date are also expressed on normal tissue at various levels, which raises toxicity concerns with “on-target off-tumor” targeting of normal tissue [14]. Therefore, the optimization of CAR selectivity and potency has been heavily studied by modulating properties of the antigen-binding domain. For instance, fine-tuning scFv affinity has been impactful in setting antigen expression thresholds required for CAR activation [15,16,17,18]. One potential avenue for decreasing toxicity concerns is reducing the affinity of the antigen-binding domain. This may increase the requirement for higher antigen density on tumor cells for optimal activation of CAR T cells, and therefore, bypass targeting of antigen-low healthy tissue. This rationale was explored by Liu and colleagues, who generated HER2-specific CARs targeting solid tumors with a 4-log range of binding affinities [15]. This study observed that the threshold for antigen density that results in CAR activation correlates with antigen-binding domain affinity. Antigen-binding domains with low nanomolar and sub-nanomolar affinity mediated T cell activation against all HER2+ cell lines tested, whereas antigen-binding domains with micromolar affinity were much more selective for tumors with higher levels of HER2 expression. This observation was confirmed using EGFR-specific CAR T cells, as both HER2 and EGFR are expressed at lower levels on several critical normal tissues. Other studies have made similar observations correlating decreased CAR binding affinity and improved selectivity for high target antigen expression [16, 17]. However, in each case, decreased affinity also correlated with lower CAR T cell-mediated cytokine secretion, even with high target antigen expression. Thus, the interplay of binding affinity, selectivity for disease-specific target antigen density, and functional activation of CAR T cells must be carefully considered when designing new CARs.
The extracellular spacer domain provides an extension from the T cell membrane and flexibility to allow the antigen-binding domain to optimally access the targeted epitope. The selected spacer can impact CAR expression, flexibility, epitope accessibility, and strength of activation outputs [19, 20], which ultimately affects CAR functionality. Most often, extracellular spacer domains are derived from natural molecules. Common examples include CD8α hinge, CD28 hinge, and IgG hinge and Fc regions. The proper spacer length for a particular binding domain—antigen pair is often empirically determined and likely depends on the target epitope location and relative level of steric hindrances present on the target cell. Notable examples of CARs requiring short spacers (CD19, CEA) [21] and long spacers (MUC1, membrane-proximal epitopes of ROR1) [19, 22] for optimal activity exist in literature. In some contexts, extracellular spacer domains can also mediate undesired effects, including antigen-independent tonic signaling [23] and interaction of IgG-derived spacers with FcγR-expressing cells [23, 24]. Importantly, these effects can be abrogated by either selecting different spacer domains or by further engineering of the spacer based on structural or functional considerations.
The transmembrane domain serves to anchor the CAR to the T cell membrane. Like the extracellular spacer domains, transmembrane domains are derived from natural proteins, with the most common versions including CD4, CD8, and CD28. The impact of the transmembrane domain on CAR activity is not well-studied as this domain is often changed as required by either the extracellular spacer domain or the intracellular signaling domains. Experiences with CARs to date show that the transmembrane domain can be active in signaling [25] and dimerization with endogenous signaling molecules [26], and can also influence CAR expression level [11].
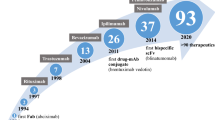
Perhaps the greatest attention in optimization strategies has surrounded the intracellular co-stimulatory signaling domain. The first version of engineered CARs in the late 1990s (termed immunoglobulin-T-cell chimeric receptor molecules) [27] were so-called “first-generation” CARs, which included an antigen-binding domain, an extracellular spacer domain and transmembrane domain, and an intracellular CD3ζ or FcRγ signaling domain. In vitro, these CAR T cells showed potent antitumor activity, yet demonstrated limited persistence and durability of therapy [28]. As these first-generation CAR T cells moved to clinical investigation, a lack of efficacy became clear in a variety of diseases. In hematologic malignancy, a phase 1 trial targeting CD20 in indolent non-Hodgkin lymphoma and mantle cell lymphoma reported safety and feasibility with modest efficacy [29]. In the context of solid tumors, a GD2-targeted first-generation CAR to treat neuroblastoma reports one patient achieving a complete response and one patient having disease cleared from the bone marrow [30]. Clinical studies targeting FRα in ovarian cancer [31], TAG72 in colorectal cancer [32], and CAIX in renal cell carcinoma [33] showed no objective clinical responses, and many of these studies remarked a lack of T cell persistence.
To address the lack of durable CAR T cell therapy, early in vivo models of B-cell malignancies illuminated the importance of co-stimulation with CD19-targeted CAR T cells [34]. In this study, durable antitumor response was observed when treating Raji Burkitt lymphoma (expressing co-stimulatory molecules CD80 and CD86) but not when treating NALM-6 pre-B-cell ALL (lacking co-stimulatory molecule expression). In vivo efficacy of CD19-CAR T cells in the second model was rescued with an engineered expression of CD80 in NALM-6 cells. Importantly, in the Raji model, CD19-CAR T cells were detected in the bone marrow of treated mice 21 days post-infusion, further accentuating the importance of co-stimulation in CAR T cell persistence. Similar phenomena were contemporaneously observed using solid tumor-directed CAR T cells targeting PSMA [28]. With this new understanding, “second-generation” CARs, which contain one co-stimulatory domain in series with the CD3ζ intracellular domain were developed [35, 36]. These CARs were able to mediate CAR T cell expansion after repeated antigen exposure while maintaining antigen-specific cytotoxic activity. The most common co-stimulatory domains added to second-generation CARs were derived from CD28 [35] and 4-1BB [36], but other domains including ICOS [37], OX40 [37], and CD27 [38] have also been explored. Clinical translation of these second-generation CAR T cells has thus far resulted in strong therapeutic responses in hematologic malignancies including chronic lymphocytic leukemia [39], B-cell acute lymphoblastic leukemia [40], diffuse large B-cell lymphoma [41], and multiple myeloma [42]. Second-generation CAR T cells have now entered clinical investigation for solid tumors, including glioblastoma [43,44,45], advanced sarcoma [46], liver metastases [47], as well as mesothelioma, ovarian cancer, and pancreatic cancer [48]. A summary of clinical trials evaluating CAR T cells for solid tumors has been recently detailed elsewhere [49].
Despite the success of second-generation CAR T cells, the hypothesis remained that co-stimulation via only one domain would lead to incomplete T cell activation. Thus, “third-generation” CARs, which incorporated two co-stimulatory domains in series with the CD3ζ, have been evaluated [50]. The most common combinations of co-stimulatory domains are CD28-OX40 and CD28-4-1BB [51]. Preclinical studies with third-generation CARs show mixed results. CARs incorporating CD28 and 4-1BB signaling demonstrated stronger cytokine production and improved in vivo antitumor response in lymphoma [52] and pulmonary metastasis [52] models relative to second-generation CARs. However, they failed to outperform a second-generation counterpart in a pancreatic cancer model [53], and resulted in decreased in vitro cytokine production and no in vivo treatment benefit relative to second-generation CARs in a leukemia model [54]. Incorporation of CD28 and OX40 signaling domains resulted in improved therapy of colon adenocarcinoma in vivo [55] and shows improved activation and cytokine production in vitro [50]. Clinical application of third-generation CAR T cells to date has been limited, but has not shown marked improvement over second-generation CAR T cells [29, 56]. However, additional investigations are warranted to define the optimal intracellular co-stimulatory domain required for CARs based on disease indication and tumor antigen target.
2.2 CAR T Cell Manufacturing
The processes used for manufacturing CAR T cells can have a profound impact on the efficacy of a clinical product. Across many clinical trials, there are significant variations to the T cell subsets chosen for CAR engineering and cell expansion protocols used. Unfortunately, due to the relatively small number of CAR T cell clinical trials completed to date, there have been few direct comparisons of manufacturing methods for a single CAR product. A recent review details many of these parameters [57].
2.3 T Cell Subsets for CAR Engineering
The majority of CAR T cell clinical trials do not select particular T cell subsets, choosing rather to isolate and engineer peripheral blood mononuclear cells (PBMCs), using stimulation methods and cytokine regimens to selectively expand T cells [57]. Early clinical trials with first-generation CARs in neurological malignancies isolated and expanded CD8+ T cell clones for manufacturing [58, 59], but this procedure led to products with low persistence in patients, likely due to exhaustion from clonal ex vivo T cell expansion. More recent innovation involves the engineering of stem-like T cell subsets [60]. Preclinical data showing the ability of central memory T cells to persist after adoptive transfer due to their stemness [61, 62] led to the use of this subset in a phase 1 clinical trial in non-Hodgkin lymphoma demonstrating safety [63] and in a unique case study of complete response in a glioblastoma patient [43]. Further, preclinical investigation of central memory T cells showed that using defined 1:1 mixtures CD4+ and CD8+ yielded more consistent potency relative to unenriched central memory T cell products [64], leading to a phase 1 clinical trial in adult B-cell acute lymphoblastic leukemia with 93% remission rate [65]. Additional phase 1 clinical trials have been applied to define CD4+/CD8+ mixtures with similar success in other B-cell diseases [66, 67]. In phase 1 clinical trial for B-cell non-Hodgkin lymphoma, CD19-CAR T cells derived from central memory T cells and naïve/memory T cells were directly compared [68]. Both arms of treatment showed efficacy in patients, but naïve/memory T cells were viewed as the superior platform because of their greater yield from apheresis as it required fewer enrichment steps, shorter ex vivo expansion time, and superior in vivo expansion. Application of naïve/memory CAR T cells in a recent phase 1 clinical trial for adult relapsed/refractory B-cell acute lymphoblastic leukemia yielded a 100% complete response rate in 13 patients treated [69]. In a retrospective study of PBMC-derived CTL019 CAR T cell products manufactured for the treatment of chronic lymphocytic leukemia, memory phenotype was correlated with complete-response in patients [70]. The impressive in vivo persistence and efficacy of CAR T cells with memory phenotype in these hematological trials motivate the application of these subsets in solid tumor indications. Further, CD8+ tumor-infiltrating lymphocytes from patient breast and melanoma tumors dominantly display memory phenotype and retain polyfunctionality despite the expression of checkpoint molecules [71]. Preclinical studies have also underscored the importance of memory phenotype in both syngeneic [72] and humanized [73] solid tumor models.
The majority of CAR T cell products are engineered from a patient’s own PBMCs or autologous products. This can lead to several issues in manufacturing, including high cost, manufacturing failures due to dysfunctional cells in the presence of disease and subsequent pre-treatment, disease progression during manufacturing, and contamination of circulating tumor cells in the apheresis product [74]. Because of these challenges, avenues for developing “off-the-shelf” or allogeneic cell-based immunotherapies, which can be obtained from healthy donors and banked, are actively being explored. Recent advances in genome modification enable engineering of healthy donor T cells or inducible pluripotent stem cells to remove endogenous HLA and TCR [75, 76]. Clinical trials are currently underway using off-the-shelf CD19-CAR T cells (NCT03939026), CD123-CAR T cells (NCT03190278), and BCMA-CAR T cells (NCT03752541). Likely, the solid tumor CAR T cell field may follow suit with evaluating allogeneic CAR T cell therapies, as another potential benefit of this approach is the removal of heterogeneity and potential immunosuppressive immune cell populations in the blood of advanced cancer patients.
2.4 Ex Vivo T Cell Expansion
Several methods for ex vivo T cell activation and expansion have been explored. Generally, isolated T cells are stimulated through the T-cell receptor, and co-stimulation through agonistic antibodies, cytokines, and/or feeder cells sustains the expansion [57]. Early protocols regularly used a monoclonal anti-CD3 antibody (OKT3) for TCR stimulation and IL-2 for T cell expansion [77]. This method was later shown to promote a more effector memory phenotype in expanded T cells, whereas the application of anti-CD28/anti-CD3 antibody-coated magnetic beads for stimulation promoted a more central memory phenotype [78]. Other studies have shown that the application of high concentrations of IL-2 in T cell culture leads to a more exhausted T cell product with poor effector function [73]. Investigations of the appropriate cytokine cocktails to sustain ex vivo expansion while maintaining memory phenotype revealed that culture with IL-7 and IL-15 cytokines increased the frequency of stem cell memory CD8+ T cells, which displayed greater antitumor activity via increased resistance to activation-induced cell death when compared to IL-2 expanded T cells [79]. A recent study showed that T cell expansion with IL-15 alone produced similar retention of stem cell memory phenotype, decreased mTORC1 activity, reduced expression of glycolytic enzymes, and improved mitochondrial fitness relative to T cells cultured with IL-2 [80].
2.5 Preconditioning and Chemotherapy Combinations to Enhance CAR T Cell Therapy
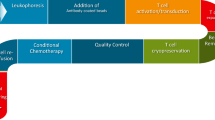
Through clinical experience with adoptive cell therapy, non-myeloablative lymphodepleting preconditioning is known to enhance outcomes. Preclinical studies have shown that the removal of host immune cells prior to adoptive cell transfer increases the in vivo availability of γc cytokines important to T cell functionality [81]. One lymphodepleting agent, cyclophosphamide, is known to enhance immune function further due to the depletion of regulatory T cells, which are hypersensitive to its effects [82]. Preclinical study has also shown that cyclophosphamide treatment can deplete myeloid-derived suppressor cells and, in combination with IL-12, increase the presence of inflammatory monocytes and neutrophils in colon cancer models [83]. A clinical comparison of preconditioning with cyclophosphamide with or without fludarabine revealed the combination approach yielded superior treatment of non-Hodgkin lymphoma, likely due to increased persistence of the engineered T cells due in part to a decreased immune response against the transgene [84]. Improved CAR T cell engraftment after preconditioning with cyclophosphamide and fludarabine was also observed in a clinical trial using first-generation CEACAM5-specific CAR T cells [85]. In addition to improving CAR T cell persistence, chemotherapies can have other synergistic effects with CAR T cell therapy. Lenalidomide, an immunomodulatory drug that has anti-multiple myeloma effects and co-stimulatory effects on T cells, enhanced CS1-targeted CAR T cell treatment in preclinical models of multiple myeloma via enhancement of the immune synapse [86]. Decitabine, a DNA methyltransferase inhibitor, enhanced CD19 expression, and thus susceptibility to CD19-targeted CAR T cell therapy, in both in vitro lymphoma models and in two treated patients [87]. The combination of temozolomide with EGFRvIII-targeted CAR T cells improved treatment of glioblastoma xenografts in mice and has been explored with an escalated dose in preclinical models as the sole lymphodepleting agent prior to CAR T cell therapy [88]. Interestingly, this study showed that the application of dose-intensified temozolomide significantly increased CAR T cell infiltration into tumors without significantly decreasing the presence of regulatory T cells. In sum, the utility of preconditioning and chemotherapy has been validated in combination with CAR T cell therapies for hematological malignancies and has become an attractive area of clinical research for the development of solid tumor CAR T cell therapies.
2.6 CAR T Cell Route of Administration
Although targeting hematological malignancies has nearly strictly required intravenous administration of CAR T cells, solid tumors introduce a unique opportunity to localize CAR T cell delivery to target tumors in selected disease sites. Two major reasons to take advantage of different routes of CAR T cell administration compared with systemic delivery are (1) to avoid the requirement of trafficking of CAR T cells to sites of disease, and (2) to direct the on-target activity of CAR T cells in tumors, thereby minimizing their opportunity to target normal tissues. Trafficking of CAR T cells in solid tumors may be hampered by their inability to penetrate tumor stroma and other physical barriers, as well as by the harsh immunosuppressive microenvironment that may impede their mobility into the tumor [89] (more details on the immunosuppressive tumor microenvironment may be found later in the chapter). Additionally, since many solid tumor antigens targeted by CAR T cells are expressed at varying levels in select normal tissue, local or regional CAR T cell delivery may mitigate the potential for on-target off-tumor toxicities [90] (more details on the selection of solid tumor antigens may be found later in the chapter).
Several examples of local or regional delivery of CAR T cells have been evaluated preclinically and in phase 1 trials. Intraperitoneal injection significantly outperformed the systemic injection of CAR T cells in preclinical models of ovarian cancer [91, 92] and peritoneal carcinomatosis [93]. On the strength of preclinical success, a phase 1 clinical trial is currently ongoing comparing intravenous and intraperitoneal infusion of MUC16-targeted CAR T cells in ovarian cancer (NCT02498912). Intravenous administration of mesothelin-targeted CAR T cells that use a murine-derived scFv for antigen recognition has yielded antibody responses against the murine component and anaphylaxis [94, 95]. To improve the efficacy of this therapy and potentially shield the CAR T cells from endogenous immunity, intrapleural delivery of CAR T cells was explored. This route of administration showed superior treatment of a preclinical orthotopic model of malignant pleural mesothelioma in both lung and extrathoracic sites compared to intravenous administration [96], leading to an ongoing phase 1 clinical trial (NCT02414269). Intrahepatic arterial delivery of CAR T cells for liver metastases has been explored preclinically, revealing the challenges of liver myeloid-derived suppressor cells to immunotherapy [97] and in phase 1 clinical trial, demonstrating safety in four patients [47]. Intraventricular administration of CAR T cells targeting HER2 in breast cancer brain metastases [98] and IL13Rα2 in glioblastoma [99] showed superior therapy relative to intravenous injection in orthotopic xenograft models. Importantly, this route of administration offers advantages over intravenous and intracranial delivery in the treatment of multifocal disease. In one patient, intraventricular infusion of IL13Rα2-targeted CAR T cells resulted in a complete response of glioblastoma [43]. Phase 1 clinical trials for intraventricular injection of CAR T cells in glioblastoma (NCT02208362, NCT03389230) and recurrent brain or leptomeningeal metastases (NCT03696030) are ongoing.
3 Barriers to Solid Tumor CAR T Cell Therapies
This chapter has highlighted multiple aspects critical to developing effective CAR T cell strategies for the treatment of solid tumors. The three most challenging areas that require attention in the development of next-generation CARs for solid tumors are (1) selective targeting of tumor antigens, (2) tumor antigen heterogeneity, and (3) the immunosuppressive tumor microenvironment. These challenges are active areas of translational research, and will likely require empirical testing for each tumor type, molecular signature, and disease stage of therapeutic intervention.
3.1 Solid Tumor Target Antigen Selection
There are nearly 300 CAR T cell clinical trials currently listed on NIH’s U.S. National Library of Medicine (ClinicalTrials.gov), with over 50 trials in solid tumors. The solid tumor antigens most frequently targeted by CAR T cell therapy include CEA, EGFR, EGFRvIII, GD2, HER2, IL13Rα2, PSCA, and PSMA [14] and more are summarized in Table 11.1. While all of these antigens are either over-expressed and/or amplified in tumors compared with normal tissue, their protein expression is not uniquely restricted to tumor cells, with the exception of EGFRvIII, a common oncogenic rearrangement in glioblastoma marked by deletion of exons 2–7 of EGFR. Therefore, unlike CD19, a B-cell restricted antigen that is expressed in many B-cell malignancies, solid tumor antigen targets pose significant toxicity concerns that may limit their utility in CAR T cell therapy.
Examples of such toxicities have been observed in clinical trials. A phase 1 trial at the NIH treated three patients using a murine TCR-expressing autologous T cell therapy targeting CEA, and although bioactivity was observed in all three patients with an objective regression in one patient, all patients developed severe transient inflammatory colitis [121]. Similar on-target toxicities were observed in a clinical trial evaluating CAIX-specific CAR T cells in patients with renal cell carcinoma, demonstrating targeting of normal bile duct epithelial cells known to express low levels of CAIX [33]. Perhaps most famously, a serious adverse event was observed in a metastatic colon cancer patient treated with a third-generation HER2-CAR T cell product at the NCI, which resulted in acute respiratory distress syndrome and death of the patient five days after treatment [56]. Two recent studies, however, have reported safety and bioactivity in two clinical trials evaluating second-generation HER2-CAR T cells in patients with advanced sarcoma and glioblastoma [45, 46]. One potential avenue for overcoming on-target off-tumor toxicity is the implementation of a suicide gene strategy, which would allow selective depletion of engineered cells via treatment with a secondary inducing agent at the onset of adverse events [122]. Apoptosis can be mediated by the expression of engineered endogenous apoptotic molecules that can be dimerized via small molecule drugs [123]. Examples of this strategy include inducible FAS [124] or inducible Caspase 9 [125, 126]. Co-expression of transmembrane-anchored proteins or peptides can mark engineered cells for destruction through monoclonal antibody therapy. Expression of full-length CD20 [127] or CD20 mimotope independently [128] or as part of the CAR construct [129, 130] enables the depletion of CAR T cells by Rituximab treatment. A truncated, non-signaling version of EGFR has been shown to facilitate CAR T cell depletion with Cetuximab therapy [131]. Importantly, while suicide gene strategies are attractive for ensuring safety, their implementation abruptly terminates therapy of potentially rapidly progressing disease. This motivates the development of other strategies to ensure safety in treatment, leaving suicide gene activation as a last resort for high-grade adverse events. One such approach was recently reported using Dasatinib, an FDA-approved tyrosine kinase inhibitor for the treatment of t(9;22) chronic myelogenous leukemia and Philadelphia chromosome + acute lymphoblastic leukemia, which suppresses T-cell activation via inhibition of proximal TCR signaling kinases, such as Src, Fyn, and Lck [132, 133]. This pharmacological approach to transiently inhibiting CAR T cell function may allow for the rescue of CAR T cell therapy once toxicities subside.
To overcome targeting tumor antigens that are also found in normal tissues, such as CEA and HER2, targeting tumor-restricted post-translational modifications may provide a unique opportunity for the development of CAR T cell therapy for solid tumors. One of the well-characterized post-translational processes that are differentially regulated in tumor cells is protein glycosylation. The most prevalent of these aberrantly glycosylated antigens are truncated O-glycans, including Tn (GalNAcα1-O-Ser/Thr) and sialyl-Tn (STn) (NeuAcα2-6-GalNAcα1-O-Ser/Thr), which are found over-expressed in many solid tumor types [134]. The four major examples that have been evaluated as CAR T cell targets are MUC1, MUC16, B7-H3, and TAG72. A report of a first-generation CAR T cell therapy for patients with colorectal cancer targeting the tumor-associated glycoprotein TAG72 [32] demonstrated safety and bioactivity, but no tumor responses were observed. Two potential explanations for the lack of therapy in this trial was the use of first-generation CARs and the observed anti-CAR immune responses. Newer versions of TAG72-CAR T cells are being investigated, including second-generation CAR T cells [92] and modifications to the scFv to avoid anti-idiotype immunogenicity [135], and will inform the field on the utility of targeting TAG72+ solid tumors with CAR T cells.
Several ongoing phase 1 clinical trials are evaluating and targeting MUC1 with CAR T cells, which is highly over-expressed and aberrantly glycosylated in many solid tumor types [22]. Given the expression of full-length MUC1 in normal tissue, however, novel engineering strategies are warranted to avoid on-target toxicities that have been observed in prior studies mentioned above. Two novel tumor-specific versions of MUC1-targeted CAR T cells are now being evaluated in early clinical trials. The first is a CAR targeting the tumor-associated Tn-glycoform of MUC1 (Tn-MUC1) [120, 136], which was shown to be highly expressed in tumor tissue, but absent in normal tissue, as compared with full-length MUC1. A similar approach was recently evaluated in mice with CAR T cells targeting the novel cleavage product, MUC1*, shown to be expressed on the cell surface of tumor cells but not in normal tissue [137]. A phase 1 trial has just begun testing MUC1*-CAR T cells for patients with breast cancer (NCT04020575). Additionally, MUC16 has been explored as a target for multiple solid tumor types, and an ongoing phase 1 trial is exploring MUC16ecto-CAR T cells for the treatment of solid tumors [138, 139] (NCT02498912). More recently, the glycoprotein B7-H3 was found to be over-expressed and aberrantly glycosylated in multiple solid tumor types. Preclinical studies have demonstrated the safety and efficacy of CAR T cells targeting B7-H3 [100,101,102], and a phase 1 clinical trial has just begun to evaluate the safety and efficacy of B7-H3-targeted CAR T cells in patients with recurrent glioblastoma (NCT04077866).
3.2 Improving Tumor Antigen Selectivity of CARs
Novel strategies have emerged in CAR design to further control the specificity and activity of CAR T cells for improved safety and antitumor efficacy. Perhaps the earliest example of this for solid tumors was investigated by Kloss and colleagues, using a combinatorial CAR targeting PSCA and PSMA in prostate cancer models. In this system, the co-stimulatory domain and the CD3ζ cytolytic domain were uncoupled and required two antigens to be simultaneously expressed on tumors for optimal CAR T cell activation [140]. Uniquely, the greatest antitumor activity in preclinical models was achieved using a first-generation PSCA-CAR, which was further affinity-tuned for optimal tumor targeting, along with a PSMA-CAR containing a 4-1BB co-stimulatory domain that lacked cytolytic activity (no CD3ζ domain). More recent versions of controlled CARs include drug-inducible platforms. One of the most promising examples of this uses an inducible MyD88/CD40 (iMC), which can be triggered in vivo with the synthetic dimerizing ligand, rimiducid, for potent co-stimulation of CAR T cells [141]. This strategy has been employed effectively in preclinical studies targeting HER2, demonstrating superiority compared with second-generation HER2-CAR T cells with CD28 co-stimulation [142]. The major improvement in this strategy may involve the ability to modulate signaling of the CAR, controlling both safety and efficacy. A phase 1 trial with this approach has been initiated in targeting PSCA+ pancreatic cancers, with interim results demonstrating safety and bioactivity in patients [143].
3.3 Tumor Antigen Heterogeneity and Escape
One of the major limitations to current CAR T cell therapies is single antigen targeting. Tumor resistance to single therapeutic agents is well-established as the majority of tumors are heterogenous, and prolonged targeting of a single drug-sensitive pathway can ultimately lead to drug-resistant tumor recurrences. Acquired or intrinsic resistance patterns following CAR T cell therapy have also been observed. CD19-CAR T cell therapy has demonstrated durable clinical remissions in 70–90% of patients with B-cell malignancies including acute lymphoblastic leukemia (ALL), however, emerging follow-up data from clinical trials show a common mechanism of resistance including loss and/or downregulation of CD19 antigen in up to 70% of patients who recur following treatment [144, 145]. Early clinical findings using CAR T cells for solid tumors have observed similar antigen escape resistance mechanisms. For instance, a phase 1 trial evaluating intravenous delivery of EGFRvIII-specific CAR T cells in patients with recurrent glioblastoma, known for its antigen heterogeneity, showed antigen loss resulting in tumor resistance [44]. A case report targeting IL13Rα2 in glioblastoma with CAR T cells demonstrated decreased IL13Rα2 expression in tumor recurrences [43], suggesting that antigen escape also may have contributed to tumor relapse. Multiple mechanisms may exist that underlay antigen escape following CAR T cell therapy. Hamieh and colleagues recently demonstrated decreased tumor target density by extracting surface expressed antigen from tumor cells by CAR T cells through a process known as trogocytosis [146]. These studies strongly suggest that treatment optimization through CAR design or the rational design of combination and/or sequential CAR T cell strategies targeting distinct tumor antigens will be necessary for effective disease control.
3.4 Multi-targeted CAR T Cells
To reduce the relapse rate in CAR T cells for the treatment of hematological malignancies, studies have emerged using dual-targeted CAR T cells. Such approaches have utilized either dual CAR constructs, or two scFvs (“OR”-gate) within a single CAR construct (known as tandem CARs) to simultaneously target different tumor antigens. Both strategies have been employed targeting CD19 and CD22 in relapsed/refractory ALL, with promising early clinical data suggesting that dual-targeting may prolong durable remission rates [147]. Additional studies are ongoing with simultaneous targeting of CD19 and CD20 with “OR”-gate CARs [148, 149], as well as CD19 and CD123 co-targeting [150] and others [151]. These approaches are also being evaluated for CAR T cells targeting multiple myeloma [152].
In solid tumors, HER2 and MUC1 tandem CARs have been evaluated in preclinical models of breast cancer with improved activity over single antigen targeting CARs [153]. Similarly, dual-targeting of HER2 and IL13Rα2 in glioblastoma has been studied in xenograft models [154]. In this study, tandem CARs were evaluated in both human xenograft and syngeneic immunocompetent mouse models of glioblastoma, and compared to T cells expressing both CARs, or pooling single-specific CAR T cells. Interestingly, tandem targeting of HER2 and IL13Rα2 resulted in superior antitumor activity, and reduced antigen escape compared with the two other dual-targeting approaches. While this finding may be specific for different antigens being targeted in solid tumors, it highlights the need to empirically define dual-targeting approaches that improve antitumor responses and potentially mitigate antigen escape mechanisms of tumor resistance.
Additional innovative approaches have been developed to target multiple antigens in attempts to overcome antigen escape in solid tumors. Recently in glioblastoma, a novel EGFRvIII-specific CAR was designed to secrete a bispecific T cell-engager (BiTE) targeting EGFR. In this study, the co-targeting of EGFRvIII and EGFR using this strategy successfully controlled heterogeneous model tumors compared with either strategy alone [155]. Another novel approach to target two tumor antigens was recently investigated in preclinical studies using oncolytic viruses to infiltrate tumors and secrete EGFR-BiTEs, in combination with CAR T cells targeting FRα, which improved antitumor activity over monotherapy [108].
Multi-targeting introduces additional toxicity concerns as each new target potentially compounds healthy tissue targeting. Newer synthetic CAR switches are being developed to circumvent this likelihood of exacerbating toxicities to normal tissue. Perhaps the most intriguing approach in recent years has been demonstrated using modular synthetic Notch receptors (synNotch) for “AND”-gate CAR T cell regulation, requiring tumor cells to express two antigens for controlling CAR T cell activation, sparing normal tissues that express either of the antigen alone [156]. This approach was further validated with co-targeting of ROR1+ tumors expressing EpCAM or B7-H3 for reduced toxicity to normal tissue [157]. One additional strategy for improved target selectivity of tumors is the use of “NOT”-gate inhibitory CAR T cells (iCARs), which use checkpoint pathway inhibition of one target while simultaneously activating CAR T cells with another target [158]. These approaches potentially provide further improvements over the previous combinatorial targeting approach mentioned above [140], and are anticipated to enter clinical testing soon for patients with solid tumors.
While requiring dual antigen expression on tumor cells for optimal activation of CAR T cells is an exciting advancement over single antigen-specific CARs, another versatile approach to engineer target specificity, called switchable or universal CARs, has recently been developed. These programmable CARs come in several forms, but each has in common the ability to redirect the specificity of CAR T cells to different antigens based on a druggable reagent. The first example of this strategy was demonstrated using CARs with an antigen-binding domain specific for the common fluorophore FITC, which controlled the activation of CAR T cells to antibodies tagged with FITC, redirecting specificity to EGFR, HER2, or CD20 [159]. Further validation of FITC-specific CAR T cells has been documented [160], as well as for biotinylated antibodies using Streptavidin-specific CAR T cells [161] and peptide neo-epitopes from the yeast transcription factor GCN4 [162, 163]. A more recent iteration of this strategy has been demonstrated using a switch, universal, and programmable (SUPRA) CAR, which employs a leucine zipper as the targeting domain on the CAR, along with an antibody tagged with the cognate leucine zipper [164]. Compared to conventional single or dual-targeted CAR T cells, these modular approaches offer improved safety with robust efficacy of CAR T cell activation, allowing for “smart” targeting of solid tumors.
3.5 Improving CAR T Cell Therapies in Immunosuppressive Solid Tumors
Another major challenge for effectively targeting solid tumors with CAR T cell therapies is the immunosuppressive tumor microenvironment. Distinct from most of the hematological malignancies that lack local immunosuppressive pathways that hamper antitumor immunity and limit adoptive T cell therapies, solid tumors can be heavily infiltrated by multiple cell types that support tumor growth, vasculature, metastasis, and may dictate therapeutic responses [165]. The most prominently studied cell types that drive immunosuppression in tumors are regulatory T cells (Tregs), M2 tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs) [166]. These immune cell infiltrates, in addition to the tumor cells themselves, drive local cytokine, chemokine, and growth factor production in solid tumors, including IL-4, IL-10, VEGF, and TGFβ, that can facilitate tumor growth and progression. Likewise, immune checkpoint pathways, including PD-1 and CTLA-4, can be highly active in tumors to dampen antitumor immunity. Considerable evidence suggests that the tumor microenvironment also controls response and resistance to immunotherapies [167], and can limit the effectiveness of CAR T cell therapy [168].
A number of recent studies have aimed to boost CAR T cell functionality by blocking immune checkpoint pathways. Multiple studies have demonstrated that following CAR T cell therapy, PD-1/PD-L1 and other checkpoint pathways are induced, thereby limiting durable therapy [169]. The simplest of these methods has been demonstrated by combining CAR T cells with immune checkpoint blockade [170,171,172]. Phase 1 clinical trials are underway evaluating this combination approach to improve response rates in hematological malignancies and solid tumors [173, 174] (NCT03545815). Novel strategies to intrinsically circumvent PD-1/PD-L1 signaling pathways to prolong CAR T cell functionality have been explored. For example, a chimeric PD1-CD28 receptor allowed for redirecting PD-1-signaling in T cells towards co-stimulation [175, 176]. Cherkasskey and colleagues evaluated multiple methods of intrinsic blockade of PD-1 in CAR T cells, including shRNA knockdown of PD-1 or a PD-1 dominant negative receptor, showing improved antitumor responses in multiple preclinical models by blunting PD-1 signaling in adoptively transferred T cells [177]. More recently, the secretion of PD1 blocking antibodies by CAR T cells was shown to similarly improve therapy [178, 179]. CRISPR/Cas9-mediated disruption of PD-1 in CAR T cells has also been explored, and clinical trials are now underway evaluating this approach in patients [180,181,182]. In the context of the most well-studied PD-1 and CTLA-4 inhibitors, it has been demonstrated that potential mechanisms of tumor resistance include compensatory upregulation of alternative immune checkpoint pathways. Therefore, it will be imperative to evaluate and overcome these resistance mechanisms in the context of combinatorial CAR T cell – immune checkpoint blockade.
Expression of TGFβ, a multi-functional cytokine that is dysregulated in many cancers, has been associated with an immune phenotype characterized by a lack of tumor T cell infiltration [183]. Hence, a recent pursuit has been dedicated to blocking TGFβ signaling in CAR T cells and in the immunosuppressive tumor microenvironment to promote adoptive and adaptive T cell antitumor immunity. Preclinical studies suggest that CAR T cells containing a CD28 co-stimulatory domain may resist TGFβ-mediated inhibitory signals predominantly through IL-2 signaling [184]. Despite recent evidence pointing to superior T cell persistence and antitumor activity, 4-1BB-containing CAR T cells may lack the ability to resist TGFβ-mediated immunosuppression. Therefore, CAR T cells engineered to be refractory to immunosuppressive factors present in the tumor microenvironment, including TGFβ, have been developed [185]. Based on these strong preclinical findings, a phase 1 clinical trial has been initiated to evaluate PSMA-targeted CAR T cells with a dominant negative TGFβ receptor in patients with metastatic castration-resistant prostate cancer (NCT03089203). Other approaches include redirecting TGFβ signaling in T cells towards 4-1BB co-stimulation [186] or IL-12 signaling [187] using chimeric receptors. Uniquely, CAR T cells targeting soluble TGFβ have also been engineered [20], which can be used in a dual-targeted CAR T cell approach to simultaneously target tumor cells and inhibit TGFβ signaling [188].
In addition to engineering CAR T cells to block inhibitory signals in the immunosuppressive tumor microenvironment, the expression of pro-inflammatory cytokines with the ability to shape the tumor microenvironment for improved T cell trafficking, survival, persistence, and antitumor functionality has been explored. The earliest example of this strategy was shown using CD19-CAR T cells engineered to secrete IL-12. In addition to increased IFNγ production, CAR T cell persistence, and overall therapeutic activity, this therapy also eliminated tumors in the absence of lymphodepleting preconditioning [189]. IL-12 secreting MUC16-directed CAR T cells also produced elevated levels of IFNγ, increased survival and persistence of CAR T cells, and improved overall therapy in xenograft models of ovarian cancer [91]. Follow-up studies in immunocompetent mice showed that IL-12-secreting MUC16-CAR T cells also shaped the immunosuppressive microenvironment in ovarian cancers by depleting tumor-associated macrophages and overcoming PD-L1-mediated T cell inhibition [190]. These preclinical studies have resulted in a clinical trial testing this approach in MUC16+ solid tumors (NCT02498912). CD19-CAR T cells have also been engineered to express IL-15 tethered to the surface of T cells (mbIL-15). mbIL-15 CAR T cells showed improved stem/memory phenotype with increased T cell persistence and durable antitumor activity [191]. Alternative platforms for intrinsic IL-15 production by CAR T cells have been investigated, including CAR T cells engineered to secrete soluble IL-15 [192], and a novel nanoparticle drug delivery platform carrying an IL-15 super-agonist complex [193]. Other approaches have introduced novel ways to redirect immunosuppressive cytokines toward pro-inflammatory pathways, including CAR T cells with chimeras in which the IL-4 receptor ectodomain is fused to the IL-7 receptor endodomain. This platform was utilized in xenograft models of pancreatic cancer using PSCA-directed CAR T cells [194]. A similar strategy was utilized to redirect IL-4 signaling towards another pro-inflammatory cytokine, IL-21 [195].
The immunosuppressive tumor microenvironment, in addition to suppressing the function of CAR T cells once they arrive at the tumor site, likely also intrinsically blocks trafficking of CAR T cells. Therefore, in addition to increasing doses of infused CAR T cells to achieve a required threshold of recruitment at the tumor site, combination approaches to amplify endogenous immunity to aid in CAR T cell responses have been explored. Oncolytic viruses (OV) can be selectively programmed to target, infect, and kill cancer cells, and genetically modified to express therapeutic genes selectively in the tumor microenvironment [196, 197]. Through cancer cell infection and lysis, OV has been used for tumor debulking, reversing tumor immunosuppression, and initiating systemic antitumor immune responses. Watanabe and colleagues showed that the combination of mesothelin-targeted CAR T cell therapy with an oncolytic adenovirus driving tumor expression of TNFα and IL-2 induced significant tumor regression in a syngeneic mouse model of pancreatic cancer. This antitumor response was accompanied by an increase in CAR T cell and endogenous T cell infiltration, pro-inflammatory M1 macrophage polarization, and dendritic cell maturation [198]. Additional studies have utilized OV to express multiple transgenes in cancer cells simultaneously, consisting of immune checkpoint inhibitors and pro-inflammatory cytokines, that, when combined with CAR T cells, showed enhanced T cell effector function [199]. These findings indicate that combining cytokine-armed oncolytic adenoviruses to enhance the efficacy of CAR T cell therapy is a promising approach to overcome the immunosuppressive tumor microenvironment and to also amplify endogenous antitumor immunity.
3.6 Pre-existing T Cell Immunity and CAR T Cell-Induced Endogenous Immunity
Current understanding suggests that the effectiveness of immunotherapy depends on the presence of pre-existing immunity and the ability to effectively modulate the baseline immune response. Clinical studies are beginning to define predictive tumor and immunological factors governing the anticancer response—one such measure is the immune classification of cancer.
The immune classification of cancer is an evolving measure that characterizes tumors with respect to their immune infiltration in two broad classifications: immunologically “hot” and immunologically “cold” tumors (Fig. 11.2). Immunologically hot, or immune-inflamed tumors, are characterized predominantly with a high infiltrate of T cells, low infiltration of immune-suppressive cells including regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSC) and include additional features like PD-L1 expression on tumor cells and tumor-associated immune cells, potential genomic instability and the presence of a pre-existing antitumor immune response. Immunologically cold, immune-excluded, or immune-deserted tumors typically have poor antitumor T cell infiltration, high immune-suppressive cell infiltration, low PD-L1 expression, with high proliferation of cancer cells and low mutational burden [167]. Studies have recently proposed a combinatorial set of parameters to augment this classification: the T cell phenotype (follicular helper T (Tfh), T helper 1 (Th1), memory and exhausted T cells) at the tumor, dependent on location (invasive margin, tumor core, and tertiary lymphoid structures), density (immune density and quantity), and functional immune orientation (chemokines, cytokines, cytotoxic factors, adhesion, attraction) [200]. These factors combine to represent cancer immune interactions for an individual patient [201], and together, they can help define immunomodulation strategies to optimize personalized treatment choices [202].
The immune landscape of solid tumors. a A representative immunologically “hot” tumor containing a high frequency of antitumor CD8 T cells, and a relatively low frequency of immunosuppressive regulatory T cells (Treg) and myeloid cell subsets including tumor-associated macrophages (TAM), mononuclear myeloid-derived suppressor cells (MO-MDSC), neutrophils, and polymorphonuclear myeloid-derived suppressor cells (PMN-MDSC), along with tumor vasculature and stromal cells.b A representative immunologically “cold” tumor containing a higher frequency of immunosuppressive cell subsets and a relatively low frequency of antitumor CD8 T cells
It has yet to be determined whether antitumor responses with CAR T cell therapy is impacted by pre-existing T cell immunity. In the context of immune checkpoint blockade, response to therapy may rely on the reactivation of pre-existing T cells, the recruitment of new T cells to the tumor, or a combination of both [203, 204]. T cell exhaustion represents a distinct state of T cell differentiation and can be driven by cell signaling, prolonged TCR engagement, co-stimulatory/inhibitory signals, soluble factors (e.g. excessive suppressive cytokines), and microenvironment features (e.g. chemokine receptor expression, adhesion molecules). Exhausted T cells acquire an epigenetic profile that is distinct from T effector cells, and despite the ability to revert to an effector using PD-1 blockade, these cells may never acquire a memory phenotype [204]. This limits the durability of immunotherapy, and an understanding of how to permanently reverse T cell exhaustion is currently incomplete. These phenotypes may heavily impact CAR T cells once they arrive at tumors, and may overcome in part by addressing immunosuppression, as covered in the section above.
The presence and density of tumor-infiltrating lymphocytes (TILs) are often interpreted as an indication of pre-existing T cell immune recognition, though recent studies have highlighted that reactivity of TILs with respect to cognate tumor antigens is rare and variable [205]. A recent study that analyzed phenotype and TCR repertoire in site matched tumors, from basal or squamous cell carcinoma patients, pre- and post-therapy showed that response to PD-1 blockade associated with the expansion of a distinct repertoire of T cell clones from pre-therapy TILs [206]. Together, these studies suggest that increasing the frequency and breadth of the tumor-specific TCR repertoire may be critical to boost the response towards immunotherapy, thereby increasing infiltration of tumor reactive T cells, and amplifying secondary immune responses. These studies also indicate that priming the tumor microenvironment prior to, and during, CAR T cell therapy may greatly impact the overall antitumor responses and provide for more durable clinical outcomes in patients.
One suggested mechanism by which adoptive T cell therapy is able to promote durable antitumor responses is through the stimulation of epitope spreading—a dynamic process that underlies the expansion of an immune response to secondary epitopes that are not targeted by therapy. In particular, epitope spreading may be initiated by the presence of a tumor-specific endogenous immune response responsible for the release of immunosuppressive mechanisms and promotion of T cell chemo-attracting cytokines at the tumor site. In the context of CAR T cell therapy, this resulting immune recruitment may confer the ability to produce a secondary immune response to cancer cells that do not express the CAR target antigen.
The potential for CAR T cells to induce epitope spreading has not been extensively studied with the exception of a few preclinical studies. In a murine CAR model targeting EGFR+ glioblastoma, mice that were cured of EGFR+ tumors later rejected EGFR-tumors when re-challenged, suggesting the generation of endogenous immunity against additional tumor antigens [207]. Pituch and colleagues showed significant changes in the tumor microenvironment and endogenous immune infiltration after IL13Rα2-CAR T cell therapy in an immunocompetent mouse model of malignant glioblastoma [208]. These changes included a decrease of immunosuppressive MDSCs and an increase in both endogenous CD4+ and CD8+ T cells, as well as CD8α+ dendritic cells. The presence of these factors along with a lack of tumor development upon re-challenge with an IL13Rα2 negative tumor, suggests these mice could acquire antitumor immunity in response to CAR T cell therapy. Modifications to the cytokine/chemokine expression of CAR T cells, namely inclusion of IL-7 and CCL19, resulted in superior antitumor activity coupled with increased endogenous immune infiltration and protection against CAR-targeted antigen-negative tumor growth [209]. These preclinical studies have underscored that CAR T cell therapy may not only modulate the immune landscape by creating a pro-inflammatory tumor microenvironment, but also recruit endogenous antitumor immunity in response to CAR T cell therapy.
Recent clinical studies have suggested that CAR T cells show evidence for inducing a secondary immune response. A first-in-human study of intravenous delivery of EGFRvIII-CAR T cells reported that the CAR T cells trafficked to the brain tumor proliferated, and exerted some bioactivity in patients with recurrent glioblastoma [44]. Although the T cell receptor clonotypes present in the CAR T product were a large fraction of the T cell repertoire infiltrating the tumor after CAR T infusion, a significant portion were not, suggesting that CAR T cell infusions could potentially increase endogenous TCR repertoire diversity to the tumor, with the potential to induce a secondary immune response targeting secondary epitopes on EGFRvIII- tumor cells [44]. CAR T cell-mediated epitope spreading was suggested in a patient with recurrent multifocal glioblastoma that received IL13Rα2-CAR T cells [43]. Following 10 intraventricular infusions, regression of all intracranial and spinal tumors with a continued clinical response in the patient for 7.5 months was observed. Evidence of endogenous T cell recruitment and stimulation in the CSF after every CAR T cell infusion was associated with increases in T cell chemo-attractants CXCL9/CXCL10, as well as IFNγ.
Together, these studies suggest that CAR T cells have the capacity to amplify an inflammatory immune response and recruit endogenous T cells to tumor sites. Increasing the frequency and breadth of the tumor-specific TCR repertoire at tumor sites may be critical to boost CAR T cell therapy by inciting a secondary immune response. This phenomenon will likely be an important component of durable clinical outcomes in patients with single or multi-targeted CAR T cell therapy to ultimately overcome resistance driven by heterogeneity and in solid tumors.
References
Couzin-Frankel J (2013) Breakthrough of the year 2013. Cancer immunotherapy. Science 342(6165):1432–1433
Wei SC, Duffy CR, Allison JP (2018) Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 8(9):1069–1086
Darvin P et al (2018) Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med 50(12):165
Wolchok J (2018) Putting the immunologic brakes on cancer. Cell 175(6):1452–1454
Boyiadzis MM et al (2018) Chimeric antigen receptor (CAR) T therapies for the treatment of hematologic malignancies: clinical perspective and significance. J Immunother Cancer 6(1):137
Priceman SJ, Forman SJ, Brown CE (2015) Smart CARs engineered for cancer immunotherapy. Curr Opin Oncol 27(6):466–474
Stoiber S et al (2019) Limitations in the design of chimeric antigen receptors for cancer therapy. Cells 8(5)
Curran KJ, Pegram HJ, Brentjens RJ (2012) Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions. J Gene Med 14(6):405–415
Salter AI, Pont MJ, Riddell SR (2018) Chimeric antigen receptor-modified T cells: CD19 and the road beyond. Blood 131(24):2621–2629
Zhang T, Lemoi BA, Sentman CL (2005) Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy. Blood 106(5):1544–1551
Zhang T, Wu MR, Sentman CL (2012) An NKp30-based chimeric antigen receptor promotes T cell effector functions and antitumor efficacy in vivo. J Immunol 189(5):2290–2299
Siegler E et al (2017) Designed ankyrin repeat proteins as Her2 targeting domains in chimeric antigen receptor-engineered T cells. Hum Gene Ther 28(9):726–736
Han X et al (2017) Adnectin-based design of chimeric antigen receptor for T cell engineering. Mol Ther 25(11):2466–2476
Wang Y et al (2017) New chimeric antigen receptor design for solid tumors. Front Immunol 8:1934
Liu X et al (2015) Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res 75(17):3596–3607
Chmielewski M et al (2004) T cell activation by antibody-like immunoreceptors: increase in affinity of the single-chain fragment domain above threshold does not increase T cell activation against antigen-positive target cells but decreases selectivity. J Immunol 173(12):7647–7653
Drent E et al (2019) Combined CD28 and 4-1BB costimulation potentiates affinity-tuned chimeric antigen receptor-engineered T cells. Clin Cancer Res 25(13):4014–4025
Hudecek M et al (2013) Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res 19(12):3153–3164
Hudecek M et al (2015) The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res 3(2):125–135
Chang ZL et al (2018) Rewiring T-cell responses to soluble factors with chimeric antigen receptors. Nat Chem Biol 14(3):317–324
Guest RD et al (2005) The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J Immunother 28(3):203–211
Wilkie S et al (2008) Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J Immunol 180(7):4901–4909
Watanabe N et al (2016) Fine-tuning the CAR spacer improves T-cell potency. Oncoimmunology 5(12):e1253656
Jonnalagadda M et al (2015) Chimeric antigen receptors with mutated IgG4 Fc spacer avoid fc receptor binding and improve T cell persistence and antitumor efficacy. Mol Ther 23(4):757–768
Guedan S et al (2018) Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight 3(1)
Bridgeman JS et al (2010) The optimal antigen response of chimeric antigen receptors harboring the CD3zeta transmembrane domain is dependent upon incorporation of the receptor into the endogenous TCR/CD3 complex. J Immunol 184(12):6938–6949
Gross G, Waks T, Eshhar Z (1989) Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A 86(24):10024–10028
Gong MC et al (1999) Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia 1(2):123–127
Till BG et al (2008) Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood 112(6):2261–2271
Pule MA et al (2008) Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 14(11):1264–1270
Kershaw MH et al (2006) A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res 12(20 Pt 1):6106–6115
Hege KM et al (2017) Safety, tumor trafficking and immunogenicity of chimeric antigen receptor (CAR)-T cells specific for TAG-72 in colorectal cancer. J Immunother Cancer 5:22
Lamers CH et al (2013) Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther 21(4):904–912
Brentjens RJ et al (2003) Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med 9(3):279–286
Maher J et al (2002) Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol 20(1):70–75
Imai C et al (2004) Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 18(4):676–684
Finney HM, Akbar AN, Lawson AD (2004) Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol 172(1):104–113
Song DG et al (2012) CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood 119(3):696–706
Porter DL et al (2011) Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365(8):725–733
Grupp SA et al (2013) Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368(16):1509–1518
Locke FL et al (2017) Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther 25(1):285–295
Brudno JN et al (2018) T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol 36(22):2267–2280
Brown CE et al (2016) Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med 375(26):2561–2569
O’Rourke DM et al (2017) A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med 9(399)
Ahmed N et al (2017) HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol 3(8):1094–1101
Ahmed N et al (2015) Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol 33(15):1688–1696
Katz SC et al (2015) Phase I hepatic immunotherapy for metastases study of intra-arterial chimeric antigen receptor-modified T-cell therapy for CEA+ liver metastases. Clin Cancer Res 21(14):3149–3159
Haas AR et al (2019) Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Mol Ther
Johnson LA, June CH (2017) Driving gene-engineered T cell immunotherapy of cancer. Cell Res 27(1):38–58
Pule MA et al (2005) A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther 12(5):933–941
van der Stegen SJ, Hamieh M, Sadelain M (2015) The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov 14(7):499–509
Zhong XS et al (2010) Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther 18(2):413–420
Abate-Daga D et al (2014) A novel chimeric antigen receptor against prostate stem cell antigen mediates tumor destruction in a humanized mouse model of pancreatic cancer. Hum Gene Ther 25(12):1003–1012
Milone MC et al (2009) Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther 17(8):1453–1464
Hombach AA, Abken H (2011) Costimulation by chimeric antigen receptors revisited the T cell antitumor response benefits from combined CD28-OX40 signalling. Int J Cancer 129(12):2935–2944
Morgan RA et al (2010) Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 18(4):843–851
Vormittag P et al (2018) A guide to manufacturing CAR T cell therapies. Curr Opin Biotechnol 53:164–181
Park JR et al (2007) Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther 15(4):825–833
Brown CE et al (2015) Bioactivity and safety of IL13Ralpha2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin Cancer Res 21(18):4062–4072
Gattinoni L, Klebanoff CA, Restifo NP (2012) Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer 12(10):671–684
Wang X et al (2011) Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood 117(6):1888–1898
Berger C et al (2008) Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest 118(1):294–305
Wang X et al (2016) Phase 1 studies of central memory-derived CD19 CAR T-cell therapy following autologous HSCT in patients with B-cell NHL. Blood 127(24):2980–2990
Sommermeyer D et al (2016) Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia 30(2):492–500
Turtle CJ et al (2016) CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 126(6):2123–2138
Turtle CJ et al (2015) Immunotherapy with CD19-specific chimeric antigen receptor (CAR)-modified T cells of defined subset composition. J Clin Oncol 33(15_suppl):3006
Gardner R et al (2016) CD19CAR T cell products of defined CD4:CD8 composition and transgene expression show prolonged persistence and durable MRD-negative remission in pediatric and young adult B-cell All. Blood 128(22):219
Popplewell L et al (2018) CD19-CAR therapy using naive/memory or central memory T cells integrated into the autologous stem cell transplant regimen for patients with B-NHL. Blood 132(Suppl 1):610
Khaled SK et al (2018) Adult patients with ALL treated with CD62L+ T Naïve/memory-enriched T cells expressing a CD19-CAR mediate potent antitumor activity with a low toxicity profile. Blood 132(Suppl 1):4016
Fraietta JA et al (2018) Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 24(5):563–571
Egelston CA et al (2018) Human breast tumor-infiltrating CD8(+) T cells retain polyfunctionality despite PD-1 expression. Nat Commun 9(1):4297
Klebanoff CA et al (2011) Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin Cancer Res 17(16):5343–5352
Gattinoni L et al (2011) A human memory T cell subset with stem cell-like properties. Nat Med 17(10):1290–1297
Graham C et al (2018) Allogeneic CAR-T cells: more than ease of access? Cells 7(10)
Torikai H, Cooper LJ (2016) Translational implications for off-the-shelf immune cells expressing chimeric antigen receptors. Mol Ther 24(7):1178–1186
Zhu H et al (2018) Concise review: human pluripotent stem cells to produce cell-based cancer immunotherapy. Stem Cells 36(2):134–145
Jensen MC et al (2000) Human T lymphocyte genetic modification with naked DNA. Mol Ther 1(1):49–55
Barrett DM et al (2014) Relation of clinical culture method to T-cell memory status and efficacy in xenograft models of adoptive immunotherapy. Cytotherapy 16(5):619–630
Xu Y et al (2014) Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood 123(24):3750–3759
Alizadeh D et al (2019) IL15 enhances CAR-T cell antitumor activity by reducing mTORC1 activity and preserving their stem cell memory phenotype. Cancer Immunol Res 7(5):759–772
Gattinoni L et al (2005) Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 202(7):907–912
Heylmann D et al (2013) Human CD4+ CD25+ regulatory T cells are sensitive to low dose cyclophosphamide: implications for the immune response. PLoS ONE 8(12):e83384
Medina-Echeverz J et al (2011) Successful colon cancer eradication after chemoimmunotherapy is associated with profound phenotypic change of intratumoral myeloid cells. J Immunol 186(2):807–815
Turtle CJ et al (2016) Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med 8(355):355ra116
Thistlethwaite FC et al (2017) The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol Immunother 66(11):1425–1436
Wang X et al (2018) Lenalidomide enhances the function of CS1 chimeric antigen receptor-redirected T cells against multiple myeloma. Clin Cancer Res 24(1):106–119
Li S et al (2019) Decitabine enhances cytotoxic effect of T cells with an anti-CD19 chimeric antigen receptor in treatment of lymphoma. Onco Targets Ther 12:5627–5638
Suryadevara CM et al (2018) Temozolomide lymphodepletion enhances CAR abundance and correlates with antitumor efficacy against established glioblastoma. Oncoimmunology 7(6):e1434464
Newick K, Moon E, Albelda SM (2016) Chimeric antigen receptor T-cell therapy for solid tumors. Mol Ther Oncolytics 3:16006
Schmidts A, Maus MV (2018) Making CAR T cells a solid option for solid tumors. Front Immunol 9:2593
Koneru M et al (2015) IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology 4(3):e994446
Murad JP et al (2018) Effective targeting of TAG72(+) peritoneal ovarian tumors via regional delivery of CAR-engineered T cells. Front Immunol 9:2268
Katz SC et al (2016) Regional CAR-T cell infusions for peritoneal carcinomatosis are superior to systemic delivery. Cancer Gene Ther 23(5):142–148
Maus MV et al (2013) T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res 1:26–31
Beatty GL et al (2014) Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res 2(2):112–120
Adusumilli PS et al (2014) Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med 6(261):261ra151
Burga RA et al (2015) Liver myeloid-derived suppressor cells expand in response to liver metastases in mice and inhibit the anti-tumor efficacy of anti-CEA CAR-T. Cancer Immunol Immunother 64(7):817–829
Priceman SJ et al (2018) Regional delivery of chimeric antigen receptor-engineered T cells effectively targets HER2(+) breast cancer metastasis to the brain. Clin Cancer Res 24(1):95–105
Brown CE et al (2018) Optimization of IL13Ralpha2-targeted chimeric antigen receptor T cells for improved anti-tumor efficacy against glioblastoma. Mol Ther 26(1):31–44
Du H et al (2019) Antitumor responses in the absence of toxicity in solid tumors by targeting B7-H3 via chimeric antigen receptor T cells. Cancer Cell 35(2):221–237e8
Majzner RG et al (2019) CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin Cancer Res 25(8):2560–2574
Nehama D et al (2019) B7-H3-redirected chimeric antigen receptor T cells target glioblastoma and neurospheres. EBioMedicine
Leuci V et al (2018) CD44v6 as innovative sarcoma target for CAR-redirected CIK cells. Oncoimmunology 7(5):e1423167
Todaro M et al (2014) CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell 14(3):342–356
Feng K et al (2016) Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci China Life Sci 59(5):468–479
Deng Z et al (2015) Adoptive T-cell therapy of prostate cancer targeting the cancer stem cell antigen EpCAM. BMC Immunol 16(1)
Kandalaft LE, Powell DJ Jr, Coukos G (2012) A phase I clinical trial of adoptive transfer of folate receptor-alpha redirected autologous T cells for recurrent ovarian cancer. J Transl Med 10:157
Wing A et al (2018) Improving CART-cell therapy of solid tumors with oncolytic virus-driven production of a bispecific T-cell engager. Cancer Immunol Res 6(5):605–616
Louis CU et al (2011) Antitumor activity and long-term fate of chimeric antigen receptor–positive T cells in patients with neuroblastoma. Blood 118(23):6050–6056
Mount CW et al (2018) Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M+ diffuse midline gliomas. Nat Med 24(5):572–579
Yvon E et al (2009) Immunotherapy of metastatic melanoma using genetically engineered GD2-specific T cells. Clin Cancer Res 15(18):5852–5860
Gao H et al (2014) Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res 20(24):6418–6428
Li K et al (2016) Adoptive immunotherapy using T lymphocytes redirected to glypican-3 for the treatment of lung squamous cell carcinoma. Oncotarget 7(3):2496–2507
Feng K et al (2017) Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell 9(10):838–847
Ahmed N et al (2007) Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Can Res 67(12):5957–5964
Sun M et al (2014) Construction and evaluation of a novel humanized HER2-specific chimeric receptor. Breast Cancer Res 16(3)
Bamdad CC et al (2018) Anti-MUC1* CAR T for solid tumors. Can Res 78(13 Supplement):2544
Priceman SJ et al (2018) Co-stimulatory signaling determines tumor antigen sensitivity and persistence of CAR T cells targeting PSCA+ metastatic prostate cancer. Oncoimmunology 7(2)
Junghans RP et al (2016) Phase I trial of anti-PSMA designer CAR-T cells in prostate cancer: possible role for interacting interleukin 2-T cell pharmacodynamics as a determinant of clinical response. Prostate 76(14):1257–1270
Posey AD Jr et al (2016) Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity 44(6):1444–1454
Parkhurst MR et al (2011) T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 19(3):620–626
Jones BS et al (2014) Improving the safety of cell therapy products by suicide gene transfer. Front Pharmacol 5:254
Fan L et al (1999) Improved artificial death switches based on caspases and FADD. Hum Gene Ther 10(14):2273–2285
Clackson T et al (1998) Redesigning an FKBP-ligand interface to generate chemical dimerizers with novel specificity. Proc Natl Acad Sci U S A 95(18):10437–10442
Straathof KC et al (2005) An inducible caspase 9 safety switch for T-cell therapy. Blood 105(11):4247–4254
Di Stasi A et al (2011) Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 365(18):1673–1683
Tasian SK et al (2017) Optimized depletion of chimeric antigen receptor T cells in murine xenograft models of human acute myeloid leukemia. Blood 129(17):2395–2407
Philip B et al (2014) A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy. Blood 124(8):1277–1287
Sommer C et al (2019) Preclinical evaluation of allogeneic CAR T cells targeting BCMA for the treatment of multiple myeloma. Mol Ther 27(6):1126–1138
Valton J et al (2018) A versatile safeguard for chimeric antigen receptor T-cell immunotherapies. Sci Rep 8(1):8972
Wang X et al (2011) A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 118(5):1255–1263
Weber EW et al (2019) Pharmacologic control of CAR-T cell function using dasatinib. Blood Adv 3(5):711–717
Mestermann K et al (2019) The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci Transl Med 11(499)
Steentoft C et al (2018) Glycan-directed CAR-T cells. Glycobiology 28(9):656–669
De Pascalis R et al (2003) In vitro affinity maturation of a specificity-determining region-grafted humanized anticarcinoma antibody: isolation and characterization of minimally immunogenic high-affinity variants. Clin Cancer Res 9(15):5521–5531
Posey AD Jr, Clausen H, June CH (2016) Distinguishing truncated and normal MUC1 glycoform targeting from Tn-MUC1-specific CAR T cells: specificity is the key to safety. Immunity 45(5):947–948
Mahanta S et al (2008) A minimal fragment of MUC1 mediates growth of cancer cells. PLoS ONE 3(4):e2054
Chekmasova AA et al (2010) Successful eradication of established peritoneal ovarian tumors in SCID-Beige mice following adoptive transfer of T cells genetically targeted to the MUC16 antigen. Clin Cancer Res 16(14):3594–3606
Koneru M et al (2015) A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric antigen receptors for recurrent ovarian cancer. J Transl Med 13:102
Kloss CC et al (2013) Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol 31(1):71–75
Foster AE et al (2017) Regulated expansion and survival of chimeric antigen receptor-modified T cells using small molecule-dependent inducible MyD88/CD40. Mol Ther 25(9):2176–2188
Mata M et al (2017) Inducible activation of MyD88 and CD40 in CAR T cells results in controllable and potent antitumor activity in preclinical solid tumor models. Cancer Discov 7(11):1306–1319
Parekh HD et al (2019) Disease characteristics and treatment outcomes of young colorectal cancer patients. J Clin Oncol 37(4_suppl): 691–691
Maude SL et al (2015) CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood 125(26):4017–4023
Majzner RG, Mackall CL (2018) Tumor antigen escape from CAR T-cell therapy. Cancer Discov 8(10):1219–1226
Hamieh M et al (2019) CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 568(7750):112–116
Gardner R et al (2018) Early clinical experience of CD19 × CD22 dual specific CAR T cells for enhanced anti-leukemic targeting of acute lymphoblastic leukemia. Blood 132(Suppl 1):278
Zah E et al (2016) T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol Res 4(6):498–508
Shah NN et al (2019) Results of a phase I study of bispecific anti-CD19, anti-CD20 chimeric antigen receptor (CAR) modified T cells for relapsed, refractory, non-Hodgkin lymphoma. J Clin Oncol 37(15_suppl):2510
Ruella M et al (2016) Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Invest 126(10):3814–3826
Shah NN et al (2019) Multi targeted CAR-T cell therapies for B-cell malignancies. Front Oncol 9:146
Lee L et al (2018) An APRIL-based chimeric antigen receptor for dual targeting of BCMA and TACI in multiple myeloma. Blood 131(7):746–758
Wilkie S et al (2012) Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J Clin Immunol 32(5):1059–1070
Hegde M et al (2016) Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J Clin Invest 126(8):3036–3052
Choi BD et al (2019) CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol 37(9):1049–1058
Roybal KT et al (2016) Engineering T cells with customized therapeutic response programs using synthetic notch receptors. Cell 167(2):419–432
Srivastava S et al (2019) Logic-gated ROR1 chimeric antigen receptor expression rescues T cell-mediated toxicity to normal tissues and enables selective tumor targeting. Cancer Cell 35(3):489–503
Fedorov VD, Themeli M, Sadelain M (2013) PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med 5(215):215ra172
Tamada K et al (2012) Redirecting gene-modified T cells toward various cancer types using tagged antibodies. Clin Cancer Res 18(23):6436–6445
Ma JS et al (2016) Versatile strategy for controlling the specificity and activity of engineered T cells. Proc Natl Acad Sci U S A 113(4):E450–E458
Lohmueller JJ et al (2017) mSA2 affinity-enhanced biotin-binding CAR T cells for universal tumor targeting. Oncoimmunology 7(1):e1368604
Rodgers DT et al (2016) Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc Natl Acad Sci U S A 113(4):E459–E468
Raj D et al (2019) Switchable CAR-T cells mediate remission in metastatic pancreatic ductal adenocarcinoma. Gut 68(6):1052–1064
Cho JH, Collins JJ, Wong WW (2018) Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell 173(6):1426–1438
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21(3):309–322
Quail DF, Joyce JA (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19(11):1423–1437
Binnewies M et al (2018) Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 24(5):541–550
Mardiana S et al (2019) Supercharging adoptive T cell therapy to overcome solid tumor-induced immunosuppression. Sci Transl Med 11(495)
Maus MV, June CH (2016) Making better chimeric antigen receptors for adoptive T-cell therapy. Clin Cancer Res 22(8):1875–1884
Yin Y et al (2018) Checkpoint blockade reverses anergy in IL-13Rα2 humanized scFv-based CAR T cells to treat murine and canine gliomas. Mol Ther Oncolytics 11:20–38
Serganova I et al (2017) Enhancement of PSMA-directed CAR adoptive immunotherapy by PD-1/PD-L1 blockade. Mol Ther Oncolytics 4:41–54
Wang H et al (2019) Immune checkpoint blockade and CAR-T cell therapy in hematologic malignancies. J Hematol Oncol 12(1):59
Cao Y et al (2019) Anti-CD19 chimeric antigen receptor T cells in combination with nivolumab are safe and effective against relapsed/refractory B-cell non-hodgkin lymphoma. Front Oncol 9:767
Chong EA et al (2017) PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood 129(8):1039–1041
Prosser ME et al (2012) Tumor PD-L1 co-stimulates primary human CD8(+) cytotoxic T cells modified to express a PD1:CD28 chimeric receptor. Mol Immunol 51(3–4):263–272
Liu X et al (2016) A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res 76(6):1578–1590
Cherkassky L et al (2016) Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest 126(8):3130–3144
Rafiq S et al (2018) Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol 36(9):847–856
Li S et al (2017) Enhanced cancer immunotherapy by chimeric antigen receptor-modified T cells engineered to secrete checkpoint inhibitors. Clin Cancer Res 23(22):6982–6992
Guo X et al (2018) Disruption of PD-1 enhanced the anti-tumor activity of chimeric antigen receptor T cells against hepatocellular carcinoma. Front Pharmacol 9:1118
Rupp LJ et al (2017) CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep 7(1):737
Baylis F, McLeod M (2017) First-in-human phase 1 CRISPR gene editing cancer trials: are we ready? Curr Gene Ther 17(4):309–319
Mariathasan S et al (2018) TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554:544
Golumba-Nagy V et al (2018) CD28-ζ CAR T cells resist TGF-β repression through IL-2 signaling, which can be mimicked by an engineered IL-7 autocrine loop. Mol Ther 26(9):2218–2230
Kloss CC et al (2018) Dominant-negative TGF-beta receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol Ther 26(7):1855–1866
Sukumaran S et al (2018) Enhancing the potency and specificity of engineered T cells for cancer treatment. Cancer Discov 8(8):972–987
Boyerinas B et al (2017) A novel TGF-β2/interleukin receptor signal conversion platform that protects CAR/TCR T cells from TGF-β2-mediated immune suppression and induces T cell supportive signaling networks. Blood 130(Suppl 1):1911
Hou AJ et al (2018) TGF-beta-responsive CAR-T cells promote anti-tumor immune function. Bioeng Transl Med 3(2):75–86
Pegram HJ et al (2012) Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 119(18):4133–4141
Yeku OO et al (2017) Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci Rep 7(1):10541
Hurton LV et al (2016) Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc Natl Acad Sci U S A 113(48):E7788–E7797
Krenciute G et al (2017) Transgenic expression of IL15 improves antiglioma activity of IL13Rα2-CAR T cells but results in antigen loss variants. Cancer Immunol Res 5(7):571–581
Tang L et al (2018) Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat Biotechnol 36(8):707–716
Mohammed S et al (2017) Improving chimeric antigen receptor-modified T cell function by reversing the immunosuppressive tumor microenvironment of pancreatic cancer. Mol Ther 25(1):249–258
Wang Y et al (2019) An IL-4/21 inverted cytokine receptor improving CAR-T cell potency in immunosuppressive solid-tumor microenvironment. Front Immunol 10:1691
Atherton MJ, Lichty BD (2013) Evolution of oncolytic viruses: novel strategies for cancer treatment. Immunotherapy 5(11):1191–1206
Kaufman HL, Bines SD (2010) OPTIM trial: a phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage III or IV melanoma. Future Oncology 6(6):941–949
Watanabe K et al (2018) Pancreatic cancer therapy with combined mesothelin-redirected chimeric antigen receptor T cells and cytokine-armed oncolytic adenoviruses. JCI insight 3(7):e99573
Shaw AR et al (2017) Adenovirotherapy delivering cytokine and checkpoint inhibitor augments CAR T cells against metastatic head and neck cancer. Mol Ther 25(11):2440–2451
Galon J, Bruni D (2019) Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov 18(3):197–218
Chen DS, Mellman I (2017) Elements of cancer immunity and the cancer-immune set point. Nature 541(7637):321–330
Blank CU et al (2016) Cancer immunology. The “cancer immunogram”. Science 352(6286):658–660
Wherry EJ, Kurachi M (2015) Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15(8):486–499
Pauken KE et al (2016) Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354(6316):1160–1165
Scheper W et al (2019) Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat Med 25(1):89–94
Yost KE et al (2019) Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med 25(8):1251–1259
Sampson JH et al (2014) EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss. Clin Cancer Res 20(4):972–984
Pituch KC et al (2018) Adoptive transfer of IL13Rα2-specific chimeric antigen receptor T cells creates a pro-inflammatory environment in glioblastoma. Mol Ther 26(4):986–995
Adachi K et al (2018) IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol 36(4):346–351
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Stern, L.A., Jonsson, V.D., Priceman, S.J. (2020). CAR T Cell Therapy Progress and Challenges for Solid Tumors. In: Lee, P., Marincola, F. (eds) Tumor Microenvironment. Cancer Treatment and Research, vol 180. Springer, Cham. https://doi.org/10.1007/978-3-030-38862-1_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-38862-1_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-38861-4
Online ISBN: 978-3-030-38862-1
eBook Packages: MedicineMedicine (R0)