Abstract
Northeastern Brazil is home to the Caatinga Forest, characterized as a Tropical Dry Forest (TDF), and the Dense Ombrophilous Forest (the Atlantic Forest) predominates near the coast. In the Caatinga, the climate is semiarid, with <600 mm of mean annual precipitation and a mean annual air temperature of 27 °C ± 6 °C. The precipitation presents a seasonal pattern, with a rainy season concentrated in the first half of the year and at least 6 months of dry season, with volumes <50 mm/month. The climate in the Atlantic Forest has a milder air temperature and a mean rainfall of 1450 mm/year. In both regions the tree species, Cedrela odorata L., widely studied in dendrochronology, presents populations in farms, with several individuals per hectare. In recent decades, 10 chronologies of C. odorata have been produced and compared to precipitation and other environmental factors, and their competition with lianas and support of the herbivorous process has been noted. This chapter presents the potential of C. odorata for dendrochronology in Northeastern Brazil; the anatomy of true rings with marginal parenchyma associated with vessels differed from the false rings by the absence of vessels. Absent rings were also observed. Nine chronologies had correlations above the critical level of 0.51 (0.51–0.79) and sensitivity between 0.547 and 0.771. The correlation between all series (rbt) of all chronologies had values between 0.27 and 0.68 and expressed population signal (EPS) above 0.85 (between 0.88 and 0.98). In both biomes, several populations of C. odorata have resulted in climate-related chronologies that showed the seasonal rainfall from May to July induces metabolism and growth rings formation. We also presented the methodology used for tropical dendroecology studies and the relationship between plant growth and environmental conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
1.1 Dendrochronology of Cedrela
Species of the genus Cedrelaoriginate in the Neotropical region, and studies of their tree rings have been producing chronologies since the last decades of the twentieth century. Several papers were published, mainly in Argentina, Brazil, and Bolivia (Chalk 1983; Détienne 1989; Vetter and Botosso 1989; Worbes 1989, 1992, 1995; Botosso et al. 2000; Brienen and Zuidema 2005, 2006a, b; Marcati et al. 2006; Villalba et al. 2006). Studies of the wood anatomy, annual formation of tree rings, and growth rates support dendrochronology and neotropical dendroecology.
One of the first studies showing the potential of Cedrela for climate reconstruction was done by Villalba et al. (1985) with populations of Cedrela angustifolia Sessé & Moc. ex DC. from forests (Salta and Jujuy states) in Argentina. From the chronology of 39 trees collected in 1982, the authors observed high sensitivity, a strong common sign in the growth and correlation with low temperatures and high precipitation during the beginning of the growing season (September and November). Two years later, Villalba et al. (1987) presented a new analysis of two chronologies of C. angustifolia. The first from Cerro Chañar (Salta) was correlated with precipitation in the spring–summer period. The second chronology from the Rio Blanco region (Jujuy) is related to summer temperatures.
In 1989, in Argentina, Boninsegna et al., analyzed the formation of annual growth rings and the increment rate of 13 tree species; one of them was Cedrela fissilis Vell., which had 15 trees studied and the annual formation of growth rings were described. The results showed it was possible that trees ages were determinate on the increment average of this species in this region.
In the 1990s, the relationship between the climate pattern and the tree growth variation for 12 chronologies of Juglans australis Griseb., C. angustifolia, and Cedrela lilloi in the Argentinean mountain forests of Northwestern Argentina was analyzed. These chronologies indicated that growth ring widths are affected by late winter and early summer climatic conditions in this region. Between 60% and 80% of the variation in annual and seasonal precipitation for the region was explained using these ring-width chronologies as predictive variables (Villalba et al. 1992).
At the beginning of the twenty-first century, Tomazello et al. (2000) indicated the potentiality of the Meliaceae family for dendrochronology in tropical and subtropical regions. A section of this paper provides a historical survey of the main research developed with Cedrela species. The next year, Tomazello et al. (2001) emphasized the potential of C. angustifolia for tropical dendrochronology, including X-ray densitometry analyses for the delimitation of growth rings.
In Brazil, Dünisch et al. (2002) studied populations of C. odorata from Central Amazonia to verify growth rings and the dynamics of intra-annual xylem growth (activation and inactivation changes). Dünisch et al. (2003) analyzed the growth rings in relation to precipitation from the central Amazon. The results show a significant correlation between tree rings and the precipitation in March and May of the previous growth period. Also in the Amazon, Dünisch (2005) used the ring widths to produce a growth curve of C. fissilis compared to El Niño-Southern Oscillation (ENSO) records showing that anomalies of this event influenced the growth variability of the studied populations, but the chronologies were not indicative of reconstruction of El-Niño events.
In Equador, the work of Bräuning et al. (2009) studied the Cedrela montana in the rainforest of the mountains in the south of the country. The chronology with extension up to 1840 showed statistical quality reached from 1910 when it included 13 individual series of trees. The authors did not find a good correlation between the chronology and the environmental factors indicating a relation between growth and temperature between January and April, and this factor influenced only 8% of the growth variation of these plants. This result led to the discussion of whether regional climatic data are representative for the studied population, since the species is subject to strong interference of the local climate due to mountainous topography. Another question was whether trees could also respond to other regional factors that were not reflected in the local climate data series.
Espinoza et al. (2014) studied the C. odorata (47 series of 27 trees) of the Peruvian Central Forest. The chronology had an extension of 215 years (1795–2009) with a close relationship between tree growth and total precipitation during the rainy season. They emphasized the influence of the precipitation of the rainy season of the previous year, the precipitation of the end of the dry season and the beginning of the rainy season of the current year.
In the same year, Becerra-Montalvo and Zevallos-Pollito (2014) used the dendrochronology of C. odorata to indicate the cutting cycle (minimum time required for new logging) of the species recorded in San Ignacio, Cajamarca region, Peru. The authors concluded that the region’s C. odorata has an ideal cutting cycle of 113 years. Andreacci et al. (2014) studied two sites with C. fissilis from the Ombrofilous Dense (plateau) and Mixed (flat land) Forests in the Atlantic Forest of Paraná, Brazil, and found a positive climatic correlation between the chronology of the plateau trees and the precipitation and temperature, but a negative correlation with the flatland population.
In Bolivia, Paredes-Villanueva et al. (2016) analyzed the relationship between growth rates of C. fissilis and C. angustifolia with precipitation data. Three sites with variable environmental impacts were selected: dry Chiquitano-Concepción (C. fissilis), Amazon transitional-Guarayos (C. fissilis), and mountain-Postrervalle forest (C. angustifolia). The populations studied were sensitive to rainfall from October to April of the current year and to the period of low temperatures between May and July. Lobão et al. (2016) analyzed the periodicity of the growth of Cedrela sp. in different conditions of development in Acre, Brazil. As they verified a significant correlation between the different growth conditions of the trees, the researchers indicated that dendrochronology can subsidize plans and decisions in sustainable forest management programs.
Köhl et al. (2017) studied 20 C. odorata trees (mean 138 years) from a rainforest in Suriname. Growth rings were used as a parameter to calculate the accumulation of carbon in the individuals’ life history. It was shown that in the final quarter of life of the trees there was an average accumulation of 39% of their total carbon stock, suggesting that the species maintains a high stock rate and accumulation in the adult phase of the trees. Baker (2017) tested the periodicity of the Cedrela sp. growth rings comparing the chronology with the radiocarbon dating. The results showed that trees from Bolivia, Ecuador, and Venezuela formed a ring per year, whereas Suriname trees formed two rings annually.
Granato-Souza et al. (2018) produced the first chronology of long-term C. odorata (1786–2016) that correlated sensitively with the humidity of the Equatorial Amazon. This chronology was used to reconstruct precipitation from February to November, mainly between the late eighteenth and nineteenth centuries. These dendroecological data of native species are important to understand the interannual and decadal variability of the rains, providing a valuable perspective on the hydrological history in the Amazon, as well as the forest structure and dynamics for this region.
Some studies have used chronologies and data from Cedrelagrowth rings for isotopic analyses in order to understand the efficient use of water by trees and annual precipitation reconstruction (Ballantyne et al. 2011; Brienen et al. 2012; Baker et al. 2015; Brienen et al. 2017). Theses were written on the dendrochronology and dendroecology of the Cedrela in Brazil, for example: reconstruction of the environmental scenario of São Paulo using dendrochronology (Ferreira 2012); management with populations of the Amazonian floodplain (Rosa 2008); dendroecology of C. fissilis from subtropical mountain forests of Brazil (Rauber 2010); dendrochronology, phenology, cambial activity, and wood quality in plants from Acre (Lobão 2011).
In the past decade, studies with C. odorata were started in the Tropical Dry Forests (TDF) of Northeastern Brazil, producing a PhD thesis with chronologies of growth rings compared to regional environmental conditions and a previous study of the 13C/12C isotopic ratio in the growth rings of TDF (Caatinga) and Atlantic Forest trees from Sergipe state (Anholletto Junior 2013). In this chapter, we aim to show some results from the studies of the C. odorata growth rings achieved by the team of the Tree Ring Laboratory of Federal University of Sergipe.
1.2 The Cedrela odorata L. (Meliaceae)
The C. odorata is known by popular names such as cedro-rosa, cedro-amargoso, cedro-cheiroso, cedro-fêmea, cedro-mogno, and cedro-vermelho. Trees reach up to 25–35 m in height, with a straight or tortuous trunk, 90–150 cm in diameter, in tropical regions where the precipitation reaches from 2500 to 4000 mm/year, and they have lower growth in drier areas, such as in the TDF (Tomazello et al. 2000). The young stems exude a characteristic odor when broken. C. odorata has composite leaves, paripinates, with opposing leaflets (10–16 leaflets), sessile leaves 8–15 cm long, and terminal inflorescences, pendant, 20–40 cm long, unissex flowers. The fruit capsule of 2.0–3.5 cm in length is the main differential characteristic of this species from C. fissilis that has larger fruit with 4.5–8.5 cm. The seeds have wings and are wind dispersed (Dávila et al. 2008).
C. odorata are deciduous, heliophyte or capable of growing under diffuse light, and are found in forests of secondary succession, usually on edges or in clearings (Amaral 2006). It flowers during the months of December–February and the fruits mature from May, when the plant is without leaves (Lorenzi 2009). In Northeastern Brazil, flowering is observed during the rainy season, the fruits mature after 4 months of flowering, and the leafless period occurs in the dry season (Fig. 3.1) (Maia 2012). The water deficit causes cambial dormancy, making the increment in tree circumference minimal or absent, forming the limits of the growth rings (Worbes 1995). The wood density is 0.66 g/cm3, it is soft and easy to cut, and used for laminates, furniture, plywood, etc.; thus, it has been exploited, reducing the natural reserves (Roweder 2011).
1.3 Distribution, Environments, and Study Sites of Cedrela odorata
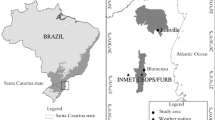
The C. odorata has great ecological range, occurring from Mexico, Central America, the Caribbean Islands, and in the countries of South America, except for Chile (Pennington 1981) (Fig. 3.2a). In Brazil, it is common in the Amazon Rainforest, the TDF-Caatinga, and the Atlantic Forest (Tomazello et al. 2000) (Fig. 3.2b). In Sergipe, the populations were found from near the coast, in the Atlantic Forest until the TDF-Caatinga region, as well as in the transitional places of these forests—Agreste (data kept in the Xiloteca ASEw) (Fig. 3.2c). Its occurrence was also recorded by the National Forest Inventory—Sergipe (Serviço Florestal Brasileiro 2017).
(a) Neotropical occurrence of Cedrela odorata L. (www.tropicos.org accessed 26/02/2019); (b) Records in Brazil (www.cria.org.br accessed 26/02/2019); (c) Records in Sergipe (xiloteca ASEw)
The Semideciduous Seasonal Forest (called Atlantic Forest) in Sergipe, Brazil occupied 40% of this territory, but at the beginning of the twenty-first century only 1% of the original vegetation was registered restricted to fragmentation (Ribeiro and Siqueira 2001). In this environment, these authors recorded 469 species, belonging to 94 families, such as Myrtaceae, Fabaceae, and Rubiaceae. The Meliaceae is represented by the C. odorata L. (cedro), Trichilia lepdota Mart., and Trichilia sp. Patrick Browne (Serviço Florestal Brasileiro 2017).
The predominant climate from the Atlantic Forestregion, Sergipe State is “As” with dry summers (according to Köppen’s climate ranking – Alvares et al. 2013). The average annual rainfall on the Atlantic coast is 1200 mm, decreasing inland up to 800 mm. This rainfall is concentrated during April and July (60% of total annual), and the dry season occurs from September to December (10% of annual rainfall). The mean annual temperature is 26 °C (18–28 °C) (Fig. 3.3). The altitude in Sergipe varies between 300 m a.s.l. (80% of area) and 600 m. Neosols predominate on the coast (Jacomine 2008–2009).
Climatic diagrams for the periods 1913 & 2004 for the Atlantic Forest (Propriá) and 1914 & 2009 for TDF-Caatinga (Porto da Folha) study sites. The diagram consists of the monthly mean temperature (°C – dashed rows) and total precipitation (mm – solid rows). (Data was downloaded from the Instituto Nacional de Pesquisas Espaciais (www.inpe.br))
The TDF are represented by biomes Cerrado, Caatinga (Savanna formation), and Campos Rupestres (Janzen 1988). The Caatinga area is 844.453 km2, present in 11% of Brazilian territory, including the northeast semi-arid region of Brazil (70% of area) and in the north of Minas Gerais State (Alves 2007). This vegetation is always subject to long dry periods and presents adaptations, such as leaf changes (Loiola et al. 2012), thorns, and small size. The Caatinga can be hyperxerophytes and hypoxerophytes. From Mariano Neto (2001), the characteristics of the hyperoxerophytic Caatinga are determined by the volume of rains below 900 mm/year, high temperatures during the dry season, aridity, and low retention of water in the soil, which is shallow and stony. In the hypoxerophytic Caatinga, the vegetation is arboreal or shrub, with species of humid forest, conferring character of transition (Alves 2008), and precipitation can reach 1200 mm/year (Cordeiro and Oliveira 2010). Common tree species in the Caatinga in Sergipe are Anadenanthera macrocarpa, Schinopsis brasiliensis, Tabebuia caraiba, C. odorata, and others.
The predominant climate in the Atlantic Forest (near the coast in Sergipe) is “As” and in the TDF-Caatinga area it is “BSh”, semi-arid with dry summers (according to Köppen’s climate ranking – Alvares et al. 2013), where the annual rainfall does not exceeds 900 mm. In Northeastern Brazil, the rainfall season occurs between May and July (45% of total annual), followed by a long dry season 7–8 months (Tsuchiya 1995; Sampaio 2010). In Sergipe State the rainfall starts in March or April and the total annual precipitation is <900 mm near the Atlantic Ocean coast (Propriá, SE) and <600 mm in the semi-arid region (Porto da Folha, SE) (Fig. 3.3). The hydrological deficit of the dry season tends to be high. The mean annual temperature is slightly higher than 26.5 °C (19.0–33.0 °C). Neosols are predominant in the northwest region (Jacomine 2008–2009).
The rains that occur in the Sergipe territory depend on several systems of atmospheric circulation (Ferreira and Mello 2005). The main mechanisms that act on the rains in the northeast region are: (i) El Niño Oscilação Sul events (ENOS); (ii) The Sea Surface Temperatures (SST) of the Atlantic Ocean, associated with the trade winds, pressure, and sea level; (iii) Intertropical Convergence Zone (ICZ) on the Atlantic Ocean; (iv) Cold Fronts; and (v) High Level Cyclonic Vortices. These authors also highlight the lines of instability and the effects of sea and land breezes on precipitation.
2 Materials and Methods
2.1 Study Sites
Samples of C. odorata were studied from 11 sites in Sergipe (Table 3.1), with 10–45 trees/sites and between 1 and 4 rays per tree. These populations of cedro are mainly on farms, and in the conservation units of Sergipe there are few records of this species. In the study sites, it was verified that these species are close to each other, near rivers or in soils with rocks.
2.2 Sampling, Polishing, and Cedrela odorata Analysis
The samplings of C. odorata were made mainly by manual core increments (Suunto e Haglof) with internal diameter of 5.15 mm, but a Stihl BT45 motor drill was also used with a core internal diameter of 10–12 mm (no destructive methods), in trees with breast height diameter (BHD) 1.30 m. Wood samples of windows or doors from houses and fences from farms were also sampled.
Sandpapers (40–1500 grains/mm2) and sanding machines with orbital and eccentric movement were used to polish the transversal surface of the samples—. The core microtome WSL was used for better visualization of the anatomical elements of the wood and distinction of the narrow rings. The samples polished with the eccentric machine showed the marginal parenchyma well contrasted with the fibers, due to the powder inside the lume of cells, when examined under a 10× magnifying glass.
The growth rings were analyzed from scanned images (with HP Deskjet 4100). The images were “.tif” with a resolution of 1200 dpi. Measurements of the radial widths of the growth rings were done with ImageProPlus software (version 4.5.0.29 for Windows). The comparison of the images with the samples seen under stereomicroscope guaranteed the correct definition of the limits of the growth rings.
These measures were exported to spreadsheets (such as Excel software), where they were compared graphically and with Pearson correlation, following the cross-dating method and the synchronization procedures. This procedure proved to be efficient for the observation of true rings, verification of missing rings, and determination of false growth rings in C. odorata. However, the quality control of these procedures was made by COFECHA software (Holmes 1983), which indicated other possible corrections to improve the correlations between the series of growth ring measurements. For this, the measured growth rings data are converted to a new file with compact format (Tucson) using the FMT program. For Cofecha analysis, the segments of series measures are adjusted to 20 years lagged successively by 10 years because the trees were not very old. Two results found at the output of the Cofecha are the intercorrelation of the synchronized series and the sensibility of the master series.
Other statistical information has also been calculated, such as the mean inter series (trees) correlations (rbar) calculating correlations for all possible pairs of individual series of the set of series of ring measurements that present a common signal (Briffa and Jones 1990). The expressed population signal (EPS), quantifies the degree to which the chronology expresses the population chronology (Wigley et al. 1984).
The chronologies were prepared from the ARSTAN software (Cook 1985; Cook and Kairiukstis 1990). To removes the variance related to factors other than climate, the individual ring-width series were analyzed with the de-trending method using linear regression, negative curves, and/or cubic smoothing spline function with a 65% cutoff filter (according Cook and Peters 1981). Standard and residual chronologies were compared with instrumental climate records, such as annual rainfall (coincident period to chronologies was 1914–2009) and to monthly rainfall data from all study site regions (data from: Secretaria do Meio Ambiente e Recursos Hidricos – SEMARH, site: https://semarh.se.gov.br), land air temperature index (http://www.ersl.noaa.gov/psd/cgi-bin/data/timeseries), and the monthly sea surface temperature (SST) of the Atlantic Ocean, spatial coordinates between Equador-20S and 10E-30W (http://www.esrl.noaa.gov/psd/data/correlation/tsa.data), among other data (OBS: only the correlation of one chronology with the annual precipitation will be present in this chapter). Chronologies were compared to climate using response and correlation functions (Blasing et al. 1984).
For the microdensitometry X-ray analysis , the radial wood samples were cut in the transverse orientation with 1.0 mm or 2.0 mm of thickness using double circular saws equipment. These thin wood samples were stored in a conditioning chamber (12 h, 20 °C, 50% relative humidity) until reaching 12% moisture content (Tomazello et al. 2008). The analyses proceeded in a Quintek Measurement Systems (QTRX-01X EUA) (Quintek Measurement Systems, 1999). The radial scanning was carried out over the transversal surface of the wood by a collimated X-ray beam. The X-ray values that cross the sample were converted into apparent density (hereafter density) recorded every 80 μm, using QMS software (Ferreira and Tomazello 2009; Castro et al. 2014; Surdi et al. 2014).
3 Results and Discussion
3.1 Growth Rings of Cedrela odorata in Sergipe
The growth rings of C. odorata are anatomical structures of the secondary xylem which, when observed in cross-section, are noted as concentric circles around the bit (IAWA Committee 1989; Larson 1994). These structures are the result of periodic changes in the activity of the cambium, which, in turn, responds to factors such as phenology, temperature, photoperiod, precipitation, and endogenous tree rhythms (Lisi et al. 2008).
The growth rings of C. odorata have their limits marked by the marginal parenchyma associated with vessels (solitary, double or triple) of larger diameter in earlywood (semiporous rings) (Botosso et al. 2000). Flat fibers also occur and with thick cellular walls at the end of the latewood and the rays extend tangentially next to the marginal parenchyma (Fig. 3.4) (Anholetto 2013). In Northeastern Brazil, the growth rings of C. odorata are well defined due to the seasonal climate of the rainy season during the summer and especially in autumn and the dry season in winter and spring, which may occur during 8–9 months of the year.
Transversal slides of Cedrela odorata from Sergipe. (a) Semiporous tree rings and high density of fibers in latewood; (b) Vessels associated with marginal parenchyma and cells of parenchyma distributed between fibers; (c) Detail of the enlargement of the ray cells along the marginal parenchyma. Scale 0.5 mm
The C. odorata from Caatinga, Sergipe, shows beige heartwood, dark pink to light brown and beige or pinkish sapwood, and has a moderate shine, characteristic odor and taste. The heartwood color shows the marginal parenchyma and facilitates the distinction of consecutive growth rings. However, when the sample has this parenchyma darker, the color blends with the remaining tissue (Fig. 3.5). One of the reasons for these differences is the anatomy of the plant, but sometimes the way the polishing of the sample is done hinders good visualization of the limits of the growth rings. Proper polishing was achieved when cell elements (vessels, fibers, and rays) are seen clearly using a 10× magnifying glass.
Many trees of C. odorata in the Caatinga present eccentricity of the trunk that hinders the anatomical visualization of the growth rings, their correct measurement, and the synchronization process of the radial measurements of the series studied in the municipality of Canhoba, Sergipe (10°08′05,8′′S; 37°03′21,1′′W). One radius of the trunk of one tree was larger than the other, so the growth rings had different radial widths (Fig. 3.6b, c, dotted line). Between trees this is more common (Fig. 3.6a, b). This eccentric growth is common, with the marginal parenchyma coming on one side of the trunk. In the cited example, it is also possible to see two other anatomical characteristics: (i) a possible absent ring (Fig. 3.6c, indicated by the small arrow in Fig. 3.6b) and (ii) a possible almost imperceptible growth ring (Fig. 3.6c, indicated by the long arrow) but visible on the other radius (Fig. 3.6b).
Samples of Cedrela odorata from Canhoba, SE. The lines bind to the corresponding growth ring boundaries. (a, b) The true growth rings (solid lines), (c) Growth rings of same tree compressed in the ray (three dotted lines). The larger arrow indicates a ring with a parenchyma difficult to see on the ray (c), and the smaller arrow indicates a growth ring present in distinct tree rays (a, b) and absent in one of the rays of a single tree (b, c)
In one disk of C. odorata, studied true rings were seen (Fig. 3.7a) and false rings identify by confluent parenchyma lines do not continue to be associated with vessels of smaller diameter in relation to the earlywood (Fig. 3.7b). It is harder to identify these false rings in samples collected with increment core because the wood section is narrow (5–12 mm). However, the comparison of two or more samples from the same tree makes it easy to identify many of these false rings, and also during the synchronization of the radial measurements of the growth rings, it is possible to separate them through graphs or statistical analysis.
(a) True growth rings; (b) false rings, with discontinuous confluent parenchyma (solid arrows) and thin parenchyma layers (open arrows); (c) traumatic tissues forming tangential lines (white arrow); (d) Cicatrization, the formation of traumatic tissues, makes the identification of growth rings difficult. Scale 3.0 mm
False growth rings are shown as thin layers of parenchyma circling the trunk, most often near the boundaries of the true ring, and sometimes discontinuous in some pieces (Fig. 3.7b). Thick bands of parenchyma were also observed parallel to a line of traumatic channels, with no change in the diffuse porosity of the growth ring (Fig. 3.7b, first two parenchyma bands from above).
Traumatic tissues have also been seen, which are cellular and intracellular channels that store substances as resin (Burger and Richter 1991). In the C. odorata studied, these channels are formed near the marginal parenchyma (Fig. 3.7c) after injuries that occurred during the inactivity of the cambium. However, traumatic tissues were also seen displaced from the boundaries of growth rings (Fig. 3.7d), when the cambium was active. Traumatic tissues are an analytical problem because the radial measurements of the growth rings are altered when made close to injuries. Another error can occur when the traumatic tissues are not in the limit between two consecutive rings, because the marginal parenchyma is not well distinguished.
Scars are frequently verified on C. odorata trunks in Northeastern Brazil (Fig. 3.7d). They are results of xylem recovery after the tree suffers mechanical shock or insect or other animal attack or human activity. In the radial tissues where the scar occurs, it is difficult to recognize the boundaries of growth rings and/or reaction wood formed. Below we show an example with the primate Callithrix jacchus L. on the growth rings of C. odorata.
3.2 Chronologies, Environment, and Climate
In Sergipe, chronologies of C. odorata collected from 10 study sites showed intercorrelation above 0.51 (Cofecha), which was calibrated for segments of 20 measures with lag of 10 measures. The descriptive statistics of the chronologies are present in Table 3.2. The express population signal (EPS) was above the critical value of 0.85 proposed by Wigley et al. (1984) for all chronologies. The 10 chronologies resulting from the work can be seen in Fig. 3.8.
Growth ring numbers (tree ages) ranged from 36 to 110 years and most plants had less than 60 growth rings. The wood of C. odorata is still used by the owners of the farms, we verified that 11 studied trees of the site S5 were cut after the sampling. There are only a few C. odoratatrees in the conservation units. Besides the difficulty of long chronologies, the conservation of the species is also difficult.
The intercorrelation results (between 0.453 and 0.796), sensitivity (between 0.547 and 0.771), and r bar (between 0.270 and 0.629) (Table 3.2) indicate that populations of C. odorata have potential for growth rings study and are sensitive to environmental conditions, especially due to the region’s low annual rainfall volumes.
Analyses of Pearson correlation (Table 3.3) indicated that only the S2 site was similar to several other study sites (S3, S4, and S8), and the values were just above 0.200. The other sites showed little similarity between them, highlighting only the sites S4 and S8 (0.597) and S9 and S10 (0.648). This may indicate two possibilities: (i) that the irregular climate of Northeastern Brazil (Ferreira and Mello 2005) causes different growth of the trees between the study sites; the rains have different volumes in each place of study; (ii) that the synchronization process needs to be improved since the false rings, for example, may not have been fully identified.
The trees of S4 are kept by the owner of the farm in an area of 50 hectares; thus, today some trees have more than 60 growth rings (reaching 110 rings) and represent a good material to study the environment of TDF-Caatinga. These ages are similar to those of Schinopsis brasiliesis (between 36 and 107 tree rings) (Cardoso 2014). He allowed us to use site S4 to exemplify the relation between the standard chronology of C. odorata and annual rainfall (Fig. 3.9). The value of 0.53 of this correlation was attributed to the irregular rains of the region, when some annual totals are much higher than those of other years (in 1983, 223 mm/year and in 1969 1087 mm/year, mean 560 mm/year), and with more dry months in the years of low precipitation. OBS: In this chapter, we chose to present only the correlations between the chronologies of site S4 with the annual precipitation. The others climatic data and correlations with others chronologies will be presented in future publications.
At site S4, woods from local trees and the region, from the time of construction of the farm were also sampled, such as wood for fences, doors, and windows of houses. Trunks of trees that died after this first step were also sawn for these uses. Sixteen pieces of wood, divided into three lots of samples, synchronized with the Master Series of live trees and correlations were: lot 01 = 0.25 (1889–1941), lot 02 = 0.45 (1902–1945), and lot 03 = 0.44 (1953–2000) (Fig. 3.10). Lot 01 is from the wood of a house on another farm, which may explain the lower correlation. Lots 02 and 03 are wood samples from the site S4, and showed higher correlations. Lot 02 has samples corresponding to the time of construction of the farm and lot 03 of a recent tree. However, lot 01 has extended the Master Series by 14 years, until 1889.
When lianas grow too much on the crown of a tree, they compete with trees for nutrients, light, and water (Gentry 1991; Brandes et al. 2010) and can decrease the number of leaves and increase the mass to be supported by the branches. The analysis of the growth rings enables us to verify decreases in the annual increments of the tree. An example of this competition can be seen in Fig. 3.11, by comparing the annual rainfall history directly with the radial measurements of the growth rings of a tree (site S2), which presented correlation 0.48, it is noted that the growth rings did not follow the annual rainfall volumes well, and that the last rings had lower values (0.65 mm/year) than the mean (2.58 mm/year).
Another interesting case is the action of the fauna on the trees. In the region of Mata Atlântica, in Sergipe, lives a primate, Callithrix jacchus L., with a habit of biting the bark of some species of trees such as C. odorata to eat gum (Castro 2003), when food resources are scarce, but plants can provide sap. The radial measures of the growth rings of a tree that showed signs of primate bites in the trunk were compared with the historical records of the annual rains. The results showed that from the juvenile phase the trees had formed wood of reaction and traumatic tissues, probably due to healing processes; however, the correlation of 0.34 was maintained for approximately 30 years. After this period, the correlation decreased (−0.07) and the mean annual increments of 3.82 mm/year decreased until mean values of 0.74 mm/year (Fig. 3.12), when many scars were seen on the trunk and branches of the tree from the continuous bites of the primates.
3.3 X-Ray Analysis
This analysis was made at the Laboratory of Wood Anatomy, Identification and X-ray Densitometry, of Forest Sciences of “Escola Superior de Agricultura Luiz de Queiroz – ESALQ/USP”. The study site was at Andaraí, Bahia (12°45′54.72′′S 41°06′51.48′′W) (sample example in Fig. 3.13). This analysis shows the apparent differences of wood ring density of earlywood and latewood. The ring boundaries of C. odorata presented an input in density in latewood because of radially flattened and concentrated fibers, and the minor density of marginal parenchyma cells in earlywood is observed. However, it is not observed in all radii analyzed.
Results of one Cedrela odorata X-ray microdensitometry analysis (blue line). Images: negative (above), positive (in the middle), and wood (below). The horizontal line (dotted line), near the wood image, indicates that the first growth rings are tangential to X-ray analysis, making it difficult to define the limits of the growth rings. Limits of the growth rings with lower density of the marginal parenchyma (vertical dotted lines). Confluent parenchyma lines corresponding to false rings (arrows)
The digital X-ray technique provides accurate negative and positive images of the sample, contributing to the definition of annual, false, and absent rings (Fig. 3.14). Marginal parenchyma is better seen in X-ray images than in wood, which makes it easier to define the limits of growth rings, as well as the distribution of vessels and fibers (darker parts).
The wood densitometry profile possibility calculates other parameters, such as mean, max, and minimum densities for every tree ring (Polge 1970; Schweingruber et al. 1978; Tomazello et al. 2008). The precise differences of wood density determined by this technique possibility define tree ring boundaries and identify the false rings. The measurement of radial dimensions of tree rings is used for the synchronization process necessary for chronologies definitions. The X-ray microdensitometry has many applications for wood technology areas, the precise values of wood apparent density are important to define the use of wood species, for example (Tomazello et al. 2008).
4 Conclusions
The Cedrela odorata from Northearstern Brazil have potential for dendrochrology. This species presents true rings and has a good relationship with climate data where the precipitation showed correlation values determined by tree rings series. Missing rings and false rings can be identified by anatomy characteristics, by synchronization of radial series of rings, and using the X-ray method. The climatic seasonality of this region, especially precipitation, with more than 6 dry months, induces the growth period in the plants and growth rings are formed. However, the irregular climate of the northeast region made it difficult to determine a good relationship between chronologies. The exploitation of timber in the past followed by protection laws allowed for the occurrence of C. odorata populations to form groups of plants on farms in this region.
References
Alvares CA, Stape JL, Sentelhas PC et al (2013) Köppen’s climate classification map for Brazil. Meteorol Z 22:711–728. https://doi.org/10.1127/0941-2948/2013/0507
Alves JJA (2007) Geoecologia da caatinga no semi-árido do nordeste brasileiro. Climatologia e Estudos de Paisagens 2(1):58–71
Alves JJA (2008) Biogeografia. João Pessoa: Editora Fotograf. 108p
Amaral VFM (2006) Multiplicação in vitro de Cedrela odorata. Dissertation, Universidade Federal de Santa Maria
Andreacci F, Botosso PC, Galvão F (2014) Sinais climáticos em anéis de crescimento de Cedrela fissilis em diferentes tipologias de florestas ombrófilas do sul do Brasil. Floresta 44(2):323–332
Anholetto Junior CR (2013) Dendroecologia e composição isotópica (δ13C) dos anéis de crescimento de árvores de Cedrela odorata, Meliaceae, na Caatinga e Mata Atlântica do Estado de Sergipe, Brasil. PHD Tesis Escola Superior de Agricultura “Luiz de Queiroz”
Anholetto CR (2013) Dendroecologia e composição isotópica (δ13C) dos anéis de crescimento de árvores de Cedrela odorata, Meliaceae, na Caatinga e Mata Atlântica do estado de Sergipe, Brasil. Dissertation, Esalq. https://doi.org/10.11606/D.11.2013.tde-30102013-105715
Baker JCA, Hunt SFP, Clerici SJ et al (2015) Oxygen isotopes in tree rings show good coherence between species and sites in Bolivia. Global Planet Change 133:298–308. https://doi.org/10.1016/j.gloplacha.2015.09.008
Baker JCA, Santos GM, Gloor M et al (2017) Does Cedrela always form annual rings? Testing ring periodicity across South America using radiocarbon dating. Trees 31(6):1999–2009. https://doi.org/10.1007/s00468-017-1604-9
Ballantyne AP, Baker PA, Chambers JQ et al (2011) Regional differences in South American monsoon precipitation inferred from the growth and isotopic composition of tropical trees. Earth Interact 15(5):1–35. https://doi.org/10.1175/2010EI277.1
Becerra-Montalvo V, Zevallos-Pollito P (2014) Determinación del turno de corta de Cedrela odorata L., Retrophyllum rospigliosii Pilger y Prumnopitys harmsiana Pilger a través del estudio dendrocronológico en San Ignacio, región Cajamarca Perú. Ceprosimad 2(2):33–47
Blasing TJ, Solomon AS, Duvick DN (1984) Response functions revisited. Tree-Ring Bull 44:1–17
Boninsegna JA, Villalba R, Amarilla L et al (1989) Studies on tree rings, growth-rates and age-size relationships of tropical tree species in Missiones, Argentina. IAWA Bull 10:161–169. https://doi.org/10.1163/22941932-90000484
Botosso PC, Vetter RE, Tomazello M (2000) Periodicidade e taxa de crescimento de árvores de cedro (Cedrela odorata L., Meliaceae), jacareúba (Calophyllum angulare A.C. Smith, Clusiaceae) e muirapiranga (Eperua bijiga Mart. ex Benth, Leg. Caesalpinioideae) de floresta de terra firme, em Manaus-AM. In: Roig FA (ed) Dendrocronología en América Latina. Ediunic, Mendoza, pp 357–380
Brandes AFN, Lisi CS, Barros CF (2010) Dendrochronology of lianas of the Leguminosae family from the Atlantic Forest, Brazil. Trees 25(1):133–144. https://doi.org/10.1007/s00468-010-0529-3
Bräuning A, Volland-Voigt F, Burchardt I et al (2009) Climatic control of radial growth of Cedrela montana in a humid mountain rainforest in southern Ecuador. Erdkunde 63:337–345. https://doi.org/10.3112/erdkunde.2009.04.04
Brienen RJW, Zuidema PA (2005) Relating tree growth to rainfall in Bolivian rain forests: a test for six species using tree ring analysis. Oecologia 146:1–12. https://doi.org/10.1007/s00442-005-0160-y
Brienen RJW, Zuidema PA (2006a) Lifetime growth patterns and ages of Bolivian rain forest trees obtained by tree ring analysis. J Ecol 94:481–493. https://doi.org/10.1111/j.1365-2745.2005.01080.x
Brienen RJW, Zuidema PA (2006b) The use of tree rings in tropical forest management: projecting timber yields of four Bolivian tree species. For Ecol Manage 226:256–267. https://doi.org/10.1016/j.foreco.2006.01.038
Brienen RJW, Helle G, Pons TL et al (2012) Oxygen isotopes in tree rings are a good proxy for Amazon precipitation and El Niño-Southern Oscillation variability. PNAS 109(42):16957–16962. https://doi.org/10.1073/pnas.1205977109
Brienen RJW, Gloor E, Clerici S et al (2017) Tree height strongly affects estimates of water-use efficiency responses to climate and CO2 using isotopes. Nat Commun 8(288):110. https://doi.org/10.1038/s41467-017-00225-z
Briffa KR, Jones PD (1990) Basic chronology statistics and assessment. In: Methods of dendrochronology: applications in the environmental sciences. Kluwer Academic Publishers, Dordrecht
Burger LM, Richter HG (1991) Anatomia da madeira. Editora Nobel, São Paulo
Cardoso DS (2014) Caracterização anatômica da madeira e potencial dendrocronológico de Schinopsis brasiliensis Engl. (Anarcadiaceae) na caatinga sergipana. Dissertation, Universidade Federal de Sergipe
Castro CSS (2003) Tamanho da área de vida e padrão de uso do espaço em grupos de saguis Callitrix jacchus (Linnaeus) (Primates Callitrichidae). Rev Bras Zool 20(1):91–96
Castro VRC, Tomazello M, Surdi PG (2014) Avaliação do perfil radial do lenho de árvores de Pinus (Pinus caribaea var. hondurensis Barr. et Golf.) por densitometria de raios X. Sci For 42(103):353–360
Chalk L (1983) Growth rings. In: Metcalfe CR, Chalk L (eds) Anatomy of dicotyledons. Oxford: Claredon Press, pp 250–265
Cook ER, Peters K (1981) The smoothing spline: a new approach to standardizing forest interior tree-ring with series for dendroclimatic studies. Tree-Ring Bull 41:45–53
Cook ER (1985) A time series analysis approach to tree-rings standardization. The University of Arizona, Tucson
Cook ER, Kairiukstis LA (1990) Methods of dendrochronology. Springer Netherlands, Dordrecht
Cordeiro JMP, Oliveira AG (2010) Levantamento fitogeográfico em trecho de Caatinga hipoxerófita – Sítio Canafístula, Sertãozinho-Paraíba, Brasil. OKARA: Geografia em debate 4(1–2):54–65
Dávila N, Honorio E, Baker T et al (2008) Fichas de identificación de espécies maderables de Loreto- Peru. Instituto de Investigaciones de La Amazônia Peruana, pp 12–13
Détienne P (1989) Appearence and periodicity of growth rings in some tropical woods. IAWA Bull 10:123–132. https://doi.org/10.1163/22941932-90000480
Dünisch O, Bauch J, Gasparotto L (2002) Formation of increment zones and intraannual growth dynamics in the xylem of Swietenia macrophylla, Carapa guianensis, and Cedrela odorata (Meliaceae). IAWA J 23(2):101–119. https://doi.org/10.1163/22941932-90000292
Dünisch O, Montoia VR, Bauch J (2003) Dendroecological investigations on Swietenia macrophylla King and Cedrela odorata L. (Meliaceae) in the central Amazon. Trees 17:244–250
Dünisch O (2005) Influence of the El-Niño southern oscillation on cambial growth of Cedrela fissilis Vell in tropical and subtropical Brazil. J Appl Bot Food Qual 79(1):5–11
Espinoza MJP, Guillen GJI, Morales MS et al (2014) Potencialidad de Cedrela odorata (Meliaceae) para estudios dendrocronológicos en la selva central del Perú. Rev Biol Trop 62(2):783–793
Ferreira AG, Mello NGS (2005) Principais sistemas atmosféricos atuantes sobre a região nordeste do Brasil e a influência dos oceanos Pacífico e Atlântico no clima da região. Revista Brasileira de Climatologia 1:15–28. https://doi.org/10.5380/abclima.v1i1.25215
Ferreira ATB, Tomazello M (2009) Caracterização dos anéis de crescimento de árvores de Pinus caribaea var. hondurensis Barr. et Golf. Por densitometria de raios X. Scientia Forestalis 37(83):287–298
Ferreira GB (2012) Análise dendroclimatológica do cedro (Cedrela fissilis L. Meliaaceae) para reconstrução do cenário ambiental recente da cidade de São Paulo, SP. Dissertation, Universidade de São Paulo. https://doi.org/10.11606/D.41.2012.tde-23072012-143643
Gentry AH (1991) The distribution and evolution of climbing plants. In: Putz FE, Mooney HA (eds) The biology of vines. Cambridge University Press, Cambridge
Granato-Souza D, Stahle DW, Barbosa AC et al (2018) Tree rings and rainfall in the equatorial Amazon. Climate Dynam 5:1–13. https://doi.org/10.1007/s00382-018-4227-y
Holmes RL (1983) Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull 43:69–78
Iawa Committee (1989) Iawa list of microscopic features for hardwood identification. IAWA Bull New Ser 10(3):219–332
Jacomine PKT (2008–2009) A nova classificação brasileira de solos. In: Anais da Academia Pernambucana de Ciência Agronômica, vol 5/6, pp 161–179
Janzen DH (1988) Tropical dry forests, the mosts endangerous major tropical ecosystem. In: Wilson EO (ed) Biodiversity. National Academy Press, Washington, DC.
Köhl M, Neupane PR, Lotfiomran N (2017) The impact of tree age on biomass growth and carbon accumulation capacity: a retrospective analysis using tree ring data of three tropical tree species grown in natural forests of Suriname. PLoS One 1:17. https://doi.org/10.1371/journal.pone.0181187
Larson PR (1994) The vascular cambium: development and structure. Springer, Berlin
Lisi CS, Tomazello-Filho M, Botosso PC et al (2008) Tree-ring formation, radial increment periodicity, and phenology of tree species from a seasonal semi-deciduous forest in southeast Brazil. IAWA J 29:189–207. https://doi.org/10.1163/22941932-90000179
Lobão MS (2011) Dendrocronologia, fenologia, atividade cambial e qualidade do lenho de árvores de Cedrela odorata L., Cedrela fissilis Vell. e Schizolobium parahyba var. amazonicum Hub. ex Ducke, no estado do Acre, Brasil. PHD Tesis Escola Superior de Agricultura “Luiz de Queiroz”
Lobão MS, Ortega GP, Amaral E et al (2016) Análise de similaridade das árvores de Cedrela sp. sob diferentes condições de crescimento no leste do estado do Acre, Brasil. Sci For 44(109):231–239. https://doi.org/10.18671/scifor.v44n109.22
Loiola MIB, Roque AA, Oliveira ACP (2012) Caatinga: vegetação do semiárido brasileiro. Ecologia 4:14–19
Lorenzi H (2009) Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas nativas do Brasil, vol 2. Instituto Plantarum, Nova Odessa
Maia GN (2012) Caatinga: árvores e arbustos e suas utilidades, vol 2. Printcolor Gráfica e Editora, Fortaleza
Marcati CR, Angyalossi V, Evert RF (2006) Seasonal variation in wood formation of Cedrela fissilis (Meliaceae). IAWA J 27(2):199–211. https://doi.org/10.1163/22941932-90000149
Mariano Neto B (2001) Ecologia e imaginário: memória cultural, natureza e submundialização. Editora Universitária/FPB, João Pessoa
Paredes-Villanueva K, López L, Cerrillo RMN (2016) Regional chronologies of Cedrela fissilis and Cedrela angustifolia in tree forest types and their relation to climate. Trees 30:1581–1593. https://doi.org/10.1007/s00468-016-1391-8
Pennington TD (1981) Meliaceae. Flora Neotrop (New York Botanical Garden) 28:359–368
Polge H (1970) The use of X-ray densitometric methods in dendrochronology. Tree-Ring Bull 30:1–10
Quintek Measurement Systems (1999) QMS tree ring analyzer model QTRS-01X. Users Guide, Knoxville
Rauber RC (2010) Dendroecologia do cedro Cedrela fissilis Vell. (Meliaceae) em um ecótono de florestas subtropicais montanas no Brasil. Dissertation, Ufrgsl
Ribeiro FE, Siqueira ER (2001) Recursos genéticos vegetais da Mata Atlântica de Sergipe. In: Siqueira ER, Ribeiro FE (eds) Mata Atlântica de Sergipe. Embrapa Tabuleiros Costeiros, Aracaju
Rosa SA (2008) Modelos de crescimento de quatro espécies madeireiras de várzea alta da Amazônia Central por meio de métodos dendrocronológicos. Dissertation, Inpa
Roweder C (2011) Germinação de sementes e desenvolvimento de plântulas de Cedro (Cedrella odorata L. – MELIACEAE) e Mogno (Swietenia macrophylla King – MELIACEAE) em diferentes condições de luminosidade, substratos e recipientes. Dissertation, Ufac
Sampaio EVSB (2010) Caraterização do Bioma Caatinga – características e potencialidades. In: Gariglio MA, Sampaio EVSB, Cestaro LA, Kageyama PY (eds) Uso sustentável e conservação dos recursos florestais da Caatinga. Serviço Florestal Brasileiro, Brasília
Schweingruber FH, Fritts HC, Braker OU et al (1978) The x-ray technique as applied to dendroclimatology. Tree-Ring Bull 38:61–91
Serviço Florestal Brasileiro (2017) Inventário florestal nacional: Sergipe: principais resultados/Serviço Florestal Brasileiro. MMA
Surdi PG, Bortoletto Júnior G, Castro VR et al (2014) Perfil de densidade do lenho utilizando métodos radiográficos. Sci For 42(102):229–236
Tomazello M, Botosso PC, Lisi CS (2000) Potencialidade da família Meliaceae para dendrocronologia em regiões tropicais e subtropicais. In: Roig FA (ed) Dendrocronología en América Latina. EDIUNC, Mendoza
Tomazello M, Botosso PC, Lisi CS et al (2001) Cedrela angustifolia Ses. et Moc. ex DC., Meliaceae: potential species for tropical endrochronology. Paleobotanist 50:47–53
Tomazello M, Brazolin S, Chagas MP et al (2008) Application of X-ray technique in nondestructive evaluation of Eucalypt wood. Maderas Cienc Tecnol 10(2):139–145
Tsuchiya A (1995) Preliminary study on the relationship between vessel growth of thorny shrubs and water balance in the semi-arid region, northeastern Brazil. Geogr Sci 50:123–131
Vetter RE, Botosso PC (1989) Remarks on age and growth rate determination of Amazonian trees. IAWA J 10(2):133–145. https://doi.org/10.1163/22941932-90000481
Villalba R, Boninsegna JA, Holmes RL (1985) Cedrela angustifolia and Juglans australis: two new tropical species useful in dendrochronology. Tree-Ring Bull 45:25–35
Villalba R, Boninsegna JA, Ripalta A (1987) Climate, site conditions and tree-growth in subtropical northwestern Argentina. Can J For Res 17(12):1527–1544. https://doi.org/10.1139/x87-235
Villalba R, Colmes RL, Boninsegna JA (1992) Spatial patterns of climate and tree-growth anomalies in subtropical northwestern Argentina. J Biogeogr 19(6):631–649. https://doi.org/10.2307/2845706
Villalba R, Delgado S, Membiela M et al (2006) Variabilidad interanual de los caracteres anatómicos en el leño de Cedrela lilloi en el noroeste de Argentina. In: Pacheco S, Brown A (eds) Interanual de los caracteres anatómicos en el leño de Cedrela lilloi en el NOA. LIEY-ProYungas, Tucumán
Wigley TM, Jones PD, Briffa K (1984) On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J Clim Appl Meteorol 23:201–213. https://doi.org/10.1175/1520-0450(1984)023<0201:OTAVOC>2.0.CO;2
Worbes M (1989) Growth rings, increment and age of trees inundation forests, savanas and amount in forest in the Neotropics. IAWA Bull 10(2):109–122. https://doi.org/10.1163/22941932-90000479
Worbes M (1992) Occurrence of seasonal climate and tree-ring research in the tropics. Lundqua Rep 34:338–342
Worbes M (1995) How to measure growth dynamics in tropical trees: a review. IAWA J 16(4):337–351. https://doi.org/10.1163/22941932-90001424
Acknowledgements
We are thankful for the support of the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and Fapitec (Fundação de Apoio à Pesquisa e a Inovação Tecnológica do Estado de Sergipe) edicts UNIVERSAL/CNPq N° 14/2011, FAPITEC/SE/FUNTEC/CNPq N° 04/2011, and UNIVERSAL– MCTI/CNPq N° 14/2014. We also thank the other team members of the UFS, ESALQ, and IANIGLA laboratories that contributed during these years to carry out these studies.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lisi, C.S. et al. (2020). Dendroecological Studies with Cedrela odorata L., Northeastern Brazil. In: Pompa-García, M., Camarero, J. (eds) Latin American Dendroecology. Springer, Cham. https://doi.org/10.1007/978-3-030-36930-9_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-36930-9_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-36929-3
Online ISBN: 978-3-030-36930-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)