Abstract
Polycystic Ovary Syndrome (PCOS) is a heterogenous, complex, genetic trait of unclear etiology, comprising of ovarian hyperandrogenism and hyperinsulinemia. It is the most common endocrine abnormality in women of reproductive age and the most common cause of anovulatory infertility. PCOS has been shown to be associated with certain autoimmune diseases like Autoimmune Thyroid Disease (AITD) and Systemic Lupus Erythematosus (SLE). Furthermore, over a hundred candidate genes have been linked to PCOS and Genome Wide Association Studies on these are ongoing. Two among these, are the most promising chromosome 9p33.3 DENND1A (DENN/MADD domain-containing protein 1A) and 2–21 THADA (Thyroid adenoma-associated) susceptibility loci. In the majority of PCOS patients, the fundamental defect is intrinsic androgenic dysfunction termed Primary Ovarian Hyperandrogenism. Primary Ovarian Hyperandrogenism is believed to be due to the rapid, high-amplitude pulsation of Gonadotropin-Releasing Hormone (GnRH) from the hypothalamus, which causes preferential release of Luteinizing Hormone (LH) over Follicle Stimulating Hormone (FSH) from the anterior pituitary gland. Hyperandrogenemia may present as hirsutism, acne or alopecia. The pathophysiology of PCOS is multifactorial but is related to insulin resistance in many cases. Hyperinsulinemia may manifest as obesity, difficulty losing weight, prediabetes or Diabetes Mellitus Type II. Many PCOS women also have irregular and anovulatory cycles, and some have polycystic ovaries on transvaginal ultrasound. Overall, PCOS encompasses a wide range of metabolic and reproductive disorders ranging from prediabetes, to infertility, endometrial hyperplasia and endometrial cancer. Treatment of PCOS is multifaceted and aims at targeting the underlying hyperinsulinemia, hyperandrogenemia and menstrual irregularity. Biguanides (i.e., Metformin) and Glucagon-like peptide 1 (GLP-1) receptor agonists are insulin sensitizers that have been studied in the treatment of PCOS.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Autoimmunity association
- PCOS
- String of pearls
- DHEA
- Oligo-anovulation
- Hyperandrogenemia
- Hirsutism
- Alopecia
- Multifactorial pathophysiology
1 Introduction
Polycystic Ovary Syndrome (PCOS) is a complex genetic trait of unclear etiology. It is the most common endocrine abnormality in women of reproductive age and the most common cause of anovulatory infertility [1]. An autoimmune basis of etiology has been suggested and studied but has not been substantiated with studies. Some reports link PCOS with Autoimmune Thyroid Disease (AITD) and Systemic Lupus Erythematosus (SLE) [2, 3]. AITD has been reported in 18–40% of PCOS patients. Several autoantibodies have also been linked with PCOS. There are ongoing studies looking into the association between autoimmunity and PCOS. American Gynecologists Irving F Stein Sr. and Michael L Leventhal first described this syndrome in 1935, as a triad of amenorrhea, hirsutism and polycystic ovaries [4]. They studied seven women with amenorrhea, hirsutism, and enlarged ovaries with multiple cysts, five of whom had hirsutism and acne and four had obesity. The syndrome was called Stein-Leventhal syndrome. However, the presence of sclerotic ovaries had been identified over 90 years prior to this. This syndrome was also known by other names, such as functional ovarian hyperandrogenism, ovarian hyperthecosis and sclerotic ovary syndrome. Currently this syndrome is known as Polycystic Ovary Syndrome which is a misnomer as it does not accurately reflect the features of this disorder and the presence of polycystic ovaries is not essential for the diagnosis of PCOS. Therefore, the Evidence Based Methodology Workshop by NIH in 2012 suggested renaming this syndrome [5]. One in ten women have PCOS, but prevalence may vary 5–15% based on ethnic predilection. Thus, PCOS is seen in roughly 4.8% Caucasians, 13% Latina/Hispanics and 8% African Americans. Twin studies have demonstrated a genetic basis of inheritance for PCOS [6]. Many PCOS adolescents have a mother with polycystic ovaries who may not manifest symptoms of PCOS, although maternal PCOS is a risk factor in daughters. 40% of PCOS patients may have an affected sister with PCOS. Familial factors associated with PCOS include metabolic syndrome, insulin resistance, and obesity, which may be seen in either parents, but more commonly in the father [6]. Over one hundred genes have been found to be associated with PCOS, but the vast majority of these have not been replicated in multiple studies. Sixteen of these genes have shown supporting evidence of replication from multiple reports. Two among these that are the most promising are chromosome 9p33.3 DENND1A (DENN/MADD domain-containing protein 1A) and 2–21 THADA (Thyroid adenoma-associated) susceptibility loci [6]. As of now, there is no genetic testing for the diagnosis of PCOS, but ongoing Genome Wide Association Studies may hold promise for future.
2 Criteria for Diagnosis
There are three diagnostic criteria for PCOS each suggested by a different group. The NICH/NICHD criteria was defined in 1992, the ESHRE/ASRM (Rotterdam Criteria) in 2004 and the Androgen Excess criteria in 2006 [6]. PCOS is a diagnosis of exclusion, and all three groups agree that other hyperandrogenic disorders like non-classical Congenital Adrenal Hyperplasia (NC-CAH), thyroid disorders, hyperprolactinemia, etc., must be excluded before a diagnosis of PCOS can be established [7]. The prevalence of PCOS varies based on the diagnostic criteria used, and according to a meta-analysis published in the Journal of Epidemiology in 2014, prevalence varied 6–9% based on the NIH criteria, 8–15% based on the Androgen excess criteria and 15–20% based on the Rotterdam Criteria [5]. The Rotterdam criteria is the most inclusive and hence the 2012 Evidence Based Methodology Workshop on PCOS by NIH suggested using this for the diagnosis of PCOS.
NIH/NICHD 1992 | ESHRE/ASRM (Rotterdam criteria) 2004 | Androgen excess society 2006 |
|---|---|---|
Exclusion of other androgen excess or related disorders | Exclusion of other androgen excess or related disorders | Exclusion of other androgen excess or related disorders |
Includes all the following: Clinical and/or biochemical hyperandrogenism | Includes two of the following: Clinical and/or biochemical hyperandrogenism | Includes all the following: Clinical and/or biochemical hyperandrogenism |
Menstrual dysfunction | ||
Oligo-ovulation or anovulation | Ovarian dysfunction and/or polycystic ovaries | |
Polycystic ovaries |
Adult Phenotypes
Specification of PCOS phenotypes was proposed by the 2012 Evidence Based Methodology Workshop by NIH for research and clinical purposes. There are four adult phenotypes listed in decreasing order of specificity and severity [8, 9]:
Phenotype 1 (Classic PCOS)- most severe
-
Clinical and/or biochemical evidence of hyperandrogenism
-
Evidence of Oligo-anovulation
-
Ultrasound evidence of polycystic ovary
Phenotype 2 (Hyperandrogenic anovulation)
-
Clinical and/or biochemical evidence of hyperandrogenism
-
Evidence of oligo-anovulation
Phenotype 3 (Ovulatory PCOS)
-
Clinical and/or biochemical evidence of hyperandrogenism
-
Ultrasound evidence of polycystic ovary
Phenotype 4 (Non-hyperandrogenic PCOS)
-
Evidence of oligo-anovulation
-
Ultrasound evidence of polycystic ovary
3 Pathophysiology
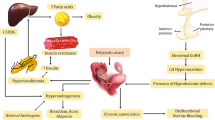
In the majority of PCOS patients, the fundamental defect is intrinsic androgenic dysfunction termed as a Primary Ovarian Hyperandrogenism . The high intraovarian androgen concentration stimulates the ovaries, causing excessive growth of the small ovarian follicles and inhibiting follicular maturation and development of a dominant follicle. It also causes premature luteinization of the follicles and hyperplasia of the thecal, stromal and cortical cells which results in anovulation and the polycystic appearance of ovaries [10]. Primary Ovarian Hyperandrogenism is believed to be due to the rapid, high-amplitude pulsation of Gonadotropin -Releasing Hormone (GnRH) from the hypothalamus, which causes preferential release of Luteinizing Hormone (LH) over Follicle Stimulating Hormone (FSH) from the anterior pituitary gland. The high LH acts on the theca cells resulting in release of high levels of androstenedione and testosterone. The testosterone is then converted in the granulosa cells by the aromatase enzyme to estradiol. The high estradiol level paradoxically inhibits follicular maturation. There is also peripheral conversion of androstenedione to estrone and testosterone, and these hormones further stimulate the LH release from the anterior pituitary. In women without PCOS, increase in LH level above steady state causes desensitization of theca cells by down regulating the LH receptors on the theca cells. This in turn inhibits ovarian steroidogenesis. In PCOS, there is partial escape from LH receptor downregulation, and ovarian steroids are hyperresponsive to LH [10, 11] (Fig. 1).
Pathophysiology of PCOS. Overall, 50–70% of women with PCOS demonstrate clinically measurable insulin resistance in vivo, above and beyond what can be expected for their body weight. Insulin directly stimulates the rapid high-amplitude pulsation of GnRH from hypothalamus. There are Insulin-like growth factor-1 (IGF-1) receptors on the ovarian theca cells, and insulin directly stimulates the theca cell to secrete androgens. Insulin also inhibits the production of hepatic sex-hormone binding globulin (SHBG), resulting in an increase in the level of free testosterone. Hypersensitivity to insulin also exists in lean women with PCOS who are not insulin-resistant. All treatments that aim at lowering insulin levels including weight loss, will improve ovarian androgen excess and promotes ovulation
Overall, 50–70% of women with PCOS demonstrate clinically measurable insulin resistance in vivo, above and beyond what can be expected for their body weight [7]. Insulin directly stimulates the rapid high-amplitude pulsation of GnRH from hypothalamus. There are Insulin-like growth factor-1 (IGF-1) receptors on the ovarian theca cells, and insulin directly stimulates the theca cell to secrete androgens. Insulin also inhibits the production of hepatic sex-hormone binding globulin (SHBG), resulting in an increase in the level of free testosterone. Hypersensitivity to insulin also exists in lean women with PCOS who are not insulin-resistant [7, 10]. All treatments that aim at lowering insulin levels including weight loss, will improve ovarian androgen excess and promotes ovulation. 30–40% of PCOS patients have adrenal hyperandrogenemia in addition to ovarian hyperandrogenemia. The rapid high-amplitude pulsation of GnRH causes the release of Adrenocorticotropic Hormone (ACTH) from the pituitary gland, which in turn stimulates the adrenal glands to release dehydroepiandrosterone sulfate (DHEAS), androstenedione, and testosterone LH [10, 12, 13].
The association of autoimmunity and inflammation in PCOS patients has been extensively studied. The following autoantibodies have been linked to PCOS: Anti-Nuclear Antibodies (ANA) were linked to PCOS in a study showing that the number of ANA positive cases increased from 8.6% to 28.6% in patients with PCOS following electrocauterization [14]. This same study reported zero cases of ANA in the control group. This result suggests there is some association between the disease and autoimmunity [14]. Another study showed significantly higher serum levels of Anti-dsDNA in patients with PCOS compared to a control group [15]. Anti-Ro (SSA) was the main subtype identified of ANAs in positive cases of PCOS [14]. Anti-thyroglobulin levels were not found to be significantly different between patients with PCOS and control groups. However, Kachuei et al. reports greater levels of anti-thyroglobulin in patients with PCOS [16]. Anti-TPO was found to be significantly higher in patients with PCOS when compared with patients in a control group [16]. This finding supports the assessment of thyroid function and autoimmunity in patients with PCOS. Antibody to protein tyrosine phosphatase was shown to be associated with a low risk of progression to type 1 diabetes [17]. Anti-histone antibody was shown to be higher in patients with PCOS than patients with unexplained fertility and healthy fertile subjects [15]. Anti-carbonic anhydrase-1 mean serum levels were found to be significantly higher in women with PCOS compared with control subjects [18]. Anti-spermatic antibody was shown to be significantly correlated with higher scores of Hirsutisms in patients with PCOS. Hirsutism is a well-known symptom of PCOS. Islet cell antibodies were associated with a low risk of progression to type 1 diabetes [17]. In the presence of other islet autoantibodies, a high risk of progression to diabetes was observed. GAD was shown to be associated with a low risk of progression to type 1 diabetes. Insulin autoantibodies was shown to be associated with a low risk of progression to type 1 diabetes [17]. However, autoimmunity as the etiological cause of PCOS has not been supported by several studies. One possibility that has been suggested that the non-organ-specific autoantibodies lead to systemic immune activation in PCOS women. This could explain the frequent association between PCOS and autoimmune diseases, especially Autoimmune Thyroid Diseases (AITD) [2].
4 Clinical Features
PCOS usually presents in adolescence, and hyperandrogenism is one of the most common presenting complaints. PCOS is responsible for 85% of androgen excess in adolescent females [19]. Hyperandrogenemia may present as hirsutism, acne or alopecia. Hirsutism is seen in 70% of women with PCOS. In hirsutism there is conversion of the female pattern vellus hair to the male pattern terminal hair and this is seen commonly on upper lip, chin, around nipple, and along the linea alba of lower abdomen. Figure 2 Some PCOs females with high testosterone level may not present with hirsutism as they lack testosterone receptors on the skin. This can be seen especially in some Asian females. Alternatively, hirsutism may occur without elevated testosterone level, which can be referred as an idiopathic hirsutism and these patients should not be labelled as having PCOS. Therefore, elevated testosterone level along with hirsutism is a more reliable indicator of hyperandrogenism. Alopecia may be seen in 10% of PCOS females [4, 6]. PCOS patients with alopecia may present with male pattern hair loss with fronto-temporal-occipital baldness or female pattern hair loss typically affecting the crown and manifesting early as a widening midline parting in a ‘Christmas tree’ pattern. Figure 3 PCOS patients are also prone to develop moderate to severe inflammatory acne in unusual locations, especially in the anterior chest and back. Figure 4 it has been estimated the incidence of acne to be 20–40% in PCOS patients [4]. Alternate cutaneous manifestations of hyperandrogenism include seborrhea (may manifest as white flaky skin in eyebrow and face), hyperhidrosis or hidradenitis suppurativa [20, 21].
Hirsutism in PCOS. Hirsutism is seen in 70% of women with PCOS. In hirsutism there is conversion of the female pattern vellus hair to the male pattern terminal hair and this is seen commonly on upper lip, chin, around nipple and along the linea alba of lower abdomen. Some PCOS females with high testosterone level may not present with hirsutism as they lack testosterone receptors on the skin. This can be seen especially in some Asian females. Alternatively, hirsutism may occur without elevated testosterone level and this is idiopathic hirsutism and these patients should not be labelled as having PCOS. Therefore, elevated testosterone level along with hirsutism is a more reliable indicator of hyperandrogenism
Female pattern hair loss in PCOS. Alopecia may be seen in 10% of PCOS females. PCOS patients with alopecia may present with male pattern hair loss with fronto-temporal-occipital baldness or female pattern hair loss typically affecting the crown and manifesting early as a widening midline parting in a ‘Christmas tree’ pattern
PCOS patients are usually obese and have a difficult time losing weight due to the underlying insulin resistance. Rapid weight gain and obesity are seen in 35–50% of PCOS females. Gestational Diabetes Mellitus is two and a half times more common in PCOS than in normal females. 10% of PCOS women develop Diabetes Mellitus Type II by age forty [22]. PCOS women may also present with acanthosis nigricans which is hyperpigmented, thick, velvety areas in skin creases, and folds. Figure 5 acanthosis nigricans is most commonly seen in the elbows, knuckles, back of the neck, or knees and is a sign of insulin resistance. Insulin resistance may cause development of skin tags in some women [20, 23].
Acanthosis nigricans in PCOS. PCOS women may also present with acanthosis nigricans which is hyperpigmented, thick, velvety areas in skin creases, and folds. Acanthosis nigricans is most commonly seen in the elbows, knuckles, back of the neck, or knees and is a sign of insulin resistance. Insulin resistance may be cause development of skin tags in some women
Abnormal menstrual cycles may be seen in 60–70% of PCOS patients and is one of the most frequent complaints of PCOS women and the reason why they seek evaluation and treatment. Abnormal menses is common during puberty, due to the immaturity of the Hypothalamic-Pituitary-Ovarian (HPO) axis. Therefore, consensus groups have urged caution before labelling hyperandrogenic adolescents as having PCOS if the menstrual abnormality has not persisted for 2 years or more. Many PCOS adolescents have delayed menarche followed by irregular menstrual cycles or they may have normal cycles at menarche which becomes abnormal with weight gain [5]. Interestingly 85–90% of women with oligomenorrhea on further work-up turn out to have PCOS and 30–40% of women with amenorrhea have PCOS. Females with PCOS may also present with menorrhagia and metrorrhagia due to the unopposed estrogen action. PCOS patients also exhibit anovulation or oligo-ovulation which leads to subfertility or infertility. Even if they do conceive, chances of multiple miscarriages are 20–40% higher than in the general obstetric population. There is also a much higher incidence of preterm birth and stillbirths in pregnant PCOS women. Many PCOS females have a higher incidence of cardiovascular risk factors like hypertension or hyperlipidemia, at a younger age [6].
5 Co-morbidities
Due to the underlying insulin resistance seen in PCOS patients, gestational diabetes is two and a half times more common in PCOS patients. Ten percent of PCOS patients develop Diabetes Mellitus Type II by age 40. DM type II is 3–5 times more common in PCOS patients. Twenty five precent of PCOS patients have metabolic syndrome and this is three times more common in PCOS patients. Sleep apnea/disordered breathing is 30–40 times more common in PCOS patients. Interestingly sleep apnea in PCOS patients is not related to the patient’s weight or androgen level but is related to the underlying insulin resistance [24]. Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH) are seen in 6.7% of PCOS patients [25]. Estrogen excess of PCOS increases the risk of developing endometrial hyperplasia, atypia and cancer. One study found that PCOS patients were three times more likely to develop endometrial cancer [26, 27]. The risk of PCOS patients developing ovarian and breast cancer has not been substantiated. Depression is four times more common in PCOS patients. One study found that the depression in PCOS patients was not related to obesity or symptoms of hyperandrogenemia [28].
The data on the direct effects of PCOS on cardiovascular disease is conflicting. However, we do know that women with PCOS are more likely to have a higher level of small dense low-density lipoprotein (LDL) particles when compared to women of similar Body Mass Index (BMI) and insulin resistance without PCOS [6]. Small dense LDL particles are strongly associated with an increased risk of coronary heart disease. There is higher incidence of coronary calcification, aortic calcification, and increased carotid intima media thickness (CIMT) in PCOS women compared to controls. Women with PCOS may also have more extensive coronary disease on angiography when compared to normal women [29]. This was illustrated in a report of women younger than 60 years of age, who were undergoing coronary angiography for assessment of chest pain or valvular disease. Dyslipidemia is highly prevalent in patients with PCOS [30] PCOS patients are also at higher risk of developing hypertension at a younger age [31]. PCOS patients who have been treated with high dose of metformin for a long period of time have an increased risk of developing vitamin B12 deficiency and therefore should have their vitamin B12 level checked at least yearly [32, 33]. A meta-analysis on pregnancy outcomes in women with PCOS demonstrated a significantly higher risk of developing gestational diabetes mellitus, pregnancy-induced hypertension, preeclampsia, and preterm birth in PCOS patients [31].
6 Diagnosis
PCOS is a diagnosis of exclusion, therefore other causes of amenorrhea and hyperandrogenemia must be excluded, before establishing the diagnosis. In patients who have signs of virilization (rapid development of deepening of voice, muscle development and clitoromegaly) adrenal or ovarian androgen secreting tumor must be considered and appropriate evaluation for these must be done. In patients without signs of virilization and with PCOS phenotype, the following work-up is recommended. In women who present with symptoms consistent with PCOS, serum total testosterone concentration provides the best overall estimate of androgen production. Even though free testosterone measurement is the single most sensitive test to diagnose hyperandrogenemia, the currently available radio immune assays are not accurate. If free testosterone test is ordered, the method used should be equilibrium dialysis. If the total testosterone level is >200 ng/dl, virializing ovarian tumor must be considered and a pelvic MRI should be ordered. Mild elevation of DHEAS is seen in 30–40% females because in addition to ovarian hyperandrogenemia, many PCOS women also have a component of adrenal hyperandrogenemia. However, if the DHEAS level is more than 700, adrenal tumor must be considered, and CT scan of the adrenal glands must be checked for evaluation [4, 6]. The dexamethasone androgen-suppression test (DAST) helps in delineating the ovarian and adrenal dysfunction of polycystic ovary syndrome (PCOS) and will help differentiate other disorders that mimic PCOS. The response of serum androgens (testosterone and DHEAS) and serum cortisol to dexamethasone are the primary outcomes measured. Serum FSH, LH and estradiol levels must be checked in women who present with amenorrhea. If FSH level is high and the estradiol level is low, this indicates premature ovarian failure (POF). If the FSH level is low and the estradiol levels is low to normal, this indicates hypothalamic amenorrhea, which is common in adolescent females. A LH/FSH ratio of 2 or 3:1 is also diagnostic of PCOS. It is also important to check Thyroid Stimulating Hormone (TSH) and prolactin levels to rule out hypothyroidism and hyperprolactinemia respectively, as the cause of amenorrhea. 17-hydroxy progesterone (17-OHP) level is used to rule out non-classical CAH, which resembles PCOS. Patients with non-classical CAH may have a combination of amenorrhea, hyperandrogenemia and polycystic ovaries (PCO) [34]. This is however prevalent in certain ethnic populations, especially in women of Eastern European Jewish (1:27 prevalence), Hispanic, Slavic or Italian descent. It is important to measure an early morning sample of 17-OHP as there is diurnal variation of this hormone. It is also essential to check an early follicular sample of 17-OHP level. A 17-OHP value of >200 ng/dL is suggestive of nonclassical CAH in an anovulatory cycle but is also compatible with recent ovulation. The high dose cosyntropin (ACTH) stimulation test is recommended to confirm the diagnosis of CAH [35]. If the serum 17-OHP is <1000 ng/dl post ACTH simulation, this excludes the diagnosis of CAH because most CAH patients will have a level of >1500 ng/dl [36].
In women who have cushingoid appearance, 24-hour urine cortisol or urine free cortisol level must be measured to rule out Cushing syndrome. In women with acromegalic phenotype, insulin-like growth factor 1 (IGF-1) level and Oral Glucose Tolerance Test (OGTT) should be checked. Transvaginal ultrasound is the best test for diagnosis of polycystic ovaries (PCO). However, this is not essential for the diagnosis of PCOS if the patient meets the other diagnostic criteria for PCOS. PCO can be seen in around 20% of females without PCOS. This includes women with hypothalamic amenorrhea, hyperprolactinemia, and in normal adolescent females [5]. Hence, consensus groups have urged against using PCO as a criterion for diagnosis of PCOS especially in adolescent females.
Sonogram Morphology for diagnosis of PCO includes the following [37]:
-
12 or more follicles in each ovary measuring 2–9 mm in diameter (25 or more follicles when using newer ultrasound machines)
-
± Increased ovarian volume of >10 mm
-
± String of Pearls (Follicles arranged in the rim of the ovary in a string of pearl fashion. This appearance is diagnostic of PCOS)
-
The presence of these findings in a single ovary is sufficient for the diagnosis of PCO (Fig. 6).
7 Treatment
Treatment of PCOS is multifaceted and aims at targeting the underlying hyperinsulinemia, hyperandrogenemia, and menstrual irregularity [6]. Lifestyle modification is a key factor in the treatment of PCOS patients [38]. It is imperative to educate patients on the importance of low carbohydrate, low glycemic diet and the need for regular exercise. For PCOS patients, consistent daily low to moderate intensity exercise is probably more beneficial than high intensity exercise done 2 or 3 days per week. Even a 5–10% weight loss will significantly help in normalization of most biochemical abnormalities of PCOS and can cause resumption of cycles. Biguanides and Glucagon-like peptide 1 (GLP-1) receptor agonists are insulin sensitizers that have been studied in the treatment of PCOS. Metformin (Biguanide) improves insulin sensitivity, the endocrine and metabolic profiles, hyperandrogenemia and in addition helps with weight loss in obese PCOS patients, according to data from multiple clinical studies [39]. The treatment is usually initiated with 500 mg dose of metformin. This dose is gradually increased by 500 mg on a weekly basis as tolerated, to a maximum dose of 2 gm/day, which is usually achieved in ‘four weeks’ time. This is the dose at which most patients experience maximum benefit. Metformin has been also been shown to increase ovulation rates when compared to placebo, in six Randomized Control Trials (RCT) [40]. The GLP-1 agonist, liraglutide has been shown to be beneficial in treating PCOS patients in several studies [41]. They are very effective with weight loss and in reducing insulin resistance in PCOS patients. One RCT showed that combination of liraglutide and metformin improved the androgen profile beyond weight reduction and was associated with better tolerability [42]. A review of the available clinical trials of GLP-1 use in PCOS, showed that exenatide and liraglutide are effective in weight reduction, reducing androgen levels, increasing menstrual frequency, and improving the glucose parameters and eating behavior [43, 44]. Hyperandrogenic features especially alopecia, acne, and hirsutism can be extremely bothersome to women with PCOS. Oral Contraceptive Pills (OCP’s) are very effective in suppressing testosterone levels and are helpful in addressing the hyperandrogenic symptoms, but the concern is that these may worsen insulin resistance in PCOS and may also increase the risk of venous thromboembolism (VTE) in obese women [45]. A good option when choosing OCP’s would be to start one containing 20 mcg of ethinyl estradiol combined with a progestin with minimal androgenicity and with less likelihood of causing VTE, like norgestimate, norethindrone or norethindrone acetate [46]. Drospirenone and desogestrel are great options for progestin with anti -androgenic properties, but are associated with increased risk of VTE, so are not recommended for PCOS patients [47]. Spironolactone can be used to treat hyperandrogenic symptoms in PCOS females. Spironolactone, an aldosterone antagonist, primarily acts by binding to the androgen receptor as an antagonist. It also inhibits adrenal and ovarian steroidogenesis, competes for androgen receptors in the hair follicles and directly inhibits 5-α-reductase activity. It may typically take around 6–8 weeks for the effects of spironolactone to become apparent [45]. Finasteride is another anti-androgenic agent that may be used to treat alopecia in PCOS. Finasteride competitively inhibits tissue and hepatic 5-α-reductase, thus preventing the conversion of testosterone to dihydrotestosterone and thereby suppressing serum dihydrotestosterone level [48]. Vaniqa (eflornithine hydrochloride cream 13.9%) is a topical drug that inhibits hair growth and is used to treat hirsutism in PCOS patients. Eflornithin acts by inhibiting the enzyme ornithine decarboxylase in the skin, which inhibits cell division and synthetic functions, and therefore reduces the rate of hair growth. It must however be used indefinitely to prevent regrowth of hair [49]. Electrolysis or laser treatment can be used to treat hirsutism in PCOS, but it is important to ensure that the testosterone level is low before starting treatment. Otherwise hirsutism may recur following expensive laser treatment [45]. Hormonal contraception and androgen receptor blockers are effective in improving menstrual irregularity, reducing serum androgens and improving hirsutism, but do not improve insulin sensitivity [6]. The chronic anovulation and hyperestrogenemia seen in PCOS are associated with an increased risk of endometrial hyperplasia and possibly endometrial cancer [26, 50]. To prevent endometrial hyperplasia, it is important to induce cyclic bleeding at least every 2 months in PCOS patients. This may be achieved by either using combination estrogen and progesterone OCP’s or progesterone only pill like minipill daily, to induce cyclic monthly bleeding. Alternatively, patients may be advised to try progesterone either as medroxyprogesterone acetate 10 mg daily or prometrium 200 mg daily, for 10–14 days each cycle to induce a withdrawal bleeding [4]. Progesterone based IUD like mirena are also effective in preventing endometrial hyperplasia. Thiazolidinediones (TZD) have been tried in the treatment of PCOS. They improve insulin resistance and menstrual frequency but have no effect on the serum testosterone level. They also have limited efficacy and there is concern for weight gain and toxicity. Statins have also been studied in the treatment of PCOS. The rationale behind using this was that it helps reduce adrenal hyperandrogenemia in PCOS [6, 45, 51, 52]. Statins do improve low-density lipoprotein cholesterol and lowers the serum testosterone slightly, but has little impact on insulin resistance, improvement in menses, hirsutism or acne [53]. There have been some clinical studies looking into the effects of using two inositol isomers, myo-inositol (MI) and D-chiro-inositol (DCI) in treatment of PCOS [54]. These have inconsistent effect on PCOS ovarian function, including improvement in insulin resistance and serum androgen levels. Some studies have investigated using fenugreek Seed Extract (Trigonella foenum-graecum, Furocyst). While fenugreek may help with resumption of cycles and reduction of ovarian volume in some women, it has no consistent effect on PCOS [55]. Therefore, consensus groups have recommended against using thiazolidinediones, statin, inositol isomers or fenugreek seed extract in the treatment of PCOS. For PCOS women who have difficulty conceiving, referral to a fertility specialist is recommended. Many PCOS patients may require ovulation induction with letrozole or clomiphene to achieve successful pregnancy [56].
8 Conclusions
PCOS is a heterogenous, complex, genetic trait of unclear etiology, comprising of ovarian hyperandrogenism and hyperinsulinemia. Over a hundred candidate genes have been linked to PCOS, and Genome Wide Association Studies on these are ongoing. The pathophysiology of PCOS is multifactorial but is related to insulin resistance in many cases. Most PCOS women demonstrate clinical and biochemical signs and symptoms of hyperinsulinemia and hyperandrogenemia. Hyperinsulinemia may manifest as obesity, difficulty losing weight, prediabetes or Diabetes Mellitus Type II. Hyperandrogenemia may manifest as acne, hirsutism or alopecia. Many PCOS women also have irregular and anovulatory cycles, and some have polycystic ovaries on transvaginal ultrasound. The complications of PCOS encompasses a wide range of metabolic and reproductive disorders ranging from prediabetes, metabolic syndrome and Diabetes Mellitus Type II, to infertility, endometrial hyperplasia and endometrial cancer. Studies have also shown a link between PCOS and Obstructive Sleep Apnea, nonalcoholic steatohepatitis and cardiovascular disease. The treatment of PCOS is multifaceted and aims to reduce insulin resistance, regulate cycles and address patient’s concern of acne, hirsutism and alopecia.
References
Practice Committee of the American Society for Reproductive Medicine. Electronic address Aao, practice Committee of the American Society for reproductive M. Role of metformin for ovulation induction in infertile patients with polycystic ovary syndrome (PCOS): a guideline. Fertil Steril. 2017;108(3):426–41.
Romitti M, Fabris VC, Ziegelmann PK, Maia AL, Spritzer PM. Association between PCOS and autoimmune thyroid disease: a systematic review and meta-analysis. Endocr Connect. 2018;7(11):1158–67.
Mobeen H, Afzal N, Kashif M. Polycystic ovary syndrome may be an autoimmune disorder. Scientifica (Cairo). 2016;2016:4071735.
Horn M, Geraci SA. Polycystic ovary syndrome in adolescents: (women’s health series). South Med J. 2013;106(10):570–6.
Hecht Baldauff N, Arslanian S. Optimal management of polycystic ovary syndrome in adolescence. Arch Dis Child. 2015;100(11):1076–83.
Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2013;6:1–13.
Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod. 2013;28(3):777–84.
Gluszak O, Stopinska-Gluszak U, Glinicki P, Kapuscinska R, Snochowska H, Zgliczynski W, et al. Phenotype and metabolic disorders in polycystic ovary syndrome. ISRN Endocrinol. 2012;2012:569862.
El Hayek S, Bitar L, Hamdar LH, Mirza FG, Daoud G. Poly cystic ovarian syndrome: an updated overview. Front Physiol. 2016;7:124.
Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian Hyperandrogenism revisited. Endocr Rev. 2016;37(5):467–520.
Abbott DH, Dumesic DA, Levine JE. Hyperandrogenic origins of polycystic ovary syndrome – implications for pathophysiology and therapy. Expert Rev Endocrinol Metab. 2019;14(2):131–43.
Tsutsumi R, Webster NJ. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr J. 2009;56(6):729–37.
Christodoulaki C, Trakakis E, Pergialiotis V, Panagopoulos P, Chrelias C, Kassanos D, et al. Dehydroepiandrosterone-sulfate, insulin resistance and ovarian volume estimation in patients with polycystic ovarian syndrome. J Family Reprod Health. 2017;11(1):24–9.
Samsami Dehaghani A, Karimaghaei N, Parsanezhad ME, Malekzadeh M, Mehrazmay M, Erfani N. Anti-nuclear antibodies in patients with polycystic ovary syndrome before and after laparoscopic Electrocauterization. Iran J Med Sci. 2013;38(2 Suppl):187–90.
Hefler-Frischmuth K, Walch K, Huebl W, Baumuehlner K, Tempfer C, Hefler L. Serologic markers of autoimmunity in women with polycystic ovary syndrome. Fertil Steril. 2010;93(7):2291–4.
Kachuei M, Jafari F, Kachuei A, Keshteli AH. Prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Arch Gynecol Obstet. 2012;285(3):853–6.
Gardner SG, Gale EA, Williams AJ, Gillespie KM, Lawrence KE, Bottazzo GF, et al. Progression to diabetes in relatives with islet autoantibodies. Is it inevitable? Diabetes Care. 1999;22(12):2049–54.
Ahmet Menteşe SG, Sümer A, Turan İ, Demir S, Karahan SC, Alver A. Serum anti-carbonic anhydrase I and II antibodies and polycystic ovary syndrome. Turk J Biochem. 2013;38:43–8.
Kyritsi EM, Dimitriadis GK, Kyrou I, Kaltsas G, Randeva HS. PCOS remains a diagnosis of exclusion: a concise review of key endocrinopathies to exclude. Clin Endocrinol. 2017;86(1):1–6.
McManus SS, Levitsky LL, Misra M. Polycystic ovary syndrome: clinical presentation in normal-weight compared with overweight adolescents. Endocr Pract. 2013;19(3):471–8.
Ramanand SJ, Ghongane BB, Ramanand JB, Patwardhan MH, Ghanghas RR, Jain SS. Clinical characteristics of polycystic ovary syndrome in Indian women. Indian J Endocrinol Metab. 2013;17(1):138–45.
Sam S. Obesity and polycystic ovary syndrome. Obes Manag. 2007;3(2):69–73.
Keen MA, Shah IH, Sheikh G. Cutaneous manifestations of polycystic ovary syndrome: a cross-sectional clinical study. Indian Dermatol Online J. 2017;8(2):104–10.
Vgontzas AN, Legro RS, Bixler EO, Grayev A, Kales A, Chrousos GP. Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance. J Clin Endocrinol Metab. 2001;86(2):517–20.
Michaliszyn SF, Lee S, Tfayli H, Arslanian S. Polycystic ovary syndrome and nonalcoholic fatty liver in obese adolescents: association with metabolic risk profile. Fertil Steril. 2013;100(6):1745–51.
Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(5):748–58.
Dumesic DA, Lobo RA. Cancer risk and PCOS. Steroids. 2013;78(8):782–5.
Dokras A, Clifton S, Futterweit W, Wild R. Increased risk for abnormal depression scores in women with polycystic ovary syndrome: a systematic review and meta-analysis. Obstet Gynecol. 2011;117(1):145–52.
Meyer ML, Malek AM, Wild RA, Korytkowski MT, Talbott EO. Carotid artery intima-media thickness in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(2):112–26.
Kim JJ, Choi YM. Dyslipidemia in women with polycystic ovary syndrome. Obstet Gynecol Sci. 2013;56(3):137–42.
Daniilidis A, Dinas K. Long term health consequences of polycystic ovarian syndrome: a review analysis. Hippokratia. 2009;13(2):90–2.
Kaya C, Cengiz SD, Satiroglu H. Obesity and insulin resistance associated with lower plasma vitamin B12 in PCOS. Reprod Biomed Online. 2009;19(5):721–6.
Lashen H. Role of metformin in the management of polycystic ovary syndrome. Ther Adv Endocrinol Metab. 2010;1(3):117–28.
Kurtoglu S, Hatipoglu N. Non-classical congenital adrenal hyperplasia in childhood. J Clin Res Pediatr Endocrinol. 2017;9(1):1–7.
Dessinioti C, Katsambas A. Congenital adrenal hyperplasia. Dermatoendocrinol. 2009;1(2):87–91.
Trapp CM, Oberfield SE. Recommendations for treatment of nonclassic congenital adrenal hyperplasia (NCCAH): an update. Steroids. 2012;77(4):342–6.
Bachanek M, Abdalla N, Cendrowski K, Sawicki W. Value of ultrasonography in the diagnosis of polycystic ovary syndrome – literature review. J Ultrason. 2015;15(63):410–22.
Norman RJ, Davies MJ, Lord J, Moran LJ. The role of lifestyle modification in polycystic ovary syndrome. Trends Endocrinol Metab. 2002;13(6):251–7.
Moghetti P, Castello R, Negri C, Tosi F, Perrone F, Caputo M, et al. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab. 2000;85(1):139–46.
Morin-Papunen L, Rantala AS, Unkila-Kallio L, Tiitinen A, Hippelainen M, Perheentupa A, et al. Metformin improves pregnancy and live-birth rates in women with polycystic ovary syndrome (PCOS): a multicenter, double-blind, placebo-controlled randomized trial. J Clin Endocrinol Metab. 2012;97(5):1492–500.
Lamos EM, Malek R, Davis SN. GLP-1 receptor agonists in the treatment of polycystic ovary syndrome. Expert Rev Clin Pharmacol. 2017;10(4):401–8.
Jensterle M, Kravos NA, Goricar K, Janez A. Short-term effectiveness of low dose liraglutide in combination with metformin versus high dose liraglutide alone in treatment of obese PCOS: randomized trial. BMC Endocr Disord. 2017;17(1):5.
Rasmussen CB, Lindenberg S. The effect of liraglutide on weight loss in women with polycystic ovary syndrome: an observational study. Front Endocrinol (Lausanne). 2014;5:140.
Kahal H, Atkin SL, Sathyapalan T. Pharmacological treatment of obesity in patients with polycystic ovary syndrome. J Obes. 2011;2011:402052.
Sheehan MT. Polycystic ovarian syndrome: diagnosis and management. Clin Med Res. 2004;2(1):13–27.
Mathur R, Levin O, Azziz R. Use of ethinylestradiol/drospirenone combination in patients with the polycystic ovary syndrome. Ther Clin Risk Manag. 2008;4(2):487–92.
de Melo AS, Dos Reis RM, Ferriani RA, Vieira CS. Hormonal contraception in women with polycystic ovary syndrome: choices, challenges, and noncontraceptive benefits. Open Access J Contracept. 2017;8:13–23.
Wu C, Jiang F, Wei K, Lin F, Jiang Z. Effects of exercise combined with finasteride on hormone and ovarian function in polycystic ovary syndrome rats. Int J Endocrinol. 2019;2019:8405796.
Kumar A, Naguib YW, Shi YC, Cui Z. A method to improve the efficacy of topical eflornithine hydrochloride cream. Drug Deliv. 2016;23(5):1495–501.
Haoula Z, Salman M, Atiomo W. Evaluating the association between endometrial cancer and polycystic ovary syndrome. Hum Reprod. 2012;27(5):1327–31.
Dereli D, Dereli T, Bayraktar F, Ozgen AG, Yilmaz C. Endocrine and metabolic effects of rosiglitazone in non-obese women with polycystic ovary disease. Endocr J. 2005;52(3):299–308.
Glintborg D, Andersen M. Thiazolinedione treatment in PCOS—an update. Gynecol Endocrinol. 2010;26(11):791–803.
Sokalska A, Piotrowski PC, Rzepczynska IJ, Cress A, Duleba AJ. Statins inhibit growth of human theca-interstitial cells in PCOS and non-PCOS tissues independently of cholesterol availability. J Clin Endocrinol Metab. 2010;95(12):5390–4.
Benelli E, Del Ghianda S, Di Cosmo C, Tonacchera M. A combined therapy with Myo-inositol and D-chiro-inositol improves endocrine parameters and insulin resistance in PCOS young overweight women. Int J Endocrinol. 2016;2016:3204083.
Swaroop A, Jaipuriar AS, Gupta SK, Bagchi M, Kumar P, Preuss HG, et al. Efficacy of a novel fenugreek seed extract (Trigonella foenum-graecum, Furocyst) in Polycystic Ovary Syndrome (PCOS). Int J Med Sci. 2015;12(10):825–31.
Mejia RB, Summers KM, Kresowik JD, Van Voorhis BJ. A randomized controlled trial of combination letrozole and clomiphene citrate or letrozole alone for ovulation induction in women with polycystic ovary syndrome. Fertil Steril. 2019;111(3):571–8.. e1
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nair, S., Nkamga, Y., Hoover-Hankerson, B. (2020). Polycystic Ovary Syndrome: An Overview of a Complex, Heterogenous Genetic Condition. In: Jain, P., Ndhlovu, L. (eds) Advanced Concepts in Human Immunology: Prospects for Disease Control. Springer, Cham. https://doi.org/10.1007/978-3-030-33946-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-33946-3_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-33945-6
Online ISBN: 978-3-030-33946-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)