Abstract
Obesity has for decades been recognised as one of the major health concerns. Recently accumulated evidence has established that obesity or being overweight is strongly linked to an increased risk of cancer. However, it is still not completely clear how adipose tissue (fat), along with other stromal connective tissues and cells, contribute to tumour initiation and progression. In the tumour microenvironment, the adipose tissue cells, in particular the adipocytes, secrete a number of adipokines, including growth factors, hormones, collagens, fatty acids, and other metabolites as well as extracellular vesicles to shape and condition the tumour and its microenvironment. In fact, the adipocytes, through releasing these factors and materials, can directly and indirectly facilitate cancer cell proliferation, apoptosis, metabolism, angiogenesis, metastasis and even chemotherapy resistance. In this chapter, the multidimensional role played by adipocytes, a major and functional component of the adipose tissue, in promoting cancer development and progression within the tumour microenvironment will be discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Tumour microenvironment
- Obesity
- Adipocytes
- Secretosomes
- Fatty acids
- Tumorigenesis and therapeutic resistance
1 Introduction
1.1 Obesity and Cancer
Changes in the environmental factors, diets, eating habits and daily lifestyles can cause an accumulation of adipose tissue in the body. This can ultimately lead to over-weight and the development of obesity, which has multiple detrimental health impacts. It is now evident that the accumulation of adipose tissue-fat, is strongly correlated with many diseases, including cardiovascular disease, type 2 diabetes, hypertension, dyslipidemia, liver disease and also cancer (Zhang and Scherer 2018; Amin et al. 2019). This has put obesity as one of the major health concerns, particularly with the steady rise in number of obese individuals (Calle and Kaaks 2004).
Obesity is defined as a body mass index (BMI) 30 kg/m2 or above and is a pathological condition where there is excessive deposition of fat due to an imbalance between the dietary intake and energy output. The excessive energy is converted into lipids and stored primarily in the adipose tissue, ultimately leading to an increase in the mass of the individual. This storage of lipids is an efficient way to put away energy for future use, because of their high caloric values. Lipids contain double the energy content (1 g = 38 Kj) as amino acids or glucose. Triglycerides (TG) are hydrophobic and insoluble in water, thus allowing the cell to store many of them as lipid droplets (Haczeyni et al. 2018). In fasting conditions, lipids are converted into energy by a process called lipolysis (Birsoy et al. 2013). The expansion of this energy-rich storage is predominantly due to an increase in volume (hypertrophy) of adipocytes (fat cells) rather than an increase in number (hyperplasia) of adipocytes (Sun et al. 2011). With increased levels of fat deposition, individuals with excessive body weight are known to have elevated risks of cancers, including head and neck (Wang et al. 2019) breast (Picon-Ruiz et al. 2017), prostate (Mistry et al. 2007), colon (Amemori et al. 2007), liver (Calle et al. 2003; Nair et al. 2002) and ovarian cancer (Olsen et al. 2013). Moreover, cancer survival outcomes are also influenced by obesity (Calle and Kaaks 2004). Although these observations are informative, the whole picture by which adipocytes in obese individuals contribute to cancer development is only beginning to unveil. In this chapter, we will discuss the potential mechanisms by which adipocytes can provide a favourable microenvironment for cancer initiation, progression and drug resistance.
1.2 The Tumour Microenvironment
Cancers are heterogeneous tissues made up of multiple components which include tumour cells and the stromal cells in the microenvironment. The stroma itself consists of connective tissues of different cell types, which function together to provide support for organs in our body (Bremnes et al. 2011). Recently, significant attention has been directed towards the interactions between the stromal and the cancer cells in the tumour microenvironment. The properties of stromal cells are known to be modified in cancer. On the other hand, these altered and sometimes deregulated stromal cells can also influence cancer progression in a positive feedforward mechanism (Hoy et al. 2017).
1.3 Adipose Tissue and Adipocytes
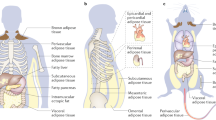
In our body, adipose tissues can broadly be classified into two main categories, the white adipose tissue (WAT) and the brown adipose tissue (BAT). The WAT is found predominantly at the subcutaneous, visceral organ and female mammary glands. Its main role is to store energy and regulate weight control. The BAT is found in supraclavicular regions and paracervical to control body temperature in response to the dietary intakes and the changes in the environmental temperature. In these adipose tissues, adipocytes are considered to be the major functional components (Duong et al. 2017) and account for over 20% of the adipose tissue cells (Suga et al. 2008). In humans, adipose tissues (fat) are found under the skin (subcutaneous fat), around internal organs (visceral fat), in bone marrow (yellow bone marrow), in muscles (muscle fat) and in the breast tissue (breast fat). This allows the adipocytes to be close to and crosstalk with many organs, such as the breast, prostate, colon and ovaries. For example, normal breast tissue is made up of mammary glands which are embedded in a stroma enriched with connective tissues (Hovey et al. 1999).
The stromal adipose tissues consist predominantly of adipocytes, but they also contain cells including preadipocytes, fibroblasts, vascular endothelial cells and immune cells, such as macrophages. These stromal adipocytes can contribute to the cancer cell development in a variety of ways (Bussard et al. 2016). Apart from being an energy provider, adipocytes also release into the tumour microenvironment various factors, such as adipokines, growth factors, hormones, collagens, fatty acids, extracellular vesicles and other metabolites, all of which can contribute to the cancer initiation, progression and therapeutic resistance (Park et al. 2011, 2013).
1.4 Cancer-Associated Adipocytes (CAAs)
In normal breast tissue, the stroma separates the mammary glands from the adipocytes. However, during tumour development, the breast tissue undergoes extracellular matrix remodelling, resulting in the adipocytes being in closer proximity to the mammary glands (Wang et al. 1975). Similar processes are also detected during the development of other solid tumours, including that of the ovarian and prostate cancer (Finley et al. 2009; Kristin et al. 2011). This close proximity between the adipocytes and the cancer cells has profound impacts on the adipocyte development (Dirat et al. 2011). In fact, adipocytes are transformed by proximal cancer cells into cancer-associated adipocytes (CAAs) to acquire an activated phenotype that contributes to cancer invasion and progression (Dirat et al. 2011). For instance, adipocytes cultivated with breast cancer cells exhibit an activated phenotype characterized by the overexpression of proteases, such as matrix metalloproteinase (MMP)-11, and proinflammatory cytokines, including interleukin (IL)-6 and IL-1β. In agreement, histological studies also showed that adipocytes situated close to the larger tumours and/or with enhanced local invasion express higher levels of the proinflammatory cytokine IL-6 (Dirat et al. 2011). Another study using an ovarian cancer and adipocyte co-culture system as well as a mouse model also showed that adipocytes promote homing, migration and invasion of ovarian cancer cells, through overexpressing adipokines, including IL-8 (Kristin et al. 2011). The study also revealed that co-culturing with adipocytes induce the ovarian cancer cells to express the fatty acid transporter FABP4, which has a key role in promoting ovarian cancer metastasis (Kristin et al. 2011).
To date, many studies have focused on determining the role of adipose tissues, in particular adipocytes, in cancer initiation and progression. However, this was only started recently in 1992, when co-transplantation studies using murine models showed that mammary carcinoma cells grow better with fat fragments. This finding suggests that adipose tissue plays an integral part in cancer development (Elliott et al. 1992). Later in 2003, another study revealed that only mature adipocytes could facilitate tumour growth in estrogen receptor positive (ER+) breast cancer cell lines using a collagen gel matrix culture system. This differentiates the mature adipocytes from pre-adipocytes in their contributions to the cancer progression, and highlights adipocytes as a key cancer promoting component in the tumour microenvironment (Manabe et al. 2003). Consistent with this finding, a further study in 2011 demonstrated that human adipocytes can promote the growth of ovarian cancer cells both in vivo and in vitro (Kristin et al. 2011). This finding narrows down further the mechanisms by which adipose tissue affects cancer growth. Likewise, similar observations were documented in the colon and prostate cancer and demonstrated a role for adipocytes (Tokuda et al. 2003; Aoki et al. 2007). Furthermore, another study also showed that CAAs contribute to breast cancer invasion (Dirat et al. 2011). However, recently in 2015, an in vivo study using breast tumours revealed that the estrogen receptor negative (ER-) breast tumours in close proximity to the adipose tissues have a low mitotic index (Han Suk et al. 2015). This observation is at odds with the results from other findings which suggested that CAAs contribute to cancer progression (Dirat et al. 2011; Kristin et al. 2011; Tokuda et al. 2003; Aoki et al. 2007). Nevertheless, the general role of the adipocytes in the cancer progression can be influenced by the tumour-type and other factors in the microenvironment.
These added layers of complexity indicate that despite plenty of evidence showing that tumour growth can be promoted by the presence of adipocytes, the complete picture of the relationships between adipocytes and cancer cells in the tumour is only beginning to be revealed.
2 Role of Adipocytes and Their Secreted Factors in Cancer Development
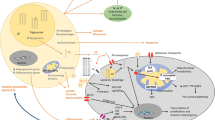
For years, adipocytes have been thought of as passive energy storage depots. However, subsequent research has revealed that adipocytes also act as the sources for endocrine and paracrine factors (Darimont et al. 1994; Amri et al. 1994). These secreted factors are also known as adipokines and they consist of cytokines, chemokines, hormones and other growth factors. Other adipocyte-derived materials include fatty acids and other metabolites, which play important parts in facilitate metabolic crosstalks between adipocytes and the cancer cells (Fig. 7.1). These factors function both locally and systemically to play distinct roles in the cancer proliferation, growth, invasion, angiogenesis and metabolism as well as therapeutic resistance (Duong et al. 2017).
Crosstalks between adipocytes and cancer cells
Adipocytes secretes various adipokines and other factors to promote cancer progression. Specifically, the ratio of leptin and adiponectin plays an important role to the survival of cancer cells. At the same time, cancer cells drive adipocytes lipolysis to generate free fatty acids for themselves
2.1 Leptin
Leptin is a hormone that helps to control energy intake and the body weight (Zhang et al. 2005). It is produced mainly by the adipocytes, released into the bloodstream, and received by the hypothalamus in the brain. High leptin levels cause a reduction in energy/food intake, while low levels stimulate an increase in energy/food intake and fat storage. In obese individuals, the secretion of leptin is elevated due to the increase in adipose tissues, but the brain becomes insensitive to high leptin levels. This high levels of leptin have also been found to be accompanied by the overexpression of leptin receptors in many cancer cells, such as breast and ovarian cancer (Ishikawa et al. 2004; Uddin et al. 2009).
Leptin has also been shown to function as a growth factor for cancer cells (Endo et al. 2011). Consistent with this idea, the elevated levels of leptin have been found to promote cancer cell proliferation through the activation of the extracellular signal-regulated kinase1/2 (ERK1/2) and c-Jun N-terminal kinase (JNK) signalling pathways (Garofalo and Surmacz 2006). This concept is further supported by three-dimensional collagen gel co-culture studies using colon cancer cells and adipocytes from leptin-deficient mice, showing that the trophic effects induced by adipocytes are abolished in leptin-deleted mice (Aoki et al. 2007). In concordance, the mammary and colorectal tumour growth is retarded in obese mice-deficient for leptin or its receptor (Endo et al. 2011; Qiao et al. 2011). Similarly, the prostate tumours induced in leptin receptor-deficient mice are significantly smaller (Ribeiro et al. 2010). However, the prostate tumours induced in the leptin-deleted mice were significantly larger. This discrepancy may be attributed to the differences in the local tumour microenvironment and the fact that leptin is also a critical regulator of the development and activation of natural killer (NK) cells (Tian et al. 1959), which have the ability to detect and kill tumour cells (Wu and Lanier 2003). Nonetheless, in breast cancer cells, leptin has been shown to promote cancer cell proliferation in the tumour microenvironment via activating the phosphoinositide 3-kinase (PI3K)-Akt signalling pathway and pyruvate kinase M2 expression, which are important for cell proliferation and epithelial-mesenchymal transition (EMT) (Wei et al. 2016; Qiao et al. 2011). Consistently, the elevated proliferative effects of adipocytes on cancer cells are lowered when leptin expression is depleted using short hairpin (sh) RNA (Amy et al. 2015). Collectively, these findings show leptin produced by adipocytes play a critical role in stimulating cancer cell proliferation.
2.2 Adiponectin
Contrary to leptin, adiponectin is an adipokine whose plasma concentrations are significantly lower in obese individuals than in non-obese subjects (Arita et al. 1999). Exposure of cancer cells to adiponectin also causes the cancer cells to cease proliferation and undergo apoptosis, suggesting an anti-tumour role in adiponectin (Kang et al. 2005; Dieudonne et al. 2006; Ishikawa et al. 2007). In agreement, adiponectin depletion was shown to promote human breast cancer growth in nude mouse xenograft models, through activating the glycogen synthase kinase-3β/β-catenin signalling pathway (Wang et al. 2006). Subsequently, it was discovered that adiponectin also restricts cancer cell growth through activating the 5’ AMP-activated protein kinase (AMPK) as well as inhibiting the AKT, ERK1/2, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kappaB) and Wnt pathways (Dalamaga et al. 2012). Interestingly, adipocytes from the tumour microenvironment are less differentiated and secrete significantly lower amounts of adiponectin than adipocytes from normal microenvironment (Fletcher et al. 2017). As both leptin and adiponectin are antagonistic adipokines secreted by the adipocytes, the ratio of these adipokines may influence the progression of the cancers. Indeed, the balance between leptin and adiponectin is often shifted in obese individuals (Al-Hamodi et al. 2014). These observations are further supported by clinical studies showing a positive correlation between leptin/adiponectin ratio and cancer risk (Ashizawa et al. 2010).
2.3 Insulin-Like Growth Factor 1 (IGF-1)
Another key cytokine secreted by adipocytes is insulin-like growth factor 1 (IGF-1). IGF-1 is strongly associated with cell proliferation and survival. As it is produced by adipocytes, the level of free IGF-1 is appropriately strongly associated with obesity (Nam et al. 1997). IGF-1 is an important regulator of energy metabolism and growth (Pollak 2008). The binding of the IGF-1 to its receptors activates primarily the PI3K-AKT and Mitogen-activated protein kinases (MAPK) pathways to promote cancer cell growth and progression (Pollak 2008). The concentration of circulating IGF-1 has also been linked to increased cancer risk (Shanmugalingam et al. 2016). Conversely, inhibition of IGF-1 receptor kinase activity limits the growth-promoting effect of adipocytes on cancer cells (D’Esposito et al. 2012). Thus, in summary, the secretion of IGF-1 by adipocytes has a direct role in stimulating the proliferation and survival of cancer cells in the tumour microenvironment and will have direct impact on cancer initiation and progression.
2.4 Vascular Endothelial Growth Factor (VEGF) and Cancer Angiogenesis
When a tumour grows beyond 1–2 mm3, it requires extra blood vessels to supply nutrients, oxygen and growth factors for continuous proliferation and survival. In the absence of new blood vessel formation, the expanding tumours may become starved of oxygen (a condition termed hypoxia), nutrients and growth factors and undergo necrosis or even apoptosis (Muz et al. 2015). Thus, the formation of the new blood vessels, a phenomenon known as angiogenesis, is crucial for tumour expansion (Nishida et al. 2006). Angiogenesis is tightly regulated in the microenvironment by both the cancer and the stromal cells. The adipocytes can promote new vessels formation by secreting adipokines (Cao 2013). VEGF is a vital mediator of angiogenesis (the formation of new blood vessels) in cancer (Fig. 7.2) (Nishida et al. 2006), and it binds VEGF receptors which are expressed on the surface of vascular endothelial cells. In response to insulin, adipocytes secrete vascular endothelial growth factor A (VEGFA) to promote angiogenesis (Mick et al. 2002). Furthermore, leptin released by adipocytes also drives endothelial cell differentiation and proliferation (Gonzalez-Perez et al. 2010). Previous studies also showed that VEGF can also be upregulated by leptin in breast cancer cells through the transcription factors HIF-1 and NF-κB (Gonzalez-Perez et al. 2010). However, unlike leptin, the effects of adiponectin in angiogenesis are not clear-cut. Some studies showed adiponectin as a pro-angiogenesis factor, while other studies demonstrated that it has the opposite effect (Rei et al. 2004; Man et al. 2010).
2.5 Free Fatty Acids, Metabolites and the Warburg Effect
Cancer cells reprogramme their energy metabolism to promote their growth, survival, proliferation, and self-renewal. The hallmark of this altered cancer metabolism (also called Warburg effect) to yield the extra energy needed is to increase glucose uptake and generate energy through glucose to lactate fermentation in anaerobic glycolysis, even in the presence of sufficient oxygen (Warburg 1956). In other words, cancer cells prefer to produce adenosine triphosphate (ATP) via glycolysis instead of oxidative phosphorylation (OXPHO). The cancer-associated adipocytes have been shown to have increased secretion of inflammatory cytokines, such as TNFα, IL-6 and IL-1β, and MMP-11, which play a key part in cancer energy metabolism by promoting metabolic switch in tumours (Ribeiro et al. 2012; Dali-Youcef et al. 2016; Dirat et al. 2011). In the tumour microenvironment, lactate and amino acids are the alternative external sources of energy available for cancer cells (Duong et al. 2017). Lately, the reverse Warburg effect has also been proposed, in which the cancer cells induce the CAFs to undergo aerobic glycolysis to produce the metabolic by-products, such as lactate and pyruvate, for the consumption of cancer cells. This induction also applies to the other stromal cells, including adipocytes, found in the tumour microenvironment (Pavlides et al. 2009). Specifically, under hypoxic conditions, lactate is secreted by these stromal cells through monocarboxylate transporters (MCTs) into the tumour microenvironment (Gonzalez-Perez et al. 2010). The secreted lactate is then imported and metabolised by the cancer cells to produce energy and other essential metabolites (Pavlides et al. 2009).
Nevertheless, many studies have now confirmed that free fatty acids (FFAs) are in fact the primary source of energy for cancer cells delivered from adipocytes. In terms of lipid and energy metabolism, the principal function of adipocytes is to store triglyceride and release fatty acids (FAs) for other cells and tissues when needed. In vitro and in vivo studies have shown that cancer cells can induce lipolysis and FA production in adipocytes. A recent study showed that CAAs have enhanced potentials to increase their lipid synthesis capacity and to breakdown the lipids by hydrolysis to release FAs, a process termed lipolysis (Balaban et al. 2017). Subsequently, the cancer cells will import the secreted FFAs from their microenvironment and use them for energy production or store them as triglycerides in lipid droplets (Young and Zechner 2013). Notably, the majority of the FFAs secreted by the adipocytes and imported by the cancer cells have been found to be long chain FAs, such as palmitic acid (Kwan et al. 2014). Interestingly, the FA transfer to cancer cells is further enhanced in “obese” adipocytes (FAs supplemented adipocytes) compared with normal adipocytes (Balaban et al. 2017). In addition to supplying the FAs, the adipocytes can stimulate the cancer cells to express higher levels of carnitine palmitoyltransferase 1A (CPT1A) and electron transport chain proteins to elevate their rates of fatty acid β-oxidation (FAO) (Fig. 7.2) (Balaban et al. 2017). Furthermore, the adipocytes also induce the cancer cells to release the stored FAs from their lipid droplets intracellularly through the adipose triglyceride lipase (ATGL)-dependent lipolytic pathway for FAO (Wang et al. 2017b).
In ovarian cancer, the uptake of FFAs is associated with an increased rate of FAO to generate a large amount of energy in the form of adenosine triphosphate (ATP) (Kristin et al. 2011). Similarly, FFAs imported have been shown to be used for FAO to generate energy to promote cell proliferation and migration in breast cancer (Balaban et al. 2017). However, recent breast cancer studies reported that the import of FFAs do not lead to the ATP production via FAO for cell proliferation or survival but cancer cell invasion (Wang et al. 2017b). In concordance with this, FFAs have been shown to enhance breast cancer cell migration through inducing the expression of plasminogen activator inhibitor-1 via SMAD4 (Byon et al. 2009). Moreover, a study following FA transfer from bone marrow adipocytes to metastatic prostate cancer cells also showed a shift in intracellular energy production from FAO to lactate production (Diedrich et al. 2016). The study also revealed that this shift to Warburg phenotype is mediated by the hypoxia-inducible factor 1α (HIF-1α) (Diedrich et al. 2016). Nevertheless, these findings collectively suggest that FFAs secreted by adipocytes into the tumour microenvironment could provide distinct effects under different conditions, but ultimately they all function to promote cancer progression and metabolism (Diedrich et al. 2016).
2.6 FABP4 (A-FABP)
Fatty acid binding protein 4 (FABP4) is a member of the FABP family of proteins involved in FA import, storage and export as well as cholesterol and phospholipid metabolism (Chmurzynska 2006; Furuhashi and Hotamisligil 2008). FABP4 expression itself is induced by high-fat diet (HFD) or obesity in adipocytes in the tumour microenvironment (Huang et al. 2017). Moreover, co-culture experiments also showed that adipocytes can also induce cancer cells to upregulate FABP4 expression (Kristin et al. 2011). FABP4 has many roles in cancer progression. FABP4 can enhance cancer progression by upregulating MMPs and stromal cell cytokine production (Huang et al. 2017). FABP4 also promotes cancer proliferation through inducing the expression of Forkhead box transcription M1 (FOXM1), a key transcription factor involved in driving cancer progression and drug resistance (Yao et al. 2018; Guaita-Esteruelas et al. 2017). The role of FABP4 in promoting cancer proliferation and migrating appears to be associated with its ability to facilitate FA metabolism in both adipocytes and cancer cells. A study showed that pharmaceutical inhibition of FABP4 in ovarian cancer cells lowered their lipid droplet accumulation and their metastatic and growth potentials. Likewise, FABP4-deletion in mice also impairs the metastatic tumour growth, indicating that FABP4 has a key role in cancer metastasis (Kristin et al. 2011). Moreover, FFAs secreted by adipocytes can elevate prostate cancer invasion, which can be reduced by pharmaceutical inhibition of the fatty acid transporter FABP4 (Herroon et al. 2013). A most recent study revealed that FABP4 also has a key role in mediating lipolysis (Hua et al. 2019). Besides FABP4, current research also showed that CAAs can also enhance lipid metabolism in breast cancer and melanoma cells by inducing the expression of another fatty acid transporter FATP1 (Lopes-Coelho et al. 2018; Zhang et al. 2018). Thus, in addition to being an energy source to fuel the Warburg effect in cancer, the adipocyte-derived FAs also function as a signalling molecule to drive the phenotypic changes in cancer to drive cancer progression within the tumour environment (Furuhashi and Hotamisligil 2008).
2.7 Matrix Metalloproteinases (MMPs)-Extracellular Matrix Remodelling
Apart from cancer growth and energy metabolism, adipocytes also support tumour progression by remodelling the extracellular matrix in the tumour microenvironment to facilitate cancer cell invasion and metastasis. Cancer cells can induce the adipocytes to secrete collagen VI which in turn promotes cancer cell survival in a paracrine positive feedback fashion (Petricoin Iii et al. 2005). Notably, a cleaved product of collagen VI, known as endotrophin, has also been shown to stimulate epithelial-mesenchymal transition (EMT) and cell metastasis (Park and Scherer 2012). To remodel the extracellular matrix, adipocytes also release degradation enzymes known as matrix metalloproteinases (MMPs) to facilitate cancer cell invasion and metastasis (Fig. 7.2) (Carine et al. 2003). Cancer cells can also induce the adipocytes to produce MMP-11 to facilitate the extracellular matrix remodelling and this enhances their invasion of the adipose tissue, highlighting the importance of MMP-11 in extracellular matrix remodelling and cancer invasion (Motrescu and Rio 2008). In support of this finding, small interfering ribonucleic acid (siRNA) mediated MMP-11 depletion causes a reduction in cancer metastasis. siRNA targeted against MMP-11 can restrict the of cancer cells to invade and metastasize to local lymph nodes (Jia et al. 2007). Indeed, high levels of MMP-11 expression are associated with cancer cell invasion and poor prognosis (Rouyer et al. 1994). Apart from secreting MMPs directly, adipocytes also release leptin to enhance the release of MMPs by the cancer cells to facilitate cancer invasion (Yeh et al. 2009).
After the local invasion, adipocytes also support cancer cells migration and seeding at the distant site. In fact, the adipose tissue is a preferential site for many metastatic cancers. This is true as adipocytes secrete many cytokines, including IL-8 in favour of the cancer cell survival (Kristin et al. 2011). An in vitro experiment using mice showed that inhibition of IL-8 receptors, particularly CXCR1 could reduce the homing of ovarian cancer cells towards adipocytes (Kristin et al. 2011). Similar observations were also found in the acute lymphoblastic leukaemia (ALL). For leukaemic cell migration, SDF-1 has been identified as a chemoattractant released by adipocytes. This leukaemic migration towards adipocytes could be blocked when SDF-1 receptors on the leukaemia cells were inhibited (Pramanik et al. 2012).
2.8 Inflammatory Cytokines-Cancer Metastasis
Metastasis is the spread and establishment of secondary cancer growths at a distal site from the primary cancer and the major cause of cancer deaths. The locations of future metastasis are predetermined microenvironments called ‘pre-metastatic niches’ (PMNs), and adipocytes also play an essential role in the homing of these metastasizing cancer cells (Peinado et al. 2017). Indeed, primary human omental adipocytes promote the homing, migration and invasion of metastasising ovarian cancer cells, through releasing adipokines including IL-6 and IL-8 (Kristin et al. 2011). The lipids, predominantly FFAs, released by resident adipocytes also serve as a source of energy to promote the growth and proliferation of the homing cancer cells (Kristin et al. 2011).
In terms of metastasis, adipocytes can induce the cancer cells to undergo an incomplete EMT, a crucial process involved in the development of an invasive and metastatic cell phenotype. In adipocyte-breast cancer co-culture studies, the cancer cells displayed reduced expression of the epithelial marker, E-cadherin, without a significant increase in mesenchymal marker expression (Dirat et al. 2011). However, subsequent experiments revealed that conditioned media from CAAs alone are enough to increase cancer cell invasiveness (Dirat et al. 2011). Experiments using the co-culture system or conditioned media with breast cancer cells also demonstrated that adipocytes have an ability to enhance the cancer cell proliferation, invasion and migration through Jak/STAT3 signalling pathway (Lapeire et al. 2014). Similar outcomes are also observed when the experiments are performed using xenograft models and 3D culture systems (Laetitia et al. 2013; Brian et al. 2014). Consistent findings were observed between adipocytes and prostate cancer cells (Abel 2012). To promote cancer invasion, adipocytes have been demonstrated to release the pro-inflammatory cytokine IL-6 into the tumour microenvironment. This level of IL-6 is directly correlated with the cancer aggressiveness, and its inhibition using an IL-6 antibody could suppress the invasiveness of the cancer cells (Dirat et al. 2011). Moreover, the secreted IL-6 can also lead to the local inflammation and activation of immune cells in the tumour microenvironment (Wright and Simone 2016). The IL-6-activated macrophages can further produce and secrete more inflammatory cytokines to promote further cancer metastasis (McNelis and Olefsky 2014). Apart from IL-6, leptin has also been identified as a promoter for breast cancer cell invasion. In fact, breast cancer-associated adipocytes also secrete proinflammatory cytokines, including IL-6, IL-8, IFNγ-inducible protein-10 (IP10, also called CXCL10), CCL2 (MCP1), and CCL5 (RANTES), to drive tumour-initiating cell abundance and metastatic progression (Picon-Ruiz et al. 2016).
2.9 Extracellular Vesicles
Tumour cells communicate with their microenvironment not only via soluble and secreted factors but also through extracellular vesicles (EVs). These EVs, which can be released by both cancer and stromal cells, are further classified into exosomes, microvesicles (MVs), and apoptotic bodies (ABs). There is a key function of extracellular vesicles in the establishment and maintenance of the tumour microenvironment (Han et al. 2017, 2019). These EVs facilitate bioactive cargo transfer between cancer and stromal cells in the tumour microenvironment and play essential roles in maintaining cell proliferation, evading growth suppression, resisting cell death, acquiring genomic instability and reprogramming stromal cell lineages and cancer cells, together contributing to the generation of a remodelled TME. For example, EVs secreted by cancer cells are involved in the transfer of IL-6 to activate the STAT3 signalling pathway to induce lipolysis and the generation of FFAs in adipocytes in lung cancer-adipocyte co-culture models (Hu et al. 2019). Conversely, Circular RNAs (circRNAs) secreted in exosomes by adipocytes promote the tumour growth by inhibiting deubiquitination in hepatocellular carcinoma (HCC) (Zhang et al. 2019).
3 Adipocytes Crosstalk with Other Stromal Cells in the Tumour Environment
Cancer-associated fibroblasts (CAFs) are known to play a vital role in cancer development and progression through the release of growth factors and chemokines and by involving in the remodelling of the extracellular matrix (Orimo et al. 2005; Buchsbaum and Oh 2016; Bochet et al. 2013). In the tumour microenvironment, the proximal localization of cancer cells to adipocytes can cause the adipocytes to undergo phenotypical changes to generate fibroblast-like cells termed adipocyte-derived fibroblasts (ADFs). These ADFs exhibit CAF-like phenotypes, including augmented fibronectin and collagen I secretion, enhanced migratory/invasive abilities, and enhanced expression of the CAF marker FSP-1 (Bochet et al. 2013). This may ultimately contribute to the number and the function of cancer-associated fibroblasts (CAFs) in the tumour environment. In addition, obese individuals also have more pre-adipocytes, macrophages and monocytes deposited in the adipose tissue. These changes to the microenvironment of obese individuals may promote cancer development (Wang et al. 2017a; Wang et al. 2017b). Adipose stem cells (ASCs) are known to alter the microenvironment and promote cancer progression. Accordingly, ASCs induce local inflammation through TGF-beta signalling pathway to recruit immune cells (Razmkhah et al. 2011). Moreover, ASCs can also promote angiogenesis by the platelet-derived growth factor BB/platelet-derived growth factor receptor-β (PDGF-BB/PDGFR-β) signalling pathway (Gehmert et al. 2010). Apart from the induction of inflammation and angiogenesis, co-culture and conditioned medium experiments revealed that ASCs also enhance EMT (Zimmerlin et al. 2011). In addition, ASCs can be differentiated into proliferation-promoting fibroblasts in a variety of cancers, including those of the breast, ovarian and lung, to accelerate cancer progression (Jotzu et al. 2010).
4 Therapeutic Resistance
Drug resistance is a major obstacle to effective cancer chemotherapy and is directly associated with limited therapeutic options and poor prognosis in cancer patients. Reduced or lack of drug responses are observed in obese cancer patients (Chen et al. 2012; Horowitz and Wright 2015), and there are plenty of examples to suggest that the cancer-associated adipocytes are involved in conferring resistance to therapeutic treatments. For instance, adipocytes secrete adipokines such as leptin and growth differentiation factor 15 (GDF15) to block the growth inhibitory action of trastuzumab in HER2-positive cancers (Griner et al. 2013). Adipocytes also produce leptin to promote melanoma drug resistance through the upregulation of pro-survival PI3K/Akt and MEK/ERK signalling pathways (Chi et al. 2014). A similar chemoprotective effect by adipocytes through leptin was also observed in colon cancer (Bartucci et al. 2010). Adipocytes impair the cytotoxic effects of the drug vincristine through elevating pro-survival signals such as Bcl-2 and Pim-2 in the leukaemic cells (Behan et al. 2009). Moreover, obesity-associated adipocytes also promote breast cancer chemotherapy resistance through releasing major vault protein (MVP), a suppressor of NF-κB signalling (Lehuede et al. 2019). L-asparaginase (ASNase) is a first-line therapy for acute lymphoblastic leukaemia (ALL) that breaks down the essential metabolic substrates asparagine and glutamine, which drive cancer metastasis (Luo et al. 2018). In this respect, adipocytes can release glutamine to the cancer microenvironment and provide the essential amino acid to the cancer cells directly to sustain proliferation and protein synthesis and lessen the cytotoxic effects of asparagine and glutamine shortage (Ehsanipour et al. 2013). Furthermore, another study reported that hypoxia conditions enhance the adipocyte-protection on breast cancer cells from chemotherapeutic toxicity (Rausch et al. 2017). Adipocytes in the bone marrow microenvironment can protect myeloma cells against chemotherapy through releasing adipokines, such as leptin and adipsin, to promote autophagy and therefore chemoresistance in the myeloma cells (Liu et al. 2015). In summary, the cancer-associated adipocytes can enhance cancer therapeutic resistance through upregulating the survival signalling pathways and DNA-repair activity (Shimizu et al. 2014) in the cancer cells and by providing metabolic substrates to boost cancer proliferation and survival.
5 Targeting Adipocyte Signalling and Other Therapeutic Perspectives
Recent data has shown that the mechanisms of energy metabolism are dysregulated in cancer. Because of the need to meet the high energy demands, tumour cells shift to the Warburg phenotype and preferentially import an excess of glucose and convert it to lactate for energy (ATP) production. At the same time, unlike their normal counterparts, the cancer cells are also less able to use lipids, like FAs, to generate ATP production via FAO (Diedrich et al. 2016; Wang et al. 2017b) because of the lower levels of FA metabolic enzymes in mitochondria of the cancer cells (Vidali et al. 1983). The low-carbohydrate, high-fat ketogenic diet is an example where our knowledge of cancer energy metabolism has been successfully translated into treatment strategies. The ketogenic diet has been designed to mimic the effects of glucose starvation and is based on the fact that cancer cells fail to adapt to glucose shortage and use lipids/fatty acids as alternative energy sources. On this diet, the cancer cells will rapidly consume intracellular energy reserves through glycolysis because of the Warburg effect. Therefore, the ketogenic diet has the potential to be an effective cancer diet therapy. In support, experiments with animal models showed that the ketogenic diet significantly reduced the growth of tumours and that the diet prolonged animal survival (Raphael Johannes et al. 2015; Kennedy et al. 2007). These findings are also well correlated with a reduction in the plasma glucose concentrations and cancer cellular proliferation markers in animal models (Martuscello et al. 2016). While the ketogenic diet works relatively well in the short term, the long-term health effects of the diet remain to be determined, as there is plenty of evidence to suggest that an accumulation of adipose tissue (fat) in the tumour microenvironment will accelerate cancer progression. Specifically, the cancer-associated adipocytes can modify the cancer cell phenotype to utilise alternative energetic nutrients to replace glucose, as well as essential metabolites, like FAs and glutamate, as energy sources to sustain cancer cell proliferation, survival and progression. In consequence, another potential strategy is to target the crosstalk between adipocytes and the cancer cells, in particular the fatty acid transporters, such as FABP4 which plays a pivotal role in fatty acid metabolism and transport in both the adipocytes and the cancer cells. Indeed, the highly specific FABP4 inhibitor, BMS309403, has been shown to be able decreased tumour cell proliferation and migration by downregulating HIF1 pathway in hepatocellular carcinoma (HCC) and reduce tumour growth in heterotopic and orthotopic xenografted mice models (Laouirem et al. 2019). In addition, prostate stromal cells can augment cancer cell invasiveness by secreting IL-8 and IL-6, which can be also abrogated by BMS309403. Thus, targeting the crosstalk between adipocytes and the cancer cells or vulnerabilities arisen as a result may provide novel strategies of cancer treatment and for overcoming drug resistance.
Moreover, FABP4 expression is frequently elevated in breast cancer and has been shown to be a potential good diagnostic and poor prognostic marker in patients with breast cancer (Cui et al. 2019).
6 Conclusion and Future Perspectives
Tumorigenesis and cancer progression require the interaction of tumour cells with the surrounding tissues in the tumour microenvironment. The contribution of adipose tissue, in particular adipocytes to the development of cancer has help us to establish a link between obesity and cancer. The proximal location of the cancer cells can dramatically modify the adipocytes to a pro-cancer phenotype. Over the past few decades the rates of obesity have risen at a dramatic rate globally (Chooi et al. 2019). Obesity and being over-weight further condition the function of the cancer-associated adipocytes. Thus, targeting the crosstalks between adipocytes and the cancer cells or vulnerabilities arisen as a result of these interactions may provide novel strategies of cancer treatment and for overcoming drug resistance. Nevertheless, further work will be required to obtain a more complete understanding of the mechanisms by which adipocytes, in conjunction with other stomal cells, promote cancer initiation, progression and drug resistance (Table 7.1). This information will allow us to identify biomarkers for early cancer risk prediction and diagnosis as well as targets and opportunities for therapeutic intervention.
References
Abel M (2012) Human periprostatic adipose tissue promotes prostate cancer aggressiveness in vitro. Cambridge Scholars Publishing, Newcastle upon Tyne
Al-Hamodi Z, Al-Habori M, Al-Meeri A, Saif-Ali R (2014) Association of adipokines, leptin/adiponectin ratio and C-reactive protein with obesity and type 2 diabetes mellitus. Diabetol Metab Syndr 6:99
Amemori S, Ootani A, Aoki S, Fujise T, Shimoda R, Kakimoto T, Shiraishi R, Sakata Y, Tsunada S, Iwakiri R, Fujimoto K (2007) Adipocytes and preadipocytes promote the proliferation of colon cancer cells in vitro. Am J Physiol Gastrointest Liver Physiol 292:G923–G929
Amin MN, Hussain MS, Sarwar MS, Rahman Moghal MM, Das A, Hossain MZ, Chowdhury JA, Millat MS, Islam MS (2019) How the association between obesity and inflammation may lead to insulin resistance and cancer. Diabetes Metab Syndr 13:1213–1224
Amri EZ, Ailhaud G, Grimaldi PA (1994) Fatty acids as signal transducing molecules: involvement in the differentiation of preadipose to adipose cells. J Lipid Res 35:930–937
Amy LS, Jason FO, Brandi AB, Lyndsay VR, Dorothy TP, Tucker HA, Claire L, Annie CB, Maria FD, Shijia Z, Jeffrey MG, Matthew EB, Bruce AB (2015) Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res 17:112
Aoki S, Tsunada S, Ootani A, Shimoda R, Sakata Y, Kakimoto T, Fujise T, Iwakiri R, Fujimoto K, Amemori S, Shiraishi R (2007) Adipocytes and preadipocytes promote the proliferation of colon cancer cells in vitro. Am J Physiol 292:G923
Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J-I, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y (1999) Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257:79–83
Ashizawa N, Yahata T, Quan J, Adachi S, Yoshihara K, Tanaka K (2010) Serum leptin–adiponectin ratio and endometrial cancer risk in postmenopausal female subjects. Gynecol Oncol 119:65–69
Balaban S, Shearer RF, Lee LS, Van Geldermalsen M, Schreuder M, Shtein HC, Cairns R, Thomas KC, Fazakerley DJ, Grewal T, Holst J, Saunders DN, Hoy AJ (2017) Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab 5:1
Bartucci M, Svensson S, Ricci-Vitiani L, Dattilo R, Biffoni M, Signore M, Ferla R, De Maria R, Surmacz E (2010) Obesity hormone leptin induces growth and interferes with the cytotoxic effects of 5-fluorouracil in colorectal tumor stem cells. Endocr Relat Cancer 17:823–833
Behan JW, Yun JP, Proektor MP, Ehsanipour EA, Arutyunyan A, Moses AS, Avramis VI, Louie SG, Butturini A, Heisterkamp N, Mittelman SD (2009) Adipocytes impair leukemia treatment in mice. Cancer Res 69:7867–7874
Birsoy K, Festuccia WT, Laplante M (2013) A comparative perspective on lipid storage in animals. J Cell Sci 126:1541–1552
Bochet L, Lehuede C, Dauvillier S, Wang YY, Dirat B, Laurent V, Dray C, Guiet R, Maridonneau-Parini I, Le Gonidec S, Couderc B, Escourrou G, Valet P, Muller C (2013) Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res 73:5657–5668
Bremnes RM, Donnem T, Al-Saad S, Al-Shibli K, Andersen S, Sirera R, Camps C, Marinez I, Busund LT (2011) The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J Thorac Oncol 6:209–217
Brian GR, Jeffrey MG, Mei S, Muralidharan A, Ryan KJ, Trivia PF, Majdouline A, Eduardo AL, Paul LF, Robert K, Ernest SC (2014) Human adipose tissue-derived stromal/stem cells promote migration and early metastasis of triple negative breast cancer xenografts. PLoS One 9:e89595
Buchsbaum RJ, Oh SY (2016) Breast cancer-associated fibroblasts: where we are and where we need to go. Cancers (Basel) 8(2):19
Bussard KM, Mutkus L, Stumpf K, Gomez-Manzano C, Marini FC (2016) Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res 18:84
Byon CH, Hardy RW, Ren C, Ponnazhagan S, Welch DR, Mcdonald JM, Chen Y (2009) Free fatty acids enhance breast cancer cell migration through plasminogen activator inhibitor-1 and SMAD4. Lab Invest 89:1221–1228
Calle EE, Kaaks R (2004) Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4:579–591
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S adults. N Engl J Med 348:1625–1638
Cao Y (2013) Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab 18:478–489
Carine C, Bernard M, Marie-Noëlle M, Stéphanie B, Patrick A, Emmanuel Van O, Sophie T-D (2003) Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J Biol Chem 278:11888–11896
Chen S, Chen C-M, Zhou Y, Zhou R-J, Yu K-D, Shao Z-M (2012) Obesity or overweight is associated with worse pathological response to neoadjuvant chemotherapy among Chinese women with breast cancer. PLoS One e41380:7
Chi M, Chen J, Ye Y, Tseng H-Y, Lai F, Tay KH, Jin L, Guo ST, Jiang CC, Zhang XD (2014) Adipocytes contribute to resistance of human melanoma cells to chemotherapy and targeted therapy. Curr Med Chem 21:1255–1267
Chmurzynska A (2006) The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet 47:39–48
Chooi YC, Ding C, Magkos F (2019) The epidemiology of obesity. Metabolism 92:6–10
Cui Y, Song M, Kim SY (2019) Prognostic significance of fatty acid binding protein-4 in the invasive ductal carcinoma of the breast. Pathol Int 69:68–75
D’esposito V, Passaretti F, Hammarstedt A, Liguoro D, Terracciano D, Molea G, Canta L, Miele C, Smith U, Beguinot F, Formisano P (2012) Adipocyte-released insulin-like growth factor-1 is regulated by glucose and fatty acids and controls breast cancer cell growth in vitro. Diabetologia 55:2811–2822
Dalamaga M, Diakopoulos KN, Mantzoros CS (2012) The role of adiponectin in cancer: a review of current evidence. Endocr Rev 33:547–594
Dali-Youcef N, Hnia K, Blaise S, Messaddeq N, Blanc S, Postic C, Valet P, Tomasetto C, Rio M-C (2016) Matrix metalloproteinase 11 protects from diabesity and promotes metabolic switch. Sci Rep 6:25140
Darimont C, Vassaux G, Ailhaud G, Negrel R (1994) Differentiation of preadipose cells: paracrine role of prostacyclin upon stimulation of adipose cells by angiotensin-II. Endocrinology 135:2030–2036
Diedrich JD, Rajagurubandara E, Herroon MK, Mahapatra G, Huttemann M, Podgorski I (2016) Bone marrow adipocytes promote the Warburg phenotype in metastatic prostate tumors via HIF-1α activation. Oncotarget 7:64854–64877
Dieudonne M-N, Bussiere M, Dos Santos E, Leneveu M-C, Giudicelli Y, Pecquery R (2006) Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem Biophys Res Commun 345:271–279
Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C (2011) Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res 71:2455–2465
Duong MN, Geneste A, Fallone F, Li X, Dumontet C, Muller C (2017) The fat and the bad: mature adipocytes, key actors in tumor progression and resistance. Oncotarget 8:57622–57641
Ehsanipour EA, Sheng X, Behan JW, Wang X, Butturini A, Avramis VI, Mittelman SD (2013) Adipocytes cause leukemia cell resistance to L-asparaginase via release of glutamine. Cancer Res 73:2998–3006
Elliott BE, Tam SP, Dexter D, Chen ZQ (1992) Capacity of adipose tissue to promote growth and metastasis of a murine mammary carcinoma: effect of estrogen and progesterone. Int J Cancer 51:416–424
Endo H, Hosono K, Uchiyama T, Sakai E, Sugiyama M, Takahashi H, Nakajima N, Wada K, Takeda K, Nakagama H, Nakajima A (2011) Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut 60:1363–1371
Finley D, Calvert V, Inokuchi J, Lau A, Narula N, Petricoin E, Zaldivar F, Santos R, Tyson D, Ornstein D (2009) Periprostatic adipose tissue as a modulator of prostate cancer aggressiveness. J Urol 182:1621–1627
Fletcher SJ, Sacca PA, Pistone-Creydt M, Colo FA, Serra MF, Santino FE, Sasso CV, Lopez-Fontana CM, Caron RW, Calvo JC, Pistone-Creydt V (2017) Human breast adipose tissue: characterization of factors that change during tumor progression in human breast cancer. J Exp Clin Cancer Res 36:26
Furuhashi M, Hotamisligil GS (2008) Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov 7:489–503
Garofalo C, Surmacz E (2006) Leptin and cancer. J Cell Physiol 207:12–22
Gehmert S, Gehmert S, Prantl L, Vykoukal J, Alt E, Song Y-H (2010) Breast cancer cells attract the migration of adipose tissue-derived stem cells via the PDGF-BB/PDGFR-β signaling pathway. Biochem Biophys Res Commun 398:601–605
Gonzalez-Perez RR, Xu Y, Guo S, Watters A, Zhou W, Leibovich SJ (2010) Leptin upregulates VEGF in breast cancer via canonic and non-canonical signalling pathways and NFκB/HIF-1α activation. Cell Signal 22:1350–1362
Griner SE, Wang KJ, Joshi JP, Nahta R (2013) Mechanisms of adipocytokine-mediated trastuzumab resistance in HER2-positive breast cancer cell lines. Curr Pharmacogen Personal Med 11:31–41
Guaita-Esteruelas S, Bosquet A, Saavedra P, Guma J, Girona J, Lam EW, Amillano K, Borras J, Masana L (2017) Exogenous FABP4 increases breast cancer cell proliferation and activates the expression of fatty acid transport proteins. Mol Carcinog 56:208–217
Haczeyni F, Bell-Anderson KS, Farrell GC (2018) Causes and mechanisms of adipocyte enlargement and adipose expansion. Obes Rev 19:406–420
Han Suk R, Han-Byoel L, Wonshik H, Dong-Young N, Hyeong-Gon M (2015) Reduced proliferation in breast cancer cells contacting the neighboring adipocytes in human breast cancer tissues. Breast Cancer Res 17:90
Han L, Xu J, Xu Q, Zhang B, Lam EW, Sun Y (2017) Extracellular vesicles in the tumor microenvironment: therapeutic resistance, clinical biomarkers, and targeting strategies. Med Res Rev 37:1318–1349
Han L, Lam EW, Sun Y (2019) Extracellular vesicles in the tumor microenvironment: old stories, but new tales. Mol Cancer 18:59
Herroon MK, Rajagurubandara E, Hardaway AL, Powell K, Turchick A, Feldmann D, Podgorski I (2013) Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget 4:2108
Horowitz NS, Wright AA (2015) Impact of obesity on chemotherapy management and outcomes in women with gynecologic malignancies. Gynecol Oncol 138:201–206
Hovey RC, Mcfadden TB, Akers RM (1999) Regulation of mammary gland growth and morphogenesis by the mammary fat pad: a species comparison. J Mammary Gland Biol Neoplasia 4:53–68
Hoy AJ, Balaban S, Saunders DN (2017) Adipocyte–tumor cell metabolic crosstalk in breast cancer. Trends Mol Med 23:381–392
Hu W, Ru Z, Zhou Y, Xiao W, Sun R, Zhang S, Gao Y, Li X, Zhang X, Yang H (2019) Lung cancer-derived extracellular vesicles induced myotube atrophy and adipocyte lipolysis via the extracellular IL-6-mediated STAT3 pathway. Biochim Biophys Acta Mol Cell Biol Lipids 1864:1091–1102
Hua TNM, Kim MK, Vo VTA, Choi JW, Choi JH, Kim HW, Cha SK, Park KS, Jeong Y (2019) Inhibition of oncogenic Src induces FABP4-mediated lipolysis via PPARgamma activation exerting cancer growth suppression. EBioMedicine 41:134–145
Huang M, Narita S, Inoue T, Koizumi A, Saito M, Tsuruta H, Numakura K, Satoh S, Nanjo H, Sasaki T, Habuchi T (2017) Fatty acid binding protein 4 enhances prostate cancer progression by upregulating matrix metalloproteinases and stromal cell cytokine production. Oncotarget 8:111780–111794
Ishikawa M, Kitayama J, Nagawa H (2004) Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res 10:4325–4331
Ishikawa M, Kitayama J, Yamauchi T, Kadowaki T, Maki T, Miyato H, Yamashita H, Nagawa H (2007) Adiponectin inhibits the growth and peritoneal metastasis of gastric cancer through its specific membrane receptors AdipoR1 and AdipoR2. Cancer Sci 98:1120–1127
Jia L, Wang S, Cao J, Zhou H, Wei W, Zhang J (2007) siRNA targeted against matrix metalloproteinase 11 inhibits the metastatic capability of murine hepatocarcinoma cell Hca-F to lymph nodes. Int J Biochem Cell Biol 39:2049–2062
Jotzu C, Alt E, Welte G, Li J, Hennessy BT, Devarajan E, Krishnappa S, Pinilla S, Droll L, Song Y-H (2010) Adipose tissue-derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor-derived factors. Anal Cell Pathol (Amst) 33:61–79
Kang JH, Lee YY, Yu BY, Yang B-S, Cho K-H, Yoon DK, Roh YK (2005) Adiponectin induces growth arrest and apoptosis of MDA-MB-231 breast cancer cell. Arch Pharm Res 28:1263–1269
Kennedy AR, Pissios P, Otu H, Roberson R, Xue B, Asakura K, Furukawa N, Marino FE, Liu FF, Kahn BB, Libermann TA, Maratos-Flier E (2007) A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab 292:E1724–E1739
Kristin MN, Hilary AK, Carla VP, Andras L, Rebecca B-G, Marion RZ, Iris LR, Mark SC, Gordon BM, Gökhan SH, Yamada SD, Marcus EP, Katja G, Ernst L (2011) Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med 17:1498–1503
Kwan HY, Fu X, Liu B, Chao X, Chan CL, Cao H, Su T, Tse AKW, Fong WF, Yu Z-L (2014) Subcutaneous adipocytes promote melanoma cell growth by activating the Akt signaling pathway: role of palmitic acid. J Biol Chem 289:30525–30537
Laetitia D, Charlotte L, Virginie D, Alice D, Hermine B, Ali M, Odile D, Marie-Paule V, Florence C-C (2013) Reciprocal interactions between breast tumor and its adipose microenvironment based on a 3D adipose equivalent model. PLoS One 8:e66284
Laouirem S, Sannier A, Norkowski E, Cauchy F, Doblas S, Rautou PE, Albuquerque M, Garteiser P, Sognigbe L, Raffenne J, Van Beers BE, Soubrane O, Bedossa P, Cros J, Paradis V (2019) Endothelial fatty liver binding protein 4: a new targetable mediator in hepatocellular carcinoma related to metabolic syndrome. Oncogene 38:3033–3046
Lapeire L, Hendrix A, Lambein K, Van Bockstal M, Braems G, Van Den Broecke R, Limame R, Mestdagh P, Vandesompele J, Vanhove C, Maynard D, Lehuede C, Muller C, Valet P, Gespach CP, Bracke M, Cocquyt V, Denys H, De Wever O (2014) Cancer-associated adipose tissue promotes breast cancer progression by paracrine oncostatin M and Jak/STAT3 signaling. Cancer Res 74:6806–6819
Lehuede C, Li X, Dauvillier S, Vaysse C, Franchet C, Clement E, Esteve D, Longue M, Chaltiel L, Le Gonidec S, Lazar I, Geneste A, Dumontet C, Valet P, Nieto L, Fallone F, Muller C (2019) Adipocytes promote breast cancer resistance to chemotherapy, a process amplified by obesity: role of the major vault protein (MVP). Breast Cancer Res 21:7
Liu Z, Xu J, He J, Liu H, Lin P, Wan X, Navone NM, Tong Q, Kwak LW, Orlowski RZ, Yang J (2015) Mature adipocytes in bone marrow protect myeloma cells against chemotherapy through autophagy activation. Oncotarget 6:34329–34341
Lopes-Coelho F, Andre S, Felix A, Serpa J (2018) Breast cancer metabolic cross-talk: fibroblasts are hubs and breast cancer cells are gatherers of lipids. Mol Cell Endocrinol 462:93–106
Luo M, Brooks M, Wicha MS (2018) Asparagine and Glutamine: Co-conspirators Fueling Metastasis. Cell Metab 27:947–949
Man K, Ng KTP, Xu A, Cheng Q, Lo CM, Xiao JW, Sun BS, Lim ZXH, Cheung JS, Wu EX, Sun CKW, Poon RTP, Fan ST (2010) Suppression of liver tumor growth and metastasis by adiponectin in nude mice through inhibition of tumor angiogenesis and downregulation of rho kinase/IFN-inducible protein 10/matrix metalloproteinase 9 signaling. Clin Cancer Res 16:967–977
Manabe Y, Toda S, Miyazaki K, Sugihara H (2003) Mature adipocytes, but not preadipocytes, promote the growth of breast carcinoma cells in collagen gel matrix culture through cancer–stromal cell interactions. J Pathol 201:221–228
Martuscello RT, Vedam-Mai V, Mccarthy DJ, Schmoll ME, Jundi MA, Louviere CD, Griffith BG, Skinner CL, Suslov O, Deleyrolle LP, Reynolds BA (2016) A supplemented high-fat low-carbohydrate diet for the treatment of glioblastoma. Clin Cancer Res 22:2482–2495
Mcnelis JC, Olefsky JM (2014) Macrophages, immunity, and metabolic disease. Immunity 41:36–48
Mick GJ, Wang X, Mccormick K (2002) White adipocyte vascular endothelial growth factor: regulation by insulin. Endocrinology 143:948–953
Mistry T, Digby JE, Desai KM, Randeva HS (2007) Obesity and prostate cancer: a role for adipokines. Eur Urol 52:46–53
Motrescu ER, Rio MC (2008) Cancer cells, adipocytes and matrix metalloproteinase 11: a vicious tumor progression cycle. Biol Chem 389:1037–1041
Muz B, De La Puente P, Azab F, Azab AK (2015) The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckland, NZ) 3:83–92
Nair S, Mason A, Eason J, Loss G, Perrillo RP (2002) Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology 36:150–155
Nam SY, Lee EJ, Kim KR, Cha BS, Song YD, Lim SK, Lee HC, Huh KB (1997) Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord 21:355–359
Nishida N, Yano H, Nishida T, Kamura T, Kojiro M (2006) Angiogenesis in cancer. Vasc Health Risk Manag 2:213–219
Olsen CM, Nagle CM, Whiteman DC, Ness R, Pearce CL, Pike MC, Rossing MA, Terry KL, Wu AH, Australian Cancer Study, Australian Ovarian Cancer Study Group, Risch HA, Yu H, Doherty JA, Chang-Claude J, Hein R, Nickels S, Wang-Gohrke S, Goodman MT, Carney ME, Matsuno RK, Lurie G, Moysich K, Kjaer SK, Jensen A, Hogdall E, Goode EL, Fridley BL, Vierkant RA, Larson MC, Schildkraut J, Hoyo C, Moorman P, Weber RP, Cramer DW, Vitonis AF, Bandera EV, Olson SH, Rodriguez-Rodriguez L, King M, Brinton LA, Yang H, Garcia-Closas M, Lissowska J, Anton-Culver H, Ziogas A, Gayther SA, Ramus SJ, Menon U, Gentry-Maharaj A, Webb PM, Ovarian Cancer Association Consortium (2013) Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocr Relat Cancer 20:251–262
Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA (2005) Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121:335–348
Park J, Scherer PE (2012) Adipocyte-derived endotrophin promotes malignant tumor progression. J Clin Invest 122:4243–4256
Park J, Euhus DM, Scherer PE (2011) Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocr Rev 32:550–570
Park J, Morley TS, Scherer PE (2013) Inhibition of endotrophin, a cleavage product of collagen VI, confers cisplatin sensitivity to tumours. EMBO Mol Med 5:935–948
Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP (2009) The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 8:3984–4001
Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, Psaila B, Kaplan RN, Bromberg JF, Kang Y, Bissell MJ, Cox TR, Giaccia AJ, Erler JT, Hiratsuka S, Ghajar CM, Lyden D (2017) Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer 17:302–317
Petricoin III EF, Liotta L, Dadachova E, Pestell RG, Lisanti MP, Bonaldo P, Scherer PE (2005) Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J Clin Investig 115:1163–1176
Picon-Ruiz M, Pan C, Drews-Elger K, Jang K, Besser AH, Zhao D, Morata-Tarifa C, Kim M, Ince TA, Azzam DJ, Wander SA, Wang B, Ergonul B, Datar RH, Cote RJ, Howard GA, El-Ashry D, Torne-Poyatos P, Marchal JA, Slingerland JM (2016) Interactions between adipocytes and breast cancer cells stimulate cytokine production and drive Src/Sox2/miR-302b-mediated malignant progression. Cancer Res 76:491–504
Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM (2017) Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin 67:378–397
Pollak M (2008) Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 8:915–928
Pramanik R, Sheng X, Ichihara B, Heisterkamp N, Mittelman SD (2012) Adipose tissue attracts and protects acute lymphoblastic leukemia cells from chemotherapy. Leuk Res 37:503–509
Qiao Z, Sarah MD, Jinling Z, Erinn D-K, Jeremy R, Stephen DH, Nathan AB, Ofer R (2011) Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocr Relat Cancer 18:491–503
Raphael Johannes M, Sepideh A-G, René Gunther F, Johannes Adalbert M, Roland L, Daniel N, Wolfgang S, Barbara K (2015) Inhibition of neuroblastoma tumor growth by ketogenic diet and/or calorie restriction in a CD1-Nu mouse model. PLoS One 10:e0129802
Rausch LK, Netzer NC, Hoegel J, Pramsohler S (2017) The linkage between breast cancer, hypoxia, and adipose tissue. Front Oncol 7:211
Razmkhah M, Jaberipour M, Erfani N, Habibagahi M, Talei A-R, Ghaderi A (2011) Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL-4, IL-10 and TGF-β1 and upregulate expression of regulatory molecules on T cells: do they protect breast cancer cells from the immune response? Cell Immunol 266:116–122
Rei S, Noriyuki O, Shinji K, Kaori S, Tohru F, Kenneth W (2004) Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of AMP-activated protein kinase signaling. J Biol Chem 279:28670–28674
Ribeiro AM, Andrade S, Pinho F, Monteiro JD, Costa M, Lopes C, Aguas AP, Monteiro MP (2010) Prostate cancer cell proliferation and angiogenesis in different obese mice models. Int J Exp Pathol 91:374
Ribeiro RJT, Monteiro CPD, Cunha VFPM, Azevedo ASM, Oliveira MJ, Monteiro R, Fraga AM, Principe P, Lobato C, Lobo F, Morais A, Silva V, Sanches-Magalhaes J, Oliveira J, Guimaraes JT, Lopes CMS, Medeiros RM (2012) Tumor cell-educated periprostatic adipose tissue acquires an aggressive cancer-promoting secretory profile. Cell Physiol Biochem 29:233–240
Rouyer N, Wolf C, Chenard MP, Rio MC, Chambon P, Bellocq JP, Basset P (1994) Stromelysin-3 gene expression in human cancer: an overview. Invasion Metastasis 14:269–275
Shanmugalingam T, Bosco C, Ridley AJ, Van Hemelrijck M (2016) Is there a role for IGF-1 in the development of second primary cancers? Cancer Med 5:3353–3367
Shimizu I, Yoshida Y, Suda M, Minamino T (2014) DNA damage response and metabolic disease. Cell Metab 20:967–977
Suga H, Matsumoto D, Inoue K, Shigeura T, Eto H, Aoi N, Kato H, Abe H, Yoshimura K (2008) Numerical measurement of viable and nonviable adipocytes and other cellular components in aspirated fat tissue. Plast Reconstr Surg 122:103–114
Sun K, Kusminski CM, Scherer PE (2011) Adipose tissue remodeling and obesity. J Clin Invest 121:2094–2101
Tian Z, Sun R, Wei H, Gao B (1959) Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem Biophys Res Commun 298:297–302
Tokuda Y, Satoh Y, Fujiyama C, Toda S, Sugihara H, Masaki Z (2003) Prostate cancer cell growth is modulated by adipocyte-cancer cell interaction. BJU Int 91:716–720
Uddin S, Bu R, Ahmed M, Abubaker J, Al-Dayel F, Bavi P, Al-Kuraya KS (2009) Overexpression of leptin receptor predicts an unfavorable outcome in middle eastern ovarian cancer. Mol Cancer 8:74
Vidali S, Aminzadeh S, Lambert B, Rutherford T, Sperl W, Kofler B, Feichtinger RG (1983) Mitochondria: the ketogenic diet—a metabolism-based therapy. Cell Biochem Funct 63:55–59
Wang Y-Y, Lehuede C, Laurent V, Dirat B, Dauvillier S, Bochet L, Le Gonidec S, Escourrou G, Valet P, Muller C (1975) Adipose tissue and breast epithelial cells: a dangerous dynamic duo in breast cancer. Cancer Lett 324:142–151
Wang Y, Lam JB, Lam KSL, Liu J, Lam MC, Hoo RLC, Wu D, Cooper GJS, Xu A (2006) Adiponectin modulates the glycogen synthase kinase-3β/β-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res 66:11462–11470
Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, Gong Z, Zhang S, Zhou J, Cao K, Li X, Xiong W, Li G, Zeng Z, Guo C (2017a) Role of tumor microenvironment in tumorigenesis. J Cancer 8:761–773
Wang YY, Attane C, Milhas D, Dirat B, Dauvillier S, Guerard A, Gilhodes J, Lazar I, Alet N, Laurent V, Le Gonidec S, Biard D, Herve C, Bost F, Ren GS, Bono F, Escourrou G, Prentki M, Nieto L, Valet P, Muller C (2017b) Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight 2:e87489
Wang K, Yu XH, Tang YJ, Tang YL, Liang XH (2019) Obesity: an emerging driver of head and neck cancer. Life Sci 233:116687
Warburg O (1956) On the origin of cancer cells. Science (NY) 123:309–314
Wei L, Li K, Pang X, Guo B, Su M, Huang Y, Wang N, Ji F, Zhong C, Yang J, Zhang Z, Jiang Y, Liu Y, Chen T (2016) Leptin promotes epithelial-mesenchymal transition of breast cancer via the upregulation of pyruvate kinase M2. J Exp Clin Cancer Res 35:166–110
Wright C, Simone N (2016) Obesity and tumor growth: inflammation, immunity, and the role of a ketogenic diet. Curr Opin Clin Nutr Metab Care 19:294–299
Wu J, Lanier LL (2003) Natural killer cells and cancer. Adv Cancer Res 90:127–156
Yao S, Fan LY, Lam EW (2018) The FOXO3-FOXM1 axis: a key cancer drug target and a modulator of cancer drug resistance. Semin Cancer Biol 50:77–89
Yeh W-L, Lu D-Y, Lee M-J, Fu W-M (2009) Leptin induces migration and invasion of glioma cells through MMP-13 production. Glia 57:454–464
Young SG, Zechner R (2013) Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes Dev 27:459–484
Zhang Z, Scherer PE (2018) Adipose tissue: the dysfunctional adipocyte—a cancer cell’s best friend. Nat Rev Endocrinol 14:132–134
Zhang F, Chen Y, Heiman M, Dimarchi R (2005) Leptin: structure, function and biology. Vitam Horm 71:345–372
Zhang M, Di Martino JS, Bowman RL, Campbell NR, Baksh SC, Simon-Vermot T, Kim IS, Haldeman P, Mondal C, Yong-Gonzales V, Abu-Akeel M, Merghoub T, Jones DR, Zhu XG, Arora A, Ariyan CE, Birsoy K, Wolchok JD, Panageas KS, Hollmann T, Bravo-Cordero JJ, White RM (2018) Adipocyte-derived lipids mediate melanoma progression via FATP proteins. Cancer Discov 8:1006–1025
Zhang H, Deng T, Ge S, Liu Y, Bai M, Zhu K, Fan Q, Li J, Ning T, Tian F, Li H, Sun W, Ying G, Ba Y (2019) Exosome circRNA secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination-related USP7. Oncogene 38:2844–2859
Zimmerlin L, Donnenberg AD, Rubin JP, Basse P, Landreneau RJ, Donnenberg VS (2011) Regenerative therapy and cancer: in vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng A 17:93–106
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jiramongkol, Y., Lam, E.WF. (2020). Multifaceted Oncogenic Role of Adipocytes in the Tumour Microenvironment. In: Serpa, J. (eds) Tumor Microenvironment . Advances in Experimental Medicine and Biology, vol 1219. Springer, Cham. https://doi.org/10.1007/978-3-030-34025-4_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-34025-4_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-34024-7
Online ISBN: 978-3-030-34025-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)