Abstract
As one of the critical intermediate compounds of the sodium roasting converter slag, sodium pyrovanadate (Na4V2O7) powder was synthesized by solid-state reaction using NaCO3 and V2O5 as raw materials in this study. The preparation was first evaluated by thermodynamic software FactSage® with the minimum Gibbs free energy principle. Effect of temperature (T) and partial pressure of carbon dioxide P(CO2) was analyzed, and the results indicated that the reaction proceeds extensively with increasing temperature and reducing P(CO2). TG-DSC was applied to further characterize the preparation process, and it can be found that the reaction proceeds extensively near 540 °C corresponding to carbon dioxide gas escaping. Non-isothermal kinetics with a single scan rate was applied to the solid-state reaction, the average apparent activation energy was obtained using Freeman–Carroll method, equal to 102 ± 6 kJ/mol by mathematic fitting. In addition, XRD further verified the phase composition of Na4V2O7, and a large number of voids were detected from SEM images caused by the gas release.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
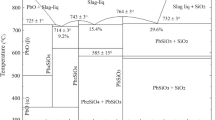
Metal vanadium (V) is frequently used in the metallurgical and chemical industries in the past few years due to its special physical and chemical properties. Almost 88% of the metal vanadium in the world is produced from the vanadium titano-magnetite ore [1,2,3]. One of the typical processes for extracting vanadium from vanadium slag, which is obtained by oxidizing hot metals that bear vanadium, is sodium roasting followed by water leaching. Sodium carbonate (Na2CO3) is used in the extraction of converter slag bearing vanadium. Vanadium slag after roasting process with Na2CO3 is regarded as the V2O5–Na2O–CaO–MgO–Fe2O3–Al2O3–MnO–Cr2O3–TiO2 multiple oxide system, and the V2O5–Na2O binary system is the critical and essential sub-system [4,5,6,7]. As one of the intermediate compounds in the V2O5–Na2O binary system, sodium metavanadate (NaVO3), its high-temperature heat capacity and phase transition kinetics have been studied in our previous study [8].
With regard to other intermediate compounds, attention on the property of the sodium pyrovanadate (Na4V2O7) should be extensively received to better understand the sodium roasting process. As for Na4V2O7, which was reported for the first time by Roscoe [9] in 1870. Then, Bjoernberg et al. [10] proposed that the crystal structure of Na4V2O7·(H2O)18 was determined from three-dimensional X-ray diffraction. The crystals are hexagonal, with space group p63/m, a = 9.2478(5), c = 16.591(2)Å, and the cell contains two formula units. Florenskij et al. [11] presented the patent entitled “Sodium pyrovanadate—is used as colour indicator for high-temperature gas redox medium”. Exploring more efficient and economic synthesis methods of Na4V2O7 is critical for further fundamental and applied research, only few methods can be found in literature. With regard to solid-state reaction, the indefinite composition of the sodium vanadate products together with the contradictory results given by some authors concerning the structure of vanadium compounds, are calling attention to the mechanism of the reaction between sodium carbonate and vanadium pentoxide. Although Kolta et al. [12] successfully prepared Na4V2O7 powder by this method, the apparent activation energy of this solid-station reaction is not well investigated, and it should be further clarified.
Herein, the preparation for Na4V2O7 powder was firstly evaluated by thermodynamic calculation. Then, the whole process was continuously recorded by TG-DSC treatment, the frequently-used Friedman–Carroll method for solid-state reaction was exacted in present study to calculate the apparent activation energy based on the mass loss of the whole process. The final products were also analyzed by XRD and SEM.
Materials and Experimental
The powder samples of NaCO3 (purity ≥ 99.99%) and V2O5 (purity ≥ 99.50%) were provided by Aladdin Co., LTD. and LiaoShuo Biological Co., LTD, respectively. The mixtures with specific molar 2:1 for NaCO3 and V2O5 were used as the raw materials to synthesize sodium pyrovanadate (Na4V2O7) powder through solid-state reaction. The general three-step procedure was introduced into the muffle, the sample was held for 10 h under Argon atmosphere with gas flow of 50 mL/min when the temperature reached at 873 K with heating rate of 10 K/min, the as-prepared sample was then cooled with muffle. The X-ray powder diffraction (XRD) patterns in the 2θ range from 10° to 50° were obtained on a PANalytical X’Pert Powder, Panalytical B.V. (Cu Kα radiation) to investigate the crystalline phases of the products. A scanning electron microscope (SEM, TESCAN VEGA 3 LMH, Czech Republic) was also employed to investigate the morphologies and particle sizes of as-prepared Na4V2O7 powder.
Methods
Thermodynamic calculation was used to evaluate the solid-state reaction, which was carried out using Factsage® software with the minimum Gibbs free energy principle. Then, Thermal Analysis Kinetics (TAK) was conducted to further understand the solid-state reaction [13]. The process was also characterized by a TG-DSC (404 F3; Netzsch) at a rate of 10 K/min with protection from high-purity argon at 50 mL/min, the changes of mass and heat flow during the preparation was recorded with detail. The frequently-used Friedman–Carroll method for solid-state reaction was exacted in present study to calculate the apparent activation energy based on the mass loss during the whole process [14,15,16].
Results and Discussion
Thermodynamic Calculation
The chemical reaction equation for the Na4V2O7 formation through solid-state reaction when heating NaCO3–V2O5 mixtures in the present study can be described as follows:
The Gibbs free energy of this reaction with various carbon dioxide pressure (PCO2) was calculated by the Reaction model of Factsage® software as shown in Fig. 1. It can be found that the Gibbs free energy P(CO2) is below 0 when carbon dioxide pressure equals 1 Pa, which indicates the reaction can be proceeded when the temperature is beyond 273 K. This solid-state reaction can be speeded bydecreasing the pressure of carbon dioxide.
Non-isothermal Kinetics Analysis
The non-isothermal kinetics analysis was extensively used in the solid-state reaction, and the conversion degree (α) can be defined as follows [17,18,19]:
where Δmt is the weight loss at specific time t and Δmo is the theoretical maxium weight loss, according to the solid-state reaction (1), the theoretical maxium weight loss equals 77%.
Figure 2 (left side) shows the changes in the mass and heat flow during the whole process. An obvious endothermic peak accompanied with mass loss of around 6% can be detected when the temperature reacts at near 100 °C, which may be caused by dehydration process of the NaCO3 powder. We carefully operated the experiment, and the addition of water inevitably introduced the mixtures when the raw materials were mixed thoroughly in an agate mortar, and the mass loss of the additional water should be deducted in the subsequent calculation. The mass loss of the mixture gradually increases with increasing the temperature, a large mass loss can be found near 540 °C, indicating that the solid-state reaction proceeds extensively in current stage corresponding to carbon dioxide gas escaping. Afterwards, the mass loss change tends to slow, and the final mass loss approximately equals 21%, which is in great agreement with the calculated value based on chemical reaction equation.
The weight loss and conversion degree are also presented as shown in Fig. 3. The conversion degree increases with the increase in the temperature, the rate of conversion degree reached at the maximum value near 540 °C.
According to the Avrami–Erofeev model [20], the differential equation can be described as follows:
where dα/dt is the reaction rate (min−1), k(T) is the reaction rate constant (min−1), and f(α) is the model function:
where A denotes the pre-exponential factor, E is the apparent activation energy, and R is the gas constant (8.314 J·mol−1·K−1). Put expression (4) into (3), the equation can be obtained as follows:
The apparent activation energy was calculated by Freeman–Carroll method [14,15,16] in this study and the express also presented as follows:
The apparent activation energy can be calculated by plotting the Δln(1-α) against Δ1/T, the average activation energy equals 102 ± 6 kJ/mol.
Characterization
XRD measurement is the basic analysis method and frequently applied to verify the phase composition evolution during the process of materials preparation and synthesis. The XRD patterns of the final products described and compared with the standard PDF Na4V2O7 (NO: 76-1462) are shown in Fig. 4a. By comparing the location and intensity of the diffraction peaks with the standard PDF card, the main phase composition of the presented sample can be checked and verified. The final phase composition was well fitted with the standard PDF cards of Na4V2O7. No additional reflection of other phases, e.g., initial reactant, was detected at the final sample. Narrow and strong reflection clearly confirmed good crystallinity of the as-prepared sample.
Surface morphology and microstructure of the as-prepared Na4V2O7 powder can be further analyzed by SEM, characteristic micrographs were obtained with four different magnifications and they are given in Fig. 4b. These micrographs reveal the agglomerates of nanoparticles shaped in small chunks of several microns in size. Form the results, we can find that the inner of the prepared sample have few hole which may be caused by the carbon dioxide released during the whole process. A large amount of this hole can extensively accelerate the solid-state reaction by improving the dynamic conditions.
Conclusions
The Na4V2O7 powder was prepared by the solid-state reaction. Thermodynamic calculation and non-isothermal kinetics with single heating rate were carried out. The following conclusions were obtained:
-
1.
Thermodynamic calculation indicated that the solid-state reaction of preparing Na4V2O7 powder using Na2CO3 and V2O5 as raw materials, proceed intensively with increasing temperature and reducing partial pressure of carbon dioxide.
-
2.
The solid-state reaction proceeds intensively at 540 °C corresponding to weight loss and endothermic peak, and the final mass loss is in good agreement with the theoretical values based on the chemical reaction equation. The activation energy was obtained by Freeman–Carroll method, the calculated average activation energy equals102 ± 6 kJ/mol.
-
3.
The XRD further verified the phase composition of preparing Na4V2O7 powder, and a large number of voids were detected from SEM images, which probably were caused by the gas release.
References
Tavakoli MR, Dreisinger DB (2014) The kinetics of oxidative leaching of vanadium trioxide. Hydrometallurgy 147–148:83–89
Xiang JY, Huang QY, Lv XW, Bai CG (2017) Multistage utilization process for the gradient-recovery of V, Fe, and Ti from vanadium-bearing converter slag, J Hazard Mater 3361
Xiang JY et al. (2019) A multi-step process for the cleaner utilization of vanadium-bearing converter slag. Paper presented at the 148st TMS Annual Meeting, San Antonio, Texas, 10–14 Mar 2019
Kolta GA, Hewaidy IF (1972) Phase diagrams of binary systems vanadium oxide-sodium carbonate and vanadium oxide-sodium sulfate 25(7):327–330
Slobodin BV, Fotiev AA (1965) Phase diagram of the Na2O-V2O5 system. Russ J Appl Chem 38(4):801–806
Wilson JR, Kerby RC (1973) Solid-liquid phase equilibria for the ternary systems V2O5-Na2O-Fe2O3, V2O5-Na2O-Cr2O3, and V2O5-Na2O-MgO, Can J Chem 51:1032–1040
Pei GS et al (2019) A literature review of heat capacity measurements method. Paper presented at the 148st TMS Annual Meeting, San Antonio, Texas, 10–14 Mar 2019
Pei GS, Xiang JY, Lv XW, Li G, Wu SS, Zhong DP, Lv W (2019) High-temperature heat capacity and phase transformation kinetics of NaVO3. J Alloy Compd 794:465–472
Roscoe HR (1870) Philos Trans R Soc 160:317
Bjoernberg A (1979) Multicomponent polyanions. 24. The crystal structure of Na4V2O7·(H2O)18. Acta Chem Scand Ser A 33:539
Florenskij K, Pkomarov B, Vvolkov V, Pnikolaeva O, Vkudryashova A, Fbashkirova A, Schajkina E A SU736751A1 pyro:vanadium-is used as colour indicator for high-temp. gas redox medium
Kolta GA, Hewaidy IF, Felix NS, Girgis NN (1973) Reactions between sodium carbonate and vanadium pentoxide. Thermochim Acta 6(2):165–177
Flynn JH (1992) Thermal analysis kinetics-past, present and future. Thermochim Acta 203:519–526
Friedman HL (1967) J Macromolecular Sci Chem 41:57
Reich L, Levi W (1968) Macromolacular review. Wiley-Interscience, New York, p 173
Ma RP, Felder RM, Ferrell JK (1988) Modelling a pilot-scale fluidized bed coal gasification reactor. Fuel Process Technol 19(3):165–290
Henderson DW (1979) Thermal analysis of non-isothermal crystallization kinetics in glass forming liquids. J Non-Cryst Solids 30:301–315
Ding CY, Lv XW, Chen Y, Bai CG (2016) Crystallization kinetics of 2CaO·Fe2O3 and CaO·Fe2O3 in the CaO-Fe2O3 system. ISIJ Int 56:1157–1163
Balamurugan GP, Maiti SN (2010) Nonisothermal crystallization kinetics of polyamide 6 and ethylene-co-butyl acrylate blends. J Appl Polym Sci 107:2414–2435
Mccune RC, Wynblatt P (1983) J Am Ceram Soc 66:111
Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFC1900500), China Postdoctoral Science Foundation (2018M640898), and Graduate Scientific Research and Innovation Foundation of Chongqing, China (Grant No. CYS19001).
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Pei, G., Xiang, J., Liu, Z., Zhong, D., Pan, F., Lv, X. (2020). Preparation of Na4V2O7 Powder by Solid-State Reaction. In: Li, B., et al. Advances in Powder and Ceramic Materials Science. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-030-36552-3_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-36552-3_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-36551-6
Online ISBN: 978-3-030-36552-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)