Abstract
In response to exercise, there are numerous alterations in body fluid and electrolyte homeostasis. These perturbations occur immediately upon initiation of exercise and can persist for hours or even days after completion of exercise. The endocrine system plays an important role in the regulation of fluid and electrolyte homeostasis that must occur with exercise. Dysregulation of the endocrine system may limit exercise activity and, in some incidences, result in debilitating morbidities or death. This chapter emphasizes responses to exercise and reviews the importance and factors involved in the maintenance of fluid and electrolyte balance. Previous reviews will be used to address the basics of effected systems; however, emphasis is placed on new data and the current discussions about performance of work and exercise.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Brain natriuretic peptide
- Atrial natriuretic peptide
- Plasma renin activity
- Renal blood flow
- Maximal exercise
Introduction
In response to exercise, there are numerous alterations in fluid and electrolyte homeostasis. These perturbations occur immediately upon initiation of exercise and can persist for hours or even days after completion of exercise. The endocrine system plays an important role in the regulation of fluid and electrolyte homeostasis that must occur with exercise. Dysregulation of the endocrine system may limit exercise activity and, in some incidences, result in debilitating morbidities or death. This chapter emphasizes responses to exercise and reviews the importance and factors involved in the maintenance of fluid and electrolyte balance. Previous reviews will be used to address the basics of effected systems; however, emphasis is placed on new data and the current discussions about performance of work and exercise.
The term exercise is an ambiguous term covering a broad range of physical activities. The term is employed to define activities such as running and cycling but is also used to cover the activities of daily living and work. Thus, when discussing responses to exercise, it is important to clarify the type of activity, the level at which it is performed, and the duration. In defining the responses to exercise, it is essential to understand the definitions of workload. The absolute workload is the level of exercise being performed, such as running on a treadmill at a defined speed. For individual subjects, this would produce a variable response depending on their level of fitness/training. Therefore, to compare exercise responses between subjects, relative workload is often employed as a normalization technique [1,2,3]. Relative workload is expressed as a percentage of the maximum capability of the individual to perform that specific exercise and is often further standardized to the heart rate or oxygen consumption of the subject.
Physiologic Responses to Exercise
A variety of conditions results from alterations in fluid and electrolytes and affects the performance of exercise and work. The disruption of the balance of fluids and electrolytes correlates with limitation of work capacity; however, the range of changes tolerated may be extended with training and repeated exposures. In general the body can undergo one of several responses to exercise: dehydration, dysnatremia, hypovolemia, or hypervolemia. The following text will review each.
Dehydration is defined as a reduction in total body water (TBW) and an increase in plasma electrolyte concentrations. Heavy exercise and extreme heat are two of the most prevalent causes of dehydration, as both are associated with exercise and subsequent loss of fluid volume due to sweating and inadequate fluid intake [4,5,6,7]. Current evidence suggest that dehydration resulting in a decrease of greater than 2% body mass will adversely affect exercise performance [6,7,8]. However, decrements in self-paced exercise may not occur until a 4% loss in body weight [9]. Regardless of the level of dehydration, a loss of TBW and an increase in plasma electrolyte concentrations are associated with limited work performance and, in extreme cases, death.
Dysnatremia covers the occurrence of both increases and decreases in plasma sodium observed with exercise [10,11,12,13,14]. Siegel et al. noted an incidence rate of dysnatremia of 32.5% in 1319 collapsed marathon runners [12]. Of these, 85% were hypernatremic and 15% hyponatremic. Both of these conditions have been associated with deaths in competitive runners.
Hypovolemia is a decrease in blood volume in the absence of changes in plasma electrolyte concentrations. This can occur with exercise or hemorrhage [15] and follows periods of water submersion to the neck or the administration of diuretics commonly used in the treatment of hypertension [16, 17]. Hypovolemia necessitates an increase in heart rate at submaximal workloads and a more rapid increase in body temperature, both indicative of limited work performance [7].
In contrast, hypervolemia is the expansion of blood volume. There is extensive literature on the expansion of blood volume by increasing the red cell mass; however, within the scope of this chapter, this term refers to expansion of the plasma volume. Plasma volume is expanded by exercise training and by acute excessive ingestion of fluids, hyperhydration [7, 18,19,20]. Warburton et al. reviewed the literature on the effect of acute expansion of plasma volume and found minimal increases in maximum oxygen consumption, but there were negligible changes in exercise endurance [20].
Modulation of Hormones in Responses to Exercise
Workload Intensity
The response of hormones to exercise is closely related to the amount of relative work performed. There are three basic patterns of hormones during exercise. The first is an increase proportional to the increase in relative workload. For example, with each increase in workload, there is a constant increase in the plasma hormone concentration of atrial natriuretic peptide (ANP) (Fig. 13.1a). The second pattern is a logarithmic/exponential increase such as that reported for plasma renin activity (PRA) (Fig. 13.1b). With increasing workloads, the level of hormone increases at an exponentially faster rate. The third pattern is related to an onset of an increase at a given threshold; this is observed for vasopressin (Fig. 13.1c). A threshold response for exercise is usually associated with the onset of anaerobic metabolism and a relative workload of about 70%. This has also been associated with the increase in stress-related hormones such as cortisol and adrenocorticotropic hormone (ACTH). These patterns of increased hormone concentrations are consistently observed in studies of acute exercise when the response is expressed relative to the workload of the task performed.
The various patterns of response of hormones to exercise. The individual dots represent the response from independent studies of exercise on a cycle ergometer with varying workloads. The variance represents differences in how the exercise was performed, state of hydration of the subjects, and difference in assay techniques. With these confounders the patterns in response to exercise are still present. The linear example is demonstrated by the response of atrial natriuretic peptide (ANP; (a) 11 studies), the logarithmic/exponential increase by plasma renin activity (PRA; (b) 20 studies), and the threshold response by vasopressin (AVP; (c) 23 studies)
Exercise Duration
The duration of exercise is also a confounding factor in the response of hormones to exercise. Extended time, rather than intensity, may have a greater influence on the levels of hormones during exercise. This is especially true of hormones involved in the regulation of fluid and electrolyte homeostasis. As exercise progresses, there is an increased metabolic heat necessitating sweating and therefore the loss of water and electrolytes. The increase in aldosterone, which regulates sodium balance, is increased twofold with acute maximal exercise (i.e., running on a treadmill) and returns to baseline levels within an hour. Extended exercise times elicit similar changes in plasma volume and sodium concentrations, but aldosterone concentration increases three to four times the basal levels and remains elevated for over 24 h [21]. The greater and enduring response to exercise of longer duration is postulated to be due to additional regulators associated with the “stress” of exercise [10, 22]. Of note, hormone concentrations may vary over time with exercise of long duration, such as during a marathon or ultra-endurance events. For example, ANP is increased by a factor of ten during the first 10 km of a marathon but subsequently decreased to levels only fivefold greater than baseline [23]. In addition, the conditions under which recovery is conducted, access to fluids or cool down exercise, are influential in the postexercise responses and need to be clarified [24, 25]. Recently, Hew-Butler et al. compared the hormonal responses to maximal exercise with a mean duration of 10–60 min of exercise at a treadmill speed equivalent to 60% of the maximum [26]. With maximal acute exercise, significant increases were reported for vasopressin (5-fold) and aldosterone (2-fold), while at submaximal effort, only aldosterone was increased (3.3-fold). Thus, both the length of time and intensity of the workload must be considered when studying the regulation and function of hormones in response to exercise.
Training
The level of training of a subject may influence the hormonal response to exercise [27, 28]. While much of the variance between subjects at absolute workloads may be due to differences in the relative workload being performed, there are still aspects of training that change the response. Individuals undergoing persistent heavy bouts of exercise training may have alterations in resting levels. In subjects doing daily long-distance runs, plasma aldosterone concentrations are elevated compared to controls [29]. However, for the majority of hormones regulating fluid and electrolyte homeostasis, training does not appear to be as an important of a factor as the intensity and duration of exercise in the response of these hormones.
Hydration Status
The initial hydration status of a subject may influence subsequent responses to exercise. Fluid intake during the performance of exercise is also an influencing factor. Dehydration or hyperhydration alters initial hormone levels; however, the subsequent response to exercise appears independent. Geelen et al. found that following dehydration, ingestion of fluid caused a rapid and pronounced reduction in vasopressin and an increase in norepinephrine that was independent of changes in plasma osmolality and volume [30]. No changes were noted in epinephrine, aldosterone, PRA, or ANP. Additional investigations reported that the greater the volume consumed, the more pronounced the decrease in vasopressin and increase in norepinephrine [24, 31,32,33]. This suggests an oropharyngeal reflex may be present and mediated by the sympathetic nervous system.
Khamnei et al. evaluated the effect of the combination of exercise and postexercise fluid intake on vasopressin [24, 32]. Subjects exercised at 50% of their maximum oxygen uptake for 30 min. Exercise resulted in a 45% increase in vasopressin which was sustained after exercise in the absence of fluid intake. In contrast, when a large volume of fluid was ingested after exercise, control levels of vasopressin were obtained within 3 min. These findings suggest fluid intake may have a profound effect on hormonal responses during exercise, independent of changes in plasma volume and osmolality. Hew-Butler has put forth the hypothesis that inappropriate increases in vasopressin during prolonged exercise in the presence of adequate fluid intake may be a contributing factor to hyponatremia and subsequent morbidity [10, 22]. This line of research awaits additional well-controlled prospective studies to fully identify underlying mechanisms.
Sex
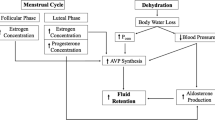
Sex of the subject is another factor with demonstrated differences. In women, the phase of the menstrual cycle in which exercise is performed may alter the hormonal responses. Resting aldosterone levels are increased during the mid-luteal phase of the cycle, and the response to exercise is amplified [34]. Further work by Stachenfeld and coworkers has demonstrated the effect of progesterone and estrogen on the levels and responses of hormones that are important in fluid and electrolyte homeostasis [35, 36]. In patients with coronary heart disease, basal levels of vasopressin were elevated in men; however, in responses to a 6 min walk test that increased vasopressin, ANP, norepinephrine, and epinephrine, there were no differences between males and females [37]. Following exercise in well-trained subjects to decrease body mass by 3%, women had a lower PRA and faster recovery of aldosterone and slower recovery of vasopressin compared to men [38]. Overall, there are minimal differences reported between male and females in resting hormone levels, and differences in response to exercise are not fully delineated [39].
Health Status
The initial health of the subject is an influencing factor in the hormonal responses to exercise and offers insights to the pathophysiology of various disease processes and in some cases a means of diagnosis and/or rehabilitation. The presence of disease represents a shift in homeostasis that requires alteration in the responsiveness of hormones important in fluid and electrolyte homeostasis. In age-matched subjects, Shim and coworkers reported that subjects with an exaggerated blood pressure response to exercise, which is indicative of a greater risk for hypertension and prevalence of cardiac hypertrophy, had elevated levels of angiotensin II at rest and an augmented increase in response to exercise [40]. However, there were no significant differences in norepinephrine, epinephrine, PRA, or aldosterone at the end of exercise. Kjaer et al. studied patients with congestive heart failure (CHF) and compared them with healthy subjects at 50 and 75% of their maximum workloads on a cycle ergometer [41]. Basal levels of ANP, brain natriuretic peptide (BNP), vasopressin, and PRA were elevated in patients with CHF. In response to exercise, ANP, arginine vasopressin (AVP), norepinephrine, and epinephrine were all increased in both groups. Even though higher absolute levels were observed in subjects with CHF, when expressed as a percent of basal concentrations group, differences were negated. BNP was increased with exercise only in patients with CHF.
Coiro et al. assessed the response of vasopressin to exercise to exhaustion on a bicycle ergometer in subjects with diabetes and controls and further segregated the groups as smokers and nonsmokers [42]. Baseline vasopressin concentrations at rest (2.1–2.6 pg/mL) were not different between groups. In all groups, there was a significant increase in vasopressin in response to exercise. While smoking was not identified as a contributing factor, there was a greater increase in vasopressin in subjects with diabetes (12–13 pg/mL) than controls (7–8 pg/mL). The difference between diabetic and normal subjects could not be attributed to cardiovascular or respiratory responses.
Other Influencing Factors
Other confounders, such as position of exercise and age, have been identified to influence hormonal responses to exertion. Wolf et al. compared supine and upright exercise on a cycle ergometer at a relative workload of 40–50% for 20 min. With supine exercise, the response of PRA and aldosterone to exercise was increased by 90% and 49%, respectively, in contrast to upright exercise [43]. These differences occurred in the absence of difference between the types of exercise in plasma osmolality or blood pressure. Perrault et al. found ANP concentrations to be increased and vasopressin, PRA, and norepinephrine to be reduced, during supine exercise on a cycle ergometer in comparison to exercise in an upright position [44]. During the performance of a marathon, subjects with a mean age of 47 years had an increase in ANP to 104 pg/mL compared to 43 pg/mL in younger subjects with a mean age of 28 years [23]. In addition, differences in hormone concentrations reported in response to exercise may be in part explained by the differing methods of measurement. The presence of such confounders in the comparison of the hormonal responses to exercise has not been systematically addressed, partially limiting our interpretation of the role of hormones in fluid and electrolyte homeostasis during exercise.
Hormone Responses to Exercise
The hormones of consequence to fluid and electrolyte balance in exercising humans are those involved in the regulation of thirst and function of the kidneys and sweat glands. The essential hormones are the catecholamines, vasopressin, the renin-angiotensin-aldosterone system, and natriuretic peptides. While these hormones have a variety of functions, the focus of the present review will be on their responses to exercise and impact on fluid and electrolyte homeostasis during and following exercise. Circulating levels of these hormones are altered during exercise as a function of changes in secretion, metabolism, and volume of distribution. The most common measurement of these hormones in association with exercise is the circulating concentrations, which will be the focus of the present effort.
Catecholamines
Catecholamines , specifically norepinephrine and epinephrine, are derived from increases in sympathetic nervous system activity and the adrenal glands [45, 46]. The kidneys are also suggested as a source of norepinephrine [47]. Levels of circulating catecholamines respond rapidly upon the onset of exercise in order to redistribute blood flow to meet metabolic demands [2, 48, 49]. In response to exercise, there is a progressive increase in circulating norepinephrine levels from 1.3 to 3.0 nmol/L at rest to 12.0 nmol/L following maximal exercise [45, 50,51,52]. The increase in epinephrine occurs later in the course of exercise and can rise from resting levels of 380–655 pmol/L to concentrations over 3000 pmol/L. The increase in the ratio of norepinephrine to epinephrine demonstrates activation of the sympathetic nervous system and is attributed to active spillover from the muscles during exercise [45, 52,53,54]. With continued exercise, there is an attenuation of the increase in the ratio of norepinephrine to epinephrine, which is indicative of an increase in the release of epinephrine predominately from the adrenal medulla under the control of hypothalamic mediation in addition to the sympathetic nervous system. Following exercise, plasma levels of catecholamines return to resting levels in a matter of minutes, as they have a short half-life due to degradation and reuptake by the sympathetic nervous system. Recent studies that inhibited the reuptake of norepinephrine have demonstrated an increase in the time necessary to complete work equal to 30 min of exercise at 75% of maximal workload [55, 56]. These studies suggest clearance from the circulation of norepinephrine plays a role in fatigue.
Vasopressin
AVP is also known as vasopressin or antidiuretic hormone (ADH). It is a neurohypophysial hormone synthesized in the hypothalamus and stored in the posterior pituitary [57, 58]. Vasopressin is a pressor that alters peripheral resistance, but its greatest effect is on the reabsorption of water in the collecting tubules of the kidneys. Secretion of vasopressin is regulated by alterations in plasma osmolality and blood pressure. Circulating concentrations of vasopressin in humans are 1–4 pg/mL [57, 59,60,61,62]. With maximal exercise, vasopressin concentrations of 4–24 pg/mL are reported. Maximum conservation of water by the kidneys is observed at vasopressin levels of 10–20 pg/mL. With progressive increases in exercise, elevation of vasopressin is not observed until 70% of maximum workload is attained, i.e., the anaerobic threshold (Fig. 13.1c). Animal experiments have demonstrated an increase in activation of hypothalamic neurons that is indicative of increased vasopressin content (production) and of performing above the anaerobic threshold [63]. Thus, the response of vasopressin appears to be associated with the onset of anaerobic metabolism, which is also related to increases in “stress hormones” such as cortisol and ACTH. An increase in vasopressin may persist for over 60 min after exercise or longer if access to fluids is restricted. Of note, at low workloads of about 25% of the anaerobic threshold, vasopressin decreases have been reported.
A variety of factors have been demonstrated to mediate the increase in vasopressin with exercise, including the increase in osmolality and reduction in intravascular volume; however, the increase in plasma osmolality appears to be the primary mediator (Fig. 13.2) [59, 64, 65]. In subjects exercising at 65% of maximum while running on a treadmill, there was a progressive increase in vasopressin with progressive workloads [66]. In subsequent tests which involved dehydration that decreased body weight by 3 and 5%, resting vasopressin levels were increased in association with the decrease in blood volume; however, in response to exercise, further increases in vasopressin were related to the magnitude of the increase in osmolality. Brandenberger et al. evaluated rehydration during exercise giving subjects no fluids, water, or an isotonic solution. Intake of water reduced osmolality but did not alter plasma volume [67]. Consumption of the isotonic solution did not change osmolality but increased plasma volume. Both methods of rehydration decreased the rise in vasopressin levels with exercise, as well as those of PRA and cortisol. Others have reported similar findings [32, 44, 68]. The independence of the increase in osmolality and blood volume, and the regulation of vasopressin in response to exercise, is similar to that reported with dehydration. Coiro and colleagues have demonstrated that the increase in vasopressin during exercise to exhaustion may be attenuated by blockade of 5-HT3 serotonergic receptors and administration of somatostatin, supporting another means of mediating the increase in vasopressin during exercise [69]. Recently, Hew-Butler et al. have questioned the relationship of vasopressin and plasma osmolality during exercise. In subjects participating in an ultramarathon, they observed 3.9-fold increase in plasma vasopressin, no significant change in plasma sodium, and a significant decrease in plasma volume [10, 22]. They also evaluated cyclists during a 109 km race and observed nearly identical changes [70]. In subjects participating in an ultramarathon, they observed a 3.9-fold increase in plasma vasopressin in the absence of a significant change in plasma sodium though plasma volume was significantly decreased. These authors and others hypothesize that under conditions of prolonged exercise, the osmotic regulation of vasopressin is overshadowed by non-osmotic stimuli, of which, the reduction in blood volume plays a minor role [14, 71, 72]. The increase in AVP was associated with elevations in cortisol, oxytocin, and BNP, which underscores the relationship of AVP release with “exercise stress.” Irrespective of the means, vasopressin is elevated by more than fourfold during acute exercise to exhaustion or intense prolonged exercise.
(a) Levels of vasopressin and (b) subjective assessment of thirst in association to plasma changes in osmolality during moderate exercise. Measurements were from subjects with different levels of fitness, under various levels of hydration. (Redrawn from Merry et al. [27])
Renin-Angiotensin-Aldosterone
The renin-angiotensin-aldosterone systems are closely coupled and increased in response to exercise. Renin is released from the kidney in response to sympathetic nerve stimulation, as well as norepinephrine spillover, resulting in increased plasma concentrations [17, 45, 52, 73,74,75,76]. Renin then converts angiotensinogen to angiotensin I, which is subsequently transformed to angiotensin II in the lung. Angiotensin II promotes the release of aldosterone from the adrenal gland.
With exercise, all aspects of this system are increased and play a variety of roles in the regulation of fluid and electrolyte homeostasis [3, 45, 60, 77, 78]. At rest, PRA has levels in the order of 0.15–0.55 ng angiotensin I/mL/h and with maximal exercise increases to levels of 1.11–1.67 ng angiotensin I/mL/h. There is an exponential increase in renin activity with increasing workloads; significant differences are reported at levels of 60–70% of maximum (Fig. 13.1b). The increase in PRA with exercise is positively associated with the increase in angiotensin II. Basal levels of angiotensin II are 15–25 ng/L, with values of 130–160 ng/L achieved with maximal exercise. Aldosterone release is regulated by angiotensin II, as well as ACTH and the plasma levels of sodium and potassium. Aldosterone concentration increases from resting levels of 80–830 pmol/L to concentrations of 250–3330 pmol/L with maximal exercise. Blockade of the conversion of angiotensin I to angiotensin II does not attenuate the response of aldosterone to maximal exercise, which supports the theory that other pertinent regulatory factors are involved [79, 80]. The elevation of aldosterone may persist for days after exercise, and levels are dependent upon the sodium and water intake [21]. In the postexercise period, the increase in aldosterone may be the product of increased water intake, which reduces the plasma sodium concentration or the persistent elevation of aldosterone—which is due to activation of the ACTH. Irrespective of the cause, the increase in aldosterone due to exercise plays a role in the conservation of sodium in the sweat glands and kidneys (Fig. 13.3).
Plasma aldosterone concentrations were compared to the urinary excretion of sodium at the end of a 2 h run (closed circles) and following 48 h of recovery with food and water ad libitum (open circles). With exercise, there was an increase in aldosterone, and over the recovery period, there was a decrease. (Adapted from Wade et al. [29]))
Natriuretic Peptides
Peptides demonstrated to elicit a natriuresis have been deemed natriuretic peptides . These include ANP, BNP, urodilatin, and adrenomedullin. These peptides appear to participate in the regulation of fluid homeostasis by protecting against volume and pressure overloads. Though these peptides have been extensively studied over the past 30 years in patients with disease such as heart failure, pulmonary hypertension, and chronic renal disease, their response to and role during exercise are not well defined. Additionally, well-designed studies in control subjects or during competitive events have yet to be undertaken.
Atrial Natriuretic Peptide
ANP is increased with exercise in a linear response (Fig. 13.1a). Resting plasma levels of 10–49 pg/mL are increased to over 100 pg/mL with acute maximal exercise [22, 25, 39, 44, 81, 82]. In response to long-duration exercise, there is initially a pronounced increase, a subsequent fall, and then a re-elevation of levels, persisting until completion of the exercise [23]. Resting levels are obtained within hours of cessation of the activity [77]. The primary stimulus for the increase in ANPwith acute exercise is an increase in atrial stretch due to an increase in venous return [62]. However as exercise progresses, atrial pressure decreases as blood flow is redistributed (cardiovascular drift) to meet the metabolic demand of active tissues and to dissipate the thermal load [48, 49]. The response of ANP to extended exercise may be increased if water is ingested, suggesting a fluid volume change directly on the heart mediating release [83, 84]. Recently, pronounced increases in ANP with exercise have been associated with increases in cardiac troponin levels, suggesting myocardial damage during heavy exercise could be a contributing factor to increases in ANP [85]. In cardiac transplant patients, ANP levels are elevated, and the response to exercise is accentuated. This suggests that in normal subjects with naturally innervated hearts, there may be neural inhibition of ANP release [44, 86, 87]. Support for this hypothesis is the observation in patients with hypertension that chronic beta-blockade substantially increases the ANP response to exercise [88]. Sodium intake appears to also affect the ANP response to exercise [89, 90]. During submaximal cycle ergometer exercise when subjects were on a low-sodium diet, ANP increased from 42 to 59 pg/mL, in contrast to a high-sodium diet where the increase was from 72 to 119 pg/mL. Thus, the increase in ANP with exercise appears to be related to a number of factors: stretch of the atrium due to volume changes, neurological inputs, and sodium intake.
Brain Natriuretic Peptide
BNP , as its name implies, was first identified in the brain and subsequently identified in other tissues, specifically in the heart [91, 92]. BNP is collocated with ANP in the heart and appears to have similar paths of regulation and actions. BNP is not consistently altered in normal subjects in response to acute exercise [41, 83, 93,94,95]. However, with long-duration exercise, such as a 100 km ultramarathon, BNP levels were increased from resting values of 3.3–18.8 fmol/mL at the end of the race. The response of BNP to exercise is altered by a number of conditions [96]. When subjects performed submaximal exercise on a low-sodium diet, an increase in BNP was not noted; however, on a high-sodium diet, a significant increase was seen. A similar finding was reported with the presence or absence of fluid intake in the course of exercise [83]. If subjects did not ingest water, there was no response to exercise, but if fluid was provided, BNP was increased with exercise. In hypertensive subjects, the increase in BNP with exercise was the same with or without beta-blockade, in contrast to the greater increase in ANP with beta-blockade [88]. This suggests that while similar mechanisms, such as atrial stretch, fluid intake, and sodium status, modify the response of both BNP and ANP to exercise, the neurological component present in the regulation of ANP is not an important factor for BNP.
Urodilatin
Urodilatin , a natriuretic hormone derived in the kidneys, has been suggested to play a role in the renal handling of sodium [97, 98]. Schmidt et al. assessed the response of urodilatin and ANP during bicycle ergometer exercise at 60% of maximum for 1 h [99]. Plasma ANP concentrations increased, and the excretion of urodilatin decreased; i.e., the hormones had a negative correlation. The decrease in urodilatin was associated with a reduction in the percent of the filtered sodium load excreted. As urodilatin increased, the amount of sodium lost also increased. These findings suggest a possible role in the regulation of sodium homeostasis during exercise that needs to be investigated further.
Adrenomedullin
Adrenomedullin is reported to have natriuretic and diuretic effects. Adrenomedullin is produced in the vascular endothelium and in smooth muscle cells. In humans, plasma concentrations are responsive to changes in blood volume [100, 101]. Furthermore, changes in adrenomedullin are correlated with changes in ANP and BNP in patients. In normotensive subjects, adrenomedullin concentrations in response to submaximal exercise of short duration were not altered, even though ANP and BNP levels were increased. In contrast, during maximal exercise, Tanaka and colleagues found adrenomedullin to be increased by 45% compared to at rest and to be negatively associated with systolic blood pressure [102]. Piquard et al. also reported that with acute maximal exercise, adrenomedullin increased from resting levels of 15–29 pmol/L at the end of exercise [103]. Yet others have found adrenomedullin to be increased with submaximal exercise and decreased with maximal exercise [104, 105]. Therefore, further investigation is warranted to elucidate the responses and actions for adrenomedullin during exercise.
Fluid and Electrolyte Regulation
The management of fluids and electrolytes is a careful balance between loss of salts and water through sweat, shifts between body compartments, and conservation by the kidneys and replenishment through ingestion [106]. While some losses are tolerated during exercise, once critical levels are exceeded, there are decrements in performance. In order to avoid these reductions, a series of compensatory mechanisms are activated that have to work in concert to maintain the milieu, to optimize performance, and to avoid subsequent morbidities and mortality.
Total Body Water
During exercise there is a loss of TBW, predominately via sweating and in part from increased respiratory loss. The reduction of TBW is tolerated until a critical level is attained. The loss of TBW during exercise is equivalent to the reduction in total body mass over the period of exercise performance. Though this assumption has been questioned, there is still a strong relationship between the decrease in TBW and body mass [107,108,109]. During long-duration exercise, the reduction in TBW may exceed 5% of body mass. In a 70 kg person, this would equate to fluid loss of 3000–4000 mL [6, 8, 110]. In laboratory experiments, a reduction of more than 2% body mass has been shown to decrease performance [110]. In contrast during competitive endurance events, a reduction of greater than 4% body mass was demonstrated to have a decrement in performance [9]. Of note, even with free access to water, a loss in TBW during exercise is observed. This water loss, in the presence of fluids to ingest, is referred to as voluntary dehydration [18, 111]. Voluntary dehydration represents about 20–30% of the total loss of body water during an activity, as 70–80% is replaced by supplemental intake over the period of exercise. During a marathon the average body mass loss was 2.3% even though fluids were available. Interestingly, in subjects finishing under 3 h, the loss was 3.1%, from 3 to 4 h 2.5%, and over 4 h 1.8% [112]. The ability to tolerate a greater decrease in TBW was inversely associated with finish time. These observations suggest that individuals who are successful in these events are able to tolerate a greater TBW loss and still perform at a high level. The loss of fluids sustained in the course of exercise is usually replaced in the subsequent 24 h [21, 29, 60, 113]. Irrespective of the TBW loss tolerance, at some point the loss of TBW will impact the performance of an individual.
The loss of TBW during exercise is not equally distributed throughout the body or between body fluid compartments. Over the course of exercise, there is a redistribution of fluids among the various compartments of the body, with a pronounced reduction in plasma volume [59, 61, 65, 114]. The reduction in plasma volume during maximal acute exercise is 8–12%, resulting in a 5–7% decrease in blood volume. This shift of fluids from the vascular space to the extravascular space has been attributed in part to increases in endothelial permeability, which could possibly be modified within specific tissues by angiotensin II, vasopressin, and norepinephrine [115,116,117]. The decrease in blood volume is compensated for by an increase in cardiac output and a redistribution of blood flow [48, 49, 118, 119]. During the performance of exercise, the redistribution of fluids within the vascular compartment is required to meet the metabolic demands of active tissues and to dissipate the thermal load resulting from the increase in metabolism. This redistribution of flow is the result of increases in local vascular resistance, which is in part due to hormonal regulation, predominately by catecholamines, angiotensin II, and vasopressin.
Sweating
The principal means of fluid and electrolyte loss during exercise is in sweat. Sweating is essential to dissipate the increased thermal load incurred by the elevation of metabolism with exercise [120]. The density of sweat pores is highly variable among subjects, as is the magnitude of sweat produced due to the subjects’ level of training and prior adaptation and acclimation to a hot environment [90, 120,121,122,123]. The rate of fluid loss by sweating can be as high as 1500 mL/h [6, 18, 108, 124]. The magnitude of fluid loss in sweat is hormonally mediated by vasopressin [125, 126]. Circulating levels of vasopressin are positively associated with the rate and composition of sweat during exercise. The rate of sweating during exercise is coupled with the changes in plasma osmolality and volume, the primary mediators of vasopressin; thus, it has been difficult to separate cause and effect [118, 119, 127, 128]. However, local subcutaneous injection of vasopressin alters the rate and composition of sweat from glands exposed to an increase in local skin temperature [129]. Plasma vasopressin concentrations have been associated with sweat sodium concentration and osmolality, suggesting vasopressin promotes water conservation in the sweat gland [125, 130]. In addition, studies involving a possible role of catecholamines on sweat rate have resulted in conflicting findings [131, 132]. However, in a study of the effect of fluid intake, it was shown that the ingestion of a large volume, >3 L, was associated with an increase in sweating, reduction in plasma concentration of norepinephrine, and an increase in skin blood flow. In contrast, the opposite effects were seen with ingestion of a small volume, >0.5 L, during long-duration submaximal exercise in the heat. Therefore, an increase in catecholamines appears to be associated with a decrease in skin blood flow that results in a decrease in sweating.
Sweat is composed of a significant amount of electrolytes [90, 120, 121, 133, 134]. Thus, during exercise the predominate means of the loss of electrolytes is through sweat. The concentration of sodium in sweat ranges from 20 to 135 mmol/L, potassium from 3 to 35 mmol/L, and chloride from 10 to 100 mmol/L, in contrast to “normal” plasma concentrations (sodium 135–145 mmol/L, potassium 3.5–5.0 mmol/L, and chloride 96–106 mmol/L) [135]. While the levels of electrolytes in sweat are lower than in plasma, the losses are significant. At a sweat rate of 1.5 L/h at a sodium concentration of 60 mmol/L, a total of 90 mmol would be lost or 3% of total body sodium. As noted above, however, the concentrations of electrolytes in sweat are highly variable. Electrolyte concentrations of sweat are decreased as a result of training and heat acclimation [65, 90, 121]. The lower concentrations reduce the tonicity of the sweat and therefore facilitate evaporation and cooling. In a comparison of 10 min of acute maximal exercise to 60 min of submaximal exercise (60% of maximum workload), minimal differences in the electrolyte concentrations were noted: sodium 70 vs. 77 mmol/L, potassium 7.7 vs. 4.8 mmol/L, and osmolality 171 vs. 172 mOSM/L for maximal and submaximal exercise, respectively. The reductions in the sodium concentration of sweat appear to be in part mediated by aldosterone [121, 136].
Fluid and Electrolyte Intake
Consumption is the primary means of replacing the fluid and electrolytes losses incurred during the course of exercise [18, 137, 138]. In the performance of long-duration exercise, 80% of the fluid lost in sweat is replaced by voluntary ingestion if free access to fluids is provided [108, 137]. The extent to which volume losses are replaced is dependent upon the composition of the ingested fluid [137,138,139,140,141]. In humans during extended exercise, the volume of fluid replacement appears to be closely regulated. In contrast, the replacement of electrolytes does not appear to be as closely titrated and is a by-product of normal nutrient intake. Takamata et al. suggested that 6–24 h after heavy exercise, salt appetite is increased in association with a decrease in plasma osmolality and sodium concentrations resulting from fluid intake [113]. Leshem et al. monitored salt intake after exercise and found a voluntary increase of 50% in the amount of salt added to food [142]. Passe et al. assessed the acceptance of hypertonic saline fluids during exercise and reported an increase in palatability of a 60 mmol/lL sodium solution, suggesting a relationship between sensory reception, hedonic response, and drink composition in the replacement of electrolytes post exercise [143]. Replacement of electrolytes may be coupled with hunger and increase in salt appetite. In animal models salt appetite is strongly associated with angiotensin II; however, this proposed relationship has yet to be definitively demonstrated in humans [11, 144, 145].
As previously noted, the replacement of fluids is closely controlled over the course of exercise and thus readily adjusted for following exercise. This tight regulation is modulated by thirst, the subjective sensation to seek and drink fluids [144,145,146,147]. The subjective sensation of thirst can persist for hours after exercise [113]. As described earlier there is a level of voluntary dehydration that can be tolerated in the performance of long-duration exercise, but the majority, about 80%, of the fluid loss is replaced by drinking. The residual loss associated with the level of voluntary dehydration is usually replaced within 24 h [21, 29, 113]. This process is associated with a variety of factors, such as the increase in plasma osmolality and reduction in blood volume, both of which are closely tied to the regulation of numerous hormones. Immediately after exercise Takamata et al. found the subjective evaluation of thirst to be immediately reduced upon ingestion of fluids yet increased hours later in spite of plasma osmolality being reduced [113]. This increase in thirst was associated with an elevation of aldosterone and presumably angiotensin II [91, 147]. If the replacement fluid is water, plasma osmolality and sodium concentration can be decreased before blood volume loss is corrected, thus presenting conflicting regulatory mechanisms resulting in a reduction in thirst [148, 149]. Merry et al. reported the subjective sensation of thirst to be associated with an increase in osmolality during moderate exercise under various levels of hydration in subject with different levels of fitness (Fig. 13.2) [27]. Osmolality was also related to an increase in vasopressin, suggesting a possible association between vasopressin and thirst. Keneflick et al. assessed the response of thirst during 1 h of walking at 50% of maximum on a treadmill in temperate (27 °C) or cold (4 °C) environments [150]. In the cold environment, the sensation of thirst was reduced by 40% and associated with lower levels of vasopressin, even though plasma osmolality was increased. The authors speculated that peripheral vasoconstriction increased central blood volume that was sensed as an actual increase in blood volume. This hypothesis is supported in part by the observation that immersion and dehydration, which increase and decrease central blood volume, respectively, alter thirst via volume-induced stimulation of the cardiopulmonary baroreceptors. Stimulation of these baroreceptors by an increase in volume results in decreased vasopressin and PRA and increased ANP [151]. In contrast dehydration causing a reduction in volume elicits the opposite responses [33]. The specific roles of these hormones in the regulation of thirst during and following exercise have yet to be clearly defined.
The ingestion of fluid during the performance of exercise has been advocated to sustain performance [4, 6, 110]. To determine fluid replacement by water ingestion during exercise, Robinson et al. had subjects perform two bouts of exercise, one with and another without fluids, on a cycle ergometer for 1 h at 85% of their maximum oxygen uptake [133]. The subjects ingested 1.5 L of water to replace the fluid loss due to sweating, which resulted in a 60% decrease in the loss of body mass. The ingestion of fluid did not alter sweat rate, the increase in body temperature, or perceived exertion. Though plasma osmolality and sodium concentrations had a greater increase in the absence of water intake, no differences in vasopressin or angiotensin II were reported. These findings were confirmed by McConell et al. who stated that ingestion of fluids had little benefit on exercise of 1 h [152]. However, others have consistently shown hypohydration to impair performance. There is an absence of data as to whether someone exercising should drink “as much as tolerable,” “to replace the weight lost during exercise,” or “ad libitum”; thus, Noakes et al. had also questioned the effects of fluid hydration during exercise [153]. The role of hormones in this debate is even more difficult to evaluate. Rehydration is shown to attenuate the response of atrial natriuretic hormone, vasopressin, and PRA to exercise [24, 32, 66]. Furthermore, the role of these hormones in the modulation of thirst during exercise is confounded. At present the data supports maintenance of an adequate hydration status to avoid the adverse effects of dehydration. The means of achieving this, and the levels needed, have yet to be defined.
In light of the present state of data in this area, an understanding of the function of hormones in the regulation of thirst is essential. Hew-Butler has reviewed the role of vasopressin in fluid balance and its possible role in dysnatremia, specifically exercise-associated hyponatremia [10]. Hyponatremia with exercise may result from water retention associated with excess fluid intake, sodium loss predominately via sweat, or more likely a combination of these factors. Put forth is the hypothesis that non-osmotic-mediated AVP release from the pituitary increases circulating levels of vasopressin leading to retention of water, even if fluid intake does not exceed recommended guidelines. This inappropriate fluid retention/overload could be a contributing factor of hyponatremia and its subsequent sequelae. The efforts from this group, in the lab and in the field, provide insights as to the contribution of vasopressin and other hormones to the regulation of fluid and electrolyte homeostasis [10, 22, 26, 70, 71].
Renal Function
The action of hormones in the regulation of kidney function is well defined due to their role in the pathophysiology of hypertension. While extensive studies have been directed at the study of hormones on kidney function during exercise, the contribution of the kidneys to fluid and electrolyte balance is limited [59, 60, 154,155,156,157]. Zambraski described the limited contribution of the kidney noting that in a normal individual, the kidneys produce about 1 mL of urine a minute or 60 mL/h [53]. This is in comparison to the loss of fluid from sweat on the order of 1000–1500 mL/h, during moderate to heavy exercise. Zambraski estimated that during exercise the renal conservation of water would only account for 4% of the loss of water and about 8% for the sodium [53]. Thus, the conservation of fluid by the kidney is hampered by the limited amounts of water and electrolyte excreted in the basal state. Nevertheless, the hormonal influences on the kidney provide insights into their role in the overall maintenance of fluid and electrolyte homeostasis during and following exercise [53, 60, 158].
Renal Blood Flow
At rest the kidney receives about 20% or approximately 1000 mL/min of the overall cardiac output. During exercise renal blood flow is reduced in relation to the intensity and duration of exercise. With mild to moderate exercise (50–70% of maximum workload), there are negligible changes, but with maximal exercise flow is decreased by 40–60% from the normal [45, 48, 53, 158,159,160]. The reduction in renal blood flow persists for over 1 h after completion of the exercise. This reduction is caused by vasoconstriction of afferent arterioles, associated with an increase in sympathetic nerve activity and circulating levels of norepinephrine derived from spillover from the kidney [45, 47, 53, 159, 161]. In animal models upon initiation of exercise, there is an immediate reduction in renal blood flow which increases over time to a steady state associated with the level of exercise [162]. This immediate decrease suggests the predominance of the neural regulatory component in the initial phase of exercise. The reduction in renal blood flow decreases the volume of fluid and electrolytes delivered to the glomeruli of the kidney and in turn contributes to regional shifts in renal blood flow within the kidneys.
Glomerular Filtration Rate
The amount of fluid moving across the membrane of the glomeruli of the kidney is termed the glomerular filtration rate . The movement of fluid is the product of the drive pressure across the membrane and oncotic pressure of the plasma. As noted above there is an increase in afferent arteriole resistance with exercise; however, this is accompanied by an increase in efferent arteriole resistance facilitating filtration. The increase in efferent arteriole resistance is controlled by angiotensin II. Changes in the rate of glomerular filtration are related to the intensity and duration of exercise and may persist for up to 24 h after exercise [163, 164]. Minimal changes in filtration are observed with exercise of less than 50% of maximum. With acute maximal exercise or long-duration exercise above 70% of maximum, the rate of filtration may be decreased by 50–70%. With heavy exercise there is also an increase in the permeability of the glomerular membrane as demonstrated by the occurrence of an increase in protein excretion [53, 165]. This alteration of permeability is suggested to be in part mediated by norepinephrine, vasopressin, and angiotensin II and results in an increase in the excretion of protein [53, 163, 166, 167].
Urine Flow Rate
Urine flow rate is the product of the amount of fluid filtered (glomerular filtration rate) and the net reabsorption of fluid in the tubules. With exercise of low intensity, there is either no change or a slight increase in urine flow rate [39, 155]. With acute maximal exercise or long-duration exercise eliciting voluntary dehydration, urine flow rates are decreased by 20–60% of the normal basal levels of 0.8–1.2 mL/min [53, 59, 60]. This minimal decrease results in the conservation of water in light of the losses due to sweating. The decrease in the amount of filtered water is predominately due to vasoconstriction of the afferent arterioles caused by norepinephrine [45, 48, 131, 161]. Exercise also causes an increase in the osmolality of urine, indicative of an increase in the reabsorption of water [57,58,59]. However, decreases have been reported in urinary osmolality indicative of an increase in free water clearance during heavy exercise [53, 59]. Therefore the role of vasopressin in the control of water reabsorption in the collecting tubule during exercise has been questioned. There may be inhibition of vasopressin or the possibility of a “washout” of the osmotic gradient in the medullary area of the kidney due to the redistribution of blood flow associated with the actions of angiotensin II. After exercise the reduction in urine flow persists and may contribute to the rectification of fluid loss along with increased drinking [21, 113].
Renal Handling of Electrolytes
At the normal rate of glomerular filtration, the amount of fluid equivalent to the TBW is filtered in 5–6 h. The filtrate contains electrolyte concentrations equivalent to those of plasma. Over the course of traversing through the kidneys, 80–99% of the filtered load of electrolytes is reabsorbed. This reabsorption is hormonally mediated for sodium and establishes an electrochemical gradient for the handling of other electrolytes and an osmotic gradient for the handling of other solutes. With acute exercise, the decrease in the excretion of electrolytes is predominately due to the reduction in glomerular filtration rate [21, 113, 156]. During and following long-duration exercise, the reabsorption of sodium is regulated by aldosterone [21, 113]. With daily heavy exercise, there is a persistent increase in aldosterone, which is strongly associated with an increase in the reabsorption of sodium (Fig. 13.3) [21].
In summary, with exercise, kidney function changes and is regulated by a number of hormonal systems. The major alterations effecting fluid and electrolyte homeostasis are a decrease in renal blood flow and an increase in the reabsorption of sodium. There are several fallacies as to the contribution of these changes in kidney function to the net maintenance of fluids and electrolytes. The primary misunderstanding is the quantitative contribution of the kidney to fluid balance and the roles of hormones in these changes.
Summary
Exercise elicits increases in a number of hormones important in the regulation of fluid and electrolyte homeostasis. The action of these hormones may persist for hours and days after completion of the exercise. While increases in hormone levels are noted, the regulation and actions of these hormones are often not well defined, specifically in relation to the changes in fluid and electrolyte balance during exercise. There are issues as to the influence by the type and duration of exercise on hormonal responses that are not often accounted for. Recent efforts employing multifactorial analysis are just beginning to define some of these factors. In addition, the role of hormones in the etiology of the detrimental effects of exercise, such as dehydration and dysnatremia, is beginning to be addressed. Finally, evidence is mounting to show that exercise plays a vital role in fluid and electrolyte homeostasis. Observations of the hormonal responses to exercise will lead to a better understanding of both exercise physiology and related disease processes.
References
Wade CE, Freund BJ. Hormonal control of blood volume during and following exercise. In: Lamb DR, Gisolfi CV, editors. Perspectives in exercise science and sports medicine, vol. 3. Carmel: Benchmark; 1990. p. 207–41.
Viru A. Plasma hormones and physical exercise. Int J Sports Med. 1992;13(3):201–9.
Viru A. Hormones in muscular activity. Boca Raton: CRC; 1985.
Cheuvront SN, Carter R III, Sawka MN. Fluid balance and endurance exercise performance. Curr Sports Med Rep. 2003;2(4):202–8.
Coris EE, Ramirez AM, Van Durme DJ. Heat illness in athletes: the dangerous combination of heat, humidity and exercise. Sports Med. 2004;34(1):9–16.
Swaka MN, Franceconi RP, Young AJ. Influence of hydration level and body fluids on exercise performance in the heat. J Am Med Assoc. 1988;252:1165–9.
Sawka MN, Montain SJ, Latzka WA. Hydration effects on thermoregulation and performance in the heat. Comp Biochem Physiol A Mol Integr Physiol. 2001;128(4):679–90.
Murray B. Hydration and physical performance. J Am Coll Nutr. 2007;26(5 Suppl):542S–8S.
Goulet ED. Effect of exercise-induced dehydration on time-trial exercise performance: a meta-analysis. Br J Sports Med. 2011;45(14):1149–56.
Hew-Butler T. Arginine vasopressin, fluid balance and exercise: is exercise-associated hyponatraemia a disorder of arginine vasopressin secretion? Sports Med. 2010;40(6):459–79.
Stachenfeld NS. Acute effects of sodium ingestion on thirst and cardiovascular function. Curr Sports Med Rep. 2008;7(4 Suppl):S7–13.
Siegel AJ, d’Hemecourt P, Adner MM, Shirey T, Brown JL, Lewandrowski KB. Exertional dysnatremia in collapsed marathon runners: a critical role for point-of-care testing to guide appropriate therapy. Am J Clin Pathol. 2009;132(3):336–40.
Siegel AJ, Januzzi J, Sluss P, et al. Cardiac biomarkers, electrolytes, and other analytes in collapsed marathon runners: implications for the evaluation of runners following competition. Am J Clin Pathol. 2008;129(6):948–51.
Verbalis JG. Renal function and vasopressin during marathon running. Sports Med. 2007;37(4–5):455–8.
Fortney SM, Nadel ER, Wenger CB, Bove JR. Effect of blood volume on sweating rate and body fluids in exercising humans. J Appl Physiol. 1981;51(6):1594–600.
Brechue WF, Stager JM. Acetazolamide alters temperature regulation during submaximal exercise. J Appl Physiol. 1990;69(4):1402–7.
Zappe DH, Helyar RG, Green HJ. The interaction between short-term exercise training and a diuretic-induced hypovolemic stimulus. Eur J Appl Physiol Occup Physiol. 1996;72(4):335–40.
Greenleaf JE. The consequences of exercise on thirst and fluid intake. In: Ramsay DJ, Booth DA, editors. Thirst. London: Springer; 1991. p. 412–21.
Nagashima K, Wu J, Kavouras SA, Mack GW. Increased renal tubular sodium reabsorption during exercise-induced hypervolemia in humans. J Appl Physiol. 2001;91(3):1229–36.
Warburton DE, Gledhill N, Quinney HA. Blood volume, aerobic power, and endurance performance: potential ergogenic effect of volume loading. Clin J Sport Med. 2000;10(1):59–66.
Wade CE, Hill LC, Hunt MM, Dressendorfer RH. Plasma aldosterone and renal function in runners during a 20-day road race. Eur J Appl Physiol Occup Physiol. 1985;54(5):456–60.
Hew-Butler T, Jordaan E, Stuempfle KJ, et al. Osmotic and nonosmotic regulation of arginine vasopressin during prolonged endurance exercise. J Clin Endocrinol Metab. 2008;93(6):2072–8.
Freund BJ, Claybaugh JR, Hashiro GM, Buono M, Chrisney S. Exaggerated ANF response to exercise in middle-aged vs. young runners. J Appl Physiol. 1990;69(5):1607–14.
Khamnei S, Alipour MR, Ahmadiasl N. The combined effects of exercise and post dehydration water drinking on plasma arginine vasopressin, plasma osmolality and body temperature in healthy males. Int J Endocrinol Metab. 2005;2:80–6.
Mandroukas A, Metaxas TI, Heller J, et al. The effect of different exercise-testing protocols on atrial natriuretic peptide. Clin Physiol Funct Imaging. 2011;31(1):5–10.
Hew-Butler T, Noakes TD, Soldin SJ, Verbalis JG. Acute changes in arginine vasopressin, sweat, urine and serum sodium concentrations in exercising humans: does a coordinated homeostatic relationship exist? Br J Sports Med. 2010;44(10):710–5.
Merry TL, Ainslie PN, Walker R, Cotter JD. Fitness alters fluid regulatory but not behavioural responses to hypohydrated exercise. Physiol Behav. 2008;95(3):348–52.
Bentzen H, Pedersen RS, Nyvad O, Pedersen EB. Influence of training habits on exercise-induced changes in plasma atrial and brain natriuretic peptide and urinary excretion of aquaporin-2 in healthy man. Scand J Clin Lab Invest. 2002;62(7):541–51.
Wade CE, Dressendorfer RH, O’Brien JC, Claybaugh JR. Renal function, aldosterone, and vasopressin excretion following repeated long-distance running. J Appl Physiol. 1981;50(4):709–12.
Geelen G, Keil LC, Kravik SE, et al. Inhibition of plasma vasopressin after drinking in dehydrated humans. Am J Phys. 1984;247(6 Pt 2):R968–71.
Melin B, Jimenez C, Savourey G, et al. Effects of hydration state on hormonal and renal responses during moderate exercise in the heat. Eur J Appl Physiol Occup Physiol. 1997;76(4):320–7.
Khamnei S, Hosseinlou A, Ebrahimi H. The effect of volume of consumed water on drinking-induced sweating and plasma levels of arginine vasopressin, epinephrine and norepinephrine. Int J Endocrinol Metab. 2004;2(1):19–28.
Maresh CM, Gabaree-Boulant CL, Armstrong LE, et al. Effect of hydration status on thirst, drinking, and related hormonal responses during low-intensity exercise in the heat. J Appl Physiol. 2004;97(1):39–44.
De Souza MJ, Maresh CM, Maguire MS, Kraemer WJ, Flora-Ginter G, Goetz KL. Menstrual status and plasma vasopressin, renin activity, and aldosterone exercise responses. J Appl Physiol. 1989;67(2):736–43.
Stachenfeld NS, Taylor HS. Effects of estrogen and progesterone administration on extracellular fluid. J Appl Physiol. 2004;96(3):1011–8.
Stachenfeld NS, DiPietro L, Kokoszka CA, Silva C, Keefe DL, Nadel ER. Physiological variability of fluid-regulation hormones in young women. J Appl Physiol. 1999;86(3):1092–6.
Radke KJ, King KB, Blair ML, Fitzpatrick PG, Eldredge DH. Hormonal responses to the 6-minute walk test in women and men with coronary heart disease: a pilot study. Heart Lung. 2005;34(2):126–35.
Stachenfeld NS, Gleim GW, Zabetakis PM, Nicholas JA. Fluid balance and renal response following dehydrating exercise in well-trained men and women. Eur J Appl Physiol Occup Physiol. 1996;72(5–6):468–77.
Freund BJ, Shizuru EM, Hashiro GM, Claybaugh JR. Hormonal, electrolyte, and renal responses to exercise are intensity dependent. J Appl Physiol. 1991;70(2):900–6.
Shim CY, Ha JW, Park S, et al. Exaggerated blood pressure response to exercise is associated with augmented rise of angiotensin II during exercise. J Am Coll Cardiol. 2008;52(4):287–92.
Kjaer A, Appel J, Hildebrandt P, Petersen CL. Basal and exercise-induced neuroendocrine activation in patients with heart failure and in normal subjects. Eur J Heart Fail. 2004;6(1):29–39.
Coiro V, Jotti GS, Volpi R, et al. Difference between diabetic and nondiabetic smokers in the pituitary response to physical exercise. Metabolism. 2004;53(9):1140–4.
Wolf JP, Nguyen NU, Dumoulin G, Berthelay S. Plasma renin and aldosterone changes during twenty minutes’ moderate exercise. Influence of posture. Eur J Appl Physiol Occup Physiol. 1986;54(6):602–7.
Perrault H, Melin B, Jimenez C, et al. Fluid-regulating and sympathoadrenal hormonal responses to peak exercise following cardiac transplantation. J Appl Physiol. 1994;76(1):230–5.
Tidgren B, Hjemdahl P, Theodorsson E, Nussberger J. Renal neurohormonal and vascular responses to dynamic exercise in humans. J Appl Physiol. 1991;70(5):2279–86.
Svedenhag J. The sympatho-adrenal system in physical conditioning. Significance for training-induced adaptations and dependency on the training state. Acta Physiol Scand Suppl. 1985;543:1–73.
Baer PG, McGiff JC. Hormonal systems and renal hemodynamics. Annu Rev Physiol. 1980;42:589–601.
Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54(1):75–159.
Boushel R. Muscle metaboreflex control of the circulation during exercise. Acta Physiol (Oxf). 2010;199(4):367–83.
Galbo H. Hormonal and metabolic adaptation to exercise. New York: Thieme-Stratton; 1983.
Galbo H, Holst JJ, Christensen NJ. Glucagon and plasma catecholamine responses to graded and prolonged exercise in man. J Appl Physiol. 1975;38(1):70–6.
Kotchen TA, Hartley LH, Rice TW, Mougey EH, Jones LG, Mason JW. Renin, norepinephrine, and epinephrine responses to graded exercise. J Appl Physiol. 1971;31(2):178–84.
Zambraski EJ. The kidney and body fluid balance during exercise. In: Buskirk ER, Puhl SM, editors. Body fluid balance: exercise and sport. Boca Raton: CRC; 1996. p. 75–95.
Peronnet F, Beliveau L, Boudreau G, Trudeau F, Brisson G, Nadeau R. Regional plasma catecholamine removal and release at rest and exercise in dogs. Am J Phys. 1988;254(4 Pt 2):R663–72.
Meeusen R, Roelands B. Central fatigue and neurotransmitters, can thermoregulation be manipulated? Scand J Med Sci Sports. 2010;20(Suppl 3):19–28.
Roelands B, Goekint M, Heyman E, et al. Acute norepinephrine reuptake inhibition decreases performance in normal and high ambient temperature. J Appl Physiol. 2008;105(1):206–12.
Wade CE. Response, regulation, and actions of vasopressin during exercise: a review. Med Sci Sports Exerc. 1984;16(5):506–11.
Weitzman R, Kleeman CR. Water metabolism and neurohypophyseal hormones. In: Maxwell MH, Kleeman CR, editors. Clinical disorders of fluid and electrolyte metabolism. New York: McGraw-Hill; 1980. p. 531–645.
Wade CE, Claybaugh JR. Plasma renin activity, vasopressin concentration, and urinary excretory responses to exercise in men. J Appl Physiol. 1980;49(6):930–6.
Wade CE, Freund BJ, Claybaugh JR. Fluid and electrolyte homeostasis during and following exercise: hormonal and non-hormonal factors. In: Claybaugh JR, Wade CE, editors. Hormonal regulation of fluids and electrolytes: environmental effects. New York: Plenum; 1989. p. 1–44.
Convertino VA, Keil LC, Bernauer EM, Greenleaf JE. Plasma volume, osmolality, vasopressin, and renin activity during graded exercise in man. J Appl Physiol. 1981;50(1):123–8.
Inder WJ, Hellemans J, Swanney MP, Prickett TC, Donald RA. Prolonged exercise increases peripheral plasma ACTH, CRH, and AVP in male athletes. J Appl Physiol. 1998;85(3):835–41.
Saito T, Soya H. Delineation of responsive AVP-containing neurons to running stress in the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2004;286(3):R484–90.
Burge J, Knechtle B, Knechtle P, Gnadinger M, Rust AC, Rosemann T. Maintained serum sodium in male ultra-marathoners—the role of fluid intake, vasopressin, and aldosterone in fluid and electrolyte regulation. Horm Metab Res. 2011;43(9):646–52.
Convertino VA, Keil LC, Greenleaf JE. Plasma volume, renin, and vasopressin responses to graded exercise after training. J Appl Physiol. 1983;54(2):508–14.
Montain SJ, Laird JE, Latzka WA, Sawka MN. Aldosterone and vasopressin responses in the heat: hydration level and exercise intensity effects. Med Sci Sports Exerc. 1997;29(5):661–8.
Brandenberger G, Candas V, Follenius M, Libert JP, Kahn JM. Vascular fluid shifts and endocrine responses to exercise in the heat. Effect of rehydration. Eur J Appl Physiol Occup Physiol. 1986;55(2):123–9.
Melin B, Eclache JP, Geelen G, et al. Plasma AVP, neurophysin, renin activity, and aldosterone during submaximal exercise performed until exhaustion in trained and untrained men. Eur J Appl Physiol Occup Physiol. 1980;44(2):141–51.
Coiro V, Maffei ML, Volta E, et al. Effect of serotonergic system on AVP secretion induced by physical exercise. Neuropeptides. 2010;44(1):53–6.
Hew-Butler T, Dugas JP, Noakes TD, Verbalis JG. Changes in plasma arginine vasopressin concentrations in cyclists participating in a 109-km cycle race. Br J Sports Med. 2010;44(8):594–7.
Hew-Butler T, Hoffman MD, Stuempfle KJ, Rogers IR, Morgenthaler NG, Verbalis JG. Changes in copeptin and bioactive vasopressin in runners with and without hyponatremia. Clin J Sport Med. 2011;21(3):211–7.
Barron JL, Noakes TD, Levy W, Smith C, Millar RP. Hypothalamic dysfunction in overtrained athletes. J Clin Endocrinol Metab. 1985;60(4):803–6.
Bouissou P, Richalet JP, Galen FX, et al. Effect of beta-adrenoceptor blockade on renin-aldosterone and alpha-ANF during exercise at altitude. J Appl Physiol. 1989;67(1):141–6.
Hespel P, Lijnen P, Vanhees L, Fagard R, Amery A. Beta-adrenoceptors and the regulation of blood pressure and plasma renin during exercise. J Appl Physiol. 1986;60(1):108–13.
Reid IA, Ganong WF. Control of aldosterone secretion. In: Genest J, Koiw E, Kuchel O, editors. Hypertension pathophysiology and treatment. New York: McGraw-Hill; 1977. p. 265–92.
Taverner D, Mackay IG, Craig K, Watson ML. The effects of selective beta-adrenoceptor antagonists and partial agonist activity on renal function during exercise in normal subjects and those with moderate renal impairment. Br J Clin Pharmacol. 1991;32(3):387–91.
Tanaka H, Shindo M, Gutkowska J, et al. Effect of acute exercise on plasma immunoreactive-atrial natriuretic factor. Life Sci. 1986;39(18):1685–93.
Gleim GW, Zabetakis PM, DePasquale EE, Michelis MF, Nicholas JA. Plasma osmolality, volume, and renin activity at the “anaerobic threshold”. J Appl Physiol. 1984;56(1):57–63.
Morris DJ. The metabolism and mechanism of action of aldosterone. Endocr Rev. 1981;2(2):234–47.
Wade CE, Ramee SR, Hunt MM, White CJ. Hormonal and renal responses to converting enzyme inhibition during maximal exercise. J Appl Physiol. 1987;63(5):1796–800.
Niessner A, Ziegler S, Slany J, Billensteiner E, Woloszczuk W, Geyer G. Increases in plasma levels of atrial and brain natriuretic peptides after running a marathon: are their effects partly counterbalanced by adrenocortical steroids? Eur J Endocrinol. 2003;149(6):555–9.
Freund BJ, Wade CE, Claybaugh JR. Effects of exercise on atrial natriuretic factor. Release mechanisms and implications for fluid homeostasis. Sports Med. 1988;6(6):364–77.
Kaka S, Mudambo MD, Coutie W, Rennie MJ. Plasma arginine vasopressin, atrial natriuretic peptide and brain natriuretic peptide responses to long-term field training in the heat: effects of fluid ingestion and acclimatization. Eur J Appl Physiol. 1997;75:219–25.
Freund BJ, Claybaugh JR, Dice MS, Hashiro GM. Hormonal and vascular fluid responses to maximal exercise in trained and untrained males. J Appl Physiol. 1987;63(2):669–75.
Ohba H, Takada H, Musha H, et al. Effects of prolonged strenuous exercise on plasma levels of atrial natriuretic peptide and brain natriuretic peptide in healthy men. Am Heart J. 2001;141(5):751–8.
Geny B, Charloux A, Lampert E, Lonsdorfer J, Haberey P, Piquard F. Enhanced brain natriuretic peptide response to peak exercise in heart transplant recipients. J Appl Physiol. 1998;85(6):2270–6.
Geny B, Richard R, Mettauer B, Lonsdorfer J, Piquard F. Cardiac natriuretic peptides during exercise and training after heart transplantation. Cardiovasc Res. 2001;51(3):521–8.
Tanaka M, Ishizaka Y, Ishiyama Y, et al. Chronic effect of beta-adrenoceptor blockade on plasma levels of brain natriuretic peptide during exercise in essential hypertension. Hypertens Res. 1996;19(4):239–45.
Wambach G, Koch J. BNP plasma levels during acute volume expansion and chronic sodium loading in normal men. Clin Exp Hypertens. 1995;17(4):619–29.
Cuneo RC, Espiner EA, Nicholls MG, Yandle TG, Joyce SL, Gilchrist NL. Renal, hemodynamic, and hormonal responses to atrial natriuretic peptide infusions in normal man, and effect of sodium intake. J Clin Endocrinol Metab. 1986;63(4):946–53.
Fitzsimons JT, Simons BJ. The effect on drinking in the rat of intravenous infusion of angiotensin, given alone or in combination with other stimuli of thirst. J Physiol. 1969;203(1):45–57.
Sudoh T, Minamino N, Kangawa K, Matsuo H. Brain natriuretic peptide-32: N-terminal six amino acid extended form of brain natriuretic peptide identified in porcine brain. Biochem Biophys Res Commun. 1988;155(2):726–32.
Yamada T, Nakao K, Morii N, et al. Central effect of atrial natriuretic polypeptide on angiotensin II-stimulated vasopressin secretion in conscious rats. Eur J Pharmacol. 1986;125(3):453–6.
Tanaka M, Ishizaka Y, Ishiyama Y, et al. Exercise-induced secretion of brain natriuretic peptide in essential hypertension and normal subjects. Hypertens Res. 1995;18(2):159–66.
Bentzen H, Pedersen RS, Nyvad O, Pedersen EB. Effect of exercise on natriuretic peptides in plasma and urine in chronic heart failure. Int J Cardiol. 2004;93(2–3):121–30.
Kato M, Kinugawa T, Ogino K, et al. Augmented response in plasma brain natriuretic peptide to dynamic exercise in patients with left ventricular dysfunction and congestive heart failure. J Intern Med. 2000;248(4):309–15.
Gerzer R, Drummer C. Is the renal natriuretic peptide urodilatin involved in the regulation of natriuresis? J Cardiovasc Pharmacol. 1993;22(Suppl 2):S86–7.
Kentsch M, Otter W, Drummer C, et al. The dihydropyridine calcium channel blocker BAY t 7207 attenuates the exercise induced increase in plasma ANF and cyclic GMP in patients with mildly impaired left ventricular function. Eur J Clin Pharmacol. 1995;49(3):177–82.
Schmidt W, Bub A, Meyer M, et al. Is urodilatin the missing link in exercise-dependent renal sodium retention? J Appl Physiol. 1998;84(1):123–8.
Nishikimi T, Saito Y, Kitamura K, et al. Increased plasma levels of adrenomedullin in patients with heart failure. J Am Coll Cardiol. 1995;26(6):1424–31.
Ishimitsu T, Nishikimi T, Saito Y, et al. Plasma levels of adrenomedullin, a newly identified hypotensive peptide, in patients with hypertension and renal failure. J Clin Invest. 1994;94(5):2158–61.
Tanaka M, Kitamura K, Ishizaka Y, et al. Plasma adrenomedullin in various diseases and exercise-induced change in adrenomedullin in healthy subjects. Intern Med. 1995;34(8):728–33.
Piquard F, Charloux A, Mettauer B, et al. Exercise-induced increase in circulating adrenomedullin is related to mean blood pressure in heart transplant recipients. J Clin Endocrinol Metab. 2000;85(8):2828–31.
Krzeminski K, Mikulski T, Kruk B, Nazar K. Plasma adrenomedullin response to maximal exercise in healthy subjects. J Physiol Pharmacol. 2003;54(2):225–32.
Krzeminski K, Mikulski T, Nazar K. Effect of prolonged dynamic exercise on plasma adrenomedullin concentration in healthy young men. J Physiol Pharmacol. 2006;57(4):571–81.
Latzka WA, Montain SJ. Water and electrolyte requirements for exercise. Clin Sports Med. 1999;18(3):513–24.
Pugh LG, Corbett JL, Johnson RH. Rectal temperatures, weight losses, and sweat rates in marathon running. J Appl Physiol. 1967;23(3):347–52.
Rogers G, Goodman C, Rosen C. Water budget during ultra-endurance exercise. Med Sci Sports Exerc. 1997;29(11):1477–81.
Tam N, Nolte HW, Noakes TD. Changes in total body water content during running races of 21.1 km and 56 km in athletes drinking ad libitum. Clin J Sport Med. 2011;21(3):218–25.
Sawaka MN, Montain SJ, Latzka WA. Body fluid balance during exercise-heat exposure. In: Buskirk ER, Puhl SM, editors. Body fluid balance: exercise and sport. Boca Raton: CRC; 1996. p. 139–57.
Greenleaf JE, Sargent F II. Voluntary dehydration in man. J Appl Physiol. 1965;20(4):719–24.
Zouhal H, Groussard C, Minter G, et al. Inverse relationship between percentage body weight change and finishing time in 643 forty-two-kilometre marathon runners. Br J Sports Med. 2011;45(14):1101–5.
Takamata A, Mack GW, Gillen CM, Nadel ER. Sodium appetite, thirst, and body fluid regulation in humans during rehydration without sodium replacement. Am J Phys. 1994;266(5 Pt 2):R1493–502.
Harrison MH. Effects on thermal stress and exercise on blood volume in humans. Physiol Rev. 1985;65(1):149–209.
Padilla J, Simmons GH, Bender SB, Arce-Esquivel AA, Whyte JJ, Laughlin MH. Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology (Bethesda). 2011;26(3):132–45.
Rush JWE, Aultman CD. Vascular biology of angiotensin and the impact o physical activity. Appl Physiol Nutr Metab. 2008;33:162–72.
Whyte JJ, Laughlin MH. Review: the effects of acute and chronic exercise on the vasculature. Acta Physiol. 2010;199:441–50.
Fortney SM, Nadel ER, Wenger CB, Bove JR. Effect of acute alterations of blood volume on circulatory performance in humans. J Appl Physiol. 1981;50(2):292–8.
Fortney SM, Wenger CB, Bove JR, Nadel ER. Effect of hyperosmolality on control of blood flow and sweating. J Appl Physiol. 1984;57(6):1688–95.
Sato K. The physiology, pharmacology, and biochemistry of the eccrine sweat gland. Rev Physiol Biochem Pharmacol. 1977;79:51–131.
Kirby CR, Convertino VA. Plasma aldosterone and sweat sodium concentrations after exercise and heat acclimation. J Appl Physiol. 1986;61(3):967–70.
Jorgenson RJ, Salinas CF, Dowben JS, St John DL. A population study on the density of palmar sweat pores. Birth Defects Orig Artic Ser. 1988;24(2):51–63.
Brown MB, McCarty NA, Millard-Stafford M. High-sweat Na+ in cystic fibrosis and healthy individuals does not diminish thirst during exercise in the heat. Am J Physiol Regul Integr Comp Physiol. 2011;301(4):R1177–85.
Wingo JE, Low DA, Keller DM, Brothers RM, Shibasaki M, Crandall CG. Skin blood flow and local temperature independently modify sweat rate during passive heat stress in humans. J Appl Physiol. 2010;109(5):1301–6.
Fasciolo JC, Totel GL, Johnson RE. Antidiuretic hormone and human eccrine sweating. J Appl Physiol. 1969;27(3):303–7.
Saini J, Geny B, Brandenberger G, et al. Training effects on the hydromineral endocrine responses of cardiac transplant patients. Eur J Appl Physiol Occup Physiol. 1995;70(3):226–33.
Coiro V, Casti A, Volta E, et al. Naloxone decreases the inhibitory effect of ethanol on the release of arginine-vasopressin induced by physical exercise in man. J Neural Transm. 2009;116(9):1065–9.
Weidmann P, Hasler L, Gnadinger MP, et al. Blood levels and renal effects of atrial natriuretic peptide in normal man. J Clin Invest. 1986;77(3):734–42.
Jougasaki M, Wei CM, Aarhus LL, Heublein DM, Sandberg SM, Burnett JC Jr. Renal localization and actions of adrenomedullin: a natriuretic peptide. Am J Phys. 1995;268(4 Pt 2):F657–63.
Gibinski K, Kozlowski S, Chwalbinska-Moneta J, Giec L, Zmudzinski J, Markiewicz A. ADH and thermal sweating. Eur J Appl Physiol Occup Physiol. 1979;42(1):1–13.
Mack GW, Shannon LM, Nadel ER. Influence of beta-adrenergic blockade on the control of sweating in humans. J Appl Physiol. 1986;61(5):1701–5.
Allen JA, Jenkinson DJ, Roddie IC. The effect of -adrenoceptor blockade on human sweating. Br J Pharmacol. 1973;47(3):487–97.
Robinson TA, Hawley JA, Palmer GS, et al. Water ingestion does not improve 1-h cycling performance in moderate ambient temperatures. Eur J Appl Physiol Occup Physiol. 1995;71(2–3):153–60.
Verde T, Shephard RJ, Corey P, Moore R. Sweat composition in exercise and in heat. J Appl Physiol. 1982;53(6):1540–5.
Robinson S, Robinson AH. Chemical composition of sweat. Physiol Rev. 1954;34(2):202–20.
Collins KJ. The action of exogenous aldosterone on the secretion and composition of drug-induced sweat. Clin Sci. 1966;30(2):207–21.
Coyle EF, Hamilton M. Fluid replacement during exercise; effects on physiological homeostasis and performance. In: Lamb DR, Gisolfi CV, editors. Perspectives in exercise science and sports medicine, vol. 3. Carmel: Benchmark; 1990. p. 281–303.
Nadel ER, Mack GW, Nose H. Influence of fluid replacement beverages on body fluid homeostasis during exercise and recovery. In: Lamb DR, Gisolfi CV, editors. Perspectives in exercise science and sports medicine, vol. 3. Carmel: Benchmark; 1990. p. 181–206.
Evans GH, Shirreffs SM, Maughan RJ. Postexercise rehydration in man: the effects of carbohydrate content and osmolality of drinks ingested ad libitum. Appl Physiol Nutr Metab. 2009;34(4):785–93.
Kavouras SA, Armstrong LE, Maresh CM, et al. Rehydration with glycerol: endocrine, cardiovascular, and thermoregulatory responses during exercise in the heat. J Appl Physiol. 2006;100(2):442–50.
Fritzsche RG, Switzer TW, Hodgkinson BJ, Lee SH, Martin JC, Coyle EF. Water and carbohydrate ingestion during prolonged exercise increase maximal neuromuscular power. J Appl Physiol. 2000;88(2):730–7.
Leshem M, Abutbul A, Eilon R. Exercise increases the preference for salt in humans. Appetite. 1999;32(2):251–60.
Passe DH, Stofan JR, Rowe CL, Horswill CA, Murray R. Exercise condition affects hedonic responses to sodium in a sport drink. Appetite. 2009;52(3):561–7.
Stricker EM, Verbalis JG. Hormones and behavior: the biology of thirst and sodium appetite. Am Scientist. 1988;76:261–7.
Stricker EM, Huang W, Sved AF. Early osmoregulatory signals in the control of water intake and neurohypophyseal hormone secretion. Physiol Behav. 2002;76(3):415–21.
Rolls BJ, Edmund TR. In: Gray J, editor. Thirst—problems in the behavioural sciences. Cambridge: Cambridge University Press; 1982.
Szczepanska-Sadowska E. Hormonal inputs to thirst. In: Ramsay DJ, Booth DA, editors. Thirst. London: Springer; 1991. p. 110–30.
Nose H, Mack GW, Shi XR, Nadel ER. Role of osmolality and plasma volume during rehydration in humans. J Appl Physiol. 1988;65(1):325–31.
Nose H, Mack GW, Shi XR, Nadel ER. Involvement of sodium retention hormones during rehydration in humans. J Appl Physiol. 1988;65(1):332–6.
Kenefick RW, Hazzard MP, Mahood NV, Castellani JW. Thirst sensations and AVP responses at rest and during exercise-cold exposure. Med Sci Sports Exerc. 2004;36(9):1528–34.
Wada F, Sagawa S, Miki K, et al. Mechanism of thirst attenuation during head-out water immersion in men. Am J Phys. 1995;268(3 Pt 2):R583–9.
McConell GK, Burge CM, Skinner SL, Hargreaves M. Influence of ingested fluid volume on physiological responses during prolonged exercise. Acta Physiol Scand. 1997;160(2):149–56.
Noakes TD. Is drinking to thirst optimum? Ann Nutr Metab. 2010;57(Suppl 2):9–17.
Castenfors J. Renal function during exercise. With special reference to exercise proteinuria and the release of renin. Acta Physiol Scand Suppl. 1967;293:1–44.
Kachadorian WA, Johnson RE. Renal responses to various rates of exercise. J Appl Physiol. 1970;28(6):748–52.
Poortmans JR. Exercise and renal function. Sports Med. 1984;1(2):125–53.
Poortmans JR, Vanderstraeten J. Kidney function during exercise in healthy and diseased humans. An update. Sports Med. 1994;18(6):419–37.
Zambraski EJ. Renal regulation of fluid homeostasis during exercise. In: Gisolfi CV, Lamb DR, editors. Perspectives in exercise science and sports medicine, vol. 3. Carmel: Benchmark; 1990. p. 247–76.
Suzuki M, Sudoh M, Matsubara S, Kawakami K, Shiota M, Ikawa S. Changes in renal blood flow measured by radionuclide angiography following exhausting exercise in humans. Eur J Appl Physiol Occup Physiol. 1996;74(1–2):1–7.
McAllister RM. Adaptations in control of blood flow with training: splanchnic and renal blood flows. Med Sci Sports Exerc. 1998;30(3):375–81.
Johnson MD, Barger AC. Circulating catecholamines in control of renal electrolyte and water excretion. Am J Phys. 1981;240(3):F192–9.
Zambraski EJ, Tucker MS, Lakas CS, Grassl SM, Scanes CG. Mechanism of renin release in exercising dog. Am J Phys. 1984;246(1 Pt 1):E71–6.
Poortmans JR, Mathieu N, De Plaen P. Influence of running different distances on renal glomerular and tubular impairment in humans. Eur J Appl Physiol Occup Physiol. 1996;72(5–6):522–7.
Tian Y, Tong TK, Lippi G, Huang C, Shi Q, Nie J. Renal function parameters during early and late recovery periods following an all-out 21-km run in trained adolescent runners. Clin Chem Lab Med. 2011;49(6):993–7.
Poortmans JR, Ouchinsky M. Glomerular filtration rate and albumin excretion after maximal exercise in aging sedentary and active men. J Gerontol A Biol Sci Med Sci. 2006;61(11):1181–5.
Lang CC, Rahman AR, Balfour DJ, Struthers AD. Effect of noradrenaline on renal sodium and water handling in euhydrated and overhydrated man. Clin Sci (Lond). 1993;85(4):487–94.
Bellinghieri G, Savica V, Santoro D. Renal alterations during exercise. J Ren Nutr. 2008;18(1):158–64.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wade, C.E. (2020). Hormonal Regulation of Fluid and Electrolyte Homeostasis During Exercise. In: Hackney, A., Constantini, N. (eds) Endocrinology of Physical Activity and Sport. Contemporary Endocrinology. Humana, Cham. https://doi.org/10.1007/978-3-030-33376-8_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-33376-8_13
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-33375-1
Online ISBN: 978-3-030-33376-8
eBook Packages: MedicineMedicine (R0)