Abstract
Salar de Llamara is situated in the north of the Atacama Desert, which is the driest desert and one of the most irradiated places on Earth. Besides, its subterranean hypersaline waters contain a high content of arsenic, among other compounds such as heavy metals that are poisonous to life in the concentrations present. Despite these extreme conditions, diverse microbial communities flourish in gypsum stratified ecosystems (microbial mats and evaporitic domes).
Here, we reviewed all the analysis carried out in these communities, involving taxonomic and functional studies by culture-independent techniques, analysis of the physicochemical parameters of the water and its relation with the microbial ecosystems, together with pigments, mineralogy, and the microscopic view.
Regarding taxonomy, the major points analyzed were: (1) the taxonomic trends at phylum level showed that Proteobacteria and Bacteroidetes were the major components of these communities. (2) A low proportion of sequences associated with the phylum Cyanobacteria were detected in all the studied samples. (3) The increased proportion of sequences that could not be affiliated with any taxonomic group that is deposited in the databases. (4) The large amount of rare phyla represented by candidate phyla, such as OD1, OP1, OD8, Hyd24-12, and NKB19.
The functional analysis, carried out in these gypsum evaporite systems, revealed that there was only a minor presence of oxygenic photosynthesizers in the community, and anoxygenic photosynthesis appears as an alternative for primary production. Since the Calvin–Benson cycle was scant, the low abundance of oxygenic photosynthesizers was also related to unusual carbon fixation pathways.
Regarding physicochemical parameters of the water, the most interesting results were: (1) a huge amount of arsenic; (2) high salinity; (3) low nutrients and high levels of some ions, such as sodium, sulfate, and calcium. The low dissolved oxygen in most of the set points was low, which was consistent with the low proportion of oxygenic photosynthesizers in all the samples studied.
Regarding the mineralogy, gypsum mainly compounded all the evaporitic domes, and the microbial mats present halite as the main mineral component.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Salar de Llamara
- Extreme environment
- Microbial diversity
- Evaporitic dome
- Microbial mats
- Functional diversity
1 Background
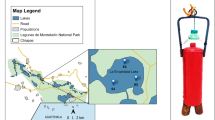
Based on the aridity index of 0.05, the Atacama Desert is considered a hyperarid area with extremely dry conditions (Wierzchos et al. 2012). Because of its aridity and UV incidence, it is largely compared to Mars, and it is also at the dry limit of microbial life (Navarro-Gonzalez et al. 2003). In addition, it is the Earth’s largest modern evaporitic regions, which comprises a large number of salt flats, with a combination of evaporitic crusts and saline lakes or playa-lakes (locally referred to as “lagunas”) (Stoertz and Ericksen 1974; Risacher et al. 2003). The Salar de Llamara is located in the north of the Atacama Desert (Fig. 11.1a). This is one of the aridest parts of the desert span toward northern Chile, and it is situated between the rain shadows of the Andes Mountains and the Coastal Cordillera (Michalski et al. 2004). Llamara 1 (L1) and Llamara 2 (L2) are two shallow wetlands at an approximately 500 m distance, containing two kinds of complex and diverse stratified microbial communities, microbial mats (Demergasso et al. 2003; Rasuk et al. 2016), and evaporitic domes (Rasuk et al. 2014, ongoing publication) (Fig. 11.1).
(a) Red square shows the location of the Salar de Llamara. (b) Location of the two water bodies L1 and L2 (marked with red), and the holes with mats (marked with yellow). (c) Photograph showing L1 lagoon. (d) Photograph showing the domes inside L2 lagoon. (e) Photograph of domes inside L1. (f) Photograph of the holes with microbial mats
The occurrence of microbial life associated with these desert environments opens up new perspectives regarding how communities adapt to and tolerate extreme environmental conditions and increases our understanding of microbial ecology and evolution. Thus, the present book chapter comprises all the available information about the stratified microbial ecosystems located in the Salar de Llamara.
2 Physicochemical Parameters in the Water from the Salar the Llamara
Physicochemical parameters in the Salar the Llamara were determined in different field campaigns (Table 11.1) (Rasuk et al. 2014, 2016, ongoing publication). Overall, similar values were obtained, and these measurements showed that the water is characterized by being moderately alkaline, having pH around 8, and a temperature between 25–30 °C. The conductivity, which is given by the charged ions dissolved in the water, is high, making these lakes hypersaline. In this sense, concentrations of the major ions were elevated in the three samples, presenting in the following order, chloride > sodium > sulfate > potassium > magnesium > calcium, except in the holes where calcium > magnesium. It is also important to highlight that nutrients including total organic nitrogen, organic matter, and phosphorus concentrations have low values in all cases; although, in L2 total phosphorous and orthophosphate had the highest values, 13.8 and 12.6 mg/L, respectively. Additionally, dissolved oxygen was markedly lower in L2 (0.7 mg/L) and in the hole waters (LL1 0.7 and LL2 1.8 mg/L) compared with L1 where the values reached 7.9 mg/L.
High arsenic content was detected in these water samples, reaching 24.6 mg/L in L2, as seems to be a feature of the Andean lakes. In fact, the major quantities of arsenic around the world were registered in those lakes (Farías et al. 2013; Rasuk et al. 2014, 2016; Rascovan et al. 2016; Ordoñez et al. 2018).
3 Unravelling the Microbial World Harboring Stratified Ecosystems in the Salar de Llamara
Two kinds of stratified microbial ecosystems were reported in the Salar de Llamara, microbial mats and evaporites.
3.1 Microbial Mats
Microbial mats are laminated structures, which are controlled by environmental factors such as light, temperature, salinity, dissolved oxygen, and the presence of sulfides. These laminations have different colorations as a result of the development of photosynthetic bacteria that contain photosynthetic pigments and, therefore, different patterns of available light. The presence of these mats is restricted to extreme environments that include coastal marine environments and hypersaline environments (Bauld 1984; Van Gemerden 1993), thermal springs (Castenholz 1984; Jorgensen and Nelson 1988), and alkaline lakes (Brock 1978). Microbial mats are considered the modern counterparts of the ancient laminated microbialites, and studying them gives interesting insights about ancient microbial life.
Up to now, four studies about the mats of the laguna Llamara were conducted:
Demergasso et al. (2003) analyzed the bacterial community of microbial mats at different sampling sites of the Salar de Llamara through microscopic and spectrophotometric techniques. They reported four heterogeneous samples along the sampling site, where the lamination of mats was different during winter and summer. Three kinds of stratification were detected. The first one was characterized by only one green layer, the second had a green and an orange layer, and the last had an additional layer with purple color. The orange layer was characterized by diatoms, the green by cyanobacteria, and the purple by anoxygenic phototrophic bacteria similar to cells of the genera Chromatium and Thiocapsa. Additionally, abundant non-photosynthetic microorganisms were detected in the mats, including unidentified cocci and bacilli. It is also important to highlight that sulfate-reducing bacteria were also present in all the sampled mats and the black layer above them was attributed to the oxidation of the sulfide generated by their metabolism.
This lamination was more developed in winter than summer, as evidenced by increased pigment and protein content, and attributed to the higher water level in winter. In addition, the pigments from the mat samples were spectrophotometrically analyzed, revealing that chlorophyll a and bacteriochlorophyll a, were the most abundant pigments. Chlorophyll a was predominant in the green layer, whereas bacteriochlorophyll a in the purple layer.
High-throughput sequencing methods enable detailed, semiquantitative analysis of entire communities in large sample sets. In addition, they provide ecological information that extends far beyond that provided by previous methods in terms of detail and magnitude. In this sense, Rasuk et al. (2016) analyzed the microbial diversity of the microbial mats using high-throughput sequencing technology and correlated it with the physicochemical parameters from water columns. They compared the samples from Llamara with sediment from other lagoons from the Atacama Desert. In this work, two kinds of mat were recognized, which were situated within holes next to L1 (Fig. 11.1b, f). One of the holes had a white suspension named LL1. In a second hole, a mat with a different appearance is noticed, with purple spots on an ocher background, this was called LL2.
In the two samples analyzed, Proteobacteria, Bacteroidetes, Spirochaetes, and sequences that could not be assigned to any taxa were the most prevalent phyla. Proteobacteria and Spirochaetes were the most abundant in LL1, while Bacteroidetes and Caldithrix, unique to LL2, were predominant in this sample. The Rhodothermaceae family was the major member of the Bacteroidetes found in both samples. It was widely detected in microbial ecosystems of Atacama Desert and other regions of the Andean Puna (Demergasso et al. 2004; Dorador et al. 2009, 2013; Farías et al. 2013; Rasuk et al. 2014; Fernandez et al. 2016; Toneatti et al. 2017; Kurth et al. 2017). Members of this family such as the genus Salinibacter sp. are characterized as requiring high salt concentrations for their growth (Anton et al. 2002; Makhdoumi-Kakhki et al. 2012). Finally, the small proportion of sequences affiliated with Cyanobacteria not only in these mat samples but also in similar geographically different samples indicate that they might not play an essential role related to carbon fixation in these extreme environments.
When they compared the diversity coupled with the physicochemical parameters between the mats and sediment samples, a higher diversity in sediment than in mat was found. They attributed this feature to the water conditions such as higher DO and more organic matter and phosphorous in the water where sediments were taken. Also, a lower conductivity was found in water with sediment samples. Finally, in the mat samples, they found to a more specific level, a community compounded by extremophiles, especially halophilic organisms, showing how environmental conditions influence the microbial composition of an ecosystem.
A more recent article (Saghaï et al. 2017) studied microbial mat fragments collected along 30 cm of a pond from the Salar de Llamara. Those fragments were obtained along strong physicochemical gradients (depth, salinity, oxygen, and temperature), and the structure of archaea, bacteria, and protist communities applying 16S/18S rRNA metabarcoding approaches was also characterized. They found that the mats were highly diverse, with a number of OTUs comparable to the most diverse environment types (soil or sediments), they included known eukaryotic and prokaryotic taxa as well as many novel lineages. Bacterial candidate divisions were more abundant in deeper layers (almost 50% of sequences), and Archaea represented up to 40% of sequences in some mat layers. Mats situated in the oxic zone were mostly composed of relatively well characterized bacterial phyla, including Bacteroidetes, Cyanobacteria, Proteobacteria, and Verrucomicrobia. The fact that these researchers found, in general, the same major phyla as the works mentioned above should be highlighted. Below the chemocline defining an oxic/anoxic and salinity transition, bacterial candidate divisions and archaea accounted for up to 75% of the sequences. Molecular phylogenetic analyses revealed six novel deeply divergent archaeal groups.
Cyanobacteria and Alphaproteobacteria, which are potential phototrophic groups were represented by 5–10% of the sequences and located in surface layers. On the other hand, potential anoxygenic phototrophs, mainly Rhodobacterales and Rhodospirillales, were detected in significant proportions in the middle zone.
Candidate divisions were particularly abundant in the two deeper mats, where the majority was Aminicenantes (former OP8), Parcubacteria (former OD1), and TA06. Also, a few OTUs from Candidate divisions were abundant and affiliated with Latescibacteria (former WS3), Saccharibacyeria (TM7), and Gracilibacteria (BD1-5). They also detected halophilic and halotolerant microbial eukaryotes, found among a variety of taxa, including especially Stramenopiles (e.g., Bacillariophyta, Bicosoecida, and Chrysophyceae), Alveolata (e.g., Ciliophora), and Fungi, being more abundant and diverse in the oxic zone mats.
Although located a few centimeters away, each studied mat fragment developed under a set of specific abiotic factors, with oxygen values dropping to zero and salinity and temperature values increased from the surface to the bottom of the pond. All these differences in environmental parameters correlated with the different structure of the prokaryotic communities.
The same mats were analyzed by WGS sequencing in the latest work (Gutiérrez-Preciado et al. 2018). This allowed further insight into the biogeochemical processes potentially taking place in the mats. The authors proposed a space-for-time substitution modeling for these mats, thus simulating the transition from early Earth conditions in the deeper mats to current oxygenic conditions in the upper mats.
Inferences from the taxonomic composition were confirmed by gene abundances. Anoxygenic photosynthesis, represented by Chloroflexi, Chlorobi, and Alpha/Gammaproteobacteria lineages, was more abundant than oxygenic photosynthesis, which could be related to Cyanobacteria and eukaryotic algae. Surprisingly, this occurred both in oxic and anoxic layers. These organisms are likely in charge of primary production in these mats. Photosynthesis genes decreased with depth, and in the lower mats, which bear increased diversity and rare phyla, primary production might be supplemented by other autotrophic metabolisms.
Carbon fixation pathways showed an inversion from the deepest, anoxic mats up to the upper oxygen-exposed mats. Ancient anoxygenic pathways, such as dicarboxylate/hydrobutyrate, 3-hydroxiproprionate/4-hydrobutyrate and, most importantly, Wood–Ljungdahl increased their abundance with depth, while the Calvin–Benson cycle markers had reduced abundance in the deeper layers.
3.2 Evaporitic Domes
Evaporites are widespread in arid locations in the world. Due to the inhospitable conditions in the desert (aridity, UV radiation, brine chemistry, and oligotrophic conditions), microbial life has been induced to search out the microhabitats most suitable for life (Albarracín et al. 2015) such as gypsum or halite pores.
Microorganisms form aggregates occupying the pore spaces inside halite, where microbial interactions occur. In this exceptional, salty, porous habitat, microbial consortia with a community structure probably acclimated to the environmental conditions occupy special microhabitats with physical and chemical properties that promote their survival (de Los Ríos et al. 2010). On the other hand, inside gypsum pores UV radiation is quenched by the selenite crystal, and it is highly hygroscopic, creating a wet UV-protected microenvironment with high access to O2 and light (Oren et al. 1995; Stivaletta et al. 2010; Farías et al. 2014). These features create a stratified system very similar to that found in microbialites and microbial mats. Thus, in hyperarid deserts, microbial life is essentially present in the form of microorganisms that take refuge in such endolithic habitats (Wierzchos et al. 2012). This kind of lifestyle is represented in the Salar de Llamara with microbial communities stratified into evaporitic structures having domal shapes.
de Los Ríos et al. (2010) characterized microbial communities inside halite evaporites from different parts of the Atacama Desert (Yungay, Salar de Llamara, and Salar Grande) using denaturing gradient gel electrophoresis (DGGE) and microscopy. Their analysis revealed that the endolithic communities harboring evaporitic halite rocks are made up predominantly of cyanobacteria, along with heterotrophic bacteria (uncultured Bacteroidetes bacterium clone from sediments of hypersaline lakes) and archaea (uncultured unidentified archaea).
4 Diversity of the Evaporitic Gypsum Domes Along of the Stations in Llamara 1
In Salar de Llamara, there are two shallow wetlands dominated by microbial ecosystems formed by dome-shaped bioherms, which presented a stratified distribution of microbial communities in color sections. Some of these domal structures are partially submerged defining an air-exposed surface and the embedded section, creating (at least) two microbial niches.
Rasuk et al. (2014) performed, for the first time, a detailed description of the microbial diversity of a dome from L1 along the stations using enhanced techniques of DNA sequencing and supported this information with the analysis of the pigments using high-performance liquid chromatography (HPLC). They also utilized SEM to view the dome sample and determined its mineralogical nature with X-ray diffraction.
The main mineral found in these domes was gypsum. Regarding biodiversity, the communities associated with these structures were analyzed by amplicon sequencing and compared between winter and summer seasons. In general, sequences related to Bacteroidetes and Proteobacteria (mainly Alfa and Gammaproteobacteria) remained as the main phylogenetic groups, and the diversity duplicated in winter (determined using Chao index, 502 in winter vs 275 in summer). The comparison of the upper (air-exposed) and bottom section (water-submerged) between the seasons showed slight variation. The upper region was dominated by Chromatiales (Gammaproteobacteria), Rhodospirillales (Alphaproteobacteria), both of them characterized by having anoxygenic photosynthesizers. Also, Sphingobacteriales (Bacteroidetes), which have halophile members such as Salinibacter sp., were significant. They have been found in microbial communities harboring salt waters and were also found in ecosystems from the Atacama Desert (Baati et al. 2008; Dorador et al. 2009, 2013; Schneider et al. 2013; Simachew et al. 2016; Toneatti et al. 2017). However, the submerged part showed marked differences between seasons, being dominated in summer by Alpha and Gammaproteobacteria and a good representation of Verrucomicrobia, but winter showed a remarkable difference being more diverse (evidenced by Chao index 435 vs 282 in summer). Even though scarce by sequence, Cyanobacteria were visually identified by SEM, which also revealed the presence of diatoms. Photosynthetic pigments related to anoxygenic bacteria, bacteriochlorophyll e, and c, together with a good representation of sequences related to Alfa and Gammaproteobacteria and a very low proportion of Cyanobacteria were found. These findings suggest that a phylognetical group other than Cyanobacteria seems to be significantly involved in carbon uptake in the Llamara domes. This was also found in the other Andean microbialite ecosystems such as Brava and Tebenquiche lakes (Fernandez et al. 2016; Farias et al. 2017). Moreover, widespread pigments such as β-carotene, lycopene, and fucoxanthin, as well as pigments related to specific groups of microorganism such as diatoxanthin (associated with diatoms) and astaxanthin (associated with Cyanobacteria) were also detected. All of them were consistent with the diversity obtained by sequencing. They proposed that the higher abundance of pigments and diversity metrics in the top layer compared to the bottom is because the upper section of the dome presents advantages over the bottom. UV would be quenched by the selenite crystals in gypsum crust and exposure in microbial communities would be minimized. In addition, gypsum is highly hygroscopic, creating a wet, UV protected microenvironment with high access to O2 and light (Oren et al. 1995). In contrast, in the bottom layer, since conditions during summer and winter are less stable, conductivity and O2 availability changes are reflected in the differences in diversity.
Although conductivity, salinity, and phosphorous were higher in summer, whereas dissolved oxygen was higher in winter (Table 11.1), it was found that there were not considerable changes in the temperature, depth, and pH of the water.
5 Characterization of the Evaporitic Gypsum Domes in Llamara 2
A detailed characterization of an evaporitic dome and the physicochemical parameters of the water from L2 were performed by Rasuk et al. (ongoing publication). Domes were similar to those found in Llamara 1 and also composed of gypsum. The study of the microbial diversity aimed to analyze the different colored horizons, and this was addressed using a combination of techniques, including amplicon sequencing of the 16S rRNA metagenomics analysis, pigment determination, and electron microscopy.
The major proportion of sequences belonged to the Bacteria domain, and just a few sequences were assigned to Archaea and Eukarya domains. Regarding phylum level, the results achieved by both approaches were consistent. In this sense, Proteobacteria, Bacteroidetes, and Firmicutes were the prevalent phyla, and rare phyla represented by unclassified bacteria and candidate phyla were also significant. On the other hand, a small amount of cyanobacterial sequences were found. The distribution of the community along the layers showed a pronounced diversity in the upper section (Fig. 11.2a), Proteobacteria (Alpha and Deltaproteobacteria) being the major component in it; in agreement with the results obtained by Rasuk et al. (2014). This also correlated with pigment analysis, where diversity was maximal in the upper photosynthetic layers. As the major member of Alphaproteobacteria, the family Rhodobacteraceae was detected (18%) in the middle section, followed by Rhodospirillaceae (11%), which was distributed in the three sections of the dome and more abundant in the middle. Another interesting finding was that the functional analysis demonstrated that anoxygenic photosynthesis was more represented than the oxygenic and mainly by Alphaproteobacteria (Rhodobacteraceae and Rhodospirillaceae). Sulfur oxidation genes were affiliated with the same taxa (Fig. 11.2b), suggesting that sulfur oxidation could be coupled with anoxygenic photosynthesis. Rhodobacteraceae and Rhodospirillaceae are purple non-sulfur bacteria (PNS) that generally use electrons from molecular hydrogen for photosynthesis, and they were thought not to use hydrogen sulfide as an electron donor while growing photoautotrophically. However, some PNS have been found using sulfide or organic compounds at lower concentrations than purple sulfur bacteria (Madigan et al. 2003).
An unexpected result found in this work was that sulfate-reduction appears to be mainly done by Deltaproteobacteria, even in the upper layers of the dome, since a high abundance not only of 16S rRNA genes was found but also the majority of genes related to this metabolism were affiliated with them. This result has also been found in the oxic zones of hypersaline microbial mats in Guerrero Negro, Solar Lake, Kiritimati Atoll, the Bahamas, and Shark Bay and in microbial ecosystems in the Salar de Atacama (Canfield and Des Marais 1991; Teske et al. 1998; Dupraz et al. 2004; Glunk et al. 2011; Arp et al. 2012; Pages et al. 2014; Farias et al. 2017).
On the other hand, the bottom layers were characterized by rare phyla, represented by sequences that could not be affiliated with any phyla (unclassified bacteria) and candidate phyla such as Hyd24-12, OP1, OP3, OD1, and SAR406. However, Firmicutes, Proteobacteria, and Lentisphaerae were also present.
Moreover, a large amount of genes related to alternative pathways of carbon fixation , such as the ancient reductive acetyl CoA, were revealed. Since the carbon monoxide dehydrogenase/acetyl-CoA synthase (key enzyme of the pathway) was even more represented than RubisCO from the classic Calvin–Benson cycle, the identified reductive acetyl-CoA pathway sequences were affiliated mainly with the sulfate-reducing Deltaproteobacteria, which are obligate anaerobic hydrogen oxidizers (Rasuk et al. ongoing publication).
This study gave a good representation of the taxonomic diversity and metabolic potentials thought to be currently used at Llamara. It might be unlikely that primary production is supported solely by Cyanobacteria, given their scarcity, and from this analysis anoxygenic photosynthesizers and Deltaproteobacteria could be significant contributors. This supports the hypothesis presented before that in these environments many of the dominating metabolisms were likely present during early earth, when conditions of the primitive land were hostile and scarce in nutrients (especially organic compounds).
6 Conclusion
This chapter presented the available studies about biodiversity in one of the driest and most irradiated environments on Earth, the Salar de Llamara. This oligotrophic system has an unexpected diversity, where oxygenic photosynthesis is not the main primary metabolism. Here, other dynamics are at work. An elevated number of genes associated with anoxygenic photosynthesis coupled with a large number of genes related to alternative carbon fixation pathways were observed. These results support the hypothesis that these poly-extreme environments would be a good model for early Earth conditions and open a universe of possibilities to elucidate these ancient metabolic pathways that provide critical information about early life in extreme environments.
References
Albarracín VH, Kurth D, Ordoñez OF et al (2015) High-up: a remote reservoir of microbial extremophiles in central Andean wetlands. Front Microbiol 6:1404. https://doi.org/10.3389/fmicb.2015.01404
Anton J, Oren A, Benlloch S et al (2002) Salinibacter ruber gen. nov., sp. nov., a novel, extremely halophilic member of the Bacteria from saltern crystallizer ponds. Int J Syst Evol Microbiol 52:485–491
Arp G, Helms G, Karlinska K et al (2012) Photosynthesis versus exopolymer degradation in the formation of microbialites on the atoll of Kiritimati, Republic of Kiribati, Central Pacific. Geomicrobiol J 29:29–65. https://doi.org/10.1080/01490451.2010.521436
Baati H, Guermazi S, Amdouni R et al (2008) Prokaryotic diversity of a Tunisian multipond solar saltern. Extremophiles 12:505–518. https://doi.org/10.1007/s00792-008-0154-x
Bauld J (1984) Microbial mats in marginal marine environments: Shark Bay, Western Australia, and Spencer Gulf, South Australia. In: Cohen Y, Castenholz R, Halvorson H (eds) Microbial mats: stromatolites. Alan R. Liss, New York, p 498
Brock TD (1978) Thermophilic microorganisms and life at high temperatures. Springer Berlin Heidelberg, New York
Canfield D, Des Marais D (1991) Aerobic sulfate reduction in microbial mats. Science 251:1471–1473. https://doi.org/10.1126/science.11538266
Castenholz R (1984) Composition of hot springmicrobial mats: a summary. In: Cohen Y, Castenholz R, Halvorson H (eds) Microbial mats: stromatolites. Alan R. Liss, New York, pp 107–119
de Los Ríos A, Valea S, Ascaso C et al (2010) Comparative analysis of the microbial communities inhabiting halite evaporites of the Atacama Desert. Int Microbiol 13:79–89
Demergasso C, Casamayor EO, Chong G et al (2004) Distribution of prokaryotic genetic diversity in athalassohaline lakes of the Atacama Desert, Northern Chile. FEMS Microbiol Ecol 48:57–69. https://doi.org/10.1016/j.femsec.2003.12.013
Demergasso C, Chong G, Galleguillos P et al (2003) Tapetes microbianos del Salar de Llamará, norte de Chile. Rev Chil Hist Nat 76:485–499. https://doi.org/10.4067/S0716-078X2003000300012
Dorador C, Meneses D, Urtuvia V et al (2009) Diversity of Bacteroidetes in high-altitude saline evaporitic basins in northern Chile. J Geophys Res 114:G00D05. https://doi.org/10.1029/2008JG000837
Dorador C, Vila I, Witzel K-P, Imhoff JF (2013) Bacterial and archaeal diversity in high altitude wetlands of the Chilean Altiplano. Fundam Appl Limnol/Arch für Hydrobiol 182:135–159. https://doi.org/10.1127/1863-9135/2013/0393
Dupraz C, Visscher PT, Baumgartner LK, Reid RP (2004) Microbe-mineral interactions: early carbonate precipitation in a hypersaline lake (Eleuthera Island, Bahamas). Sedimentology 51:745–765. https://doi.org/10.1111/j.1365-3091.2004.00649.x
Farías ME, Contreras M, Rasuk MC et al (2014) Characterization of bacterial diversity associated with microbial mats, gypsum evaporites and carbonate microbialites in thalassic wetlands: Tebenquiche and La Brava, Salar de Atacama, Chile. Extremophiles 18:311–329. https://doi.org/10.1007/s00792-013-0617-6
Farías ME, Rascovan N, Toneatti DM et al (2013) The discovery of stromatolites developing at 3570 m above sea level in a high-altitude volcanic lake Socompa, Argentinean Andes. PLoS One 8:e53497. https://doi.org/10.1371/journal.pone.0053497
Farias ME, Rasuk MC, Gallagher KL et al (2017) Prokaryotic diversity and biogeochemical characteristics of benthic microbial ecosystems at La Brava, a hypersaline lake at Salar de Atacama, Chile. PLoS One 12:e0186867. https://doi.org/10.1371/journal.pone.0186867
Fernandez AB, Rasuk MC, Visscher PT et al (2016) Microbial diversity in sediment ecosystems (evaporites domes, microbial mats, and crusts) of Hypersaline Laguna Tebenquiche, Salar de Atacama, Chile. Front Microbiol 7:1284. https://doi.org/10.3389/fmicb.2016.01284
Glunk C, Dupraz C, Braissant O et al (2011) Microbially mediated carbonate precipitation in a hypersaline lake, Big Pond (Eleuthera, Bahamas). Sedimentology 58:720–736. https://doi.org/10.1111/j.1365-3091.2010.01180.x
Gutiérrez-Preciado A, Saghaï A, Moreira D et al (2018) Functional shifts in microbial mats recapitulate early Earth metabolic transitions. Nat Ecol Evol. 2(11):1700–1708. https://doi.org/10.1038/s41559-018-0683-3
Jorgensen BB, Nelson DC (1988) Bacterial zonation, photosynthesis, and spectral light distribution in hot spring microbial mats of Iceland. Microb Ecol 16:133–147. https://doi.org/10.1007/BF02018909
Kurth D, Amadio A, Ordoñez OF et al (2017) Arsenic metabolism in high altitude modern stromatolites revealed by metagenomic analysis. Sci Rep 7:1024. https://doi.org/10.1038/s41598-017-00896-0
Madigan MT, Martinko JM, Parker J (2003) Brock biology of microorganisms. Prentice Hall/Pearson Education, Upper Saddle River, NJ
Makhdoumi-Kakhki A, Amoozegar MA, Ventosa A (2012) Salinibacter iranicus sp. nov. and Salinibacter luteus sp. nov., isolated from a salt lake, and emended descriptions of the genus Salinibacter and of Salinibacter ruber. Int J Syst Evol Microbiol 62:1521–1527. https://doi.org/10.1099/ijs.0.031971-0
Michalski G, Böhlke JK, Thiemens M (2004) Long term atmospheric deposition as the source of nitrate and other salts in the Atacama Desert, Chile: new evidence from mass-independent oxygen isotopic compositions. Geochim Cosmochim Acta 68:4023–4038. https://doi.org/10.1016/J.GCA.2004.04.009
Navarro-Gonzalez R, Rainey FA, Molina P et al (2003) Mars-like soils in the Atacama Desert, Chile, and the dry limit of microbial life. Science 302:1018–1021. https://doi.org/10.1126/science.1089143
Ordoñez OF, Rasuk MC, Soria MN et al (2018) Haloarchaea from the Andean Puna: biological role in the energy metabolism of arsenic. Microb Ecol 76:1–11. https://doi.org/10.1007/s00248-018-1159-3
Oren a, Kuhl M, Karsten U (1995) An endoevaporitic microbial mat within a gypsum crust: zonation of phototrophs, photopigments, and light penetration. Mar Ecol Prog Ser 128:151–159. https://doi.org/10.3354/meps128151
Pages A, Welsh DT, Teasdale PR et al (2014) Diel fluctuations in solute distributions and biogeochemical cycling in a hypersaline microbial mat from Shark Bay, WA. Mar Chem 167:102–112. https://doi.org/10.1016/j.marchem.2014.05.003
Rascovan N, Maldonado J, Vazquez MP, Eugenia Farías M (2016) Metagenomic study of red biofilms from Diamante Lake reveals ancient arsenic bioenergetics in haloarchaea. ISME J 10(2):299–309. https://doi.org/10.1038/ismej.2015.109
Rasuk MC, Fernández AB, Kurth D et al (2016) Bacterial diversity in microbial mats and sediments from the Atacama Desert. Microb Ecol 71:44–56. https://doi.org/10.1007/s00248-015-0649-9
Rasuk MC, Kurth D, Flores MR et al (2014) Microbial characterization of microbial ecosystems associated to evaporites domes of gypsum in Salar de Llamara in Atacama desert. Microb Ecol 68:483–494. https://doi.org/10.1007/s00248-014-0431-4
Risacher F, Alonso H, Salazar C (2003) The origin of brines and salts in Chilean salars: a hydrochemical review. Earth Sci Rev 63:249–293. https://doi.org/10.1016/S0012-8252(03)00037-0
Saghaï A, Gutiérrez-Preciado A, Deschamps P et al (2017) Unveiling microbial interactions in stratified mat communities from a warm saline shallow pond. Environ Microbiol 19:2405–2421. https://doi.org/10.1111/1462-2920.13754
Schneider D, Arp G, Reimer A et al (2013) Phylogenetic analysis of a microbialite-forming microbial mat from a hypersaline lake of the Kiritimati atoll, Central Pacific. PLoS One 8:e66662. https://doi.org/10.1371/journal.pone.0066662
Simachew A, Lanzén A, Gessesse A, Øvreås L (2016) Prokaryotic community diversity along an increasing salt gradient in a soda ash concentration pond. Microb Ecol 71:326. https://doi.org/10.1007/s00248-015-0675-7
Stivaletta N, López-García P, Boihem L et al (2010) Biomarkers of endolithic communities within gypsum crusts (southern Tunisia). Geomicrobiol J 27:101–110. https://doi.org/10.1080/01490450903410431
Stoertz GE, Ericksen GE (1974) Geology of salars in northern Chile. US Geological Survey professional paper, Washington, DC
Teske A, Ramsing NB, Habicht K et al (1998) Sulfate-reducing bacteria and their activities in cyanobacterial mats of solar lake (Sinai, Egypt). Appl Environ Microbiol 64:2943–2951
Toneatti DM, Albarracín VH, Flores MR et al (2017) Stratified bacterial diversity along physico-chemical gradients in high-altitude modern stromatolites. Front Microbiol 8:646. https://doi.org/10.3389/fmicb.2017.00646
Van Gemerden H (1993) Microbial mats: a joint venture. Mar Geol 113:3–25
Wierzchos J, de los Ríos A, Ascaso C (2012) Microorganisms in desert rocks: the edge of life on Earth. Int Microbiol 15:173–183. https://doi.org/10.2436/20.1501.01.170
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rasuk, M.C., Contreras Leiva, M., Kurth, D., Farías, M.E. (2020). Complete Characterization of Stratified Ecosystems of the Salar de Llamara (Atacama Desert). In: Farías, M. (eds) Microbial Ecosystems in Central Andes Extreme Environments. Springer, Cham. https://doi.org/10.1007/978-3-030-36192-1_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-36192-1_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-36191-4
Online ISBN: 978-3-030-36192-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)