Abstract
Preventing infections and the transmission of disease in the radiology environment can be challenging due to the number of patients seen, the amount of equipment in use, and the rapid pace of care delivery in this setting. Hospital-acquired infections pose a threat to patients and are costly to treat. Infection prevention and control strategies mitigate risks to both patients and healthcare workers. Advanced practice nurses can provide leadership in developing a culture of teamwork and safety for both patients and healthcare workers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Advances in radiology benefit patients who now have many more minimally invasive diagnostic and treatment options. Higher volumes of patients, some of them critically ill, undergo complex procedures in radiology rooms. The potential to cause harm to patients through infection transmission exists in radiology departments whether the patient is undergoing noninvasive imaging or an invasive procedure. Although healthcare becomes ever safer, the Centers for Disease Control and Prevention (CDC) estimates that 5–10% of hospital patients suffer from hospital-acquired infections (HAIs) in US hospitals every year, resulting in nearly 100,000 deaths, and with an associated cost of $20 billion [1]. HAIs result from invasive procedures, contact with the contaminated hands of healthcare workers (HCWs) or equipment, and overuse or misuse of antibiotics [1].
Infection prevention and control has been practiced throughout history, even when medical knowledge was limited. In medieval times victims of bubonic plaque were isolated in their own homes and their belongings burned. If they died of the disease, their bodies were buried in mass graves away from town [2]. Over time, less drastic measures to prevent the transmission of infectious diseases have been developed: hand hygiene, standard precautions, contact precautions, and sterile techniques for invasive procedures. By the 1950s most operating suites worldwide had adopted surgical clothing and sterile drapes [3]. Unfortunately, the radiology procedural suite has been slower to adopt similar techniques. In 2007, less than 60% of practicing radiologists wore hats, masks, and sterile gowns, or used full sterile drapes during central line placement [4, 5]. Infection control has recently become a major focus in many radiology departments [6]. Proper practices have the ability to prevent transmission of nosocomial pathogens and to save lives. Infection preventionists and healthcare epidemiologists provide expertise in advising and educating staff [7]. This chapter provides the advance practice nurse and nurse manager with foundational knowledge in infection prevention and control for both general imaging and interventional radiology.
2 Standard Precautions
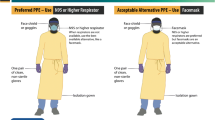
The radiology department is a potentially pathogen-rich environment. Almost every patient encounters radiology at some point, creating a unique level of risk. The CDC [8] defines standard precautions as a set of practices to be used whether or not the patient is diagnosed with an infection (Table 11.1). Radiology personnel must be vigilant in the use of personal protective equipment (PPE) to protect both themselves and the other patients they come into contact with on a daily basis [5]. Gloves should be worn for all interactions where there is potential for contact with blood or bodily fluids, mucous membranes, or non-intact skin. Gloves should fit well. Gloves that are too large may slip off and provide an incomplete barrier; gloves that are too small may tear more easily [5]. Gloves should never be used as a substitute for hand washing, as micro perforations can contaminate hands even when gloves are worn.
Use of non-sterile gowns as well as eye and mouth protection are needed when there is any chance of spray, splash, or splatter of blood or other body fluids. Some masks include eye protection. Eyeglasses are not considered complete protection against splash. Eye shields or goggles can be reused if they are disinfected in between patient encounters. Many radiology departments now opt for disposable eye shields. (This differs from lead eyewear to protect against radiation exposure though these can be used for both purposes). PPE should be removed when leaving the patient care area.
The use of sterile gowns is required for any personnel having direct contact with a sterile field (see “Surgical Attire” below). The CDC, the Institute for Healthcare Improvement (IHI), and American College of Radiology (ACR) all agree that sterile gowns are an essential infection prevention strategy when performing procedures such as abscess drainage or arterial puncture [6]. The CDC and the Association for Professionals in Infection Control and Prevention (APIC) also recommend that surgical masks be worn when performing spinal injection (e.g., myelography) or intracapsular procedures (e.g., arthrography) that include injection of material or insertion of a catheter [9, 10], as absence of mask use has been associated with iatrogenic bacterial meningitis and septic arthritis due to oropharyngeal microflora, presumably due to cross-contamination from respiratory droplets [11, 12].
3 Hand Hygiene
Hand hygiene provides the foundation for infection control. The earliest evidence of this dates backs to the 1840s when Semmelweiss and Holmes each observed that mortality rates decreased significantly when physicians washed their hands with antiseptic [13]. Unfortunately getting HCWs to perform hand hygiene remains a challenge in the modern era. The CDC [14] estimates that HCWs wash their hands about half the times they should.
The question of why HCWs miss opportunities to cleanse their hands is an interesting one. Transmission of bacteria is an invisible process, so there is no evidence “in the moment” when it occurs. Researchers have found that factors influencing compliance with hand hygiene can be divided into two categories: motivational factors and work environment [15]. Motivational factors include the actions of senior medical and nursing staff, the amount of self-perceived risk in a given patient care situation, the acuity of patient care, and the number of cues present to prompt hand hygiene. Work environment factors included availability of hand hygiene products, organizational commitment to compliance with hand hygiene, and educating HCWs [15]. Skin damage as a result of frequent washing has also been cited as a deterrent to frequent hand washing [16].
Alcohol-based hand rubs and soap and water are the two methods for hand hygiene available in the healthcare setting. A surgical scrub is done for sterile procedures. Alcohol-based hand rubs are the most effective at killing most fungi, viruses, and bacteria, including multidrug-resistant organisms (MDRO) such as methicillin-resistant Staphylococcus aureus (MRSA). These products are also less likely to cause skin irritation [16]. However, alcohol-based hand rubs are not the best choice when dealing with spore-forming bacteria such as Clostridioides difficile (formerly known as Clostridium difficile) and certain viruses such as noroviruses [17,18,19]. When performing hand hygiene for patients with infections due to alcohol-resistant pathogens or when hands are visibly soiled, handwashing with soap and water is recommended [18] (Table 11.2). For the emerging pathogen Candida auris, the CDC recommends adhering to standard guidelines (alcohol-based hand rubs unless hands are visibly soiled) [18]. As more information becomes available about Candida auris new guidelines may be developed [20]. Timely and effective communication between radiology personnel, infection prevention personnel, and primary clinicians that includes information about contact precautions needed and the recommended method of hand hygiene for a given patient are critical for optimal infection control practice in radiology departments.
Some studies show that even when HCWs perform hand hygiene, the correct technique may not be used. Common shortcomings include not cleaning for long enough and not cleaning all of the hand’s surfaces (Table 11.3) [19, 21]. Alcohol-based hand rub dispensers and sinks for soap and water washing need to be readily available. Lotions compatible with the products used should be available to HCWs to prevent avoidance of hand hygiene caused by dermatitis. Nails should be no longer than a ¼” and artificial nail use is restricted as these have been shown to harbor bacteria [22].
The World Health Organization (WHO) has a wealth of slide set presentations, posters, and other teaching material available to assist in educating HCWs [23]. Another technique to educate staff about hand hygiene utilizes fluorescent gel products that mimic the way bacteria spread between healthcare workers’ hands to patients or surfaces [24]. The fluorescent gel product is applied to hands and then the participants are led through different exercises such as shaking hands and handling equipment. A black light is used to illuminate fluorescent residue (representing “germs”) on surfaces and hands. This exercise provides a powerful visual demonstration of how easy it is to spread organisms from dirty hands.
Observational audits are the most common way of measuring compliance with hand hygiene [16, 19]. HCWs are observed to see if they perform hand hygiene when entering or exiting a patient room, as proxy measures of hand hygiene opportunities before or after touching a patient and/or their surroundings, respectively. The audits can be performed by infection preventionists or by unit personnel. The advantage to this method is that the quality of hand hygiene can be assessed as well. The main disadvantage is that staff may be aware that they are being audited and this can skew the results. Change in behavior due to an awareness of being observed is termed the Hawthorne effect [25]. Another important limitation of observational audits performed outside a patient room is that opportunities for hand hygiene that occur in the room during patient care may not be assessed. Examples include before performing a clean or aseptic procedure or after a potential body fluid exposure. Hand hygiene compliance can be posted on units and used as educational and quality improvement tools. Sample audit tool templates are available on the WHO website [23].
Recently electronic monitoring devices have been brought to market as an alternative to traditional, observer-based auditing programs. These technologies have the potential to measure compliance with hand hygiene opportunities that occur throughout the workday, including nights, weekends, holidays, and other times that are challenging to audit using traditional methods. Moreover, these products have the ability to markedly increase the frequency of audits without the expense of personnel to perform manual audits. Finally, these systems also may have an advantage of minimizing the Hawthorn effect. However, installation of these devices is expensive and may be tied to certain products, making it difficult for organizations to switch products [16]. Some studies have also reported that HCWs and patients may not regard the technology positively, and it remains to be determined whether these systems improve hand hygiene compliance or help reduce HAIs [26, 27]. A final option to assess hand hygiene is measuring product usage. Although this method doesn’t provide any data on how well the hand hygiene was performed, it has been more accurately associated with HAI trends than observational audits and is less time-consuming [16].
4 Environmental Cleaning and Disinfection
It has been well established that patients can acquire infections from the hospital environment [28, 29]. Organisms such as MRSA, vancomycin-resistant Enterococcus (VRE), and C. difficile can remain viable on surfaces for days to months [28, 30]. Items that come into physical contact with patients need to be either disinfected or sterilized based on their use (Table 11.4). All equipment cleaning should take into account the manufacturer’s instructions. This chapter will include low-level disinfection only as it applies to all areas of radiology. It should be noted that ultrasound probes that contact body cavities and non-intact skin require high-level disinfection, even when a sterile sleeve is used to cover the probe. This includes probes used for ultrasound guided punctures. For more information on sterilization and high-level disinfection, please refer to CDC guidelines.
Equipment and surfaces in radiology rooms need disinfection in between patients. This includes mattresses, control panels, tables, carts, positioning devices, and pillows. For interventional cases, planning ahead to have necessary devices, wires, and equipment on hand helps to prevent frequent room entry and exit and thereby avoids contaminating items unnecessarily. Items such as radiographic markers and lead aprons may be overlooked. Cleaning protocols for these items should be understood and practiced by HCWs, including clinicians and environmental service personnel [31, 32]. Use of keyboards and computers in radiology for documentation in electronic medical records is now commonplace. These items should be included in room turnovers [33].
Knowing how to use disinfectants is essential knowledge for radiology teams. Chlorine-based products, quaternary ammonium compounds, or phenolics are examples of liquids used for low-level disinfection [28]. These products are usually packaged as wipes. The manufacturer of each product will have a contact time listed on the packaging to instruct how long the liquid must remain in contact with the surface in order for successful disinfection to occur. Chlorine-based products are often used for point-of-care cleaning in areas occupied by patients with C. difficile or other pathogens intrinsically resistant to quaternary ammonium disinfectants, such as non-enveloped viruses (e.g., norovirus). HCWs should be well acquainted with different products available to them and be aware of the contact time for each product. HCWs should also be educated to make sure lids on wipe canisters are shut to avoid drying them out. Environmental cleaning staff play an integral role in patient safety and should be acknowledged for their work.
No-touch decontamination of patient care areas is a more recent development in environmental cleaning. Ultraviolet light or hydrogen peroxide mist can be used as an adjunct to regular cleaning for patients with C. difficile, MRSA, or other resistant infections. The devices are programmed according to the size of the room and have proven effective in eliminating pathogenic bacteria from the environment. However, since these technologies are expensive and can take several hours to complete, they are not a viable option for routine room turnovers but can be considered for terminal cleaning at the end of the day [28].
Surfaces can appear perfectly clean but still harbor pathogenic organisms. How do we know that surfaces are truly disinfected? How can we quantify cleanliness? ATP (adenosine triphosphate) bioluminescence testing was developed in the food industry and is now used in the healthcare environment [34]. ATP identifies organic material on surfaces although it does not specifically identify what organic material is present. A commercial swab is used on surfaces to be tested and the swab is placed in a machine (luminometer) which detects the presence of ATP (Fig. 11.1). ATP is the energy source for all organic materials and remains stable in the environment over time, making this a reliable indicator of cleanliness [35]. Different brands of luminometers have different ranges for cleanliness thresholds. Results can be posted in a graph format and discussed with staff (Fig. 11.2). Fluorescent gels, discussed earlier, can also be used to audit cleaning practices between room turnovers. The CDC provides guidance on what surfaces should be tested in patient care areas [36].
5 Transmission-Based Isolation Precautions
Transmission-based precautions are used for any communicable disease. Multidrug-resistant organisms (MDROs) have been on the rise all over the world. These organisms cause infections that can be difficult and potentially impossible to treat. Although identifying these patients is important so that staff can practice the appropriate precautions, it is important to remember that patients may not yet be identified as having been colonized or infected with an MDRO; consequently, standard precautions should always be used to protect both patients and staff. There should be clear communication about isolation precautions needed for patients traveling to the radiology setting. Patients undergoing procedures should also be clearly identified while in the radiology suite to avoid an accidental break in precautions. Posting signs on doorways can alert staff that isolation precautions apply and make clear the preferred method of hand hygiene.
Modes of transmission are contact (direct or indirect), droplets, or airborne (Table 11.5). Some diseases can be transmitted in more than one way. Airborne isolation requires not only the use of PPE, but also a special air handling system to prevent infectious respiratory particles from traveling to other areas of the hospital [37]. For patients requiring airborne isolation their case should be delayed until treatment has been completed if this does not pose a threat to the patient. If the case cannot be delayed, a negative pressure room should be used. High-efficiency particulate air (HEPA) filters do not take the place of negative pressure rooms but are sometimes used to mitigate the risk in locations without negative pressure [38]. Some organizations try to schedule isolation cases at the end of the day. The number of patients on isolation precautions is on the rise, so this may not always be a practical strategy.
6 Surgical Attire
The use of surgical scrubs in the operating room dates back to the 1930s and 1940s. Prior to this, street clothing was covered by a sterile gown [3]. The purpose of specialized surgical garments is twofold: prevention of surgical site infection from extra procedural bacteria and reduction in contamination of clothing worn outside the surgical suite [3, 5, 6]. As recently as 2008, many interventional radiology departments did not require scrub attire in the procedural suite [4]. This practice is changing, as the IR suite more closely resembles an operating suite in terms of infection prevention practices and with the advent of hybrid interventional/OR rooms. Indeed, this is the recommendation of the Society of Interventional Radiology (SIR), the Association of perioperative Registered Nurses (AORN), and the Association for Radiologic and Imaging Nursing (ARIN) [6]. Hospitals should provide and launder specific garb for wearing in surgical suites; this garb is not to be worn outside the hospital or laundered at home. Some hospitals identify these scrubs with a distinctive color.
Maximum barrier precautions are recommended when performing any sterile procedure such as thoracentesis, central line insertion, or percutaneous endovascular aneurysm repair. This includes the use of hats and masks, sterile gowns, sterile gloves, and full sterile draping of the patient. Sterile gloves and masks should be worn during joint injection or lumbar punctures as oral flora can cause septic arthritis and other infections [12]. There is little data currently to support the use of shoe covers as an infection prevention tool; shoe covers are recommended to prevent splash contamination for shoes that will be worn outside of a procedure area [6]. Traffic in the room during the procedure should be minimized, and others in the room should also be wearing hats and masks [6].
7 Preparing the Patient’s Skin
The patient’s skin provides a natural barrier to infection. Skin preparation is done with an antimicrobial agent for invasive procedures. If necessary, hair removal should be done with clippers prior to applying the antimicrobial agent. Chlorhexidine gluconate is most commonly used because of its broad range of antimicrobial activity, ease of application, and proven efficacy in preventing surgical site infections [6]. If the patient has an allergy to chlorhexidine gluconate, other agents, such as povidone-iodine, can be used as a substitute.
8 Antibiotic Prophylaxis
The Society of Interventional Radiology (SIR) separates procedures performed in the interventional radiology area into categories from clean (such as diagnostic arteriogram) to dirty (such as abscess drainage or placement of gastrostomy tubes). Recommendations for antibiotic prophylaxis from the SIR continue to evolve as new data becomes available. The accompanying table is accurate as of 2018 (Table 11.6). While antibiotics are no longer recommended for most procedures, pre-procedural antimicrobial prophylaxis still has a place in management. Prolonged antibiotic therapy is no longer recommended. Procedures listed in the table are the only procedures for which antibiotic prophylaxis is recommended. Prophylaxis for routine angiography, closure device deployment, routine biopsy (except transrectal biopsy), radioembolization, and fistulography is no longer recommended [39]. Timing of the administration of any antibiotic should be per current guidelines and institutional policy.
9 Summary
The modern-day radiology suite typically sees a large volume of patients ranging from the ambulatory to the critically ill. This is a likely environment for the potential transmission of pathogenic organisms. Factors that play a role in decreasing the occurrence of HAIs in radiology include having clear infection prevention and control guidelines and easy access to disinfectants, alcohol hand rubs, sinks, and PPE. On the organizational level, having senior clinicians demonstrate best practices and promoting a culture of teamwork creates an environment that emphasizes safety for both patients and HCWs. Advanced practice nurses can partner with infection preventionists and hospital epidemiologists, as well as other clinicians and administration to provide leadership regarding implementation of guidelines from the CDC, the Association for Radiologic and Imaging Nursing (ARIN), and SIR and have these embraced by staff.
References
Centers for Disease Control and Prevention. Healthcare-associated infections. https://www.cdc.gov/hai/index.html. 2018. Accessed 1 Mar 2019.
Smith PW, Watkin K, Hewlett A. Infection control through the ages. Am J Infect Control. 2012;40:35–42.https://ajicjournal.org. https://doi.org/10.1016/j.ajjic.2011.02.019.
Adams LW, Aschenbrenner CA, Houle TT, Roy RC. Uncovering the history of operating room attire through photographs. Anesthesiology. 2016;124(1):19–24. https://doi.org/10.1097/ALN.0000000000000932.
Reddy P, Liebovitz D, Chrisman H, Nemcek AA, Noskin GA. Infection control practices among interventional radiologists: results of an online survey. J Vasc Interv Radiol. 2009;20:1070–4.
Mizra SK, Tragon TR, Fukui MB, Hartman MS, Hartman AL. Microbiology for radiologists: how to minimize infection transmission in the radiology department. Radiographics. 2015;35:1231–44. https://radiographics.rsna.org. https://doi.org/10.1148/rg.2015140034.
Chan D, Downing D, Keough CE, Saad WA, Annamalai G, Janned’Othee B, et al. Joint practice guideline for sterile technique during vascular and interventional radiology procedures: from the Society of Interventional Radiology, Association of periOperative Registered Nurses, and Association for Radiologic and Imaging Nursing, for the Society of Interventional Radiology (Wael Saad, MD, Chair), Standards of Practice Committee, and Endorsed by the Cardiovascular Interventional Radiological Society of Europe and the Canadian Interventional Radiology Association. J Radiol Nurs. 2012;31(4):130–43.
Kok HK, Torreggiani WC, Nihill DM. Imaging services and radiation oncology. In: Text of infection control and epidemiology. Association for Professionals in Infection Control and Epidemiology. 2014. http://text.apic.org/toc/infection-prevention-for-practice-settings-and-service-specific-patient-care-areas/imaging-services-and-radiation-oncology. Accessed 1 Mar 2019.
Centers for Disease Control and Prevention. Standard precautions for all patient care. https://www.cdc.gov/infectioncontrol/basics/standard-precautions.html 2018. Accessed 1 Mar 2019.
Dolan SA, Arias KM, Felizardo G, Barnes S, Kraska S, Patric M, et al. APIC position paper: safe injection, infusion, and medication vial practices in health care. Am J Infect Control. 2016;44:750–7.
Siegel JD, Rhinehart E, Jackson M, Chiarello L, Health Care Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(Suppl 2):S65–164.
Veringa E, van Belkum A, Scheliekens H. Iatrogenic meningitis by Streptococcus salivarius following lumbar puncture. J Hosp Infect. 1995;29(4):316–8.
Cain SM, Enfield KB, Giannetta ET, Sifri CD, Lewis JD. Septic arthritis due to oral streptococci following intra-articular injection: a case series. Am J Infect Control. 2018;46(11):1301–3.
Lane HJ, Blum N, Fee E. Oliver Wendell Holmes (1809–1894) and Ignaz Philipp Semmelweis (1818–1865): preventing the transmission of puerperal fever. Am J Public Health. 2010;100(6):1008–9.
Centers for Disease Control and Prevention. Hand hygiene in health care settings. 2018. https://www.cdc.gov/handhygiene/science/index.html. Accessed 15 Mar 2019.
Smiddy MP, O'Connell R, Creedon SA. Systematic qualitative literature review of health care workers' compliance with hand hygiene guidelines. Am J Infect Control. 2015;43(3):269–74.
Gould D. Auditing hand hygiene practice. Nurs Stand. 2010;25(2):50–6.
Lawson PA, Citron DM, Tyrrell KL, Finegold SM. Reclassification of Clostridium difficile as Clostridioides difficile (Hall and O’Toole 1935) Prévot 1938. Anaerobe. 2016;40:95–9.
Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431–55.
Centers for Disease Control and Prevention. Guidelines for hand hygiene in healthcare. 2018. https://www.cdc.gov/mmwr/PDF/rr/rr5116.pdf. Accessed 15 Mar 2019.
Ku TS, Walraven CJ, Lee SA. Candida auris: disinfectants and implications for infection control. Front Microbiol. 2018;9:1–12.
Hass J. Hand hygiene. In: Text of infection control and epidemiology. Association for Professionals in Infection Control and Epidemiology. 2014. http://text.apic.org/toc/basic-principles-of-infection-prevention-practice/hand-hygiene. Accessed 1 Mar 2019.
Hedderwick SA, McNeil SA, Lyons MJ, Kauffman CA. Pathogenic organisms associated with artificial fingernails worn by healthcare workers. Infect Control Hosp Epidemiol. 2000;21(8):505–9.
World Health Organization. https://www.who.int/gpsc/5may/tools/training_education/en/. 2019. Accessed 1 Mar 2019.
Fishbein A, Tellez I, Lin H, Sullivan C, Groll M. Glow gel hand washing in the waiting room: a novel approach to improving hand hygiene education. Infect Control Hosp Epidemiol. 2011;32(7):661–6.
Hagel S, Reischke J, Kesselmeier M, Winning J, Gastmeier P, Brunkhorst FM, et al. Quantifying the Hawthorne effect in hand hygiene compliance through comparing direct observation with automated hand hygiene monitoring. Infect Control Hosp Epidemiol. 2015;36(8):957–62.
Ellingson K, Polgreen PM, Schneider A, Kaldjian LC, Wright D, Thomas GW, et al. Healthcare personnel perceptions of hand hygiene monitoring technology. Infect Control Hosp Epidemiol. 2011;32(11):1091–6.
McGuckin M, Govednik J. A review of electronic hand hygiene monitoring:considerations for hospital management in data collection, healthcare worker supervision, and patient perception. J Health Manag. 2015;60(5):348–61.
Rutala WA, Weber DJ. Disinfection and sterilization: an overview. Am J Infect Control. 2013;41(Supp 5):S2–5.
Weber DJ, Anderson D, Rutala WA. The role of the surface environment in healthcare-associated infections. CurrOpin Infect Dis. 2013;26(4):338–44.
Shelly MJ, Scanlon TG, Ruddy R, Hannan MM, Murray JG. Methicillin-resistant Staphylococcus aureus (MRSA) environmental contamination in a radiology department. Clin Radiol. 2011;66:861–4.
Boyle H, Strudwick RM. Do lead aprons pose an infection risk? Radiography. 2010;16:297–303.
Tugwell J, Maddison A. Radiographic markers – a reservoir for bacteria? Radiography. 2010;17:115–20.
Rutala WA, White MS, Gergen MF, Weber DJ. Bacterial contamination of keyboards: efficacy and functional impact of disinfectants. Infect Control Hosp Epidemiol. 2006;27(4):372–7.
Amodio E, Dino C. Use of ATP bioluminescence for assessing the cleanliness of hospital surfaces: a review of the published literature (1990–2012). J Infect Public Health. 2014;7(2):92–8.
Alfa MJ, Olson N, Murray B. Adenosine tri-phosphate (ATP)-based cleaning monitoring in health care: how rapidly does environmental ATP deteriorate? J Hosp Infect. 2015;90(1):59–65.
Centers for Disease Control and Prevention. Options for evaluating environmental cleaning. 2018. https://www.cdc.gov/hai/toolkits/appendices-evaluating-environ-cleaning.html. Accessed Mar 1 2019.
Fiutem C. Risk factors facilitating transmission of infectious agents. In: Text of infection control and epidemiology. Association for Professionals in Infection Control and Epidemiology. 2014. http://text.apic.org/toc/microbiology-and-risk-factors-for-transmission/risk-factors-facilitat. Accessed 1 March 2019.
Lee JY. Tuberculosis infection control in health-care facilities: environmental control and personal protection. Tuberc Respir Dis. 2016;79:234–40.
Monzer AC, Thakor AS, Tulin-Silver S, Connolly BL, Cahill AM, Ward TJ, et al. Adult and pediatric antibiotic prophylaxis during vascular and IR procedures: A Society of Interventional Radiology practice parameter update endorsed by the Cardiovascular and Interventional Radiological Society of Europe and the Canadian Association for Interventional Radiology. JVIR. 2018;29(11):1483–501.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
McDaniel, C., Schwaner, S.L., Sifri, C.D. (2020). Infection Prevention in Radiology. In: Gross, K. (eds) Advanced Practice and Leadership in Radiology Nursing. Springer, Cham. https://doi.org/10.1007/978-3-030-32679-1_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-32679-1_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-32678-4
Online ISBN: 978-3-030-32679-1
eBook Packages: MedicineMedicine (R0)