Abstract
The modulation of cognitive functions by noninvasive stimulation of the human brain has gained increasing attention over the last few decades. Transcranial direct current stimulation (tDCS) is an easy-to-use, painless, and affordable modulatory technique with no or minimal side effects. However, studies show no consistency in outcome measures due to high variability in terms of the methodological approach. This review starts with an enumeration and critical discussion of all the influencing parameters regarding (1) the tDCS protocol itself, (2) the targeted behavioral task, (3) the characteristics of the study group, and (4) the outcome measures used. First, the use of different settings with different combinations of parameters that might co-interfere hampers the comparability between studies. A focus on within instead of between subjects can give a better insight in which parameters to use in which conditions. Second, the area of stimulation has to depend on the used behavioral task and may crucially influence the therapeutic outcome and thereby its efficiency. Different brain networks play a role in language and motor speech, such as the prefrontal cortex, the inferior frontal gyrus, and the superior temporal gyrus. That means that therapists need to know which functional network is addressed by a given behavioral task and how this network can be targeted the most efficiently with tDCS taking into account the sustained neural damage. Third, different tasks might be differentially sensitive to performance changes induced by tDCS, depending on, e.g., task complexity. Finally, speech-language therapy is a type of neurorehabilitation that focuses not only on the rehabilitation of an impairment but also searches for compensating strategies aiming to improve functional communication. Therefore, this chapter also provides a road map summing up all the variables and linking them in a patient-centered virtuous circle, since speech-language therapy should be an iterative process where the clinician is in constant dialogue with the patient.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
The modulation of cognitive functions by noninvasive stimulation of the human brain has gained increasing attention over the last few decades. The two most known neuromodulation techniques are transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). The popularity of tDCS compared to TMS is due to its safety, portability, and cost-effectiveness. Moreover tDCS is an easy-to-use, painless, tolerable corticomotor modulation technique with no or minimal side effects (Bolognini, Pascual-Leone, & Fregni, 2009). While TMS implicates more artefacts such as acoustic noise and muscle twitching, only minor adverse effects are reported from tDCS (Fertonani, Ferrari, & Miniussi, 2015; Poreisz, Boros, Antal, & Paulus, 2007).
tDCS can be used to probe and modulate cortical plasticity (Prehn & Flöel, 2015) that is defined as the capacity of the brain to develop new neuronal-synaptic interconnections and thereby develop and adapt new functions or reorganize/compensate for changes. During tDCS, weak polarizing direct currents are delivered to the cortex via two electrodes placed on the scalp. The current induces changes in the resting membrane potential of the neurons. This means that tDCS does not directly elicit action potentials but changes the amount of additional input needed to generate an action potential in neuronal populations. In other words, tDCS changes the likelihood that an incoming action potential will result in postsynaptic firing both immediately during stimulation and a short period of time after stimulation. Therefore, tDCS has an impact on two neurophysiological mechanisms: (1) subthreshold alterations of the resting membrane potential involving ionic concentration shifts within the extracellular fluid (“primary effect”) and (2) the synaptic plasticity of glutamatergic connections (i.e., N-methyl-d-aspartate (NMDA) receptor-dependent processes) (“aftereffect”) (Prehn & Flöel, 2015). Since tDCS acts upon the resting membrane potential and NMDA-receptor activity, it promotes synaptic plasticity of glutamatergic connections (namely, synaptic long-term potentiation (LTP)-/long-term depression (LTD)-like mechanisms) that can outlast the duration of stimulation for several hours (Stagg & Nitsche, 2011). In general, the resting membrane potential is lowered underneath the anode, inducing higher excitability, while it is heightened underneath the cathode, inducing lower excitability. While these neurophysiological effects are well understood, little is known about the long-term effects, especially with respect to cognitive enhancement (Holland, Leff, Penny, Rothwell, & Crinion, 2016).
Studies show a high variability in terms of the methodological approach, the characteristics of the study group, the targeted cognitive functions (Cappon, Jahanshahi, & Bisiacchi, 2016), and the outcome measures used. To the best of our knowledge, there is no standard protocol to evaluate the impact of tDCS. A standard protocol is arguable considering the differential impact and the diversity of the research, but a road map focusing on different parameters and their impact might be a valuable starting point (Jacobson, Koslowsky, & Lavidor, 2012). In this chapter, we provide a critical review on all the influencing parameters, along with a draft for such a road map. In the Appendix in the Back matter of this book, a non-exhaustive overview of studies using tDCS to study/boost language functions in healthy (Appendix A: Tables A1 and A2) and patient (Appendix B: Tables B1, B2, and B3) populations is included, which will give the reader a general idea of the demographic characteristics of the targeted population (Tables A1 and B1) and the methodological (Tables A2 and B2) and therapeutic (Table B3) approach of the current studies.
6.2 Variability in Methodological Approach
6.2.1 tDCS Protocol
Zooming in on the methodological approach, many parameters pertaining to the tDCS device may influence its impact: (1) the stimulation schedule (frequency, duration, and type), (2) the current of the stimulation (intensity, density, and total charge), (3) the targeted area of stimulation (left/right, frontal/temporal), (4) the used electrodes (montage, material, sizes, and shape), and (5) the combination of tDCS and therapy (online or offline stimulation, impact of stimulation on task performance).
6.2.1.1 Stimulation Schedule
The stimulation schedule has three general dimensions: frequency, duration, and type. Frequency pertains to the amount of tDCS sessions a participant gets. In literature, one session is often used for healthy participants and repeated sessions are used in participants with speech-language impairments. In research, sessions are often separated by at least four hours since the cortical excitability alterations can last for over an hour after the end of the stimulation (Westwood & Romani, 2017). In practice, sessions are often separated by a minimum of 24 h or even one week. In clinical practice, daily sessions are recommended to evoke a cumulative and long-term effect. Repeated stimulations after a short interval of 20 min (i.e., during the aftereffects of stimulation) result in initially reduced yet ongoing excitability enhancement (LTP-like plasticity), while temporally contiguous stimulation and repeated stimulation after a prolonged time interval (i.e., after the aftereffects have disappeared) might result in a reversal of neuroplasticity (Monte-Silva et al., 2013). This suggests that, in a clinical population, studies need to focus not only on the frequency but also on the interval time between consecutive sessions, so that optimal neuroplasticity effects can be induced.
By duration we mean the total number of time one session takes. This parameter ranges in the literature from 6 to 30 min, with a mean duration of 20 min.
Different types of stimulation can be used: anodal tDCS (atDCS), cathodal tDCS (ctDCS), and placebo (sham). From a neurophysiological point of view, the type of stimulation refers to the polarity of the current and thereby to the way neurons are influenced. However, a nonlinear system like the brain is unlikely to have a linear response to an externally applied electric current (Westwood & Romani, 2017). In general, anodal stimulation increases cortical excitability, whereas cathodal stimulation decreases it (Fiori et al., 2011). In literature, however, there is a consensus about the stimulation effect of atDCS (e.g., Alberto Pisoni et al., 2015; Jacobson et al., 2012), but there is no consensus about the effect of ctDCS. Sham is the placebo stimulation where the electrodes are also attached on the head, but the current is turned on for a maximum period of one minute. The current is quickly ramped up and down in the beginning (and in some studies in the end as well) of each stimulation session. This technique is useful within a research context, since it blinds the participant from knowing whether they are actively stimulated or not, by giving them the initial sensation of the current building up (Gandiga, Hummel, & Cohen, 2006). Bastani and Jaberzadeh (2012) state that the application of tDCS is associated with minimal or no somatosensory input, implicating that the stimulation remains imperceptible by most people during its application. However, some participants do report an itching sensation beneath both electrodes during the early rising phase of the current. This tingling sensation elicited on the scalp lasts only for the first few seconds and then disappears (Nitsche et al., 2003). However, the research of O’Connell et al. (2012) showed that participants receiving both types of stimulation can easily discriminate between real and sham stimulation. When participants only received one type of stimulation, blinding was much more reliable (Russo, Wallace, Fitzgerald, & Cooper, 2013). Unreliable blinding may play a role in data variability present in current tDCS literature. In a research context, often different types of stimulation are used with the order of sessions counterbalanced across participants to control for learning effects. However, in clinical practice, repeated sessions of one type of stimulation are the most favorable approach in order to accumulate the most positive effect. The assumption that atDCS enhances and ctDCS diminishes cortical excitability (Nitsche & Paulus, 2000) has been mainly supported by studies that focus on the effects of tDCS on motor functions. However, a meta-analysis of tDCS studies found that the probability of achieving this classical “anodal-facilitatory/cathodal-inhibitory” effect on motor outcomes was only 0.67 and for cognitive outcomes only 0.16 (Jacobson et al., 2012). The underlying explanation might be that the anodal electrode increases further neuronal firing of a previously activated region, contributing to a greater facilitation of (cognitive) performance of this area. Decreased neural firing, resulting from the cathodal electrode, cannot generate sufficient inhibition when the initial rate is already high, since subjects are engaged in cognitive tasks. Moreover, since cognitive functions are not restricted to a specific brain area but rather to a brain network, these functions may be immune to inhibitory stimulation.
6.2.1.2 Stimulation Current
The applied current can be defined by its intensity, density, and total charge. The current intensity varies from 1 to 2 mA. Although stimulation with a stronger current over a longer period of time is more intense, it is unknown whether stimulation is also more effective (Prehn & Flöel, 2015). Vöröslakos et al. (2018) found that current intensities conventionally used in tDCS studies are insufficient to affect neuronal circuits directly, suggesting that reported behavioral and cognitive effects result from indirect mechanisms. The current intensity has an impact on local (i.e., modulation of endogenous low-frequency oscillations) brain areas (Hartwigsen, 2015), within network connectivity (Meinzer et al., 2012), as well as on functionally connected, remote brain areas (i.e., spreading via excitatory and inhibitory neural pathways (Polanía, Nitsche, Korman, Batsikadze, & Paulus, 2012)); the exact amount of the impact is still questionable. Therefore, researchers often define the current density, i.e., the stimulation intensity (mA) per area of stimulating electrode size (cm2). This density is independent of the duration of the stimulation. Densities below 25 mA/cm2 are considered safe, since 25 mA/cm2 is the threshold for brain tissue damage in rats. Electrode sizes of 25–35 cm2 are commonly used with a constant current of 2 mA intensity, resulting in a current density of 0.080–0.057 mA/cm2 at the skin which will not induce brain tissue damage. Nonetheless, the intensity of current that reaches and affects the cortex below the electrodes is difficult to determine. It is typically inferred from physiologic outcomes such as functional imaging, which is not necessarily linear or even monotonic with local current intensity, or from behavioral changes, where the relationship with regional current flow is yet less clear (Edwards et al., 2013). Moreover, Sandars, Cloutman, and Woollams (2016) reported that even though current density is uniform, between 41% and 61% of the applied current does not penetrate the skull. This limited spatial accuracy is a potential limitation of tDCS (Raffin & Siebner, 2014). In order to overcome this limitation, high-density (HD) tDCS is required, which can be achieved by using smaller electrodes, in configurations that yield more focal stimulation (Datta, Baker, Bikson, & Fridriksson, 2011). While the exact amount and the spreading of the current are hard to define, the maximum effect may not be below the electrode pads as assumed, making it more complicated to choose the appropriate stimulation site. Precise modeling studies might be essential for future research to employ stimulation parameters that optimize current density distribution. Therefore, researchers also report the total charge of the current, i.e., the amount of current that is applied over the head during the session and is determined by the duration of the session and the current intensity.
6.2.1.3 Area of Stimulation
The next important question is the area of stimulation, since therapeutic goals and outcomes of tDCS are linked to the targeted brain regions. For this parameter as well, there is no consensus about the optimal location of the electrodes. The placement of the tDCS electrodes is usually guided by the 10–20 EEG system, since this is a helpful and easy-to-use technique facilitating the incorporation of tDCS in day-to-day clinical practice (Meinzer, Darkow, Lindenberg, & Flöel, 2016). As outlined earlier, the stimulated area is a window onto a large-scale functional network, rather than on an isolated site (e.g., Bikson, Datta, Rahman, & Scaturro, 2010; Manjaly et al., 2005; Moliadze, Antal, & Paulus, 2010). So the question is: which is the ideal network to stimulate in order to obtain the maximum out of tDCS? Many researchers stimulate the left frontal cortex, since this includes the area of Broca, which is important for speech production, i.e., speech repetition, reading, writing, and naming (Bashir & Howell, 2017). The frontal cortex is 1/3 of the cortex and electrodes are 25–35 cm2, so a more specific spot needs to be chosen. For example, Meinzer et al. (2014) have shown that tDCS over the primary motor cortex (M1, C3) induces long-lasting changes in cortical excitability and can improve word retrieval in healthy participants. Besides the primary motor cortex, the left dorsolateral prefrontal cortex (DLPFC, Fp1/AF3) (Manenti et al., 2015; Saidmanesh, Pouretemad, Amini, Nillipour, & Ekhtian, 2012; Shah-Basak et al., 2015; Wirth et al., 2011) or the inferior frontal gyrus (F5) (e.g., Campana, Caltagirone, & Marangolo, 2015; Fiori et al., 2013; Pisoni, Papagno, & Cattaneo, 2012; Vestito, Rosellini, Mantero, & Bandini, 2014) are frequently targeted areas of stimulation. Depending on the behavioral task administered during stimulation, researchers also stimulate other brain areas beyond the frontal cortex, such as the left temporal region. Sparing, Dafotakis, Meister, Thirugnanasambandam, and Fink (2008), for example, applied tDCS over the posterior perisylvian region, i.e., an area which includes Wernicke’s area (T5), and reported an impact on lexical-phonological retrieval. Besides the therapeutic goals and outcomes, interindividual variability may also influence the impact of tDCS. Therefore, studies that employed similar areas of stimulation resulted in highly variable stimulation effects (de Aguiar, Paolazzi, & Miceli, 2015). Klaus and Schutter (2018) argue that the placement of the active electrode over the targeted region and the reference electrode over the contralateral supraorbital region yields the highest field strengths anterior to the targeted region as well as additional frontal effects in the right hemisphere. These wide electrical field distributions may cause collateral activation of surrounding tissue and contribute to the heterogeneous findings reported in previous studies. It remains to be tested whether additional modifications of the montages (e.g., by using smaller electrodes or a HD tDCS setup) further reduce induced field strengths in regions peripheral to the targeted region. The area chosen for stimulation also depends on the location of the reference electrode. Most researchers place the reference electrode on the contralateral (right) supraorbital region (e.g., Buchwald et al., 2019), whereas others place it on the contralateral (right) homologue area (e.g., Marangolo et al., 2013).
6.2.1.4 Electrodes
Different electrodes, i.e., different montages, materials, shapes, and sizes, vary across studies. Different montages are available: (a) bipolar (two cephalic electrodes) (e.g., Marangolo et al., 2013) or (b) unipolar (one cephalic and one extracephalic electrode) (e.g., Nitsche & Paulus, 2000). The magnitude of the tDCS-elicited changes in cortical excitability depends on the electrode montage, due to the interdependence between neuronal orientation and the orientation of the induced current (Wagner et al., 2007). At present, different electrode arrangements have been evaluated – this has been done mainly for stimulation of the primary motor cortex, less for non-motor areas. Since language is a complex cognitive task involving language networks, not specific areas, in recent literature, researchers have investigated the added benefits of bilateral/bipolar stimulation over unilateral stimulation (e.g., Li et al., 2015; Moliadze et al., 2010). Regarding the material of the electrodes, two types are most often used: nonmetallic, conductive rubber electrodes, covered by saline-soaked sponges or rubber electrodes used with conductive gel (Prehn & Flöel, 2015), minimizing chemical reactions at the electrode-skin interface.
Looking at the size and shape of the electrodes, two large electrode pads with areas of several tens of cm2 are used (Saturnino, Antunes, & Thielscher, 2015). This conventional tDCS electrode montage results in very diffuse brain current flow, with areas of clustering (“hot spots”) and ineffective pervasion of the targeted area (Edwards et al., 2013). When using the conventional large stimulation electrodes (i.e., 25–35 cm2), tDCS is less suitable to investigate functional-anatomic subdivisions within language areas, but it might be preferable for therapeutic, longitudinal purposes (Monti et al., 2013).
6.2.1.5 Combination of tDCS and Behavioral Task
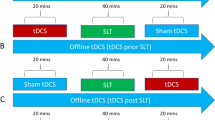
The fifth variable concerns the combination of tDCS and the behavioral task; researchers distinguish between online and offline tDCS. Online tDCS implicates that the tDCS stimulation is given during a therapy session, therefore potentially optimizing the effects of language therapy, whereas offline tDCS implicates that the tDCS stimulation is given before a therapy session, potentially priming the language system in preparation for the task used during treatment (de Aguiar, Paolazzi, & Miceli, 2015). Behavioral priming results in improved performance due to repeated encounters with the same or related stimuli and is caused by a reduction in task-dependent neural activity (Holland et al., 2011). Neural priming is the neurophysiological explanation of the cumulative effect of tDCS on behavior. Since the human brain consists of dense neuronal tissue, it operates on limited neural resources and thereby consists of overlapping neural networks (Brem, Unterburger, Speight, & Jäncke, 2014). In cognitive and neurobiological models, cognitive functions are supported by distributed, interconnected, overlapping, and highly parallel processing networks (Hebb, 1949; Horwitz, Heng, & Quazi, 2003). In these networks, higher-order cortices can be involved in a flexible, context-dependent manner in different functions (Behrens & Sporns, 2012; Bressler & Menon, 2010). For example, the language and motor action systems feature tight functional connections and share neural resources (Willems & Hagoort, 2007). This implicates that enhancement of cognitive functions by means of tDCS is never isolated. The facilitated switching between the overlapping neural systems involved during the behavioral task explains the improved behavioral performance afterwards. In this way, priming cortical excitability using tDCS optimizes the learning processes involved in language therapy and leads to more distinct and long-term functional communication gains (Bolognini et al., 2009). However, the exact cumulative mechanism of the externally applied tDCS, the internal modulation of neuronal activity, and the impact on an individual’s behavior has yet to be determined (Holland et al., 2016). Since Monti et al. (2008) suggest the absence of effects of offline perilesional atDCS, recent studies have focused on online atDCS in elderly participants with or without aphasia (e.g., Binney et al., 2018).
Based on the results of the literature, we believe that the following tDCS parameters should result in the most effective outcome: multiple stimulation sessions (>5) should be used, each lasting for at least 20 min, with a short time interval (~24 h), so that neurons are triggered effectively. The current strength is set at 2 mA and the 25–35 cm2−electrodes are placed in saline-soaked sponges to obtain an optimal current flow. The area and the type of stimulation are linked to the training/therapeutic goal (Table 6.1). This leads to determining the influencing parameters of one specific behavioral task.
6.2.2 Behavioral Task
To the best of our knowledge, there is no consensus on what the behavioral task, i.e., the speech-language therapy, should be. Nonetheless, selecting the correct pairing between the area of stimulation and the behavioral task may crucially influence the therapeutic outcome and thereby its efficiency. The goals of speech-language therapy may be better achieved if tDCS is delivered to an area putatively involved in the task at hand, as this ensures that electrical stimulation is paired with ongoing synaptic activation, a seemingly necessary factor for lasting effects (Fritsch et al., 2010).
In an ideal cumulative situation, tDCS enhances corticomotor excitability and augments the efficacy of therapeutic approaches inducing lasting neurobiological effects (Hummel & Cohen, 2006). Different tasks might be differentially sensitive to performance changes induced by tDCS. Moreover, there is an economical argument as well: therapists have less time to treat patients and limited funding is available. Therefore, clinicians are looking for the most ideal therapy program to maximize the patient’s communicative skills in as little time as possible and at the lowest possible cost, aiming for the most effective outcome (Maas et al., 2012).
Considering the literature on the cumulative effects of tDCS and behavioral speech-language therapy, researchers have reported cognitive enhancement in healthy participants for different cognitive (e.g., sustained attention, working memory, information processing, and language) and executive (e.g., inhibition and planning) functions. This chapter will focus on language and on motor speech, the two main domains of speech-language therapy.
6.2.2.1 tDCS and Language
Most studies focus on phonological and semantic aspects of oral language production, examining the effects of tDCS on picture naming (Fertonani, Brambilla, Cotelli, & Miniussi, 2014; Holland et al., 2011; Indefrey, 2011), verbal fluency (e.g., Iyer et al., 2005), or picture-word interference (Indefrey & Levelt, 2004). Few studies focus on the cumulative effect of tDCS and semantic aspects of language comprehension, using lexical decision (Brückner & Kammer, 2017), semantic judgment (McDermott, Petersen, Watson, & Ojemann, 2003), or ambiguous words (Peretz & Lavidor, 2013). Two studies have focused on the syntactic aspects of language (e.g., Cattaneo, Pisoni, & Papagno, 2011; De Vries et al., 2010). Two studies (Dick, Goldin-Meadow, Hasson, Skipper, & Small, 2009; Manuel & Schnider, 2016) have focused on tDCS and nonverbal communication, and three studies have combined tDCS with functional communication (Campana et al., 2015; Marangolo et al., 2014; Marangolo, Fiori, Calpagnano, et al., 2013) (Appendix A).
With respect to phonology and semantics, several researchers have reported the cumulative effect of tDCS on picture naming (Fertonani et al., 2014; Holland et al., 2011; Indefrey, 2011). However, no consensus has been reached on the stimulation site, since a large left frontotemporal network plays an important role in a naming task, including phonological and semantic skills. This network supports many cognitive processes, i.e., word-retrieval processes and different cognitive control processes, the initiation and sequencing of speech, and the motor speech act (Crosson, 2013; Dick, Bernal, & Tremblay, 2014; Eickhoff, Heim, Zilles, & Amunts, 2009). As Westwood and Romani (2017) state, picture naming necessitates cortical excitation (word retrieval) as well as inhibition (fending off alternative competitors). This network consists of the dorsal stream (i.e., left frontal hemisphere, the mapping of sensory input, and phonological information on the articulatory network) and the ventral stream (i.e., bilateral temporal hemispheres, the mapping of sounds onto meanings and meanings onto spoken output) (Hickok & Poeppel, 2000; Sandars et al., 2016). Studies have reported significant effects from applying atDCS over the left superior temporal gyrus and DLPFC on object and action naming (Fertonani et al., 2014; Fertonani, Rosini, Cotelli, Rossini, & Miniussi, 2010; Sparing et al., 2008), with tDCS mostly affecting naming latencies, rather than error rates (Table 6.2).
Another frequently examined language task pertains to verbal fluency (e.g., Iyer et al., 2005; Meinzer, Flaisch, et al., 2012). This usually involves a short test in which participants are required to generate as many words as possible from a semantic category (i.e., “semantic fluency,” which is a more common task, since we organize our daily lives in semantic categories) or beginning with a specific letter (i.e., “phonemic fluency,” a more complex and less familiar task) within a limited period of time. Many cognitive processes are involved in verbal fluency. In order to name as many examples as possible, one has to search the word content, retrieve it, monitor it, and select the appropriate word form from among competing alternatives (Fertonani et al., 2010). Considering its cognitive complexity, many brain areas are involved: (1) the prefrontal cortex (“switching,” i.e., changing from subcategories, as seen when one goes from providing examples of one subcategory to another, a more controlled process), (2) the inferior frontal gyrus (finding words), and (3) the superior temporal gyrus (“clustering” of words, i.e., the contiguous generation of words, a more automatic process) (Hirshorn & Thompson-Schill, 2006) (Table 6.2). To make it even more complex, phonemic and semantic word fluency involve partially different neural networks: semantic fluency is associated with a greater activation of the left inferior temporal lobe, reflecting the site of stored information being retrieved (Heim, Eickhoff, & Amunts, 2008). The inferior frontal gyrus is likely to subserve common processes critical for both semantic and phonemic tasks (Costafreda et al., 2006). Clustering and switching processes are also dependent on a number of participant characteristics, such as age and level of education (Vannorsdall et al., 2016). Evidence suggests that older healthy participants switch less frequently on semantic fluency tasks and produce larger clusters on phonemic fluency tasks than younger participants (Troyer, Moscovitch, & Winocur, 1997). While some studies report increased verbal fluency during or after tDCS (inferior frontal gyrus: Cattaneo et al., 2011; Iyer et al., 2005; Penolazzi, Pastore, & Mondini, 2013; Pisoni et al., 2018; DLPFC: Vannorsdall et al., 2012), other studies have not obtained such an effect (inferior frontal gyrus: Cattaneo et al., 2011; Ehlis, Haeussinger, Gastel, Fallgatter, & Plewnia, 2016; Vannorsdall et al., 2016; DLPFC: Cerruti & Schlaug, 2009). A third, less frequently reported language production task is a picture-word interference task. Participants are asked to name pictures while ignoring a visually or aurally presented distractor word. The relatedness of the target and the distractor is systematically varied. Typically, a semantically related distractor increases naming latencies compared to an unrelated distractor, while a phonologically related distractor speeds up naming latencies. Lexical-semantic processing has been associated with the left medial temporal gyrus, while phonological processing has been located in the left superior temporal gyrus (Indefrey & Levelt, 2004) (Table 6.2). Semantically related distractors are examined in more detail in semantic blocking tasks. In such a task, naming latencies are compared between semantically homogeneous (i.e., containing words from the same semantic category) and heterogeneous (i.e., semantically unrelated words) blocks. Retrieving and producing semantically related words in a row typically results in longer naming latencies compared to producing semantically unrelated words. This effect is called “the semantic interference effect” and underlines the competitive selection of target responses. This process is believed to rely on the left temporal cortex (Indefrey, 2011). Therefore, Indefrey (2011) and Wirth et al. (2011) focused on semantic interference during spoken word production using continuous and blocked cyclic naming paradigms (Damian, Vigliocco, & Levelt, 2001; Howard, Nickels, Coltheart, & Cole-Virtue, 2006). The underlying explanation is that in order to map a conceptual representation onto a speech representation, lexical-semantic encoding needs to take place (Belke & Stielow, 2013). However, on the one hand, researchers still have to unravel the precise functionality of the human brain. For example, Indefrey (2011) suggests that more research is needed to disentangle the precise role of subregions of the left inferior frontal gyrus and of the inferior parietal cortex in word production. On the other hand, the setup of the language task itself is still open for debate. Belke and Stielow (2013) demonstrate that the study of semantic context effects on object naming has proven to be a powerful tool for investigations in language production, although the persistency of semantic context effects still remains to be elucidated.
While there is an abundance of literature on language production, less is known on language comprehension, on syntax, on nonverbal communication, or on functional communication. Looking at language comprehension, Brückner and Kammer (2017) focused on the relationship between a lexical decision task and ctDCS across the left posterior superior temporal gyrus. McDermott et al. (2003) focused on semantic judgment and the specific role for the left inferior frontal gyrus, while Yang, Fuller, Khodaparast, and Krawczyk (2010) reported a positive effect of atDCS over Broca’s area while performing a figurative language comprehension task (Table 6.2). Peretz and Lavidor (2013) focused on ambiguous words while using a semantic decision task. De Vries et al. (2010) and Cattaneo et al. (2011) focused on syntax : they combined implicit artificial grammar learning with atDCS of the inferior frontal gyrus.
Only one study (Dick et al., 2009) focused on nonverbal communication. They reported the cumulative effect of gesture-language interplay, in which the inferior frontal gyrus plays a critical role.
Looking at functional communication, Marangolo, Fiori, Campana, et al. (2014); Marangolo et al. (2013); and Campana et al. (2015) combined tDCS with activity-based intensive conversational therapy. They used short video clips to set up a natural conversation and encouraged the individual to use a broad range of communicative means (e.g., gestures, drawings, orthographic or phonological cues) to exchange salient information about the video clip. They concluded that atDCS delivered over Broca’s area improved informative speech, i.e., individuals used more and more communicative units and the improvement persisted after 1 month (Table 6.2).
6.2.2.2 tDCS and Motor Speech Act
Recently tDCS has also been used in normal motor control (e.g., Grimaldi et al., 2016; Kang, Summers, & Cauraugh, 2016; Lefaucheur, 2016). The literature on the effects of tDCS on the motor speech act is far more scarce compared to tDCS and language. Five studies have combined tDCS with repeating orally presented words (Bashir & Howell, 2017; Buchwald et al., 2019; Chesters, Hsu, Bishop, Watkins, & Mottonen, 2017; Fiori, Cipollari, Caltagirone, & Marangolo, 2014; Simione, Fregni, & Green, 2018) and only one study has combined tDCS with oral reading (Wong, Chan, Ng, & Zhu, 2019). Their overall focus was on maximizing speech motor performance, i.e., the fluent and accurate articulation of sequential sounds in words, measured by acquisition, retention, and generalization of speech motor performance (e.g., Maas, 2015; Marangolo, Fiori, Campana, et al., 2014). Although speech production is a habitual and unique form of human daily communication, it is a complex behavior requiring the integration of concurrent linguistic, cognitive, attentional, and sensorimotor processes (Oh, Duerden, & Pang, 2014; Simione et al., 2018). When speaking, one should carefully plan and program precise muscle instructions, and oral movements must be highly coordinated.
During the last two decades, studies (Adams & Page, 2000; Bislick, Weir, Spencer, Kendall, & Yorkston, 2012; Ito, Coppola, & Ostry, 2016; Jones & Croot, 2016; Lisman & Sadagopan, 2013; Steinhauer & Grayhack, 2000; Wong, Whitehill, Ma, & Masters, 2013) have primarily focused on the integration of training principles in the nonspeech domains of motor learning, i.e., principles of motor learning (PML) (Schmidt, 1988; Schmidt & Lee, 2005). These PML are derived from relatively easy motor tasks, implicating that they cannot be directly translated to such complex motor tasks as the speech motor act, which possibly depends on a separate and unique motor system (Ziegler, 2003). PML specify (1) the structure of the practice, i.e., practice amount, distribution, variability and schedule, attentional focus, and target complexity, and (2) the nature of feedback, i.e., type, frequency, and timing, in order to enhance the learning capabilities for novel movements (Bislick et al., 2012). Although the application of these PML in speech motor learning has shown positive results in a healthy population, further investigation is warranted (Bislick et al., 2012; Maas, 2015), including replication of current research, extension of investigations of young healthy participants to older healthy participants, extension of investigations to motor speech disorders (e.g., ataxic dysarthria), and investigations of additional PML in both healthy participants and participants with motor speech disorders. In this way, PML can function as a theoretical framework, generating specific hypotheses that need to be investigated in more detail in different populations.
Recently, Buchwald et al. (2019) found that atDCS over the left motor cortex (C3) can improve speech motor learning in an offline condition, which makes it a possible stimulation target to enhance the performance in pure speech motor processing, such as syllable repetition or nonword repetition (Fuertinger, Horwitz, & Simonyan, 2015). Fiori et al. (2014) have confirmed critical involvement of the left premotor region (BA 6) , including Broca’s area (BA 44/45), in speech repetition (e.g., Baddeley, 2010; Trost & Gruber, 2012). They showed that speech accuracy and vocal reaction times while repeating tongue twisters during atDCS significantly improved during and 1 h after the stimulation. On the contrary, ctDCS significantly reduced speech articulation performance, while sham had no influence on speech articulation. Moreover, they showed generalization effects to untreated language production skills, which underlined the fact that speech engages motor and linguistic networks (Simione et al., 2018).
6.2.2.3 tDCS and Task Complexity
Besides the specific speech-language task, the complexity of the task influences the effect of tDCS and thereby the functional outcome. de Aguiar, Paolazzi, and Miceli (2015) reported that atDCS may be more suitable for easy tasks, while ctDCS may be more appropriate when the task is difficult. The question remains which task is easy and which task is not. Difficulty is not a one-way scale from “easy” to “complex ”, and different multi-way parameters have an impact on the level of complexity in different ways. First, the input, i.e., the way the task is delivered, might affect task complexity and thus the effectiveness of tDCS. A visually presented task, such as a reading task, impacts other, more occipital brain areas than an aural task, such as a repetition task, which impacts more temporal brain areas (Church, Coalson, Lugar, Petersen, & Schlaggar, 2008). For example, in a recent study, Rollans, Cheema, Georgiou, and Cummine (2017) suggested that the left inferior fronto-occipital fasciculus is more sensitive to overt response times that reflect slower and nonautomatic processes. Secondly, cueing and feedback, i.e., the way the task is supported, can be defined in different ways to influence the level of complexity. Miniussi, Harris, and Ruzzoli (2013) created a cueing strategy for a picture naming task, while Peach and Chapey (2008) postulated a cueing hierarchy of different semantic, orthographic, and phonological cues for the same task. Thirdly, the output, i.e., the way the task is performed, will impact which brain regions will be involved. For example, an oral response induces activity in other brain regions than a written response, the same for a verbal response versus a nonverbal response. Finally, the training material itself might impact task complexity. A consistent finding has been that when naming objects in context with other items from the same semantic category, response time increases compared to naming in unrelated contexts (Gauvin, Meinzer, & de Zubicaray, 2017).
6.2.3 Study Group
Besides stimulation- and task-related parameters, one should also take into account interindividual variability. Individual cortical susceptibility to stimulation may differ, inducing different levels of excitability among participants (Krause & Cohen Kadosh, 2014; Parazzini, Fiocchi, Liorni, & Ravazzani, 2015).
Mattson (2015) and Rabipour, Wu, Davidson, and Iacoboni (2018) report a list of interindividual differences: (1) general physiognomic differences, such as the morphology of the individual’s brain (Kim et al., 2014); (2) cognitive differences, such as information processing capacity, processing speed, attention, episodic memory, decision making, executive control functions, emotion processing, regulation, and lifelong cognitive stimulation; (3) demographic differences, such as age, gender (Madhavan, McQueeny, Howe, Shear, & Szaflarski, 2014), and level of education (El Hachioui et al., 2013); (4) social differences, such as social support and lifestyle factors; (5) medical differences, such as diabetes, overweight, or the use of medication; (6) physical differences, such as physical activity; and (7) psychological differences, such as motivation, expectations of outcomes, and affect (Table 6.3).
6.2.3.1 Physiognomic Differences
Regarding physiognomic differences, interindividual differences in cranial and brain anatomy can influence the impact of tDCS by inducing variability in the actual current received by the brain, even when the same electrical dose is administered. Some examples of these physiognomic differences are skull thickness and cerebrospinal fluid thickness (Opitz, Paulus, Will, Antunes, & Thielscher, 2015), subcutaneous fat (Truong, Magerowski, Blackburn, Bikson, & Alonso-Alonso, 2013), gyral pattern (Datta, Truong, Minhas, Parra, & Bikson, 2012), local tissue heterogeneities (Shahid, Wen, & Ahfock, 2014), and orientation of neurons (Arlotti, Rahman, Minhas, & Bikson, 2012). Anatomical factors do not always have the expected influence. For example, Opitz et al. (2015) demonstrated that a thicker skull resulted in a more complex relationship between skull thickness and current density.
6.2.3.2 Cognitive Differences
As for cognitive differences, Smith and Clithero (2009) demonstrate that both atDCS and ctDCS over the left DLPFC can enhance performance in attention tasks, working memory, planning abilities, information processing capacity, and speed. For example, sustained attention is an influencing factor in the rehabilitation process, since it is a prerequisite of cognitive relearning.
6.2.3.3 Demographic Differences
With respect to demographic differences, such as aging , researchers often start with healthy, young participants. Nevertheless, Summers, Kang, and Cauraugh (2016) underline that the neuroplasticity of the elder brain differs from that of a younger brain. They reported that it is more difficult for older participants to retrieve proper names in a naming task and that their verbal fluency is slowing down. This implicates that test results of a younger population cannot be extrapolated to an older population. At the neurophysiological level, aging negatively impacts gray and white matter integrity and neurotransmitter activity (Gutchess, 2014). The impact ranges from a loss of neurons and cortical thinning over impaired neurotransmitter-receptor binding and signaling and an accumulation of neurofibrillary tangles and amyloid plaques to altered concentrations of various brain metabolites (Jagust, 2013). Tatti, Rossi, Innocenti, Rossi, and Santarnecchi (2016) suggested this results in reduced hemispheric lateralization in cognitive aging, which leads to a complex relationship between functional overactivation, structural integrity, and cognitive abilities. Meinzer, Lindenberg, Antonenko, Flaisch, and Floel (2013) showed that elderly participants present with greater bilateral prefrontal activation than young adults and that this correlated with poorer performance in semantic word generation. In word-retrieval studies, decreased accuracy (Meinzer et al., 2009) and increased reaction times (Wierenga et al., 2008) have been noted for older populations. Right frontal activity has only been found in more demanding tasks, i.e., when the older participants produced fewer correct responses compared to the young adults (Meinzer et al., 2009; Meinzer, Flaisch, et al., 2012). This increased bilateral activity is explained by enhanced cognitive demands. According to Meinzer et al. (2014), there might be a co-interference of aging and challenging task conditions. They reported enhanced activity in right prefrontal areas in healthy older compared to younger participants when performing a language task. This enhanced activity might be due to an age-related phenomenon or it might reflect task difficulty effects. Moreover, control processes may have been more challenged in the older group due to deterioration of specialized neural populations in left frontal areas (Park & Reuter-Lorenz, 2009) or medial temporal structures (Pihlajamäki et al., 2000). Changes in hippocampal functions (Pihlajamäki et al., 2000) may also explain selectively impaired semantic word generation in healthy older participants. This may result in local changes in brain activity and also disruption of coordinated activity between different brain regions. Bennett and Madden (2014) have also demonstrated widespread changes in structural connectivity in aging, which has been linked to behavioral impairment and changes in functional networks, such as frontoparietal attention networks. Taking this into account, researchers agreed that atDCS is a viable tool to improve language function in aging (Fertonani et al., 2014; Perceval, Flöel, & Meinzer, 2016).
Moreover, cognitive performance declines with age (Mattson, 2015), although this decline does not affect all individuals equally (Berryhill & Jones, 2012). This decline is due to structural changes, i.e., neural atrophy from prefrontal and parietal regions, as well as functional changes, i.e., the recruitment of additional resources to maintain cognitive task performance (Davis, Dennis, Daselaar, Fleck, & Cabeza, 2008). Besides the impact of age, the level of education might impact the functional outcome after tDCS. For example, Katz et al. (2017) show that cognitive training may be more beneficial to those who already have strong cognitive abilities. The advantage of tDCS seemed to increase proportionally with decreasing baseline ability and conferred little additional advantage to a participant who had already performed high at baseline. It is still unclear which specific cognitive ability, such as higher levels of numeracy, literacy, or academic attainment, may mediate the interaction between stimulation and low baseline performance.
6.2.3.4 Social Differences
Social differences may also affect responsiveness to therapy. Patients with better social support experience better and faster recovery (Glass, Matchar, Belyea, & Feussner, 1993). To the best of our knowledge, the specific impact of social differences on tDCS efficacy has not been examined yet, but it is a factor that needs to be taken into account when using tDCS as a therapeutic aid.
6.2.3.5 Medical Differences
As for medical differences, besides concomitant diabetes or overweight, medication as well can impact the effect of tDCS. Prehn and Flöel (2015) focused on the interference of dopaminergic and serotonergic agents and tDCS, which could change the outcome of the stimulation. More research is necessary to investigate the value of additional biomarkers, such as learning relevant candidate genes, inflammatory markers, neurotransmitter concentrations, markers of cortical excitability and neurodegeneration, as well as neuronal activation patterns in predicting the therapeutic efficacy of tDCS.
6.2.3.6 Physical Differences
Regarding physical differences, even something as seemingly minor as hair thickness may impact the outcome of tDCS, since poorer electrode contact can reduce the amount of current passing through the scalp and skull. Other more obvious differences, such as poorer motor coordination or postural control, might influence the impact of tDCS on functional outcome (Uehara, Coxon, & Byblow, 2015).
6.2.3.7 Psychological Differences
tDCS studies rarely examine psychological differences such as motivation, expectations of outcome, affect, and attitude which may influence tDCS responsiveness through placebo-like effects. Two findings in the literature have underlined the importance of examining psychological differences in tDCS studies: evidence for the influence of (1) expectations on cognitive interventions (e.g., Foroughi, Monfort, Paczynski, McKnight, & Greenwood, 2016) and performance (e.g., Schwarz, Pfister, & Büchel, 2016) and (2) factors such as emotional state (Sarkar, Dowker, & Cohen Kadosh, 2014) and motivation (Jones, Stephens, Alam, Bikson, & Berryhill, 2015) on responsiveness to tDCS.
6.2.3.8 Brain Lesions
Besides these seven interindividual differences in a healthy population, an extra category of differences is linked to the brain lesion underlying an acquired speech-language disorder. Focusing on a participant with a brain lesion, a variety of factors has the potential to influence the outcome of speech-language therapy. Relevant roles can be played by:
-
1.
Stroke severity (Pedersen, Vinter, & Olsen, 2004): for example, Maas et al. (2012) reported the negative influence of a larger lesion on post-stroke aphasia recovery.
-
2.
Lesion characteristics such as site, size (Maas et al., 2012), and type (El Hachioui et al., 2013): looking at the lesion site, lesions of the left hemisphere might provide cortical disinhibition in perilesional structures, thereby increasing activity in left areas involved in language, with this perilesional activation associated with good recovery. However, this lesion can also disrupt the balance of interhemispheric competition. Whether increased right hemisphere activation is beneficial or maladaptive is controversial (Hamilton, Chrysikou, & Coslett, 2011).
-
3.
Characteristics of the speech-language disorder: less severe overall aphasic deficits (Pedersen et al., 2004) and sparing of phonological skills (El Hachioui et al., 2013) are significant predictors of recovery.
Several stroke studies showed that participants with larger deficits and less surviving brain structures, assessed by lesion size (Bolognini et al., 2015), white matter tract integrity (Bradnam, Stinear, & Byblow, 2013), or level of impairment (Saucedo Marquez, Zhang, Swinnen, Meesen, & Wenderoth, 2013), appeared to experience less benefit from tDCS. Bradnam et al. (2013) reported that ctDCS on the contralesional hemisphere in severely impaired patients could even have a negative effect. The underlying explanation might be that the contralesional activity is having a compensatory effect rather than impairing recovery of the lesioned hemisphere (O’Shea et al., 2014). Other factors such as time post-onset (Saucedo Marquez et al., 2013) and increased baseline functional connectivity (Rosso et al., 2014) might also confer better responsiveness to tDCS. Knowledge of the mechanisms underlying spontaneous recovery and of those underlying the effect of tDCS is yet insufficient to constrain neurostimulation strategies in participants with post-stroke aphasia.
6.2.4 Outcome Measures
A final methodological variable is the used outcome measure. The reported measures vary across studies and are often not ideal for evaluating the therapeutic goals. Speech-language therapy is a type of neurorehabilitation that focuses not only on the rehabilitation of an impairment but also searches for compensating strategies, implicating a progress in functional communication. Therefore, in a picture naming task, researchers should not only evaluate the impairment (i.e., using a picture naming task after naming therapy), but they should also focus on transfer (i.e., does the patient’s naming also improve on non-trained words) and generalization (i.e., does the patient use the trained words in functional communication) of the naming abilities. Moreover, researchers should not only focus on naming accuracy but also on the reaction time, since higher reaction times are associated with more fluent language output, which maintains the flow of the conversation. This implicates that they should focus on the functional outcome and on the impact on the participant’s quality of life. An impairment-based outcome measure has an advantage for the researcher, since it assesses the interplay between the neurophysiological effects of tDCS and levels of cortical excitability. For the clinician and the patient, however, the impairment-based focus is less crucial; they are more focused on improving the functional communication and the patient’s quality of life.
Moreover, the outcome measures should not only be evaluated immediately after therapy, but follow-up measures should be included as well to determine whether treatment effects endure after treatment. Some studies (e.g., Shah-Basak et al., 2015) have shown only a trend towards improvement immediately after the combination of atDCS and naming therapy, but showed significant improvement at 2 months’ follow-up.
6.3 tDCS in Patients with Language/Speech Disorders
6.3.1 Aphasia
The clinical application of tDCS in participants with aphasia was first reported in August 2006 (Hummel & Cohen, 2006). Since then, 44 studies have been published, investigating the therapeutic potential of the technique. These studies have included 394 participants with aphasia due to a vascular lesion. About 76% of the participants (n = 301) had chronic aphasia, 17% (n = 68) (Hesse et al., 2007; Kang, Kim, Sohn, Cohen, & Paik, 2011; Jung, Lim, Kang, Sohn, & Paik, 2011) had subacute aphasia (4–8 weeks post-stroke), and 6% (n = 25) (Rosso et al., 2014) were in the lesion phase (3–6 months post-stroke). To exclude spontaneous recovery, most studies zoom in on participants with chronic aphasia. However, in clinical practice it might be better to combine tDCS with aphasia therapy in the lesion phase so that the cumulative effect of spontaneous recovery and therapy-induced recovery can merge into the most optimal recovery for the individual participant with aphasia. Zooming in on the tDCS parameters, most of these 44 studies use: (1) multiple tDCS sessions (ranging from 1 to 30 sessions, mean: 7.1 sessions); (2) with a current strength of 1.5 mA (ranging from 1 to 2 mA); (3) the active electrode is most often placed on the left inferior frontal gyrus and the reference electrode is placed on the right supraorbital region; (4) a unipolar montage is used; (5) current is transferred by nonmetallic electrodes covered in saline-soaked sponges of 35 cm2; and (6) tDCS is combined with online therapy. Most studies focused solely on patients with non-fluent aphasia (n = 172; 44%), i.e., Broca’s aphasia, global aphasia, and transcortical motor aphasia (e.g., Marangolo et al., 2014; Marangolo, Fiori, Campana, et al., 2014; Saidmanesh et al., 2012). Fridriksson, Richardson, Baker, and Rorden (2011) reported a study focusing solely on patients with fluent aphasia (n = 8; 2%), i.e., Wernicke’s aphasia, amnestic aphasia, and transcortical sensory aphasia. Other studies combine patient groups: non-fluent aphasia (n = 161; 40.5%), fluent aphasia (n = 47; 12%) and mixed aphasia (n = 6; 1.5%) (Baker, Rorden, & Fridriksson, 2010; de Aguiar, Paolazzi, & Miceli, 2015; Jung et al., 2011; Kang et al., 2011; Lee, Cheon, Yoon, Chang, & Kim, 2013; Vestito et al., 2014; Volpato et al., 2013).
With these different methodological approaches in mind, determining a specific pathway for participants with aphasia is far from simple. Aphasia is among the most devastating consequences of stroke, as it affects vocational integration, social life, and psychological well-being on the individual level and places major burdens on the healthcare system. Intensive and deficit-oriented treatment can alleviate aphasia even in the chronic stage, but treatment effect sizes are often modest (e.g., Brady, Kelly, Godwin, & Enderby, 2012; Wilssens et al., 2015); hence there is a pressing need to explore new strategies to enhance treatment efficacy in chronic aphasia. The combination of tDCS and behavioral therapy might be important in evidence-based language therapy (Marangolo, 2017). Multisession tDCS has been shown to induce more permanent behavioral and neural modulation (Meinzer, Darkow, et al., 2016). Therefore, interest in tDCS as a therapeutic tool for aphasia is booming (e.g., Crinion et al., 2006; Holland & Crinion, 2012; Tippett, Niparko, & Hillis, 2015), based on its potential to guide neuroplasticity in recovery and thereby facilitate learning during behavioral therapy.
Recent neuroimaging and behavioral data have indicated that considerable changes in the cortical representation of language processing can occur in the days, weeks, and months following stroke in the left hemisphere, and the degree of language recovery after stroke depends significantly on the degree of neuroplasticity in the participant’s brain (Hamilton et al., 2011). Three kinds of changes in neural activity after stroke may be most relevant for aphasia recovery: (1) recruitment of perilesional left hemisphere regions for language-related tasks; (2) acquisition, unmasking, or refinement of language processing ability in the nondominant right hemisphere; and (3) dysfunctional activation of the nondominant hemisphere that may interfere with language recovery. Evidence indicates that unilateral injury, such as left hemispheric stroke, can lead to cortical disinhibition in at least two regions: (1) neighboring ipsilesional cortical areas and (2) contralesional homotopic areas connected via the corpus callosum (Bütefisch, Kleiser, & Seitz, 2006).
It is well established that activity in one cerebral hemisphere affects activity in the other one via a rich network of interhemispheric connections and that these interactions represent a dynamic process that can be flexibly modulated based on task demands or by exogenous stimulation (Chrysikou & Hamilton, 2011). The inhibitory interplay between homologous hemispheric regions likely contributes to normal performance on a variety of tasks and can be manipulated with tDCS. Unilateral stroke gives rise to maladaptive patterns of interhemispheric competition. In the healthy brain, there is a mutual inhibitory control between the two hemispheres, mediated by transcallosal connections (Bütefisch, Weβling, Netz, Seitz, & Hömberg, 2008). Thus, a unilateral left-side lesion reduces transcallosal inhibition of the right hemisphere by the left hemisphere and therefore increases activity in the intact right hemisphere. Since the right hemisphere can still send transcallosal inhibitory impulses to the left hemisphere, activation in the damaged left hemisphere is further reduced (de Aguiar et al., 2015; de Aguiar, Paolazzi, & Miceli, 2015).
Appendix A shows an overview of the heterogeneous literature on tDCS and aphasia. The first reports focused on the safety of the tDCS device in participants with aphasia (e.g., Hesse et al., 2007). Since 2011, the focus has moved from the most ideal stimulation site over to the most ideal task and the most ideal stimulation schedule. Unfortunately, researchers do not formulate a clear step-by-step protocol that fits all patients, due to all the interrelated methodological variables. In Sect. 6.4, we will present a road map for determining a tDCS protocol that can be used in therapy.
6.3.2 Motor Speech: Dysarthria/Apraxia of Speech (AoS)
As in the case of aphasia , it is complex to specify a pathway for participants with isolated motor speech disorders, i.e., dysarthria (Duffy, 2013) or apraxia of speech (AoS), or for individuals with motor speech disorders in the presence of aphasia (Wambaugh, Duffy, McNeil, Robin, & Rogers, 2006).
In daily clinical practice, many treatment approaches have been developed to remediate motor speech disorders. For dysarthria, the application of PML can be interesting as a therapeutic approach (Austermann Hula, Robin, Maas, Ballard, & Schmidt, 2008; Ballard, Maas, & Robin, 2007; Kaipa & Peterson, 2016; Van der Merwe, 2011; Wambaugh et al., 2017, 2018; Wambaugh, Nessler, Cameron, & Mauszycki, 2013; Wambaugh, Nessler, Wright, & Mauszycki, 2014; Whitfield & Goberman, 2017). Systematic reviews (Mitchell, Bowen, Tyson, Butterfint, & Conroy, 2017) have indicated that people with AoS benefit from articulatory treatment at the impairment level. However, no data are available about the impact of motor speech disorders and/or motor speech disorder therapy on functional communication (Wambaugh & Mauszycki, 2010) and well-being.
To the best of our knowledge, no research data exist about the impact of tDCS on speech motor performance for participants with dysarthria. For AoS, Marangolo and colleagues are the only researchers who have studied the impact of tDCS in this patient population (Marangolo et al., 2016, 2011; Marangolo, Fiori, Cipollari, et al., 2013). Marangolo et al. (2011) treated three right-handed native Italian speakers (2 M, 1 F, mean age 66 years) with stroke-induced (ischemic lesion, n = 2; hemorrhagic lesion, n = 1) chronic non-fluent aphasia and severe AoS. All three had a lesion in the left hemisphere involving damage to (1) structures functionally connected with Broca and (2) the insula. None of them showed damage to the inferior frontal gyrus. In a randomized double-blinded experiment, all three underwent online intensive articulatory training with online tDCS for 2 weeks. The tDCS protocol involved sessions (atDCS , 20 min, 1 mA) followed by a 6-day intersession interval and another five sessions (sham tDCS, 20 min, 1 mA) or vice versa. The behavioral task was an aurally presented repetition task of speech stimuli (n = 40 per condition) ranging from consonant-vowel (CV) syllables to CVCCV disyllabic words. The word list was adapted for each participant according to their own specific motor speech disorder. Training was delivered in five different steps: (1) the patient could watch the articulatory movements of the clinician; (2 and 3) the patient repeated this focusing on syllable-segmentation, vowel-sound prolonging and exaggerating the articulatory gestures; and (4 and 5) the patient fluently repeated this. The stimulating electrode (atDCS) was positioned over the left inferior frontal gyrus (inferior frontal gyrus, F7), and the cathodal electrode was placed over the contralateral supraorbital region. The accuracy of training was measured pre- and posttreatment. Results showed a significant acquisition effect indicated by a higher accuracy of syllable/word repetition in the post-training phase in both tDCS conditions. Moreover, the mean percentage of correct responses was greater after atDCS than after sham. Language measures showed generalization effects to oral (i.e., reading aloud) and written (i.e., writing to dictation) production tasks. None of the participants improved on oral naming. Marangolo et al. (2011) conducted three follow-ups (1 week, 1 and 2 months), which resulted in significantly better retention effects after atDCS than after sham. However, study results established significant improvements on acquisition, retention, and generalization of training in both atDCS and sham.
Marangolo and colleagues replicated these findings in a group of 17 right-handed subjects (9 M, 8 F, mean age 56.71 years) with chronic, ischemic stroke. All 17 were native Italian speakers with non-fluent aphasia and severe AoS (Marangolo et al., 2016; Marangolo, Fiori, Cipollari, et al., 2013). All 17 had a lesion in the left hemisphere involving damage to (1) structures functionally connected with Broca and (2) the insula. These studies as well resulted in improved accuracy of speech articulation and generalization effects. The studies of 2013 and 2016 differ at some methodological variables from the study of 2011:
-
1.
tDCS parameters, i.e., (a) the intensity of the current is now set at 2 mA (instead of 1 mA); (b) the treatment intensity is augmented from five to 15 sessions; and (c) bihemispheric stimulation (instead of unihemispheric stimulation) is used. This means that the anode was placed over the ipsilateral and the cathode over the contralateral IFG (F7 and F6).
-
2.
The outcome measures: two tasks, i.e., vocal reaction times and picture description, were added and only one follow-up (after 1 week) was performed. Vocal reaction times declined after atDCS and 11 out of 17 patients improved on the picture description task after atDCS.
In the 2016 study, Marangolo et al. (2016) included fMRI data and showed that atDCS boosted the recovery process by increasing functional connectivity in the left lesioned cerebral hemisphere. After sham stimulation, on the other hand, functional connectivity increased in the right intact cerebral hemisphere.
6.4 Road Map
In Fig. 6.1 we have constructed a road map, summing up all the variables and linking them in a patient-centered virtuous circle. We believe that speech-language therapy should be an iterative process where the clinician is in constant dialogue with the patient. (A–B) The clinician should formulate shared, monitored, accessible, relevant, transparent, evolving, and relationship-centered (SMARTER: Hersh, Worrall, Howe, Sherratt, & Davidson, 2012) therapeutic goals taking into account interindividual differences and the patient’s expressed needs (Table 6.3). (C) These goals should be linked to therapy, identifying the therapeutic material that is relevant for each particular patient in each particular stage of their rehabilitation. Meanwhile, the clinician should consider how they will present the material to the patient (nonverbal, oral, or written input), how they will support the patient (cueing and feedback), and how the patient should respond (nonverbal, oral, or written output). (D) The clinician should choose the area of stimulation, identifying the brain regions that are involved in the language processes in question (Table 6.2). Here, a specific brain area or a language network can be targeted. To optimize the effects of tDCS on impaired networks and to choose the relevant targeted area and polarity, clinicians need a better understanding of brain reorganization, the time course of the reorganization, and the involvement of perilesional and contralesional cortices, in addition to the precise molecular mechanisms associated with tDCS (Biou et al., 2019). Moreover, the right Broca homologue and supplementary motor area seem to be involved in the subacute phase of stroke, and language reorganization needs these divergent processes before a normalization and reshifting of cortex activity towards the left can occur at the chronic stage (Saur, 2006). Klaus and Schutter (2018) showed in their meta-analysis a small, but reliable effect of online tDCS on language production. On the other hand, Westwood and Romani (2017) found no effect of tDCS on performance in language production and reading tasks. (E) The clinician has to determine the type of stimulation, taking into account the location and the severity of the stroke. Different strategies can be used through inhibition of interfering areas or excitation of compensating/perilesional tissue. This will depend on several different factors (e.g., timing of stimulation and area of stimulation). (F) The clinician has to establish the stimulation parameters, determining the intensity, current, and area of stimulation; the placement, type, size, and shape of the electrodes; and linking these settings with the therapeutic goal (Table 6.1). (G) The most sensitive outcome measure should be chosen with a focus on the impairment, the transfer, or the generalization to functional communication. (H) Follow-up is needed to evaluate if the progression remains or possibly augments. Here, it is also important to take into account the patient’s subjective feeling of progress and well-being. In close consultation with the patient, new therapeutic goals can be set, thereby repeating the circle.
6.5 Discussion
These heterogeneous influencing parameters illustrate the difficulties associated with tDCS in anticipating the direction and the magnitude of its behavioral effects (Jacobson et al., 2012; Oldrati & Schutter, 2017). Recent publications have highlighted substantial variability among reported stimulation effects in healthy participants (e.g., Wiethoff, Hamada, & Rothwell, 2014), criticized methodological reasons (Antal, Keeser, Priori, Padberg, & Nitsche, 2015), or even questioned the potential of tDCS to induce behavioral effects on cognition and on motor function (Horvath, Forte, & Carter, 2015). They have motivated reflections on the use and the efficacy of tDCS and prompted urgent calls for more rigorous methodology (e.g., within-subject instead of between-subject designs), including replication studies (Fertonani & Miniussi, 2017) and extension of investigations to older participants, to other language disorders (e.g., semantics or syntax) and other motor speech disorders (e.g., ataxic dysarthria), and to specific behavioral tasks (e.g., investigations of additional PML in participants with motor speech disorders). However, the more parameters one can distinguish as impacting the functional outcome, the more complex it becomes to find homogeneous groups in order to unravel the cumulative effect of speech-language therapy and tDCS.
Moreover, the complexity of the brain network which controls speech and language remains largely unknown. Functional MRI (fMRI) data highlight (1) the existence of a shared core network of segregated local neural communities in the primary sensorimotor and parietal regions, in which the left primary motor cortex plays an important role in the speech network organization; (2) the flexibility of these strong interconnected local neural communities based on their participation in several functional domains across different networks; and (3) the capacity to adaptively switch long-range functional connectivity, depending on the nature of the task. This means that each behavioral task addresses a different functional network which is related to a different neural community structure (Fuertinger et al., 2015). For example, the motor speech production network and the real-life language network share high-strength neural communities but also recruit function-specific non-shared network nodes. Predicting the efficacy of tDCS over a specific region will therefore depend on our knowledge about the exact involvement of that region in the task that will be used in combination with tDCS. Especially in patients, the underlying neural mechanisms are usually not easy to determine or understand. Therefore, besides as an interventional tool, tDCS should also be used as a research tool to complete neuroimaging approaches, neurophysiological parameters, and behavioral measures and thereby unravel the mechanism of neuroplasticity (Hartwigsen, 2015). This more fundamental methodological approach could be developed in parallel with clinical practice, in which therapy goals should be carefully planned and training should be impairment, activity, or participation oriented.
6.6 Conclusion
Considering patients with aphasia, atDCS over the left inferior frontal gyrus (F5) associated with naming therapy can result in higher naming accuracy for right-handed participants with chronic non-fluent vascular aphasia. Patients with severe AoS and chronic non-fluent vascular aphasia might benefit more from atDCS associated with articulation therapy, i.e., oral repetition. However, the generalizability of the tDCS findings to other aphasic symptoms or to other speech motor impairments in other stages of rehabilitation might be limited. Concerning tDCS parameters, bihemispheric stimulation might be more efficient than unihemispheric stimulation (Marangolo et al., 2016; Marangolo, Fiori, Cipollari, et al., 2013). These findings are in line with the interhemispheric inhibition hypothesis and confirm the importance of activating perilesional brain tissue for enhanced speech-language outcome. The stimulation schedule should include repeated sessions of tDCS that might induce more permanent behavioral and neural long-term effects in the stimulated network (Meinzer et al., 2014; Meinzer, Darkow, et al., 2016; Reis et al., 2009). To determine the appropriate behavioral task, reactivation or (mal-)adaptation strategies should be monitored. More evidence from behavioral treatment studies in speech motor learning could help in exploring the impact of tDCS. However, the impact of tDCS with modality- and task-specific speech-language therapy remains limited and equivocal. Interindividual differences should be taken into account; in tDCS studies only demographic information about age, gender, and education has been well reported. Since it is known that the neuroplasticity of the elder brain differs from that of a younger brain, further research is warranted in all age categories of a healthy population (Summers et al., 2016).
However, it is impossible to set up a standard protocol since many parameters co-interfere with the behavioral task and interindividual variability and therefore hamper comparability between studies. While there is study-specific evidence for the efficacy of tDCS in language production research, the methodological variability between studies is large. Therefore, a patient-centered road map has been described in this chapter, which can be used as a guideline to determine the tDCS protocol with the most potential for each individual patient. This road map has been constructed as a virtuous circle since the needs of the patient and the neural complexity of the damaged network will change over time. This also allows for timely evaluation of the efficacy of the tDCS protocol and makes it possible to adjust if needed.
In general, further research should bridge the gap between tDCS and neuroimaging, neurophysiological, and behavioral findings in speech-language therapy, while using a more homogeneously constructed research methodology. The implementation of tDCS in the clinical speech-language therapy is promising, but remains experimental. Many research questions still need to be addressed: (1) More research is required to study the advantages of high definition tDCS, which can be used to stimulate more focally. (2) More research is needed for specific patient populations. There is evidence to promote online tDCS in participants with chronic non-fluent aphasia (combined with severe AoS) , but up until now, there is less to no evidence to promote online tDCS in other patient populations, such as individuals with dysarthria. And (3) research should focus more on functional communication, well-being, and follow-up results, instead of focusing only on the impairment.
Abbreviations
- AoS:
-
Apraxia of speech
- atDCS:
-
Anodal transcranial direct current stimulation
- BA:
-
Brodmann area
- ctDCS:
-
Cathodal transcranial direct current stimulation
- DLPFC:
-
Dorsolateral prefrontal cortex
- F:
-
Female
- h:
-
Hours
- HD tDCS:
-
High-density transcranial direct current stimulation
- IFG:
-
Inferior frontal gyrus
- ITG:
-
Inferior temporal gyrus
- LTD:
-
Long-term depression
- LTP:
-
Long-term potentiation
- M:
-
Male
- M1:
-
Primary motor cortex
- min:
-
Minutes
- MTG:
-
Middle temporal gyrus
- NMDA:
-
N-Methyl-d-aspartate
- PFC:
-
Prefrontal cortex
- PML:
-
Principles of motor learning
- pSTG:
-
Posterior superior temporal gyrus
- STG:
-
Superior temporal gyrus
- tDCS:
-
Transcranial direct current stimulation
- TMS:
-
Transcranial magnetic stimulation
References
Adams, S. G., & Page, A. D. (2000). Effects of selected practice and feedback variables on speech motor learning. Journal of Medical Speech-Language Pathology, 8(4), 215–220.
Antal, A., Keeser, D., Priori, A., Padberg, F., & Nitsche, M. A. (2015). Conceptual and procedural shortcomings of the systematic review evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: A systematic review by Horvath and co-workers. Brain Stimulation, 8(4), 846.
Arlotti, M., Rahman, A., Minhas, P., & Bikson, M. (2012). Axon terminal polarization induced by weak uniform DC electric fields: A modeling study (pp. 4575–4578). 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, pp. 4575–4578.
Austermann Hula, S. N., Robin, D. A., Maas, E., Ballard, K. J., & Schmidt, R. A. (2008). Effects of feedback frequency and timing on acquisition, retention, and transfer of speech skills in acquired apraxia of speech. Journal of Speech, Language, and Hearing Research, 51(5), 1088–1113.
Baddeley, A. (2010). Working memory. Current Biology, 20(4), R136–R140.
Baker, J. M., Rorden, C., & Fridriksson, J. (2010). Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke, 41(6), 1229–1236.
Ballard, K. J., Maas, E., & Robin, D. A. (2007). Treating control of voicing in apraxia of speech with variable practice. Aphasiology, 21(12), 1195–1217.
Bashir, N., & Howell, P. (2017). P198 tDCS stimulation of the left inferior frontal gyrus in a connected speech task with fluent speakers. Clinical Neurophysiology, 128(3), e111.
Bastani, A., & Jaberzadeh, S. (2012). Does anodal transcranial direct current stimulation enhance excitability of the motor cortex and motor function in healthy individuals and subjects with stroke: a systematic review and meta-analysis. Clinical Neurophysiology, 123(4), 644–657.
Behrens, T. E., & Sporns, O. (2012). Human connectomics. Current Opinion in Neurobiology, 22(1), 144–153.
Belke, E., & Stielow, A. (2013). Cumulative and non-cumulative semantic interference in object naming: Evidence from blocked and continuous manipulations of semantic context. The Quarterly Journal of Experimental Psychology, 66(11), 2135–2160.
Bennett, I. J., & Madden, D. J. (2014). Disconnected aging: Cerebral white matter integrity and age-related differences in cognition. Neuroscience, 276, 187–205.
Berryhill, M. E., & Jones, K. T. (2012). tDCS selectively improves working memory in older adults with more education. Neuroscience Letters, 521(2), 148–151.
Bikson, M., Datta, A., Rahman, A., & Scaturro, J. (2010). Electrode montages for tDCS and weak transcranial electrical stimulation: Role of “return” electrode’s position and size. Clinical Neurophysiology, 121(12), 1976–1978.
Binney, R. J., Zuckerman, B. M., Waller, H. N., Hung, J., Ashaie, S. A., & Reilly, J. (2018). Cathodal tDCS of the bilateral anterior temporal lobes facilitates semantically-driven verbal fluency. Neuropsychologia, 111, 62–71.
Biou, E., Cassoudesalle, H., Cogné, M., Sibon, I., De Gabory, I., Dehail, P., … Glize, B. (2019). Transcranial direct current stimulation in post-stroke aphasia rehabilitation: A systematic review. Annals of Physical and Rehabilitation Medicine, 62(2), 104–121.
Bislick, L. P., Weir, P. C., Spencer, K., Kendall, D., & Yorkston, K. M. (2012). Do principles of motor learning enhance retention and transfer of speech skills? A systematic review. Aphasiology, 26(5), 709–728.
Bolognini, N., Convento, S., Banco, E., Mattioli, F., Tesio, L., & Vallar, G. (2015). Improving ideomotor limb apraxia by electrical stimulation of the left posterior parietal cortex. Brain, 138(2), 428–439.
Bolognini, N., Pascual-Leone, A., & Fregni, F. (2009). Using non-invasive brain stimulation to augment motor training-induced plasticity. Journal of NeuroEngineering and Rehabilitation, 6(1), 8. https://doi.org/10.1186/1743-0003-6-8
Bradnam, L. V., Stinear, C. M., & Byblow, W. D. (2013). Ipsilateral motor pathways after stroke: implications for non-invasive brain stimulation. Frontiers in Human Neuroscience, 7. https://doi.org/10.3389/fnhum.2013.00184
Brady, M. C., Kelly, H., Godwin, J., & Enderby, P. (2012). Speech and language therapy for aphasia following stroke. The cochrane collaboration: John Wiley & Sons, Ltd..
Brem, A.-K., Unterburger, E., Speight, I., & Jäncke, L. (2014). Treatment of visuospatial neglect with biparietal tDCS and cognitive training: A single-case study. Frontiers in Systems Neuroscience, 8, 180.
Bressler, S. L., & Menon, V. (2010). Large-scale brain networks in cognition: Emerging methods and principles. Trends in Cognitive Sciences, 14(6), 277–290.
Brückner, S., & Kammer, T. (2017). Both anodal and cathodal transcranial direct current stimulation improves semantic processing. Neuroscience, 343, 269–275. https://doi.org/10.1016/j.neuroscience.2016.12.015
Buchwald, A., Calhoun, H., Rimikis, S., Lowe, M. S., Wellner, R., & Edwards, D. J. (2019). Using tDCS to facilitate motor learning in speech production: The role of timing. Cortex, 111, 274–285. https://doi.org/10.1016/j.cortex.2018.11.014
Bütefisch, C. M., Kleiser, R., & Seitz, R. J. (2006). Post-lesional cerebral reorganisation: Evidence from functional neuroimaging and transcranial magnetic stimulation. Journal of Physiology-Paris, 99(4–6), 437–454.
Bütefisch, C. M., Weβling, M., Netz, J., Seitz, R. J., & Hömberg, V. (2008). Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabilitation and Neural Repair, 22(1), 4–21. https://doi.org/10.1177/1545968307301769
Campana, S., Caltagirone, C., & Marangolo, P. (2015). Combining voxel-based lesion-symptom mapping (VLSM) with A-tDCS language treatment: Predicting outcome of recovery in nonfluent chronic aphasia. Brain Stimulation, 8(4), 769–776.
Cappon, D., Jahanshahi, M., & Bisiacchi, P. (2016). Value and efficacy of transcranial direct current stimulation in the cognitive rehabilitation: A critical review since 2000. Frontiers in Neuroscience, 10. https://doi.org/10.3389/fnins.2016.00157
Cattaneo, Z., Pisoni, A., & Papagno, C. (2011). Transcranial direct current stimulation over Broca’s region improves phonemic and semantic fluency in healthy individuals. Neuroscience, 183, 64–70. https://doi.org/10.1016/j.neuroscience.2011.03.058
Cerruti, C., & Schlaug, G. (2009). Anodal transcranial direct current stimulation of the prefrontal cortex enhances complex verbal associative thought. Journal of Cognitive Neuroscience, 21(10), 1980–1987.
Chesters, J., Hsu, J. H., Bishop, D. V. M., Watkins, K. E., & Mottonen, R. (2017). Comparing effectiveness of three TDCS protocols on online and offline components of speech motor learning. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation, 10(4), e27–e28.
Chrysikou, E. G., & Hamilton, R. H. (2011). Noninvasive brain stimulation in the treatment of aphasia: Exploring interhemispheric relationships and their implications for neurorehabilitation. Restorative Neurology and Neuroscience, 6, 375–394. https://doi.org/10.3233/RNN-2011-0610
Church, J. A., Coalson, R. S., Lugar, H. M., Petersen, S. E., & Schlaggar, B. L. (2008). A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cerebral Cortex, 18(9), 2054–2065. https://doi.org/10.1093/cercor/bhm228
Costafreda, S. G., Fu, C. H. Y., Lee, L., Everitt, B., Brammer, M. J., & David, A. S. (2006). A systematic review and quantitative appraisal of fMRI studies of verbal fluency: Role of the left inferior frontal gyrus. Human Brain Mapping, 27(10), 799–810. https://doi.org/10.1002/hbm.20221
Crinion, J., Turner, R., Grogan, A., Hanakawa, T., Noppeney, U., Devlin, J. T., … Price, C. J. (2006). Language control in the bilingual brain. Science, 312(5779), 1537–1540. https://doi.org/10.1126/science.1127761
Crosson, B. (2013). Thalamic mechanisms in language: A reconsideration based on recent findings and concepts. Brain and Language, 126(1), 73–88. https://doi.org/10.1016/j.bandl.2012.06.011
Damian, M. F., Vigliocco, G., & Levelt, W. J. M. (2001). Effects of semantic context in the naming of pictures and words. Cognition, 81(3), B77–B86. https://doi.org/10.1016/S0010-0277(01)00135-4
Datta, A., Baker, J. M., Bikson, M., & Fridriksson, J. (2011). Individualized model predicts brain current flow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain Stimulation, 4(3), 169–174. https://doi.org/10.1016/j.brs.2010.11.001
Datta, A., Truong, D., Minhas, P., Parra, L. C., & Bikson, M. (2012). Inter-individual variation during transcranial direct current stimulation and normalization of dose using MRI-derived computational models. Frontiers in Psychiatry, 3. https://doi.org/10.3389/fpsyt.2012.00091
Davis, S. W., Dennis, N. A., Daselaar, S. M., Fleck, M. S., & Cabeza, R. (2008). Que PASA? The posterior-anterior shift in aging. Cerebral Cortex, 18(5), 1201–1209. https://doi.org/10.1093/cercor/bhm155
de Aguiar, V., Bastiaanse, R., Capasso, R., Gandolfi, M., Smania, N., Rossi, G., & Miceli, G. (2015). Can tDCS enhance item-specific effects and generalization after linguistically motivated aphasia therapy for verbs? Frontiers in Behavioral Neuroscience, 9. https://doi.org/10.3389/fnbeh.2015.00190
de Aguiar, V., Paolazzi, C. L., & Miceli, G. (2015). tDCS in post-stroke aphasia: The role of stimulation parameters, behavioral treatment and patient characteristics. Cortex, 63, 296–316. https://doi.org/10.1016/j.cortex.2014.08.015
De Vries, M. H., Barth, A. C., Maiworm, S., Knecht, S., Zwitserlood, P., & Flöel, A. (2010). Electrical stimulation of Broca’s area enhances implicit learning of an artificial grammar. Journal of Cognitive Neuroscience, 22(11), 2427–2436.
Dick, A. S., Bernal, B., & Tremblay, P. (2014). The language connectome: New pathways, new concepts. The Neuroscientist, 20(5), 453–467. https://doi.org/10.1177/1073858413513502
Dick, A. S., Goldin-Meadow, S., Hasson, U., Skipper, J. I., & Small, S. L. (2009). Co-speech gestures influence neural activity in brain regions associated with processing semantic information. Human Brain Mapping, 30(11), 3509–3526. https://doi.org/10.1002/hbm.20774
Duffy, J. R. (2013). Motor speech disorders-E-book: Substrates, differential diagnosis, and management. Elsevier Health Sciences.
Edwards, D., Cortes, M., Datta, A., Minhas, P., Wassermann, E. M., & Bikson, M. (2013). Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: A basis for high-definition tDCS. NeuroImage, 74, 266–275. https://doi.org/10.1016/j.neuroimage.2013.01.042
Ehlis, A.-C., Haeussinger, F. B., Gastel, A., Fallgatter, A. J., & Plewnia, C. (2016). Task-dependent and polarity-specific effects of prefrontal transcranial direct current stimulation on cortical activation during word fluency. NeuroImage, 140, 134–140. https://doi.org/10.1016/j.neuroimage.2015.12.047
Eickhoff, S. B., Heim, S., Zilles, K., & Amunts, K. (2009). A systems perspective on the effective connectivity of overt speech production. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 367(1896), 2399–2421. https://doi.org/10.1098/rsta.2008.0287
El Hachioui, H., Lingsma, H. F., van de Sandt-Koenderman, M. W. M. E., Dippel, D. W. J., Koudstaal, P. J., & Visch-Brink, E. G. (2013). Long-term prognosis of aphasia after stroke. Journal of Neurology, Neurosurgery & Psychiatry, 84(3), 310–315. https://doi.org/10.1136/jnnp-2012-302596
Fertonani, A., Brambilla, M., Cotelli, M., & Miniussi, C. (2014). The timing of cognitive plasticity in physiological aging: A tDCS study of naming. Frontiers in Aging Neuroscience, 6. https://doi.org/10.3389/fnagi.2014.00131
Fertonani, A., Ferrari, C., & Miniussi, C. (2015). What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clinical Neurophysiology, 126(11), 2181–2188. https://doi.org/10.1016/j.clinph.2015.03.015
Fertonani, A., & Miniussi, C. (2017). Transcranial electrical stimulation. The Neuroscientist, 23(2), 109–123.
Fertonani, A., Rosini, S., Cotelli, M., Rossini, P. M., & Miniussi, C. (2010). Naming facilitation induced by transcranial direct current stimulation. Behavioural Brain Research, 208(2), 311–318. https://doi.org/10.1016/j.bbr.2009.10.030
Fiori, V., Cipollari, S., Caltagirone, C., & Marangolo, P. (2014). “If two witches would watch two watches, which witch would watch which watch?” tDCS over the left frontal region modulates tongue twister repetition in healthy subjects. Neuroscience, 256, 195–200.
Fiori, V., Cipollari, S., Di Paola, M., Razzano, C., Caltagirone, C., & Marangolo, P. (2013). tDCS stimulation segregates words in the brain: Evidence from aphasia. Frontiers in Human Neuroscience, 7. https://doi.org/10.3389/fnhum.2013.00269
Fiori, V., Coccia, M., Marinelli, C. V., Vecchi, V., Bonifazi, S., Ceravolo, M. G., … Marangolo, P. (2011). Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasic subjects. Journal of Cognitive Neuroscience, 23(9), 2309–2323. https://doi.org/10.1162/jocn.2010.21579
Foroughi, C. K., Monfort, S. S., Paczynski, M., McKnight, P. E., & Greenwood, P. M. (2016). Placebo effects in cognitive training. Proceedings of the National Academy of Sciences, 113(27), 7470–7474. https://doi.org/10.1073/pnas.1601243113
Fridriksson, J., Richardson, J. D., Baker, J. M., & Rorden, C. (2011). Transcranial direct current stimulation improves naming reaction time in fluent aphasia: A double-blind, sham-controlled study. Stroke, 42(3), 819–821. https://doi.org/10.1161/STROKEAHA.110.600288
Fritsch, B., Reis, J., Martinowich, K., Schambra, H. M., Ji, Y., Cohen, L. G., & Lu, B. (2010). Direct current stimulation promotes BDNF-dependent synaptic plasticity: Potential implications for motor learning. Neuron, 66(2), 198–204. https://doi.org/10.1016/j.neuron.2010.03.035
Fuertinger, S., Horwitz, B., & Simonyan, K. (2015). The functional connectome of speech control. PLoS Biology, 13(7), e1002209.
Gandiga, P. C., Hummel, F. C., & Cohen, L. G. (2006). Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clinical Neurophysiology, 117(4), 845–850. https://doi.org/10.1016/j.clinph.2005.12.003
Gauvin, H. S., Meinzer, M., & de Zubicaray, G. I. (2017). tDCS effects on word production: Limited by design? Comment on Westwood et al. (2017). Cortex, 96, 137–142. https://doi.org/10.1016/j.cortex.2017.06.017
Glass, T. A., Matchar, D. B., Belyea, M., & Feussner, J. R. (1993). Impact of social support on outcome in first stroke. Stroke, 24(1), 64–70. https://doi.org/10.1161/01.STR.24.1.64
Grimaldi, G., Argyropoulos, G. P., Bastian, A., Cortes, M., Davis, N. J., Edwards, D. J., … Celnik, P. (2016). Cerebellar transcranial direct current stimulation (ctDCS) a novel approach to understanding cerebellar function in health and disease. The Neuroscientist, 22(1), 83–97.
Gutchess, A. (2014). Plasticity of the aging brain: New directions in cognitive neuroscience. Science, 346(6209), 579–582. https://doi.org/10.1126/science.1254604
Hamilton, R. H., Chrysikou, E. G., & Coslett, B. (2011). Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain and Language, 118(1–2), 40–50. https://doi.org/10.1016/j.bandl.2011.02.005
Hartwigsen, G. (2015). The neurophysiology of language: Insights from non-invasive brain stimulation in the healthy human brain. Brain and Language, 148, 81–94. https://doi.org/10.1016/j.bandl.2014.10.007
Hebb, D. O. (1949). The organization of behavior. New York, NY: John Wiley & Sons, Ltd..
Heim, S., Eickhoff, S. B., & Amunts, K. (2008). Specialisation in Broca’s region for semantic, phonological, and syntactic fluency? NeuroImage, 40(3), 1362–1368.
Hersh, D., Worrall, L., Howe, T., Sherratt, S., & Davidson, B. (2012). SMARTER goal setting in aphasia rehabilitation. Aphasiology, 26(2), 220–233. https://doi.org/10.1080/02687038.2011.640392
Hesse, S., Werner, C., Schonhardt, E. M., Bardeleben, A., Jenrich, W., & Kirker, S. G. B. (2007). Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: A pilot study. Restorative Neurology and Neuroscience, 25, 9–15.
Hickok, G., & Poeppel, D. (2000). Towards a functional neuroanatomy of speech perception. Trends in Cognitive Sciences, 4(4), 131–138. https://doi.org/10.1016/S1364-6613(00)01463-7
Hirshorn, E. A., & Thompson-Schill, S. L. (2006). Role of the left inferior frontal gyrus in covert word retrieval: Neural correlates of switching during verbal fluency. Neuropsychologia, 44(12), 2547–2557. https://doi.org/10.1016/j.neuropsychologia.2006.03.035
Holland, R., & Crinion, J. (2012). Can tDCS enhance treatment of aphasia after stroke? Aphasiology, 26(9), 1169–1191. https://doi.org/10.1080/02687038.2011.616925
Holland, R., Leff, A. P., Josephs, O., Galea, J. M., Desikan, M., Price, C. J., … Crinion, J. (2011). Speech facilitation by left inferior frontal cortex stimulation. Current Biology, 21(16), 1403–1407. https://doi.org/10.1016/j.cub.2011.07.021
Holland, R., Leff, A. P., Penny, W. D., Rothwell, J. C., & Crinion, J. (2016). Modulation of frontal effective connectivity during speech. NeuroImage, 140, 126–133. https://doi.org/10.1016/j.neuroimage.2016.01.037
Horvath, J. C., Forte, J. D., & Carter, O. (2015). Quantitative review finds no evidence of cognitive effects in healthy populations from single-session transcranial direct current stimulation (tDCS). Brain Stimulation, 8(3), 535–550. https://doi.org/10.1016/j.brs.2015.01.400
Horwitz, F. M., Heng, C. T., & Quazi, H. A. (2003). Finders, keepers? Attracting, motivating and retaining knowledge workers. Human Resource Management Journal, 13(4), 23–44. https://doi.org/10.1111/j.1748-8583.2003.tb00103.x
Howard, D., Nickels, L., Coltheart, M., & Cole-Virtue, J. (2006). Cumulative semantic inhibition in picture naming: Experimental and computational studies. Cognition, 100(3), 464–482.
Hummel, F. C., & Cohen, L. G. (2006). Non-invasive brain stimulation: A new strategy to improve neurorehabilitation after stroke? The Lancet Neurology, 5(8), 708–712. https://doi.org/10.1016/S1474-4422(06)70525-7
Indefrey, P. (2011). The spatial and temporal signatures of word production components: A critical update. Frontiers in Psychology, 2. https://doi.org/10.3389/fpsyg.2011.00255
Indefrey, P., & Levelt, W. J. M. (2004). The spatial and temporal signatures of word production components. Cognition, 92(1–2), 101–144. https://doi.org/10.1016/j.cognition.2002.06.001
Ito, T., Coppola, J. H., & Ostry, D. J. (2016). Speech motor learning changes the neural response to both auditory and somatosensory signals. Scientific Reports, 6(1). https://doi.org/10.1038/srep25926
Iyer, M. B., Mattu, U., Grafman, J., Lomarev, M., Sato, S., & Wassermann, E. M. (2005). Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology, 64, 872–875.
Jacobson, L., Koslowsky, M., & Lavidor, M. (2012). tDCS polarity effects in motor and cognitive domains: A meta-analytical review. Experimental Brain Research, 216(1), 1–10. https://doi.org/10.1007/s00221-011-2891-9
Jagust, W. (2013). Vulnerable neural systems and the borderland of brain aging and neurodegeneration. Neuron, 77(2), 219–234. https://doi.org/10.1016/j.neuron.2013.01.002
Jones, K., & Croot, K. (2016). The effect of blocked, random and mixed practice schedules on speech motor learning of tongue twisters in unimpaired speakers. Motor Control, 20(4), 350–379.
Jones, K. T., Stephens, J. A., Alam, M., Bikson, M., & Berryhill, M. E. (2015). Longitudinal neurostimulation in older adults improves working memory. PLOS ONE, 10(4), e0121904. https://doi.org/10.1371/journal.pone.0121904
Jung, I.-Y., Lim, J. Y., Kang, E. K., Sohn, H. M., & Paik, N.-J. (2011). The factors associated with good responses to speech therapy combined with transcranial direct current stimulation in post-stroke aphasic patients. Annals of Rehabilitation Medicine, 35(4), 460. https://doi.org/10.5535/arm.2011.35.4.460
Kaipa, R., & Peterson, A. M. (2016). A systematic review of treatment intensity in speech disorders. International Journal of Speech-Language Pathology, 18(6), 507–520.
Kang, E. K., Kim, Y. K., Sohn, H. M., Cohen, L., & Paik, N. (2011). Improved picture naming in aphasia patients treated with cathodal tDCS to inhibit the right Broca’s homologue area. Restorative Neurology and Neuroscience, 3, 141–152. https://doi.org/10.3233/RNN-2011-0587
Kang, N., Summers, J. J., & Cauraugh, J. H. (2016). Transcranial direct current stimulation facilitates motor learning post-stroke: A systematic review and meta-analysis. Journal of Neurology, Neurosurgery & Psychiatry, 87(4), 345–355. https://doi.org/10.1136/jnnp-2015-311242
Katz, B., Au, J., Buschkuehl, M., Abagis, T., Zabel, C., Jaeggi, S. M., & Jonides, J. (2017). Individual differences and long-term consequences of tDCS-augmented cognitive training. Journal of Cognitive Neuroscience, 29(9), 1498–1508. https://doi.org/10.1162/jocn_a_01115
Kim, W., Jung, S., Oh, M., Min, Y., Lim, J., & Paik, N. (2014). Effect of repetitive transcranial magnetic stimulation over the cerebellum on patients with ataxia after posterior circulation stroke: A pilot study. Journal of Rehabilitation Medicine, 46(5), 418–423. https://doi.org/10.2340/16501977-1802
Klaus, J., & Schutter, D. J. L. G. (2018). Non-invasive brain stimulation to investigate language production in healthy speakers: A meta-analysis. Brain and Cognition, 123, 10–22. https://doi.org/10.1016/j.bandc.2018.02.007
Krause, B., & Cohen Kadosh, R. (2014). Not all brains are created equal: The relevance of individual differences in responsiveness to transcranial electrical stimulation. Frontiers in Systems Neuroscience, 8(25). https://doi.org/10.3389/fnsys.2014.00025
Lee, S. Y., Cheon, H.-J., Yoon, K. J., Chang, W. H., & Kim, Y.-H. (2013). Effects of dual transcranial direct current stimulation for aphasia in chronic stroke patients. Annals of Rehabilitation Medicine, 37(5), 603. https://doi.org/10.5535/arm.2013.37.5.603
Lefaucheur, J.-P. (2016). A comprehensive database of published tDCS clinical trials (2005–2016). Neurophysiologie Clinique/Clinical Neurophysiology, 46(6), 319–398. https://doi.org/10.1016/j.neucli.2016.10.002
Li, L., Abutalebi, J., Zou, L., Yan, X., Liu, L., Feng, X., … Ding, G. (2015). Bilingualism alters brain functional connectivity between “control” regions and “language” regions: Evidence from bimodal bilinguals. Neuropsychologia, 71, 236–247. https://doi.org/10.1016/j.neuropsychologia.2015.04.007
Lisman, A. L., & Sadagopan, N. (2013). Focus of attention and speech motor performance. Journal of Communication Disorders, 46(3), 281–293. https://doi.org/10.1016/j.jcomdis.2013.02.002
Maas, E. (2015). Optimalisering van spraaktherapie: De toepassing van trainingsprincipes voor het leren van motorische vaardigheden. Stem-, Spraak-, en Taalpathologie, 20, 44–70.
Maas, M. B., Lev, M. H., Ay, H., Singhal, A. B., Greer, D. M., Smith, W. S., … Furie, K. L. (2012). The prognosis for aphasia in stroke. Journal of Stroke and Cerebrovascular Diseases, 21(5), 350–357. https://doi.org/10.1016/j.jstrokecerebrovasdis.2010.09.009
Madhavan, K. M., McQueeny, T., Howe, S. R., Shear, P., & Szaflarski, J. (2014). Superior longitudinal fasciculus and language functioning in healthy aging. Brain Research, 1562, 11–22. https://doi.org/10.1016/j.brainres.2014.03.012
Manenti, R., Petesi, M., Brambilla, M., Rosini, S., Miozzo, A., Padovani, A., … Cotelli, M. (2015). Efficacy of semantic–phonological treatment combined with tDCS for verb retrieval in a patient with aphasia. Neurocase, 21(1), 109–119. https://doi.org/10.1080/13554794.2013.873062
Manjaly, Z. M., Marshall, J. C., Stephan, K. E., Gurd, J. M., Zilles, K., & Fink, G. R. (2005). Context-dependent interactions of left posterior inferior frontal gyrus in a local visual search task unrelated to language. Cognitive Neuropsychology, 22(3–4), 292–305. https://doi.org/10.1080/02643290442000149
Manuel, A. L., & Schnider, A. (2016). Effect of prefrontal and parietal tDCS on learning and recognition of verbal and non-verbal material. Clinical Neurophysiology, 127(7), 2592–2598. https://doi.org/10.1016/j.clinph.2016.04.015
Marangolo, P. (2017). The potential effects of transcranial direct current stimulation (tDCS) on language functioning: Combining neuromodulation and behavioral intervention in aphasia. Neuroscience Letters. https://doi.org/10.1016/j.neulet.2017.12.057
Marangolo, P., Fiori, V., Calpagnano, M. A., Campana, S., Razzano, C., Caltagirone, C., & Marini, A. (2013). tDCS over the left inferior frontal cortex improves speech production in aphasia. Frontiers in Human Neuroscience, 7. https://doi.org/10.3389/fnhum.2013.00539
Marangolo, P., Fiori, V., Campana, S., Antonietta Calpagnano, M., Razzano, C., Caltagirone, C., & Marini, A. (2014). Something to talk about: Enhancement of linguistic cohesion through tdCS in chronic non fluent aphasia. Neuropsychologia, 53, 246–256. https://doi.org/10.1016/j.neuropsychologia.2013.12.003
Marangolo, P., Fiori, V., Cipollari, S., Campana, S., Razzano, C., Di Paola, M., … Caltagirone, C. (2013). Bihemispheric stimulation over left and right inferior frontal region enhances recovery from apraxia of speech in chronic aphasia. European Journal of Neuroscience, 38(9), 3370–3377. https://doi.org/10.1111/ejn.12332
Marangolo, P., Fiori, V., Di Paola, M., Cipollari, S., Razzano, C., Oliveri, M., & Caltagirone, C. (2013). Differential involvement of the left frontal and temporal regions in verb naming: A tDCS treatment study. Restorative Neurology and Neuroscience, 1, 63–72. https://doi.org/10.3233/RNN-120268
Marangolo, P., Fiori, V., Gelfo, F., Shofany, J., Razzano, C., Caltagirone, C., & Angelucci, F. (2014). Bihemispheric tDCS enhances language recovery but does not alter BDNF levels in chronic aphasic patients. Restorative Neurology and Neuroscience, 2, 367–379. https://doi.org/10.3233/RNN-130323
Marangolo, P., Fiori, V., Sabatini, U., De Pasquale, G., Razzano, C., Caltagirone, C., & Gili, T. (2016). Bilateral transcranial direct current stimulation language treatment enhances functional connectivity in the left hemisphere: Preliminary data from aphasia. Journal of Cognitive Neuroscience, 28(5), 724–738. https://doi.org/10.1162/jocn_a_00927
Marangolo, P., Marinelli, C. V., Bonifazi, S., Fiori, V., Ceravolo, M. G., Provinciali, L., & Tomaiuolo, F. (2011). Electrical stimulation over the left inferior frontal gyrus (IFG) determines long-term effects in the recovery of speech apraxia in three chronic aphasics. Behavioural Brain Research, 225(2), 498–504.
Mattson, M. P. (2015). Lifelong brain health is a lifelong challenge: From evolutionary principles to empirical evidence. Ageing Research Reviews, 20, 37–45. https://doi.org/10.1016/j.arr.2014.12.011
McDermott, K. B., Petersen, S. E., Watson, J. M., & Ojemann, J. G. (2003). A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia, 41(3), 293–303. https://doi.org/10.1016/S0028-3932(02)00162-8
Meinzer, M., Darkow, R., Lindenberg, R., & Flöel, A. (2016). Electrical stimulation of the motor cortex enhances treatment outcome in post-stroke aphasia. Brain, 139(4), 1152–1163. https://doi.org/10.1093/brain/aww002
Meinzer, M., Flaisch, T., Seeds, L., Harnish, S., Antonenko, D., Witte, V., … Crosson, B. (2012). Same modulation but different starting points: Performance modulates age differences in inferior frontal cortex activity during word-retrieval. PLOS ONE, 7(3), e33631. https://doi.org/10.1371/journal.pone.0033631
Meinzer, M., Flaisch, T., Wilser, L., Eulitz, C., Rockstroh, B., Conway, T., … Crosson, B. (2009). Neural signatures of semantic and phonemic fluency in young and old adults. Journal of Cognitive Neuroscience, 21(10), 2007–2018. https://doi.org/10.1162/jocn.2009.21219
Meinzer, M., Lindenberg, R., Antonenko, D., Flaisch, T., & Floel, A. (2013). Anodal transcranial direct current stimulation temporarily reverses age-associated cognitive decline and functional brain activity changes. Journal of Neuroscience, 33(30), 12470–12478. https://doi.org/10.1523/JNEUROSCI.5743-12.2013
Meinzer, M., Lindenberg, R., Sieg, M. M., Nachtigall, L., Ulm, L., & Floel, A. (2014). Transcranial direct current stimulation of the primary motor cortex improves word-retrieval in older adults. Frontiers in Aging Neuroscience, 6. https://doi.org/10.3389/fnagi.2014.00253
Miniussi, C., Harris, J. A., & Ruzzoli, M. (2013). Modelling non-invasive brain stimulation in cognitive neuroscience. Neuroscience & Biobehavioral Reviews, 37(8), 1702–1712. https://doi.org/10.1016/j.neubiorev.2013.06.014
Mitchell, C., Bowen, A., Tyson, S., Butterfint, Z., & Conroy, P. (2017). Interventions for dysarthria due to stroke and other adult-acquired, non-progressive brain injury. Cochrane Database of Systematic Reviews. https://doi.org/10.1002/14651858.CD002088.pub3
Moliadze, V., Antal, A., & Paulus, W. (2010). Electrode-distance dependent after-effects of transcranial direct and random noise stimulation with extracephalic reference electrodes. Clinical Neurophysiology, 121(12), 2165–2171. https://doi.org/10.1016/j.clinph.2010.04.033
Monte-Silva, K., Kuo, M.-F., Hessenthaler, S., Fresnoza, S., Liebetanz, D., Paulus, W., & Nitsche, M. A. (2013). Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimulation, 6(3), 424–432. https://doi.org/10.1016/j.brs.2012.04.011
Monti, A., Cogiamanian, F., Marceglia, S., Ferrucci, R., Mameli, F., Mrakic-Sposta, S., … Priori, A. (2008). Improved naming after transcranial direct current stimulation in aphasia. Journal of Neurology, Neurosurgery & Psychiatry, 79(4), 451–453. https://doi.org/10.1136/jnnp.2007.135277
Monti, A., Ferrucci, R., Fumagalli, M., Mameli, F., Cogiamanian, F., Ardolino, G., & Priori, A. (2013). Transcranial direct current stimulation (tDCS) and language. Journal of Neurology, Neurosurgery & Psychiatry, 84(8), 832–842. https://doi.org/10.1136/jnnp-2012-302825
Nitsche, M. A., & Paulus, W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology, 527(3), 633–639.
Nitsche, M. A., Schauenburg, A., Lang, N., Liebetanz, D., Exner, C., Paulus, W., & Tergau, F. (2003). Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. Journal of Cognitive Neuroscience, 15(4), 619–626.
O’Connell, N. E., Cossar, J., Marston, L., Wand, B. M., Bunce, D., Moseley, G. L., & De Souza, L. H. (2012). Rethinking clinical trials of transcranial direct current stimulation: Participant and assessor blinding is inadequate at intensities of 2mA. PLOS ONE, 7(10), e47514. https://doi.org/10.1371/journal.pone.0047514
Oh, A., Duerden, E. G., & Pang, E. W. (2014). The role of the insula in speech and language processing. Brain and Language, 135, 96–103. https://doi.org/10.1016/j.bandl.2014.06.003
Oldrati, V., & Schutter, D. J. L. G. (2017). Targeting the human cerebellum with transcranial direct current stimulation to modulate behavior: A meta-analysis. The Cerebellum. https://doi.org/10.1007/s12311-017-0877-2
Opitz, A., Paulus, W., Will, S., Antunes, A., & Thielscher, A. (2015). Determinants of the electric field during transcranial direct current stimulation. NeuroImage, 109, 140–150.
O’Shea, J., Boudrias, M.-H., Stagg, C. J., Bachtiar, V., Kischka, U., Blicher, J. U., & Johansen-Berg, H. (2014). Predicting behavioural response to TDCS in chronic motor stroke. NeuroImage, 85, 924–933. https://doi.org/10.1016/j.neuroimage.2013.05.096
Parazzini, M., Fiocchi, S., Liorni, I., & Ravazzani, P. (2015). Effect of the interindividual variability on computational modeling of transcranial direct current stimulation. Computational Intelligence and Neuroscience, 2015, 1–9. https://doi.org/10.1155/2015/963293
Park, D. C., & Reuter-Lorenz, P. (2009). The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology, 60(1), 173–196. https://doi.org/10.1146/annurev.psych.59.103006.093656
Peach, R. K., & Chapey, R. (2008). Global aphasia: Identification and management. In Language intervention strategies in aphasia and related neurogenic communication disorders (pp. 583–588).
Pedersen, P. M., Vinter, K., & Olsen, T. S. (2004). Aphasia after stroke: Type, severity and prognosis. Cerebrovascular Diseases, 17(1), 35–43. https://doi.org/10.1159/000073896
Penolazzi, B., Pastore, M., & Mondini, S. (2013). Electrode montage dependent effects of transcranial direct current stimulation on semantic fluency. Behavioural Brain Research, 248, 129–135. https://doi.org/10.1016/j.bbr.2013.04.007
Perceval, G., Flöel, A., & Meinzer, M. (2016). Can transcranial direct current stimulation counteract age-associated functional impairment? Neuroscience & Biobehavioral Reviews, 65, 157–172. https://doi.org/10.1016/j.neubiorev.2016.03.028
Peretz, Y., & Lavidor, M. (2013). Enhancing lexical ambiguity resolution by brain polarization of the right posterior superior temporal sulcus. Cortex, 49(4), 1056–1062. https://doi.org/10.1016/j.cortex.2012.03.015
Pihlajamäki, M., Tanila, H., Hänninen, T., Könönen, M., Laakso, M., Partanen, K., … Aronen, H. J. (2000). Verbal fluency activates the left medial temporal lobe: A functional magnetic resonance imaging study. Annals of Neurology, 47(4), 470–476. https://doi.org/10.1002/1531-8249(200004)47:4<470::AID-ANA10>3.0.CO;2-M
Pisoni, A., Papagno, C., & Cattaneo, Z. (2012). Neural correlates of the semantic interference effect: New evidence from transcranial direct current stimulation. Neuroscience, 223, 56–67. https://doi.org/10.1016/j.neuroscience.2012.07.046
Pisoni, A., Mattavelli, G., Papagno, C., Rosanova, M., Casali, A. G., & Romero Lauro, L. J. (2018). Cognitive enhancement induced by anodal tDCS drives circuit-specific cortical plasticity. Cerebral Cortex, 28(4), 1132–1140. https://doi.org/10.1093/cercor/bhx021
Pisoni, A., Turi, Z., Raithel, A., Ambrus, G. G., Alekseichuk, I., Schacht, A., … Antal, A. (2015). Separating recognition processes of declarative memory via anodal tDCS: Boosting old item recognition by temporal and new item detection by parietal stimulation. PLOS ONE, 10(3), e0123085. https://doi.org/10.1371/journal.pone.0123085
Polanía, R., Nitsche, M. A., Korman, C., Batsikadze, G., & Paulus, W. (2012). The importance of timing in segregated theta phase-coupling for cognitive performance. Current Biology, 22(14), 1314–1318. https://doi.org/10.1016/j.cub.2012.05.021
Poreisz, C., Boros, K., Antal, A., & Paulus, W. (2007). Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Research Bulletin, 72(4–6), 208–214. https://doi.org/10.1016/j.brainresbull.2007.01.004
Prehn, K., & Flöel, A. (2015). Potentials and limits to enhance cognitive functions in healthy and pathological aging by tDCS. Frontiers in Cellular Neuroscience, 9. https://doi.org/10.3389/fncel.2015.00355
Rabipour, S., Wu, A. D., Davidson, P. S. R., & Iacoboni, M. (2018). Expectations may influence the effects of transcranial direct current stimulation. Neuropsychologia, 119, 524–534. https://doi.org/10.1016/j.neuropsychologia.2018.09.005
Raffin, E., & Siebner, H. R. (2014). Transcranial brain stimulation to promote functional recovery after stroke. Current Opinion in Neurology, 27(1), 54–60. https://doi.org/10.1097/WCO.0000000000000059
Reis, J., Schambra, H. M., Cohen, L. G., Buch, E. R., Fritsch, B., Zarahn, E., … Krakauer, J. W. (2009). Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proceedings of the National Academy of Sciences, 106(5), 1590–1595. https://doi.org/10.1073/pnas.0805413106
Rollans, C., Cheema, K., Georgiou, G. K., & Cummine, J. (2017). Pathways of the inferior frontal occipital fasciculus in overt speech and reading. Neuroscience, 364, 93–106. https://doi.org/10.1016/j.neuroscience.2017.09.011
Rosso, C., Perlbarg, V., Valabregue, R., Arbizu, C., Ferrieux, S., Alshawan, B., … Samson, Y. (2014). Broca’s area damage is necessary but not sufficient to induce after-effects of cathodal tDCS on the unaffected hemisphere in post-stroke aphasia. Brain Stimulation, 7(5), 627–635. https://doi.org/10.1016/j.brs.2014.06.004
Russo, R., Wallace, D., Fitzgerald, P. B., & Cooper, N. R. (2013). Perception of comfort during active and sham transcranial direct current stimulation: A double blind study. Brain Stimulation, 6(6), 946–951. https://doi.org/10.1016/j.brs.2013.05.009
Saidmanesh, M., Pouretemad, H. R., Amini, A., Nillipour, R., & Ekhtian, H. (2012). Effects of transcranial direct current stimulation on working memory in patients with non-fluent aphasia disorder. Research Journal of Biological Sciences, 7(7), 290–296.
Sandars, M., Cloutman, L., & Woollams, A. M. (2016). Taking sides: An integrative review of the impact of laterality and polarity on efficacy of therapeutic transcranial direct current stimulation for anomia in chronic poststroke aphasia. Neural Plasticity, 2016, 8428256. https://doi.org/10.1155/2016/8428256
Sarkar, A., Dowker, A., & Cohen Kadosh, R. (2014). Cognitive enhancement or cognitive cost: Trait-specific outcomes of brain stimulation in the case of mathematics anxiety. Journal of Neuroscience, 34(50), 16605–16610. https://doi.org/10.1523/JNEUROSCI.3129-14.2014
Saturnino, G. B., Antunes, A., & Thielscher, A. (2015). On the importance of electrode parameters for shaping electric field patterns generated by tDCS. NeuroImage, 120, 25–35.
Saucedo Marquez, C. M., Zhang, X., Swinnen, S. P., Meesen, R., & Wenderoth, N. (2013). Task-specific effect of transcranial direct current stimulation on motor learning. Frontiers in Human Neuroscience, 7. https://doi.org/10.3389/fnhum.2013.00333
Saur, D. (2006). Dynamics of language reorganization after stroke. Brain, 129(6), 1371–1384. https://doi.org/10.1093/brain/awl090
Schmidt, R. A. (1988). Motor and action perspectives on motor behaviour. Advances in Psychology, 50, 3–44.
Schmidt, R. A., & Lee, T. D. (2005). Motor learning and control: A behavioral emphasis. Champaign, IL: Human Kinetics.
Schwarz, K. A., Pfister, R., & Büchel, C. (2016). Rethinking explicit expectations: Connecting placebos, social cognition, and contextual perception. Trends in Cognitive Sciences, 20(6), 469–480. https://doi.org/10.1016/j.tics.2016.04.001
Shah-Basak, P. P., Norise, C., Garcia, G., Torres, J., Faseyitan, O., & Hamilton, R. H. (2015). Individualized treatment with transcranial direct current stimulation in patients with chronic non-fluent aphasia due to stroke. Frontiers in Human Neuroscience, 9. https://doi.org/10.3389/fnhum.2015.00201
Shahid, S., Wen, P., & Ahfock, T. (2014). Assessment of electric field distribution in anisotropic cortical and subcortical regions under the influence of tDCS: Impact of Brain anisotropy on electric field. Bioelectromagnetics, 35(1), 41–57. https://doi.org/10.1002/bem.21814
Simione, M., Fregni, F., & Green, J. R. (2018). The effect of transcranial direct current stimulation on jaw motor function is task dependent: Speech, syllable repetition and chewing. Frontiers in Human Neuroscience, 12. https://doi.org/10.3389/fnhum.2018.00033
Smith, D. V., & Clithero, J. A. (2009). Manipulating executive function with transcranial direct current stimulation. Frontiers in Integrative Neuroscience, 3(26). https://doi.org/10.3389/neuro.07.026.2009
Sparing, R., Dafotakis, M., Meister, I. G., Thirugnanasambandam, N., & Fink, G. R. (2008). Enhancing language performance with non-invasive brain stimulation—A transcranial direct current stimulation study in healthy humans. Neuropsychologia, 46(1), 261–268. https://doi.org/10.1016/j.neuropsychologia.2007.07.009
Stagg, C. J., & Nitsche, M. A. (2011). Physiological basis of transcranial direct current stimulation. The Neuroscientist, 17(1), 37–53. https://doi.org/10.1177/1073858410386614
Steinhauer, K., & Grayhack, J. P. (2000). The role of knowledge of results in performance and learning of a voice motor task. Journal of Voice, 14(2), 137–145. https://doi.org/10.1016/S0892-1997(00)80020-X
Summers, J. J., Kang, N., & Cauraugh, J. H. (2016). Does transcranial direct current stimulation enhance cognitive and motor functions in the ageing brain? A systematic review and meta- analysis. Ageing Research Reviews, 25, 42–54. https://doi.org/10.1016/j.arr.2015.11.004
Tatti, E., Rossi, S., Innocenti, I., Rossi, A., & Santarnecchi, E. (2016). Non-invasive brain stimulation of the aging brain: State of the art and future perspectives. Ageing Research Reviews, 29, 66–89. https://doi.org/10.1016/j.arr.2016.05.006
Tippett, D. C., Niparko, J. K., & Hillis, A. E. (2015). Aphasia. Current Concepts in Theory and Practice, 2(1), 1042.
Trost, S., & Gruber, O. (2012). Evidence for a double dissociation of articulatory rehearsal and non-articulatory maintenance of phonological information in human verbal working memory. Neuropsychobiology, 65(3), 133–140. https://doi.org/10.1159/000332335
Troyer, A. K., Moscovitch, M., & Winocur, G. (1997). Clustering and switching as two components of verbal fluency: Evidence from younger and older healthy adults. Neuropsychology, 11(1), 138–146. https://doi.org/10.1037//0894-4105.11.1.138
Truong, D. Q., Magerowski, G., Blackburn, G. L., Bikson, M., & Alonso-Alonso, M. (2013). Computational modeling of transcranial direct current stimulation (tDCS) in obesity: Impact of head fat and dose guidelines. NeuroImage: Clinical, 2, 759–766. https://doi.org/10.1016/j.nicl.2013.05.011
Uehara, K., Coxon, J. P., & Byblow, W. D. (2015). Transcranial direct current stimulation improves ipsilateral selective muscle activation in a frequency dependent manner. PLOS ONE, 10(3), e0122434. https://doi.org/10.1371/journal.pone.0122434
Van der Merwe, A. (2011). A speech motor learning approach to treating apraxia of speech: Rationale and effects of intervention with an adult with acquired apraxia of speech. Aphasiology, 25(10), 1174–1206.
Vannorsdall, T. D., Schretlen, D. J., Andrejczuk, M., Ledoux, K., Bosley, L. V., Weaver, J. R., … Gordon, B. (2012). Altering automatic verbal processes with transcranial direct current stimulation. Frontiers in Psychiatry, 3. https://doi.org/10.3389/fpsyt.2012.00073
Vannorsdall, T. D., Van Steenburgh, J. J., Schretlen, D. J., Jayatillake, R., Skolasky, R. L., & Gordon, B. (2016). Reproducibility of tDCS results in a randomized trial: Failure to replicate findings of tDCS-induced enhancement of verbal fluency. Cognitive and Behavioral Neurology, 29(1), 11–17.
Vestito, L., Rosellini, S., Mantero, M., & Bandini, F. (2014). Long-term effects of transcranial direct-current stimulation in chronic post-stroke aphasia: A pilot study. Frontiers in Human Neuroscience, 8. https://doi.org/10.3389/fnhum.2014.00785
Volpato, C., Cavinato, M., Piccione, F., Garzon, M., Meneghello, F., & Birbaumer, N. (2013). Transcranial direct current stimulation (tDCS) of Broca’s area in chronic aphasia: A controlled outcome study. Behavioural Brain Research, 247, 211–216. https://doi.org/10.1016/j.bbr.2013.03.029
Vöröslakos, M., Takeuchi, Y., Brinyiczki, K., Zombori, T., Oliva, A., Fernández-Ruiz, A., … Berényi, A. (2018). Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nature Communications, 9(1). https://doi.org/10.1038/s41467-018-02928-3
Wagner, T., Fregni, F., Fecteau, S., Grodzinsky, A., Zahn, M., & Pascual-Leone, A. (2007). Transcranial direct current stimulation: A computer-based human model study. NeuroImage, 35(3), 1113–1124. https://doi.org/10.1016/j.neuroimage.2007.01.027
Wambaugh, J. L., Duffy, J. R., McNeil, M. R., Robin, D. A., & Rogers, M. A. (2006). Treatment guidelines for acquired apraxia of speech: A synthesis and evaluation of the evidence. Journal of Medical Speech-Language Pathology, 14(2), xv–xv.
Wambaugh, J. L., & Mauszycki, S. C. (2010). Sound production treatment: Application with severe apraxia of speech. Aphasiology, 24(6–8), 814–825.
Wambaugh, J. L., Nessler, C., Cameron, R., & Mauszycki, S. C. (2013). Treatment for acquired apraxia of speech: Examination of treatment intensity and practice schedule. American Journal of Speech-Language Pathology, 22(1), 84–102.
Wambaugh, J. L., Nessler, C., Wright, S., & Mauszycki, S. C. (2014). Sound production treatment: Effects of blocked and random practice. American Journal of Speech-Language Pathology, 23(2), S225–S245.
Wambaugh, J. L., Nessler, C., Wright, S., Mauszycki, S. C., DeLong, C., Berggren, K., & Bailey, D. J. (2017). Effects of blocked and random practice schedule on outcomes of sound production treatment for acquired apraxia of speech: Results of a group investigation. Journal of Speech, Language, and Hearing Research, 60(6S), 1739–1751.
Wambaugh, J. L., Wright, S., Boss, E., Mauszycki, S. C., DeLong, C., Hula, W., & Doyle, P. J. (2018). Effects of treatment intensity on outcomes in acquired apraxia of speech. American Journal of Speech-Language Pathology, 27(1S), 306–322.
Westwood, S. J., & Romani, C. (2017). Transcranial direct current stimulation (tDCS) modulation of picture naming and word reading: A meta-analysis of single session tDCS applied to healthy participants. Neuropsychologia, 104, 234–249. https://doi.org/10.1016/j.neuropsychologia.2017.07.031
Whitfield, J. A., & Goberman, A. M. (2017). Speech motor sequence learning: Acquisition and retention in parkinson disease and normal aging. Journal of Speech, Language, and Hearing Research, 60(6), 1477–1492. https://doi.org/10.1044/2016_JSLHR-S-16-0104
Wierenga, C. E., Benjamin, M., Gopinath, K., Perlstein, W. M., Leonard, C. M., Rothi, L. J. G., … Crosson, B. (2008). Age-related changes in word retrieval: Role of bilateral frontal and subcortical networks. Neurobiology of Aging, 29(3), 436–451. https://doi.org/10.1016/j.neurobiolaging.2006.10.024
Wiethoff, S., Hamada, M., & Rothwell, J. C. (2014). Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimulation, 7(3), 468–475. https://doi.org/10.1016/j.brs.2014.02.003
Willems, R. M., & Hagoort, P. (2007). Neural evidence for the interplay between language, gesture, and action: A review. Brain and Language, 101(3), 278–289. https://doi.org/10.1016/j.bandl.2007.03.004
Wilssens, I., Vandenborre, D., van Dun, K., Verhoeven, J., Visch-Brink, E., & Mariën, P. (2015). Constraint-Induced aphasia therapy versus intensive semantic treatment in fluent aphasia. American Journal of Speech-Language Pathology, 24(2), 281. https://doi.org/10.1044/2015_AJSLP-14-0018
Wirth, M., Rahman, R. A., Kuenecke, J., Koenig, T., Horn, H., Sommer, W., & Dierks, T. (2011). Effects of transcranial direct current stimulation (tDCS) on behaviour and electrophysiology of language production. Neuropsychologia, 49(14), 3989–3998. https://doi.org/10.1016/j.neuropsychologia.2011.10.015
Wong, A. W.-K., Whitehill, T. L., Ma, E. P.-M., & Masters, R. (2013). Effects of practice schedules on speech motor learning. International Journal of Speech-Language Pathology, 15(5), 511–523. https://doi.org/10.3109/17549507.2012.761282
Wong, M. N., Chan, Y., Ng, M. L., & Zhu, F. F. (2019). Effects of transcranial direct current stimulation over the Broca’s area on tongue twister production. International Journal of Speech-Language Pathology, 21(2), 182–188.
Yang, F. G., Fuller, J., Khodaparast, N., & Krawczyk, D. C. (2010). Figurative language processing after traumatic brain injury in adults: A preliminary study. Neuropsychologia, 48(7), 1923–1929.
Ziegler, W. (2003). Speech motor control is task-specific: Evidence from dysarthria and apraxia of speech. Aphasiology, 17(1), 3–36.
Further Reading
Biou, E., Cassoudesalle, H., Cogné, M., Sibon, I., De Gabory, I., Dehail, P., … Glize, B. (2019). Transcranial direct current stimulation in post-stroke aphasia rehabilitation: A systematic review. Annals of Physical and Rehabilitation Medicine, 62(2), 104–121.
de Aguiar, V., Paolazzi, C. L., & Miceli, G. (2015). tDCS in post-stroke aphasia: The role of stimulation parameters, behavioral treatment and patient characteristics. Cortex, 63, 296–316. https://doi.org/10.1016/j.cortex.2014.08.015
Monti, A., Ferrucci, R., Fumagalli, M., Mameli, F., Cogiamanian, F., Ardolino, G., & Priori, A. (2013). Transcranial direct current stimulation (tDCS) and language. Journal of Neurology, Neurosurgery & Psychiatry, 84(8), 832–842. https://doi.org/10.1136/jnnp-2012-302825
Sandars, M., Cloutman, L., & Woollams, A. M. (2016). Taking sides: An integrative review of the impact of laterality and polarity on efficacy of therapeutic transcranial direct current stimulation for anomia in chronic poststroke aphasia. Neural Plasticity, 2016(8428256). https://doi.org/10.1155/2016/8428256
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Vandenborre, D., Wilssens, I., van Dun, K., Manto, M. (2020). Transcranial Direct Current Stimulation (tDCS) and Language/Speech: Can Patients Benefit from a Combined Therapeutic Approach?. In: Argyropoulos, G.P.D. (eds) Translational Neuroscience of Speech and Language Disorders. Contemporary Clinical Neuroscience. Springer, Cham. https://doi.org/10.1007/978-3-030-35687-3_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-35687-3_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-35686-6
Online ISBN: 978-3-030-35687-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)