Abstract
Bone defects are common and are associated with a significant burden of disease threatening the health of many people around the globe. Since the last decade, data obtained from case studies have demonstrated that 20% of patients who experience an osteoporotic hip break are unable to endure the primary year after medical treatment. Many similar cases suggest that there is a huge requirement for better treatment of unhealthy and broken bones. Human bone comprises of about 70% of calcium phosphate (CaP) mineral, therefore CaPs are possible alternative materials to fix a broken bone. CaP is broadly utilized for bone fixation because of its bioactive properties like osteoinductivity, osteoconductivity, and biodegradability. Therefore, examination of these properties and the impact of their different affecting factors are crucial for balancing CaP during the fabrication procedure to maximally fulfill required clinical prerequisites. The aim of this chapter is to highlight the systems behind the CaP-assisted bone development in the initial phase, specifically as a biocompatible bone graft substitute. In this study, the latest developments in the biological properties of CaP biomaterials, including hydroxyapatite (HA), tricalcium phosphate (TCP), and biphasic CaP (BCP), have been summarized. Moreover, recent advances on how their properties are altered by different factors are reviewed. Finally, perspectives regarding future developments of CaP materials are provided.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Medical advances have definitely paved a way in increasing our life span. However, increasing longevity raises new challenges. Age-related diseases result in significant reductions in the quality of our life. The loss of a skeletal tissue that accompanies trauma, injury, disease can result in significant morbidity as well as significant socioeconomic cost. It, thus, emphasizes the need for new and more reliable skeletal regeneration strategy. Today, hundreds of people across the globe are diagnosed with musculoskeletal diseases such as arthritis, osteoporosis, bone fractures, bone tumors, back ache, and other cerebrospinal disorders [1, 2]. To address this dire need of bone augmentation and tissue regeneration, regenerative medicine has come to the forefront in recent years with new advances in neoskeletal tissue formation. The successful outcome of this approach requires pluripotent stem cells, novel scaffolds, and growth factors that assure bone regeneration strategies for improved life quality. This chapter demonstrates various characteristic aspects of osteoconductive and osteoinductive biomaterials, and advances in their applications in the field of tissue engineering to address various bone defect problems. Damaged bone has to be regenerated naturally or it needs to be substituted with a prosthesis, or a bone material from another body part by surgery [3]. At present, demineralized bone , HA and other graft substitutes are developed and have been used to facilitate osteogenesis at damaged bony tissue parts. However, they have failed to bring about satisfactory results in the regeneration of tissues. Recently, growth factors, such as bone morphogenic factors (BMF) [4], platelet-derived growth factors (PDGF) [5], insulin-like growth factors (IGF) [6], and cytokines [7] have been reported to be very useful in the regeneration of bone tissues.
The application of bone graft substitutes is conducted by autograft and allograft methods. However, grafting methods suffer from several problems, such as limited availability of grafts [8]. Moreover, there always exists a chance of bacterial infection and blood loss during surgical processes. In addition, the areas from which the grafts are taken experience poor structural stability [9]. The autograft method has an advantage over allografts with respect to donor availability because they are obtained from allo-doners, but osteoinductive potential of allogenic bones are far inferior than autogenous, which makes it suitable for temporary support only. In order to circumvent these problems, active research has been directed to the development of bone graft substitutes which possess excellent biomechanical properties of metal grafts and biological properties of bone grafts.
CaP ceramics are the most widely used bone substitutes for clinical applications of bone grafting and orthopedics [10]. However, not a wide variety of CaP ceramics are responsible for influencing better biological performance in vivo [11,12,13]. On the other hand, most of the CaP ceramics are osteoconductive in nature, just a few specific groups are osteoinductive in nature [13]. These little differences in their intrinsic characteristics which in turn enhance osteoblast differentiation are identified with little difference in the physical and chemical properties of CaP ceramics. For instance, chemical properties, such as, surface chemistry and charge can impact biological phenomenon like protein adsorption [10], which can in this manner to effect osteoblastic differentiation by means of cell–extracellular matrix (ECM) interactions [14, 15]. In this manner, physical properties, for example, surface topography (roughness) can facilitate cell differentiation by helping cellular attachment on the material surface [16]. Moreover, some other surface characteristics of CaP ceramics can enhance the recruitment of important cell-attaching proteins and in this manner give conditions favorable to the development of fixed focal adhesive compounds [17]. Along these lines, understanding the exact roles that material properties play for regulating the cell material interaction process is a primary step toward designing osteoinductive CaP materials. This chapter describes the physical as well as chemical characteristics of CaP ceramics and its influence with regards to bone tissue engineering (BTE). Specifically, it clarifies the variation in CaP ceramic properties like surface roughness, solubility and crystallinity, related to and contrasted with osteoconductivity and osteoinductivity (Fig. 1).
Bone and Its Properties
Bone is a mineralized connective tissue that shows four kinds of cells: osteoblasts , bone-covering cells, osteocytes, and osteoclasts [1, 2]. In spite of its inactive appearance, bone is an exceedingly powerful organ that is persistently resorbed by osteoclasts and reformed by osteoblasts. In this section, we address the present information about bone cell science, the bone network, and the variables that impact the bone rebuilding process.
Hierarchical Design of Bone
In order to investigate the mechanical properties of bone tissue, it is very much essential to have a fair understanding of the constituent mineral phases of bone, and the macrostructural co-relationship between them at different levels of hierarchical morphological arrangements [18,19,20]. These organizations are:
-
I.
Basic macro-architecture: cancellous bone and cortical bone;
-
II.
Basic micro-architecture (10–500 μm): haversian canals and osteons;
-
III.
Sub-micro-architecture (1–10 μm): lamellae;
-
IV.
Nanostructure (100 nm–1 μm): fibrillar collagen and embedded mineral; and
-
V.
Sub-nanostructure (<100 nm): molecular organization of constituent mineral components, like collagen, and non-collagenous organic proteins.
This hierarchically arrangement of bone has an intricate, but optimized structural orientation of the components, making the bone material heterogeneous and anisotropic (Fig. 2).
Hierarchical architecture of bone [21]
Composition of Bone Materials
The CaP biomaterial is constantly talked about in connection with bone repair as CaP is the fundamental inorganic component of bone. In spite of the fact that the shape of bone changes in various pieces of the body, the physicochemical structure of bone for the different shape is biochemically similar. Bone tissue can be viewed as a composite material developed by a collagen biopolymer and CaP bioceramic. Normal bone comprises of 69% CaP bioceramic, considered as the standard bone material. The natural part (22%) built in by proteins, type I collagen (90%), and some other non-collagenous proteins, such as proteoglycans, lipids, and osteogenic stimulus (this is meant to be enhancement factors, such as bone morphogenetic proteins (BMPs) and vascular endothelial growth factors (VEGFs)) [22]. The other remaining 9% is filled up by water molecules. Table 1 [23] describes the characteristic properties of bone tissues and their capabilities in bone mineralization events.
Bone Cells
Despite its strength and hardness, bone is a dynamic living tissue. Bone is composed of a series of complex events altogether arranged by different types of bone cells associated with each other and also with the ECM. The bone cells comprise of four types of cells namely (1) osteoblasts, (2) osteoclasts, (3) osteocytes, and (4) bone-covering cells. Osteoblasts are cells that are responsible for the creation and mineralization of the bone grid; whereas osteoclasts are accountable for bone resorption. Osteoblasts are derived from mesenchymal stem cells (MSCs). The dedication of MSCs toward the osteoprogenitor lineage requires the expression of specific genes, which follow modified steps, including the synthesis of BMPs and also members from the Wingless (Wnt) pathways. Run related translation factor 2 (Runx2) is the ace gene of osteoblast differentiation. Runx2 is also vital for osteoblast differentiation [22, 24]. Additionally, Runx2 has been shown to upregulate osteoblast-related qualities , for example, collagen ColIA1, alkaline phosphatase (ALP), bone sialoprotein (BSP), and osteocalcin (OCN).
Structure of Bone Grafts
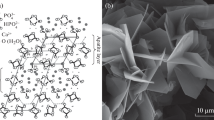
Although bone has its own capacity to repair, the capacity declines with age, and is constrained to small defects. So far, grafts are important to help bone repair when bone loss is too enormous. A few grafts can be the choices in the clinic for bone repair. An autograft, which is gathered from the patient’s own body, has no issue with biocompatibility and immune response [25]. Autografts might be cortical, cancellous, or cortico-cancellous (scanning ultra-micrographs of various bone grafts are introduced in Fig. 3). The cortical bone has higher mineral substance than the trabecular or cancellous bone [18]. The compressive solidness and quality of the cortical bone are a lot higher than those of the cancellous bone. In choosing a graft, the specialist must know about these major contrasts in bony structures [26, 27].
Cortical bone grafts are utilized for the most part for structural help and strength, and cancellous bone grafts for osteogenesis. Cancellous bone grafts are ordinarily utilized in break non-association, dental imperfections, maxillofacial deformities, spinal combination, and other little bone deformities [28, 29]. These grafts need mechanical stability; however, the permeable structure of cancellous bone grafts can upgrade bone cell ingrowth and improve the healing process, permitting quicker revascularization [30]. Cortical bone grafts are used less regularly, and they might be utilized as only as grafts [31]. An allograft, which is given by the donor, has osteogenic capacity yet the supply of allografts is constrained. The inadequacies of autografts and allografts legitimize the improvement of artificial bone joint biomaterials.
Bone Porosity
Interconnecting porosity is an important physicochemical property of bone. The size of pores and interconnection within bone determine the internal vascularization as well as tissue ingrowth [24, 32,33,34,35]. Bone pore sizes in a typical cortical bone territory vary from 1 to 100 μm while trabecular bone has pores extending from 200 to 400 μm [36]. The size range, degree, and interconnectivity of the pores are basic variables influencing dispersion of supplements, cell adhesion, migration and expression, and tissue ingrowth that are important for bone arrangement and repair or recovery [37].
Bone Strength
The high level of combination and introduction of the mineral and natural segments gives bone its mechanical strength. The property that is frequently used to describe the mechanical behavior of bone substitutes is their compressive strength. Since these materials are proposed to be utilized as bone substitutes, it is vital to remember that the compressive strength of human cortical bone ranges somewhere in the range of 90 and 230 MPa (with elastical strength from 90 to 190 MPa), while the compressive strength of cancellous bone ranges somewhere in the range of 2 and 45 MPa [38]. Table 2 describes the comparative mechanical strength of CaP with metals used as a biocompatible material.
Types of CaP Derivatives Present in the Body
The existence of CaP in the vertebrate has been reported to be in the form of apatite. The mineral present in teeth and bones is appreciated as calcium HA, Ca10(PO4)6(OH)2 [39] with minor segments of carbonate (CO3), magnesium (Mg), and sodium (Na). The crystal structure of apatite in enamels is well understood among all of the apatites in our body. It was found to be platelet-like in shape (lengths and widths (30–45 nm) and thickness around 5 nm) embedded in collagen nanofibrils [40]. As shown in the XRD profile of enamel apatite [41] and a lot more extensive diffraction peaks of either bone or dentin apatite (Fig. 4), it is evident that apatite present in enamel possesses a bigger crystal size (around 2000 nm) as compared to any other apatite in our body .
Many other biological non-apatitic CaPs exist in our body, which are equally responsible for the regeneration of bone, as summarized in Table 3.
Categories of CaPs
Based on structural composition, CaPs for bone and teeth regeneration are classified as: (1) calcium-deficient apatite, CDA (i.e., Ca/P molar ratio less than the stoichiometric value of 1.67 for pure HA), (2) HA, Ca10(PO4)6(OH)2, (3) beta-tricalcium phosphate (β-TCP), Ca3(PO4)2, and (4) biphasic CaP (BCP), an intimate mixture of HA and β-TCP of varying HA/β-TCP weight ratios, are available commercially (Table 4).
Hydroxyapatite (HA)
HA is broadly used as an alternative inorganic filler material in bone tissue engineering because of its compositional similarities with that of the inorganic counterpart of bone [43]. HA material is the most stable phase among various other forms of CaP, specifically it is the most steady in a dissolve stage [44, 45]. In spite of the fact that not exceedingly solvent, the surface of HA mineral is favorable as a nucleation site in culture medium (continuously soaked with calcium and phosphate particles) for the precipitation of apatite crystal [46]. In addition, stoichiometrically HA(Ca5(PO4)3)OH contain a Ca/P ratio of 1.67 and is believed to be as osteoconductive not osteoinductive [47].
Tricalcium Phosphate (TCP)
Proportion of Ca/P ratio in TCP is 1.5 and it is most likely to exit in two different phases, namely α-TCP phase and β-TCP phase; these two phases have indistinguishable different crystal structures [48]. The two phases are less steady than HA [49]. The α-TCP phase is believed to be osteoconductive as well as osteoinductive properties. It can encourage the formation of an apatite layer when incubated in biological fluid containing different ionic arrangements [50]. β-TCP is the more utilized form of TCP than α-TCP in bone regeneration .
Biphasic CaP (BCP)
This type of CaP belongs to a bone substitute group that consists of an intimate mixture of two ceramics with varying ratios. BCP powder is synthesized by the homogeneous mixing of HA and TCP powder by means of physical grinding, or by high temperature sintering of calcium-deficient HA (CDHA) above 700 °C, resulting in a composition of two individual phases [51]. The Ca/P ratio in BCPs mainly depends upon the calcium deficiency of CDHA and sintering temperature of CDHA, which generally falls in the range between pure β-TCP and HA. Furthermore, in biomedical applications, BCP, is known as a potential candidate for bone regeneration, drug delivery vehicle and carrier of growth factors .
Solubility of CaP
Cell-mediated biodegradation happens under acidic conditions [53]. Therefore, in vitro dissolution studies of CaP biomaterials may be predictive of their in vivo dissolution or biodegradation [25]. Monocalcium phosphate monohydrate (MCPM) is the most acidic and soluble CaP among all other CaPs. MCPM isn't biocompatible because of its very acidic nature and high solubility. Bone apatite is like CDHA apart from the presence of carbonate (CO3−2 ) and the other components, for example, Na+, K+, Mg2+, Sr2+, and Zn2+ [54,55,56]. HA and β-TCP are considered to be the most useful CaPs compared to other CaPs due to their osteogenic potential and also their ability to form reliable attachment with host bone tissues near the defect site. The dissolution property of β-TCP is greater than HA which makes it beneficial as a bioresorbable agent [57, 58]. The advancement of biphasic CaP (BCP)-based biomaterials comprising of HA and β-TCP [26,27,28] are likewise important to control degradation properties. Table 5 presents an overview of various CaPs and their properties .
Bioactivity and Resorbability of CaP Materials
The justification in the advanced applications of CaPs has been lying in their close proximity in composition and properties to that of bone, like osteoconductivity as well as bioactivity. To discover its huge potential as an artificial bone substitute, biomaterial researchers need an understanding of the basic properties of CaP material, like biological and mechanical properties. Some of the relevant properties of CaP biomaterials in terms of osteoinductivity, osteoconductivity, biodegradability, and the potential factors which influence these inherent properties of CaP biomaterials in hard tissue recovery utilizing tissue design are discussed in the following section.
Cell Signalling in CaP Mediated Osteoinductivity
As of now, most metallic implants do not possess osteogenetic characteristics , whereas some CaP ceramics used encourage osteogenesis without adding any more osteogenic agents from the outside. This type of characteristic is defined as osteoinduction. Osteoinduction implies the recruitment of immature cells and the stimulation of these cells to develop into a pre-osteoblasts lineage. Osteoconductivity of CaP has been studied in the literature but the mechanism behind has not been well explored [64]. Release of ions from CaP materials is thought to be the main contributor to this phenomenon. However, some osteogenic growth factors including TGF-β and BMPs appear to play an important role in the osteoinduction process via related signalling pathways [65]. Thus, to understand the detailed mechanism behind the osteoinduction process, researchers need to focus their study into the molecular level for a detailed osteoinduction signalling pathway. A schematic illustration of feasible signalling pathways can be created in Fig. 5.
Transforming growth factor-beta (TGF-β) is appearing to play a major role in the formation of bone cells from MSCs during mammalian development. In brief, the TGF-β superfamily is comprised of over forty members of proteins, such as TGF-βs, nodal, activin, and BMPs [66]. In this signalling event, first the signal was internalized across the plasma membrane through the formation of heteromeric complexes of specific type I and type II serine/threonine kinase receptors. After that, a specific type II receptor is activating via phosphorylation of type I receptors. Next, phosphorylation of some specific proteins, called Smad and R-mad initiate the signalling pathway by the help of the activated type I receptor. The transcriptional process starts inside nucleus as activated R-Smads translocate into the nucleolus by forming a complex with co-Smad and Smad4.
There are other signalling pathways, like BMPs, Wnt which are also capable of modulating new bone formation through this osteoinduction procedure [67]. This signalling route exhibits various regulatory functions in enhancing various processes during osteogenesis (like signal transduction, gene expression, and osteoblast differentiation ).
Osteoconductibility of CaP Materials
Osteoconductivity is a process, where bioactive materials are implanted inside a defect bone, and consequently bone cells will adhere or attach on the material’s outer surface and at a later time point, bone cells will invade inside the pore of the implanted material; this process is also defined as bone conduction [68]. These large amount of bone cells that occupy the implant material surface and internal pores, clearly indicate their osteoinductive property. Both material-dependent factors and defect sites are two decisive factors which influence the osteoconduction process during osteogenesis. Yu et al. showed in his work that material properties can be factor for inducing osteogenesis. The results clearly indicate that vascularization was different for different channel diameters in CaP scaffolds (Fig. 6), higher expression of the PLGF (placental growth factor), angiogenic factors HIF1 alpha (hypoxia-inducible factor 1), and migration factor CXCR4 (C-X-C chemokine receptor type 4), which are responsible for starting the development of micro vessels, was seen inside the CaP porous scaffolds with a channel diameter of 250 p.m. Whereas, the diameter of the 500 pm channel in the same CaP scaffold gradually increased the expression of VEGF-A (vascular endothelial growth factor A), which initiated the formation of macro vessels [69]. However, apart from the size of the interconnecting channels, macroporosity (pores >50 μm) and microporosity (porosity <50 μm) are thought to have a prominent function in cellular attachment on the material implant. For example, macroporosity influences cell adhesion and accordingly vascular growth as well as the development of bone tissue. On the other hand, the microporosity surface of bioceramic enhances protein adsorption, which in turn escalates cell attachment on the material.
Graphical representation of the angiogenesis strategy within a CaP scaffold internal channel pore: (a) different channel diameters influence different blood vessel formation and (b) the increased HIF1a expression in the internal pore channels influence the formation of blood vessels into its host [61]
Studies have also shown that BCP ceramics coated with HA and seeded with MSCs were augmented and have shown enhanced formation of new bone tissue in the BCP ceramics after 12 weeks of implantation inside rabbit tibia [70]. Based on the other literature references, it can be confirmed that CaP performs well in human patients. Still, not many studies have shown the osteoconductive properties of CaP ceramics in human patients. That is why researchers need to explore more about CaP osteoconductibility in humans with suitable approaches.
Biodegradability of CaP Materials
The essential properties of a perfect bioactive bone substitute is that substitute materials have to disintegrate at a similar rate at which the osteoblast cells start to develop into new bone cells on the material surfaces, until the substitute material is fully supplemented by new, active bone tissue, although, biomaterials are believed to exhibit identical biomechanical as well as biochemical characteristics and regenerate bone tissue in a similar fashion as autologous bone [59]. Till date, various biomaterials have been explored to determine their feasibility to be used as an absorbable implant. In case of metals (Ti, Co-Cr-Mo) and synthetic polymers (polylactic acid or PLA, polylactic-co-glycolic acid or PLGA), which were not degradable legitimately with time after implantation has not been accounted for superior biodegradability as implant material. On the other hand, CaP ceramics, particularly TCP, have shown very good biodegradability. The mechanism of CaP biodegradability can be explained in two ways, one is, “dispersing material into particles” and another way says “dissolving material into ions”. The first idea is based on the belief that material first disintegrates in some small tiny particles which are engulfed by macrophages or osteoclast cells; this process is called phagocytosis [71]. The explanation behind the second idea is that the reinforced material disintegrates and dissolves as Ca2+ and HPO4−2 ions, which are then accumulated by the bone forming cells for proliferation, differentiation, and the development of regenerated bone [72]. Moreover, in the study by Sheikh et al. [73], the in vivo degradation event of biomaterials is categorized by three reactions: such as physical reaction, chemical reaction, and biological reaction. It also involves the stages featuring the complete degradation of biomaterial and its assimilation by cells.
In a physical reaction, biomaterials degrade by dissolving of material, and consequently an apatite-like layer is formed on the surface of the biomaterial, which is believed to be developed by dissolving, depositing, ion exchange events occurring on the material surface during the early degradation period until the material at last is crushed into tiny particles. Mechanical stability of biomaterials decreases rapidly during this time period. Biological reaction means degradation and adsorption or microscopic segregation of biomaterials in a biological fluid by the shared support of various bone forming cells including osteoclasts [74], osteoblasts, macrophages [75], fibroblasts, and multinucleated monster cells (Fig. 7) [76].
Function of osteoclasts on the surface of CaP [77]
Recently, Wang et al. [78] demonstrated that biodegradation and osteoinductive ability of a BCP material were correlated to each other. Moreover, there are some specific mechanisms and much complexity in between both processes, which should be addressed in the future to develop promising biomaterials for bone repair and regeneration.
Characteristics of Osteoinductive Materials
Till date, among all materials that are currently used as bone grafts, CaP materials hold the most promise to be utilized in the clinic for bone tissue designing, because of its biocompatibility, osteoconductivity, and osteoinductivity. Additionally, alongside a 3D permeable structure and some specific basic intrinsic characteristics for CaP ceramic production, they are important to new bone regeneration. In the accompanying sections, we will talk in detail about the impact of different material attributes of CaP materials on osteoinductivity.
Effect of Crystallinity
A number of studies related to material characterization of CaP have suggested that crystallinity is an important factor in inducing bone formation. The concentration of ions in the culture medium and pH of the culture medium can be affected by crystallinity and solubility of CaP ceramics, which in turn are responsible for cell adhesion on a material surface. Hu et al. showed that BMSCs from rabbit adhered better on HA (higher crystallinity) and it was better than amorphous HA (lower crystallinity) [65]. In another study, Berube et al. showed that the adhesion of osteoblasts from rat calvarias, was better on higher crystalline HA surfaces than on lower crystalline HA surfaces [79]. In addition, Knabe et al. investigated the reasons behind the lower attachment of BMSC from rat bone onto CaKPO4 pellet samples in comparison with α-TCP pellet samples, and explained that as the release of phosphate and potassium ion from CaKPO4 samples decreased the concentration of calcium ion from the culture medium caused lower attachment of cells onto material surfaces [77]. Moreover, the authors suggested that the formation of an apatite layer on the material surface played an important role in influencing cell attachment and proliferation on the material surfaces.
Overall, the research on the crystallinity of materials indicates that crystalline and solubility of released ions from CaP ceramics may develop stable surfaces for cell adhesion and proliferation in physiological conditions.
Effect of Solubility
The adsorption of proteins on the surfaces of CaP ceramic materials depends on surface charge and solubility of the material, which in turn influences cell behavior by changing the concentration of ions in the solution [65]. In a recent investigation by Lee et al., it was observed that, CaP ceramics nanoparticles in a polypropylene fumarate [80] and poly(lactic-co-glycolic acid) matrix enhances the adsorption of proteins compared to without CaP ceramic samples [81]. Overall, these results indicated that higher ion concentrations and changes in pH near the surface of soluble CaP ceramics (e.g., HA, TCP, and BCP ) promote cell adhesion by facilitating protein adsorption on the surfaces of the materials.
Effect of Surface Roughness
Although integrin binding and cell adhesion on the material surfaces can be influenced by material surfaces having nano and sub-micron level roughness properties [82]. As an example, Zhou et al. demonstrated that rabbit BMSCs attached on HA surfaces (with an Ra value of 11.9 nm) was more prominent than on surfaces having an Ra value of 54.2 nm, where the particle sizes were different for two nonidentical materials [83]. In addition, Dulgar-Tulloch et al. investigated these events: small grains in the range of ~50–100 nm influenced (decrease) the attachment of human BMSCs in comparison with large grain (200 nm) [84]. Altogether, these studies indicated that surface roughness (nano and micro) and crystal grain size less than 100 nm can promote protein adsorption as well as cell adhesion .
Effect of Surface Charge
Surface charge is another important factor along with crystallinity and solubility may influence protein adsorption as well as cellular attachment by significantly varying the charge concentration near material surfaces. In addition, cationic charged surfaces could have assumed a positive function in cell attachment by promoting the adsorption of proteins on the surface of the material. In a recent study by Feng et al., it has been shown that calcium-coated titanium implant surfaces increase osteoblast attachment compared to phosphate-coated titanium implant surfaces [85]. The authors further suggested that apatite-coated implants gave better cellular adhesion compared to calcium-coated implants. Overall, the results indicated that calcium-coated surface provided positively charged ions that appear to significantly increase the adsorption of negatively charged glycoproteins (e.g., fibronectin, vitronectin).
Expert Opinion and Five-Year View
An incredible test is to develop a biomaterial that carries on in a route identical to an autograft. The material of the scaffold needs to dissolve in a fashion, parallel to that of bone tissue regeneration. On the chance that resorption happens too rapidly, pseudoarthrosis may happen. On the other hand, if the rate of resorption happens very slowly, then bone ingrowth might be hindered and pushing again to pseudoarthrosis.
To these reasons, numerous design and fabrication processes have been adopted to modify and develop chemical and phase composition of bioactive CaP materials so that they should be ready to release of particular ions from the bone scaffold material into the surrounding space. This might influence the osteointegration process of the cell-scaffold construct. In addition, advancement in the design and fabrication of 3D porous bioactive ceramics is still needed to encourage control of graft material resorption and bone tissue regeneration in a desirable manner. Nanotechnology can give an alternative method for fabricating CaP bioceramics with increased mechanical properties and higher bioactivity, as well as resorbability. Nano-biotechnology has the capability to work with material parameters on a nuclear, subatomic, and supra-subatomic dimension. It is evident from the literature that grain size (in the nano level) of biomaterials might be a decisive factor for the enhancement of its mechanical performance. In addition, CaP bioceramics created with nanograin microstructures are characterized by prevalent bioactivity compared with traditional micrograin bioceramics.
In this manner, properties like dissolution and protein adsorption on CaP biomaterials, which are subject to surface science, energy, and roughness, can be improved to upregulate cell adhesion, proliferation, and differentiation, which in turn are collectively responsible for the osteoinductivity and osteoconductivity of CaP biomaterials.
Current Challenges and Future Directions
The mechanical stability and osteointegrity of scaffolds that must bear loads long-term are critical problems. Insufficient vascularization of the interior of thick bone substitutes, limiting cell ingrowth and survivability, is associated with poor osseointegration. Mechanical strength is heavily dependent on porosity and geometry of the scaffold, and pores and strut dimensions. Therefore, CaP biomaterials have to address this problem in a manner to solve the mechanical as well as biological issues related to scaffold tissue engineering.
CaP ceramics present a category that possesses osteoconductivity and osteoinductivity properties, making them ideal materials for bone regeneration process. The osteoinductive limit of CaP ceramics in vivo is influenced by the solubility of surface particles of CaP materials. In this perspective, both β-TCP and amorphous CaP put an impression of being osteoinductive and increase bone cell ingrowth quicker than a slowly dissolving HA.
Although the major advancement happened toward understanding the possible mechanism of CaP osteoinduction, still much work needs to be done within CaP materials toward inducing bone tissue regeneration. Primarily, tuning the physical properties of CaP free of its compositional chemistry. Secondarily, the morphological behavior of MSCs varies in the presence and absence of osteogenic supplements. CaP in the presence of osteogenic media hardly influences the osteogenic property of materials. In this regards, research involving the in vitro osteoinductive capacity of CaP without osteogenic media can give a thorough technique for investigation which assists in the interpretation of the in vivo nature of CaP materials.
In this manner, a lot of work needs to be done in understanding the adsorption of cell-glue proteins, for example, fibronectin onto CaP ceramic surfaces. Successful clinical applications of bone substitutes require an interplay among cells, biological signals, and biomaterials. Many unanswered questions and unexplored frontiers remain for the optimal use of nanostructured materials. Fundamental advances in life and materials sciences are required.
References
Woolf AD, Pfleger B (2003) Burden of major musculoskeletal conditions. Bull World Health Organ 81:646–656
Brooks PM (2002) Impact of osteoarthritis on individuals and society: how much disability? Social consequences and health economic implications. Curr Opin Rheumatol 14(5):573–577
Mistry AS, Mikos AG (2005) Tissue engineering strategies for bone regeneration. In: Regenerative medicine II. Springer, Berlin, pp 1–22
Burt DW, Law AS (1994) Evolution of the transforming growth factor-beta superfamily. Prog Growth Factor Res 5(1):99–118
Ross R, Raines EW, Bowen-Pope DF (1986) The biology of platelet-derived growth factor. Cell 46(2):155–169
Humbel RE (1990) Insulin-like growth factors I and II. Eur J Biochem 190(3):445–462
Opal SM, Depalo VA (2000) Anti-inflammatory cytokines. Chest 117(4):1162–1172
Springer IN et al (2004) Particulated bone grafts–effectiveness of bone cell supply. Clin Oral Implants Res 15(2):205–212
Naran S, Menard RM (2015) Bone grafting: physiology and techniques. In: Ferraro’s fundamentals of maxillofacial surgery. Springer, Berlin, pp 115–133
Giannoudis PV, Dinopoulos H, Tsiridis E (2005) Bone substitutes: an update. Injury 36(3):S20–S27
Radin S, Ducheyne P (1993) The effect of calcium phosphate ceramic composition and structure on in vitro behavior. II. Precipitation. J Biomed Mater Res 27(1):35–45
Nandi S et al (2010) Orthopaedic applications of bone graft & graft substitutes: a review. Indian J Med Res 132(1):15–30
Ferna E et al (1999) Calcium phosphate bone cements for clinical applications. Part I: solution chemistry. J Mater Sci Mater Med 10(3):169–176
Zeng H, Chittur KK, Lacefield WR (1999) Analysis of bovine serum albumin adsorption on calcium phosphate and titanium surfaces. Biomaterials 20(4):377–384
Zhu X et al (2010) Effect of phase composition and microstructure of calcium phosphate ceramic particles on protein adsorption. Acta Biomater 6(4):1536–1541
Boyan BD et al (1996) Role of material surfaces in regulating bone and cartilage cell response. Biomaterials 17(2):137–146
Wang C et al (2004) Phenotypic expression of bone-related genes in osteoblasts grown on calcium phosphate ceramics with different phase compositions. Biomaterials 25(13):2507–2514
Rho J-Y, Kuhn-Spearing L, Zioupos P (1998) Mechanical properties and the hierarchical structure of bone. Med Eng Phys 20(2):92–102
Gao H (2006) Application of fracture mechanics concepts to hierarchical biomechanics of bone and bone-like materials. Int J Fract 138(1-4):101
Huang S et al (2014) A novel model for porous scaffold to match the mechanical anisotropy and the hierarchical structure of bone. Mater Lett 122:315–319
Reznikov N, Shahar R, Weiner S (2014) Bone hierarchical structure in three dimensions. Acta Biomater 10(9):3815–3826
Lemons J (1993) Inorganic and organic composition for treatment of bone lesions. Google Patents
Szabo CM, Martin MB, Oldfield E (2002) An investigation of bone resorption and Dictyostelium discoideum growth inhibition by bisphosphonate drugs. J Med Chem 45(14):2894–2903
Inanç B, Elcin AE, Elcin YM (2007) Effect of osteogenic induction on the in vitro differentiation of human embryonic stem cells cocultured with periodontal ligament fibroblasts. Artif Organs 31(11):792–800
Xia Z et al (2006) In vitro biodegradation of three brushite calcium phosphate cements by a macrophage cell-line. Biomaterials 27(26):4557–4565
Choi K et al (1990) The elastic moduli of human subchondral, trabecular, and cortical bone tissue and the size-dependency of cortical bone modulus. J Biomech 23(11):1103–1113
Boivin G, Meunier PJ (2003) Methodological considerations in measurement of bone mineral content. Osteoporos Int 14(5):22–28
Fennis J, Stoelinga P, Jansen J (2004) Mandibular reconstruction: a histological and histomorphometric study on the use of autogenous scaffolds, particulate cortico-cancellous bone grafts and platelet rich plasma in goats. Int J Oral Maxillofac Surg 33(1):48–55
Silva R et al (2005) The use of hydroxyapatite and autogenous cancellous bone grafts to repair bone defects in rats. Int J Oral Maxillofac Surg 34(2):178–184
Moore WR, Graves SE, Bain GI (2001) Synthetic bone graft substitutes. ANZ J Surg 71(6):354–361
Finkemeier CG (2002) Bone-grafting and bone-graft substitutes. JBJS 84(3):454–464
Weiner S, Wagner HD (1998) The material bone: structure-mechanical function relations. Annu Rev Mater Sci 28(1):271–298
Nakase T et al (1994) Alterations in the expression of osteonectin, osteopontin and osteocalcin mRNAs during the development of skeletal tissues in vivo. Bone Miner 26(2):109–122
Florencio-Silva R et al (2015) Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int 2015:421746
Ferro F et al (2010) Biochemical and biophysical analyses of tissue-engineered bone obtained from three-dimensional culture of a subset of bone marrow mesenchymal stem cells. Tissue Eng Part A 16(12):3657–3667
Klein M et al (2009) Pore characteristics of bone substitute materials assessed by microcomputed tomography. Clin Oral Implants Res 20(1):67–74
Karageorgiou V, Kaplan D (2005) Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 26(27):5474–5491
Hannink G, Arts JC (2011) Bioresorbability, porosity and mechanical strength of bone substitutes: what is optimal for bone regeneration? Injury 42:S22–S25
Vallet-Regi M, González-Calbet JM (2004) Calcium phosphates as substitution of bone tissues. Prog Solid State Chem 32(1-2):1–31
Wopenka B, Pasteris JD (2005) A mineralogical perspective on the apatite in bone. Mater Sci Eng C 25(2):131–143
Legeros RZ (1981) Apatites in biological systems. Progr Crystal Growth Character 4(1-2):1–45
Dorozhkin SV, Epple M (2002) Biological and medical significance of calcium phosphates. Angew Chem Int Ed 41(17):3130–3146
Sun F, Zhou H, Lee J (2011) Various preparation methods of highly porous hydroxyapatite/polymer nanoscale biocomposites for bone regeneration. Acta Biomater 7(11):3813–3828
Ferreira A, Oliveira C, Rocha F (2003) The different phases in the precipitation of dicalcium phosphate dihydrate. J Cryst Growth 252(4):599–611
Tang R et al (2003) Constant composition dissolution of mixed phases: II. Selective dissolution of calcium phosphates. J Colloid Interface Sci 260(2):379–384
Nancollas G, Tomazic B (1974) Growth of calcium phosphate on hydroxyapatite crystals. Effect of supersaturation and ionic medium. J Phys Chem 78(22):2218–2225
Zhao J et al (2014) Rietveld refinement of hydroxyapatite, tricalcium phosphate and biphasic materials prepared by solution combustion method. Ceram Int 40(2):3379–3388
Carrodeguas RG, De Aza S (2011) α-Tricalcium phosphate: synthesis, properties and biomedical applications. Acta Biomater 7(10):3536–3546
Kamitakahara M, Ohtsuki C, Miyazaki T (2008) Behavior of ceramic biomaterials derived from tricalcium phosphate in physiological condition. J Biomater Appl 23(3):197–212
Cicek G et al (2011) Alpha-tricalcium phosphate (α-TCP): solid state synthesis from different calcium precursors and the hydraulic reactivity. J Mater Sci Mater Med 22(4):809–817
Nilsson M et al (2002) Characterization of a novel calcium phosphate/sulphate bone cement. J Biomed Mater Res 61(4):600–607
LeGeros RZ (2008) Calcium phosphate-based osteoinductive materials. Chem Rev 108(11):4742–4753
Yamada S et al (1997) Osteoclastic resorption of biphasic calcium phosphate ceramic in vitro. J Biomed Mater Res 37(3):346–352
Berry E (1967) The structure and composition of some calcium-deficient apatites. J Inorg Nucl Chem 29(2):317–327
Hutchens SA et al (2006) Biomimetic synthesis of calcium-deficient hydroxyapatite in a natural hydrogel. Biomaterials 27(26):4661–4670
Ravi ND, Balu R, Sampath Kumar T (2012) Strontium-substituted calcium deficient hydroxyapatite nanoparticles: synthesis, characterization, and antibacterial properties. J Am Ceram Soc 95(9):2700–2708
Klein CP et al (1990) Studies of the solubility of different calcium phosphate ceramic particles in vitro. Biomaterials 11(7):509–512
Yamada S et al (1997) Osteoclastic resorption of calcium phosphate ceramics with different hydroxyapatite/β-tricalcium phosphate ratios. Biomaterials 18(15):1037–1041
Klein C et al (1983) Biodegradation behavior of various calcium phosphate materials in bone tissue. J Biomed Mater Res 17(5):769–784
Groot KD (1988) Effect of porosity and physicochemical properties on the stability, resorption, and strength of calcium phosphate ceramics. Ann N Y Acad Sci 523(1):227–233
de Groot K (2018) Ceramics of calcium phosphates: preparation and properties. In: Bioceramics calcium phosphate. CRC, Boca Raton, FL, pp 99–114
Sun L et al (2010) Preparation and properties of nanoparticles of calcium phosphates with various Ca/P ratios. J Res Natl Inst Standards Technol 115(4):243
ŚAlósarczyk A et al (1996) Calcium phosphate materials prepared from precipitates with various calcium: phosphorus molar ratios. J Am Ceram Soc 79(10):2539–2544
Eyckmans J et al (2010) A clinically relevant model of osteoinduction: a process requiring calcium phosphate and BMP/Wnt signalling. J Cell Mol Med 14(6b):1845–1856
Samavedi S, Whittington AR, Goldstein AS (2013) Calcium phosphate ceramics in bone tissue engineering: a review of properties and their influence on cell behavior. Acta Biomater 9(9):8037–8045
Zayzafoon M (2006) Calcium/calmodulin signalling controls osteoblast growth and differentiation. J Cell Biochem 97(1):56–70
Barradas AM et al (2012) A calcium-induced signalling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials 33(11):3205–3215
LeGeros RZ (2002) Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res 395:81–98
Lu J, Yu H, Chen C (2018) Biological properties of calcium phosphate biomaterials for bone repair: a review. RSC Adv 8(4):2015–2033
Li S et al (2003) Macroporous biphasic calcium phosphate scaffold with high permeability/porosity ratio. Tissue Eng 9(3):535–548
Kitsugi T et al (1993) Four calcium phosphate ceramics as bone substitutes for non-weight-bearing. Biomaterials 14(3):216–224
Ambard AJ, Mueninghoff L (2006) Calcium phosphate cement: review of mechanical and biological properties. J Prosthodont 15(5):321–328
Sheikh Z et al (2015) Mechanisms of in vivo degradation and resorption of calcium phosphate based biomaterials. Materials 8(11):7913–7925
Benahmed M et al (1996) Biodegradation of synthetic biphasic calcium phosphate by human monocytes in vitro: a morphological study. Biomaterials 17(22):2173–2178
Aparicio JL et al (2016) Effect of physicochemical properties of a cement based on silicocarnotite/calcium silicate on in vitro cell adhesion and in vivo cement degradation. Biomed Mater 11(4):045005
Heymann D, Pradal G, Benahmed M (1999) Cellular mechanisms of calcium phosphate ceramic degradation. Histol Histopathol 14(3):871–877
Sun H et al (2006) Proliferation and osteoblastic differentiation of human bone marrow-derived stromal cells on akermanite-bioactive ceramics. Biomaterials 27(33):5651–5657
Wang J et al (1998) Biological evaluation of biphasic calcium phosphate ceramic vertebral laminae. Biomaterials 19(15):1387–1392
Yang X (2017) Hydroxyapatite: design with nature. In: Orthopedic biomaterials. Springer, Berlin, pp 141–165
Venkatesan J et al (2015) Alginate composites for bone tissue engineering: a review. Int J Biol Macromol 72:269–281
Du C et al (2000) Formation of calcium phosphate/collagen composites through mineralization of collagen matrix. J Biomed Mater Res 50(4):518–527
Mendonça G et al (2008) Advancing dental implant surface technology–from micron-to nanotopography. Biomaterials 29(28):3822–3835
Hu Q et al (2007) Effect of crystallinity of calcium phosphate nanoparticles on adhesion, proliferation, and differentiation of bone marrow mesenchymal stem cells. J Mater Chem 17(44):4690–4698
Dulgar-Tulloch A, Bizios R, Siegel R (2009) Human mesenchymal stem cell adhesion and proliferation in response to ceramic chemistry and nanoscale topography. J Biomed Mater Res A 90(2):586–594
Xu B et al (2012) RhoA/ROCK, cytoskeletal dynamics, and focal adhesion kinase are required for mechanical stretch-induced tenogenic differentiation of human mesenchymal stem cells. J Cell Physiol 227(6):2722–2729
Acknowledgements
The authors thank the Department of Ceramic Engineering and Biotechnology and Medical Engineering (NIT Rourkela, India), Dr. Sudip Dasgupta (Department of Ceramic Engineering, NIT Rourkela) for a critical review of the manuscript and for providing valuable suggestions.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Maji, K., Mondal, S. (2020). Calcium Phosphate Biomaterials for Bone Tissue Engineering: Properties and Relevance in Bone Repair. In: Li, B., Moriarty, T., Webster, T., Xing, M. (eds) Racing for the Surface. Springer, Cham. https://doi.org/10.1007/978-3-030-34471-9_20
Download citation
DOI: https://doi.org/10.1007/978-3-030-34471-9_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-34470-2
Online ISBN: 978-3-030-34471-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)