Abstract
In this study, glass-ceramics was prepared with coal fly ash by direct sintering to solve the problem of resource utilization of coal fly ash. The effects of the sintering temperature on volume density, water absorption, and open and closed porosity from 1000 to 1200 °C were investigated. The sintering process of glass-ceramics was also investigated by XRD and SEM. The data indicate that the sintering process was accomplished by dissolving the solid amorphous phases, hematite, and portion quartz of coal fly ash to form anorthite. During the process, the open porosity of the material gradually tended to closure. The glass-ceramics fired at 1180 °C for 30 min featured good strengths, with compressive strength of 122 MP and bending strength of 34 MP. The obtained relatively low density of 1.94 g cm−3 and moderate water absorption of about 1% would make these materials suitable for lightweight construction tiles. Besides, owing to the abundance of coal fly ash produced from thermal power plants, the present technology should be suitable for large-scale manufacturing of glass-ceramics, with economic benefits and possible solutions to environmental and wastes disposal concerns.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
China is one of the few countries that use coal as major energy source, accounting for about 63% of China’s primary energy consumption. Coal would still be the primary energy source in the near future. Currently, large amounts of fly ash have been produced from thermal power plants, consuming about 45% of total coal outcome each year [1]. The emissions of coal fly ash exceed 580 million tons per year in China. On the other hand, only 70% of China’s coal fly ash is taken into account while the remaining causes serious environmental pollution, including haze and smog issued from random accumulation [2, 3].
Oxides like silica, alumina, calcium oxide, and iron oxide in coal fly ash are considered as low-cost raw materials for preparing glass-ceramics. As a result, various types of glass-ceramics are prepared from fly ash by controlling nucleation and crystallization process through traditional melting and sintering methods with addition of natural wastes and raw materials, such as shell, tailings, waste glasses, red mud, and silica [4,5,6,7]. It has to be kept in mind that direct sintering is similar to traditional sintering but favored by scholars due to its low energy consumption.
Sintered samples made from direct sintering are referred as glass-ceramics owing to phase transformation during the sintering process [8,9,10]. In fact, coal fly ash generated at high temperature followed by sudden cooling contains more than 50% amorphous glassy phase [11]. During direct sintering, amorphous glassy phase will melt and react with each other to form aluminosilicate crystals. Therefore, coal fly ash can be used to prepare glass-ceramics by direct sintering process. For instance, Lu et al. prepared glass-ceramics by direct sintering from mixtures of magnesia, waste glass, and fly ash, and investigated the influence of particle size on sinterability, crystallization kinetics, and flexural strength of the resulting glass-ceramics [12, 13], while the amounts of coal fly ash did not exceed 50%. Due to the large amount of coal ash produced each year, producing glass-ceramics is a useful approach for the consumption of fly ash.
In this work, direct sintering was used to prepare sintered glass-ceramics from coal fly ash. The sintering process and characterization of glass-ceramics sintered at different temperatures were studied by XRD, SEM, and Archimedes’ method. The sintering activation energies were calculated. These investigations are helpful to understand the application of coal fly ash in the preparation of glass-ceramics.
Experimental
Coal fly ash used in this study was taken from a power plant in Mianyang, Sichuan, China, and labeled as CAF. The chemical compositions of CFA were measured by X-ray fluorescence (XRF, PANalytical), and the results are listed in Table 1. The CFA contained large amounts of alumina and silica with small amounts of calcium and iron. The loss of ignition (LOI) of coal combustion residues was estimated to 3.02%. The particle size of CFA was analyzed by a laser particle size analyzer (Zetasizer Nano Zs90, Fig. 1). The CFA powders showed broad particle size distribution ranging from 1 to 100 um, with a mean size of about 12.62 um.
The samples were formed into disks of 25 mm in diameter from a mixture of 3 g CFA with 5 wt% additional PVA (polyvinyl alcohol) at pressure of 10 MPa applied for 30 s. The PVA was used as a binder for the disks for increasing the green strength and decreasing the initial porosity. The samples were sintered in an electric box furnace at 1000, 1100, 1150, 1160, 1170, 1180, 1190, and 1200 °C, respectively, for 10, 20, 30, 40, 50, and 60 min with heating rate of 10 °C/min. After sintering, the samples were naturally cooled down to room temperature. The obtained sintered specimens were labeled as CFA-sinter temperature-holding time. The schematic representation of glass-ceramics is shown in Fig. 2. Water absorption (W) was measured using standard tests in boiling distilled water for 1 h. The bulk density (ρb) and open porosity (ρo) were measured by the Archimedes method. The true densities (ρt) were measured by gas (Ar) pycnometer. The results were used to estimate the total (ρa) and closed (ρc) porosities using Eqs. (1) and (2).
With respect to other properties, three-point bending strength was measured using rectangular bars of 5 × 10 × 60 mm3 at 0.5 mm/min displacement. The sintering kinetics of glass-ceramics was based on bulk density. The empirical Eqs. (3) and (4) were employed to calculate the sintering activation energies.
where D is the bulk density, C is a characteristic constant of the powders, K is the reaction rate constant, t is sintering time, Q is the activation energy, R is gas constant, T is the absolute temperature, and A is a constant [14, 15].
The crystalline structures of glass-ceramics were determined by X-ray diffraction (XRD, PANalytical, 2θ range 3°–80°, step 0.03°). The microstructures of glass-ceramics were observed using scanning electron microscopy (SEM, Ultra 55) operating at 15 kV at magnifying multiple ranges of 1000–500. The samples were crushed and gold sputtered, and both surface and fractured surface were then analyzed.
Results and Discussion
Figure 3 showed that the sintering process started at 1000 °C while overfiring was observed at 1190–1200 °C. The glass-ceramics CFA1180-60 showed the minimum water absorption of 4.4% (Fig. 3a), which rose to higher water absorption due to overfiring. On the other hand, bulk density revealed an opposite trend, with maximum bulk density of 1.86 g/m3 at the same temperature. Also, bulk density declined due to overfiring. The same tendency can be seen in Fig. 3b: a regular decrease in open porosity was observed as a function of temperature, which still affected by about 8% of open porosity after sintering at 1180 °C. A regular increase in closed porosity was observed at temperatures below 1180 °C. The plot in Fig. 3b depicted a typical sintering behavior, with decreased open porosity and increased permanence of very few closed porosities [16]. The 4.4% water absorption estimated for CFA-1180-60 was caused by the 8% open porosity. After sintering at 1180 °C, the outer surface of each sample showed open porosity due to overfiring, which can cause deformation. The water absorption showed an increasing trend and bulk density declined. In general, the sintering process stared at 1000 °C and as sintering progressed, the open porosity gradually transformed into closed or vanished porosity. Also, water absorption of the samples gradually reduced and bulk density rose due to increased viscous flow caused by melting material in CFA.
To meet suitability for applications in lightweight construction tiles with low water absorption, the effects of holding time at temperatures of 1160, 1170, and 1180 °C were investigated and the results are shown in Fig. 4. The increase in holding time led to similar trend as the increase in sintering temperature (Fig. 4a) since water absorption first declined and then increased due to overfiring. It will be noted that the sintering process of CFA1180 reached completion after 30 min and sintered samples CFA-1180-30 showed lowest water absorption values (1.00%). Interestingly, water absorption reached about 7% after 50 min at 1160 °C, 4% after 40 min at 1170 °C, and 1% after 30 min at 1180 °C. The latter could be attributed to the similar trends observed for open porosity (Fig. 4b).
Sintering was driven by the increased viscous flow caused by melting of non-crystalline and crystalline material in CFA. Hence, raising the holding time and especially sintering temperature may reduce the viscosity of the glassy phase causing collapse of structure and densification of sintered samples [15]. Especially densification the surface of the samples, because the surface is generally hotter than the rest for the electric furnace heating [17].
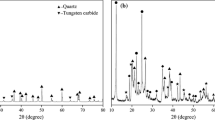
To explain the sintering process of glass-ceramics, the samples were analyzed by XRD and SEM and their sintering activation energies were determined at different sintering temperatures. Main phase transformations observed at different temperatures are gathered in Fig. 5. CFA contained mainly quartz, mullite, and hematite with amorphous metastable glassy phase forms. These data were consistent with those published in other literature [3, 18, 19]. The sample treated at 1000 °C demonstrated slight decrease in quartz and amorphous metastable glassy phases. Also, anorthite began to appear and hematite phase totally vanished. At 1100 and 1180 °C, the decrease in quartz and amorphous metastable glassy phase became significant while anorthite amounts increased. In other words, quartz and mullite as residual minerals and anorthite were reconstructed from amorphous metastable glassy phase, hematite and quartz during the firing process from CFA. The diffuse diffraction background of CFA in XRD data was related to CaO content [20] and aluminum silicate glassy phase. The excess energy fixed in amorphous metastable glassy phase made them inclined to release excess energy by crystallization [21]. As sintering temperature rose, the XRD background levels and intensity of quartz decreased due to portion dissolution [22]. The intensities of hematite vanished and those of anorthite increased indicating less amorphous glassy phase and shift to anorthite. Qin et al. [23] reported that the synthesis of anorthite at low temperatures was challenging except for small particle size raw materials. The powder particle size analysis estimated d50 of CFA to 12.62 μm (Fig. 1). Hence, low-temperature fabrication of anorthite was successfully achieved by CFA at 1180 °C.
Figure 6 shows the SEM micrograph of both surface and fracture surface of sintered samples at different temperatures. The sintering densification process occurred as temperature increased. At 1000 °C, both the surface and fracture surface appeared uneven and granular (Fig. 6a, d). The increase in firing temperature to 1100 °C resulted in smoother surfaces, and fracture surface shown in Fig. 6b, e, indicated melting and sintering processes. As firing temperature further rose, porosity of fracture surfaces of sintered samples increased and showed changes from unconnected to interconnected shapes. Samples at low temperatures illustrated unconnected and irregular sized pores (5–10 um) caused by porosity and incomplete reaction of coal ash itself (Fig. 6d, e). By contrast, samples at relatively high temperatures depicted larger and interconnected sized pores of 5–30 um (Fig. 6f). Here, porosity was determined by sinterability [19], which should be ascribed to the lower viscosity of liquid phase caused by high temperatures of the densification process. By contrast, surface porosity of sintered samples greatly decreased as firing temperature increased consistent with water absorption data (Fig. 3a). Figure 6 showed typical sintering behavior, with open porosity of surface decreased. Meanwhile, the closed porosity of fracture surface increased.
Equation (3) revealed that the reaction rate constant (k) was determined by the slope of the plot: D (bulk density) versus logt (logarithmic scale of sintering time shown in Fig. 7a). Figure 4 suggested that the sintering process reached completion after 30 min for CFA1180, 40 min for CFA1170, and 50 min for CFA1160. The plot D versus logt was consistent with Fig. 4. Also, the sintering activation energy can be calculated from the slop: ln K versus 1/T graphs of glass-ceramics shown in Fig. 7b. The sintering activation energy of glass-ceramics was estimated to 127 kJ/mol, which was lower than that reported by Lu et al. [12] for glass-ceramics sintered from fine powders. This may be attributed to less time used during the sintering process. In other words, coal fly ash could easily be sintered not only for high special surface energy of fine powder but also for time during sintering process.
To acquire sintered samples suitable for lightweight construction tiles with limited water absorption, CFA1180-30 with least water absorption was produced. The physical and mechanical properties of the sample are listed in Table 2. The 1% water absorption obtained for CFA 1180-30 was caused by absorption from sample’s side without any dense surface layer [17]. The density of CFA 1180-30 was estimated to only 1.94 g cm−3, much was lower than those of other materials reported in the literature [6, 12]. This made it suitable for applications in lightweight construction tiles, especially for tiles that can be placed vertically for coverage of internal walls and manufacturing of ventilated facades. The compressive and bending strengths were issued from the compactness of the samples, while values of glass-ceramics were quite lower than those prepared by Yoon et al. [6] (compressive strength of 238 MPa and bending strength of 94 MPa) due to porosity.
The strength between the porous material (\( \delta \)) and the same material without porosity (\( \delta _{0} \)) can be calculated by the formula proposed by Rice: \( \frac{\sigma }{{\sigma_{0} }} = (1 - AP)^{n} \) [24]. The porosity of CFA1180-30 was estimated to 26% (P = 0.26), with n = 3 and A = 1 (for spherical pores) [17]. The compressive and bending strength of glass-ceramics produced by direct sintering should be 98 MPa and 38 MPa, respectively. The measured compressive strength was hence larger than the estimated, while the measured bending strength was lower. This could be due to different locations of remarkable stress concentrations.
Conclusions
Glass-ceramics were prepared from coal fly ash by direct sintering to solve the environmental problems and offering large-scale manufacturing. The XRD and SEM analyses of sintered samples indicated that increased sintering temperature from 1000 to 1180 °C dissolved the amorphous metastable glassy phase, hematite, and portion quartz to form anorthite during sintering densification process. The sintering activation energy of glass-ceramics from CFA was estimated to 127 kJ/mol indicating CFA could easily be sintered not only due to high special surface energy of CFA powder but also to the short sintering time. The water absorption, bulk density, compressive and bending strength of samples sintered at 1180 °C for 30 min were estimated to 1.0%, 1.94 g cm−3, 122 MPa, and 34 MPa, respectively. These values appeared promising for potential applications in lightweight construction tiles, such as coverage of internal wall and manufacturing of ventilated facades. Overall, this study did not only investigate how to maximize used fly ash to provide industrial production method of glass-ceramics but also established comprehensive utilization of similar solid wastes containing amorphous glassy phase and valuable oxide resources of glass-ceramics.
References
Qi L, Yuan Y (2011) Characteristics and the behavior in electrostatic precipitators of high-alumina coal fly ash from the Jungar power plant, Inner Mongolia, China. J Hazard Mater 192:222–225
Liu J, Dong Y, Dong X et al (2016) Feasible recycling of industrial waste coal fly ash for preparation of anorthite-cordierite based porous ceramic membrane supports with addition of dolomite. J Eur Ceram Soc 36:1059–1071
Luo Y, Ma S, Liu C et al (2017) Effect of particle size and alkali activation on coal fly ash and their role in sintered ceramic tiles. J Eur Ceram Soc 37:1847–1856
Kim JM, Kim HS (2004) Processing and properties of a glass-ceramic from coal fly ash from a thermal power plant through an economic process. J Eur Ceram Soc 24:2825–2833
Li B, Deng L, Zhang X et al (2013) Structure and performance of glass-ceramics obtained by bayan obo tailing and fly ash. J Non-Cryst Solids 380:103–108
Yoon SD, Lee JU, Lee JH et al (2013) Characterization of wollastonite glass-ceramics made from waste glass and coal fly ash. J Mater Sci Technol 29(2):149–153
Erol M, Küçükbayrak S, Ersou-Meriçboyu A (2007) Production of glass-ceramics obtained from industrial wastes by means of controlled nucleation and crystallization. Chem Eng J 132:335–343
Francis AA, Rawlings RD, Sweeney R et al (2002) Processing of coal ash into glass ceramic products by powder technology and sintering. Glass Technol 42(2):58–62
Dimech C, Cheeseman CR, Cook S et al (2008) Production of sintered materials from air pollution control residues from waste incineration. J Mater Sci 43:4143–4151
Binhussain MA, Marangoni M, Bernardo E et al (2014) Sintered and glazed glass-ceramics from natural and waste raw materials. Ceram Int 40:3543–3551
Dai S, Zhao L, Peng S et al (2010) Abundances and distribution of minerals and elements in high-alumina coal fly ash from the Jungar powder plant, Inner Mongolia China. Int J Coal Geol 81:320–332
Lu J, Lu Z, Peng C et al (2014) Influence of particle size on sinterability, crystallisation kinetics and flexural strength of wollastonite glass-ceramics from waste glass and fly ash. Mater Chem Phys 148:449–456
Lu Z, Lu J, Li X et al (2016) Effect of MgO addition on sinterability, crystallization kinetics, and flexural strength of glass–ceramics from waste materials. Ceram Int 42:3452–3459
Yürüyen S, Toplan HÖ (2009) The sintering kinetics of porcelain bodies made from waste glass and fly ash. Ceram Int 35:2427–2433
Demirkiran AS, Artir R, Avci E (2008) Effect of natural zeolite addition on sintering kinetics of porcelain bodies. J Mater Process Tech 203:465–470
Andreola F, Barbieri L, Karamanova E et al (2008) Recycling of CRT panel glass as fluxing agent in the porcelain stoneware tile production. Ceram Int 34:1289–1295
Bernardo E, Lazzari MD, Colombo P et al (2010) Lightweight porcelain stoneware by engineered CeO2 addition. Adv Eng Mater 12:65–70
Lin B, Li S, Hou X et al (2015) Preparation of high performance mullite ceramics from high-aluminum fly ash by an effective method. J Alloy Compd 623:359–361
Wei Z, Hou J, Zhu Z (2016) High-aluminum fly ash recycling for fabrication of cost-effective ceramic membrane supports. J Alloy Compd 683:474–480
Ilic M, Cheeseman C, Sollars C, et al (2003) Mineralogy and microstructure of sintered lignite coal fly ash. Fuel 82:331–336
Zhang Z, Zhang L, Li A (2015) Development of a sintering process for recycling oil shale fly ash and municipal solid waste incineration bottom ash into glass ceramic. Waste Manage 38:185–193
Mukhopadhyay TK, Ghosh S, Ghosh J et al (2010) Effect of fly ash on the physico-chemical and mechanical properties of a porcelain composition. Ceram Int 36:1055–1062
Qin J, Cui C, Cui X et al (2015) Recycling of lime mud and fly ash for fabrication of anorthite ceramic at low sintering temperature. Ceram Int 41:5648–5655
Rice RW (1998) Porosity of ceramics. Marcel Decker Inc., New York
Acknowledgements
This work was financially supported by the Longshan academic talent research and Innovation Team Project of SWUST (Grant No. 17LZXT11).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Zeng, L., Sun, H., Peng, T., Zheng, W. (2020). Sintering Process and Characteristics of Glass-Ceramics from Coal Fly Ash. In: Li, B., et al. Advances in Powder and Ceramic Materials Science. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-030-36552-3_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-36552-3_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-36551-6
Online ISBN: 978-3-030-36552-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)