Abstract
There is growing evidence linking physical activity with stress and depression. The hypothalamic-pituitary-adrenal (HPA) axis is a major modulator of such anxiogenic and depressive behaviors. The aim of the present chapter is to review the current state of knowledge on how different types of physical activities performed by distinct groups of individuals, at determined intensities and volumes, influence the activation of the HPA axis. Animal and human studies will respectively be used to clarify the mechanistic and clinical aspects through which exercise influences the HPA axis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
The HPA Axis
Introduction
Over the last decades, important discoveries have allowed exercise science to bloom as a research field. Practical applications in kinesiology influence a wide range of populations including individuals with diverse degrees of disabilities to high-performance athletes. Important advances include the optimization of training techniques, biomechanics, motor skills, periodization, and injury prevention. Sports psychology is another emerging discipline recognized to have a profound impact on active individuals in terms of adherence and compliance to a training program as well as physical improvements and raw performance. As for injury prevention, it is now generally accepted that physical activity must be performed in an equilibrated way in order to maximize the desire to pursue while reducing the risks of nonadherence, of non-compliance, and of developing psychological disorders. Since its discovery, the hypothalamic-pituitary-adrenal (HPA) axis was shown to play a major role in the control of anxiogenic and depressive behaviors. A growing evidence indicate that exercise exerts acute and chronic effects on the HPA axis. However, the mechanisms through which it influences the HPA axis, and vice versa, remain to be clarified. To add to the complexity, a wide range of HPA axis responses are reported in different populations. These are generally proposed to depend on the type of physical activity, the intensity, and the volume at which it is achieved. Hence, overtraining and the dynamic progression of performance could also influence the relationship between exercise and the HPA axis. The present chapter will review the current state of knowledge to clarify how exercise influences the HPA axis.
Defining the HPA Axis
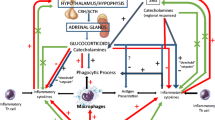
The HPA axis consists of three structurally independent components including the hypothalamus, the anterior pituitary, and the adrenal cortex (see Fig. 3.1). These structures are intimately interacting through the release of neuroendocrine messengers. In the medial parvocellular and the magnocellular parts of the paraventricular nucleus of the hypothalamus (PVH), corticotropin-releasing factor [CRF, a 41-amino acid (aa) peptide] and arginine vasopressin (AVP, expressed in approximately half of the CRF neurons) are synthesized [1]. CRF neurons project to the exterior layer of the median eminence and release CRF into the portal circulation until they subsequently reach corticotroph cells from the anterior pituitary to stimulate the secretion of adrenocorticotropic hormone (ACTH). In turn, ACTH is released and transported via the general circulation to activate the adrenal secretion of glucocorticoids. Importantly, it is known that glucocorticoids negatively control pituitary corticotrophs and PVH CRF neurons through direct or hippocampus-mediated feedback inhibition mechanisms [2, 3].
In mammals, the CRF system is not limited to PVH CRF neurons. The system also comprises two CRF receptor types (CRF-R1 and CRF-R2) [4], a CRF-binding protein [5] and endogenous CRF receptor ligands, that include mammalian peptides CRF [6], urocortin (UCN) [7], UCN II [8, 9], and UCN III [9, 10]. In the brain, the broad distribution of CRFergic cells, UCNergic neurons, and CRF receptors is compatible with the main functions attributed to the CRF system [11]. Central administration of CRF evokes autonomic responses [2, 3], general arousal [12], as well as anxiety-like behaviors [3, 13]. Furthermore, central CRF injections also activate the sympathetic while inhibiting the parasympathetic branches of the autonomic nervous system by stimulating cardiorespiratory functions [14] and reducing the activity of the digestive system [15]. Because of their selectivity for CRF-R2, UCN II and UCN III [10] (also referred to stresscopin in humans) have been described as “stress-coping” peptides capable of exerting anxiolytic effects [9].
AVP is a 9-amino acid (aa) peptide with a disulfide bridge that is mainly secreted from the magnocellular cells of the supraoptic nucleus and the PVH and transported to the circulation to exert its effects on kidneys and blood vessels [16, 17]. In addition, AVP’s expression is also reported in the parvocellular neurons of the bed nucleus of the stria terminalis, the medial amygdala, the suprachiasmatic nucleus, and the PVH [18,19,20]. Three major types of AVP receptors are known: AVPR1a, AVPR1b, and AVPR2 [21, 22]. The activation of AVPR1b in the anterior pituitary stimulates the release of ACTH [23], while AVPR1a and AVPR2 are mainly expressed in the kidneys and blood vessels [24].
ACTH is a 39 aa peptide derived from the proteolytic cleavage of the proopiomelanocortin (POMC) gene [25,26,27]. The expression of ACTH is modulated positively by CRF and AVP, naloxone, interleukins (IL) IL-1 and IL-6, as well as leukemia inhibitory factor (LIF), but negatively by glucocorticoids [28,29,30,31,32]. However, other factors such as pituitary adenylate cyclase-activating peptide (PACAP), catecholamines, ghrelin, nitric oxide synthase (NOS), dihydroxyphenylalanine (DOPA), serotonin, and γ-aminobutyric acid (GABA) are also suspected to influence ACTH secretion through still ill-defined mechanisms [33,34,35]. ACTH is released in a pulsatile manner and has been shown to be regulated through a calcium-dependent mechanism [36]. It is subsequently transported in the circulation to activate the melanocortin type 2 receptor (MC2R) from the adrenal glands [37, 38] and, ultimately, stimulate species-specific glucocorticoid (either cortisol in human, nonhuman primates, pigs, and dogs or corticosterone in laboratory rodents such as rats and mice) synthesis and secretion [39]. In a matter of seconds to minutes, the release of glucocorticoids from adrenal glands will activate glucocorticoid receptors (GR), stimulate annexin 1 (ANXA1) production, and, consequently, block CRF-induced ACTH secretion [40, 41]. It is however suggested that the level of complexity of the direct and indirect mechanisms through which glucocorticoids exert their repressive effects on the HPA is much higher than what was anticipated during the 1980s [1].
Other mediators of the HPA axis were identified over the last decades. For instance, the gut microbiota is now proposed to influence anxiogenic and depressive behaviors via its effects on the HPA axis. Germ-free (absence of gut microbiota) chronically restrained mice display antianxiety behaviors but increased CRF, ACTH, cortisol, and aldosterone levels in hypothalamic tissues compared to specific pathogen-free microbiota mice [42,43,44,45]. Although the microbial mechanisms influencing these effects remain ill-defined, it is proposed to regulate glucocorticoid receptor sensitivity (Fkbp5), steroidogenesis (MC2R, StaR, Cyp11a1), and catecholamine synthesis (TH, PNMT) [46]. Hence, colon expression of 11-β hydroxysteroid dehydrogenase 1 (11HSD-1), CRF, urocortin II and its receptor, and CRFR2 as well as cytokines TNFα, INFγ, IL-4, IL-5, IL-6, IL-10, IL-13, and IL-17 is also reported to be modulated by the microbiota.
As recently evidenced, there is an intimate link between the regulation of the HPA axis and inflammatory cytokines [47]. For instance, interleukin 1β (IL-1β) is reported to influence the release of CRF in the hypothalamus, ACTH in the pituitary, and glucocorticoids in the adrenal cortex [48,49,50,51,52,53]. It was also reviewed that IL-6 and TNF-α promote the activation of the HPA axis [54]. Some of these effects are mediated through the activation of cyclooxygenase enzymes (prostaglandins) as well as by brain nitric oxide, noradrenaline, and serotonin production [55]. Interestingly, the translocation of endotoxins (derived from Gram-negative microbial components such as lipopolysaccharides/LPS and others) was previously shown to activate the HPA axis through the release of IL-1, IL-6, and TNF-α [56]. This reinforces the existence of an intimate relation between the gut (and the microbiota) and the brain for the regulation of the HPA axis.
Brain-derived neurotrophic factor (BDNF) is another factor with an influence on the HPA axis. For example, a single bout of exercise was shown to stimulate hippocampal BDNF expression in mice [57]. In humans, carriers of the Val66Met BDNF allele (prevalence of up to 50% and 32% in Asians and Caucasians, respectively [58]) were shown to display increased HPA axis activity through a higher cortisol response to stress [59, 60]. Expression of BDNF is co-localized with CRF and AVP in the PVH and the lateral ventricle [61]. Hence, BDNF administrations increased the expression of CRF while exerting the opposite effect on AVP in the parvocellular and magnocellular PVH portions. Hence this treatment was likely to promote CRF secretion since its levels were decreased, while those of AVP were higher in the hypothalamus. This hypothesis is supported by the fact that the administration of BDNF also upregulated ACTH and corticosterone plasma concentrations.
The HPA Axis and Exercise
Endurance Training
The effect of endurance training on the activation of the HPA axis has been investigated extensively in animal and human models. In pigs submitted to a high-fat diet, a 200% increase in free fatty acid (FFA) levels is related to a 40% decrease in ACTH concentrations in response to stress [62]. In the same study, pigs submitted to an endurance training program displayed a 60% increase in ACTH following a stress challenge; this effect was associated with a 56% decrease in FFA without other changes in body composition and insulin sensitivity. In another study, rats confined to a cage that allowed voluntary wheel running, corticosterone responses to various stimulatory challenges of the HPA axis were shown to be significantly higher than in untrained animals [63, 64]. Interestingly, this enhanced adrenal sensitivity to ACTH was completely restored to normal following 5–8 weeks of exercise training. In an ovine model, ACTH levels were found to rise in response to exercise, even though the animals had been previously submitted to a CRF infusion [65]. The latter suggests that ACTH release could be stimulated by other factor than CRF, and the authors suggested AVP as a plausible candidate. Endurance training upregulated mRNA expression of BDNF and its receptor TrkB in the hippocampus, midbrain, and striatum while increasing BDNF levels in the hippocampus and striatum in rats [66]. On the other hand, sprint interval training was more effective to enhance BDNF brain content than intensive endurance training in rats [67]. These increased BDNF levels in the brain were also shown to be associated with reduced anxiety- and depression-like behaviors in tested animals.
In human studies, the activation of the HPA axis in response to physical activity has been abundantly reported. For instance, individuals submitted to chronic endurance training displayed higher hair cortisol [68]. In endurance-trained men, after a day without physical exercise, ACTH and cortisol concentrations were similar to those of untrained controls [69]. For most of these athletes, dexamethasone (a synthetic agonist of the glucocorticoid receptor) was not found to influence the activity of the HPA axis; however, in contrast to untrained subjects, a subsequent administration of CRF was shown to increase cortisol levels. On the other hand, obese adolescents submitted to a chronic physical activity program displayed a marginal decrease in glucocorticoid sensitivity and increased levels of glucocorticoid receptor-α (GR-α) expression in blood mononuclear cells [70]. In young men who were previously undergoing a strength training program, cortisol responses were significantly increased when submitted to higher frequencies of endurance training [71]. Twenty weeks of endurance training were also shown to decrease basal cortisol levels [72]. Hence, the magnitude of the reduction in cortisol levels was significantly associated with increases in local skeletal muscle endurance. As observed in animals, endurance training also significantly upregulated basal BDNF circulating levels in healthy sedentary or physically active males, and the authors suggest that this effect could promote brain health in these populations [73, 74].
The influence of an acute bout of endurance exercise on HPA axis activity has also been investigated in a multitude of studies. In response to a walk on a treadmill until exhaustion at 40 °C, circulating levels of cortisol were higher in trained than in untrained individuals, while those of ACTH were not different [75]. Interestingly, in response to the same challenge in trained and untrained individuals, ACTH, norepinephrine, and dehydroepiandrosterone-sulfate (DHEA-S) levels were significantly increased, while those of growth hormones (GHs), aldosterone and epinephrine, were initially elevated but reached a maximal value (plateau) at 38.5 °C. In athletes submitted to a strenuous exercise, CRF and cortisol responses to HPA activation were not blunted by physiological endogenous hypercortisolism, and this suggests that pituitary sensitivity is decreased in response to the feedback inhibition induced by cortisol [76]. As noted, acute physical activity has been reported to influence HPA axis activity; however, the relevance of considering other physiological conditions should not be neglected. In fasting subjects submitted to physical exhaustion, ACTH and cortisol levels significantly increased in hypoglycemic conditions, but this effect was abolished when pretest glycemic levels were maintained [77]. This also suggests the relevance of further examining the HPA axis activation under hypoglycemia.
While the abovementioned information indicates that HPA axis activity is modulated by chronic and acute training, it is also important to evaluate the effect of a recuperation phase. In runners, it has been observed that cortisol and ACTH levels are significantly lower 2 days following a marathon, while whole body 11β-HSD-1 and ghrelin levels are upregulated [78]. Also, the suppression of cortisol in response to a dexamethasone challenge is strongly increased after 6 weeks of reduced training.
Resistance Training
Although the effect of endurance training on the activation of the HPA axis is abundantly described, fewer studies have evaluated the effect of resistance training. Resistance training can be defined as any exercise program using one or multiple training strategies (own body mass, free weights, or diverse exercising machines), to enhance health, fitness, and performance [79]. In healthy untrained men submitted to acute resistance training, cortisol concentrations were not modulated [80]. However, in the same subjects, catecholamines, lactate, TNF-α, IL-2, and epidermal growth factor (EGF) levels increased, while monocyte chemotactic protein-1 (MCP-1) concentrations decreased. Furthermore, a positive correlation was observed between the concentrations of cortisol and TNF-α. Interestingly, the type and the intensity at which resistance training is performed are suggested to influence the HPA axis. In competitive athletes performing in muscular power disciplines (alpine ski, bodybuilding, and volleyball), an isokinetic exercise induced higher acute increases in ACTH, cortisol, and lactate than in endurance athletes (marathon, triathlon, cross-country skiing, and rowing) [81]. However, this effect was not observed during the recovery period. The type of training is reported to influence the activation of the HPA axis; however the effects of the intensity and volume of resistance training needed to be clarified. Interestingly, significantly lower cortisol levels were measured after a single bout of high-intensity resistance training (HIT) then after performing a traditional 3-set protocol in male college students [82].
Age, gender, circadian rhythm, and body composition are other factors that are often reported to influence hormonal secretions (see Copeland, Chap. 23 in this book). Studies were conducted to clarify the effects of age, gender, circadian rhythm, and body composition on the activation of the HPA axis. Young and middle-aged men were submitted to an 8-week resistance training program which was shown to decrease both basal cortisol and ACTH levels [83]. However, age did not have a significant influence on the results. In contrast, 9 weeks of combined endurance and resistance training was shown to increase cortisol levels by 23% in young sedentary women, but this effect was not observed in their male counterparts [84]. This suggested that women undergoing physical training are more sensitive to the activation of the HPA axis than males. To determine the role of the circadian rhythm on the activation of the HPA axis, trained subjects were instructed to perform the same resistance training session at three time periods over different days. Cortisol levels were higher in the morning but decreased 3 min and up to 48 h after performing their bout of exercise [85]. This indicated the importance of considering the time at which blood samples are collected before, during, and after undergoing a session of resistance training. Contrastingly, after submitting untrained young males to 11 weeks of resistance training, the time of the day at which exercises were performed did not influence the levels of hormones of the HPA axis [86]. However, the same authors reported that postexercise cortisol levels were lower than basal concentrations. To determine the effects of body composition on the activation of the HPA axis, normal weight and obese individuals were submitted to resistance training. Cortisol levels were significantly different between normal weight and obese individuals [87]. This suggested that body composition may also modulate the HPA axis.
Different types of resistance training promote skeletal muscle hypertrophy or strength. Untrained young male and female adults were recruited to clarify the different effects of the two types of resistance training on the HPA axis. While performing the experimental protocol, significantly higher BDNF levels were measured during the exercise designed to promote hypertrophy than the one intended to increase strength [88]. In trained men, BDNF levels increased similarly in response to the different intensity and volume levels of resistance training [89]. In older adults submitted to various loads of resistance training, BDNF levels increased in male participants, while no effects could be detected in female individuals [90]. These data support the hypothesis that the HPA axis activation might be influenced by the type of training, the intensity, the post-training period, and body mass but not by age or the time of the day at which it is performed. These latter issues are in need of further investigations to clarify aspects of the contradictory results.
Intensity of Physical Activity and HPA Axis Activation
It is profusely reported that the HPA axis is activated in response to physical activity, and different levels of exercise intensity were also shown to have an important impact. In mice submitted to acute psychological stress, high-intensity physical activity increased cortisol, IL-1β, IL-2, and IL-6 while decreasing ACTH-positive cells in the pituitary [91]. Although collected in animals, these results indicated the relevance of considering the intensity of physical activity, and this was also investigated in human models. For instance, it was initially proposed that cortisol levels are increased by 60 min of running on a treadmill at a threshold intensity of 60% of the VO2max [92]. Moderately trained men also displayed a significant increase in cortisol after performing 30 min of exercise at 60% and 80% of their VO2max, while ACTH levels were only elevated at the highest intensity [93]. In endurance-trained males, 30 min of exercise on a cycle ergometer, significant increases in cortisol were only observed at 80% of the VO2max both in saliva serum [94]. Interestingly, the same authors observed that peak cortisol levels were only monitored 30 min after the cessation of the physical activity. When compared to low-intensity, high-intensity cycling caused similar increases in BDNF and cortisol levels in both participants with or without depression [95]. In trained athletes submitted to a prolonged high-intensity exercise, increased plasma concentrations of cortisol, ACTH, CRF, and AVP were observed [96]. It was also reported that the rise in osmolality observed during exercise correlates with increases in plasma AVP. Furthermore, for a given type of physical activity, high-intensity and prolonged duration respectively increased AVP and CRF levels. In healthy participants administered with dexamethasone (4 mg), performing physical activity at the highest intensity (90% vs. 100% maximal aerobic capacity) caused a significant raise in ACTH, cortisol, and AVP circulating levels [97]. Interestingly, this response was shown to be amplified in women with regard to the one observed in men. Interestingly, high-intensity interval training was shown to increase BDNF levels to a higher magnitude than continuous moderate-intensity exercises in obese individuals [98]. This suggests that short and intense bouts of exercise could exert beneficial effects to individuals intending to design and/or perform physical activity programs.
While the effect of exercise intensity was evaluated in response to distinct physical activities, another group compared occupational differences between workers performing high-intensity duties (slaughterhouse workers) and others achieving low-intensity tasks (office workers) [99]. Slaughterhouse workers displayed higher levels of ACTH, total peroxides, antioxidant capacity, oxidative stress index, and c-reactive protein (CRP), while their levels of endogenous peroxidase activity, polyphenols, and BDNF were reduced. These results were even affected by the duration of the work shifts in slaughterhouse workers since higher CRP and lower BDNF levels were measured after completing 12 h vs. 8 h shifts.
Results presented in this section clearly indicate that the intensity and the volume of a physical activity, the fitness level, and the type of exercise performed by an individual have a direct impact on the activation of the HPA axis. In turn, this should be taken into consideration when elaborating training programs.
Highly Trained and Elite Athletes
Overall, the increased activity of the HPA axis in highly trained athletes could have important implications on their somatic and mental health. During a progressive stress test until exhaustion on a treadmill, cortisol levels were higher from baseline to the initiation of recuperation in professional athletes than in controls [100]. Interestingly, hormonal levels were regularized over the recuperation period. In ultramarathon runners, cortisol levels were at their highest at the completion of a 622 km race, and levels were only normalized after 6 days of recovery [101]. In highly trained athletes, the morning surge in ACTH and cortisol was observed earlier, and ACTH levels were significantly higher than in normal individuals [102]. In addition, the stimulation of CRF and ACTH release was more pronounced in highly trained athletes than in untrained individuals following the administration of the nonselective opioid receptor antagonist naloxone [32]. Altered HPA axis functions were also observed in elite athletes. For instance, artistic gymnasts competing at the European Championships displayed higher salivary cortisol concentrations and more important levels of psychological stress than controls [103]. In addition, higher psychological stress and saliva cortisol levels were also observed in female vs. male athletes. In elite junior soccer players, nonfunctional overreaching performances were associated with higher scores of depression and angriness, whereas resting GH and ACTH concentrations after maximal effort were diminished [104]. These observations could be associated with the decreased expression of GR-α mRNA in highly trained individuals and with lower increases in atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) levels in response to exercise [105,106,107,108,109,110]. These elements suggest the influence of the HPA axis on stress and emotional status. Ultimately these factors could also have a major incidence on sportive performances in elite athletes.
Overtraining
The available information regarding altered HPA axis functions in athletes suggests the relevance of considering potentially for pathological conditions such as overtraining. In rats submitted to daily swimming bouts of 45 min 5 days per week for 2, 4, or 6 weeks, corticosterone gradually increased. In parallel both basal ACTH and corticosterone plasma levels increased until they reached a plateau after 6 weeks of swimming [111]. Hence, in the PVN and the pituitary of the same animals, mRNA expression of the glucocorticoid receptor decreased, while the one of CRF transiently increased. While these results are interesting, it is difficult to determine whether the important volume of exercise to which rats were submitted can be considered as overtraining. These results raise important questions since cortisol levels were significantly below normal in overtrained Standardbred racehorses [112, 113]. Interestingly, these discrepancies may be species-specific or be related to the duration overtraining in the animals. In other words, rats submitted to swimming may still have the capacity to produce corticosterone, while Standardbred racehorses may have been submitted to a chronic overactivation of the HPA axis which led to impairments in their capacities to secrete cortisol before being diagnosed. In different populations of human athletes, several alternative methods such as a CRF stimulation test (evaluation of basal ACTH concentrations and GH pulsatility), free testosterone over cortisol concentrations, low basal cortisol levels, as well as HPA responses to two standardized exercise tests were proposed for the diagnostic of overtraining [114,115,116]. For instance, in response to two acute bouts of exercise, increased prolactin (PRL) levels and decreased ACTH concentrations are reported in overtrained athletes [117,118,119]. These effects could be mediated by the repetitive occurrence of muscle and skeletal trauma resulting in local inflammation and, consequently, in a systemic inflammatory responses which, in turn, could yield to impairments of athletic performances [115].
Postexercise Recuperation
Depending on the type of physical activity and its intensity and volume, it is critical to allow the body to recuperate, replenish its energy reserves, and resynthesize injured tissues in order to improve athletic performance. Recuperation is well-characterized in nutrition and physiology; however, it is another factor to take into consideration when considering the effects of exercise on the HPA axis. For instance, it was shown that the carbohydrate/electrolyte consumption right after performing a bout of high-intensity physical activity significantly reduced blood cortisol levels in male athletes [120]. However, the hydration status, per se, was not associated with an alteration of circulating cortisol concentrations [121]. In rugby players submitted to a magnesium supplementation, significantly higher ACTH but decreased cortisol levels were observed compared to the same type of participants given a placebo [122]. In addition, magnesium supplementation abolished the post-game increase in IL-6 while reducing the increase in neutrophil/lymphocyte ratio.
Memory, Defeat, Fear, and Cognitive Functions
During a physical activity, the capacity to remember how to optimally perform an exercise as well as the bad feelings and the fear of defeat or mishaps occurring during the event may have profound effects on an individual’s performance. Because of obvious ethical reasons, this is difficult to investigate in humans. However, rodent models were used to investigate the effect of the HPA axis on memory, defeat, and fear. In rats administered with metyrapone (a corticosterone synthesis disruptor), impaired traces of fear conditioning have been observed [123]. A number of studies also evaluated the effects of CRF on defeat conditioning as well as on memory. The central administration of anti-sauvagine-30 (a CRF-R2 receptor antagonist) reduced submissive and defensive behaviors induced by territorial aggression conditioning in Syrian hamsters [124]. However this effect was not observed in response to neither metyrapone nor CP-154,526 (a CRF-R1 antagonist) administrations in the lateral ventricle of rats increased spatial memory through a β-adrenergic-dependent mechanism [125]. Also, central administrations of NBI30775 (CRF-R1 antagonist) prevented stress-induced hippocampal dendritic spine loss while restoring stress-impaired cognitive functions [126]. This suggests that stress-induced central effects are mediated through the activation of CRF-R1. These discoveries are important since they could allow the implementation of targeted interventions or pharmacological treatments to reduce the fear of defeat or the occurring of an injury in individuals or athletes who previously encountered such negative experiences. In turn, this would allow preventing the adverse outcomes on their athletic performances.
Conclusion
The last paragraphs have underlined the importance of the HPA axis on the regulation of moods and behaviors in animals and humans undergoing physical activity. Depending on the population of interest and the objectives to be reached, it is critical to adapt training programs to maximize their benefits while minimizing the risk of developing anxiety and depression. This has to be applied to athletes as well as other populations with different levels of fitness and/or degrees of disabilities. For instance, professional or Olympic athletes are particularly at risk of overtraining, and their moods, cognitive functions, and confidence levels have an important impact on their performances. Physical activity is also an important element of a healthy lifestyle to prevent and/or counteract the dreadful effects of obesity and ensuing metabolic dysfunctions that have reached epidemic levels in North American populations. In obese individuals, adherence and compliance to training programs remain major obstacles. For athletes or obese individuals, a better understanding on how exercise modulates the HPA axis will provide essential tools to develop novel training approaches. As one consequence of this, it will be essential to exhaustively characterize individuals undergoing an exercise program in order to determine their levels of fitness; the type, intensity, and volume of physical activity required; as well as the window of time over which objectives need to be achieved. It is also important to constantly monitor exercising individuals since adaptation to the training planification will be required as soon as anxiogenic or depressive behaviors will be present. Hence, distinct factors of the HPA axis may be used as sensitive biomarkers to detect disorders before clinical symptoms can be detected. Globally, this indicates the relevance of including parameters of the HPA axis as modulators of anxiety and depressive behaviors in exercising individuals. In sum, while it remains a precarious equilibrium, it suggests that elements of the HPA axis must be taken into consideration along with the assessment of an individual’s physical capacities when designing a training program. Consequently, while the planification and periodization must be optimized, it is important to adapt the program accordingly in function of early signs of anxiogenic or depressive behaviors. Ultimately, this will be more effective at yielding improvements in athletic performance and health benefits than the simple addition of strenuous exercises that could provoke the premature interruption or to slow down physical training program for various clinical reasons.
Our current knowledge of the relationship between the HPA axis and physical exercise reviewed in the above paragraphs clearly highlights the importance of adequate preparation for exercise. Also, a number of data indicate that complex molecular and cellular mechanisms intervene in highly trained athletes that do not occur in normal individuals. As a whole, the above information suggests that the HPA axis importantly influences stress-induced functions and that the intensity of HPA axis activation is intimately related to the type of training, the intensity, and the volume at which it is performed. This strongly suggests the relevance of considering the impact of the HPA axis when elaborating training programs in different types of individuals.
References
Watts AG. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: a complexity beyond negative feedback. Front Neuroendocrinol. 2005;26:109–30. https://doi.org/10.1016/j.yfrne.2005.09.001.
Brown MR, Fisher LA. In: Nemeroff CB, De Souza EB, editors. Corticotropin-releasing factor: basic and clinical studies of a neuropeptide. CRC. Taylor & Francis group 1990. p. 291–8.
Heinrichs SC, Tache Y. Therapeutic potential of CRF receptor antagonists: a gut-brain perspective. Expert Opin Investig Drugs. 2001;10:647–59. https://doi.org/10.1517/13543784.10.4.647.
Muller MB, Wurst W. Getting closer to affective disorders: the role of CRH receptor systems. Trends Mol Med. 2004;10:409–15. https://doi.org/10.1016/j.molmed.2004.06.007.
Linton EA, Behan DP, Saphier PW, Lowry PJ. Corticotropin-releasing hormone (CRH)-binding protein: reduction in the adrenocorticotropin-releasing activity of placental but not hypothalamic CRH. J Clin Endocrinol Metab. 1990;70:1574–80.
Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–7.
Vaughan J, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–92. https://doi.org/10.1038/378287a0.
Reyes TM, et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98:2843–8. https://doi.org/10.1073/pnas.051626398.
Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7:605–11. https://doi.org/10.1038/87936.
Lewis K, et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–5. https://doi.org/10.1073/pnas.121165198.
Turnbull AV, Rivier C. Corticotropin-releasing factor (CRF) and endocrine responses to stress: CRF receptors, binding protein, and related peptides. Proc Soc Exp Biol Med. 1997;215:1–10.
Koob GF, Cole BJ, Swerdlow NR, Le Moal M, Britton KTS. performance, and arousal: focus on CRF. NIDA Res Monogr. 1990;97:163–76.
Krysiak R, Obuchowicz E, Herman ZS. Role of corticotropin-releasing factor (CRF) in anxiety. Pol J Pharmacol. 2000;52:15–25.
Fisher LA, et al. Corticotropin-releasing factor (CRF): central effects on mean arterial pressure and heart rate in rats. Endocrinology. 1982;110:2222–4.
Taché Y, Gunion MM, Stephens R. In: Nemeroff CB, De Souza EB, editors. Corticotropin-releasing factor: basic and clinical studies of a neuropeptide. CRC. Taylor & Francis group 1990. p. 299–307.
Brownstein MJ, Russell JT, Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980;207:373–8.
Nishimura H, Fan Z. Regulation of water movement across vertebrate renal tubules. Comp Biochem Physiol A Mol Integr Physiol. 2003;136:479–98.
Dogterom J, Snijdewint FG, Buijs RM. The distribution of vasopressin and oxytocin in the rat brain. Neurosci Lett. 1978;9:341–6.
Buijs RM. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res. 1978;192:423–35.
DeVries GJ, Buijs RM, Van Leeuwen FW, Caffe AR, Swaab DF. The vasopressinergic innervation of the brain in normal and castrated rats. J Comp Neurol. 1985;233:236–54. https://doi.org/10.1002/cne.902330206.
Michell RH, Kirk CJ, Billah MM. Hormonal stimulation of phosphatidylinositol breakdown with particular reference to the hepatic effects of vasopressin. Biochem Soc Trans. 1979;7:861–5.
Jard S, Lombard C, Marie J, Devilliers G. Vasopressin receptors from cultured mesangial cells resemble V1a type. Am J Phys. 1987;253:F41–9.
Antoni FA, Holmes MC, Makara GB, Karteszi M, Laszlo FA. Evidence that the effects of arginine-8-vasopressin (AVP) on pituitary corticotropin (ACTH) release are mediated by a novel type of receptor. Peptides. 1984;5:519–22.
Bankir L. Antidiuretic action of vasopressin: quantitative aspects and interaction between V1a and V2 receptor-mediated effects. Cardiovasc Res. 2001;51:372–90.
Bicknell AB. The tissue-specific processing of pro-opiomelanocortin. J Neuroendocrinol. 2008;20:692–9. https://doi.org/10.1111/j.1365-2826.2008.01709.x.
Oliver RL, Davis JR, White A. Characterisation of ACTH related peptides in ectopic Cushing’s syndrome. Pituitary. 2003;6:119–26.
Raffin-Sanson ML, de Keyzer Y, Bertagna X. Proopiomelanocortin, a polypeptide precursor with multiple functions: from physiology to pathological conditions. Eur J Endocrinol. 2003;149:79–90.
Papadimitriou A, Priftis KN. Regulation of the hypothalamic-pituitary-adrenal axis. Neuroimmunomodulation. 2009;16:265–71. https://doi.org/10.1159/000216184.
Itoi K, Jiang YQ, Iwasaki Y, Watson SJ. Regulatory mechanisms of corticotropin-releasing hormone and vasopressin gene expression in the hypothalamus. J Neuroendocrinol. 2004;16:348–55. https://doi.org/10.1111/j.0953-8194.2004.01172.x.
Mastorakos G, Chrousos GP, Weber JS. Recombinant interleukin-6 activates the hypothalamic-pituitary-adrenal axis in humans. J Clin Endocrinol Metab. 1993;77:1690–4.
Crofford LJ, et al. Circadian relationships between interleukin (IL)-6 and hypothalamic-pituitary-adrenal axis hormones: failure of IL-6 to cause sustained hypercortisolism in patients with early untreated rheumatoid arthritis. J Clin Endocrinol Metab. 1997;82:1279–83.
Inder WJ, et al. Elevated basal adrenocorticotropin and evidence for increased central opioid tone in highly trained male athletes. J Clin Endocrinol Metab. 1995;80:244–8.
White A. Adrenocorticotropic hormone. Endocrinology. Publisher Elsevier Saunders; 2005:323–39.
Arvat E, et al. Endocrine activities of ghrelin, a natural growth hormone secretagogue (GHS), in humans: comparison and interactions with hexarelin, a nonnatural peptidyl GHS, and GH-releasing hormone. J Clin Endocrinol Metab. 2001;86:1169–74.
Jankord R, McAllister RM, Ganjam VK, Laughlin MH. Chronic inhibition of nitric oxide synthase augments the ACTH response to exercise. Am J Physiol Regul Integr Comp Physiol. 2009;296:R728–34. https://doi.org/10.1152/ajpregu.90709.2008.
Gambacciani M, et al. Intrinsic pulsatility of ACTH release from the human pituitary in vitro. Clin Endocrinol. 1987;26:557–63.
Xia Y, Wikberg JE. Localization of ACTH receptor mRNA by in situ hybridization in mouse adrenal gland. Cell Tissue Res. 1996;286:63–8.
Gorrigan RJ, Guasti L, King P, Clark AJ, Chan LF. Localisation of the melanocortin-2-receptor and its accessory proteins in the developing and adult adrenal gland. J Mol Endocrinol. 2011;46:227–32. https://doi.org/10.1530/JME-11-0011.
Chan LF, Metherell LA, Clark AJ. Effects of melanocortins on adrenal gland physiology. Eur J Pharmacol. 2011;660:171–80. https://doi.org/10.1016/j.ejphar.2010.11.041.
John CD, Gavins FN, Buss NA, Cover PO, Buckingham JC. Annexin A1 and the formyl peptide receptor family: neuroendocrine and metabolic aspects. Curr Opin Pharmacol. 2008;8:765–76. https://doi.org/10.1016/j.coph.2008.09.005.
Buckingham JC. Glucocorticoids: exemplars of multi-tasking. Br J Pharmacol. 2006;147(Suppl 1):S258–68. https://doi.org/10.1038/sj.bjp.0706456.
Crumeyrolle-Arias M, et al. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology. 2014;42:207–17. https://doi.org/10.1016/j.psyneuen.2014.01.014.
Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014;19:146–8. https://doi.org/10.1038/mp.2013.65.
Wong ML, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry. 2016;21:797–805. https://doi.org/10.1038/mp.2016.46.
Huo R, et al. Microbiota modulate anxiety-like behavior and endocrine abnormalities in Hypothalamic-Pituitary-Adrenal axis. Front Cell Infect Microbiol. 2017;7:489. https://doi.org/10.3389/fcimb.2017.00489.
Vodicka M, et al. Microbiota affects the expression of genes involved in HPA axis regulation and local metabolism of glucocorticoids in chronic psychosocial stress. Brain Behav Immun. 2018;73:615–24. https://doi.org/10.1016/j.bbi.2018.07.007.
Papargyri P, et al. Links between HPA axis and adipokines: clinical implications in paradigms of stress-related disorders. Expert Rev Endocrinol Metab. 2018;13:317–32. https://doi.org/10.1080/17446651.2018.1543585.
Goshen I, Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol. 2009;30:30–45. https://doi.org/10.1016/j.yfrne.2008.10.001.
Mohn CE, et al. Adrenal gland responses to lipopolysaccharide after stress and ethanol administration in male rats. Stress. 2011;14:216–26. https://doi.org/10.3109/10253890.2010.532254.
Gadek-Michalska A, Bugajski J. Interleukin-1 (IL-1) in stress-induced activation of limbic-hypothalamic-pituitary adrenal axis. Pharmacol Rep. 2010;62:969–82.
Gadek-Michalska A, Tadeusz J, Rachwalska P, Spyrka J, Bugajski J. Effect of repeated restraint on homotypic stress-induced nitric oxide synthases expression in brain structures regulating HPA axis. Pharmacol Rep. 2012;64:1381–90.
Gibb J, Hayley S, Poulter MO, Anisman H. Effects of stressors and immune activating agents on peripheral and central cytokines in mouse strains that differ in stressor responsivity. Brain Behav Immun. 2011;25:468–82. https://doi.org/10.1016/j.bbi.2010.11.008.
Marquez C, Nadal R, Armario A. The hypothalamic-pituitary-adrenal and glucose responses to daily repeated immobilisation stress in rats: individual differences. Neuroscience. 2004;123:601–12.
Dunn AJ. Cytokine activation of the HPA axis. Ann N Y Acad Sci. 2000;917:608–17.
Gadek-Michalska A, Tadeusz J, Rachwalska P, Bugajski J. Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol Rep. 2013;65:1655–62.
Beishuizen A, Thijs LG. Endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis. J Endotoxin Res. 2003;9:3–24. https://doi.org/10.1179/096805103125001298.
Venezia AC, Quinlan E, Roth SM. A single bout of exercise increases hippocampal Bdnf: influence of chronic exercise and noradrenaline. Genes Brain Behav. 2017;16:800–11. https://doi.org/10.1111/gbb.12394.
Balkaya M, Cho S. Genetics of stroke recovery: BDNF val66met polymorphism in stroke recovery and its interaction with aging. Neurobiol Dis. 2018;126:36. https://doi.org/10.1016/j.nbd.2018.08.009.
Jiang R, et al. Brain-derived neurotrophic factor (BDNF) Val66Met polymorphism interacts with gender to influence cortisol responses to mental stress. Psychoneuroendocrinology. 2017;79:13–9. https://doi.org/10.1016/j.psyneuen.2017.02.005.
Schule C, et al. Brain-derived neurotrophic factor Val66Met polymorphism and dexamethasone/CRH test results in depressed patients. Psychoneuroendocrinology. 2006;31:1019–25. https://doi.org/10.1016/j.psyneuen.2006.06.002.
Givalois L, et al. A single brain-derived neurotrophic factor injection modifies hypothalamo-pituitary-adrenocortical axis activity in adult male rats. Mol Cell Neurosci. 2004;27:280–95. https://doi.org/10.1016/j.mcn.2004.07.002.
Jankord R, Ganjam VK, Turk JR, Hamilton MT, Laughlin MH. Exercise training alters effect of high-fat feeding on the ACTH stress response in pigs. Appl Physiol Nutr Metab. 2008;33:461–9. https://doi.org/10.1139/H08-022.
Campbell JE, Rakhshani N, Fediuc S, Bruni S, Riddell MC. Voluntary wheel running initially increases adrenal sensitivity to adrenocorticotrophic hormone, which is attenuated with long-term training. J Appl Physiol. 2009;106:66–72. https://doi.org/10.1152/japplphysiol.91128.2008.
Fediuc S, Campbell JE, Riddell MC. Effect of voluntary wheel running on circadian corticosterone release and on HPA axis responsiveness to restraint stress in Sprague-Dawley rats. J Appl Physiol. 2006;100:1867–75. https://doi.org/10.1152/japplphysiol.01416.2005.
Smoak B, Deuster P, Rabin D, Chrousos G. Corticotropin-releasing hormone is not the sole factor mediating exercise-induced adrenocorticotropin release in humans. J Clin Endocrinol Metab. 1991;73:302–6.
Chalimoniuk M, Chrapusta SJ, Lukacova N, Langfort J. Endurance training upregulates the nitric oxide/soluble guanylyl cyclase/cyclic guanosine 3′,5′-monophosphate pathway in the striatum, midbrain and cerebellum of male rats. Brain Res. 2015;1618:29–40. https://doi.org/10.1016/j.brainres.2015.05.020.
TaheriChadorneshin H, Cheragh-Birjandi S, Ramezani S, Abtahi-Eivary SH. Comparing sprint and endurance training on anxiety, depression and its relation with brain-derived neurotrophic factor in rats. Behav Brain Res. 2017;329:1–5. https://doi.org/10.1016/j.bbr.2017.04.034.
Skoluda N, Dettenborn L, Stalder T, Kirschbaum C. Elevated hair cortisol concentrations in endurance athletes. Psychoneuroendocrinology. 2012;37:611–7. https://doi.org/10.1016/j.psyneuen.2011.09.001.
Duclos M, Corcuff JB, Pehourcq F, Tabarin A. Decreased pituitary sensitivity to glucocorticoids in endurance-trained men. Eur J Endocrinol. 2001;144:363–8.
Faria CD, et al. Impact of prolonged low-grade physical training on the in vivo glucocorticoid sensitivity and on glucocorticoid receptor-alpha mRNA levels of obese adolescents. Horm Res Paediatr. 2010;73:458–64. https://doi.org/10.1159/000313591.
Jones TW, Howatson G, Russell M, French DN. Performance and endocrine responses to differing ratios of concurrent strength and endurance training. J Strength Cond Res. 2016;30:693–702. https://doi.org/10.1519/JSC.0000000000001135.
Grandys M, et al. The importance of the training-induced decrease in basal cortisol concentration in the improvement in muscular performance in humans. Physiol Res. 2016;65:109–20.
Seifert T, et al. Endurance training enhances BDNF release from the human brain. Am J Physiol Regul Integr Comp Physiol. 2010;298:R372–7. https://doi.org/10.1152/ajpregu.00525.2009.
Zoladz JA, et al. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J Physiol Pharmacol. 2008;59(Suppl 7):119–32.
Wright HE, Selkirk GA, McLellan TM. HPA and SAS responses to increasing core temperature during uncompensable exertional heat stress in trained and untrained males. Eur J Appl Physiol. 2010;108:987–97. https://doi.org/10.1007/s00421-009-1294-0.
Duclos M, et al. Corticotroph axis sensitivity after exercise in endurance-trained athletes. Clin Endocrinol. 1998;48:493–501.
Tabata I, Ogita F, Miyachi M, Shibayama H. Effect of low blood glucose on plasma CRF, ACTH, and cortisol during prolonged physical exercise. J Appl Physiol. 1991;71:1807–12.
Bobbert T, et al. Adaptation of the hypothalamic-pituitary hormones during intensive endurance training. Clin Endocrinol. 2005;63:530–6. https://doi.org/10.1111/j.1365-2265.2005.02377.x.
Ratel S. High-intensity and resistance training and elite young athletes. Med Sport Sci. 2011;56:84–96. https://doi.org/10.1159/000320635.
Fatouros I, et al. Acute resistance exercise results in catecholaminergic rather than hypothalamic-pituitary-adrenal axis stimulation during exercise in young men. Stress. 2010;13:461–8. https://doi.org/10.3109/10253891003743432.
Minetto MA, et al. Corticotroph axis sensitivity after exercise: comparison between elite athletes and sedentary subjects. J Endocrinol Invest. 2007;30:215–23.
Cintineo HP, et al. Acute physiological responses to an intensity-and time-under-tension-equated single- vs. multiple-set resistance training bout in trained men. J Strength Cond Res. 2018;32:3310–8. https://doi.org/10.1519/JSC.0000000000002872.
Arazi H, Damirchi A, Asadi A. Age-related hormonal adaptations, muscle circumference and strength development with 8 weeks moderate intensity resistance training. Ann Endocrinol (Paris). 2013;74:30–5. https://doi.org/10.1016/j.ando.2012.11.004.
Kyrolainen H, et al. Effects of combined strength and endurance training on physical performance and biomarkers of healthy young women. J Strength Cond Res. 2018;32:1554–61. https://doi.org/10.1519/JSC.0000000000002034.
Ammar A, et al. Acute and delayed responses of steroidal hormones, blood lactate and biomarkers of muscle damage after a resistance training session: time-of-day effects. J Sports Med Phys Fitness. 2018;58:980–9. https://doi.org/10.23736/S0022-4707.17.07048-7.
Sedliak M, et al. Morphological, molecular and hormonal adaptations to early morning versus afternoon resistance training. Chronobiol Int. 2018;35:450–64. https://doi.org/10.1080/07420528.2017.1411360.
Sheikholeslami-Vatani D, Ahmadi S, Salavati R. Comparison of the effects of resistance exercise orders on number of repetitions, serum IGF-1, testosterone and cortisol levels in normal-weight and obese men. Asian J Sports Med. 2016;7:e30503. https://doi.org/10.5812/asjsm.30503.
Marston KJ, et al. Intense resistance exercise increases peripheral brain-derived neurotrophic factor. J Sci Med Sport. 2017;20:899–903. https://doi.org/10.1016/j.jsams.2017.03.015.
Church DD, et al. Comparison of high-intensity vs. high-volume resistance training on the BDNF response to exercise. J Appl Physiol (1985). 2016;121:123–8. https://doi.org/10.1152/japplphysiol.00233.2016.
Nuvagah Forti L, et al. High versus low load resistance training: the effect of 24 weeks detraining on serum Brain Derived-Neurotrophic Factor (BDNF) in older adults. J Frailty Aging. 2017;6:53–8. https://doi.org/10.14283/jfa.2017.2.
Wang J, et al. The impact of water-floating and high-intensity exercise on rat’s HPA axis and interleukins concentrations. Acta Physiol Hung. 2012;99:261–70. https://doi.org/10.1556/APhysiol.99.2012.3.3.
Davies CT, Few JD. Effects of exercise on adrenocortical function. J Appl Physiol. 1973;35:887–91. https://doi.org/10.1152/jappl.1973.35.6.887.
Hill EE, et al. Exercise and circulating cortisol levels: the intensity threshold effect. J Endocrinol Invest. 2008;31:587–91. https://doi.org/10.1007/BF03345606.
VanBruggen MD, Hackney AC, McMurray RG, Ondrak KS. The relationship between serum and salivary cortisol levels in response to different intensities of exercise. Int J Sports Physiol Perform. 2011;6:396–407.
Ross RE, Saladin ME, George MS, Gregory CM. High-intensity aerobic exercise acutely increases brain-derived neurotrophic factor. Med Sci Sports Exerc. 2019;51(8):1698–709. https://doi.org/10.1249/MSS.0000000000001969.
Inder WJ, Hellemans J, Swanney MP, Prickett TC, Donald RA. Prolonged exercise increases peripheral plasma ACTH, CRH, and AVP in male athletes. J Appl Physiol. 1998;85:835–41.
Deuster PA, et al. High intensity exercise promotes escape of adrenocorticotropin and cortisol from suppression by dexamethasone: sexually dimorphic responses. J Clin Endocrinol Metab. 1998;83:3332–8. https://doi.org/10.1210/jcem.83.9.5110.
Rodriguez AL, et al. Acute high-intensity interval exercise induces greater levels of serum brain-derived neurotrophic factor in obese individuals. Exp Biol Med (Maywood). 2018;243(14):1153–60. https://doi.org/10.1177/1535370218812191.
Zelzer S, et al. Work intensity, low-grade inflammation, and oxidative status: a comparison between office and slaughterhouse workers. Oxidative Med Cell Longev. 2018;2018:2737563. https://doi.org/10.1155/2018/2737563.
Popovic B, et al. Acute response to endurance exercise stress: focus on catabolic/anabolic interplay between cortisol, testosterone, and sex hormone binding globulin in professional athletes. J Med Biochem. 2019;38:6–12. https://doi.org/10.2478/jomb-2018-0016.
Choi ES, et al. Changes in hormone levels of participants in a 622-km ultramarathon race based on distance and recovery period. J Sports Med Phys Fitness. 2018;59(4):700–7. https://doi.org/10.23736/S0022-4707.18.08533-X.
Wittert GA, Livesey JH, Espiner EA, Donald RA. Adaptation of the hypothalamopituitary adrenal axis to chronic exercise stress in humans. Med Sci Sports Exerc. 1996;28:1015–9.
Georgopoulos NA, et al. Abolished circadian rhythm of salivary cortisol in elite artistic gymnasts. Steroids. 2011;76:353–7. https://doi.org/10.1016/j.steroids.2010.10.013.
Schmikli SL, de Vries WR, Brink MS, Backx FJ. Monitoring performance, pituitary-adrenal hormones and mood profiles: how to diagnose non-functional over-reaching in male elite junior soccer players. Br J Sports Med. 2012;46:1019. https://doi.org/10.1136/bjsports-2011-090492.
Wisen AG, Ekberg K, Wohlfart B, Ekman R, Westrin A. Plasma ANP and BNP during exercise in patients with major depressive disorder and in healthy controls. J Affect Disord. 2011;129:371–5. https://doi.org/10.1016/j.jad.2010.09.002.
Carr BR, Mason JI. The effects of alpha-human atrial natriuretic polypeptide on steroidogenesis by fetal zone cells of the human fetal adrenal gland. Am J Obstet Gynecol. 1988;159:1361–5.
Crandall ME, Gregg CM. In vitro evidence for an inhibitory effect of atrial natriuretic peptide on vasopressin release. Neuroendocrinology. 1986;44:439–45.
Strohle A, Holsboer F. Stress responsive neurohormones in depression and anxiety. Pharmacopsychiatry. 2003;36(Suppl 3):S207–14. https://doi.org/10.1055/s-2003-45132.
Strohle A, Kellner M, Holsboer F, Wiedemann K. Atrial natriuretic hormone decreases endocrine response to a combined dexamethasone-corticotropin-releasing hormone test. Biol Psychiatry. 1998;43:371–5.
Bonifazi M, et al. Glucocorticoid receptor mRNA expression in peripheral blood mononuclear cells in high trained compared to low trained athletes and untrained subjects. J Endocrinol Invest. 2009;32:816–20. https://doi.org/10.3275/6428.
Park E, et al. Changes in basal hypothalamo-pituitary-adrenal activity during exercise training are centrally mediated. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1360–71. https://doi.org/10.1152/ajpregu.00103.2005.
de Graaf-Roelfsema E, Keizer HA, van Breda E, Wijnberg ID, van der Kolk JH. Hormonal responses to acute exercise, training and overtraining. A review with emphasis on the horse. Vet Q. 2007;29:82–101.
Cayado P, et al. Hormone response to training and competition in athletic horses. Equine Vet J Suppl. 2006;38:274–8.
Banfi G, Dolci A. Free testosterone/cortisol ratio in soccer: usefulness of a categorization of values. J Sports Med Phys Fitness. 2006;46:611–6.
Angeli A, Minetto M, Dovio A, Paccotti P. The overtraining syndrome in athletes: a stress-related disorder. J Endocrinol Investig. 2004;27:603–12.
Uusitalo AL, Huttunen P, Hanin Y, Uusitalo AJ, Rusko HK. Hormonal responses to endurance training and overtraining in female athletes. Clin J Sport Med. 1998;8:178–86.
Meeusen R, et al. Hormonal responses in athletes: the use of a two bout exercise protocol to detect subtle differences in (over)training status. Eur J Appl Physiol. 2004;91:140–6. https://doi.org/10.1007/s00421-003-0940-1.
Lehmann M, Foster C, Dickhuth HH, Gastmann U. Autonomic imbalance hypothesis and overtraining syndrome. Med Sci Sports Exerc. 1998;30:1140–5.
Lehmann MJ, et al. Training and overtraining: an overview and experimental results in endurance sports. J Sports Med Phys Fitness. 1997;37:7–17.
Mor A, Kayacan Y, Ipekoglu G, Arslanoglu E. Effect of carbohydrate-electrolyte consumption on insulin, cortisol hormones and blood glucose after high-intensity exercise. Arch Physiol Biochem. 2019;125(4):344–50. https://doi.org/10.1080/13813455.2018.1465098.
Svendsen IS, Killer SC, Gleeson M. Influence of hydration status on changes in plasma cortisol, leukocytes, and antigen-stimulated cytokine production by whole blood culture following prolonged exercise. ISRN Nutr. 2014;2014:561401. https://doi.org/10.1155/2014/561401.
Dmitrasinovic G, et al. ACTH, cortisol and IL-6 levels in athletes following magnesium supplementation. J Med Biochem. 2016;35:375–84. https://doi.org/10.1515/jomb-2016-0021.
Burman MA, Hamilton KL, Gewirtz JC. Role of corticosterone in trace and delay conditioned fear-potentiated startle in rats. Behav Neurosci. 2010;124:294–9. https://doi.org/10.1037/a0018911.
Cooper MA, Huhman KL. Blocking corticotropin-releasing factor-2 receptors, but not corticotropin-releasing factor-1 receptors or glucocorticoid feedback, disrupts the development of conditioned defeat. Physiol Behav. 2010;101:527–32. https://doi.org/10.1016/j.physbeh.2010.08.003.
Row BW, Dohanich GP. Post-training administration of corticotropin-releasing hormone (CRH) enhances retention of a spatial memory through a noradrenergic mechanism in male rats. Neurobiol Learn Mem. 2008;89:370–8. https://doi.org/10.1016/j.nlm.2007.10.008.
Chen Y, et al. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc Natl Acad Sci U S A. 2010;107:13123–8. https://doi.org/10.1073/pnas.1003825107.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
St-Pierre, D.H., Richard, D. (2020). The Effect of Exercise on the Hypothalamic-Pituitary-Adrenal Axis. In: Hackney, A., Constantini, N. (eds) Endocrinology of Physical Activity and Sport. Contemporary Endocrinology. Humana, Cham. https://doi.org/10.1007/978-3-030-33376-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-33376-8_3
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-33375-1
Online ISBN: 978-3-030-33376-8
eBook Packages: MedicineMedicine (R0)