Abstract
Metastasis is the major cause of mortality in patients with breast cancer; however, the mechanisms of tumor cell dissemination and metastasis formation are not well established yet. The study of circulating tumour cells (CTCs), the metastatic precursors of distant disease, may help in this search. CTCs can be found in the blood of cancer patients as single cells or as tumor cell aggregates, known as CTC clusters. CTC clusters have differential biological features such as an enhanced survival and metastatic potential, and they hold great promises for the evaluation of prognosis, diagnosis and therapy of the metastatic cancer. The analysis of CTC clusters offers new insights into the mechanism of metastasis and can guide towards the development of new diagnostic and therapeutic strategies to suppress cancer metastasis. This has become possible thanks to the development of improved technologies for detection of CTCs and CTC clusters. However, more efficient methods are needed in order to address important questions regarding the metastatic potential of CTC and future clinical applications. In this chapter, we explore the current knowledge on the role of CTC clusters in breast cancer metastasis, their origin, metastatic advantages and clinical importance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Breast cancer
- Circulating tumor cells (CTCs)
- CTC clusters
- Metastatic potential
- Homotypic CTC clusters
- Heterotypic CTC clusters
7.1 Introduction
Breast cancer (BC) is the most common cancer in women worldwide. It exceeded 2 million new cases diagnosed in 2018, representing about 25% of all cancers in women [1]. Despite advances in prevention, diagnosis and treatment, about 5–10% of patients show metastasis at the time of diagnosis and near a 30% will develop metastasis throughout the course of treatment [2]. The metastatic stage remains an incurable malignant disease, accounting for more than 625,000 deaths per year worldwide (WHO). The ultimate responsible of seeding cancer metastasis are the circulating tumor cells (CTCs), which hematogenous spread was reported as early as in the nineteenth century. CTCs are found in the blood of cancer patients as single cells, although small groups of these cells named CTC clusters have also been detected, and very importantly, their presence is associated with an earlier onset of metastatic disease [3,4,5,6].
The advancement on the development of technological platforms able to isolate and to identify individual CTCs and CTC clusters from blood samples has been key to overcome the difficulties of working with a population of rare cells, extremely infrequent in the case of CTC clusters. It is estimated that only a 3,4% of CTCs are clusters, and that about 50% of patients with metastatic breast cancer (MBC) have at least one cluster [4]. For this reason, most of the knowledge gained about the contribution of CTC clusters to metastasis and their clinical implications has been achieved in the last decade, and it suggests that CTC clusters may represent one of the key mechanisms initiating the metastasis process. However, the biological features of CTC clusters such as genesis and the underlying molecular mechanisms of their metastatic competency, remain largely unknown.
In this chapter, we will discuss the role of CTC clusters in breast cancer metastasis, focusing on their biological features and clinical implications.
7.2 Insights on the Existence of CTC Clusters
The first experimental evidences of the presence of tumor cells in blood circulation were made by Langenbeck’s in 1841 [7], followed by the observations in 1869 by the Australian physician and pathologist Thomas Ashword, who also reported the presence of tumor cells in the blood of a male patient with metastatic cancer [8]. The vast majority of CTCs in circulation are found as single cells, and just a small proportion are represented by CTC clusters. By definition, a cluster of CTCs is a group of more than two tumor cells detected in the blood of a cancer patient, moreover the size of the clusters can vary from 2 tumor cells up to >100 cells [5]. Different names have been used in the literature to describe aggregates of tumor cell. CTC clusters have also been referred as circulating tumor microemboli (CTM), circulating micrometastases, circulating tumor aggregates, and tumor cell clumps [4, 5, 9, 10]. For an easy understanding on the development of this chapter, we will refer to them as CTC clusters.
The existence of clusters of circulating tumor cells was already predicted by Rudolf Virchow, in 1858. In his theory about the dissemination of tumor cells, he hypothesized that metastasis could be explained simply by the arrest of tumor-cell emboli in the vasculature [11]. It was almost a century later that some initial studies emerged acknowledging the role of CTC clusters in metastasis, although at the time the term emboli, and not cells, was widely used to describe these tumor aggregates [12, 13]. In 1954, the pioneering work by Watanabe showed that clumps of viable carcinoma cells injected intravenously in mice were able to form lung metastases much more efficiently than single cells suspensions, when injected at equal numbers [14]. Watanabe showed that the total number of cells injected was, apparently, not an important factor and he suggested that clumps of cells have a survival advantage as compared to single cells. This data represents the initial indication of the greater predisposition of CTC clusters to form distal metastasis than single CTCs. Short after, in the 1970s, similar results were obtained in preclinical studies employing metastatic models to lung of melanoma, fibrosarcoma, and mammary tumor cells, corroborating the highest efficiency of CTC clusters to form distant metastasis [10, 15,16,17]. These studies also showed that the success rate of CTC clusters on the formation of metastases partially depends on the size and the concentration of the clusters found in the blood. Moreover, they revealed that CTC clusters are able of passaging through the circulation vessel in the lungs of experimental animals [17,18,19,20]. Similarly to these preclinical studies, the entrapment of CTC clusters in the microvasculature of patients has also been reflected more recently in the literature. Commonly, these tumor cell aggregates are found during autopsy of cancer patients [21, 22]. Thus, lungs might retain a substantial number of CTCs, and clusters of tumor cells (named tumor cell emboli) were observed in three out of eight patients with MBC [22]. Furthermore, a post-mortem analysis of a patient with metastatic Triple Negative breast cancer showed the presence of tumor emboli in diverse metastatic locations such as brain and lungs [21].
Despite of these initial findings, the advance on the understanding on the behavior and biology of CTC clusters and the appreciation of their full contribution to the process of metastasis was hampered mainly for one reason, the lack of technology available with the sufficient sensitivity to detect small populations of CTCs, including CTC clusters, and able to distinguish them from blood cells. Even nowadays, a key challenge is to develop enrichment technologies capable of capturing intact CTC clusters avoiding breaking them apart [23]. In this regard, it is worth reminding that if the presence of CTCs in the blood of cancer patients is rare, the presence of CTC clusters is extremely rare [24], representing only 2–5% of all CTCs, according to clinical and preclinical studies [4, 6].
On the other hand, the established view of the metastatic process as described by the clonal evolution model by Peter Nowell in 1976 [25], by which metastatic tumors arise from the proliferation of individual CTCs disseminated into distant organs [26, 27], has also contributed to the slow advancement on the study of CTC clusters.
Although the existence of CTC clusters has also been known for decades, it was in the 1990’s that the first studies isolated CTC clusters from the blood of patients with prostate, colorectal, breast, lung cancer and clear cell renal cell carcinoma [3, 5, 28,29,30,31]. A summary of the studies in which CTC clusters have been investigated in the blood of patients from different cancer types is shown in Table 7.1. Since then, recent technological advances, developed mainly in the last two decades, have enabled a more efficient isolation of CTCs and CTC clusters from the blood of cancer patients and thus, the significance of CTC clusters has emerged as a functional entity in the metastatic process (Fig. 7.1).
7.3 Challenging the Traditional View of the Dissemination Process
The traditional view on the development of metastasis believed that the establishment of metastatic tumors is due to the proliferation of individual CTCs released by the primary tumor into distant organs [26, 27]. Within this scenario, if the “seed” of the metastasis is a single CTC, then the resulting tumor will be clonal. However, this conventional model of cancer metastasis has been challenged by data extracted from recent genomic studies tracking the evolutionary histories of tumor cell clones along the metastasis progression, which show that metastases can be composed of multiple genetically distinct clones. Thus, in murine models of breast cancer, pancreas and small cell carcinoma [6, 45, 66], and in patients with metastatic prostate cancer [67], the presence in high frequency of polyclonal metastases has been observed. Along with these evidences, the isolation from the blood of cancer patients of CTC clusters and their capacity to seed distant metastasis (later discussed) suggest that, if a CTC cluster is the “seed”, the resulting metastasis can be polyclonal. These observations together with some other experimental evidences suggest that different clones of tumor cells can show cooperative behavior, a concept called “clonal cooperation”, promoting their mutual survival and metastatic capacity [68,69,70]. Therefore, it is feasible to speculate that CTC clusters can be formed by the combination of different clones harboring diverse biological properties regarding survival and growth.
In this sense, the mouse as a preclinical model, combined with the performance of lineage tracing experiments, has been a valuable tool to probe the seeding of polyclonal metastases by CTC clusters, in particular in breast cancer. By establishing primary tumors using color coded tumor cells, expressing fluorescent proteins of diverse colors, four independent groups have tested whether metastases arise by accumulation of single CTCs or by the direct seeding of CTC clusters [6, 45, 46, 63]. In the experimental setup involving lineage tracing and tumor transplantation, single colored metastases will arise either from seeding of single CTC and clusters composed by only one color (monoclonal). On the other hand, multicolored metastases will be the result of seeding by CTC clusters composed of more than one color (polyclonal). Three of these mouse experiments have been conducted in breast cancer models, including patient-derived xenografts (PDXs) models, and the fourth one in a pancreatic cancer model. As a result of these experiments, all groups have found evidences of multicolored metastases, indicating that CTC clusters can seed polyclonal metastases [6, 45, 46, 63]. However, as it will be discussed in the next section, the mechanism by which tumor cells give rise to CTC clusters seems to differ in some cases. The experimental design of these studies however, did not address whether a cooperative behavior was happening between clones. In addition, a similar experiment developed, in which two melanoma cell lines with different metastatic potential were mixed and injected into the flank of nude mice, has shown the presence of polyclonal CTC clusters and polyclonal metastases. Unlike previous studies, this work showed that the cell lines within the CTC clusters cooperated on the development of metastases and that tumor cells with lower metastatic potential acquired higher metastatic capability when grouping together [71].
7.4 Origin of CTC Clusters
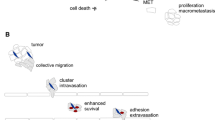
An important question still under debate is the origin of CTC clusters. Mainly two models are under evaluation; (i) CTC cluster can be directly derived from the primary tumor due to the cohesive unit of tumor cells in an orchestrated phenomenon where tumor cells cooperate and collectively migrate, and (ii) CTC cluster can arise from the aggregation and proliferation of individual CTCs in the bloodstream (Fig. 7.2).
Two previously mentioned studies in breast cancer experimentally addressed this question. By injecting breast cancer color-tagged tumor cells into mice at two different locations (mammary fat pads at opposite flanks), they were able to prove that intravascular aggregation of individual CTCs was not the cause of CTC cluster formation, supportive of the existence of a mechanism of collective cell migration and shedding of CTC clusters into the circulation from the primary tumor [6, 46]. Evidences in support of this have been also shown for pancreatic cancer [45]. The molecular mechanisms linked to the formation of clusters, at least in breast cancer, are connected to two proteins, plakoglobin (JUP) and keratin 14 (KRT14), found to be critical for CTC cluster formation. Both proteins are associated with desmosomes and hemidesmosomes, and involved in cell-cell junctions, necessary for the maintenance of the integrity of CTC clusters. In the same line of thought, experimental evidences gathered on an in vitro platform mimicking the bloodstream have shown that the unfavorable conditions present in the bloodstream will not support the intravascular aggregation and proliferation of individual CTCs [72].
However, a recent report developed with PDXs mouse models bearing metastatic breast cancer showed evidences for the presence of clustered tumor cells both in migration and circulation as the result of aggregation of individual tumor cells rather than collective migration and cohesive shedding to the bloodstream [63]. Using intravital multiphoton microscopic imaging, it was shown that cells expressing the stem cell marker CD44 are capable of aggregating into clusters in the circulation or lung vasculature, and that this marker is required for the formation of metastases. This evidence goes in agreement with an earlier study showing the formation of multicellular aggregates at the sites of their primary attachment to the endothelia previous to metastases formation [73], although intravascular cell proliferation of individual CTCs attached to the endothelium has also been reported as an initial step for lung metastasis formation, without need of extravasation and tissue parenchyma invasion [74]. All evidences point towards a possible combined action of both mechanisms in the formation of CTC clusters, and it allows to speculate about the existence of an interplay or even synergy between both mechanisms [63].
A third model for the origin of CTC clusters has also been recently proposed, called “cell jamming”. According to this model, the increasing confinement from the growing mass of tumor or higher density of extracellular matrix (ECM) may promote grouping of the cells, and therefore facilitate CTC cluster formation [75]. This hypothesis or model is supported by in vitro evidences showing that ECM density affects how tumor cells invade. Thus, when ECM density is high, mesenchymal tumor cells show a preference for collective invasion, and single cell invasion is observed under low ECM density conditions [76].
7.5 CTC Clusters Isolation Technologies
Technologies developed for the capture of CTCs could be in principle also applicable for the capture of CTC clusters. However, in almost all cases they have not been designed with this specific purpose in mind, which translates into a low efficiency of recovery, inability to separate CTC clusters from single CTCs, and often causing cluster damage and break up during separation. Therefore the main challenge it is not to separate CTC clusters from blood cells but to separate them from individual CTCs without affecting their integrity. From the research point of view, a platform for the isolation of CTC clusters should be able to isolate clusters of different sizes in an epitope independent manner, with short processing times, and able to preserve the integrity of the clusters as well as the recovery of viable cells; but from the clinical standpoint, such a platform should demonstrate reproducibility and clinical validity. A summary of the technologies used for CTC clusters isolation and detection is shown in Table 7.2. CTC clusters are usually small groups of cells, from 2 to 19 cells [44], although CTC clusters bigger than 100 cells have been reported. In this sense, size exclusion methods are the best approach to isolate CTC clusters, yielding a good recovery. In particular, filtration technologies are popular given their easy use and high throughput. However, given the physical properties of CTC clusters, those strategies that exclusively rely on size-based separation might loss a significant fraction of CTC clusters [24]. It is because of this that researchers have devoted efforts to improve and develop technologies for the detection of CTC clusters, mainly combining microfluidics with size exclusion approaches. An example, it is the development of the Cluster-Chip, a microfluidic device designed specifically to capture CTC clusters from whole blood [44]. The Cluster-Chip uses triangular micropillars arrays forming bifurcating traps for the capture of clusters, without compromising their integrity. This chip detected CTC clusters in 30–40% of patients with metastatic breast or prostate cancer, or melanoma; however, it showed some limitations regarding the recovery of the clusters immobilized on micropillar arrays. In response to this problem, the inventors have developed a new microfluidic device relying on a two-stage deterministic lateral displacement (DLD) approach [77]. This system sorts cell clusters based on size and asymmetry, and allows for a high recovery efficiency of viable cells with minimal cluster dissociation. This system remains to be tested in the clinical setting with cancer patient blood samples. An in detail discussion and revision of methodologies used form CTC clusters isolation can be found in the following reference [24].
7.6 Metastatic Features of CTC Clusters
In addition to the preclinical evidences published in the 1970s [10, 15,16,17], more recent studies mainly developed in breast cancer have demonstrated the high predisposition of CTC clusters to generate distant metastases than single CTCs. There are strong evidences in support of the high metastatic potential of CTC clusters as compared to individual CTCs. Despite of the reported low frequency of CTC clusters both in the blood of breast cancer patients and in the blood of breast tumor mouse models, it has been shown that CTC clusters are responsible for seeding between 50 and 97% of metastatic tumors in mouse models [6, 46]. Aceto et al. using a mouse xenograft model of MDA-MB-231 LM2 cell line, have reported that CTC clusters have an estimated metastatic potential 23–50 times higher than single CTCs [6]. Interestingly, this work has shown the self-seeding potential of CTC clusters within the primary tumor, as well as their oligoclonal origin. In the same way, and making use of the Confetti and Rainbow mice MMTV-PyMT model, Cheung et al. have estimated the metastatic potential of clusters to be >100 times increased relative to single cells [46]. These two studies support the formation of clusters of tumor cells at the primary tumor and their shedding into the bloodstream as a group. Similarly, a study by Liu et al. using triple negative patient-derived breast cancer models (PDXs) shows that CTC clusters have a higher efficiency in mediating metastasis formation than single CTCs [63]. Interestingly, evidences for a higher efficiency of CTC clusters than single CTCs in forming metastases have also been found in pancreatic cancer and colon cancer models [45, 89].
Despite of the demonstration of the increased metastatic potential of CTC clusters compared to individual CTCs, and the frequent polyclonal seeding occurring from the primary tumor to secondary sites suggesting that different clonal combinations in the cluster could have different properties with respect to growth, it still remains under debate whether the tumor cells within a CTC cluster harbor different metastatic potentials. In support of this, a study in melanoma showed that tumor cells with lower metastatic potential can acquire a higher metastatic capability when grouping together with cells with a higher metastatic potential [71]. On the other hand, it was previously reported, that when injecting melanoma cells with different metastatic properties as cellular aggregates, the presence of metastatic cells did not change the inability of non-metastatic cells to proliferate in a distant organ [90]. This last piece of evidence suggests that the metastatic potential of a CTC cluster may depend on the most malignant tumor cells. Further experimental evidences are needed in order to determine whether cooperation between heterogeneous clones making up tumor cell clusters is really happening, and also what is the significance for the metastatic potential of CTC clusters. At this point, it is worth reminding that it is now well accepted and demonstrated the existence of heterogeneous populations of CTCs, with a differential contribution to the metastatic process in prostate, lung and breast cancer [91,92,93]. In this regard, CTC cluster show both epithelial and mesenchymal traits at the same time, in breast cancer and other tumor types [38, 94]. CTC clusters isolated form breast cancer patients have been found to be positive for mesenchymal markers such as fibronectin, N-cadherin or PAI-1 and weakly positive for endothelial markers (EpCAM or cytokeratins). These findings could indicate a possible cooperative behavior between mesenchymal CTCs and epithelial CTCs within the same cluster, although it has not been formally probed. In order to address this question, cells from an individual CTC clusters should be individualized and analyzed at single cell level, proving the existence of a heterogeneous population of CTCs expressing either epithelial markers or mesenchymal markers.
7.7 Survival and Proliferative Advantage of CTC Clusters
Metastasis is regarded as a highly inefficient process. The vast majority of tumor cells shed into the bloodstream do not survive. It is only a small fraction of CTCs that are viable and capable of surviving, seeding distant organs, and eventually giving rise to overt metastatic disease. This argues that only those CTCs able to survive the transit in the bloodstream will stand a chance in order to contribute to the development of metastases.
CTC clusters have a survival advantage over single CTCs, and we nowadays partially understand some of the underlying reasons. An important feature of the CTCs forming the clusters is that they have strong cell–cell contacts linking them together [95]. It is well established that loss of adhesion-dependent survival signals by epithelial cells when transitioning in the bloodstream leads to anoikis, being therefore causative of CTCs death [96]. This goes in support of the idea that strong cell–cell interactions in the clusters can provide survival stimuli favoring their metastatic spread [75, 97]. Indeed, the interaction between the proteins circulating galectin-3 and cancer-associated mucin1 (MUC1), as well as CD44-mediated signaling pathways, promote homotypic tumor cell aggregation and cluster formation, and prevents CTCs in circulation from anoikis in breast and colon cancer [63, 98], enhancing metastases formation potential.
CTC clusters seem to have a shorter half-life in circulation than single CTCs (6–10 min and 25–30 min, respectively) [6], what may help them to survive favoring the outgrowth into micrometastases [99]. Mouse studies in breast cancer showed that CTCs clusters are more resistant to apoptosis at distal metastatic sites than individual CTCs, allowing them to expand more rapidly. Thus, disseminated tumor cells in the lungs of mice injected with CTC cluster did not undergo apoptosis, opposite to disseminated cells from mice injected with single cells which underwent massive apoptosis [6].
The protection of CTC clusters against apoptosis was also shown in patients with small-cell lung cancer; while a 57% of patients showed apoptotic single CTCs (from 0.2 to 20% of CTCs), none of the patients presenting CTC clusters have apoptotic cells within the clusters [5]. Likewise, a study of triple negative breast cancer patients found that only a 0.4% of the cells in the clusters (4 cells out 943 in a total of 194 clusters) were apoptotic, as opposed to a 20% of apoptotic single CTCs (1674 cells out of 8393 single CTC) [43]. These clinical evidences clearly support the protection of CTCs forming the clusters to apoptosis.
Other factors possibly modulating the survival of CTC clusters while transitioning in the bloodstream have been proposed. In patients with MBC the hybrid epithelial-mesenchymal phenotype observed in CTC clusters, which confers a substantial plasticity to these aggregates, has been put forward as a feature for survival advantage [38]. Mesenchymal traits favoring a migratory phenotype together with the preservation of cell–cell junctions of epithelial cells, seem to be the underlying mechanism [100]. Furthermore, methylation and gene expression analyses in CTC clusters from both breast cancer patients and breast cancer xenograft models revealed an enrichment on genes related to cell-cell junction, proliferation and DNA replication [61]. Indeed, CTC clusters show an increase in the percentage of CTCs expressing the marker Ki67 compared to single CTCs, indicative of a higher proliferation rate. Also, CTC clusters seem to share several properties that commonly feature stem cell biology [61, 63]. These features may play a relevant role in the intravasation, enhanced adaptation to new microenvironments and facilitate metastasis initiation by CTC clusters. Interestingly, the epigenetic signature found in CTC clusters, hypomethylated regions enriched with embryonic stem cell transcription factor binding sites, correlates with an enhanced metastatic phenotype and with poor prognosis in patients with breast cancer [61].
7.8 CTC Clusters, a Small Portion of Tumor Microenvironment
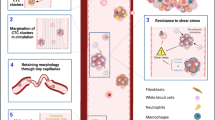
It has been suggested that the presence within the CTC clusters of immune cells, platelets and stroma-derived cells and factors, known as heterotypic clusters, may be of benefit for the survival and metastatic outgrowth of CTC clusters [100] (Fig. 7.3). Although the role of tumor microenvironment components within CTC cluster remains largely uncharacterized, some evidences are starting to emerge.
Platelets coating CTCs and CTC clusters in the bloodstream act as a physical shield protecting them from the shear forces [101] and immune attacks [102], but also protecting them through the paracrine secretion of factors such as transforming growth factor β (TGF-β), a known inducer of EMT [38]. Staining of CTC clusters isolated from the blood of MBC patients showed an abundance of attached platelets, what goes in support of the strong TGF-β signatures found in mesenchymal CTC clusters [38].
Cancer associated fibroblasts (CAFs) similarly to cancer cells, can disseminate through the circulation to secondary sites, suggesting a role for these cells in the metastatic process [103]. Indeed, the presence of CAFs in heterotypic CTC clusters enables an enhanced survival of tumor cells and also provides growth advantage to them after seeding at distant sites. This has been proved in an experimental setup in mice in which depletion of fibroblasts in the clusters reduced their capacity to form lung metastases [104]. However, even though it has been suggested that CAFs promote tumor growth and metastasis, new evidences also support antitumor actions; meaning, at least, that the role of CAFs within heterotypic CTC clusters need to be further investigated.
Among the white blood cells (WBC) found forming clusters with CTCs, neutrophils seem to play an important role on CTC clusters mediated-metastasis [84]. The direct interaction of neutrophils with breast cancer CTCs shapes the transcriptional profile of tumor cells supporting cell cycle progression in circulation and accelerating metastasis seeding. Moreover, neutrophils are actively involved in the genesis of CTC clusters, as their depletion in BC animal models reveled a delayed shedding of CTCs and CTC–neutrophil clusters from the primary tumor, a delayed metastasis development, and a shorter overall survival of the mice. Of note, those BC patients in whom at least a single neutrophil-containing CTC cluster was found had a worse progression-free survival than patients with ≥5 CTCs in 7.5 ml of peripheral blood [84].
7.9 Prognostic Value of CTC Clusters in Metastatic Breast Cancer
Enumeration of CTCs by CellSearch® platform has been extensively proved to be an independent predictor of survival in patients with MBC [105, 106]. Importantly, the prognostic value of CTCs has also been proved in patients with early breast cancer [107]. Despite the demonstration of the prognostic value of CTC enumeration in breast cancer, it took a decade to demonstrate the prognostic value of CTC clusters (A summary of studies in breast cancer in which the prognostic value of CTC clusters has been investigated is shown in Table 7.3); although initial evidences for this were previously shown in liver cancer and small-cell lung cancer [5, 32].
A prospective randomized phase II trial determined the number of CTC clusters and evaluated its predictive value in a cohort of 32 metastatic Triple Negative Breast Cancer patients (TNBC), on samples collected at baseline, and follow-up after initiation of therapy. This work demonstrated that the persistent presence of CTC clusters detected by CellSearch® at follow-up, but not baseline, was associated with shorter patient survival [43]. This goes in agreement with a previous study in patients with small-cell lung cancer (SCLC) showing that the presence of CTC clusters was significantly associated with worse prognosis [5]. In addition, it was previously shown that the presence of CTC clusters in patients with progressing metastatic breast cancer (79 patients) also correlates with poor prognosis, although in this occasion the technology used for CTC cluster identification was the HBCTC-Chip [6]. This chip has a high efficiency capturing both small and large clusters [33], it isolates CTC clusters based on the expression of EpCAM, HER2, and the mesenchymal marker CDH11. Interestingly the study included patients with different breast cancer subtypes, and showed that the persistent presence of CTC clusters in the blood of these patients was associated with an adverse clinical outcome [6]. Likewise, the authors reproduced these data on a cohort of prostate cancer patients. Taken together, these studies demonstrate the prognostic value of CTC cluster in advanced breast cancer regardless the technology used for their identification.
Furthermore, in recent years a few other studies have evaluated and corroborated the prognostic value of CTC clusters in breast cancer. It is important to notice that in all following studies the presence of CTC clusters was evaluated using the CellSearch® platform. Indeed, these studies have shown that CTC cluster evaluation added additional prognostic value to CTC enumeration alone [42, 50, 51, 80]. Thus, a prospective study involving 115 advance breast cancer patients (stage III and IV), from all subtypes, has shown that CTC cluster evaluation allows for the stratification of patients with elevated baseline CTCs into different survival groups [42]. It also reported that the prognostic value of CTC-clusters appeared to be more pronounced in patients with inflammatory breast cancer, and showed evidences for a yet unreported worse prognosis for patients with CTC clusters present at baseline. A latter work in a cohort of 52 MBC patients from all subtypes undergoing first-line systemic therapy, also showed a poorer prognosis in terms of progression-free survival and overall survival for those patients in which CTC clusters were present in peripheral blood during treatment [51]. This effect was independent of other prognostic factors such as CTC numbers and breast cancer subtype. Similarly, a study in a cohort of 156 MBC patients starting first-line systemic therapy, including all subtypes, showed that longitudinal evaluation of CTC clusters improves prognostication and monitoring. Again this work indicates the added prognostic value of CTC clusters to CTC enumeration alone, and showed no association between breast cancer subtype and presence of CTC clusters [80]. On the other hand, the prognostic value of CTC clusters at baseline is still under debate with evidences building up in both senses [42, 43, 50, 51, 80].
In addition, these clinical studies are also shedding light on the biology of CTC clusters. Thus, a link between CTC cluster size and patient prognosis has been established [50]. Longitudinal data collected from 128 MBC patients at baseline and before starting a new therapy revealed that patients with CTC clusters composed of 3 cells have a pronounce decrease in OS compared to patients with 2-cell CTC clusters. These findings are in line with preclinical evidences previously reported [10, 15]. Moreover, evaluation of the expression of the stem cell marker CD44 in CTC clusters showed that patients with CD44+ CTC clusters had a lower OS than patients with CD44− CTC clusters [63]. Finally, these studies indicate that CTC cluster are more often found in TNBC and HER-2 positive patients than in hormone receptor-positive patients [43, 51].
In summary, these studies clearly demonstrate that CTC cluster counts it is an independent prognostic factor, as the presence of CTC clusters adds significant prognostic value to CTC enumeration alone in patients with high CTC counts.
7.10 Therapeutic Implications: Targeting CTC Clusters
Given the importance of CTC cluster to the development of metastasis, research efforts are being directed to identify possible vulnerabilities of clusters in order to target them. In this sense, the advancement on the knowledge of the biology of these cells through their molecular phenotyping is crucial to find or design specific treatments. In this regard a few proof of concept studies have been published.
The identification of plakoglobin as a gene highly overexpressed in CTCs from clusters relative to single CTCs as well as its expression in primary breast tumors associated with a significantly reduced distant metastasis-free survival led to investigate its potential as a therapeutic target. Knockdown of this gene in breast cancer cell lines injected into mice led to a diminished presence of CTC clusters in the blood as well as a decreased metastasis formation, suggesting that is a key mediator in tumor cell clustering, without altering primary tumor growth [6]. Similarly, keratin 14 has also been identified to be highly enriched in some CTCs of the clusters as well as in micrometastases, relative to primary tumors or macrometastases [46]. In this case, the knockdown of keratin 14 in the primary tumor led to a decrease in metastasis formation. As both proteins, plakoglobin and keratin 14, are involved in cell-cell junctions necessary for the maintenance of the integrity of CTC clusters, a link can be established between cluster integrity and metastasis seeding, suggesting that the disruption or disaggregation of CTC clusters could be a valid therapeutic strategy. This idea is further supported by recent data showing that the knockdown of CD44 or the use of an anti-CD44 neutralizing antibody disrupted tumor cell aggregation and diminished metastasis formation by CTC clusters [63].
In this sense, the treatment of breast tumor bearing mice with the thrombolytic agent urokinase, exerted and antimetastatic effect by dissociating CTC Clusters [108]. Importantly, these mice showed a 20% increase in survival upon urokinase treatment relative to control animals. More recently, a screening for compounds able to dissociate CTC clusters found that Na+/K+ ATPase inhibitors can efficiently reduce cluster size [61]. Further analysis of the Na+/K+ ATPase inhibitor ouabain in a breast cancer model, showed that the administration of this compound to mice is able to in vivo suppresses the ability of tumors to shed CTC clusters (while increasing the frequency of single CTCs), leading to a remarkable reduction on overall metastasis formation.
As previously mentioned, heterotypic CTC clusters may have enhanced metastatic potential as to that of homotypic clusters, suggesting that the targeting of stromal components within the clusters might be a successful strategy to limit the metastasis seeding capacity. An initial indirect indication for this showed that the depletion of CAFs, which spontaneously metastasize along with cancer cells, in a metastasis mouse model of lung cancer, reduced the number of lung metastases [104]. More recently, it has been shown that the molecule VCAM1 has an important role in mediating the interaction between CTCs and neutrophils, and that the targeting of this molecule prevents the formation of CTC–neutrophil clusters which have an enhanced metastasis seeding capacity [84].
Although so far limited in number, these evidences support a model by which targeting CTC clusters could be a valuable therapeutic approach. Indeed, they support two possible different therapeutic strategies that could be of benefit for cancer patients (at least in breast cancer), i) Preventing CTC cluster formation at early stage for the treatment of cancer while a localized disease and before it disseminates (neoadjuvant and adjuvant treatment), and ii) Disassembling of CTC clusters while in circulation for the treatment of late disease stages cancer to prevent metastasis from seeding other metastases.
7.11 Remaining Questions and Opportunities
Based on current evidences, CTC clusters seem to be responsible for the formation of tumor metastasis. Despite of their origin, whether they are formed by collective shedding to the blood stream or by intravascular aggregation, these tumor cell aggregates have and enhanced survival capacity and improved secondary tumor growth. Interesting features are now known about the biology of CTC clusters; i.e. a hybrid epithelial-mesenchymal profile, a stemnes phenotype, and heteroptypic composition. Most importantly, these features are being correlated to a worse prognosis in breast cancer patients, suggestive of the many clinical implication of CTC clusters. But this knowledge raises important questions that needed to be answered. It remains to be determined whether the oligoclonal/polyclonal nature of CTC clusters is the result of an oncogenic cooperative behavior between tumor subclones. Whether CTC clusters hold tumor cells with diverse molecular phenotypes conferring a differential metastatic capacity. It is yet elusive whether the hybrid epithelial-mesenchymal phenotype observed in CTC clusters is due to the combination of cells with a heterogeneous EMT phenotype or rather a mixture of cells bearing either epithelial or mesenchymal features. If the later, evidences are needed of a cooperative behavior between mesenchymal CTCs and epithelial CTCs within the cluster. Moreover, finding out the specific influence of other cell types, such as tumor-associated macrophages, fibroblasts, or leukocytes, on the CTCs within a heterotypic cluster, grants further mechanistic investigation. In this sense, technological aid is paramount. The advancement on the development of more efficient CTC clusters isolation technologies and their combination with under development single cell genomic, transcriptomic, and proteomic analyses is key to address these important questions.
The increased knowledge on the biology of CTC clusters brings about new therapeutic opportunities to interfere with the process of metastasis. Current experimental evidences indicate that CTC cluster disaggregation, as a therapeutic approach, seems quite plausible. However, this strategy may entail some risks (at least for urokinase treatment), since it may increase the invasiveness of tumor cells and therefore metastatic spreading, resulting in the opposite effect [109]. Alternatively, interfering with non-tumor cells associated to CTCs in the clusters may provide a new therapeutic approach, as recently showed [84]. But in this case, more mechanistic insights on how these cells affect tumor cells during different steps of metastasis are needed. Lastly, further knowledge about what are the therapeutic implications of tumor cell clusters, remains to be acquired. CTC clusters represent a challenge because they could contain tumor cells with different drug uptake and resistance properties [110, 111], and even it is now suggested that cluster “compactness” may predict early treatment response in different cancer types including breast cancer [112]. Emerging methods for the ex vivo culture of CTCs are very valuable tools for the assessment of drug response and resistance, but they will also help to address some of the question mentioned above.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. https://doi.org/10.3322/caac.20073.
Brandt B, Junker R, Griwatz C, Heidl S, Brinkmann O, Semjonow A, et al. Isolation of prostate-derived single cells and cell clusters from human peripheral blood. Cancer Res. 1996;56(20):4556–61.
Cho EH, Wendel M, Luttgen M, Yoshioka C, Marrinucci D, Lazar D, et al. Characterization of circulating tumor cell aggregates identified in patients with epithelial tumors. Phys Biol. 2012;9(1):016001. https://doi.org/10.1088/1478-3975/9/1/016001.
Hou JM, Krebs MG, Lancashire L, Sloane R, Backen A, Swain RK, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol. 2012;30(5):525–32. https://doi.org/10.1200/JCO.2010.33.3716.
Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–22. https://doi.org/10.1016/j.cell.2014.07.013.
Langenbeck B. On the development of cancer in the vein s, and the transmission of cancer from man to the lower animals. Edinb Med Surg J. 1841;55(147):251–3.
Ashworth TR. A case of cancer in which cells similar to those in the Tumours were seen in the blood after death. Aust Med J. 1869; https://doi.org/10.1111/j.1528-1167.2011.03138.x.
Denis MG, Tessier MH, Dreno B, Lustenberger P. Circulating micrometastases following oncological surgery. Lancet. 1996;347(9005):913. https://doi.org/10.1016/s0140-6736(96)91402-6.
Liotta LA, Kleinerman J, Saidel GM. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 1976; https://doi.org/10.1097/INF.0b013e31818ec288.
Virchow R. Cellular pathology. As based upon physiological and pathological histology. Philadelphia: J B Lippincott; 1863. https://doi.org/10.5962/bhl.title.32770.
Coman DR, Delong RP, McCutcheon M. Studies on the mechanisms of metastasis. The distribution of tumors in various organs in relation to the distribution of arterial emboli. Cancer Res. 1951;11(8):648–51.
Zeidman I. Metastasis: a review of recent advances. Cancer Res. 1957;17(3):157–62.
Watanabe S. The metastasizability of tumor cells. Cancer. 1954;7(2):215–23. https://doi.org/10.1002/1097-0142(195403)7:2<215::aid-cncr2820070203>3.0.co;2-6.
Fidler IJ. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer. 1973; https://doi.org/10.1016/S0014-2964(73)80022-2.
Thompson SC. The colony forming efficiency of single cells and cell aggregates from a spontaneous mouse mammary tumour using the lung colony assay. Br J Cancer. 1974; https://doi.org/10.1038/bjc.1974.201.
Liotta LA, Kleinerman J, Saidel GM. Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Cancer Res. 1974;34(5):997–1004.
Knisely WH, Mahaley MS. Relationship between size and distribution of “spontaneous” metastases and three sizes of intravenously injected particles of VX2 carcinoma. Cancer Res. 1958;18(8 Part 1):900–5.
Zeidman I, Buss JM. Transpulmonary passage of tumor cell emboli. Cancer Res. 1952;12(10):731–3.
Lione A, Bosmann HB. Quantitative relationship between volume of tumour cell units and their intravascular survival. Br J Cancer. 1978; https://doi.org/10.1038/bjc.1978.33.
Tsoi DT, Rowsell C, McGregor C, Kelly CM, Verma S, Pritchard KI. Disseminated tumor embolism from breast cancer leading to multiorgan failure. J Clin Oncol. 2010; https://doi.org/10.1200/JCO.2009.25.1009.
Peeters DJE, Brouwer A, Van den Eynden GG, Rutten A, Onstenk W, Sieuwerts AM, et al. Circulating tumour cells and lung microvascular tumour cell retention in patients with metastatic breast and cervical cancer. Cancer Lett. 2015; https://doi.org/10.1016/j.canlet.2014.10.039.
Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. J Cell Biol. 2011;192(3):373–82. https://doi.org/10.1083/jcb.201010021.
Au SH, Edd J, Haber DA, Maheswaran S, Stott SL, Toner M. Clusters of circulating tumor cells: a biophysical and technological perspective. Curr Opin Biomed Eng. 2017;3:13–9. https://doi.org/10.1016/j.cobme.2017.08.001.
Nowell PC. The clonal evolution of tumor cell populations. Science. 1976; https://doi.org/10.1126/science.959840.
Poste G, Fidler IJ. The pathogenesis of cancer metastasis. Nature. 1980;283(5743):139–46. https://doi.org/10.1038/283139a0.
Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–8. https://doi.org/10.1038/nrc1098.
Molnar B, Ladanyi A, Tanko L, Sreter L, Tulassay Z. Circulating tumor cell clusters in the peripheral blood of colorectal cancer patients. Clin Cancer Res. 2001;7(12):4080–5.
Brandt B, Roetger A, Heidl S, Jackisch C, Lelle RJ, Assmann G, et al. Isolation of blood-borne epithelium-derived c-erbB-2 oncoprotein-positive clustered cells from the peripheral blood of breast cancer patients. Int J Cancer. 1998;76(6):824–8.
Hou JM, Krebs M, Ward T, Sloane R, Priest L, Hughes A, et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol. 2011;178(3):989–96. https://doi.org/10.1016/j.ajpath.2010.12.003.
Kats-Ugurlu G, Roodink I, De Weijert M, Tiemessen D, Maass C, Verrijp K, et al. Circulating tumour tissue fragments in patients with pulmonary metastasis of clear cell renal cell carcinoma. J Pathol. 2009; https://doi.org/10.1002/path.2613.
Vona G, Estepa L, Béroud C, Damotte D, Capron F, Nalpas B, et al. Impact of cytomorphological detection of circulating tumor cells in patients with liver cancer. Hepatology. 2004; https://doi.org/10.1002/hep.20091.
Stott SL, Hsu CH, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci. 2010;107(43):18392–7. https://doi.org/10.1073/pnas.1012539107.
Mascalchi M, Falchini M, Maddau C, Salvianti F, Nistri M, Bertelli E, et al. Prevalence and number of circulating tumour cells and microemboli at diagnosis of advanced NSCLC. J Cancer Res Clin Oncol. 2016; https://doi.org/10.1007/s00432-015-2021-3.
Krebs MG, J-m H, Sloane R, Lancashire L, Priest L, Nonaka D, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol. 2012;7(2):306–15. https://doi.org/10.1097/JTO.0b013e31823c5c16.
Khoja L, Backen A, Sloane R, Menasce L, Ryder D, Krebs M, et al. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer. 2012;106(3):508–16. https://doi.org/10.1038/bjc.2011.545.
Wendel M, Bazhenova L, Boshuizen R, Kolatkar A, Honnatti M, Cho EH, et al. Fluid biopsy for circulating tumor cell identification in patients with early-and late-stage non-small cell lung cancer: a glimpse into lung cancer biology. Phys Biol. 2012; https://doi.org/10.1088/1478-3967/9/1/016005.
Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–4. https://doi.org/10.1126/science.1228522.
Hosokawa M, Kenmotsu H, Koh Y, Yoshino T, Yoshikawa T, Naito T, et al. Size-based isolation of circulating tumor cells in lung cancer patients using a microcavity array system. PLoS One. 2013;8(6) https://doi.org/10.1371/journal.pone.0067466.
Warkiani ME, Guan G, Luan KB, Lee WC, Bhagat AAS, Kant Chaudhuri P, et al. Slanted spiral microfluidics for the ultra-fast, label-free isolation of circulating tumor cells. Lab Chip. 2014; https://doi.org/10.1039/c3lc50617g.
Harouaka RA, Zhou MD, Yeh YT, Khan WJ, Das A, Liu X, et al. Flexible micro spring array device for high-throughput enrichment of viable circulating tumor cells. Clin Chem. 2014;60(2):323–33. https://doi.org/10.1373/clinchem.2013.206805.
Mu Z, Wang C, Ye Z, Austin L, Civan J, Hyslop T, et al. Prospective assessment of the prognostic value of circulating tumor cells and their clusters in patients with advanced-stage breast cancer. Breast Cancer Res Treat. 2015;154(3):563–71. https://doi.org/10.1007/s10549-015-3636-4.
Paoletti C, Li Y, Muñiz MC, Kidwell KM, Aung K, Thomas DG, et al. Significance of circulating tumor cells in metastatic triple-negative breast cancer patients within a randomized, phase II trial: TBCRC 019. Clin Cancer Res. 2015; https://doi.org/10.1158/1078-0432.CCR-14-2781.
Sarioglu AF, Aceto N, Kojic N, Donaldson MC, Zeinali M, Hamza B, et al. A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nat Methods. 2015;12:685–91. https://doi.org/10.1038/nmeth.3404.
Maddipati R, Stanger BZ. Pancreatic cancer metastases harbor evidence of polyclonality. Cancer Discov. 2015;5(10):1086–97. https://doi.org/10.1158/2159-8290.CD-15-0120.
Cheung KJ, Padmanaban V, Silvestri V, Schipper K, Cohen JD, Fairchild AN, et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Natl Acad Sci U S A. 2016;113(7):E854–63. https://doi.org/10.1073/pnas.1508541113.
Long E, Ilie M, Bence C, Butori C, Selva E, Lalvée S, et al. High expression of TRF2, SOX10, and CD10 in circulating tumor microemboli detected in metastatic melanoma patients. A potential impact for the assessment of disease aggressiveness. Cancer Med. 2016;5:1022–33. https://doi.org/10.1002/cam4.661.
Chen JY, Tsai WS, Shao HJ, Wu JC, Lai M, Lu SH, et al. Sensitive and specific biomimetic lipid coated microfluidics to isolate viable circulating tumor cells and microemboli for cancer detection. PLoS One. 2016;11(3):e0149633. https://doi.org/10.1371/journal.pone.0149633.
Kulasinghe A, Tran THP, Blick T, O’Byrne K, Thompson EW, Warkiani ME, et al. Enrichment of circulating head and neck tumour cells using spiral microfluidic technology. Sci Rep. 2017; https://doi.org/10.1038/srep42517.
Wang C, Mu Z, Chervoneva I, Austin L, Ye Z, Rossi G, et al. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res Treat. 2017;161(1):83–94. https://doi.org/10.1007/s10549-016-4026-2.
Jansson S, Bendahl PO, Larsson AM, Aaltonen KE, Rydén L. Prognostic impact of circulating tumor cell apoptosis and clusters in serial blood samples from patients with metastatic breast cancer in a prospective observational cohort. BMC Cancer. 2016;16(1):1–15. https://doi.org/10.1186/s12885-016-2406-y.
Reddy RM, Murlidhar V, Zhao L, Grabauskiene S, Zhang Z, Ramnath N et al. Basic science: lung cancer Pulmonary venous blood sampling significantly increases the yield of circulating tumor cells in early-stage lung cancer. 2016 March; https://doi.org/10.1016/j.jtcvs.2015.09.126.
Chang MC, Chang YT, Chen JY, Jeng YM, Yang CY, Tien YW, et al. Clinical significance of circulating tumor microemboli as a prognostic marker in patients with pancreatic ductal adenocarcinoma. Clin Chem. 2016;62(3):505–13. https://doi.org/10.1373/clinchem.2015.248260.
Zhang D, Zhao L, Zhou P, Ma H, Huang F, Jin M, et al. Circulating tumor microemboli (CTM) and vimentin+ circulating tumor cells (CTCs) detected by a size-based platform predict worse prognosis in advanced colorectal cancer patients during chemotherapy. Cancer Cell Int. 2017;17:6. https://doi.org/10.1186/s12935-016-0373-7.
Zheng X, Fan L, Zhou P, Ma H, Huang S, Yu D, et al. Detection of circulating tumor cells and circulating tumor microemboli in gastric cancer. Transl Oncol. 2017;10(3):431–41. https://doi.org/10.1016/j.tranon.2017.02.007.
Lee M, Kim EJ, Cho Y, Kim S, Chung HH, Park NH, et al. Predictive value of circulating tumor cells (CTCs) captured by microfluidic device in patients with epithelial ovarian cancer. Gynecol Oncol. 2017;145(2):361–5. https://doi.org/10.1016/j.ygyno.2017.02.042.
Fanelli MF, Oliveira TB, Braun AC, Corassa M, Abdallah EA, Nicolau UR, et al. Evaluation of incidence, significance, and prognostic role of circulating tumor microemboli and transforming growth factor-β receptor I in head and neck cancer. Head Neck. 2017;39(11):2283–92. https://doi.org/10.1002/hed.24899.
Hayashi M, Zhu P, McCarty G, Meyer CF, Pratilas CA, Levin A, et al. Size-based detection of sarcoma circulating tumor cells and cell clusters. Oncotarget. 2017;8(45):78965–77. https://doi.org/10.18632/oncotarget.20697.
Murlidhar V, Reddy RM, Fouladdel S, Zhao L, Ishikawa MK, Grabauskiene S, et al. Poor prognosis indicated by venous circulating tumor cell clusters in early-stage lung cancers. Cancer Res. 2017;77(18):5194–206. https://doi.org/10.1158/0008-5472.CAN-16-2072.
Xu Y, Qin T, Li J, Wang X, Gao C, Xu C, et al. Detection of circulating tumor cells using negative enrichment immunofluorescence and an in situ hybridization system in pancreatic cancer. Int J Mol Sci. 2017;18(4) https://doi.org/10.3390/ijms18040622.
Gkountela S, Castro-Giner F, Szczerba BM, Vetter M, Landin J, Scherrer R, et al. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell. 2019;176(1–2):98–112.e14. https://doi.org/10.1016/j.cell.2018.11.046.
Krol I, Castro-Giner F, Maurer M, Gkountela S, Szczerba BM, Scherrer R, et al. Detection of circulating tumour cell clusters in human glioblastoma. Br J Cancer. 2018;114(4):487–91. https://doi.org/10.1038/s41416-018-0186-7.
Liu X, Taftaf R, Kawaguchi M, Chang YF, Chen W, Entenberg D, et al. Homophilic CD44 interactions mediate tumor cell aggregation and polyclonal metastasis in patient-derived breast cancer models. Cancer Discov. 2019;9(1):96–113. https://doi.org/10.1158/2159-8290.CD-18-0065.
Kulasinghe A, Zhou J, Kenny L, Papautsky I, Punyadeera C. Capture of circulating tumour cell clusters using straight microfluidic chips. Cancers. 2019; https://doi.org/10.3390/cancers11010089.
Abdallah EA, Braun AC, Flores BCTCP, Senda L, Urvanegia AC, Calsavara V, et al. The potential clinical implications of circulating tumor cells and circulating tumor microemboli in gastric cancer. Oncologist. 2019; https://doi.org/10.1634/theoncologist.2018-0741.
McFadden DG, Papagiannakopoulos T, Taylor-Weiner A, Stewart C, Carter SL, Cibulskis K, et al. Genetic and clonal dissection of murine small cell lung carcinoma progression by genome sequencing. Cell. 2014; https://doi.org/10.1016/j.cell.2014.02.031.
Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JMC, Papaemmanuil E, et al. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520(7547):353–7. https://doi.org/10.1038/nature14347.
Marusyk A, Tabassum DP, Altrock PM, Almendro V, Michor F, Polyak K. Non-cell-autonomous driving of tumour growth supports sub-clonal heterogeneity. Nature. 2014;514(7520):54–8. https://doi.org/10.1038/nature13556.
Tabassum DP, Polyak K. Tumorigenesis: it takes a village. Nat Rev Cancer. 2015;15(8):473–83. https://doi.org/10.1038/nrc3971.
Cleary AS, Leonard TL, Gestl SA, Gunther EJ. Tumour cell heterogeneity maintained by cooperating subclones in Wnt-driven mammary cancers. Nature. 2014; https://doi.org/10.1038/nature13187.
Küsters B, Kats G, Roodink I, Verrijp K, Wesseling P, Ruiter DJ, et al. Micronodular transformation as a novel mechanism of VEGF-A-induced metastasis. Oncogene. 2007; https://doi.org/10.1038/sj.onc.1210360.
Hong Y, Fang F, Zhang Q. Circulating tumor cell clusters: what we know and what we expect (review). Int J Oncol. 2016;49(6):2206–16. https://doi.org/10.3892/ijo.2016.3747.
Glinsky VV, Glinsky GV, Glinskii OV, Huxley VH, Turk JR, Mossine VV, et al. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003;63(13):3805–11.
Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med. 2000;6(1):100–2. https://doi.org/10.1038/71429.
Giuliano M, Shaikh A, Lo HC, Arpino G, De Placido S, Zhang XH, et al. Perspective on circulating tumor cell clusters: why it takes a village to metastasize. Cancer Res. 2018;78:845–52.
Haeger A, Krause M, Wolf K, Friedl P. Cell jamming: collective invasion of mesenchymal tumor cells imposed by tissue confinement. Biochim Biophys Acta Gen Subj. 2014; https://doi.org/10.1016/j.bbagen.2014.03.020.
Au SH, Edd J, Stoddard AE, Wong KHK, Fachin F, Maheswaran S, et al. Microfluidic isolation of circulating tumor cell clusters by size and asymmetry. Sci Rep. 2017;7:2433. https://doi.org/10.1038/s41598-017-01150-3.
Marrinucci D, Bethel K, Kolatkar A, Luttgen MS, Malchiodi M, Baehring F, et al. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys Biol. 2012;9(1):016003. https://doi.org/10.1088/1478-3975/9/1/016003.
Desitter I, Guerrouahen BS, Benali-Furet N, Wechsler J, Jänne PA, Kuang Y, et al. A new device for rapid isolation by size and characterization of rare circulating tumor cells. Anticancer Res. 2011;31(2):427–41.
Larsson AM, Jansson S, Bendahl PO, Levin Tykjaer Jörgensen C, Loman N, Graffman C, et al. Longitudinal enumeration and cluster evaluation of circulating tumor cells improve prognostication for patients with newly diagnosed metastatic breast cancer in a prospective observational trial. Breast Cancer Res. 2018;20(1):48. https://doi.org/10.1186/s13058-018-0976-0.
Ozkumur E, Shah AM, Ciciliano JC, Emmink BL, Miyamoto DT, Brachtel E, et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci Transl Med. 2013;5(179):179–47. https://doi.org/10.1126/scitranslmed.3005616.
Karabacak NM, Spuhler PS, Fachin F, Lim EJ, Pai V, Ozkumur E, et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat Protoc. 2014;9(3):694–710. https://doi.org/10.1038/nprot.2014.044.
Friedlander TW, Ngo VT, Dong H, Premasekharan G, Weinberg V, Doty S, et al. Detection and characterization of invasive circulating tumor cells derived from men with metastatic castration-resistant prostate cancer. Int J Cancer. 2014;134(10):2284–93. https://doi.org/10.1002/ijc.28561.
Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019; https://doi.org/10.1038/s41586-019-0915-y.
Bhagwat N, Dulmage K, Pletcher CH, Wang L, DeMuth W, Sen M, et al. An integrated flow cytometry-based platform for isolation and molecular characterization of circulating tumor single cells and clusters. Sci Rep. 2018;8(1):5035. https://doi.org/10.1038/s41598-018-23217-5.
Cheng SB, Xie M, Xu JQ, Wang J, Lv SW, Guo S, et al. High-efficiency capture of individual and cluster of circulating tumor cells by a microchip embedded with three-dimensional poly(dimethylsiloxane) scaffold. Anal Chem. 2016;88(13):6773–80. https://doi.org/10.1021/acs.analchem.6b01130.
Cheng SB, Xie M, Chen Y, Xiong J, Liu Y, Chen Z, et al. Three-dimensional scaffold chip with thermosensitive coating for capture and reversible release of individual and cluster of circulating tumor cells. Anal Chem. 2017;89(15):7924–32. https://doi.org/10.1021/acs.analchem.7b00905.
Zhou J, Kulasinghe A, Bogseth A, O’Byrne K, Punyadeera C, Papautsky I. Isolation of circulating tumor cells in non-small-cell-lung-cancer patients using a multi-flow microfluidic channel. Microsyst Nanoeng. 2019;5:8. https://doi.org/10.1038/s41378-019-0045-6.
Topal B, Roskams T, Fevery J, Penninckx F. Aggregated colon cancer cells have a higher metastatic efficiency in the liver compared with nonaggregated cells: an experimental study. J Surg Res. 2003;112(1):31–7. https://doi.org/10.1016/S0022-4804(03)00140-9.
Fidler IJ, Talmadge JE. Evidence that intravenously derived murine pulmonary melanoma metastases can originate from the expansion of a single tumor cell. Cancer Res. 1986; https://doi.org/10.1158/0008-5472.CAN-09-0167.Glioma.
Chen JF, Ho H, Lichterman J, Lu YT, Zhang Y, Garcia MA, et al. Subclassification of prostate cancer circulating tumor cells by nuclear size reveals very small nuclear circulating tumor cells in patients with visceral metastases. Cancer. 2015;121(18):3240–51. https://doi.org/10.1002/cncr.29455.
de Wit S, van Dalum G, Lenferink ATM, Tibbe AGJ, Hiltermann TJN, Groen HJM, et al. The detection of EpCAM+ and EpCAM– circulating tumor cells. Sci Rep. 2015;5(1):12270. https://doi.org/10.1038/srep12270.
Bulfoni M, Turetta M, Del Ben F, Di Loreto C, Beltrami AP, Cesselli D. Dissecting the heterogeneity of circulating tumor cells in metastatic breast cancer: going far beyond the needle in the haystack. Int J Mol Sci. 2016;17(10):1–25. https://doi.org/10.3390/ijms17101775.
Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015; https://doi.org/10.1038/nature16064.
Fabisiewicz A, Grzybowska E. CTC clusters in cancer progression and metastasis. Med Oncol. 2017;34(1):1–10. https://doi.org/10.1007/s12032-016-0875-0.
Yao X, Choudhury AD, Yamanaka YJ, Adalsteinsson VA, Gierahn TM, Williamson CA, et al. Functional analysis of single cells identifies a rare subset of circulating tumor cells with malignant traits. Integr Biol (United Kingdom). 2014; https://doi.org/10.1039/c3ib40264a.
Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta. 2013;1833(12):3481–98.
Zhao Q, Barclay M, Hilkens J, Guo X, Barrow H, Rhodes JM, et al. Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol Cancer. 2010;9:1–12. https://doi.org/10.1186/1476-4598-9-154.
Micalizzi DS, Maheswaran S, Haber DA. A conduit to metastasis: circulating tumor cell biology. Genes Dev. 2017;31(18):1827–40. https://doi.org/10.1101/gad.305805.117.
Aceto N, Toner M, Maheswaran S, Haber DA. En route to metastasis: circulating tumor cell clusters and epithelial-to-mesenchymal transition. Trends Cancer. 2015;1(1):44–52. https://doi.org/10.1016/j.trecan.2015.07.006.
Dasgupta A, Lim AR, Ghajar CM. Circulating and disseminated tumor cells: harbingers or initiators of metastasis? Mol Oncol. 2017;11(1):40–61.
Sharma D, Brummel-Ziedins KE, Bouchard BA, Holmes CE. Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. J Cell Physiol. 2014;229(8):1005–15. https://doi.org/10.1002/jcp.24539.
Olivier De W, Mieke Van B, Marc M, An H, Marc B. Carcinoma-associated fibroblasts provide operational flexibility in metastasis. Semin Cancer Biol. 2014;25:33–46. https://doi.org/10.1016/j.semcancer.2013.12.009.
Duda DG, Duyverman AMMJ, Kohno M, Snuderl M, Steller EJA, Fukumura D, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A. 2010;107(50):21677–82. https://doi.org/10.1073/pnas.1016234107.
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–91. https://doi.org/10.1056/NEJMoa040766.
Bidard FC, Peeters DJ, Fehm T, Nolé F, Gisbert-Criado R, Mavroudis D, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014; https://doi.org/10.1016/S1470-2045(14)70069-5.
Zhang L, Riethdorf S, Wu G, Wang T, Yang K, Peng G, et al. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res. 2012; https://doi.org/10.1158/1078-0432.CCR-12-1587.
Choi JW, Kim JK, Yang YJ, Kim P, Yoon KH, Yun SH. Urokinase exerts antimetastatic effects by dissociating clusters of circulating tumor cells. Cancer Res. 2015;75(21):4474–82. https://doi.org/10.1158/0008-5472.CAN-15-0684.
Mirshahi S, Pujade-Lauraine E, Soria C, Pocard M, Mirshahi M, Soria J. Urokinase antimetastatic effects-letter. Cancer Res. 2016;76(16):4909. https://doi.org/10.1158/0008-5472.CAN-16-0138.
Bithi SS, Vanapalli SA. Microfluidic cell isolation technology for drug testing of single tumor cells and their clusters. Sci Rep. 2017;7:41707. https://doi.org/10.1038/srep41707.
Cheung KJ, Ewald AJ. A collective route to metastasis: seeding by tumor cell clusters. Science (New York, NY). 2016;352:167–9. https://doi.org/10.1126/science.aaf6546.
Balakrishnan A, Koppaka D, Anand A, Deb B, Grenci G, Viasnoff V, et al. Circulating tumor cell cluster phenotype allows monitoring response to treatment and predicts survival. Sci Rep. 2019;9(1):7933. https://doi.org/10.1038/s41598-019-44404-y.
Acknowledgements
The work of the authors is supported by Roche-Chus Joint Unit (IN853B 2018/03) funded by GAIN, “Consellería de Economía, Emprego e Industria”. IMP is funded by the Training Programme for Academic Staff (FPU) fellowship, from the Ministry of Education and Vocational Training, Spanish Government.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Piñeiro, R., Martínez-Pena, I., López-López, R. (2020). Relevance of CTC Clusters in Breast Cancer Metastasis. In: Piñeiro, R. (eds) Circulating Tumor Cells in Breast Cancer Metastatic Disease. Advances in Experimental Medicine and Biology, vol 1220. Springer, Cham. https://doi.org/10.1007/978-3-030-35805-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-35805-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-35804-4
Online ISBN: 978-3-030-35805-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)