Abstract
Given the population increase in the catchment to Chilika Lake and the related changes in land use policies, agricultural practices, and water resource management, this lake has been subjected to increasing anthropogenic influence. As a consequence, the unique biodiversity and primary production within the lagoon decreased, while eutrophication and siltation increased. As a counter-initiative it was decided to artificially open the lake to the sea by dredging. To help trace and quantify the anthropologic effects on Chilika Lake, a combined sedimentologic, chemical, and isotopic study of the lagoon and its sediments is in progress. The results from two campaigns during the monsoon and consecutive dry season suggest that the large gradients in salinity, sediment and nutrient inputs, as well as primary productivity within the lagoon are controlled by variable fluxes of water, sediment, and nutrients from the three separate catchments to the lagoon. Trends in changes of salinity, H- and O-isotope compositions of waters, but also of concentrations and C- and/or N-isotope compositions of the dissolved inorganic carbon (DIC), particulate organic matter (POM), and aquatic plants indicate that mixing in the lagoon occurs between new freshwater inputs and evaporated water within the basin itself. Except for the outer channel, mixing with seawater is limited. In contrast, the C-isotope compositions of the organic matter in the sediments support a higher overall proportion of “marine” or estuarine POM during the past. The latter may be important during the dry season, coupling salinity increase to the changes in DIC and POM carbon isotope compositions. The salinity, DIC, H-, O-, and C-isotope compositions of water are compatible with evaporation as the main driver for a salinity increase, rather than admixtures with seawater.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
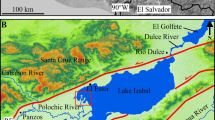
Chilika Lake, the largest lagoon on the Asian continent and second-largest lagoon on Earth, is located on the east coast of India, just south of the Bay of Bengal. Between the wet (monsoon) and the dry (summer) season Chilika Lake has a surface area of 1160 km2 or 900 km2, respectively, with an average depth of only about 1.2 m (between 40 cm to 1.4 m maximum) (Jayaraman et al. 2007; Ghosh and Pattnaik 2005). It is separated from the Bay of Bengal by a sandbar of about 100 m to 1.5 km width, 30 km length, with a channel behind this sandbar that connects the lagoon naturally to the sea. This sandbar developed only during the Late Holocene (3000–4000 years B.P. and possibly as late as 350 years B.P.) The result was a change of Chilika Lake having been a bay open to the sea with abundant mangrove forests to a lagoon that became more isolated with time. While the lagoon thus became more influenced by abundant freshwater input during the monsoon season, it was finally closed off completely from the sea from 1992 to the beginning of 2000 (Khandelwal et al. 2008; Zachmann et al. 2009). Given the freshwater influence from three different drainage basins (northern, central, and southern sectors; Fig. 5.1) and the fact that the natural tidal mouth gets annually displaced towards the sea and hence is adversely affecting the tidal exchange with the sea, the lagoon is subjected to large temporal and spatial salinity gradients (Jayaraman et al. 2007). As such, the lagoon has a characteristic estuarine ecosystem that may still be influenced by seasonal variations of the seawater influx. It has a large and unique biodiversity and genetic ecological biodiversity, representing the largest wintering ground for migratory birds of the Asian subcontinent. It also has an important traditional fishing industry (with about 8000 to 12,000 tons of fish and prawns caught annually in the area) and is of touristic, cultural, religious and spiritual importance. However, by analogy with other lakes and estuarine systems, the anthropogenic influence on Chilika Lake has substantially changed over the past century (Ghosh and Pattnaik 2005): (A) the overall hydrology of the lake changed with the main supply of freshwater from the Mahanadi river basin (Northern Sector) being diverted for hydroelectric power production and because the river is used as a water resource for the cities of Bhubaneswar and Cuttack; (B) the partial compensation of this change in hydrology through the natural closure of the lagoon to the sea, with the adverse effect of a decrease in salinity during the monsoon period over the past few decades and increased silt sedimentation (Pal and Mohanty 2002), increased nutrient supply from the drainage basins, including untreated waste waters, and hence also increased growth of invasive freshwater macrophytes and local eutrophication; (C) changes in land-use policies and agricultural activity with increased rice production and use of pesticides in the main drainage basin (Northern Sector); (D) decreasing biodiversity within the lagoon including fish production with a decrease to about 2000 tons annually during the 1990s with the natural closure to the sea, but a dramatic increase in catch from the year 2000 onwards due to the dredged, man-made opening of the lagoon directly to the sea; (E) increased population pressure and resulting contamination with plastics, oil, waste waters, among others (Pal and Mohanty 2002; Kannan et al. 2005; Baliarsingh et al. 2014).
It is hence clear that the anthropogenic influence on Chilika Lake has substantially changed over the past century (Ghosh and Pattnaik 2005) and this opens questions on the changes in the ecology associated with this evolution. In view of the setting of Chilika Lake and in order to better evaluate the human impacts on the ecology of this shallow water lagoon, three priorities of research were identified:
-
A.
To trace and quantify the hydrological cycle of Chilika Lake.
-
B.
To evaluate the nutrient supply and extent of eutrophication along the shores of the lake, that is to distinguish the autochthonous from allochthonous contributions of organic matter.
-
C.
To reflect on the sedimentologic and ecologic evolution of Chilika Lake by analysis of the sedimentary records.
A comparison of the geochemical compositions measured within the present-day water column to those measured in the sediments allows for a reflection on possible changes in the ecology of the lagoon with time. The three research priorities can be addressed via the geochemical and isotopic compositions of water as well as the particulate organic matter within the water column of the lagoon and the organic matter within the sediments.
The variations in the hydrogen and oxygen isotope compositions of water in a lake or a lagoon can be used as conservative tracers of the mixing processes of the different waters entering the lake or lagoon, as well as tracers for the effects of evaporation that may influence the lake itself (Clark and Fritz 1997; Hoefs 2009). The isotopic compositions of water in each of the drainage basins are, in turn, a function of the mean ambient air temperature of precipitation as well as the so-called “rain-out” effects on the moisture carried by the air mass that supplies the precipitation, including its source of moisture and the ultimate transport distance of the moisture in the air mass (distance from the sea or the point of origin of the evaporated moisture) and/or the atmospheric circulation patterns (Clark and Fritz 1997; Teranes and McKenzie 2001), that is the “continentality” of the precipitation, the altitude of condensation, as well as other factors such as relative local humidity, all of which may lead to condensation and rain formation and thus separation of liquid water from the residual water vapor in the air mass. Ultimately, it is the isotopic fractionation between liquid and vapor water molecules, which changes as a function of temperature, that is responsible for the changes in isotopic composition of rainwater and hence surface water. As a result, the isotopic composition of rainwater is largely positively correlated with mean air temperature and negatively correlated with the amount of rainout experienced by the moisture carried in the air mass (Clark and Fritz 1997).
The amount and the carbon isotope composition of dissolved inorganic carbon (DIC) are controlled by the source of the DIC (lithogenic – by dissolution of carbonate-bearing rocks – or biologic – by respiration of organic matter – or atmospheric uptake in surface and groundwater), the primary production within the water column that uses the DIC as a nutrient, and by equilibrium exchange with atmospheric CO2 as a function of the partial pressures of CO2 in the water column (Clark and Fritz 1997; Hoefs 2009).
The chemical and isotopic compositions of the organic matter within the water column and of organic matter within the sediment are commonly used for (paleo)ecological studies (Jeffrey et al. 1983; Altabet and McCarthy 1985; Kennicutt et al. 1987; Cifuentes et al. 1988; Bernasconi et al. 1997; Hodell and Schelske 1998; Schelske and Hodell 1995; Brenner et al. 1999; Meyers 2003; Lehmann et al. 2004; Michener and Lajtha 2007). The organic matter is a complex mixture of particulate organic remains and living organic tissue. The complexity derives from the multitude of sources and source organisms, the different biosynthetic pathways of the organisms, as well as transformations that occur during the decomposition of the dead organic matter. In aqueous systems in general, the carbon isotope composition of the particulate organic matter (POM) reflects largely that of the living plankton within the euphotic zone. However, depending on the depth of the water column (or residence time in the water column), the redox conditions, pH, and temperature, the POM may undergo changes in its chemical and isotopic composition due to biological reworking (Hodell and Schelske 1998; Bernasconi et al. 1997; Lehmann et al. 2004). This may also apply to the nitrogen isotope composition as well as the nitrogen concentrations suggesting that these effects may be related to the loss of, for example, the more labile amino acids that are relatively rich in 13C and 15N compared to the more refractory but isotopically light lipid fraction (Altabet and McCarthy 1985; Cifuentes et al. 1988; Hodell and Schelske 1998; Bernasconi et al. 1997). However, given that the magnitude of these effects is a direct function of the above-mentioned environmental conditions, a comparison of the chemical and isotopic composition of POM in the water column to that within the sediments allows for an evaluation of the trophic state of the aqueous system. As a corollary, any changes in the chemical and isotopic composition of the organic matter in the stratified sediments deposited over the history of the aqueous system allow for an interpretation on paleoecological changes.
In addition, despite these possible changes in the POM during its passage through the water column and early diagenesis within the sediment, in many aqueous systems a difference in the C/N ratios as well as the isotopic compositions of the POM produced in the euphotic zone of a lake and that of the POM introduced by terrestrial erosion is retained (Meyers 2003). This difference is related to the different carbon and nitrogen cycles in terrestrial and aqueous systems such that, for example, the marine primary production results in autochthonous organic matter with an isotopic composition that is about 7 permil higher in its δ13C value compared to the terrestrially fixed carbon. Similar effects are also noted for the nitrogen system with generally higher δ15N values for primary producers in aqueous compared to terrestrial systems (Michener and Lajtha 2007). Hence, these differences are often used to distinguish marine (or also freshwater) versus terrestrial organic matter sources in sediments, but also help to distinguish the autochthonous from the allochthonous contributions to the total organic matter in the water column and sediments, and hence allows for an interpretation of the local bio-productivity (Sackett and Thompson 1963; Hilton et al. 2008).
5.2 Methods
Samples for this study were taken from the same locations (within the precision of the GPS-coordinates for each location) during two consecutive seasons:
-
1.
The monsoon season from the 7th to the 15th of September 2013, where a total of 188 samples from 56 locations have been collected; 94 of water, 32 of sediments, 53 of plant and 21 of fish (9 sampled during this season, another 12 sampled during the following dry season). 56 locations were sampled for water within the lake, as well as 15 locations for rivers.
-
2.
The dry season during the first week of February 2014. About 46 locations were sampled for water only as some locations previously sampled fell dry during this season. Sediment sampling was also reduced to 32 stations only.
For the water samples all standard parameters (conductivity, temperature, pH, oxygen content) were measured directly in the field, while the chemical and isotopic composition of the water samples (major anions and cations, DIC, particulate organic matter in suspension (filtered to retain the fraction larger than 0.7 μm) was analyzed in the laboratories of the University of Lausanne using standard analytical procedures. In addition, the mineralogy, grain size, qualitative analyses of the ostracods and foraminifera, as well as the C- and N-concentrations and stable isotopic compositions of the organic (and inorganic) fractions within the sediments and sediment cores have been analyzed. In this chapter, the relevant isotopic compositions are discussed in preference, but all measurements are discussed in more detail as part of Masters project studies that focused on different aspects of the lagoon (Delavy and Ecuyer 2014; Lange 2014; Bourgeois 2015; Hostettler 2015).
5.3 Results
5.3.1 Chemical and Isotopic Composition of the Water Column
The hydrological cycle of Chilika Lake can be assessed through stable isotope measurements of water in conjunction with already existing routine measurements (coordinated by the CDA) of the major anions and cations, oxygen concentrations, salinity, and temperature. The latter are routinely analyzed for the 30 monitoring stations covering the different sectors of the lagoon (Northern, Central, Southern Sectors and the Outer Channel), but also for a number of surface and ground waters that enter the lagoon from three different catchments.
The different sectors of Chilika Lake have different average salinities and average stable H- and O-isotope compositions of their waters, both during the monsoon season (Fig. 5.2a) and during the consecutive dry season (Fig. 5.2b). During the monsoon season, this difference is also apparent for the catchments adjacent to the lagoon. The surface water (rivers and ponds) from the Mahanadi catchment that extends well into the interior of India towards the Himalayas, has lower values (δ18Oavg = −4.3 ± 1.2‰, n = 8) compared to the local rainfall and hence surface waters draining into the lagoon in the western (δ18Oavg = −2.9 ± 0.8‰, n = 8) and southern catchments (−1.2 ± 2.2‰, n = 2). Interestingly, this pattern changes somewhat during the dry season as the Northern Sector now has higher average values compared to the Central Sector, while the Southern Sector changes less and retains the highest average values. The order of relative enrichment in the heavy isotopes in the sectors of the lagoon is also paralleled by changes in the order of enrichment in the catchment surface waters (δ18Oavg = −2.8 ± 1.3‰, n = 6; −4.0 ± 1.1‰, n = 5; +0.6‰, n = 1, respectively for northern, central and southern catchments). It has been estimated that about 50% of the freshwater to the lagoon is derived from the northern catchment of the Mahanadi river, about 39% from the western, local catchment and only about 10% from direct precipitation runoff, all largely during the monsoon season, the same season that will also deliver the maximum sediment loads to the lagoon (Zachmann et al. 2009).
It is also clear from Fig. 5.2 that the waters do not plot along the local meteoric water line (LMWL; after Kumar et al. 2010) but rather along lines with a lower slope compared to the LMWL, suggesting that the waters were subjected to evaporation. The evaporation effect is similar for all of the waters in the lagoon as well as those in the catchments, and the regression line through all of these points does not pass through the measurements made for seawater sampled off the Bay of Bengal. An important implication of this is that the water within the lagoon does not represent a mixture of seawater and freshwater from the variable catchments. Instead, all waters have been driven towards higher values in δD and δ18O through evaporative processes and that they are entirely of freshwater origin (Clark and Fritz 1997; Hoefs 2009).
This is also given by differences in salinity that parallel those of the stable isotope composition of water, as well as by differences in carbon isotope composition of DIC (Fig. 5.3). While the concentration and isotopic composition of DIC also change in parallel, the DIC also differs in its composition between the relative drainage basins: δ13CDIC values of –11.1‰, s.d. = 1.31, n = 6, for the northern, agriculturally intensively cultivated terrain, and –12.9‰, s.d. = 0.69, n = 7, for the western catchment rivers draining a largely forested terrain, and –4.8‰ for the Palur canal). Hence, the average C-isotope compositions of the DIC in the sectors are also different. In addition, as a nutrient in the carbon cycle, the DIC is consumed by photosynthetic activity of aquatic plants, further changing the isotopic composition of DIC towards higher 13C content as the isotopically light fraction is preferentially taken up by the biologic material. This has further implications for the carbon cycle in each sector (see below).
The relationship between salinity and oxygen isotope composition and that between salinity and C-isotope composition of DIC, as well as the variations in hydrogen and oxygen isotope compositions of water all indicate trends that are not simple mixing lines with seawater (see the point for the Bay of Bengal), but rather evaporation and CO2 degassing lines. For the C-isotope compositions the trends may also suggest increased primary productivity and biomineralization of the carbon within the lagoon, away from the freshwater sources that introduce the carbon and other nutrients. In the case of the Outer Channel, mixing with seawater is indicated by the trends in salinity, oxygen and hydrogen isotope composition and DIC (Fig.’s 5.2 and 5.3).
5.3.2 Chemical and Isotopic Composition of Particulate Organic Matter in the Water Column and Surface Sediments
To examine the sedimentological and ecological evolution of Chilika Lake the mineralogical, geochemical, and isotopic composition of the sediments as well as the geochemical and isotopic composition of the organic matter within sediments and also for reference that of the particulate organic matter within the water column of the lake were investigated in detail during two field seasons (Delavy and Ecuyer 2014; Bourgeois 2015; Hostettler 2015). The mineralogy and grain size distribution of the sediments is directly related to the erosional evolution of the catchment and the dispersal of the sediment within the lake. As outlined above, the abundance, chemical and stable isotope composition of organic matter can also help to interpret the paleo-environmental and ecological conditions. In addition, calcareous fossils such as ostracods and/or foraminifera can be exploited as sensitive ecological indicators (Hostettler 2015). The ostracod species and their abundance, as well as the geochemical and isotopic composition of their carbonate shells (Sr, Mg, and Ca concentrations as well as C- and O-isotope compositions of calcite shells), have been shown to be indicators of oxygenation, salinity, and temperature of the water column in marine and brackish systems and have been studied for Chilika Lake too (Hostettler 2015), but because of limited space are not discussed further here.
The C-isotope compositions of the POM measured for the two seasons so far have a range that is typical for estuarine systems, notably because of the large range in compositions (Fig. 5.4) (e.g., Sackett and Thompson 1963; Altabet and McCarthy 1985; Cifuentes et al. 1988; Michener and Lajtha 2007). This is to be expected as terrestrially derived particulate carbon either as detritus or as living organic tissue formed within a freshwater-dominated system tends towards δ13C values of about −25‰ for a typical C3 type of vegetation (Sackett and Thompson 1963; Kennicutt et al. 1987; Finlay and Kendall 2007; Michener and Lajtha 2007). In contrast, autotrophic organic matter in freshwater and marine systems may have a wide range of values, from −16 down to −35‰ not being uncommon (Finlay and Kendall 2007). However, in marine systems values normally cluster closer to −23‰ for organic matter formed in surface waters, while lower values are more characteristic of deeper marine waters and or methanotrophic systems (Michener and Kaufman 2007). The principle reason for the large range in C-isotope composition of autotrophic organic matter in both fresh and marine systems is the range in nutrient type and its range in isotopic composition (CO2 or DIC or even CH4 as principle source of carbon and its respective origin – soil horizons, respiration, atmospheric, lithogenic – being the major control for the compositions).
The δ13C values of the SOM (sediment organic matter) are, however, higher than those of the POM sampled during the monsoon and dry seasons in the water column (Fig. 5.4b). The reason for this difference could either be a dominant terrestrial organic carbon source for the sediment organic matter, or a higher proportion of “normal” marine-derived input of carbon that averages about −23‰. A more important marine influence on the organic matter would require that the bulk of the sediment-derived organic matter is relatively old, hence was introduced during periods where the potential marine influence could have been higher as the lagoon was still naturally open to the sea (Khandelwal et al. 2008). As was argued above, todays hydrologic system does not indicate an important entry of seawater to the lagoon, hence also excluding marine-derived nutrients to enter the system (with the exception of the outer channel). Alternatively, proportionally higher sedimentation of organic matter during the summer season (not sampled yet) and dry season could also be indicated by the higher δ13C values of SOM. As indicated in Tables 5.1 and 5.2 and Fig. 5.5a, b, average δ13C values of DIC, as an important nutrient during the dry season where limited terrestrially derived organic matter can enter the lagoon via the riverine input, are higher than those during the monsoon season. As such higher δ13C values for POM using the DIC as major nutrient source during the dry season would be expected (Tables 5.1 and 5.2). Increased sedimentation of such organic matter in conjunction with a decreased freshwater input can also explain the trends in the salinity-δ13CDIC value relationship as well as that between δ18OH2O and salinity. Evaporation as the main driver for a salinity increase is thus accompanied by an increase in δ13CDIC due to continued autotrophic primary productivity and degassing of CO2 during the warm summer season because of a decrease in the solubility of CO2 with increasing water temperature.
The relationship between isotopic composition of DIC and that of the POM is illustrated for the two different seasons in Fig. 5.5a, b. For both seasons the isotopic compositions of the inorganic and organic pools of carbon more or less track each other, with an average difference between these carbon pools of 19–22‰, Δ(DIC-POM). However, there is a considerable spread of values across this line of constant offset. The reason for this spread in values across the line of 20‰ for Δ(DIC-POM) could be a matter of the actual time of measurement of the DIC relative to the period of biosynthesis of the POM. The DIC was sampled over the course of several days only for each season. In contrast, while the POM was sampled at the same time, the material likely represents several days or even weeks of bio-productivity in the water column in addition to an admixture of allochthonous POM from distinct terrestrial sources. Figure 5.5 also indicates that, except for parts of the Northern Sector for which the terrestrial inputs are also likely to be the highest, the 20‰ difference in average δ13C values for the DIC and POM is more tightly correlated during the dry season, even though the large range in values is still preserved.
For the plants within the lagoon, a number of seagrasses were analysed (Eichornia crassipes, Halophila ovalis, Hydrilla verticillate, Potamogeton Pectinatus, Salvinia molesta, Scirpus sp, Vallisneria spiralis, (Delavy and Ecuyer 2014)), including transects in the coastal regions with abundant invasive macrophytes (Phragmites karka). The δ13C values of plants have a range between −12 to −34‰; Fig. 5.6). A variation of several permil has also been measured for individual parts of the same plant (Delavy and Ecuyer 2014). This large range in values suggests large seasonal changes in nutrient sources, possibly also including localized sources of methanogenic carbon and complete reduction of nitrate to ammonia (see below) related to anoxic conditions measured in some shallow water coastal parts.
The N-isotope composition of organic-bound nitrogen in the sediment is similar to that of the plants within the lagoon, with a range in values of between +0.4 to +5.4‰ (+6.6‰; plants) for their δ15N values (relative to Air; Figs. 5.7 and 5.8 (Delavy and Ecuyer 2014)). There is little or no difference between the different sectors though, which is unlike the variation for carbon. These values, together with the relatively low C/N ratios are typical for autochthonous, aquatic primary production of the organic matter (Cifuentes et al. 1988; Meyers 2003; Lehmann et al. 2004; Finlay and Kendall 2007, Michener and Kaufman 2007) rather than allochthonous organic matter of terrestrial origin. However, for the plants a number of transects were sampled in the coastal regions with abundant invasive macrophytes. In some transects, anoxic conditions lead to an abundance of ammonium in the shallow water coastal parts. The plants have values of between +4 up to +6.6‰ in their δ15N values. This suggests large seasonal changes with possible local sources of methanogenic carbon and/or complete reduction of nitrate to ammonium during the growth of the plants. Oxygen levels measured in these waters were below 2 mg/ltr. Given the density of the population along the western lake shores and the associated wastewaters as well as the agricultural activities, much of the nutrients causing the eutrophication along the lake shore may be of anthropogenic origin. This work on the nitrogen cycle is also of importance to an evaluation of the invasive macrophytes that proliferate along the shores and are seen as a potential environmental hazard but may actually be effective filters for excess nitrate and nutrient fluxes into the lagoon.
Plants and SOM from the Outer Channel have higher overall δ15N values, indicative of different sources of N, likely also a larger influence of marine nitrogen sources from the Bay of Bengal. This is compatible with interpretations based on the H- and O-isotope compositions of water, the DIC and salinity relationships for the Outer Channel.
5.3.3 Chemical and Isotopic Composition of Organic Matter in the Sediment Core
A reconnaissance core was taken during the first sampling campaign in September 2013. The sediment core has been investigated for its sedimentological features, mineralogy, fossil contents of ostracods and/or foraminifera and attempts were made to date the top of the core with the radioactive tracer of 137Cs. Given the information on previous cores by Zachmann et al. (2009) and Khandelwal et al. (2008), the present sediment core with about 1.5 to 2 m of sediment, corresponds ideally to the last 2000 years. The last several thousand of years are of particular interest to biogeochemical studies as previous work on pollen by Khandelwal et al. (2008) has shown substantial changes in the biological communities, some of them likely anthropogenic in origin. In addition the agricultural practices have changed drastically and as a result processes of siltation and the nutrient cycles may have been impacted (Ghosh and Pattnaik 2005).
The geochemical results for this core are summarized in Fig. 5.9. The 137Cs activities measured are compatible with a date back to about 1950 for the top 50 cm’s of the core. Figure 5.9 illustrates that while most geochemical parameters remain relatively constant in values over the top 40 cm’s, there is a gradual increase in the amount of organic carbon and nitrogen bound to organic matter. In parallel, the δ13C values of the organic matter preserved in the sediments decrease towards the top while the C/N ratios increase. In view of the above discussion of similar values measured for the SOM in the surface sediments throughout the lagoon, these trends would be compatible with increasing terrestrial input of organic carbon and nitrogen as nutrients over the last 50 or more years (Meyers 2003; Finlay and Kendall 2007; Michener and Lajtha 2007). If the dates for the core can be confirmed, these changes in geochemical records could be related to changes in land use policies and increased agricultural importance within the Chilika Lake drainage basin. This may lead to an increased sedimentation rate, which is required in order to account for the relatively rapid accumulation of the top 40 cm’s of the core in only 40 to 50 years (c.f. the work of Khandelwal et al. 2008).
Sediment core sampled from the Central sector of Chilika Lake in September 2013. Changes in geochemical parameters are given relative to the depth in the core that was sampled. (Figure taken from Bourgeois (2015))
5.4 Conclusions
Present results from two consecutive sampling campaigns representing the monsoon (wet) and the dry season, indicate that the chemical composition of the lagoon waters, including the nutrient supply, are largely controlled by terrestrial inputs from three different drainage basins to the Northern Sector, the Central Sector and the Southern Sector. Mixing of the different waters entering Chilika Lake and within the lagoon is very limited and a seawater influx is only important in the Outer Channel but very limited through the newly dredged seaward channel for the rest of the lagoon. All salinity changes as well as isotopic changes within the lagoon can be accounted for by evaporation and internal bio-productivity as well as degassing of the water column in CO2. As a consequence, there are relatively large seasonal variations in both the isotopic composition of the waters as well as the dissolved organic and inorganic carbon content and the autochthonous produced organic carbon within the lagoon.
While average concentrations and isotopic compositions of both C and N of organic matter in the sediments are relatively constant at the present surface sediments compared to much larger variations in the particulate organic matter within the water column, larger changes are obvious over the last 50 years or so within sediment cores. Hence, in order to avoid unwanted changes in the ecological functioning of the lagoon, the three different drainage basins should be monitored/controlled if further environmental impact by the increased population and agricultural activities on the lagoon are to be limited.
Based on the limited exchange of water between the open marine system via both the Outer Channel and the newly dredged channel, any increased fish catch that may be related to the opening of the new mouth in 2000 is likely to be the result of marine fish that migrates into the lagoon and is readily caught within the shallow waters of the lagoon, rather than an increased nutrient supply and/or oxygenation or circulation of the lagoon waters themselves.
References
Altabet MA, McCarthy JJ (1985) Temporal and spatial variations in the natural abundance of 15N in POM from a warm-core ring. Deep Sea Res 32:755–772
Baliarsingh SK et al (2014) Oil pollution in Chilika lagoon: an anthropogenic threat to biodiversity. Curr Sci 104:516–517
Bernasconi SM, Barbieri A, Simona M (1997) Carbon and nitrogen isotope variations in sedimenting organic matter in Lake Lugano. Limnol Oceanogr 42:1755–1765
Bourgeois G (2015) Historical sediment record in Chilika Lake, India: geochemical, mineralogical and micropaleontological study. Master thesis, Faculty of Geoscience and Environment, University of Lausanne, 81 p
Brenner M, Whitmore T, Curtis J, Hodell D, Schelske C (1999) Stable isotope (δ13C and δ15N) signatures of sedimented organic matter as indicators of historic lake trophic state. J Paleolimnol 22:205–221
Cifuentes LA, Sharp JH, Fogel ML (1988) Stable carbon and nitrogen isotope biogeochemistry in the Delaware estuary. Limnol Oceanogr 33:1102–1115
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. CRC Press, New York
Delavy K, Ecuyer M (2014) Isotopic study of the spatial and temporal origin, source and dynamic of organic matter in Chilika Lake’s estuarine ecosystem, Master thesis, Faculty of Geoscience and Environment, University of Lausanne, 161
Finlay JC, Kendall C (2007) Stable isotope tracing of temporal and spatial variability in organic matter sources to freshwater ecosystems. In: Michener R, Lajtha K (eds) Stable isotopes in ecology. Blackwell Publishing, Malden, pp 283–333
Ghosh KA, Pattnaik AK (2005) Chilika lagoon: experience and lessons learned brief. http://www.iwlearn.net/publications/ll/chilikalagoon_2005.pdf
Hilton RG, Galy A, Hovius N (2008) Riverine particulate organic carbon from an active mountain belt: importance of landslides. Glob Biogeochem Cycles 22:GB1017
Hodell DA, Schelske CL (1998) Production, sedimentation, and isotopic composition of organic matter in Lake Ontario. Limnol Oceanogr 43:200–214
Hoefs J (2009) Isotope geochemistry, 6th edn. Springer, Berlin
Hostettler C (2015) Ecology and geochemistry of living ostracods in Chilika Lake, Odisha, India. Master thesis, Faculty of Geoscience and Environment, University of Lausanne, 72 p
Jayaraman G, Rao AD, Dube A, Mohanty PK (2007) Numerical simulation of circulation and salinity structure in Chilika Lagoon. J Coast Res 23:861–877
Jeffrey AWA, Pflaum RC, Brooks JM, Sackett WM (1983) Vertical trends in particulate organic carbon 13C: 12C ratios in the upper water column. Deep Sea Res Part A Oceanogr Res Pap 30:971–983
Kannan K, Ramu K, Kajiwara N, Sinha RK, Tanabe S (2005) Organochlorine pesticides, polychlorinated biphenyls, and Polybrominated diphenyl ethers in Irrawaddy dolphins from India. Arch Environ Contam Toxicol 49:415–420
Kennicutt MC, Barker C, Brooks JM, DeFreitas DA, Zhu GH (1987) Selected organic matter source indicators in the Orinoco, Nile and Changjiang deltas. Org Geochem 11:41–51
Khandelwal A, Mohanti M, Garcia-Rodriguez F, Scharf BW (2008) Vegetation history and sea level variations during the last 13,500¬†years inferred from a pollen record at Chilika Lake, Orissa, India. Veg Hist Archaeobotany 17:335–344
Kumar B, Rai SP, Saravana Kumar U, Verma SK, Garg P, Vijaya Kumar SV, Jaiswal R, Purendra BK, Kumar SR, Pande NG (2010) Isotopic characteristics of Indian precipitation. Water Resour Res 46:W12548. http://sci-hub.tw/10.1029/2009WR008532
Lange P (2014) Water dynamics in Chilika Lake (India). Master thesis, Faculty of Geoscience and Environment, University of Lausanne, 66 p
Lehmann MF et al (2004) Seasonal variation of the δ13C and δ15N of particulate and dissolved carbon and nitrogen in Lake Lugano: constraints on biogeochemical cycling in a eutrophic lake. Limnol Oceanogr 49:415–429
Meyers PA (2003) Applications of organic geochemistry to paleolimnological reconstructions: a summary of examples from the Laurentian Great Lakes. Org Geochem 34:261–289
Michener RH, Kaufman L (2007) Stable isotope tracers in marine food webs: an update. In: Michener R, Lajtha K (eds) Stable isotopes in ecology. Blackwell Publishing, Malden, pp 238–282
Michener R, Lajtha K (2007) Stable isotopes in ecology. Blackwell Publishing, Malden
Pal SR, Mohanty PK (2002) Use of IRS-1B data for change detection in water quality and vegetation of Chilka lagoon, east coast of India. Int J Remote Sens 23:1027–1042
Sackett WM, Thompson RR (1963) Isotopic organic carbon composition of recent continental derived clastic sediments of eastern Gulf Coast, Gulf of Mexico. AAPG Bull 47:525–528
Schelske CL, Hodell DA (1995) Using carbon isotopes of bulk sedimentary organic matter to reconstruct the history of nutrient loading and eutrophication in Lake Erie. Limnol Oceanogr 40:918–929
Teranes JL, McKenzie JA (2001) Lacustrine oxygen isotope record of 20th-century climate change in central Europe: evaluation of climatic controls on oxygen isotopes in precipitation. J Paleolimnol 26:131–146
Zachmann DW, Mohanti M, Treutler HC, Scharf B (2009) Assessment of element distribution and heavy metal contamination in Chilika Lake sediments (India). Lakes Reserv Res Manag 14:105–125
Acknowledgments
This work would not have been possible without the dedicated investment of time and effort by the “team” of MSc students (Géraldine Bourgeois, Kelly Delavey, Michèle Ecuyer, Caroline Hostettler, Pauline Lange) and researchers (Prakash Kumar Sahoo), which profited from the financial support of the “Fonds d’Investissement” (FINV) of the Faculty of Geoscience and Environment of the University of Lausanne and a student/researcher academic exchange program established between KIIT University in Bhubaneswar and the University of Lausanne. The first FINV was granted to Dr. Jean-Luc Epard, whose help and support for this project is greatly appreciated. The study was also generously supported, financially and logistically, by the Chilika Development Authority (CDA) of Orissa, India, as well as the staff and students of the School of Biotechnology of the KIIT University of Bhubaneswar, India. Finally, Dr. Jorge Spangenberg and Dr. Thierry Adatte are thanked for the assistance with many of the analyses made for these studies. The authors also appreciate the thorough and thoughtful review by an anonymous reviewer.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Vennemann, T. et al. (2020). Sedimentologic, Chemical, and Isotopic Constraints on the Anthropogenic Influence on Chilika Lake, India. In: Finlayson, C., Rastogi, G., Mishra, D., Pattnaik, A. (eds) Ecology, Conservation, and Restoration of Chilika Lagoon, India. Wetlands: Ecology, Conservation and Management, vol 6. Springer, Cham. https://doi.org/10.1007/978-3-030-33424-6_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-33424-6_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-33423-9
Online ISBN: 978-3-030-33424-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)