Abstract
Nerve transfers involve taking an expendable motor or sensory nerve and anastomosing it to a damaged nerve for restoration of critical motor function or sensation. The use of nerve transfers, while rare in compressive neuropathies, can be considered in cases of severe proximal compression leading to axonal loss or in cases of iatrogenic injury from previous surgery. Nerve transfers are classically described using end to end nerve coaptation; however, in cases of nerve compression we would recommend the use of distal end to side transfers as it allows for the augmentation of proximal native nerve recovery while allowing for early reinnervation. Recent data suggests that nerve transfers can help to restore motor function and protective sensibility in cases of compressive ulnar neuropathy. Here we describe the principles of nerve transfers and strategies for both motor and sensory transfers in cases of median and ulnar compressive neuropathies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Nerve transfers involve the division of an expendable motor or sensory nerve and anastomosing it to the distal intact portion of an injured nerve. Nerve coaptation can be performed in an end to end (ETE), or end to side (ETS) fashion beyond the zone of injury. The nerve transfer is ideally performed close to the end organ (muscle or skin); thus minimizing the time required for innervation from the donor transferred nerve. Historically, nerve transfers have been utilized for the treatment of traumatic proximal motor nerve injuries, particularly those involving the brachial plexus. Nerve transfers are most commonly used as an alternative to direct nerve repair and nerve grafting when the site of nerve damage is very proximal, precluding re-innervation of distal target muscles or when the area of injury is extensive, necessitating long intercalated nerve grafts with low potentials for meaningful recovery [1].
The use of nerve transfers, while rare in the majority of compressive neuropathies, can be considered in cases of severe proximal compression leading to axonal loss or in cases of iatrogenic injury following surgery. While most nerve transfers have classically been performed as ETE repairs, in this chapter we will advocate for the use of ETS transfers. ETS transfer allows for the preservation of recovering native nerve fibers while also allowing for the benefits of a distal ETS transfer. Recent data suggests that the benefits of ETS transfers can include preservation of motor end plates, improved native nerve recovery and additional axonal fiber ingrowth from the donor nerve [2, 3]. Such ETS transfers have been referred to as “supercharged” end to side transfers (SETS) and can help to preserve muscle function, restore protective sensation and potentially accelerate the recovery of native ingrowing nerve fibers [2, 4, 5]. Here we describe the principles and strategies for nerve transfers to restore sensation and muscle function in cases of severe median and ulnar compressive neuropathies.
Nerve Transfer Principles
Nerve transfers are performed as an alternative to direct nerve repair and nerve grafting. The procedure involves transfer a local, uninjured and expendable nerve or fascicle to the injured nerve close to the recipient end organ (muscle or skin). It effectively converts a proximal nerve injury into a distal injury. The classic example is that of the Oberlin transfer where a motor fascicle of the ulnar nerve is transferred to the biceps for restoration of elbow flexion following an upper trunk brachial plexus injury [6, 7]. The Oberlin procedure is illustrative of the potential advantages of nerve transfer procedures as the donor nerve is attached close to the biceps muscle, thus minimizing the time for innervation. Decreasing the time to innervation will theoretically decreasing the loss of motor end plates and fatty replacement of the denervated muscle [8]. The second major benefit is that the transfer can be performed out of the zone of injury. Theoretically one transferred nerve can innervate multiple muscle groups, such as the anterior interosseous nerve transferred to the motor branch of the ulnar nerve. Finally, nerve transfers, (if successful), can reestablish the native tendon muscle interaction which can restore independent finger motion, in comparison to tendon transfers which link muscle groups together.
More recent evidence has suggested that end to side motor transfers provide the added benefit of releasing neurotropic factors to the proximal and distal ends of the injured nerve which can stimulate the recovery of proximal native nerve fibers [2]. MacKinnon and colleagues have developed the term supercharging or SETS transfer, as a way to describe an ETS nerve transfers, which augment native nerve healing. The SETS transfer has been shown to add regenerating motor axons across the ETS coaptation to reinnervate the neuromuscular junction. Farber and colleagues looked at nerve recovery in an incomplete sciatic nerve injury model in mice, in which the tibial nerve was cut and a nerve allograft was interposed [2]. This was compared to those mice where the peroneal nerve was sutured in a SETS fashion in addition to the tibial nerve isograft. During nerve regeneration, they found axonal contribution from both the partially injured tibial nerve and axonal sprouting from the SETS peroneal nerve [2]. This correlated to increased function and a higher muscle specific force during sciatic nerve stimulation of the gastrocnemius muscle compared to the mice without the SETS transfer. When the peroneal nerve contribution was removed by cutting the coaptation, the increased force was no longer significant [2]. The authors believe that the SETS transfer acts to “babysit” the muscle and protect it from atrophy by providing axons at an earlier time point in addition to donating additional motoneurons to achieve target muscle reinnervation [2].
Nerve transfers are contraindicated when there is end-organ unresponsiveness, motor denervation for greater than 12 months, or loss of the musculotendinous unit due to trauma or fibrosis. A healthy donor nerve, as evident by a strong donor muscle, is required for a successful nerve transfer, typically greater than a Modified British Medical Research Council (BMRC) graded 4. In addition, there should be a large proportion of the type of axons required for the function you wish to restore (i.e.: large number of purely motor axons for motor reconstruction). Thus, one should strive for a pure motor or sensory nerve with greater than M4 or S4 at the time of transfer.
Joint releases, if necessary, should be performed prior to nerve transfer and passive motion of all effected joints maximized. If tendon transfers have been performed in addition to nerve transfers, then the tendon transfer rehabilitation will dictate the post-operative protocol [9]. The time required for functional recovery following nerve transfer can vary from 6 to 12 months or longer [6, 10].
Diagnosis
Nerve transfer for median or ulnar nerve compression should be reserved for cases exhibiting signs of pre-operative axonal injury. Compressive neuropathies progress in severity and early cases will present with intermittent symptoms and this is often a sign of dynamic reversible nerve ischemia. If compression is persistent, nerve ischemia can progress to demyelination; in these cases, the nerve conduction studies will show slowing conduction velocities. If compression is severe or long standing, nerve injury progresses from demyelination to axonal injury. These patients classically have signs of motor weakness on physical exam and evidence of muscle atrophy. For cases of severe ulnar nerve compression, we would expect to see intrinsic wasting, inability to cross the fingers, a positive Froment sign and clawing. For cases of severe median nerve compression at the level of the wrist one should see thenar atrophy and loss of palmar abduction of the thumb. These physical findings can be cooperated with the use of nerve conduction studies where one will see decrease amplitude. The EMG will show signs of fibrillations during the resting phase which is a definitive sign for denervation. If motor unit action potentials are seen during the recruitment phase of the EMG, this indicates collateral sprouting and evidence of attempted recovery.
In regard to chronic ulnar neuropathy, recent findings from Power and colleagues show that a reduction in compound muscle action potentials (CMAP) in the first dorsal interosseous muscle is a reliable indicator of axonal loss and substantial nerve compression [11]. In these cases, the nerve will recover at 1 mm per day in an ideal environment. This means that if the compression has occurred at the level of the elbow it may be over a year until the intrinsic muscles in the hand will receive re-innervation. Based on the time line of injury this may result in poor recovery of motor function without the aid of a SETS transfer.
SETS transfer should be reserved for patients with electro diagnostic evidence of denervation. Fibrillation in the intrinsic muscles in the hand points to recent denervation and the potential for re-innervation. CMAPs should be of low amplitude reflecting the severity of axonal involvement; however absence of CMAPs suggests that the amount of nerve injury has been present too long to allow for meaningful recovery [11]. Davidge and colleagues have shown that patients who showed little improvement following SETS transfer had absence of fibrillation and CMAPs preoperatively [12]. As a corollary, CMAPs of good amplitude suggest that recovery of function may occur with simple decompression and SETS may not be required. To summarize, the best patients for transfer will:
-
1.
Have signs of ulnar atrophy/ or median nerve atrophy.
-
2.
Have evidence of fibrillations on EMG.
-
3.
Decreased, but not absent, CMAPs.
We will now examine the details of specific transfers for both ulnar and median nerve compression.
Ulnar Nerve Compression
Ulnar Nerve Compression Below the Elbow
In a low ulnar nerve palsy, the flexor carpi ulnaris (FCU) and flexor digitorum profundus (FDP) to the ring and little finger are spared. Thus, the deep motor branch to the intrinsic hand muscles is affected, in addition to the volar sensation to the little finger, half of the ring finger and the palm. Intrinsic motor loss can lead to a claw deformity. Coordinated flexion is lost as the lumbrical muscles first flex the metacarpophalangeal joints (MCPJ) when making a fist, followed by activation of the extrinsic flexor tendons. With intrinsic loss, the extrinsic flexors will initiate movement and finger flexion will begin at the interphalangeal joints (IPJs), which results in a rolling motion where the fingers prematurely close before they reach the palm [13]. Additionally, with the loss of the adductor pollicus, 1st dorsal interosseous, and deep head of the flexor pollicis brevis (FPB) key pinch is weakened. Due to loss of finger adduction from the interossei muscles, the little finger can develop an abducted posture due to the unopposed pull of the extensor digiti minimi, producing the Wartenberg’s sign [13]. While most cases of compression do not display all of these signs we would advocate for SETS transfers for those with evidence of 1st dorsal interosseous atrophy and decreased CMAPs.
Motor SETS Transfer in Cases of Severe Lower Ulnar Compression
The target nerve for motor reconstruction of low ulnar nerve palsy is the deep motor branch of the ulnar nerve. This is usually found between the dorsal cutaneous branch of the ulnar nerve (DCBUN) and the sensory branch of the ulnar nerve [14]. The common donor nerve transfers are the anterior interosseous nerve (AIN) from the median nerve [4, 12, 15], and end to side from the thenar motor branch using a bridging nerve graft [16] (See Table 18.1 for list of possible nerve donors and recipients).
The AIN transfer can be done as ETE transfer or an end-to-side SETS “supercharge” transfer into the ulnar motor branch. The ETE transfer is typically performed if there is no chance of recovery of the ulnar nerve from a proximal injury. If there is an incomplete or mixed injury, as in the case of severe compression, where some motor recovery is expected, then a SETS transfer may be beneficial. This can help to reinnervate the distal targets quicker and preserve motor plate function while the native ulnar nerve recovers [2, 12, 14].
The SETS AIN to deep motor transfer has been shown to improve recovery of intrinsic function with complete ulnar nerve injury. Davidge and colleagues in 2015, reported the outcomes of their SETS AIN to ulnar motor transfer in a mixed group of patients with severe motor and sensory ulnar nerve dysfunction [12]. At an average of 8 months following SETS transfer, 70% of patients had BMRC >M3, as opposed to 15% pre-operatively. Motor improvement was seen in 50% of patients between 3 and 12 months, which was attributed to the SETS transfer [12]. Those with evidence of AIN injury or absent motor unit potentials on pre-operative EMG had worse recovery. Following sacrifice of the AIN they did not report any limitations in forearm pronation.
While the use of SETS transfers in chronic ulnar nerve compression is still controversial several studies have noted promising results. Barbour and colleagues suggest that the use of the SETS AIN transfer is an important adjunct in those patients with severe or failed cubital tunnel surgery [14]. In Davidge’s study, there were 15 patients with a compression neuropathy at the elbow who underwent both SETS AIN to ulnar motor transfer in conjunction with an anterior transposition and Guyon’s release; seven of these patients had a rapid recovery [12]. Unfortunately, they did not perform any subgroup analysis separating the compressive group from the other traumatic injuries. Baltzer and colleagues evaluated the use of the SETS AIN transfer in a matched cohort study comparing ulnar nerve repair alone with ulnar nerve repair and SETS AIN transfer, in addition to ulnar nerve release alone with ulnar nerve release and SETS AIN transfer. They found that the SETS AIN transfer improved intrinsic recovery, with 84% recovery with the transfer compared to 38% without. In this study, recovery of motor function was more rapid with SETS transfer occurring at an average of 3.4 months versus 12.0 months in those without the transfer [4]. It is important to note that the conventional group that received a cubital tunnel and Guyon’s canal release and those with an added SETS transfer had a 67% chance long term of recovering intrinsic function [4]. While results with traumatic injuries are encouraging, more study will be needed to determine which patients with compressive neuropathies are the best candidates for SETS AIN transfer. Since the donor site morbidity is so low, we still recommend this procedure for patients with evidence of atrophy and low amplitude CMAP response in the first dorsal interosseous muscle (Fig. 18.1).
A case of a 61 year old man with signs of severe ulnar nerve compression on EMG and nerve conduction studies. The site of compression is localized to the cubital tunnel. CMAPs were reduced in the ADM muscle and 1st dorsal interosseous muscle. (a) The patient’s hand shows signs of clawing pre-operatively. (b) Incisions are planned for full ulnar nerve release with submuscular transposition, AIN distal SETS transfer, and cross bridging sensory graft from median to ulnar sensory nerve at the level of the wrist. (c) AIN transfer completed in an ETS fashion to ulnar motor branch. (d) Bridging allografts or “cross-cross grafts” were used to go from median sensory nerve to 3rd web space and long finger to both sensory fascicles of the ulnar nerve going to ring and small finger
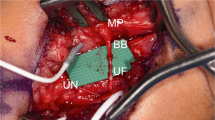
As an alternative to the AIN SETS transfer, Sherif and Amr looked at using a nerve graft “bridge” sutured end to side into both the median and ulnar nerve in four patients with either a high median or ulnar nerve injury [16]. All cases were combined with grafting of the proximal injured nerve. The authors found early intrinsic hand muscle reinnervation with these high injuries, suggesting that reinnervation occurred through the bridge graft. In all patients, EMG studies had activation of the recipient nerve’s intrinsic muscles through the donor nerve and bridge graft and not from the repaired ulnar nerve [16]. We find this a good option when severe compression has occurred at the level of Guyon’s canal or in cases of severe proximal median nerve compression (Fig. 18.2).
An alternative to AIN SETS transfer in patients with pre-existing injury to AIN or injury distal to Guyon’s canal is a bridge graft performed from in an ETS fashion from the median motor branch to the deep motor branch of the ulnar nerve. (a–f) A case of a 12 year old boy with an injury to the ulnar nerve at the level of Guyon’s canal and inadequate motor recover despite nerve grafting at the site of injury. Image (a) shows scar over Guyon’s canal and (b) shows persistent evidence of clawing and intrinsic weakness. Images (c, d) show palmar incision with isolation of recurrent thenar motor branch beneath superior blue vessel loop. Lower vessel loop surrounds ulnar motor branch. Image (e) shows nerve graft sew ETS into thenar motor branch. Image (f) shows completed graft going from median motor to ulnar motor branch. Figure (g) shows another case utilizing allograft for bridge grafting
Surgical Technique: AIN to Deep Ulnar Motor Branch SETS Technique
The ulnar nerve is exposed in the distal forearm through either a curvilinear or Bruner-style incision (Fig. 18.3). The flexor carpi ulnaris (FCU ) tendon and muscle belly is retracted ulnarly to identify the ulnar artery and nerve. The branch point of the DCBUN is identified. The most ulnar fascicle distal to this level will represent the deep motor branch, while the radial fascicle is the sensory nerve. If needed, the ulnar nerve and deep motor branch can be identified distally with a Guyon’s canal release and traced proximally to determine the correct ulnar nerve topography. The ulnar motor branch can be stimulated to ensure there is no recovery or function prior to transfer. A formal internal neurolysis is not typically necessary or recommended. The point of the coaptation is typically just distal to the DCBUN branch point.
Technique for AIN transfer. Isolation of ulnar motor branch in forearm should be performed distal to dorsal sensory branch take off. (a) Intraoperative case showing red vessel loop around more radial sensory component while arrow points to motor branch. If there is any question of orientation, dissection can be performed retrograde after release of Guyon’s canal. (b, c) A spate case showing isolation and dissection of AIN through the cut portion of the pronator quadratus muscle. The nerve should be dissected terminally to obtain longus possible graft length. (d) An End to side neuroraphy is performed with aid of operating room microscope. Figure (e) shows AIN transfer (small arrow) and deep course of motor branch as it enters Guyon’s canal
The finger flexor muscle bellies are retracted radially to expose the pronator quadratus (PQ) and the AIN motor branch and anterior interosseous artery as they enter the proximal aspect of the pronator (Fig. 18.3). The AIN is dissected distally by using bipolar cautery to release the muscle as it lies on top of the AIN. The AIN is dissected until it begins branching, ensuring to maximize the diameter of the nerve for coaptation. The AIN is then divided at this point and dissected proximally until it is able to transpose ulnarly to the ulnar motor nerve area. A distal portion of the insertion of the flexor digitorum profundus can be released to improve the reach of the AIN. The area of coaptation is identified and an epineurial and perineurial window is made on the ulnar aspect of the ulnar motor branch. This will avoid the need for internal neurolysis and potential coaptation into the sensory branch of the ulnar nerve. The AIN is sutured end to side to the deep ulnar motor branch using 9-0 nylon sutures and is then bathed in fibrin glue (Fig. 18.3). Fibrin glue should be placed along the margins of the PQ dissection to prevent bleeding. The tension on the coaptation should be checked with range of motion of the wrist and elbow.
Sensory Reconstruction
The target nerves for reconstruction of the low ulnar nerve palsy sensory deficits are the branch to the 4th webspace [6], the ulnar digital nerve to the little finger [17] or the ulnar sensory nerve proper [6]. The common donor are the 3rd webspace nerve from the median nerve [6, 18, 19], the radial digital nerve of the middle or ring finger [20], the lateral antebrachial cutaneous nerve (LABC) [17, 18, 21], the DCBUN (in low palsies), the palmar cutaneous branch of the median or ulnar nerve [17] and end to side into the median nerve [6]. In addition, Mackinnon has recently reported a side to side bridging nerve graft, similar to that described Sherif and Amr, to restore sensation in the ulnar digits [5] (Fig. 18.1).
A commonly described transfer combination to restore full ulnar sensation is as follows: (1) median nerve branch to the 3rd webspace end-to-end to the ulnar sensory branch (2) DCBUN end-to-side to the median nerve (3) distal stump of the 3rd webspace donor nerve placed end-to-side back to the median nerve to minimize the donor deficit [6, 19, 22, 23]. This is typically performed in conjunction with an AIN transfer to the deep motor branch and can be performed at the wrist level to avoid painful scars in the palm or injury to the palmar arches. The third webspace branch can be readily identified and neurolysed from the median nerve to provide length for transfer, ensuring to preserve interfascicular branches [22] (Fig. 18.4). Another option to restore sensation to the volar aspect of the ulnar nerve would be to cut the ulnar sensory nerve and take the distal stump end-to-side to the 3rd webspace median nerve fascicle, which would be the most ulnar aspect of the median nerve. Few studies report on the outcomes of these transfers. It is felt that they provide protective sensation and may take up to 2 years to reach maximal benefit [19].
The use of the radial digital nerve of the middle or ring finger to the ulnar digital nerve was studied by Brunelli, who achieved S2+ (tactile sensation with associated allodynia) in two patients and S1 (protective sensation) in a third patient. Bertelli described the transfer of the palmar cutaneous branch of the median nerve to the ulnar digital nerve of the little finger in patients with lower brachial plexus injuries to restore sensation [17]. Two patients had an additional transfer from the ulnar digital nerve of the index. They achieved recovery of two-point discrimination <10 mm in three of eight patients (S3+), five had >S3 recovery (perceived contact from a 33.1-g/mm2 monofilament) [17]. Oberlin described the LABC transfer to the DCBUN with an interpositional graft to restore protective sensation in patients with a lower brachial plexus injury [24]. Felder et al. have introduced the idea of the bridging nerve graft for recovery of sensation, which they term a “cross-cross graft” [5]. In their series, 48 patients had one or more bridging nerve grafts, consisting of both allograft and autograft, placed between the ulnar and median sensory components in the palm. In this study 20 of these patients had severe cubital tunnel syndrome, 60% of which were revision cases. Of the 24 patients with complete data, 21 (87%) recovered protective sensation within 1 year [5]. Due to the limited donor site morbidity of this procedure, this is our transfer of choice in patients with severe ulnar nerve compression and severe sensory loss (Figs. 18.1 and 18.5).
Examples of cross-cross grafts for restoration of ulnar nerve function. Image (a) shows a case of severe ulnar nerve compression occurring at the elbow in a 58 year old man with >12 mm pre-operative 2 point sensation in small and ring finger. (a) Arrow points to visible compression of nerve following nerve release at cubital tunnel. (b, c) Show image of cross-cross graft with arrow pointing to proximal allograft running from median sensory of 3rd web to the ulnar aspect of the ulnar sensory nerve and the distal graft (arrowhead) running to radial component of ulnar sensory nerve. (d) Severe damage to the ulnar nerve may necessitate the use of an ETE nerve transfer of the 3rd web space branch of the median nerve to the sensory branch to the small and ring finger. Image (d) shows such a transfer. The sensory component of the median nerve has been isolated with a green micro background from larger median nerve (Solid arrow head). 3rd web space branch is sewn end to end into the sensory component of ulnar nerve (black arrow), while the distal sensory stump of the median nerve sensory branch with be sewn ETS into the main sensory branch of the median nerve (Clear arrowhead)
High Ulnar Neuropathy
In high ulnar nerve injuries, in addition to the deficits of a low injury, there is a loss of FCU and FDP function. There is loss of sensation of the dorsal hand and digits from the DCBUN. This sensation can be lost in a low ulnar nerve palsy, as well, if the injury is proximal to the takeoff of the DCBUN. The claw deformity is less severe as there is reduced pull from the ulnar FDP muscle belly [13].
Motor Reconstruction
The same options are present as for a low ulnar nerve palsy, and the SETS AIN to deep motor branch transfer is still considered the gold standard. Additional reconstruction of the FCU or FDP branches of the ulnar nerve are not typically attempted, as the FCR tendon can adequately power wrist flexion, and a side-to-side tenorrhaphy of the ring and little finger FDP tendons to the long finger FDP tendon (excluding the index to preserve independent function) produces adequate function.
Sallam et al. performed an AIN to ulnar motor branch in conjunction with sensory transfers from the 3rd webspace branch of the median nerve to the ulnar sensory branch with an end-to-side transfer of the DCBUN and the donor nerve stump to the median nerve in patients with high ulnar nerve injuries [23]. This group was compared to a group where nerve grafting of the nerve injury was performed without transfers. They had 83% M3 or greater recovery of intrinsic function in the nerve transfer group versus 57% in those with only nerve grafts. There was no difference in sensory recovery, with 58% and 54% of patients achieving S3 or greater sensation in the transfer and graft group, respectively [23]. In terms of sensory donor morbidity, two patients were aware of the loss of sensation and all patients regained at least protective sensation (S1).
Sensory Reconstruction
The same options would be available for a high ulnar nerve lesion, as described previously. A high ulnar nerve injury would definitively affect the DCBUN, which may or may not be affected in a low palsy. The reconstruction of the DCBUN is not considered a critical sensory area, but can be important as the ulnar side of the hand acts as a support while the radial side performs manipulation [17].
Median Nerve Compression
Low Median Neuropathy
A low median neuropathy produces a loss of motor function distal to the branch point of the AIN; thus, the motor function of the pronator teres, FCR, FDS, palmaris longus (PL) are preserved. The palmar cutaneous branch is also spared. Weakness of loss of thumb thenar function results in limited thumb opposition, palmar abduction and pronation. Some function of the flexor pollicus brevis may remain due to ulnar nerve innervation, but pinch strength has been shown to be limited to 60% and 70% of the contralateral side [25]. The sensory loss in these injuries is extremely disabling due to the use of the radial digits for fine manipulation. Sensory loss to the thumb can result in a 20% global functional loss to the entire hand [26]. There are few reports in the literature commenting on SETS transfers in cases of severe median neuropathies, however in the section below we present the nerve transfers which can be used to resort both motor and sensory function.
Motor Reconstruction
The recipient nerve for reconstruction of intrinsic hand function after a low median nerve injury is the recurrent motor branch of the median nerve. The typical donor nerves are the AIN [6], FDS, FCR, PL, abductor digiti minimi (ADM) branch of the ulnar nerve [25], or the 3rd lumbrical branch from the ulnar nerve [27].
The AIN and recurrent motor branch have comparable axon counts of 900 and 1050, respectively, and given that it is predominately a motor branch at this level, it is a good choice as a donor nerve. However, it is necessary to perform an interpositional graft. It is approached through an extended carpal tunnel release with neurolysis of the recurrent motor branch as proximal as possible, after which the resulting gap is grafting with an interpositional graft. Wang and Zhu transferred the AIN to the recurrent motor branch in 14 patients with a low median nerve palsy at an average of 5 years and 8 months, with 3 patients obtaining M5, 6 with M4, 3 with M3 and 2 with M2 [28]. The transfer from ulnar nerve branches of the ADM or 3rd lumbrical can allow direct coaptation due to their proximity and are beneficial in high median nerve injuries when the AIN is not a usable donor and nerve recovery may be prolonged with nerve repair or grafting (Fig. 18.6). This transfer will be discussed further as a SETS transfer in the high median nerve injury section.
A case of an ADM SETS transfer to median nerve motor branch in 15 year old who suffered supracondylar fracture with no thenar function at 4 months and evidence of decreased CMAPs to that muscle. (a) The median nerve was explored at the elbow. (b) Exploration of nerve and intraoperative nerve stimulation revealed intact nerve with evidence of partial axonal injury (arrow points to area of severe nerve contusion). (c) ADM motor branch transfer was performed to help preserve thenar function. (d) ADM was isolated and motor branch dissected back to the deep motor branch of the ulnar nerve. Arrow points to ADM motor branch. The median nerve motor branch was identified after carpal tunnel release (e) (arrow points to motor branch). And SETS transfer was then performed as close to thenar muscle as possible (f)
Sensory Reconstruction
To restore critical median nerve sensation, the radial digital nerve of the index and ulnar digital nerve of the thumb should be restored [6]. Common donor nerves are the 4th webspace branches of the ulnar nerve (ulnar digital nerve of the ring and radial digital nerve of the little finger) [6, 26], the DCBUN [22], and the superficial branch of the radial nerve [26, 29,30,31], which are less critical sensory areas. The 4th webspace branch is identified in the palm around the metacarpal head and transferred end-to-end to the 1st webspace branches of the median nerve, while the distal stump of the 4th webspace branch of the ulnar nerve is transferred end-to-side back to intact sensory branches to attempt to preserve donor site sensation [6]. A similar technique can be performed with the DCBUN by tracing it as distal as possible, transferring it to the thumb and radial aspect of the index sensory branches, and transferring the distal stump end to side to intact ulnar nerve fascicles [22]. To restore sensation to the second and third webspace, these can be transferred end-to-side to the ulnar digital nerve of the little finger. Brunelli performed a transfer of the DCBUN to the 1st webspace in two patients with a brachial plexus injury, however, both did not regain any sensation (S0) [20]. When he used the 4th webspace nerve to the 1st webspace in two patients, they regained tactile sensation (S2) [20].
The use of the radial nerve was first reported by Harris in 1921 to restore median nerve sensation in with a low median nerve injury with recovery beginning around 3 months post-operative [31]. Brunelli performed 12 cases of transfer from the radial sensory branches of the 1st webspace to the thumb and index finger at the 1st webspace and 2 similar transfers in the wrist in patients with a brachial plexus injury [20]. He had 6 patients with S2+, 6 with S2, 1 with S1 (protective sensation) and 1 patient with S0 (no sensation). Additionally, he had three patients where he transferred digital nerves from the ring and little to the thumb and index, in which they achieved S2 function [20]. Ozkan et al. performed various digital nerve transfers for both median and ulnar nerve injuries. They were able to achieve two-point discrimination of less than 10 mm in 15 of their 25 patients [26]. Of these patients, 7 had a median nerve injury of which all recovered >S3 sensation after a transfer to either an index or thumb digital nerve from the digital nerves of the ring or long finger [26].
High Median Neuropathy
In addition to the motor deficits of a low median neuropathy of the thenar musculature, a high median neuropathy also affects pronator teres, FCR, FDS, PL and AIN (FDP, pronator quadratus and flexor pollicis longus) function. The AIN and the branches to pronator teres now become a priority to provide proper hand function as tendon transfers to restore thumb opposition are well described and successful, while there are few successful tendon transfers to restore pronation [32]. The sensory deficit of a high median neuropathy includes the palmar cutaneous branch of the median nerve. It can be more difficult to regain sensation in a high median nerve injury versus a low injury. In a low injury, the sensation can be restored more predictably through direct suturing or repair with nerve grafting. With a high injury, there is a longer distance to reinnervate the target area and thus less chance of recovering protective sensation or motor function [29]. It can also be more difficult to line up the proper fascicular pattern to reinnervate distal targets when the injury is more proximal.
Motor Reconstruction
In addition to reinnervating the recurrent motor branch, the AIN and pronator teres branch are additional targets in a high median nerve injury and are no longer available donors. The common transfers are as follows: (1) ECRB, brachioradialis, supinator or FCU transferred to the pronator teres branch (2) Supinator, ECRB or brachialis branch of the musculocutaneous nerve to the AIN [22]. The use of ECRB for pronator teres is a synergistic transfer typically performed end-to-end and is preferred by some authors [6]. Hsiao et al. described a case of a high median nerve injury after a humeral fracture where a supinator to AIN and ECRB to pronator teres was performed [32]. The patient progressed from M0 AIN and pronator function to M4+ pronator teres and flexor pollicis longus and M4-FDP function at 18 months [32].
If the injury is not a complete high median neuropathy, then FCR, FDS or PL branches can be used to reconstruct the AIN, if available. As stated previously reconstruction of high median nerve injuries can be performed using a bridging nerve graft sutured end to side into both the median and ulnar nerve [16]. This allows axon sprouting between the nerves and recovery of the damaged recipient nerve function through the donor nerve both clinically and on EMG studies has been demonstrated.
Shultz and Aiache described the transfer of the ulnar nerve fascicle to the 3rd lumbrical to the recurrent motor branch in a patient with a high median nerve laceration [6]. The ulnar nerve branch was detached just proximal to the myoneural junction and the recurrent motor was neurolysed to allow tension free coaptation as close to the thenar musculature as possible. The patient recovered thumb abduction at 11 weeks post-operatively and regained pinch motion between his thumb, index and small fingers. EMG studies showed conduction from the ulnar nerve into the abductor pollicis brevis [6]. Bertelli et al. performed a cadaveric study and case series describing the transfer of the ADM branch of the ulnar nerve, which is the first branch of the deep motor branch of the ulnar nerve, to the recurrent motor branch of the median nerve. This was performed in five patients with a high median nerve injury, three of which had a concomitant ECRB to AIN transfer. All patients recovered M4 thumb and index finger flexion and an average grasp and pinch strength of 77% and 75% of the contralateral hand, respectively. The ABP strength improved from an average of M1.8 to M4 [25]. Our experience with this transfer has also been favorable (Fig. 18.6).
Sensory Reconstruction
As with low median nerve palsies, the target for reconstruction is the 1st webspace branches of the median nerve. Typically, the additional deficit of the palmar branch of the median nerve is not a priority for reconstruction as ulcers and injuries occur usually at the fingertips and not on the palm [29]. It has been reported that protective sensation to the palm and proximal aspects of the thumb and long finger are typically preserved by the palmar branches of the radial nerve after a median nerve injury and reconstruction should be focused on the tips of the thumb and index [20]. The options exist as per a low median nerve palsy, using the radial sensory nerve, the 4th webspace, DCBUN or LABC. Bertelli and Ghizoni performed transfer of the dorsal branches of the radial sensory nerve to the proper digital nerves of the index and thumb at the digital level in eight patients with a high median nerve injury [29]. Within 3–4 months, all thumbs recovered protective sensation and at 6 months, the index fingers recovered protective sensation in seven of eight patients (ability to feel a 2.0 g Semmes-Weinstein monofilament). At 12 months, three of eight patients had normal sensation in the thumb (ability to feel a 0.05 g filament) with no donor site deficit [29].
Summary
The indications for nerve transfers in cases of severe nerve compression are evolving. The use of SETS transfers may offer the benefit of more rapid motor recovery and improved sensory recovery long term. While not all patients with compressive neuropathies are candidates, we would recommend considering SETS transfers for patients with ulnar nerve compression at the elbow with evidence of decreased CMAP to the hand intrinsic muscles. Crossing bridge grafts may also be a means of improving sensation with little donor site morbidity. The use of nerve transfers in median nerve compression requires additional study; however, transfers can be considered in cases of high median nerve compression where critical sensation and thenar function are significantly affected (Fig. 18.7).
(a) A case of a 63 year old woman with history of rheumatoid arthritis and previous total elbow replacement with recurrent ulnar nerve compression following release done elsewhere. (b, c) Pre-operatively the hand had evidence of intrinsic atrophy and decreased CMAPs within the ulnar intrinsic muscles to the hand. (d) At time of cubital tunnel release the patient was found to have significant scaring and fibrosis around the nerve. (e) AIN SETS transfer was performed in conjunction with a proximal neurolysis. Good intrinsic function was obtained at 5 months following the surgery and continued to improve for 12 months (f–h)
References
Tung TH, Barbour JR, Gontre G, Daliwal G, Mackinnon SE. Transfer of the extensor digiti minimi and extensor carpi ulnaris branches of the posterior interosseous nerve to restore intrinsic hand function: case report and anatomic study. J Hand Surg Am. 2013;38(1):98–103.
Farber SJ, Glaus SW, Moore AM, Hunter DA, Mackinnon SE, Johnson PJ. Supercharge nerve transfer to enhance motor recovery: a laboratory study. J Hand Surg Am. 2013;38(3):466–77.
Isaacs J, Patel G, Mallu S, et al. Effect of reverse end-to-side (supercharging) neurotization in long processed acellular nerve allograft in a rat model. J Hand Surg Am. 2019;44(5):419.e1–419.e10.
Baltzer H, Woo A, Oh C, Moran SL. Comparison of ulnar intrinsic function following supercharge end-to-side anterior interosseous-to-ulnar motor nerve transfer: a matched cohort study of proximal ulnar nerve injury patients. Plast Reconstr Surg. 2016;138(6):1264–72.
Felder JM, Power H, Hill E, Hasak J, Mackinnon SE. Cross-cross sensory nerve grafts to enhance sensory recovery in complex ulnar neuropathy [abstract]. Paper presented at: American Society of Peripheral Nerve Annual Meeting, Phoenix, Arizona; 12–14 Jan 2018.
Brown JM, Mackinnon SE. Nerve transfers in the forearm and hand. Hand Clin. 2008;24(4):319–40, v.
Moran SL, Steinmann SP, Shin AY. Adult brachial plexus injuries: mechanism, patterns of injury, and physical diagnosis. Hand Clin. 2005;21(1):13–24.
Gordon T, Tyreman N, Raji MA. The basis for diminished functional recovery after delayed peripheral nerve repair. J Neurosci. 2011;31(14):5325–34.
Sammer DM, Chung KC. Tendon transfers: part I. Principles of transfer and transfers for radial nerve palsy. Plast Reconstr Surg. 2009;123(5):169e–77e.
Schreiber JJ, Byun DJ, Khair MM, Rosenblatt L, Lee SK, Wolfe SW. Optimal axon counts for brachial plexus nerve transfers to restore elbow flexion. Plast Reconstr Surg. 2015;135(1):135e–41e.
Power HA, Sharma K, El-Haj M, Moore AM, Patterson MM, Mackinnon SE. Compound muscle action potential amplitude predicts the severity of cubital tunnel syndrome. J Bone Joint Surg Am. 2019;101(8):730–8.
Davidge KM, Yee A, Moore AM, Mackinnon SE. The supercharge end-to-side anterior interosseous-to-ulnar motor nerve transfer for restoring intrinsic function: clinical experience. Plast Reconstr Surg. 2015;136(3):344e–52e.
Woo A, Bakri K, Moran SL. Management of ulnar nerve injuries. J Hand Surg Am. 2015;40(1):173–81.
Barbour J, Yee A, Kahn LC, Mackinnon SE. Supercharged end-to-side anterior interosseous to ulnar motor nerve transfer for intrinsic musculature reinnervation. J Hand Surg Am. 2012;37(10):2150–9.
Novak CB, Mackinnon SE. Distal anterior interosseous nerve transfer to the deep motor branch of the ulnar nerve for reconstruction of high ulnar nerve injuries. J Reconstr Microsurg. 2002;18(6):459–64.
Magdi Sherif M, Amr AH. Intrinsic hand muscle reinnervation by median-ulnar end-to-side bridge nerve graft: case report. J Hand Surg Am. 2010;35(3):446–50.
Bertelli JA. Distal sensory nerve transfers in lower-type injuries of the brachial plexus. J Hand Surg Am. 2012;37(6):1194–9.
Ray WZ, Chang J, Hawasli A, Wilson TJ, Yang L. Motor nerve transfers: a comprehensive review. Neurosurgery. 2016;78(1):1–26.
Brown JM, Yee A, Mackinnon SE. Distal median to ulnar nerve transfers to restore ulnar motor and sensory function within the hand: technical nuances. Neurosurgery. 2009;65(5):966–77; discussion 977-968
Brunelli GA. Sensory nerves transfers. J Hand Surg Br. 2004;29(6):557–62.
Ruchelsman DE, Price AE, Valencia H, Ramos LE, Grossman JA. Sensory restoration by lateral antebrachial cutaneous to ulnar nerve transfer in children with global brachial plexus injuries. Hand (N Y). 2010;5(4):370–3.
Moore AM, Franco M, Tung TH. Motor and sensory nerve transfers in the forearm and hand. Plast Reconstr Surg. 2014;134(4):721–30.
Sallam AA, El-Deeb MS, Imam MA. Nerve transfer versus nerve graft for reconstruction of high ulnar nerve injuries. J Hand Surg Am. 2017;42(4):265–73.
Oberlin C, Teboul F, Severin S, Beaulieu JY. Transfer of the lateral cutaneous nerve of the forearm to the dorsal branch of the ulnar nerve, for providing sensation on the ulnar aspect of the hand. Plast Reconstr Surg. 2003;112(5):1498–500.
Bertelli JA, Soldado F, Rodrigues-Baeza A, Ghizoni MF. Transfer of the motor branch of the abductor digiti quinti for thenar muscle reinnervation in high median nerve injuries. J Hand Surg Am. 2018;43(1):8–15.
Ozkan T, Ozer K, Gulgonen A. Restoration of sensibility in irreparable ulnar and median nerve lesions with use of sensory nerve transfer: long-term follow-up of 20 cases. J Hand Surg Am. 2001;26(1):44–51.
Schultz RJ, Aiache A. An operation to restore opposition of the thumb by nerve transfer. Arch Surg. 1972;105(5):777–9.
Wang Y, Zhu S. Transfer of a branch of the anterior interosseus nerve to the motor branch of the median nerve and ulnar nerve. Chin Med J. 1997;110(3):216–9.
Bertelli JA, Ghizoni MF. Very distal sensory nerve transfers in high median nerve lesions. J Hand Surg Am. 2011;36(3):387–93.
Turnbull F. Restoration of digital sensation after transference of nerves. J Neurosurg. 1963;20:238–40.
Harris RI. The treatment of irreparable nerve injuries. Can Med Assoc J. 1921;11(11):833–41.
Hsiao EC, Fox IK, Tung TH, Mackinnon SE. Motor nerve transfers to restore extrinsic median nerve function: case report. Hand (N Y). 2009;4(1):92–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gillis, J.A., Moran, S.L. (2020). Nerve Transfers for Neuropathies of the Median and Ulnar Nerve. In: Sotereanos, D., Papatheodorou, L. (eds) Compressive Neuropathies of the Upper Extremity. Springer, Cham. https://doi.org/10.1007/978-3-030-37289-7_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-37289-7_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-37288-0
Online ISBN: 978-3-030-37289-7
eBook Packages: MedicineMedicine (R0)