Abstract
In recent few decades, our understanding in the field of nanobiotechnology is increased dramatically. A number of nanoparticles (NPs) are synthesized by physical, chemical, as well as relatively safer green synthesis method. NPs seem to be the substitute for their respective bulk particles because of better action and sustained release. In agriculture production NPs could replace the use of chemical fertilizer in bulk, which otherwise deteriorates the soil health in the long term. However, because of unregulated use, it has posed negative impacts on ecosystems. NPs in the soil interact with the belowground microbes. The interaction reported till date is positive, negative, or neutral. For the application of engineered NPs in the agricultural field, it is of prime importance to understand the interaction among NPs, soil microbes, and plant roots. However, being a relatively new field, this understating is still at its beginning. This chapter reports about the interaction of NPs with symbiotic microbes, e.g., arbuscular mycorrhizal (AM) fungi, Rhizobia, etc. The mechanisms behind the reported interactions till date are generalized with the help of artwork. The chapter ends with the future perspectives of NPs along with the symbiotic soil microbes in increasing agronomy and soil heath.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Nanoparticles (NPs) are defined as the particles with a size of 1–100 nm in diameter (Auffan et al. 2009). Because of their high surface-to-volume ratio, they are very reactive. They can pass even through the cell membrane. These fascinating characteristics make them unique and hence, overwhelming researches have been carried out to explore their possible roles in biotechnology and agriculture production (Singh et al. 2016; Mishra et al. 2017; Shweta et al. 2017, 2018; Arif et al. 2018; Vishwakarma et al. 2018; Kehri et al. 2019; Rastogi et al. 2019a, b; Singh et al. 2019). NPs have promising application in the field of nanofertilizer, nanopesticide, nanoherbicide, nanosensor and as a delivery system for the sustained release of agrochemicals (Mishra et al. 2017; Koul et al. 2018; Singh et al. 2018; Tripathi et al. 2018; Vishwakarma et al. 2018). It was of thought that NPs could be used for boosting the agricultural economic growth in the near future (Sabourin and Ayande 2015). But, unregulated use of NPs has threw the ecosystems at the brink of risk caused by toxicity of these NPs (Hong et al. 2014). Although NPs have gained the rapid attention of plant scientists during the recent few years, their fates in relation to sustainable agriculture are still unexplored.
Despite of achievements in the field of NPs in agronomy, their role is still in a topsy-turvy stage (Yang et al. 2017). Different types of plants behave NPs differently. Also, same plant behaves different NPs differently. The response of NPs on plants as well as microbes depends upon the nature and concentration of NPs, nature of coating material, nature of growth media, mode of application, part of the plants, and even stage of plant development (Yang et al. 2017). As most of the NPs are made up of heavy metals, their elevated concentration in the biological systems could be detrimental to both aboveground and belowground flora as well.
Yang et al. (2017) have concluded the interaction of NPs with plants and microbes in their review. They have discussed pros and cons of NPs in details. But unfortunately, no conclusion could be established about the effect of NPs on symbioses in plants. In this chapter, extensive literature mining has been carried out to collect all the reports on interactions where effects of NPs have been studied on plants in relation to the symbiotic bacteria and/or fungi. Although there are very limited reports on tripartite interaction, it has been tried to find out the nature of interaction. Also, the possible mechanism behind these interactions is elucidated with the help of artwork. Eventually, this chapter puts forward the future perspectives and direction of research to study the behavior of NPs with reference to plant-microbe symbioses in greater depth.
4.2 Nanoparticles Versus Plant Growth
First stage of NP and plant interaction is the uptake of NPs inside the plant cell. Usually, plants can accumulate NPs of 40–50 nm dimension (Sabo-Attwood et al. 2012; Taylor et al. 2014). Besides the size, morphology, chemical nature, and coating properties play crucial roles in the absorption of NPs in plants (Raliya et al. 2016).
After absorption of NPs through roots, they follow either apoplastic pathway (extracellular spaces and xylem vessels) or symplastic pathway (across the living cell and through plasmodesmata). On foliar application, most of NPs are absorbed through the stomata (Pérez-de-Luque 2017). After absorption NPs are transported to the sink and internalized into the cell by pore formation, carrier proteins, and plasmodesmata or through the ion channels (Pérez-de-Luque 2017).
Fe3O4, TiO2, and carbon NPs were reported to inhibit the seed germination rate, root elongation, and germination index in cucumber plant (Mushtaq 2011). Cu NPs were found inhibitory on emerging root length, and Ag NPs decreased the plant biomass and transpiration (Stampoulis et al. 2009). ZnO NPs caused cytotoxic (lipid peroxidation) and genotoxic effects (decreasing mitotic index, increasing micronuclei and chromosomal aberration index) in Allium cepa (Kumari et al. 2011). CuO NPs have DNA-damaging effect in Raphanus sativus, Lolium perenne, and Lolium rigidum (Atha et al. 2012).
On the contrary to above findings, carbon nanotubes (CNTs) were reported to increase the seed germination and growth in tomato (Khodakovskaya et al. 2009). TiO2 NPs improved the energy utilization and conversion efficiency in D1/D2/Cyt b559 complex in spinach (Su et al. 2009). Stampoulis et al. (2009) reported no negative effects of multi-walled carbon nanotubes (MWCNTs) and Ag, Cu, ZnO, and Si NPs on seed germination in Cucurbita pepo. Lee et al. (2010) reported no negative or positive effect of Al2O3 NPs on Arabidopsis thaliana. TiO2 NPs were absorbed by the Triticum aestivum, Brassica napus, and Arabidopsis thaliana but did not affect their germination and root elongation (Larue et al. 2011).
Despite enormous researches carried out on NP-plant interaction, no clear conclusion has been made till date. NPs are reported to play positive, negative, as well as neutral roles on plant growth. Various fates of NPs in plants and their interaction have been reviewed in details by Pérez-de-Luque (2017). Accordingly, NPs alter the physiology, biochemistry, and genetics of plants. The interaction depends upon various factors, e.g., nature and concentration of NPs, nature of coating material, nature of growth media, mode of application, part of the plants, and even stage of plant development (Yang et al. 2017).
4.3 Nanoparticles Versus Soil Microorganisms
Plants are immobile organisms. They are dependent upon root system for water and nutrient absorption. In the vicinity of root systems, millions of microbes including plant growth-promoting bacteria (PGPR) dwell. They affect the root systems through their activity and themselves get affected by the exudates of roots (Philippot et al. 2013; Zoomi et al. 2017). More often, after continuous absorption, nutrient and water adjacent to the root systems get depleted. Because of the limited growth of roots, they cannot extract their need from soil from farther distance. In such conditions it is arbuscular mycorrhizal (AM) fungi and other microbes play key roles in the survival of plants (Zoomi et al. 2017; Kehri et al. 2018). NPs in the soil also interact with these microbes, and this interaction can control the growth and survival of aboveground plants. Therefore, the consequences of NPs on belowground biodiversity are of prime importance to understand.
The research study carried out on the effect of NPs on soil microbes is still in the beginning phase. NPs are reported as a good stimulator of soil microbes, but their harmful effect cannot be neglected (Rajput et al. 2018). Some research studies, however, report NPs as soil microbe neutral. The harmful aspect of NPs on soil microorganisms has been reviewed by Rajput et al. (2018) in details. The interaction of NPs with soil microbes depends upon particle configuration and coating (McKee and Filser 2016). McKee and Filser (2016) summarized the morphological nature of bacteria with reference to the NPs. In most of the reports, they have found gram-negative bacteria as less sensitive than gram-positive bacteria. However, any general conclusion could not be drawn. Further, toxicity may be related to specific nanoscale properties of the NPs.
McKee and Filser (2016) concluded that Ag- and Cu-based NPs exhibit antimicrobial activities. Suresh et al. (2013) described the toxic effects of Ag, Al2O3, TiO2, CeO2, CuO, CdSe, CdTe, FePt, and ZnO NPs. 1.0 g/l citrate-coated Ag NPs (c-Ag NPs) inhibited the growth of E. coli by 90 ± 5% (Doody et al. 2014). Simonin et al. (2016) reported very strong negative impact of TiO2 NPs on nitrification enzyme activities and the abundances of ammonia-oxidizing microorganism just after 90 days of exposure to even the lowest, realistic concentration. High concentration of TiO2 (Du et al. 2011; Ge et al. 2011; Simonin et al. 2015) and ZnO (Du et al. 2011; Ge et al. 2011) reported to inhibit microbial respiration and enzyme activity in the soil. CuO and Fe2O3 NPs were reported as potentially harmful to soil environment (Frenk et al. 2013). Besides the negative effects, NPs were also reported to pose positive effects on soil microorganisms. 1.0 g/l citrate-coated Ag NPs (c-Ag NPs) enhanced the growth of Bacillus subtilis by 127 ± 23% (Doody et al. 2014). Also, Si, Fe, Au, Pd, Ag2S, and Pt NPs exhibit no or little effect on soil microbes (Suresh et al. 2013).
4.4 Nanoparticles Versus Symbioses
4.4.1 ZnO Nanoparticle Versus Symbioses
AM fungi reduced the Zn accumulation in plants (Wang et al. 2016; Li et al. 2015). Under low phosphorus condition, AM fungi reduced translocation, uptake, and accumulation of Zn (Jing et al. 2016; Wang et al. 2014, 2018) in plant. AM fungi-mediated reduction in the soil pH is responsible for decreased Zn bioavailability (Jing et al. 2016). Further, under low phosphorus conditions, AM fungi reduce the translocation and accumulation of Zn released from Zn NP (Jing et al. 2016). AM fungi secrete a group of glycoproteins called glomalin-related soil proteins (GRSPs) in the soil. GRSPs bind ZnO NP in the soil (Ghasemi Siani et al. 2017), making them immobilized. The efficiency of AM fungi is further increased under organic phosphorus (Wang et al. 2018) and low phosphorus conditions (Jing et al. 2016). Under low phosphorus condition, the efficiency of AM fungi is at maximum. High level of phosphorus in the soil inhibits the AM colonization, as plants get adequate phosphorus and the mutualism starts shifting toward parasitism. Under ZnSO4 treatment the efficiency of AM fungi becomes more responsive toward the ZnO NP (Li et al. 2015). It is because AM fungi perceive heavy metal toxicity more quickly. Other mechanism of amelioration of ZnO NP toxicity in plants is the AM fungi-mediated increase in the accumulation of P, N, K, Fe, and Cu (Wang et al. 2014, 2016, 2018; Jing et al. 2016). Decrease in ZnO NP toxicity was confirmed by reduced ROS production in AM fungi-colonized plants (Wang et al. 2016).
ZnO NPs enhance the growth performance in plants inhabited by non-AM symbiotic microbes (Medina-Velo et al. 2017; Singhal et al. 2017; Bandyopadhyay et al. 2015). ZnO nanorods stimulate the number of fungal pellets, spore size, early sporulation, and thick hyphae in Piriformospora indica. This caused increased crop productivity (Singhal et al. 2017). ZnO NPs increased the growth performance in leguminous plants by accumulation of essential elements – sulfur and magnesium – assisted by higher nodule formation in the roots.
Contrary to above findings, ZnO NPs were found bactericidal to Sinorhizobium meliloti (Bandyopadhyay et al. 2012). However, it is less toxic than ZnCl2 for Medicago sativa L. – Sinorhizobium meliloti association (Bandyopadhyay et al. 2015). Ultra-high-resolution scanning transmission electron microscopy (STEM) revealed that ZnO NPs were accumulated on the bacterial cell wall and internalized into the periplasmic space. ZnO NPs also altered the polysaccharide structures of extracellular polymeric substances on bacterial cell wall (Bandyopadhyay et al. 2012). ZnO NPs also reduced the root shoot biomass (Bandyopadhyay et al. 2015).
4.4.2 Ag Nanoparticle Versus Symbioses
Ag NPs over 0.01 mg/kg enhanced the growth and ecological behavior and also decreased the antioxidants’ activity in AM fungi-associated plants by decreasing the Ag content in plants (Feng et al. 2013). They through X-ray microcomputed tomography found that AM fungi decreased the Ag content in plants.
Ag NPs in low concentration (50 ppm) improved growth parameters in leguminous plant (Pallavi et al. 2016). They have found that it increased the total bacteria, nitrogen fixer, and phosphate solubilizer count. Also, Ag NPs increase root nodulation in the roots of legumes. However, higher concentration (75 ppm) of Ag NPs was found toxic to nitrogen fixers and other bacteria (Pallavi et al. 2016).
4.4.3 CeO2 Nanoparticle Versus Symbioses
CeO2 NPs were found as bacteriostatic to the symbiotic N2-fixer Sinorhizobium meliloti . It was accumulated on the surface of bacterial cell. CeO2 NPs altered the protein and polysaccharide structures of extracellular polymeric substances on bacterial cell wall (Bandyopadhyay et al. 2012).
4.4.4 Fe3O4 Nanoparticle Versus Symbioses
Fe3O4 NPs in higher concentration reduced the biomass production in mycorrhizal clover (Feng et al. 2013). It also decreases the bacterial abundance and dissolved organic content (DOC) in maize plant rhizosphere (Cao et al. 2016) and shifted the community composition. At high concentration Fe3O4 NPs reduced the root mycorrhizal colonization, soil GRSP content, and alkaline phosphatase activity. This also decreased the availability of P nutrition in plants (Cao et al. 2017).
AM fungi reduced the negative effect of Fe3O4 NPs on the plant-microbe interaction (Cao et al. 2016). They have found that AM fungi enhanced the growth of plant and organic matter release from root (Cao et al. 2016).
4.5 Conclusions
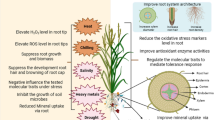
On the account of controversial results obtained on the interaction of NPs with plants, soil microbes, and symbionts, it is hard to draw any straightforward mechanism of interaction. However, several common behaviors could be attributed to this interaction. NPs in higher concentration cause adverse effects on plants as well as their microbial symbionts including AM fungi. Higher concentration of NPs reduces AM colonization, soil GRSP content, and alkaline phosphatase activity (Fig. 4.1a). This decreases the availability of P nutrition in plants (Fig. 4.1a). NPs in higher concentration are bacteriostatic and sometimes toxic to nitrogen fixers and other symbiotic bacteria.
In symbiotically associated plants, the effect of NPs toxicity is significantly lower as compared to the plants without symbionts (Fig. 4.1b). AM fungal and bacterial symbionts reduce the NP toxicity to the plants by the mechanism yet to be much understood. AM fungi have more than one mechanism through which they dilute the NP toxicity in host plants (Fig. 4.1b). These include (1) immobilization and, hence, reduced accumulation of heavy metallic NP by secretion of GRSPs, (2) reduction in NP accumulation by altering the soil pH and production of more DOC, (3) accumulation of more nutrient resulting in more biomass accumulation, and (4) reduction in reactive oxygen species in plants (Fig. 4.1b).
Besides the negative roles, regulated dose of NPs has positive roles in plants, especially those inhabited by endophytic microbes and/or nitrogen fixers. The interaction among non-AM symbionts, NPs, and plants is also poorly understood. However, based on researches carried out, several conclusions could be drawn. Accordingly, NPs stimulate the plant growth by (1) increased accumulation of essential elements – sulfur and magnesium – assisted by higher nodule formation in the roots; (2) stimulation in the number of fungal pellets, spore size, early sporulation, and thick hyphae in endophytes; and (3) increasing the total bacteria, nitrogen fixer, and phosphate solubilizer count.
4.6 Future Perspectives
Use of engineered NPs in soil which is increasing day by day may pose detrimental effects on belowground biodiversity. Our understating on the interaction of NPs with belowground biodiversity is at its preliminary phase. For sustainable use of NPs in agriculture production, there is a need of extensive research to explore the effects of various NPs and their optimum dose, coating material, carrier, mode, time, and frequency of application on different symbiotic microbes with reference to the host plant. Extensive research is also needed to trace and study the movement and localization of NPs in symbiotic microbes. Also, consequences of NPs internalization on gene expression and cellular functions should be studied.
Abbreviations
- AM:
-
Arbuscular mycorrhiza
- CNT:
-
Carbon nanotubes
- DOC:
-
Dissolved organic carbon
- GRSPs:
-
Glomalin-related soil proteins
- MWCNTs:
-
Multi-walled carbon nanotubes
- NP:
-
Nanoparticles
- ROS:
-
Reactive oxygen species
References
Arif N, Yadav V, Singh S, Tripathi DK, Dubey NK, Chauhan DK, Giorgetti L (2018) Interaction of copper oxide nanoparticles with plants: uptake, accumulation, and toxicity. In: Nanomaterials in plants, algae, and microorganisms. Academic Press, London, pp 297–310
Atha DH, Wang H, Petersen EJ et al (2012) Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ Sci Technol 46(3):1819–1827. https://doi.org/10.1021/es202660k
Auffan M, Rose J, Bottero JY et al (2009) Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol 4(10):634–641. https://doi.org/10.1038/nnano.2009.242
Bandyopadhyay S, Peralta-Videa JR, Plascencia-Villa G et al (2012) Comparative toxicity assessment of CeO2 and ZnO nanoparticles towards Sinorhizobium meliloti, a symbiotic alfalfa associated bacterium: use of advanced microscopic and spectroscopic techniques. J Hazard Mater 241–242:379–386. https://doi.org/10.1016/j.jhazmat.2012.09.056
Bandyopadhyay S, Plascencia-Villa G, Mukherjee A et al (2015) Comparative phytotoxicity of ZnO NPs, bulk ZnO, and ionic zinc onto the alfalfa plants symbiotically associated with Sinorhizobium meliloti in soil. Sci Total Environ 515–516:60–69. https://doi.org/10.1016/j.scitotenv.2015.02.014
Cao J, Feng Y, Lin X et al (2016) Arbuscular mycorrhizal fungi alleviate the negative effects of iron oxide nanoparticles on bacterial community in rhizospheric soils. Front Environ 4:10. https://doi.org/10.3389/fenvs.2016.00010
Cao J, Feng Y, Lin X et al (2017) Iron oxide magnetic nanoparticles deteriorate the mutual interaction between arbuscular mycorrhizal fungi and plant. J Soils Sediments 17(3):841–851. https://doi.org/10.1007/s11368-016-1561-8
Doody M, Bais H, Jin Y (2014) Effects of citrate-coated silver nanoparticles on interactions between soil bacteria and the major crop plant Zea mays. In: EGU general assembly conference abstracts, Vienna, Austria, 27 April – 2 May, 2014
Du W, Sun Y, Ji R et al (2011) TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J Environ Monit 13(4):822–828. https://doi.org/10.1039/c0em00611d
Feng Y, Cui X, He S et al (2013) The role of metal nanoparticles in influencing arbuscular mycorrhizal fungi effects on plant growth. Environ Sci Technol 47(16):9496–9504. https://doi.org/10.1021/es402109n
Frenk S, Ben-Moshe T, Dror I et al (2013) Effect of metal oxide nanoparticles on microbial community structure and function in two different soil types. PLoS One 8(12):e84441. https://doi.org/10.1371/journal.pone.0084441
Ge Y, Schimel JP, Holden PA (2011) Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environ Sci Technol 45(4):1659–1664. https://doi.org/10.1021/es103040t
Ghasemi Siani N, Fallah S, Pokhrel LR et al (2017) Natural amelioration of Zinc oxide nanoparticle toxicity in fenugreek (Trigonella foenum-graecum) by arbuscular mycorrhizal (Glomus intraradices) secretion of glomalin. Plant Physiol Biochem 112:227–238. https://doi.org/10.1016/j.plaphy.2017.01.001
Hong J, Peralta-Videa JR et al (2014) Evidence of translocation and physiological impacts of foliar applied CeO2 nanoparticles on cucumber (Cucumis sativus) plants. Environ Sci Technol 48(8):4376–4385. https://doi.org/10.1021/es404931g
Jing XX, Su ZZ, Xing HE et al (2016) Biological effects of ZnO nanoparticles as influenced by arbuscular mycorrhizal inoculation and phosphorus fertilization. Huan Jing Ke Xue 37(8):3208–3215. https://doi.org/10.13277/j.hjkx.2016.08.049
Kehri HK, Akhtar O, Zoomi I et al (2018) Arbuscular Mycorrhizal Fungi: Taxonomy and its Systematics. Int J Life Sci Res 6(4):58–71
Kehri HK, Zoomi I, Singh U et al (2019) Review on biogenic synthesis of nanoparticles and their therapeutic applications: List of novel eco-friendly approaches. J Nanosci Tech 5(4):810–816
Khodakovskaya M, Dervishi E, Mahmood M et al (2009) Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 3(10):3221–3227. https://doi.org/10.1021/nn900887m
Koul A, Kumar A, Singh VK, Tripathi DK, Mallubhotla S (2018) Exploring plant-mediated copper, iron, titanium, and cerium oxide nanoparticles and their impacts. In: Nanomaterials in plants, algae, and microorganisms. Academic Press, London, pp 175–194
Kumari M, Khan SS, Pakrashi S et al (2011) Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. J Hazard Mater 190(1–3):613–621. https://doi.org/10.1016/j.jhazmat.2011.03.095
Larue C, Khodja H, Herlin-Boime N et al (2011) Investigation of titanium dioxide nanoparticles toxicity and uptake by plants. J Phys Conf Ser 304:012057. https://doi.org/10.1088/1742-6596/304/1/012057
Lee CW, Mahendra S, Zodrow K et al (2010) Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ Toxicol Chem 29(3):669–675. https://doi.org/10.1002/etc.58
Li S, Liu X, Wang F et al (2015) Effects of ZnO nanoparticles, ZnSO4 and arbuscular mycorrhizal fungus on the growth of maize. Huan Jing Ke Xue 36(12):4615–4622. http://www.ncbi.nlm.nih.gov/pubmed/27012001
McKee MS, Filser J (2016) Impacts of metal-based engineered nanomaterials on soil communities. Environ Sci Nano 3(3):506–533. https://doi.org/10.1039/C6EN00007J
Medina-Velo IA, Barrios AC, Zuverza-Mena N et al (2017) Comparison of the effects of commercial coated and uncoated ZnO nanomaterials and Zn compounds in kidney bean (Phaseolus vulgaris) plants. J Hazard Mater 332:214–222. https://doi.org/10.1016/j.jhazmat.2017.03.008
Mishra S, Keswani C, Abhilash PC et al (2017) Integrated approach of agri-nanotechnology: challenges and future trends. Front Plant Sci 8:471. https://doi.org/10.3389/fpls.2017.00471
Mushtaq YK (2011) Effect of nanoscale Fe3O4, TiO2 and carbon particles on cucumber seed germination. J Environ Sci Health A 46(14):1732–1735. https://doi.org/10.1080/10934529.2011.633403
Pallavi, Mehta CM, Srivastava R et al (2016) Impact assessment of silver nanoparticles on plant growth and soil bacterial diversity. 3 Biotech 6(2):254. https://doi.org/10.1007/s13205-016-0567-7
Pérez-de-Luque A (2017) Interaction of nanomaterials with plants: what do we need for real applications in agriculture? Front Environ Sci 5:12. https://doi.org/10.3389/fenvs.2017.00012
Philippot L, Raaijmakers JM, Lemanceau P et al (2013) Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol 11(11):789–799. https://doi.org/10.1038/nrmicro3109
Rajput VD, Minkina T, Sushkova S et al (2018) Effect of nanoparticles on crops and soil microbial communities. J Soils Sediments 18(6):2179–2187. https://doi.org/10.1007/s11368-017-1793-2
Raliya R, Franke C, Chavalmane S et al (2016) Quantitative understanding of nanoparticle uptake in watermelon plants. Front Plant Sci 7:1288. https://doi.org/10.3389/fpls.2016.01288
Rastogi A, Tripathi DK, Yadav S, Chauhan DK, Živčák M, Ghorbanpour M, El-Sheery NI, Brestic M (2019a) Application of silicon nanoparticles in agriculture. 3 Biotech 9(3):90
Rastogi A, Zivcak M, Tripathi DK, Yadav S, Kalaji HM, Brestic M (2019b) Phytotoxic effect of silver nanoparticles in Triticum aestivum: improper regulation of photosystem I activity as the reason for oxidative damage in the chloroplast. Photosynthetica 57(1):209–216
Sabo-Attwood T, Unrine JM, Stone JW et al (2012) Uptake, distribution and toxicity of gold nanoparticles in tobacco (Nicotiana xanthi) seedlings. Nanotoxicology 6(4):353–360. https://doi.org/10.3109/17435390.2011.579631
Sabourin V, Ayande A (2015) Commercial opportunities and market demand for nanotechnologies in agribusiness sector. J Technol Manag Innov 10(1):40–51. https://doi.org/10.4067/S0718-27242015000100004
Shweta, Vishwakarma K, Sharma S, Narayan RP, Srivastava P, Khan AS, Dubey NK, Tripathi DK, Chauhan DK (2017) Plants and carbon nanotubes (CNTs) interface: present status and future prospects. In: Nanotechnology. Springer Singapore, Singapore, pp 317–340
Shweta, Tripathi DK, Chauhan DK, Peralta-Videa JR (2018) Availability and risk assessment of nanoparticles in living systems: a virtue or a peril? In: Nanomaterials in plants, algae, and microorganisms. Academic Press, London, pp 1–31
Simonin M, Guyonnet JP, Martins JMF et al (2015) Influence of soil properties on the toxicity of TiO2 nanoparticles on carbon mineralization and bacterial abundance. J Hazard Mater 283:529–535. https://doi.org/10.1016/j.jhazmat.2014.10.004
Simonin M, Richaume A, Guyonnet JP et al (2016) Titanium dioxide nanoparticles strongly impact soil microbial function by affecting archaeal nitrifiers. Sci Rep 6(1):33643. https://doi.org/10.1038/srep33643
Singh S, Tripathi DK, Dubey NK, Chauhan DK (2016) Effects of nano-materials on seed germination and seedling growth: striking the slight balance between the concepts and controversies. Mater Focus 5(3):195–201
Singh A, Singh NB, Hussain I et al (2018) Plant-nanoparticle interaction: an approach to improve agricultural practices and plant productivity. Int J Pharm Sci Invent 4(8):25–40
Singh J, Vishwakarma K, Ramawat N, Rai P, Singh VK, Mishra RK, Kumar V, Tripathi DK, Sharma S (2019) Nanomaterials and microbes’ interactions: a contemporary overview. 3 Biotech 9(3):68
Singhal U, Khanuja M, Prasad R et al (2017) Impact of synergistic association of ZnO-nanorods and symbiotic fungus Piriformospora indica DSM 11827 on Brassica oleracea var. botrytis (Broccoli). Front Microbiol 8:1909. https://doi.org/10.3389/fmicb.2017.01909
Stampoulis D, Sinha SK, White JC (2009) Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol 43(24):9473–9479. https://doi.org/10.1021/es901695c
Su M, Liu H, Liu C et al (2009) Promotion of nano-anatase TiO2 on the spectral responses and photochemical activities of D1/D2/Cyt b559 complex of spinach. Spectrochim Acta A Mol Biomol Spectrosc 72(5):1112–1116. https://doi.org/10.1016/j.saa.2009.01.010
Suresh AK, Pelletier DA, Doktycz MJ (2013) Relating nanomaterial properties and microbial toxicity. Nanoscale 5(2):463–474. https://doi.org/10.1039/C2NR32447D
Taylor AF, Rylott EL, Anderson CWN et al (2014) Investigating the toxicity, uptake, nanoparticle formation and genetic response of plants to gold. PLoS One 9(4):e93793. https://doi.org/10.1371/journal.pone.0093793
Tripathi DK, Ahmad P, Sharma S, Chauhan DK, Dubey NK (eds) (2018) Nanomaterials in plants, algae, and microorganisms: concepts and controversies, vol 1. Academic Press, London
Vishwakarma K, Upadhyay N, Kumar N, Tripathi DK, Chauhan DK, Sharma S, Sahi S (2018) Potential applications and avenues of nanotechnology in sustainable agriculture. In: Nanomaterials in plants, algae, and microorganisms. Academic Press, London, pp 473–500
Wang WZ, Wang FY, Li S et al (2014) Arbuscular mycorrhizal symbiosis influences the biological effects of nano-ZnO on maize. Huan Jing Ke Xue 35(8):3135–3141. http://www.ncbi.nlm.nih.gov/pubmed/25338390
Wang F, Liu X, Shi Z et al (2016) Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants – a soil microcosm experiment. Chemosphere 147:88–97. https://doi.org/10.1016/j.chemosphere.2015.12.076
Wang F, Jing X, Adams CA et al (2018) Decreased ZnO nanoparticle phytotoxicity to maize by arbuscular mycorrhizal fungus and organic phosphorus. Environ Sci Pollut Res 25(24):23736–23747. https://doi.org/10.1007/s11356-018-2452-x
Yang J, Cao W, Rui Y (2017) Interactions between nanoparticles and plants: phytotoxicity and defense mechanisms. J Plant Interact 12(1):158–169. https://doi.org/10.1080/17429145.2017.1310944
Zoomi I, Narayan RP, Akhtar O et al (2017) Role of plant growth promoting rhizobacteria in reclamation of wasteland. In: Microbial biotechnology. Springer Singapore, Singapore, pp 61–80. https://doi.org/10.1007/978-981-10-6847-8_3
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Akhtar, O., Zoomi, I., Pandey, D., Kehri, H.K., Narayan, R.P. (2020). Tripartite Interaction Among Nanoparticles, Symbiotic Microbes, and Plants: Current Scenario and Future Perspectives. In: Bhushan, I., Singh, V., Tripathi, D. (eds) Nanomaterials and Environmental Biotechnology. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-34544-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-34544-0_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-34543-3
Online ISBN: 978-3-030-34544-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)